Bacterial Glycocalyx Integrity Impacts Tolerance of Myxococcus xanthus to Antibiotics and Oxidative-Stress Agents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Cell Culture
2.2. Disc-Diffusion Assay
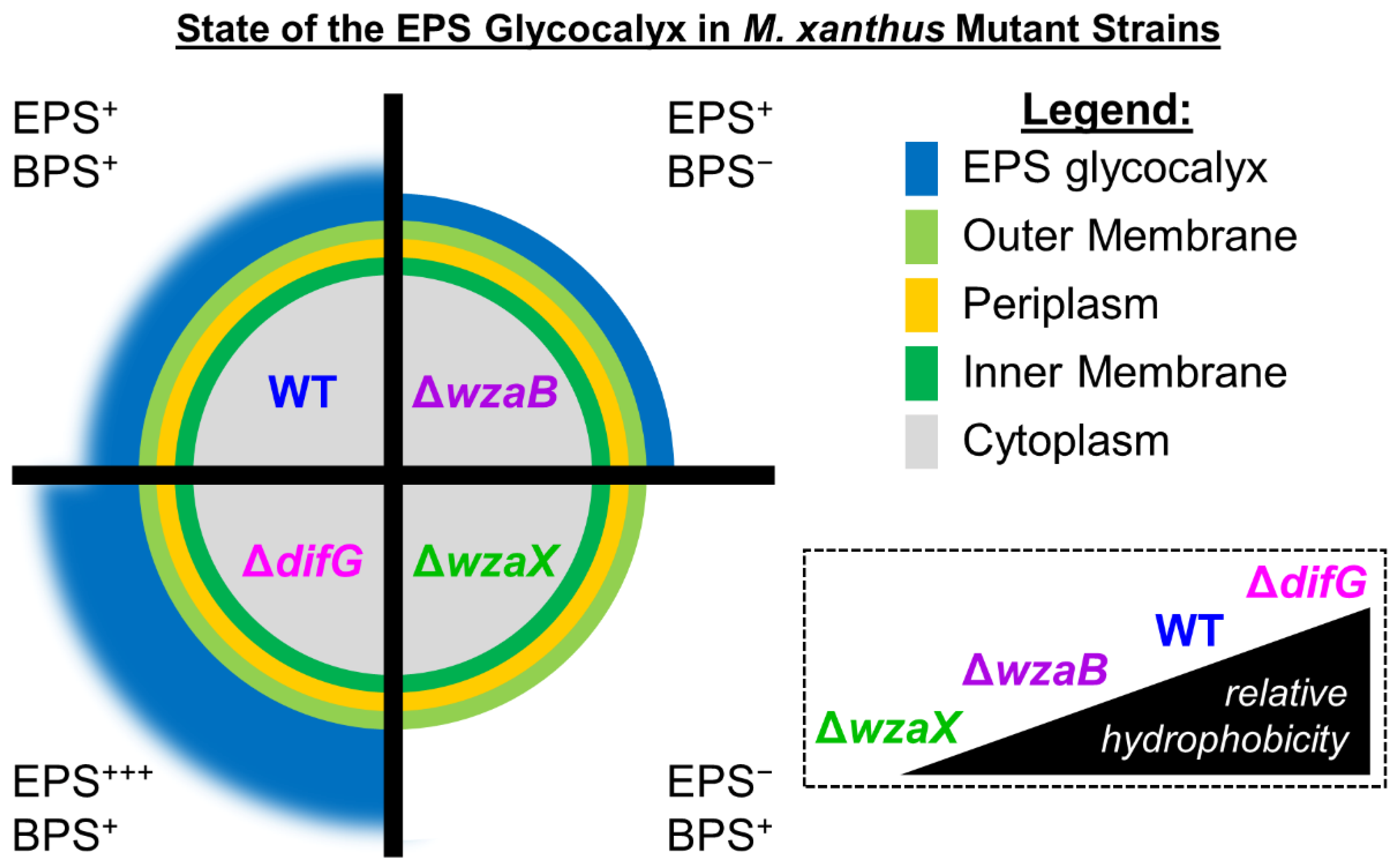
3. Results
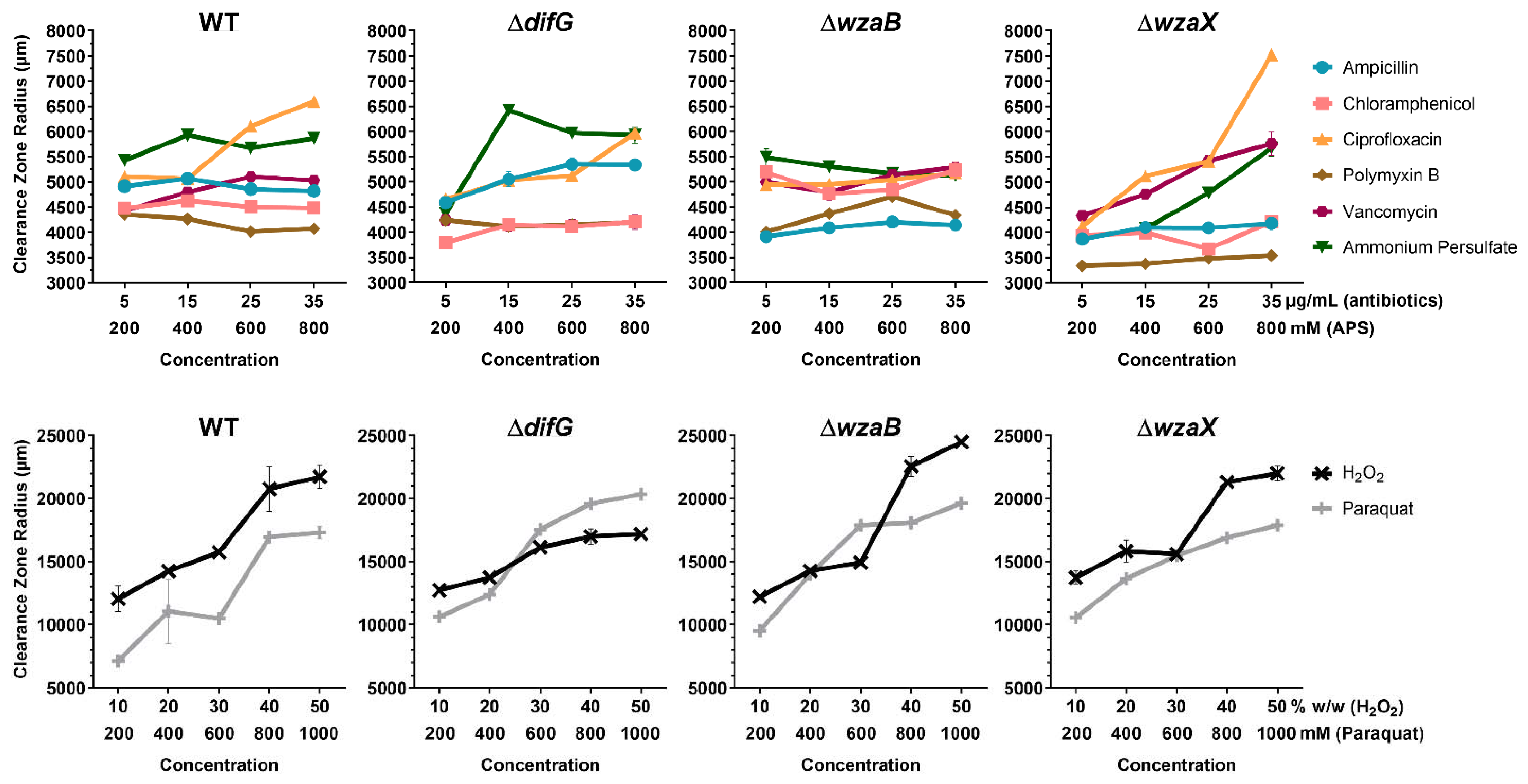
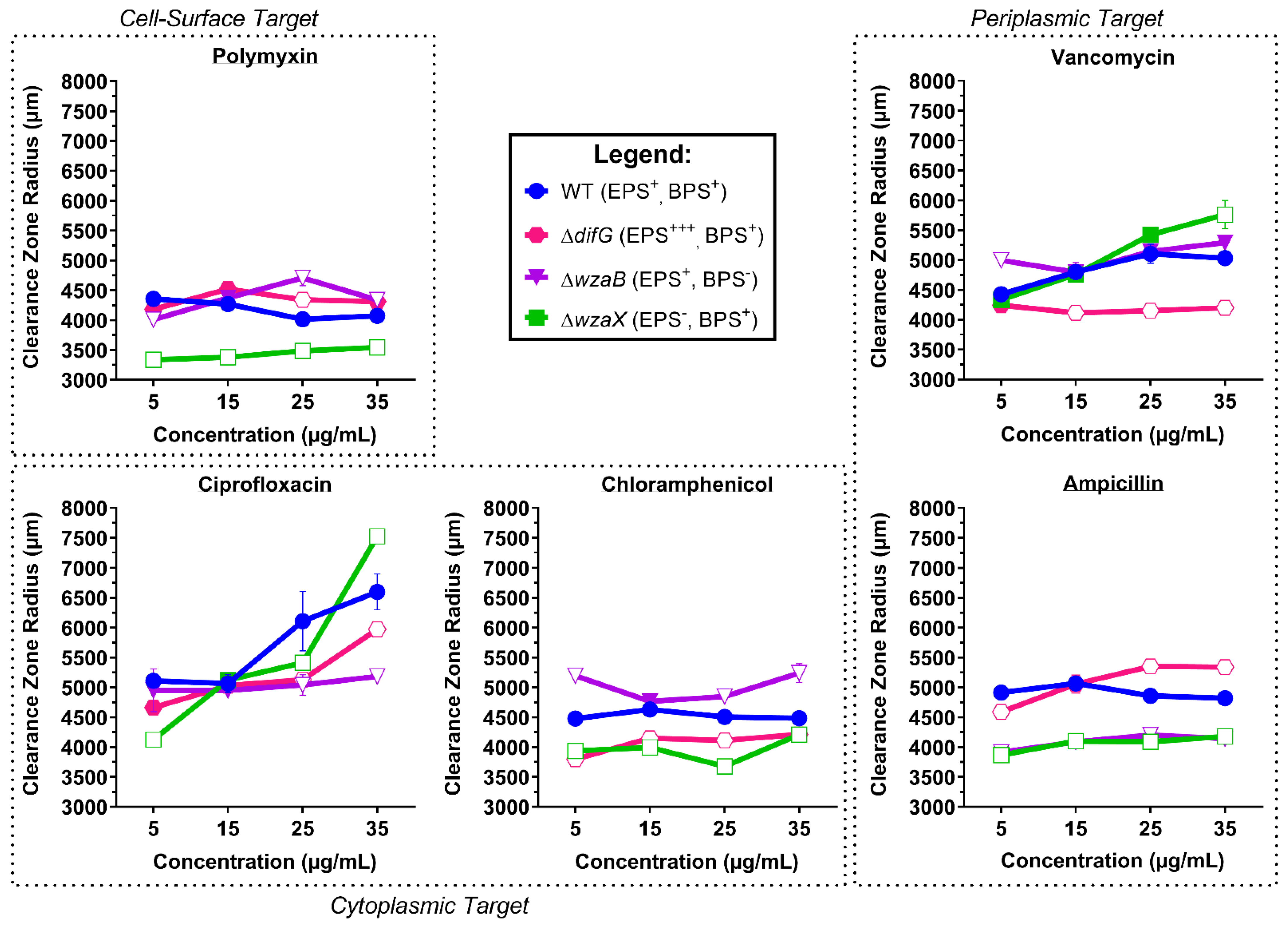
3.1. Tolerance to Antibiotics
3.1.1. Tolerance of Cells That Produce a Thicker EPS Glycocalyx
3.1.2. Tolerance of Cells That Produce a Non-Disrupted EPS Glycocalyx
3.1.3. Tolerance of Cells That Do Not Produce an EPS Glycocalyx
3.2. Tolerance to Reactive Oxygen Species
3.2.1. Tolerance of Cells That Produce a Thicker EPS Glycocalyx
3.2.2. Tolerance of Cells That Produce a Non-Disrupted EPS Glycocalyx
3.2.3. Tolerance of Cells That Do Not Produce an EPS Glycocalyx
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
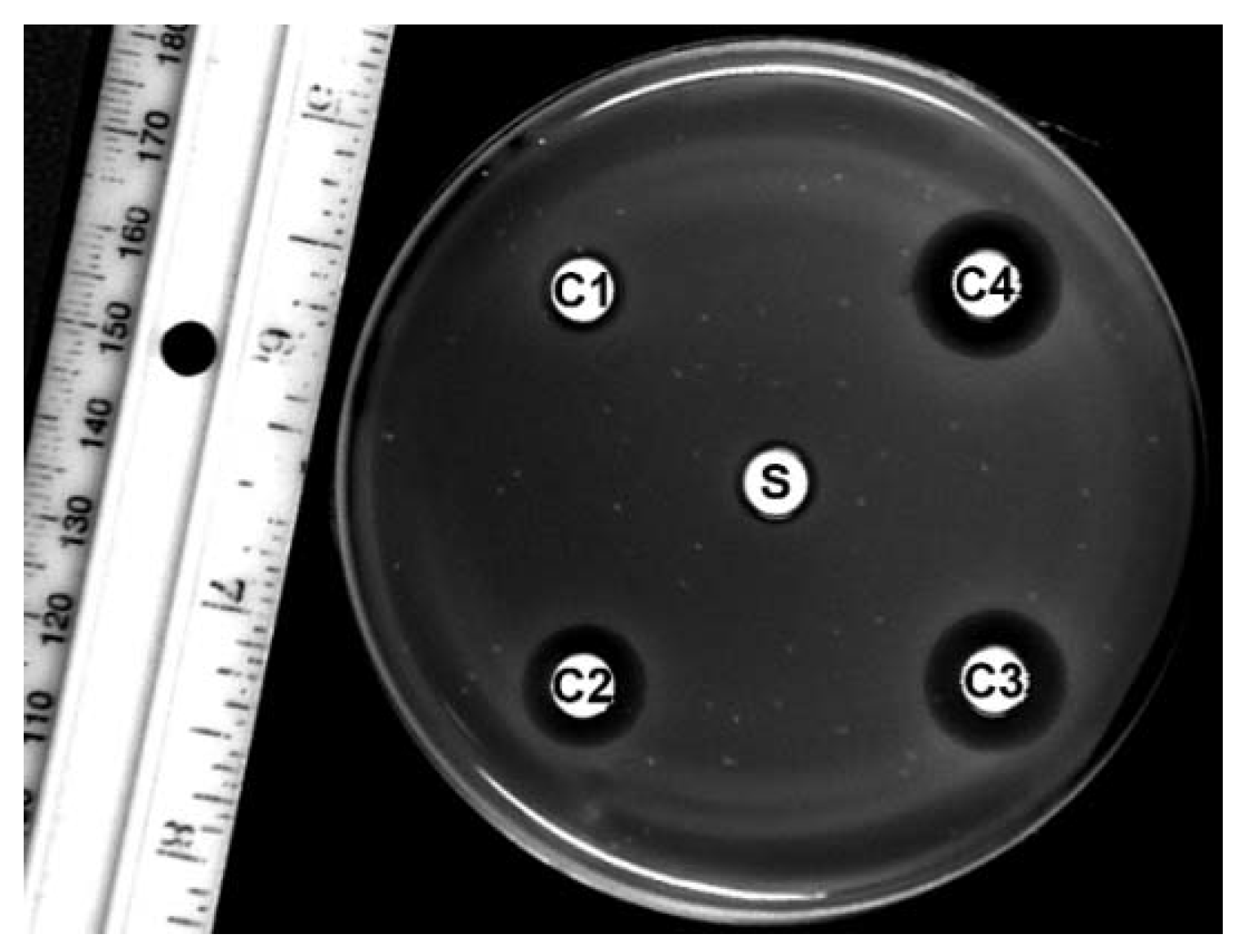
Appendix B
References
- Costerton, J.W.; Geesey, G.; Cheng, K.J. How bacteria stick. Sci. Am. 1978, 238, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Olsen, I. Biofilm-specific antibiotic tolerance and resistance. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 877–886. [Google Scholar] [CrossRef]
- Muñoz-Dorado, J.; Marcos-Torres, F.J.M.; García-Bravo, E.; Moraleda-Munoz, A.; Pérez, J. Myxobacteria: Moving, Killing, Feeding, and Surviving Together. Front. Microbiol. 2016, 7, 781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seef, S.; Herrou, J.; de Boissier, P.; My, L.; Brasseur, G.; Robert, D.; Jain, R.; Mercier, R.; Cascales, E.; Habermann, B.H.; et al. A Tad-like apparatus is required for contact-dependent prey killing in predatory social bacteria. eLife 2021, 10, e72409. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.T.; Mignot, T. The mysterious nature of bacterial surface (gliding) motility: A focal adhesion-based mechanism in Myxococcus xanthus. Semin. Cell. Dev. Biol. 2015, 46, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Faure, L.M.; Fiche, J.-B.; Espinosa, L.; Ducret, A.; Anantharaman, V.; Luciano, J.; Lhospice, S.; Islam, S.T.; Tréguier, J.; Sotes, M.; et al. The mechanism of force transmission at bacterial focal adhesion complexes. Nature 2016, 539, 530–535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islam, S.T.; Alvarez, I.V.; Saïdi, F.; Guiseppi, A.; Vinogradov, E.; Sharma, G.; Espinosa, L.; Morrone, C.; Brasseur, G.; Guillemot, J.-F.; et al. Modulation of bacterial multicellularity via spatio-specific polysaccharide secretion. PLoS Biol. 2020, 18, e3000728. [Google Scholar] [CrossRef]
- Saïdi, F.; Jolivet, N.Y.; Lemon, D.J.; Nakamura, A.; Belgrave, A.M.; Garza, A.G.; Veyrier, F.J.; Islam, S.T. Bacterial glycocalyx integrity drives multicellular swarm biofilm dynamism. Mol. Microbiol. 2021, 116, 1151–1172. [Google Scholar] [CrossRef]
- Pérez-Burgos, M.; Søgaard-Andersen, L. Biosynthesis and function of cell-surface polysaccharides in the social bacterium Myxococcus xanthus. Biol. Chem. 2020, 401, 1375–1387. [Google Scholar] [CrossRef]
- Hu, W.; Lux, R.; Shi, W. Analysis of Exopolysaccharides in Myxococcus xanthus Using Confocal Laser Scanning Microscopy. Bact. Cell Surf. 2012, 966, 121–131. [Google Scholar] [CrossRef]
- Smaldone, G.T.; Jin, Y.; Whitfield, D.L.; Mu, A.Y.; Wong, E.C.; Wuertz, S.; Singer, M. Growth of Myxococcus xanthus in continuous-flow-cell bioreactors as a method for studying development. Appl. Environ. Microbiol. 2014, 80, 2461–2467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Burgos, M.; García-Romero, I.; Jung, J.; Schander, E.; Valvano, M.A.; Søgaard-Andersen, L. Characterization of the exopolysaccharide biosynthesis pathway in Myxococcus xanthus. J. Bacteriol. 2020, 202, e00335-20. [Google Scholar] [CrossRef] [PubMed]
- Holkenbrink, C.; Hoiczyk, E.; Kahnt, J.; Higgs, P.I. Synthesis and assembly of a novel glycan layer in Myxococcus xanthus spores. J. Biol. Chem. 2014, 289, 32364–32378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islam, S.T.; Fieldhouse, R.J.; Anderson, E.M.; Taylor, V.L.; Keates, R.A.B.; Ford, R.C.; Lam, J.S. A cationic lumen in the Wzx flippase mediates anionic O-antigen subunit translocation in Pseudomonas aeruginosa PAO1. Mol. Microbiol. 2012, 84, 1165–1176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islam, S.T.; Eckford, P.D.W.; Jones, M.L.; Nugent, T.; Bear, C.E.; Vogel, C.; Lam, J.S. Proton-dependent gating and proton uptake by Wzx support O-antigen-subunit antiport across the bacterial inner membrane. mBio. 2013, 4, e00678-13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islam, S.T.; Taylor, V.L.; Qi, M.; Lam, J.S. Membrane topology mapping of the O-antigen flippase (Wzx), polymerase (Wzy), and ligase (WaaL) from Pseudomonas aeruginosa PAO1 reveals novel domain architectures. mBio. 2010, 1, e00189-10. [Google Scholar] [CrossRef] [Green Version]
- Islam, S.T.; Gold, A.C.; Taylor, V.L.; Anderson, E.M.; Ford, R.C.; Lam, J.S. Dual conserved periplasmic loops possess essential charge characteristics that support a catch-and-release mechanism of O-antigen polymerization by Wzy in Pseudomonas aeruginosa PAO1. J. Biol. Chem. 2011, 286, 20600–20605. [Google Scholar] [CrossRef] [Green Version]
- Islam, S.T.; Huszczynski, S.M.; Nugent, T.; Gold, A.C.; Lam, J.S. Conserved-residue mutations in Wzy affect O-antigen polymerization and Wzz-mediated chain-length regulation in Pseudomonas aeruginosa PAO1. Sci. Rep. 2013, 3, 3441. [Google Scholar] [CrossRef] [Green Version]
- Islam, S.T.; Lam, J.S. Synthesis of bacterial polysaccharides via the Wzx/Wzy-dependent pathway. Can. J. Microbiol. 2014, 60, 697–716. [Google Scholar] [CrossRef] [Green Version]
- Whitfield, C.; Wear, S.S.; Sande, C. Assembly of bacterial capsular polysaccharides and exopolysaccharides. Annu. Rev. Microbiol. 2020, 74, 521–543. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, J.; Clarke, B.R.; Seidel, L.; Bolla, J.R.; Ward, P.N.; Zhang, P.; Robinson, C.V.; Whitfield, C.; Naismith, J.H. The molecular basis of regulation of bacterial capsule assembly by Wzc. Nat. Commun. 2021, 12, 4349. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Beis, K.; Nesper, J.; Brunkan-LaMontagne, A.L.; Clarke, B.R.; Whitfield, C.; Naismith, J.H. Wza the translocon for E. coli capsular polysaccharides defines a new class of membrane protein. Nature 2006, 444, 226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nickerson, N.N.; Mainprize, I.L.; Hampton, L.; Jones, M.L.; Naismith, J.H.; Whitfield, C. Trapped translocation intermediates establish the route for export of capsular polysaccharides across Escherichia coli outer membranes. Proc. Natl. Acad. Sci. USA 2014, 111, 8203–8208. [Google Scholar] [CrossRef] [Green Version]
- Saïdi, F.; Mahanta, U.; Panda, A.; Jolivet, N.Y.; Bitazar, R.; John, G.; Martinez, M.; Mellouk, A.; Calmettes, C.; Chang, Y.-W.; et al. Bacterial outer-membrane polysaccharide export (OPX) proteins occupy three structural classes with selective β-barrel porin requirements for polymer secretion. bioaRxiv 2022. [Google Scholar] [CrossRef]
- Mori, Y.; Maeda, M.; Takegawa, K.; Kimura, Y. PhpA, a tyrosine phosphatase of Myxococcus xanthus, is involved in the production of exopolysaccharide. Microbiology 2012, 158, 2546–2555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, R.; Habermann, B.H.; Mignot, T. Complete genome assembly of Myxococcus xanthus strain DZ2 using long high-fidelity (hifi) reads generated with PacBio technology. Microbiol. Resour. Announc. 2021, 10, e00530-21. [Google Scholar] [CrossRef]
- Li, Y.; Sun, H.; Ma, X.; Lu, A.; Lux, R.; Zusman, D.; Shi, W. Extracellular polysaccharides mediate pilus retraction during social motility of Myxococcus xanthus. Proc. Natl. Acad. Sci. USA 2003, 100, 5443–5448. [Google Scholar] [CrossRef] [Green Version]
- Campos, J.M.; Zusman, D.R. Regulation of development in Myxococcus xanthus: Effect of 3’:5’-cyclic AMP, ADP, and nutrition. Proc. Natl. Acad. Sci. USA 1975, 72, 518–522. [Google Scholar] [CrossRef] [Green Version]
- Ducret, A.; Valignat, M.-P.; Mouhamar, F.; Mignot, T.; Theodoly, O. Wet-surface–enhanced ellipsometric contrast microscopy identifies slime as a major adhesion factor during bacterial surface motility. Proc. Natl. Acad. Sci. USA 2012, 109, 10036–10041. [Google Scholar] [CrossRef] [Green Version]
- Kapoor, G.; Saigal, S.; Elongavan, A. Action and resistance mechanisms of antibiotics: A guide for clinicians. J. Anaesthesiol. Clin. Pharmacol. 2017, 33, 300–305. [Google Scholar] [CrossRef]
- Linley, E.; Denyer, S.P.; McDonnell, G.; Simons, C.; Maillard, J.-Y. Use of hydrogen peroxide as a biocide: New consideration of its mechanisms of biocidal action. J. Antimicrob. Chemother. 2012, 67, 1589–1596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vecbiskena, L.; Rozenberga, L. Nanocelluloses obtained by ammonium persulfate (APS) oxidation of bleached kraft pulp (BKP) and bacterial cellulose (BC) and their application in biocomposite films together with chitosan. Holzforschung 2017, 71, 659–666. [Google Scholar] [CrossRef]
- Khademian, M.; Imlay, J.A. How microbes evolved to tolerate oxygen. Trends Microbiol. 2021, 29, 428–440. [Google Scholar] [CrossRef] [PubMed]
- Imlay, J.A. Transcription factors that defend bacteria against reactive oxygen species. Annu. Rev. Microbiol. 2015, 69, 93–108. [Google Scholar] [CrossRef] [Green Version]
- D’Haeze, W.; Glushka, J.; De Rycke, R.; Holsters, M.; Carlson, R.W. Structural characterization of extracellular polysaccharides of Azorhizobium caulinodans and importance for nodule initiation on Sesbania rostrata. Mol. Microbiol. 2004, 52, 485–500. [Google Scholar] [CrossRef]
- Cycoń, M.; Mrozik, A.; Piotrowska-Seget, Z. Antibiotics in the soil environment—degradation and their impact on microbial activity and diversity. Front. Microbiol. 2019, 10, 338. [Google Scholar] [CrossRef]
- Yu, G.-H.; Kuzyakov, Y. Fenton chemistry and reactive oxygen species in soil: Abiotic mechanisms of biotic processes, controls and consequences for carbon and nutrient cycling. Earth Sci. Rev. 2021, 214, 103525. [Google Scholar] [CrossRef]
- Mueller, U.G.; Sachs, J.L. Engineering Microbiomes to Improve Plant and Animal Health. Trends Microbiol. 2015, 23, 606–617. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saïdi, F.; Bitazar, R.; Bradette, N.Y.; Islam, S.T. Bacterial Glycocalyx Integrity Impacts Tolerance of Myxococcus xanthus to Antibiotics and Oxidative-Stress Agents. Biomolecules 2022, 12, 571. https://doi.org/10.3390/biom12040571
Saïdi F, Bitazar R, Bradette NY, Islam ST. Bacterial Glycocalyx Integrity Impacts Tolerance of Myxococcus xanthus to Antibiotics and Oxidative-Stress Agents. Biomolecules. 2022; 12(4):571. https://doi.org/10.3390/biom12040571
Chicago/Turabian StyleSaïdi, Fares, Razieh Bitazar, Nicholas Y. Bradette, and Salim T. Islam. 2022. "Bacterial Glycocalyx Integrity Impacts Tolerance of Myxococcus xanthus to Antibiotics and Oxidative-Stress Agents" Biomolecules 12, no. 4: 571. https://doi.org/10.3390/biom12040571
APA StyleSaïdi, F., Bitazar, R., Bradette, N. Y., & Islam, S. T. (2022). Bacterial Glycocalyx Integrity Impacts Tolerance of Myxococcus xanthus to Antibiotics and Oxidative-Stress Agents. Biomolecules, 12(4), 571. https://doi.org/10.3390/biom12040571







