α-Synuclein Fibrils as Penrose Machines: A Chameleon in the Gear
Abstract
:Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
References
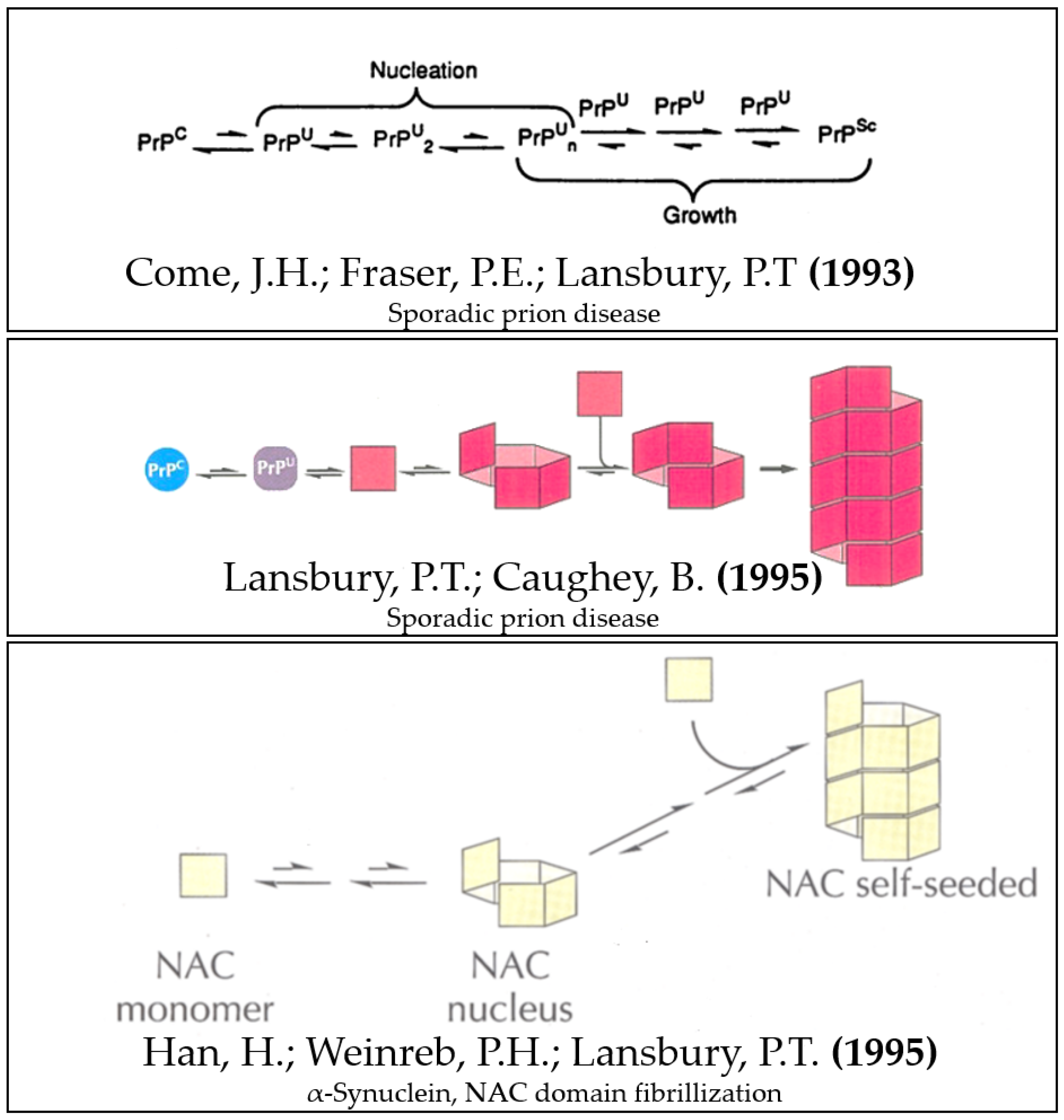
- Come, J.H.; Fraser, P.E.; Lansbury, P.T. A Kinetic Model for Amyloid Formation in the Prion Diseases: Importance of Seeding. Proc. Natl. Acad. Sci. USA 1993, 90, 5959–5963. [Google Scholar] [CrossRef] [Green Version]
- Lansbury, P.T. Mechanism of Scrapie Replication. Science 1994, 265, 1510. [Google Scholar] [CrossRef]
- Han, H.; Weinreb, P.H.; Lansbury, P.T. The Core Alzheimer’s Peptide NAC Forms Amyloid Fibrils Which Seed and Are Seeded by Beta-Amyloid: Is NAC a Common Trigger or Target in Neurodegenerative Disease? Chem. Biol. 1995, 2, 163–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lansbury, P.T.; Caughey, B. The Chemistry of Scrapie Infection: Implications of the “ice 9” Metaphor. Chem. Biol. 1995, 2, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ueda, K.; Fukushima, H.; Masliah, E.; Xia, Y.; Iwai, A.; Yoshimoto, M.; Otero, D.A.C.; Kondo, J.; Ihara, Y.; Saitoh, T. Molecular Cloning of cDNA Encoding an Unrecognized Component of Amyloid in Alzheimer Disease. Proc. Natl. Acad. Sci. USA 1993, 90, 11282–11286. [Google Scholar] [CrossRef] [Green Version]
- Alper, T.; Cramp, W.A.; Haig, D.A.; Clarke, M.C. Does the Agent of Scrapie Replicate without Nucleic Acid? Nature 1967, 214, 764–766. [Google Scholar] [CrossRef]
- Griffith, J.S. Self-Replication and Scrapie. Nature 1967, 215, 1043–1044. [Google Scholar] [CrossRef]
- Prusiner, S.B. Novel Proteinaceous Infectious Particles Cause Scrapie. Science 1982, 216, 136–144. [Google Scholar] [CrossRef] [Green Version]
- Weinreb, P.H.; Zhen, W.; Poon, A.W.; Conway, K.A.; Lansbury, P.T. NACP, a Protein Implicated in Alzheimer’s Disease and Learning, Is Natively Unfolded. Biochemistry 1996, 35, 13709–13715. [Google Scholar] [CrossRef] [PubMed]
- Pauling, L. A Theory of the Structure and Process of Formation of Antibodies*. J. Am. Chem. Soc. 1940, 62, 2643–2657. [Google Scholar] [CrossRef]
- Uversky, V.N. A Protein-Chameleon: Conformational Plasticity of Alpha-Synuclein, a Disordered Protein Involved in Neurodegenerative Disorders. J. Biomol. Struct. Dyn. 2003, 21, 211–234. [Google Scholar] [CrossRef] [PubMed]
- Bessen, R.A.; Kocisko, D.A.; Raymond, G.J.; Nandan, S.; Lansbury, P.T.; Caughey, B. Non-Genetic Propagation of Strain-Specific Properties of Scrapie Prion Protein. Nature 1995, 375, 698–700. [Google Scholar] [CrossRef] [PubMed]
- Brandner, S.; Isenmann, S.; Raeber, A.; Fischer, M.; Sailer, A.; Kobayashi, Y.; Marino, S.; Weissmann, C.; Aguzzi, A. Normal Host Prion Protein Necessary for Scrapie-Induced Neurotoxicity. Nature 1996, 379, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Meyer, R.K.; McKinley, M.P.; Bowman, K.A.; Braunfeld, M.B.; Barry, R.A.; Prusiner, S.B. Separation and Properties of Cellular and Scrapie Prion Proteins. Proc. Natl. Acad. Sci. United States Am. 1986, 83, 2310. [Google Scholar] [CrossRef] [Green Version]
- Spillantini, M.G.; Schmidt, M.L.; Lee, V.M.-Y.; Trojanowski, J.Q.; Jakes, R.; Goedert, M. Alpha-Synuclein in Lewy Bodies. Nature 1997, 388, 839–840. [Google Scholar] [CrossRef] [PubMed]
- Caughey, B.; Lansbury, P.T. Protofibrils, Pores, Fibrils, and Neurodegeneration: Separating the Responsible Protein Aggregates from the Innocent Bystanders. Annu. Rev. Neurosci. 2003, 26, 267–298. [Google Scholar] [CrossRef]
- Waugh, D.F. A Mechanism for the Formation of Fibrils from Protein Molecules. J. Cell. Physiology. Suppl. 1957, 49, 145–164. [Google Scholar] [CrossRef]
- Naiki, H.; Higuchi, K.; Nakakuki, K.; Takeda, T. Kinetic Analysis of Amyloid Fibril Polymerization in Vitro. Lab. Investig. A J. Tech. Methods Pathol. 1991, 65, 104–110. [Google Scholar]
- Zhabotinsky, A.M.; Zaikin, A.N. Autowave Processes in a Distributed Chemical System. J. Theor. Biol. 1973, 40, 45–61. [Google Scholar] [CrossRef]
- Jouaville, L.S.; Ichas, F.; Holmuhamedov, E.L.; Camacho, P.; Lechleiter, J.D. Synchronization of Calcium Waves by Mitochondrial Substrates in Xenopus Laevis Oocytes. Nature 1995, 377, 438–441. [Google Scholar] [CrossRef]
- Ichas, F.; Jouaville, L.S.; Mazat, J.P. Mitochondria Are Excitable Organelles Capable of Generating and Conveying Electrical and Calcium Signals. Cell 1997, 89, 1145–1153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lartigue, L.; Medina, C.; Schembri, L.; Chabert, P.; Zanese, M.; Tomasello, F.; Dalibart, R.; Thoraval, D.; Crouzet, M.; Ichas, F.; et al. An Intracellular Wave of Cytochrome c Propagates and Precedes Bax Redistribution during Apoptosis. J. Cell Sci. 2008, 121, 3515–3523. [Google Scholar] [CrossRef] [Green Version]
- Kushnirov, V.V.; Ter-Avanesyan, M.D. Structure and Replication of Yeast Prions. Cell 1998, 94, 13–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kushnirov, V.V.; Dergalev, A.A.; Alexandrov, A.I. Amyloid Fragmentation and Disaggregation in Yeast and Animals. Biomolecules 2021, 11, 1884. [Google Scholar] [CrossRef] [PubMed]
- Tittelmeier, J.; Sandhof, C.A.; Ries, H.M.; Druffel-Augustin, S.; Mogk, A.; Bukau, B.; Nussbaum-Krammer, C. The HSP110/HSP70 Disaggregation System Generates Spreading-competent Toxic A-synuclein Species. EMBO J. 2020, 39, e103954. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, S.O.; Uzunoğlu, G.; Nussbaum-Krammer, C. α-Synuclein Strains: Does Amyloid Conformation Explain the Heterogeneity of Synucleinopathies? Biomolecules 2021, 11, 931. [Google Scholar] [CrossRef] [PubMed]
- Knowles, T.P.; Fitzpatrick, A.W.; Meehan, S.; Mott, H.R.; Vendruscolo, M.; Dobson, C.M.; Welland, M.E. Role of Intermolecular Forces in Defining Material Properties of Protein Nanofibrils. Science 2007, 318, 1900–1903. [Google Scholar] [CrossRef]
- Xue, W.F.; Hellewell, A.L.; Gosal, W.S.; Homans, S.W.; Hewitt, E.W.; Radford, S.E. Fibril Fragmentation Enhances Amyloid Cytotoxicity. J. Biol. Chem. 2009, 284, 34272–34282. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, M.; Chien, P.; Naber, N.; Cooke, R.; Weissman, J.S. Conformational Variations in an Infectious Protein Determine Prion Strain Differences. Nature 2004, 428, 323–328. [Google Scholar] [CrossRef]
- Bousset, L.; Pieri, L.; Ruiz-Arlandis, G.; Gath, J.; Jensen, P.H.; Habenstein, B.; Madiona, K.; Olieric, V.; Böckmann, A.; Meier, B.H.; et al. Structural and Functional Characterization of Two Alpha-Synuclein Strains. Nat. Commun. 2013, 4, 1–13. [Google Scholar] [CrossRef] [Green Version]
- De Giorgi, F.; Laferrière, F.; Zinghirino, F.; Faggiani, E.; Lends, A.; Bertoni, M.; Yu, X.; Grélard, A.; Morvan, E.; Habenstein, B.; et al. Novel Self-Replicating α-Synuclein Polymorphs That Escape ThT Monitoring Can Spontaneously Emerge and Acutely Spread in Neurons. Sci. Adv. 2020, 6, eabc4364. [Google Scholar] [CrossRef]
- Penrose, L.S.; Penrose, R. A Self-Reproducing Analogue. Nature 1957, 179, 1183. [Google Scholar]
- Withrington, R.E. Research Film Show, Glasgow, August 1958. J. Photogr. Sci. 1958, 6, 185–187. [Google Scholar] [CrossRef]
- Penrose, L. Proceedings of the X International Congress of Genetics = Comptes Rendus Du Xe Congres International de Genetique: 20-27 August 1958, McGill University, Montreal, 10th ed.; University of Toronto Press: Toronto, ON, Canada, 1958; Volume 2, p. 10. [Google Scholar]
- Krinsky, V.I. Autowaves: Results, Problems, Outlooks. In Self-Organization Autowaves and Structures Far from Equilibrium; Springer Series in Synergetics; Springer: Berlin/Heidelberg, Germany, 1984; Volume 28, pp. 9–19. [Google Scholar]
- Holmuhamedov, E.L. Oscillating Dissipative Structures in Mitochondrial Suspensions. Eur. J. Biochem. 1986, 158, 543–546. [Google Scholar] [CrossRef] [PubMed]
- Holmuhamedov, E.L.; Evtodienko, Y.V. Spatial Structures in Mitochondrial Suspension Induced by Cation Efflux. FEBS Lett. 1986, 195, 339–343. [Google Scholar] [CrossRef] [Green Version]
- Braak, H.; del Tredici, K.; Rüb, U.; de Vos, R.A.I.; Jansen Steur, E.N.H.; Braak, E. Staging of Brain Pathology Related to Sporadic Parkinson’s Disease. Neurobiol. Aging 2003, 24, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Y.; Englund, E.; Holton, J.L.; Soulet, D.; Hagell, P.; Lees, A.J.; Lashley, T.; Quinn, N.P.; Rehncrona, S.; Björklund, A.; et al. Lewy Bodies in Grafted Neurons in Subjects with Parkinson’s Disease Suggest Host-to-Graft Disease Propagation. Nat. Med. 2008, 14, 501–503. [Google Scholar] [CrossRef]
- Kordower, J.H.; Chu, Y.; Hauser, R.A.; Freeman, T.B.; Olanow, C.W. Lewy Body-like Pathology in Long-Term Embryonic Nigral Transplants in Parkinson’s Disease. Nat. Med. 2008, 14, 504–506. [Google Scholar] [CrossRef]
- Luk, K.C.; Kehm, V.; Carroll, J.; Zhang, B.; O’Brien, P.; Trojanowski, J.Q.; Lee, V.M.Y. Pathological α-Synuclein Transmission Initiates Parkinson-like Neurodegeneration in Nontransgenic Mice. Science 2012, 338, 949–953. [Google Scholar] [CrossRef] [Green Version]
- Masuda-Suzukake, M.; Nonaka, T.; Hosokawa, M.; Oikawa, T.; Arai, T.; Akiyama, H.; Mann, D.M.A.; Hasegawa, M. Prion-like Spreading of Pathological α-Synuclein in Brain. Brain 2013, 136, 1128–1138. [Google Scholar] [CrossRef] [PubMed]
- Uversky, V.N.; Li, J.; Fink, A.L. Evidence for a Partially Folded Intermediate in α-Synuclein Fibril Formation. J. Biol. Chem. 2001, 276, 10737–10744. [Google Scholar] [CrossRef] [Green Version]
- Uversky, V.N.; Li, J.; Fink, A.L. Pesticides Directly Accelerate the Rate of Alpha-Synuclein Fibril Formation: A Possible Factor in Parkinson’s Disease. FEBS Lett. 2001, 500, 105–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manning-Bog, A.B.; McCormack, A.L.; Li, J.; Uversky, V.N.; Fink, A.L.; di Monte, D.A. The Herbicide Paraquat Causes Up-Regulation and Aggregation of Alpha-Synuclein in Mice: Paraquat and Alpha-Synuclein. J. Biol. Chem. 2002, 277, 1641–1644. [Google Scholar] [CrossRef] [Green Version]
- Uversky, V.N.; Li, J.; Fink, A.L. Metal-Triggered Structural Transformations, Aggregation, and Fibrillation of Human Alpha-Synuclein. A Possible Molecular NK between Parkinson’s Disease and Heavy Metal Exposure. J. Biol. Chem. 2001, 276, 44284–44296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uversky, V.N.; Li, J.; Bower, K.; Fink, A.L. Synergistic Effects of Pesticides and Metals on the Fibrillation of Alpha-Synuclein: Implications for Parkinson’s Disease. Neurotoxicology 2002, 23, 527–536. [Google Scholar] [CrossRef]
- Li, J.; Uversky, V.N.; Fink, A.L. Effect of Familial Parkinson’s Disease Point Mutations A30P and A53T on the Structural Properties, Aggregation, and Fibrillation of Human Alpha-Synuclein. Biochemistry 2001, 40, 11604–11613. [Google Scholar] [CrossRef]
- Li, J.; Uversky, V.N.; Fink, A.L. Conformational Behavior of Human Alpha-Synuclein Is Modulated by Familial Parkinson’s Disease Point Mutations A30P and A53T. Neurotoxicology 2002, 23, 553–567. [Google Scholar] [CrossRef] [PubMed]
- Uversky, V.N.; Li, J.; Fink, A.L. Trimethylamine-N-Oxide-Induced Folding of Alpha-Synuclein. FEBS Lett. 2001, 509, 31–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munishkina, L.A.; Phelan, C.; Uversky, V.N.; Fink, A.L. Conformational Behavior and Aggregation of Alpha-Synuclein in Organic Solvents: Modeling the Effects of Membranes. Biochemistry 2003, 42, 2720–2730. [Google Scholar] [CrossRef]
- Uversky, V.N. Mysterious Oligomerization of the Amyloidogenic Proteins. FEBS J. 2010, 277, 2940–2953. [Google Scholar] [CrossRef]
- Uversky, V.N.; Lee, H.J.; Li, J.; Fink, A.L.; Lee, S.J. Stabilization of Partially Folded Conformation during Alpha-Synuclein Oligomerization in Both Purified and Cytosolic Preparations. J. Biol. Chem. 2001, 276, 43495–43498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohlberg, J.A.; Li, J.; Uversky, V.N.; Fink, A.L. Heparin and Other Glycosaminoglycans Stimulate the Formation of Amyloid Fibrils from Alpha-Synuclein in Vitro. Biochemistry 2002, 41, 1502–1511. [Google Scholar] [CrossRef]
- Goers, J.; Uversky, V.N.; Fink, V.L. Polycation-Induced Oligomerization and Accelerated Fibrillation of Human Alpha-Synuclein in Vitro. Protein Sci. A Publ. Protein Soc. 2003, 12, 702–707. [Google Scholar] [CrossRef]
- Goers, J.; Manning-Bog, A.B.; McCormack, A.L.; Millett, I.S.; Doniach, S.; di Monte, D.A.; Uversky, V.N.; Fink, A.L. Nuclear Localization of Alpha-Synuclein and Its Interaction with Histones. Biochemistry 2003, 42, 8465–8471. [Google Scholar] [CrossRef] [PubMed]
- Uversky, V.N.; Cooper, E.M.; Bower, K.S.; Li, J.; Fink, A.L. Accelerated Alpha-Synuclein Fibrillation in Crowded Milieu. FEBS Lett. 2002, 515, 99–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uversky, V.N.; Li, J.; Souillac, P.; Millett, I.S.; Doniach, S.; Jakes, R.; Goedert, M.; Fink, A.L. Biophysical Properties of the Synucleins and Their Propensities to Fibrillate: Inhibition of Alpha-Synuclein Assembly by Beta- and Gamma-Synucleins. J. Biol. Chem. 2002, 277, 11970–11978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uversky, V.N.; Yamin, G.; Souillac, P.O.; Goers, J.; Glaser, C.B.; Fink, A.L. Methionine Oxidation Inhibits Fibrillation of Human Alpha-Synuclein in Vitro. FEBS Lett. 2002, 517, 239–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamin, G.; Uversky, V.N.; Fink, A.L. Nitration Inhibits Fibrillation of Human Alpha-Synuclein in Vitro by Formation of Soluble Oligomers. FEBS Lett. 2003, 542, 147–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamin, G.; Glaser, C.B.; Uversky, V.N.; Fink, A.L. Certain Metals Trigger Fibrillation of Methionine-Oxidized Alpha-Synuclein. J. Biol. Chem. 2003, 278, 27630–27635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uversky, V.N. Looking at the Recent Advances in Understanding α-Synuclein and Its Aggregation through the Proteoform Prism. F1000Research 2017, 6, 525. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hall, C.K. Seeding and Cross-Seeding Fibrillation of N-Terminal Prion Protein Peptides PrP(120–144). Protein Sci. 2018, 27, 1304–1313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peduzzo, A.; Linse, S.; Buell, A.K. The Properties of α-Synuclein Secondary Nuclei Are Dominated by the Solution Conditions Rather than the Seed Fibril Strain. ACS Chem. Neurosci. 2020, 11, 909–918. [Google Scholar] [CrossRef] [PubMed]
- Bhak, G.; Lee, J.; Kim, T.H.; Lee, S.; Lee, D.; Paik, S.R. Molecular Inscription of Environmental Information into Protein Suprastructures: Temperature Effects on Unit Assembly of α-Synuclein Oligomers into Polymorphic Amyloid Fibrils. Biochem. J. 2014, 464, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Gathagan, R.J.; Covell, D.J.; Medellin, C.; Stieber, A.; Robinson, J.L.; Zhang, B.; Pitkin, R.M.; Olufemi, M.F.; Luk, K.C.; et al. Cellular Milieu Imparts Distinct Pathological α-Synuclein Strains in α-Synucleinopathies. Nature 2018, 557, 558–563. [Google Scholar] [CrossRef]
- Martinez-Valbuena, I.; Visanji, N.P.; Kim, A.; Lau, H.H.C.; So, R.W.L.; Alshimemeri, S.; Gao, A.; Seidman, M.A.; Luquin, M.R.; Watts, J.C.; et al. Alpha-Synuclein Seeding Shows a Wide Heterogeneity in Multiple System Atrophy. Transl. Neurodegener. 2022, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Maury, C.P.J. Origin of Life. Primordial Genetics: Information Transfer in a Pre-RNA World Based on Self-Replicating Beta-Sheet Amyloid Conformers. J. Theor. Biol. 2015, 382, 292–297. [Google Scholar] [CrossRef] [Green Version]
- Maury, C.P.J. Amyloid and the Origin of Life: Self-Replicating Catalytic Amyloids as Prebiotic Informational and Protometabolic Entities. Cell. Mol. Life Sci. 2018, 75, 1499–1507. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Giorgi, F.; Uversky, V.N.; Ichas, F. α-Synuclein Fibrils as Penrose Machines: A Chameleon in the Gear. Biomolecules 2022, 12, 494. https://doi.org/10.3390/biom12040494
De Giorgi F, Uversky VN, Ichas F. α-Synuclein Fibrils as Penrose Machines: A Chameleon in the Gear. Biomolecules. 2022; 12(4):494. https://doi.org/10.3390/biom12040494
Chicago/Turabian StyleDe Giorgi, Francesca, Vladimir N. Uversky, and François Ichas. 2022. "α-Synuclein Fibrils as Penrose Machines: A Chameleon in the Gear" Biomolecules 12, no. 4: 494. https://doi.org/10.3390/biom12040494






