The Functional Interplay between Ethylene, Hydrogen Sulfide, and Sulfur in Plant Heat Stress Tolerance
Abstract
:1. Introduction
1.1. Heat Stress: Impact and Consequences
1.2. Physiological and Molecular Responses to Heat Stress
1.3. Regulation of Heat Stress by Phytohormones
1.4. Sulfur-Containing Compounds in Heat Stress Tolerance
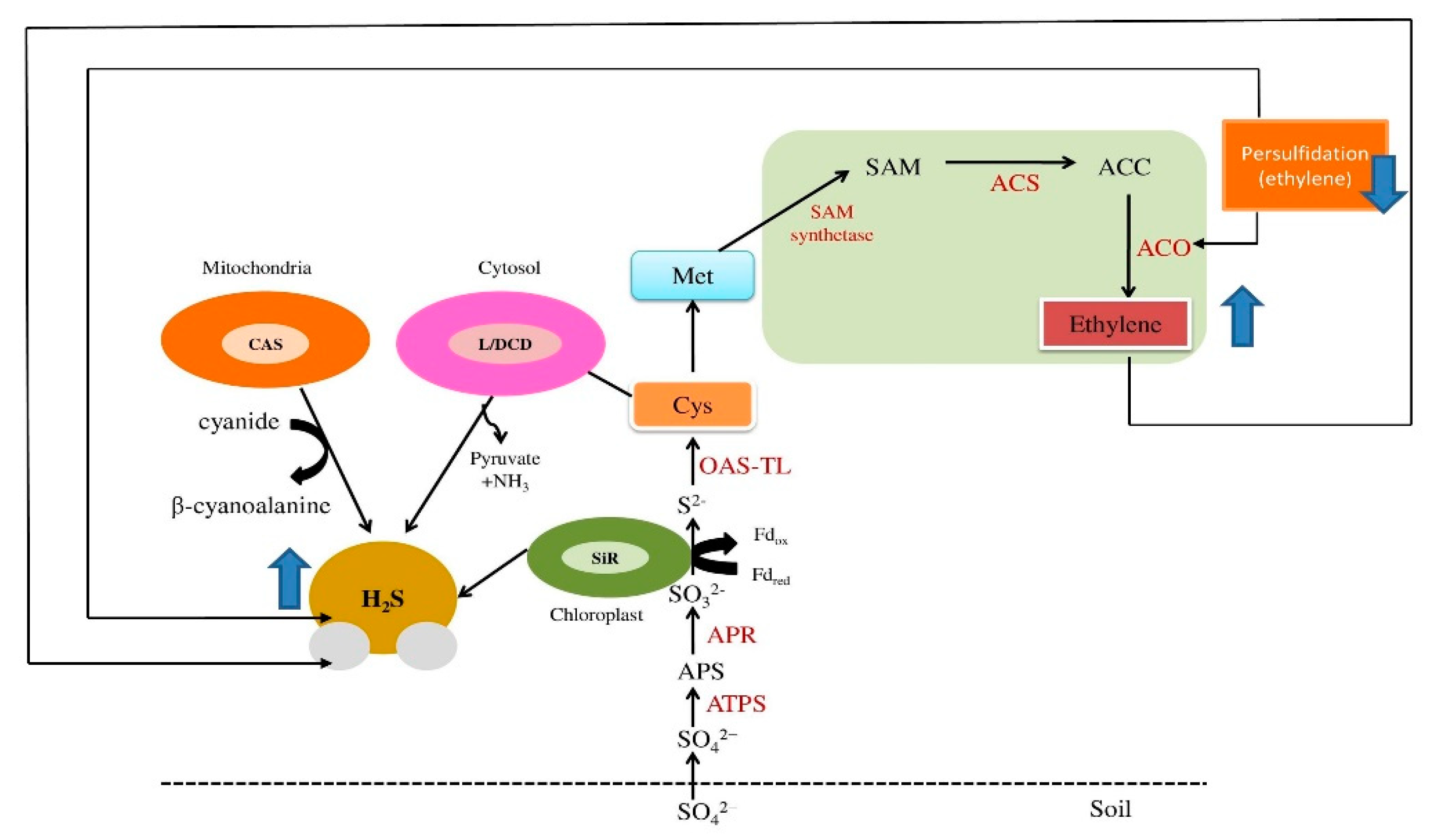
1.5. Sulfur and Hydrogen Sulfide in Heat Stress Tolerance and Their Interrelationship with Ethylene
2. Ethylene and H2S Synthesis: Involvement of the Sulfur Assimilation Pathway
3. The Crucial Roles of Ethylene, H2S, and S in Heat Stress Tolerance
3.1. Potential Role of Ethylene in Heat Stress Tolerance
3.2. Role of H2S in Heat Stress Tolerance
3.3. Potential Role of Sulfur/S Compounds in Heat Stress Tolerance
4. Post-Translational Modification of Ethylene- and H2S-Associated Proteins under Heat Stress
4.1. Ethylene and Related Post-Translational Modifications
4.2. H2S and Related Post-Translational Modifications
5. Crosstalk between Ethylene and H2S for Heat Stress Tolerance through the Involvement of Sulfur
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tardieu, F.; Tuberosa, R. Dissection and modelling of abiotic stress tolerance in plants. Curr. Opin. Plant Biol. 2010, 13, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Saud, S.H.A.H.; Chen, Y.; Long, B.; Fahad, S.H.A.H.; Sadiq, A.R.O.O.J. The different impact on the growth of cool season turf grass under the various conditions on salinity and draught stress. Int. J. Agric. Sci. Res. 2013, 3, 77–84. [Google Scholar]
- Battisti, D.S.; Naylor, R.L. Historical warnings of future food insecurity with unprecedented seasonal heat. Science 2009, 323, 240–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- IPCC. The Climate Change 2021: The Physical Science Basis. In Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: New York, NY, USA, 2021. [Google Scholar]
- Tito, R.; Vasconcelos, H.L.; Feeley, K.J. Global climate change increases risk of crop yield losses and food insecurity in the tropical Andes. Glob. Change Biol. 2014, 24, 592–602. [Google Scholar] [CrossRef]
- Aryal, J.P.; Sapkota, T.B.; Khurana, R.; Khatri-Chhetri, A.; Rahut, D.B.; Jat, M.L. Climate change and agriculture in South Asia: Adaptation options in smallholder production systems. Environ. Dev. Sustain. 2020, 22, 5045–5075. [Google Scholar] [CrossRef] [Green Version]
- Parry, M.L.; Rosenzweig, C.; Iglesias, A.; Livermore, M.; Fischer, G. Effect of climate change on global food production under SRES emissions and socio-economic scenarios. Glob. Environ. Chang. 2004, 14, 53–67. [Google Scholar] [CrossRef]
- Ortiz, R.; Sayre, K.D.; Govaerts, B.; Gupta, R.; Subbarao, G.V.; Ban, T.; Reynolds, M. Climate change: Can wheat beat the heat? Agricul. Ecosyst. Environ. 2008, 126, 46–58. [Google Scholar] [CrossRef]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Asthir, B. Protective mechanisms of heat tolerance in crop plants. J. Plant Inter. 2015, 10, 202–210. [Google Scholar] [CrossRef]
- Hemmati, H.; Gupta, D.; Basu, C. Molecular physiology of heat stress responses in plants. In Elucidation of Abiotic Stress Signaling in Plants; Springer: New York, NY, USA, 2015; pp. 109–142. [Google Scholar]
- Li, L.; Wu, J.; Luo, M.; Sun, Y.; Wang, G. The effect of heat stress on gene expression, synthesis of steroids, and apoptosis in bovine granulosa cells. Cell Stress Chaperones 2016, 21, 467–475. [Google Scholar] [CrossRef] [Green Version]
- Fatma, M.; Iqbal, N.; Sehar, Z.; Alyemeni, M.N.; Kaushik, P.; Khan, N.A.; Ahmad, P. Methyl jasmonate protects the ps ii system by maintaining the stability of chloroplast d1 protein and accelerating enzymatic antioxidants in heat-stressed wheat plants. Antioxidants 2021, 10, 1216. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, N.; Sehar, Z.; Fatma, M.; Umar, S.; Sofo, A.; Khan, N.A. Nitric Oxide and Abscisic Acid Mediate Heat Stress Tolerance through Regulation of Osmolytes and Antioxidants to Protect Photosynthesis and Growth in Wheat Plants. Antioxidants 2022, 11, 372. [Google Scholar] [CrossRef] [PubMed]
- Howarth, C.J. Genetic improvements of tolerance to high temperature. In Abiotic Stresses–Plant Resistance through Breeding and Molecular Approaches; Ashraf, M., Harris, P.J.C., Eds.; Haworth Press Inc.: New York, NY, USA, 2005; pp. 277–300. [Google Scholar]
- Schöffl, F.; Prandl, R.; Reindl, A. Molecular responses to heat stress. In Molecular Responses to Cold, Drought, Heat and Salt Stress in Higher Plants; Shinozaki, K., Yamaguchi-Shinozaki, K., Eds.; R.G. Landes Co.: Austin, TX, USA, 1999; pp. 81–98. [Google Scholar]
- Bhattacharya, A. Effect of High Temperature on Crop Productivity and Metabolism of Macro Molecules, 1st ed.; Academic Press: New York, NY, USA, 2019; pp. 1–628. [Google Scholar]
- Barnabás, B.; Jäger, K.; Fehér, A. The effect of drought and heat stress on reproductive processes in cereals. Plant Cell Environ. 2008, 31, 11–38. [Google Scholar] [CrossRef] [PubMed]
- Wise, R.R.; Olson, A.J.; Schrader, S.M.; Sharkey, T.D. Electron transport is the functional limitation of photosynthesis in field-grown Pima cotton plants at high temperature. Plant Cell Environ. 2004, 27, 717–724. [Google Scholar] [CrossRef]
- Gautam, H.; Sehar, Z.; Rehman, M.T.; Hussain, A.; AlAjmi, M.F.; Khan, N.A. Nitric oxide enhances photosynthetic nitrogen and sulfur-use efficiency and activity of ascorbate-glutathione cycle to reduce high temperature stress-induced oxidative stress in rice (Oryza sativa L.) plants. Biomolecules 2021, 11, 305. [Google Scholar] [CrossRef]
- Morales, D.; Rodríguez, P.; Dell’Amico, J.; Nicolas, E.; Torrecillas, A.; Sánchez-Blanco, M.J. High-temperature preconditioning and thermal shock imposition affects water relations, gas exchange and root hydraulic conductivity in tomato. Biol. Plantar. 2019, 47, 203–208. [Google Scholar] [CrossRef]
- Ruan, Y.L.; Jin, Y.; Yang, Y.J.; Li, G.J.; Boyer, J.S. Sugar input, metabolism, and signaling mediated by invertase: Roles in development, yield potential, and response to drought and heat. Mol. Plant 2010, 3, 942–955. [Google Scholar] [CrossRef]
- Camejo, D.; Jiménez, A.; Alarcón, J.J.; Torres, W.; Gómez, J.M.; Sevilla, F. Changes in photosynthetic parameters and antioxidant activities following heat-shock treatment in tomato plants. Funct. Plant Biol. 2006, 33, 177–187. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Iqbal, N.; Masood, A.; Per, T.S.; Khan, N.A. Salicylic acid alleviates adverse effects of heat stress on photosynthesis through changes in proline production and ethylene formation. Plant Signal. Behav. 2013, 8, e26374. [Google Scholar] [CrossRef] [Green Version]
- Gautam, H.; Fatma, M.; Sehar, Z.; Iqbal, N.; Albaqami, M.; Khan, N.A. Exogenously-Sourced Ethylene Positively Modulates Photosynthesis, Carbohydrate Metabolism, and Antioxidant Defense to Enhance Heat Tolerance in Rice. Int. J. Mol. Sci. 2022, 23, 1031. [Google Scholar] [CrossRef]
- Sung, D.Y.; Kaplan, F.; Lee, K.J.; Guy, C.L. Acquired tolerance to temperature extremes. Trends Plant Sci. 2003, 8, 179–187. [Google Scholar] [CrossRef]
- Renata Szyma´nska Lesak, I.; Orzechowska, A.; Kruk, J. Physiological and biochemical responses to high light and temperature stress in plants. Environ. Exp. Bot. 2017, 139, 165–177. [Google Scholar]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Roychowdhury, R.; Fujita, M. Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef] [PubMed]
- Parankusam, S.; Adimulam, S.S.; Bhatnagar-Mathur, P.; Sharma, K.K. Nitric oxide (NO) in plant heat stress tolerance: Current knowledge and perspectives. Front. Plant Sci. 2017, 8, 1582. [Google Scholar] [CrossRef] [PubMed]
- Allakhverdiev, S.I.; Kreslavski, V.D.; Klimov, V.V.; Los, D.A.; Carpentier, R.; Mohanty, P. Heat stress: An overview of molecular responses in photosynthesis. Photosynth. Res. 2008, 98, 541–550. [Google Scholar] [CrossRef]
- Li, X.; Cai, C.; Wang, Z.; Fan, B.; Zhu, C.; Chen, Z. Plastid translation elongation factor Tu is prone to heat-induced aggregation despite its critical role in plant heat tolerance. Plant Physiol. 2018, 176, 3027–3045. [Google Scholar] [CrossRef] [Green Version]
- Xalxo, R.; Yadu, B.; Chandra, J.; Chandrakar, V.; Keshavkant, S. Alteration in Carbohydrate Metabolism Modulates Thermotolerance of Plant under Heat Stress. In Heat Stress Tolerance in Plants: Physiological, Molecular and Genetic Perspectives, 1st ed.; Wani, S.H., Kumar, V., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2020; pp. 77–115. [Google Scholar]
- Liu, J.; Zhang, R.; Xu, X.; Fowler, J.C.; Miller, T.E.X.; Dong, T. Effect of summer warming on growth, photosynthesis and water status in female and male Populus cathayana: Implications for sex-specific drought and heat tolerances. Tree Physiol. 2020, 40, 1178–1191. [Google Scholar] [CrossRef]
- Wang, L.; Ma, K.B.; Lu, Z.G.; Ren, S.X.; Jiang, H.R.; Cui, J.W.; Chen, G.; Teng, N.J.; Lam, H.M.; Jin, B. Differential physiological, transcriptomic and metabolomic responses of Arabidopsis leaves under prolonged warming and heat shock. BMC Plant Biol. 2020, 20, 86. [Google Scholar] [CrossRef]
- Slimen, I.B.; Najar, T.; Ghram, A.; Dabbebi, H.; Mrad, M.B.; Abdrabbah, M. Reactive oxygen species, heat stress and oxidative-induced mitochondrial damage. A review. Int. J. Hyperthermia. 2014, 30, 513–523. [Google Scholar] [CrossRef]
- Zhao, J.; Lu, Z.; Wang, L.; Jin, B. Plant Responses to Heat Stress: Physiology, Transcription, Noncoding RNAs, and Epigenetics. Int. J. Mol. Sci. 2021, 22, 117. [Google Scholar] [CrossRef]
- He, X.; Guo, S.; Wang, Y.; Wang, L.; Shu, S.; Sun, J. Systematic identification and analysis of heat-stress-responsive lncRNAs, circRNAs and miRNAs with associated co-expression and ceRNA networks in cucumber (Cucumis sativus L.). Physiol. Plant. 2020, 168, 736–754. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, W.; Xia, Y.; Li, R.; Bai, G.; Siddique, K.H.M.; Guo, P. Non-coding RNAs: Functional roles in the regulation of stress response in Brassica crops. Genomics 2020, 112, 1419–1424. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Qiu, K.; Sun, W.; Yang, T.; Wu, T.; Song, T.; Zhang, J.; Yao, Y.; Tian, J. A long non-coding RNA functions in high-light-induced anthocyanin accumulation in apple by activating ethylene synthesis. Plant Physiol. 2022, 189, 66–83. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gao, L.; Li, J.; Zhu, B.; Zhu, H.; Luo, Y.; Wang, Q.; Zuo, J. Analysis of long-non-coding RNAs associated with ethylene in tomato. Gene 2018, 674, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Iranbakhsh, A.; Ardebili, Z.O.; Ardebili, N.O. Gene regulation by H2S in plants. In Hydrogen Sulfide in Plant Biology; Singh, S., Singh, V., Tripathi, D., Prasad, S., Chauhan, D., Dubey, N.K., Eds.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Iba, K. Acclimative response to temperature stress in higher plants: Approaches of gene engineering for temperature tolerance. Ann. Rev. Plant Biol. 2002, 53, 225–245. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Euring, D.; Cha, J.Y.; Lin, Z.; Lu, M.; Huang, L.J.; Kim, W.Y. Plant Hormone-Mediated Regulation of Heat Tolerance in Response to Global Climate Change. Front. Plant Sci. 2020, 11, 2318. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.M.; Cristescu, S.M.; Miersch, O.; Harren, F.J.; Wasternack, C.; Mur, L.A. Jasmonates act with salicylic acid to confer basal thermotolerance in Arabidopsis thaliana. New Phytol. 2009, 182, 175–187. [Google Scholar] [CrossRef]
- Hu, X.; Liu, R.; Li, Y.; Wang, W.; Tai, F.; Xue, R.; Li, C. Heat shock protein 70 regulates the abscisic acid-induced antioxidant response of maize to combined drought and heat stress. Plant Growth Regul. 2010, 60, 225–235. [Google Scholar] [CrossRef]
- Poór, P.; Nawaz, K.; Gupta, R.; Ashfaque, F.; Khan, M.I.R. Ethylene involvement in the regulation of heat stress tolerance in plants. Plant Cell Rep. 2021, 41, 675–698. [Google Scholar] [CrossRef]
- Firon, N.; Pressman, E.; Meir, S.; Khoury, R.; Altahan, L. Ethylene is involved in maintaining tomato (Solanum lycopersicum) pollen quality under heat-stress conditions. AoB Plants 2012, 2012, pls024. [Google Scholar] [CrossRef]
- Huang, D.; Huo, J.; Liao, W. Hydrogen sulfide: Roles in plant abiotic stress response and crosstalk with other signals. Plant Sci. 2021, 302, 110733. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Mahmud, J.A.; Nahar, K.; Mohsin, S.M.; Parvin, K.; Fujita, M. Interaction of sulfur with phytohormones and signaling molecules in conferring abiotic stress tolerance to plants. Plant Signal. Behav. 2018, 13, e1477905. [Google Scholar] [CrossRef] [PubMed]
- Capaldi, F.R.; Gratão, P.L.; Reis, A.R.; Lima, L.W.; Azevedo, R.A. Sulfur metabolism and stress defense responses in plants. Trop. Plant Biol. 2015, 8, 60–73. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.I.R.; Asgher, M.; Khan, N.A. Alleviation of salt-induced photosynthesis and growth inhibition by salicylic acid involves glycinebetaine and ethylene in mungbean (Vigna radiata L.). Plant Physiol. Biochem. 2014, 80, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Biswal, B.; Raval, M.K.; Biswal, U.C.; Joshi, P. Response of photosynthetic organelles to abiotic stress: Modulation by sulfur metabolism. In Sulfur Assimilation and Abiotic Stress in Plants; Khan, N.A., Singh, S., Umar, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 167–191. [Google Scholar]
- Rather, B.A.; Mir, I.R.; Masood, A.; Anjum, N.A.; Khan, N.A. Ethylene-nitrogen synergism induces tolerance to copper stress by modulating antioxidant system and nitrogen metabolism and improves photosynthetic capacity in mustard. Environ. Sci. Poll. Res. 2022, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Mobin, M.; Khan, M.N.; Abbas, Z.K.; Ansari, H.R.; Al-Mutairi, K.A. Significance of sulfur in heat stressed cluster bean (Cymopsis tetragonoloba L. Taub) genotypes, responses of growth, sugar and antioxidative metabolism. Arch. Agron. Soil Sci. 2017, 63, 288–295. [Google Scholar] [CrossRef]
- Wolak, N.; Kowalska, E.; Kozik, A.; Rapala-Kozik, M. Thiamine increases the resistance of baker’s yeast Saccharomyces cerevisiae against oxidative, osmotic and thermal stress, through mechanisms partly independent of thiamine diphosphate-bound enzymes. FEMS Yeast Res. 2014, 14, 1249–1262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, S.; Hjernø, K.; Larsen, M.; Wingsle, G.; Larsen, P.; Fey, S.; Roepstorff, P.; Pais, M.S. Proteome profiling of Populus euphratica upon heat stress. Ann. Bot. 2006, 98, 361–377. [Google Scholar] [CrossRef] [Green Version]
- Du, H.; Wang, Z.; Yu, W.; Liu, Y.; Huang, B. Differential metabolic responses of perennial grass Cynodon transvaalensis × Cynodon dactylon (C4) and Poa pratensis (C3) to heat stress. Physiol. Plant. 2011, 141, 251–264. [Google Scholar] [CrossRef]
- Heckathorn, S.A.; Downs, C.A.; Sharkey, T.D.; Coleman, J.S. The small, methionine-rich chloroplast heat-shock protein protects photosystem II electron transport during heat stress. Plant Physiol. 1998, 116, 439–444. [Google Scholar] [CrossRef] [Green Version]
- Sagor, G.H.M.; Berberich, T.; Takahashi, Y.; Niitsu, M.; Kusano, T. The polyamine spermine protects Arabidopsis from heat stress-induced damage by increasing expression of heat shock-related genes. Transgenic Res. 2013, 22, 595–605. [Google Scholar] [CrossRef]
- Noctor, G.; Mhamdi, A.; Chaouch, S.; Han, Y.; Neukermans, J.; Marquez-Garcia, B.; Queval, G.; Foyer, C.H. Glutathione in plants: An integrated overview. Plant Cell Environ. 2012, 35, 454–484. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Chattopadhyay, S. Glutathione modulates the expression of heat shock proteins via the transcription factors BZIP10 and MYB21 in Arabidopsis. J. Exp. Bot. 2018, 69, 3729–3743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Datta, R.; Kumar, D.; Sultana, A.; Hazra, S.; Bhattacharyya, D.; Chattopadhyay, S. Glutathione Regulates 1-Aminocyclopropane-1-Carboxylate Synthase Transcription via WRKY33 and 1-Aminocyclopropane-1-Carboxylate Oxidase by Modulating Messenger RNA Stability to Induce Ethylene Synthesis during Stress. Plant Physiol. 2015, 169, 2963–2981. [Google Scholar] [PubMed]
- Yoshida, S.; Tamaoki, M.; Ioki, M.; Ogawa, D.; Sato, Y.; Aono, M.; Kubo, A.; Saji, S.; Saji, H.; Satoh, S.; et al. Ethylene and salicylic acid control glutathione biosynthesis in ozone-exposed Arabidopsis thaliana. Physiol. Plant. 2009, 136, 284–298. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Wang, L.; Yang, Y.; Wang, P.; Guo, T.; Kang, G. Abscisic acid enhances tolerance of wheat seedlings to drought and regulates transcript levels of genes encoding ascorbate-glutathione biosynthesis. Front. Plant Sci. 2015, 6, 458. [Google Scholar] [CrossRef] [PubMed]
- Chae, H.B.; Moon, J.C.; Shin, M.R.; Chi, Y.H.; Jung, Y.J.; Lee, S.Y.; Kang, C.H. Thioredoxin reductase type C (NTRC) orchestrates enhanced thermotolerance to Arabidopsis by its redox-dependent holdase chaperone function. Mol. Plant. 2013, 6, 323–336. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Ballesta, M.C.; Moreno, D.A.; Carvajal, M. The physiological importance of glucosinolates on plant response to abiotic stress in Brassica. Int. J. Mol. Sci. 2013, 14, 11607–11625. [Google Scholar] [CrossRef] [Green Version]
- Filipovic, M.R.; Jovanovic, V.M. More than just an intermediate: Hydrogen sulfide signalling in plants. J. Exp. Bot. 2017, 68, 4733–4736. [Google Scholar] [CrossRef]
- Hou, Z.; Wang, L.; Liu, J.; Hou, L.; Liu, X. Hydrogen sulfide regulates ethylene-induced stomatal closure in Arabidopsis thaliana. J. Int. Plant Biol. 2013, 55, 277–289. [Google Scholar] [CrossRef]
- Liu, J.; Hou, L.; Liu, G.; Liu, X.; Wang, X. Hydrogen sulfide induced by nitric oxide mediates ethylene-induced stomatal closure of Arabidopsis thaliana. Chin. Sci. Bull. 2011, 56, 3547–3553. [Google Scholar] [CrossRef] [Green Version]
- Ge, Y.; Hu, K.D.; Wang, S.S.; Hu, L.Y.; Chen, X.Y.; Li, Y.H.; Zhang, H. Hydrogen sulfide alleviates postharvest ripening and senescence of banana by antagonizing the effect of ethylene. PLoS ONE 2017, 12, e0180113. [Google Scholar] [CrossRef] [PubMed]
- Husain, T.; Suhel, M.; Prasad, S.M.; Singh, V.P. Ethylene and hydrogen sulphide are essential for mitigating hexavalent chromium stress in two pulse crops. Plant Biol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Kaya, C.; Ashraf, M. The mechanism of hydrogen sulfide mitigation of iron deficiency-induced chlorosis in strawberry (Fragaria × ananassa) plants. Protoplasma 2019, 256, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.G.; Jin, J.Z. Hydrogen sulfide partly mediates abscisic acid-induced heat tolerance in tobacco (Nicotiana tabacum L.) suspension cultured cells. Plant Cell Tissue Organ Cult. 2016, 125, 207–214. [Google Scholar] [CrossRef]
- Hancock, J.T. Hydrogen sulfide and environmental stresses. Environ. Exp. Bot. 2019, 161, 50–56. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Ashfaque, F.; Chhillar, H.; Irfan, M.; Khan, N.A. The intricacy of silicon, plant growth regulators and other signaling molecules for abiotic stress tolerance: An entrancing crosstalk between stress alleviators. Plant Physiol. Biochem. 2021, 162, 36–47. [Google Scholar] [CrossRef]
- Pavlíková, D.; Neuberg, M.; Žižková, E.; Motyka, V.; Pavlík, M. Interactions between nitrogen nutrition and phytohormone levels in Festulolium plants. Plant Soil Environ. 2012, 58, 367–372. [Google Scholar] [CrossRef] [Green Version]
- Asgher, M.; Khan, N.A.; Khan, M.I.R.; Fatma, M.; Masood, A. Ethylene production is associated with alleviation of cadmium-induced oxidative stress by sulfur in mustard types differing in ethylene sensitivity. Ecotoxicol. Environ. Saf. 2014, 106, 54–61. [Google Scholar] [CrossRef]
- Jahan, B.; Rasheed, F.; Sehar, Z.; Fatma, M.; Iqbal, N.; Masood, A.; Khan, N.A. Coordinated role of nitric oxide, ethylene, nitrogen, and sulfur in plant salt stress tolerance. Stresses 2021, 1, 181–199. [Google Scholar] [CrossRef]
- Jahan, B.; Sehar, Z.; Masood, A.; Anjum, N.A.; Khan, M.I.R.; Khan, N.A. Sulfur availability potentiates phytohormones-mediated action in plants. In Plant Signaling Molecules; Khan, M.I.R., Reddy, P.S., Ferrante, A., Khan, N.A., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 287–301. [Google Scholar]
- Jahan, B.; Iqbal, N.; Fatma, M.; Sehar, Z.; Masood, A.; Sofo, A.; Khan, N.A. Ethylene supplementation combined with split application of nitrogen and sulfur protects salt-inhibited photosynthesis through optimization of proline metabolism and antioxidant system in mustard (Brassica juncea L.). Plants 2021, 10, 1303. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, J.; Liu, J.; Liu, T.; Xue, S. Hydrogen sulfide (H2S) signaling in plant development and stress responses. Abiotech 2021, 2, 32–63. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Huang, L.; Zhang, Y.; Liu, L.; Sun, C.; Lin, X. Sulfur deficiency exacerbates phytotoxicity and residues of imidacloprid through suppression of thiol-dependent detoxification in lettuce seedlings. Environ. Poll. 2021, 291, 118221. [Google Scholar] [CrossRef] [PubMed]
- Astolfi, S.; Celletti, S.; Vigani, G.; Mimmo, T.; Cesco, S. Interaction between sulfur and iron in plants. Front. Plant Sci. 2021, 12, 670308. [Google Scholar] [CrossRef]
- Feldman-Salit, A.; Veith, N.; Wirtz, M.; Hell, R.; Kummer, U. Distribution of control in the sulfur assimilation in Arabidopsis thaliana depends on environmental conditions. New Phytolol. 2019, 222, 1392–1404. [Google Scholar] [CrossRef]
- Samanta, S.; Singh, A.; Roychoudhury, A. Involvement of sulfur in the regulation of abiotic stress tolerance in plants. Protective chemical agents in the amelioration of plant abiotic stress. Biochem. Mol. Perspect. 2020, 11, 437–466. [Google Scholar]
- Filipovic, M.R.; Zivanovic, J.; Alvarez, B.; Banerjee, R. Chemical biology of H2S signaling through persulfidation. Chem. Rev. 2018, 118, 1253–1337. [Google Scholar] [CrossRef]
- Arif, M.S.; Yasmeen, T.; Abbas, Z.; Ali, S.; Rizwan, M.; Aljarba, N.H.; Abdel-Daim, M.M. Role of exogenous and endogenous hydrogen sulfide (H2S) on functional traits of plants under heavy metal stresses: A recent perspective. Front. Plant Sci. 2021, 11, 2063. [Google Scholar] [CrossRef]
- Jez, J.M. Structural biology of plant sulfur metabolism: From sulfate to glutathione. J. Exp. Bot. 2019, 70, 4089–4103. [Google Scholar] [CrossRef]
- García-Mata, C.; Lamattina, L. Hydrogen sulphide, a novel gasotransmitter involved in guard cell signalling. New Phytol. 2010, 188, 977–984. [Google Scholar] [CrossRef]
- Gotor, C.; García, I.; Aroca, Á.; Laureano-Marín, A.M.; Arenas-Alfonseca, L.; Jurado-Flores, A.; Romero, L.C. Signaling by hydrogen sulfide and cyanide through post-translational modification. J. Exp. Bot. 2019, 70, 4251–4265. [Google Scholar] [CrossRef] [PubMed]
- Rather, B.A.; Mir, I.R.; Sehar, Z.; Anjum, N.A.; Masood, A.; Khan, N.A. The outcomes of the functional interplay of nitric oxide and hydrogen sulfide in metal stress tolerance in plants. Plant Physiol. Biochem. 2020, 155, 523–534. [Google Scholar] [CrossRef] [PubMed]
- Khanna, K.; Sharma, N.; Kour, S.; Ali, M.; Ohri, P.; Bhardwaj, R. Hydrogen Sulfide: A Robust Combatant against Abiotic Stresses in Plants. Hydrogen 2021, 2, 319–342. [Google Scholar] [CrossRef]
- Fu, Y.; Tang, J.; Yao, G.F.; Huang, Z.Q.; Li, Y.H.; Han, Z.; Zhang, H. Central role of adenosine 5′-phosphosulfate reductase in the control of plant hydrogen sulfide metabolism. Front. Plant Sci. 2018, 9, 1404. [Google Scholar] [CrossRef]
- Wilson, L.G.; Bressan, R.A.; Filner, P. Light-dependent emission of hydrogen sulfide from plants. Plant Physiol. 1978, 61, 184–189. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Deng, Y.; Liu, Z.; Liao, W. Hydrogen Sulfide in Plants: Crosstalk with Other Signal Molecules in Response to Abiotic Stresses. Int. J. Mol. Sci. 2021, 22, 12068. [Google Scholar] [CrossRef]
- Corpas, F.J. Hydrogen sulfide: A new warrior against abiotic stress. Trends Plant Sci. 2019, 24, 983–988. [Google Scholar] [CrossRef]
- Thakur, M.; Anand, A. Hydrogen sulfide: An emerging signaling molecule regulating drought stress response in plants. Physiol. Plantar. 2021, 172, 1227–1243. [Google Scholar] [CrossRef]
- Kolluru, G.K.; Shen, X.; Bir, S.C.; Kevil, C.G. Hydrogen sulfide chemical biology: Pathophysiological roles and detection. Nitric oxide 2013, 35, 5–20. [Google Scholar] [CrossRef] [Green Version]
- Ishigami, M.; Hiraki, K.; Umemura, K.; Ogasawara, Y.; Ishii, K.; Kimura, H. A source of hydrogen sulfide and a mechanism of its release in the brain. Antioxid. Redox Signal. 2009, 11, 205–214. [Google Scholar] [CrossRef] [Green Version]
- Kamoun, P. Endogenous production of hydrogen sulfide in mammals. Amino Acids 2004, 26, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Peter, E.A.; Bir, S.; Wang, R.; Kevil, C.G. Analytical measurement of discrete hydrogen sulfide pools in biological specimens. Free Radic Biol. Med. 2012, 52, 2276–2283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, N.; Gaddam, S.R.; Singh, D.; Trivedi, P.K. Regulation of arsenic stress response by ethylene biosynthesis and signaling in Arabidopsis thaliana. Environ. Exp. Bot. 2021, 185, 104408. [Google Scholar] [CrossRef]
- Maruyama-Nakashita, A.; Nakamura, Y.; Tohge, T.; Saito, K.; Takahashi, H. Arabidopsis SLIM1 is a central transcriptional regulator of plant sulfur response and metabolism. Plant Cell 2006, 18, 3235–3251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wawrzyńska, A.; Sirko, A. To control and to be controlled: Understanding the Arabidopsis SLIM1 function in sulfur deficiency through comprehensive investigation of the EIL protein family. Front. Plant Sci. 2014, 5, 575. [Google Scholar] [PubMed]
- Djanaguiraman, M.; Prasad, P.V. Ethylene production under high temperature stress causes premature leaf senescence in soybean. Funct. Plant Biol. 2010, 37, 1071–1084. [Google Scholar] [CrossRef]
- Hays, D.; Mason, E.; Do, J.H.; Menz, M.; Reynolds, M. Expression quantitative trait loci mapping heat tolerance during reproductive development in wheat (Triticum aestivum). In Wheat Production in Stressed Environments. Developments in Plant Breeding; Buck, H.T., Nisi, J.E., Salomón, N., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 373–382. [Google Scholar]
- Wu, Y.S.; Yang, C.Y. Ethylene-mediated signaling confers thermo-tolerance and regulates transcript levels of heat shock factors in rice seedlings under heat stress. Bot. Stud. 2019, 60, 23. [Google Scholar] [CrossRef] [PubMed]
- Larkindale, J.; Knight, M.R. Protection against heat stress-induced oxidative damage in Arabidopsis involves calcium, abscisic acid, ethylene, and salicylic acid. Plant Physiol. 2002, 128, 682–695. [Google Scholar] [CrossRef]
- Jegadeesan, S.; Chaturvedi, P.; Ghatak, A.; Pressman, E.; Meir, S.; Faigenboim, A.; Firon, N. Proteomics of heat-stress and ethylene-mediated thermotolerance mechanisms in tomato pollen grains. Front. Plant Sci. 2018, 9, 1558. [Google Scholar] [CrossRef] [Green Version]
- Larkindale, J.; Huang, B. Thermotolerance and antioxidant systems in Agrostis stolonifera: Involvement of salicylic acid, abscisic acid, calcium, hydrogen peroxide, and ethylene. J. Plant Physiol. 2004, 161, 405–413. [Google Scholar] [CrossRef]
- Korkmaz, A. Ameliorative effects of ethylene precursor and polyamines on the high temperature inhibition of seed germination in lettuce (Lactuca sativa L.) before and after seed storage. Seed Sci. Technol. 2006, 34, 465–474. [Google Scholar] [CrossRef]
- Shinohara, T.; Martin, E.A.; Leskovar, D.I. Ethylene regulators influence germination and root growth of globe artichoke seedlings exposed to heat stress conditions. Seed Sci. Technol. 2017, 45, 167–178. [Google Scholar] [CrossRef]
- Huberman, M.; Riov, J.; Goldschmidt, E.E.; Apelbaum, A.; Goren, R. The novel ethylene antagonist, 3-cyclopropyl-1-enyl-propanoic acid sodium salt (CPAS), increases grain yield in wheat by delaying leaf senescence. Plant Growth Regul. 2014, 73, 249–255. [Google Scholar] [CrossRef] [Green Version]
- Jegadeesan, S.; Pressman, E.; Beery, A.; Singh, V.; Peres, L.E.P.; Shabtai, S.; Firon, N. An ethylene over-producing mutant of tomato (Solanum lycopersicum), epinastic, exhibits tolerance to high temperature conditions. Amer. J. Plant Sci. 2021, 12, 487–497. [Google Scholar] [CrossRef]
- Kaur, H.; Ozga, J.A.; Reinecke, D.M. Balancing of hormonal biosynthesis and catabolism pathways, a strategy to ameliorate the negative effects of heat stress on reproductive growth. Plant Cell Environ. 2021, 44, 1486–1503. [Google Scholar] [CrossRef]
- Jespersen, D.; Yu, J.; Huang, B. Metabolite responses to exogenous application of nitrogen, cytokinin, and ethylene inhibitors in relation to heat-induced senescence in creeping bentgrass. PLoS ONE 2015, 10, e0123744. [Google Scholar] [CrossRef]
- Müller, M.; Munné-Bosch, S. Ethylene response factors: A key regulatory hub in hormone and stress signaling. Plant Physiol. 2015, 169, 32–41. [Google Scholar] [CrossRef] [Green Version]
- Cheng, M.C.; Liao, P.M.; Kuo, W.W.; Lin, T.P. The Arabidopsis Ethylene Response Factor1 regulates abiotic stress-responsive gene expression by binding to different cis-acting elements in response to different stress signals. Plant Physiol. 2013, 162, 1566–1582. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.; Ma, A.; Yang, S.; Liu, X.; Zhao, T.; Zhang, J.; Xu, R. Transcriptome analysis and weighted gene co-expression network reveals potential genes responses to heat stress in turbot Scophthalmus maximus. Comp. Biochem. Physiol. D Genom. Proteom. 2020, 33, 100632. [Google Scholar] [CrossRef]
- Busch, W.; Wunderlich, M.; Schöffl, F. Identification of novel heat shock factor-dependent genes and biochemical pathways in Arabidopsis thaliana. Plant J. 2005, 41, 1–14. [Google Scholar] [CrossRef]
- Wang, W.; Vinocur, B.; Shoseyov, O.; Altman, A. Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends Plant Sci. 2004, 9, 244–252. [Google Scholar] [CrossRef]
- Li, Z.G.; Gong, M.; Xie, H.; Yang, L.; Li, J. Hydrogen sulfide donor sodium hydrosulfide-induced heat tolerance in tobacco (Nicotiana tabacum L.) suspension cultured cells and involvement of Ca2+ and calmodulin. Plant Sci. 2012, 185, 185–189. [Google Scholar] [CrossRef]
- Yang, X.; Zhu, W.; Zhang, H.; Liu, N.; Tian, S. Heat shock factors in tomatoes: Genome-wide identification, phylogenetic analysis and expression profiling under development and heat stress. Peer J. 2016, 4, e1961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, P.; Luo, Q.; Wang, R.; Xu, J. Hydrogen sulfide toxicity inhibits primary root growth through the ROS-NO pathway. Sci. Rep. 2017, 7, 868. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Hu, Y.; Fan, T.; Li, J. Hydrogen sulfide modulates actin-dependent auxin transport via regulating ABPs results in changing of root development in Arabidopsis. Sci. Rep. 2015, 5, 8251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.G.; Ding, X.J.; Du, P.F. Hydrogen sulfide donor sodium hydrosulfide-improved heat tolerance in maize and involvement of proline. J. Plant Physiol. 2013, 170, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Christou, A.; Manganaris, G.A.; Papadopoulos, I.; Fotopoulos, V. Hydrogen sulfide induces systemic tolerance to salinity and non-ionic osmotic stress in strawberry plants through modification of reactive species biosynthesis and transcriptional regulation of multiple defence pathways. J. Exp. Bot. 2013, 64, 1953–1966. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, N.; Masood, A.; Khan, M.I.R.; Asgher, M.; Fatma, M.; Khan, N.A. Cross-talk between sulfur assimilation and ethylene signaling in plants. Plant Signal. Behav. 2013, 8, e22478. [Google Scholar] [CrossRef] [Green Version]
- Min, Y.A.N.G.; Qin, B.P.; Ping, W.A.N.G.; Li, M.L.; Chen, L.L.; Chen, L.T.; Yin, Y.P. Foliar application of sodium hydrosulfide (NaHS), a hydrogen sulfide (H2S) donor, can protect seedlings against heat stress in wheat (Triticum aestivum L.). J. Integr. Agric. 2016, 15, 2745–2758. [Google Scholar]
- Christou, A.; Manganaris, G.A.; Fotopoulos, V. Systemic mitigation of salt stress by hydrogen peroxide and sodium nitroprusside in strawberry plants via transcriptional regulation of enzymatic and non-enzymatic antioxidants. Environ. Exp. Bot. 2014, 107, 46–54. [Google Scholar] [CrossRef]
- Li, Z.G.; Yi, X.Y.; Li, Y.T. Effect of pretreatment with hydrogen sulfide donor sodium hydrosulfide on heat tolerance in relation to antioxidant system in maize (Zea mays L.) seedlings. Biologia 2014, 69, 1001–1009. [Google Scholar] [CrossRef]
- Li, Z.G.; Xie, L.R.; Li, X.J. Hydrogen sulfide acts as a downstream signal molecule in salicylic acid-induced heat tolerance in maize (Zea mays L.) seedlings. J. Plant Physiol. 2015, 177, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.G.; Luo, L.J.; Zhu, L.P. Involvement of trehalose in hydrogen sulfide donor sodium hydrosulfide-induced the acquisition of heat tolerance in maize (Zea mays L.) seedlings. Bot. Stud. 2014, 55, 20. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, A.; Roychoudhury, A. Roles of hydrogen sulfide in regulating temperature stress response in plants. In Plant Growth Regulators; Springer: Cham, Switzerland, 2021; pp. 207–215. [Google Scholar]
- Li, Z.G.; Long, W.B.; Yang, S.Z.; Wang, Y.C.; Tang, J.H.; Chen, T. Involvement of sulfhydryl compounds and antioxidant enzymes in H2S-induced heat tolerance in tobacco (Nicotiana tabacum L.) suspension-cultured cells. Vitr. Cell. Dev. Biol. Plant 2015, 51, 428–437. [Google Scholar] [CrossRef]
- Ribeiro, R.V.; Machado, E.C.; Oliveira, R.F.D. Temperature response of photosynthesis and its interaction with light intensity in sweet orange leaf discs under non-photorespiratory condition. Ciência Agrotecnologia 2006, 30, 670–678. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Akram, N.A.; Ashraf, M.; Alyemeni, M.N.; Wijaya, L.; Ahmad, P. Plant responses to environmental stresses—from gene to biotechnology. AoB Plants 2017, 9, plx025. [Google Scholar] [CrossRef] [Green Version]
- Ihsan, M.Z.; Daur, I.; Alghabari, F.; Alzamanan, S.; Rizwan, S.; Ahmad, M.; Shafqat, W. Heat stress and plant development: Role of sulphur metabolites and management strategies. Acta Agric. Scand. Sect. B Soil Plant Sci. 2019, 69, 332–342. [Google Scholar] [CrossRef]
- Bashir, H.; Ibrahim, M.M.; Bagheri, R.; Ahmad, J.; Arif, I.A.; Baig, M.A.; Qureshi, M.I. Influence of sulfur and cadmium on antioxidants, phytochelatins and growth in Indian mustard. AoB Plants 2015, 7, plv001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- North, K.A.; Kopriva, S. Sulfur in resistance to environmental stresses. In Sulfur in Plants. An Ecological Perspective; Springer: Dordrecht, The Netherlands, 2007; pp. 143–168. [Google Scholar]
- Martins, L.; Knuesting, J.; Bariat, L.; Dard, A.; Freibert, S.A.; Marchand, C.H.; Riondet, C. Redox modification of the iron-sulfur glutaredoxin GRXS17 activates holdase activity and protects plants from heat stress. Plant Physiol. 2020, 184, 676–692. [Google Scholar] [CrossRef]
- Brunel-Muguet, S.; d’Hooghe, P.; Bataillé, M.P.; Larré, C.; Kim, T.H.; Trouverie, J.; Dürr, C. Heat stress during seed filling interferes with sulfur restriction on grain composition and seed germination in oilseed rape (Brassica napus L.). Front. Plant Sci. 2015, 6, 213. [Google Scholar] [CrossRef] [Green Version]
- Waraich, E.A.; Hussain, A.; Ahmad, Z.; Ahmad, M.; Barutçular, C. Foliar application of sulfur improved growth, yield and physiological attributes of canola (Brassica napus L.) under heat stress conditions. J. Plant Nutr. 2022, 45, 369–379. [Google Scholar] [CrossRef]
- Waraich, E.A.; Ahmad, M.; Soufan, W.; Manzoor, M.T.; Ahmad, Z.; Habib-Ur-Rahman, M.; Sabagh, A.E. Seed priming with sulfhydral thiourea enhances the performance of Camelina sativa L. under heat stress conditions. Agronomy 2021, 11, 1875. [Google Scholar] [CrossRef]
- Ali, M.M.; Waleed Shafique, M.; Gull, S.; Afzal Naveed, W.; Javed, T.; Yousef, A.F.; Mauro, R.P. Alleviation of heat stress in tomato by exogenous application of sulfur. Horticulturae 2021, 7, 21. [Google Scholar] [CrossRef]
- Altaf, A.; Zhu, X.; Zhu, M.; Quan, M.; Irshad, S.; Xu, D.; Zada, A. Effects of Environmental Stresses (Heat, Salt, Waterlogging) on Grain Yield and Associated Traits of Wheat under Application of Sulfur-Coated Urea. Agronomy 2021, 11, 2340. [Google Scholar] [CrossRef]
- Gomord, V.; Faye, L. Post-translational modification of therapeutic proteins in plants. Curr. Opin. Plant Biol. 2004, 7, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, K.; Neuhaus, J.M. Cis-elements of protein transport to the plant vacuoles. J. Exp. Bot. 1999, 50, 165–174. [Google Scholar] [CrossRef]
- Park, H.J.; Yun, D.J. New insights into the role of the small ubiquitin-like modifier (SUMO) in plants. Int. Rev. Cell Mol. Biol. 2013, 300, 161–209. [Google Scholar]
- Kosová, K.; Vítámvás, P.; Urban, M.O.; Prášil, I.T.; Renaut, J. Plant abiotic stress proteomics: The major factors determining alterations in cellular proteome. Front. Plant Sci. 2018, 9, 122. [Google Scholar] [CrossRef] [Green Version]
- Kurepa, J.; Walker, J.M.; Smalle, J.; Gosink, M.M.; Davis, S.J.; Durham, T.L.; Vierstra, R.D. The small ubiqui tin-like modifier (SUMO) protein modification system in Arabidopsis: Accumulation of SUMO1 and-2 conjugates is increased by stress. J. Biol. Chem. 2003, 278, 6862–6872. [Google Scholar] [CrossRef] [Green Version]
- Saracco, S.A.; Miller, M.J.; Kurepa, J.; Vierstra, R.D. Genetic analysis of SUMOylation in Arabidopsis: Conjugation of SUMO1 and SUMO2 to nuclear proteins is essential. Plant Physiol. 2007, 145, 119–134. [Google Scholar] [CrossRef] [Green Version]
- Mishra, N.; Sun, L.; Zhu, X.; Smith, J.; Prakash Srivastava, A.; Yang, X.; Zhang, H. Overexpression of the rice SUMO E3 ligase gene OsSIZ1 in cotton enhances drought and heat tolerance, and substantially improves fiber yields in the field under reduced irrigation and rainfed conditions. Plant Cell Physiol. 2017, 58, 735–746. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.K.; Zhang, C.; Caine, R.S.; Gray, J.; Sadanandom, A. Rice SUMO protease Overly Tolerant to Salt 1 targets the transcription factor, Osb ZIP 23 to promote drought tolerance in rice. Plant J. 2017, 92, 1031–1043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Zhuang, K.; Wang, S.; Lv, J.; Ma, N.N.; Meng, Q. A novel tomato SUMO E3 ligase, SlSIZ1, confers drought tolerance in transgenic tobacco. J. Integr. Plant Biol. 2017, 59, 102–117. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Wang, M.; Xia, Z. Overexpression of a maize SUMO conjugating enzyme gene (ZmSCE1e) increases Sumoylation levels and enhances salt and drought tolerance in transgenic tobacco. Plant Sci. 2019, 281, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Rytz, T.C.; Miller, M.J.; McLoughlin, F.; Augustine, R.C.; Marshall, R.S.; Juan, Y.T.; Vierstra, R.D. SUMOylome profiling reveals a diverse array of nuclear targets modified by the SUMO ligase SIZ1 during heat stress. Plant Cell 2018, 30, 1077–1099. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, S.; Lv, J.; Liu, Z.; Wang, Y.; Ma, N.; Meng, Q. SUMO E3 ligase SlSIZ1 facilitates heat tolerance in tomato. Plant Cell Physiol. 2018, 59, 58–71. [Google Scholar] [CrossRef] [Green Version]
- Niskanen, E.A.; Palvimo, J.J. Chromatin sumoylation in heat stress: To protect, pause and organise? Sumo stress response on chromatin. BioEssays 2017, 39, 1600263. [Google Scholar] [CrossRef]
- Lima, J.C.D.; Loss-Morais, G.; Margis, R. MicroRNAs play critical roles during plant development and in response to abiotic stresses. Gene. Mol. Biol. 2012, 35, 1069–1077. [Google Scholar] [CrossRef] [Green Version]
- Khraiwesh, B.; Zhu, J.K.; Zhu, J. Role of miRNAs and siRNAs in biotic and abiotic stress responses of plants. Biochim. Biophys. Acta (BBA) Gene Regul. Mech. 2012, 1819, 137–148. [Google Scholar] [CrossRef] [Green Version]
- Sunkar, R.; Kapoor, A.; Zhu, J.K. Posttranscriptional induction of two Cu/Zn superoxide dismutase genes in Arabidopsis is mediated by downregulation of miR398 and important for oxidative stress tolerance. Plant Cell 2006, 18, 2051–2065. [Google Scholar] [CrossRef] [Green Version]
- Kotak, S.; Larkindale, J.; Lee, U.; von Koskull-Döring, P.; Vierling, E.; Scharf, K.D. Complexity of the heat stress response in plants. Curr. Opin. Plant Biol. 2007, 10, 310–316. [Google Scholar] [CrossRef]
- Yu, X.; Wang, H.; Lu, Y.; de Ruiter, M.; Cariaso, M.; Prins, M.; van Tunen, A.; He, Y. Identification of conserved and novel microRNAs that are responsive to heat stress in Brassica rapa. J. Exp. Bot. 2012, 63, 1025–1038. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.R.; Pathak, H.; Sharma, S.K.; Kala, Y.K.; Nirjal, M.K.; Singh, G.P.; Goswami, S.; Rai, R.D. Novel and conserved heat-responsive microRNAs in wheat (Triticum aestivum L.). Funct. Integr. Geno. 2015, 15, 323–348. [Google Scholar] [CrossRef] [PubMed]
- Reyes, J.L.; Chua, N.H. ABA induction of miR159 controls transcript levels of two MYB factors during Arabidopsis seed germination. Plant J. 2007, 49, 592–606. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sun, F.; Cao, H.; Peng, H.; Ni, Z.; Sun, Q.; Yao, Y. TamiR159 directed wheat TaGAMYB cleavage and its involvement in anther development and heat response. PLoS ONE 2012, 7, e48445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, Y.; Ni, Z.; Peng, H.; Sun, F.; Xin, M.; Sunkar, R.; Zhu, J.K.; Sun, Q. Non-coding small RNAs responsive to abiotic stress in wheat (Triticum aestivum L.). Funct. Integr. Geno. 2010, 10, 187–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, H.; Gaubert, H.; Bucher, E.; Mirouze, M.; Vaillant, I.; Paszkowski, J. An siRNA pathway prevents transgenerational retrotransposition in plants subjected to stress. Nature 2011, 472, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, J.; Liu, Z.; Li, X.; Wu, F.; He, Y. Heat-induced tas1 target1 mediates thermotolerance via heat stress transcription factor A1a–directed pathways in Arabidopsis. Plant Cell 2014, 26, 1764–1780. [Google Scholar] [CrossRef] [Green Version]
- Wierzbicki, A.T. The role of long non-coding RNA in transcriptional gene silencing. Curr. Opin. Plant Biol. 2012, 15, 517–522. [Google Scholar] [CrossRef]
- Song, X.; Liu, G.; Huang, Z.; Duan, W.; Tan, H.; Li, Y.; Hou, X. Temperature expression patterns of genes and their coexpression with LncRNAs revealed by RNA-Seq in non-heading Chinese cabbage. BMC Geno. 2016, 17, 297. [Google Scholar] [CrossRef] [Green Version]
- Savada, R.P.; Ozga, J.A.; Jayasinghege, C.; Waduthanthri, K.D.; Reinecke, D.M. Heat stress differentially modifies ethylene biosynthesis and signaling in pea floral and fruit tissues. Plant Mol. Biol. 2017, 95, 313–331. [Google Scholar] [CrossRef] [PubMed]
- Valluru, R.; Reynolds, M.P.; Davies, W.J.; Sukumaran, S. Phenotypic and genome-wide association analysis of spike ethylene in diverse wheat genotypes under heat stress. New Phytol. 2017, 214, 271–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, C.J.; Seo, Y.S. Heat shock proteins: A review of the molecular chaperones for plant immunity. Plant Pathol. J. 2015, 31, 323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathur, S.; Agrawal, D.; Jajoo, A. Photosynthesis: Response to high temperature stress. J. Photochem. Photobiol. B Biol. 2014, 137, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Zhang, H.; Ma, Q.; Fan, F.; Fu, R.; Ahammed, G.J.; Shi, K. Role of ethylene biosynthesis and signaling in elevated CO2-induced heat stress response in tomato. Planta 2019, 250, 563–572. [Google Scholar] [CrossRef]
- Rickey, T.M.; Belknap, W.R. Comparison of the expression of several stress-responsive genes in potato tubers. Plant Mol. Biol. 1991, 16, 1009–1018. [Google Scholar] [CrossRef]
- Salman, A.; Filgueiras, H.; Cristescu, S.; Lopez-Lauri, F.; Harren, F.; Sallanon, H. Inhibition of wound-induced ethylene does not prevent red discoloration in fresh-cut endive (Cichorium intybus L.). Eur. Food Res. Technol. 2009, 228, 651–657. [Google Scholar] [CrossRef]
- Jacob, P.; Hirt, H.; Bendahmane, A. The heat-shock protein/chaperone network and multiple stress resistance. Plant Biotechnol. J. 2017, 15, 405–414. [Google Scholar] [CrossRef]
- Raz, V.; Ecker, J.R. Regulation of differential growth in the apical hook of Arabidopsis. Development 1999, 126, 3661–3668. [Google Scholar] [CrossRef]
- Kruszka, K.; Pacak, A.; Swida-Barteczka, A.; Nuc, P.; Alaba, S.; Wroblewska, Z.; Karlowski, W.; Jarmolowski, A.; Szweykowska-Kulinska, Z. Transcriptionally and post-transcriptionally regulated microRNAs in heat stress response in barley. J. Exp. Bot. 2014, 65, 6123–6135. [Google Scholar] [CrossRef] [Green Version]
- Lü, P.; Yu, S.; Zhu, N.; Chen, Y.R.; Zhou, B.; Pan, Y.; Tzeng, D.; Fabi, J.P.; Argyris, J.; Garcia-Mas, J.; et al. Genome encode analyses reveal the basis of convergent evolution of fleshy fruit ripening. Nat. Plants 2018, 4, 784–791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.Q.; Wang, J.; Wu, Y.Y.; Li, D.W.; Allan, A.C.; Yin, X.R. Genome-wide analysis of coding and non-coding RNA reveals a conserved miR164-NAC regulatory pathway for fruit ripening. New Phytol. 2020, 225, 1618–1634. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Xiao, T.; Zhou, H.; Xie, Y.; Shen, W. Hydrogen sulfide: A versatile regulator of environmental stress in plants. Acta Physiol. Plant. 2016, 38, 16. [Google Scholar] [CrossRef]
- Ye, X.Y.; Qiu, X.M.; Sun, Y.Y.; Li, Z.G. Interplay between hydrogen sulfide and methylglyoxal initiates thermotolerance in maize seedlings by modulating reactive oxidative species and osmolyte metabolism. Protoplasma 2020, 257, 1415–1432. [Google Scholar] [CrossRef]
- Mustafa, A.K.; Gadalla, M.M.; Sen, N.; Kim, S.; Mu, W.; Gazi, S.K.; Snyder, S.H. H2S signals through protein S-sulfhydration. Sci. Signal. 2009, 2, ra72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aroca, Á.; Serna, A.; Gotor, C.; Romero, L.C. S-sulfhydration: A cysteine posttranslational modification in plant systems. Plant Physiol. 2015, 168, 334–342. [Google Scholar] [CrossRef] [Green Version]
- Aroca, A.; Schneider, M.; Scheibe, R.; Gotor, C.; Romero, L.C. Hydrogen sulfide regulates the cytosolic/nuclear partitioning of glyceraldehyde-3-phosphate dehydrogenase by enhancing its nuclear localization. Plant Cell Physiol. 2017, 58, 983–992. [Google Scholar] [CrossRef]
- Sevilla, F.; Camejo, D.; Ortiz-Espín, A.; Calderón, A.; Lázaro, J.J.; Jiménez, A. The thioredoxin/peroxiredoxin/sulfiredoxin system: Current overview on its redox function in plants and regulation by reactive oxygen and nitrogen species. J. Exp. Bot. 2015, 66, 2945–2955. [Google Scholar] [CrossRef] [Green Version]
- Wedmann, R.; Onderka, C.; Wei, S.; Szijártó, I.A.; Miljkovic, J.L.; Mitrovic, A.; Filipovic, M.R. Improved tag-switch method reveals that thioredoxin acts as depersulfidase and controls the intracellular levels of protein persulfidation. Chem. Sci. 2016, 7, 3414–3426. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Zhou, M.; Zhou, H.; Zhao, D.; Gotor, C.; Romero, L.C.; .Xie, Y. Hydrogen sulfide, a signaling molecule in plant stress responses. J. Integ. Plant Biol. 2021, 63, 146–160. [Google Scholar] [CrossRef]
- Kitajima, S.; Kurioka, M.; Yoshimoto, T.; Shindo, M.; Kanaori, K.; Tajima, K.; Oda, K. A cysteine residue near the propionate side chain of heme is the radical site in ascorbate peroxidase. FEBS J. 2008, 275, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Begara-Morales, J.C.; Sánchez-Calvo, B.; Chaki, M.; Valderrama, R.; Mata-Pérez, C.; López-Jaramillo, J.; Barroso, J.B. Dual regulation of cytosolic ascorbate peroxidase (APX) by tyrosine nitration and S-nitrosylation. J. Exp. Bot. 2014, 65, 527–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kesherwani, V.; Nandi, S.S.; Sharawat, S.K.; Shahshahan, H.R.; Mishra, P.K. Hydrogen sulfide mitigates homocysteine-mediated pathological remodeling by inducing miR-133a in cardiomyocytes. Mol. Cell. Biochem. 2015, 404, 241–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, H.; Chen, S.; Liu, D.; Liesche, J.; Shi, C.; Wang, J.; Li, J. Ethylene-induced hydrogen sulfide negatively regulates ethylene biosynthesis by persulfidation of ACO in tomato under osmotic stress. Front. Plant Sci. 2018, 9, 1517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Hao, D.D.; Zhang, J.S.; Zhu, Y.C. Hydrogen sulphide inhibits cardiomyocyte hypertrophy by up-regulating miR-133a. Biochem. Biophys. Res. Commun. 2011, 413, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Pei, Y.; Cao, Q.; Wang, R. MicroRNA-21 represses human cystathionine gamma-lyase expression by targeting at specificity protein-1 in smooth muscle cells. J. Cell. Physiol. 2012, 227, 3192–3200. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Noctor, G. Redox homeostasis and antioxidant signaling: A metabolic interface between stress perception and physiological responses. Plant Cell 2005, 17, 1866–1875. [Google Scholar] [CrossRef] [Green Version]
- Szalai, G.; Kellős, T.; Galiba, G.; Kocsy, G. Glutathione as an antioxidant and regulatory molecule in plants under abiotic stress conditions. J. Plant Growth Regul. 2009, 28, 66–80. [Google Scholar] [CrossRef]
- De Kok, L.J. Sulfur metabolism in plants exposed to atmospheric sulphur. In Sulfur Nutrition and Sulfur Assimilation in Higher Plants: Fundamental, Environmental and Agricultural Aspects; Renneberg, H., Brunold, C., De Kok, L.J., Stulen, I., Eds.; Backhuys Publishers: Leiden, The Netherlands, 1990; pp. 111–130. [Google Scholar]
- De Kok, L.J.; Yang, L.; Stuiver, C.E.E.; Stulen, I. Negative vs. positive functional plant responses to air pollution: A study establishing cause–effect relationships of SO2 and H2S. Dev. Environ. Sci. 2009, 9, 121–135. [Google Scholar]
- Koralewska, A.; Stuiver, C.E.E.; Posthumus, F.S.; Kopriva, S.; Hawkesford, M.J.; De Kok, L.J. Regulation of sulfate uptake, expression of the sulfate transporters Sultr1; 1 and Sultr1; 2, and APS reductase in Chinese cabbage (Brassica pekinensis) as affected by atmospheric H2S nutrition and sulfate deprivation. Funct. Plant Biol. 2008, 35, 318–327. [Google Scholar] [CrossRef]
- Schuetz, T.J.; Gallo, G.J.; Sheldon, L.; Tempst, P.; Kingston, R.E. Isolation of a cDNA for HSF2: Evidence for two heat shock factor genes in humans. Proc. Natl. Acad. Sci. USA 1991, 88, 6911–6915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Álvarez, C.; Ángeles Bermúdez, M.; Romero, L.C.; Gotor, C.; García, I. Cysteine homeostasis plays an essential role in plant immunity. New Phytol. 2012, 193, 165–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gotor, C.; Álvarez, C.; Bermúdez, M.Á.; Moreno, I.; García, I.; Romero, L.C. Low abundance does not mean less importance in cysteine metabolism. Plant Signal. Behav. 2010, 5, 1028–1030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wawrzynska, A.; Moniuszko, G.; Sirko, A. Links between ethylene and sulfur nutrition—a regulatory interplay or just metabolite association? Front. Plant Sci. 2015, 6, 1053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masood, A.; Khan, M.I.R.; Fatma, M.; Asgher, M.; Per, T.S.; Khan, N.A. Involvement of ethylene in gibberellic acid-induced sulfur assimilation, photosynthetic responses, and alleviation of cadmium stress in mustard. Plant Physiol. Biochem. 2016, 104, 1–10. [Google Scholar] [CrossRef]
- Nazar, R.; Khan, M.I.R.; Iqbal, N.; Masood, A.; Khan, N.A. Involvement of ethylene in reversal of salt-inhibited photosynthesis by sulfur in mustard. Physiol. Plantar. 2014, 152, 331–344. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Nazir, F.; Asgher, M.; Per, T.S.; Khan, N.A. Selenium and sulfur influence ethylene formation and alleviate cadmium-induced oxidative stress by improving proline and glutathione production in wheat. J. Plant Physiol. 2015, 173, 9–18. [Google Scholar] [CrossRef]
- Bloem, E.; Haneklaus, S.; Schnug, E. Comparative effects of sulfur and nitrogen fertilization and post-harvest processing parameters on the glucotropaeolin content of Tropaeolum majus L. J. Sci. Food Agric. 2007, 87, 1576–1585. [Google Scholar] [CrossRef]
- Koprivova, A.; North, K.A.; Kopriva, S. Complex signaling network in regulation of adenosine 5′-phosphosulfate reductase by salt stress in Arabidopsis roots. Plant Physiol. 2008, 146, 1408–1420. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Chi, Q.; Liu, Q.; Wang, D.; Zhang, Y.; Li, S. Atmospheric H2S triggers immune damage by activating the TLR-7/MyD88/NF-κB pathway and NLRP3 inflammasome in broiler thymus. Chemosphere 2019, 237, 124427. [Google Scholar] [CrossRef]
- Iqbal, N.; Umar, S.; Khan, N.A.; Corpas, F.J. Nitric oxide and hydrogen sulfide coordinately reduce glucose sensitivity and decrease oxidative stress via ascorbate-glutathione cycle in heat-stressed wheat (Triticum aestivum L.) plants. Antioxidants 2021, 10, 108. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S. Recent advancements in the mechanism of nitric oxide signaling associated with hydrogen sulfide and melatonin crosstalk during ethylene-induced fruit ripening in plants. Nitric Oxide 2019, 82, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liang, X.; Huang, J.; Zhang, D.; Lu, H.; Liu, Z.; Bi, Y. Involvement of ethylene and hydrogen peroxide in induction of alternative respiratory pathway in salt-treated Arabidopsis calluses. Plant Cell Physiol. 2010, 51, 1754–1765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sehar, Z.; Jahan, B.; Masood, A.; Anjum, N.A.; Khan, N.A. Hydrogen peroxide potentiates defense system in presence of sulfur to protect chloroplast damage and photosynthesis of wheat under drought stress. Physiol. Plant. 2021, 172, 922–934. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Wu, X.; Sun, M.; Peng, F. Hydrogen sulfide alleviates waterlogging-induced damage in peach seedlings via enhancing antioxidative system and inhibiting ethylene synthesis. Front. Plant Sci. 2020, 11, 696. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yang, B.; Hao, Z.; Zhu, J.; Zhang, Y.; Xu, T. Exogenous hydrogen sulfide ameliorates seed germination and seedling growth of cauliflower under lead stress and its antioxidant role. J. Plant Growth Regul. 2018, 37, 5–15. [Google Scholar] [CrossRef]

| S. No. | Plant | Ethylene Source/Concentration | Temperature Range | Response | Reference |
|---|---|---|---|---|---|
| 1. | Agrostis stolonifera | 100 µmol L−1 ACC | 35 °C | Increased activity of ascorbate peroxidase, superoxide dismutase, and catalase and regulated thermotolerance | [110] |
| 2. | Cynara cardunculus | 30 µmol L−1 ETH | 30 °C | Improved seed germination, root growth, and seed vigor | [112] |
| 3. | Lactuca sativa | 10 μM ACC | 35 °C | Improved seed germination performance | [111] |
| 4. | Oryza sativa | 10 μM ACC | 45 °C | Decreased oxidative stress, upregulated antioxidant defense system, and reduced ion leakage | [107] |
| 5. | Solanum lycopersicum | 1 μL L−1 ETH | 50 °C | Promoted expression of ethylene-induced responsive genes and improved pollen quality | [47] |
| 6. | Solanum lycopersicum | 1 μL L−1 ETH | 50 °C | Alleviated oxidative stress and maintained redox homeostasis | [109] |
| 7. | Oryza sativa | 1.6 mM ETH | 40 °C | Stimulated antioxidant defense system, improved carbohydrate metabolism, and increased photosynthetic and growth attributes | [20] |
| S. No. | Plant | H2S Source | Temperature Range | Response | References |
|---|---|---|---|---|---|
| 1. | Fragaria | 100 µM NAHS | 42 °C | Increased activity of antioxidant enzymes and increased expression of antioxidant enzymes | [131] |
| 2. | Nicotiana tabacum | 50 µM NAHS | 42 °C | Increased vitality of cells and alleviated electrolyte leakage | [122] |
| 3. | Nicotiana tabacum | 50 µM NAHS | 43 °C | Increased S-containing compounds such as cysteine and glutathione as well as antioxidant enzymes | [132] |
| 4. | Zea mays | 1.2 mmol NAHS | 47 °C | Decreased oxidative stress and upregulated antioxidant defense system | [133] |
| 5. | Zea mays | 1.5 mmol NAHS | 38 °C | Increased proline biosynthesis | [134] |
| 6. | Zea mays | 0.5 mmol NAHS | 47 °C | Increased betaine accumulation | [135] |
| 7. | Zea mays | 500 µM NAHS | 48 °C | Increased endogenous H2S accumulation | [132] |
| S. No. | Plant | Sulfur Concentration | Temperature Range | Response | Reference |
|---|---|---|---|---|---|
| 1. | Brassica napus | 8.7 μM | 33 °C | Improved grain quality and enhanced nutritional compounds | [142] |
| 2. | Brassica napus | 500 ppm | 28 °C | Improved growth, yield, and physiological characteristics | [143] |
| 3. | Brassica napus | 500 ppm | 28 °C | Improved physiological and yield characteristics | [144] |
| 4. | Cymopsis tetragonoloba | 100 mg S kg−1 soil | 45 °C | Enhanced carbohydrate metabolism and mitigated oxidative damage | [140] |
| 5. | Solanum lycopersicum | 2–8 ppm | 45 °C | Improved growth, photosynthesis, and biochemical attributes | [145] |
| 6. | Triticum aestivum | 130 kg ha−1 S-coated urea | 33 °C | Improved growth rate, yield, physiological parameters, and N content | [146] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sehar, Z.; Gautam, H.; Iqbal, N.; Alvi, A.F.; Jahan, B.; Fatma, M.; Albaqami, M.; Khan, N.A. The Functional Interplay between Ethylene, Hydrogen Sulfide, and Sulfur in Plant Heat Stress Tolerance. Biomolecules 2022, 12, 678. https://doi.org/10.3390/biom12050678
Sehar Z, Gautam H, Iqbal N, Alvi AF, Jahan B, Fatma M, Albaqami M, Khan NA. The Functional Interplay between Ethylene, Hydrogen Sulfide, and Sulfur in Plant Heat Stress Tolerance. Biomolecules. 2022; 12(5):678. https://doi.org/10.3390/biom12050678
Chicago/Turabian StyleSehar, Zebus, Harsha Gautam, Noushina Iqbal, Ameena Fatima Alvi, Badar Jahan, Mehar Fatma, Mohammed Albaqami, and Nafees A. Khan. 2022. "The Functional Interplay between Ethylene, Hydrogen Sulfide, and Sulfur in Plant Heat Stress Tolerance" Biomolecules 12, no. 5: 678. https://doi.org/10.3390/biom12050678
APA StyleSehar, Z., Gautam, H., Iqbal, N., Alvi, A. F., Jahan, B., Fatma, M., Albaqami, M., & Khan, N. A. (2022). The Functional Interplay between Ethylene, Hydrogen Sulfide, and Sulfur in Plant Heat Stress Tolerance. Biomolecules, 12(5), 678. https://doi.org/10.3390/biom12050678











