Brassinosteroids in Plants: Crosstalk with Small-Molecule Compounds
Abstract
:1. Introduction
2. Discovery and Development of Brassinosteroids
2.1. Discovery and Biosynthesis
2.2. The Roles of BR in Growth, Development, and Stress Response
3. Brassinosteroids and Nitric Oxide
4. Brassinosteroids and Ethylene
5. Brassinosteroids and Hydrogen Peroxide
6. Brassinosteroids and Hydrogen Sulfide
7. Brassinosteroids and Sphingolipids
8. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, K.; Zhou, X.M.; Sun, X.P.; Li, G.H.; Hou, L.; Zhao, S.Z.; Zhao, C.Z.; Ma, C.L.; Li, P.C.; Wang, X.J. Coordination between midasin 1-mediated ribosome biogenesis auxin for modulating plant development. J. Exp. Bot. 2021, 72, 2501–2513. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.J.; Zhang, M.L.; Wei, S.H.; Zhang, J.; Liao, W.B. Roles of nitric oxide in heavy metal stress in plants: Cross-talk with phytohormones protein s-nitrosylation. Environ. Pollut. 2020, 259, 113943. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.H.; Zhang, L.; Sun, Y.P.; Zheng, S.; Wang, J.; Zhang, T.G. Hydrogen peroxide is involved in strigolactone induced low temperature stress tolerance in rape seedlings (brassica rapa L.). Plant Physiol. Biochem. 2020, 157, 402–415. [Google Scholar] [CrossRef]
- Singh, H.; Bhat, J.A.; Singh, V.P.; Corpas, F.J.; Yadav, S.R. Auxin metabolic network regulates the plant response to metalloids stress. J. Hazard. Mater. 2020, 405, 124250. [Google Scholar]
- Dehghana, M.; Balouchib, H.; Yadavib, A.; Zare, E. Improve wheat (Triticum aestivum) performance by brassinolide application under different irrigation regimes. S. Afr. J. Bot. 2020, 130, 259–267. [Google Scholar]
- Soares, T.; Dias, D.; Oliveira, A.; Ribeiro, D.M.; Dias, L. Exogenous brassinosteroids increase lead stress tolerance in seed germination seedling growth of Brassica juncea L. Ecotoxicol. Environ. Saf. 2020, 193, 110296. [Google Scholar]
- Demissie, Z.A.; Huang, F.; Song, H.; Todd, A.T.; Loewen, M.C. Barley “uzu” wheat “uzu-like” brassinosteroid receptor BRI1 kinase domain variations modify phosphorylation activity in Vitro. Biochemistry 2020, 59, 2986–2997. [Google Scholar] [CrossRef]
- Nolan, T.M.; Vukašinović, N.; Liu, D.; Yin, Y.H. Brassinosteroids: Multidimensional regulators of plant growth, development, stress responses. Plant Cell 2020, 32, 295–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alam, M.N.; Zhang, L.; Yang, L.; Islam, M.R.; Liu, Y.; Luo, H.; Yang, P.; Wang, Q.; Chan, Z. Transcriptomic profiling of tall fescue in response to heat stress improved thermotolerance by melatonin 24-epibrassinolide. BMC Genomi. 2018, 19, 224. [Google Scholar]
- Li, J.; Wen, J.Q.; Lease, K.A.; Doke, J.T.; Tax, F.E.; Walker, J.C. BAK1, an Arabidopsis IRR receptor-like protein kinase, interacts with BRI1 modulates brassionosteroid signaling. Cell 2002, 110, 213. [Google Scholar] [CrossRef] [Green Version]
- She, J.; Han, Z.; Kim, T.W.; Wang, J.; Cheng, W.; Chang, J.; Shi, S.; Wang, J.; Yang, M.; Wang, Z.Y.; et al. Structural insight into brassinosteroid perception by BRI1. Nature 2011, 474, 472–476. [Google Scholar] [CrossRef] [Green Version]
- Jablonka, E. Inheritance systems the evolution of new levels of individuality. J. Theor. Biol. 1994, 170, 301–309. [Google Scholar] [CrossRef]
- Niu, L.J.; Yu, J.; Liao, W.B.; Xie, J.M.; Yu, J.H.; Lv, J.; Wu, Y. Proteomic investigation of S-nitrosylated proteins during NO-induced adventitious rooting of cucumber. Int. J. Mol. Sci. 2019, 20, 5363. [Google Scholar] [CrossRef] [Green Version]
- Wei, L.J.; Wang, C.L.; Liao, W.B. Hydrogen sulfide improves the vase life quality of cut roses chrysanthemums. J. Plant Growth Regul. 2021, 40, 2532–2547. [Google Scholar] [CrossRef]
- Wang, L.; Suo, X.; Liu, Y.; Liu, C.; Luo, M. Sphingosine promotes embryo biomass in upland cotton: A biochemical transcriptomic analysis. Biomolecules 2021, 11, 525. [Google Scholar] [CrossRef]
- Wei, L.J.; Zhang, J.; Wang, C.L.; Liao, W.B. Recent progress in the knowledge on the alleviating effect of nitric oxide on heavy metal stress in plants. Plant Physiol. Biochem. 2019, 147, 161–171. [Google Scholar] [CrossRef]
- Xu, X.T.; Jin, X.; Liao, W.B.; Dawuda, M.M.; Li, X.P.; Wang, M.; Niu, L.J.; Ren, P.J.; Zhu, Y.C. Nitric oxide is involved in ethylene-induced adventitious root development in cucumber (Cucumis sativus L.) explants. Sci. Hortic. 2017, 215, 65–71. [Google Scholar] [CrossRef]
- Li, S.; Zheng, H.; Lin, L.; Wang, F.; Sui, N. Roles of brassinosteroids in plant growth abiotic stress response. Plant Growth Regul. 2020, 93, 29–38. [Google Scholar] [CrossRef]
- Jiroutová, P.; Mikulík, J.; Novák, O.; Strnad, M.; Oklestkova, J. Brassinosteroids induce strong, dose-dependent inhibition of etiolated pea seedling growth correlated with ethylene production. Biomolecules 2019, 9, 849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, Y.; Min, F.; Qin, Z.; Lv, H.; Bai, M.Y. Hydrogen peroxide positively regulates brassinosteroid signaling through oxidation of the brassinazole-resistant1 transcription factor. Nat. Commun. 2018, 9, 1063. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.L.; Shao, L.H.; Zhang, W.; Zheng, F.X. Hydrogen sulfide induced by hydrogen peroxide mediates brassinosteroid-induced stomatal closure of Arabidopsis thaliana. Funct. Plant Biol. 2020, 48, 195–205. [Google Scholar] [CrossRef]
- Peres, A.L.; Soares, J.S.; Tavares, R.G.; Righetto, G.; Zullo, M.A.; Mandava, N.B.; Menossi, M. Brassinosteroids, the sixth class of phytohormones: A molecular view from the discovery to hormonal interactions in plant development stress adaptation. Int. J. Mol. Sci. 2019, 20, 331. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.Z.; Wang, C.L.; Wang, N.; Wei, L.J.; Li, W.F.; Yao, Y.D.; Liao, W.B. Roles of small-molecule compounds in plant adventitious root development. Biomolecules 2019, 9, 420. [Google Scholar] [CrossRef] [Green Version]
- Grove, M.D.; Spencer, G.F.; Rohwedder, W.K.; Mandava, N.; Cook, J.L.C. Brassinolide, a plant growth-promoting steroid isolated from Brassica napus pollen. Nature 1979, 281, 216–217. [Google Scholar] [CrossRef]
- Li, T.; Lei, W.; He, R.; Tang, X.; Zhang, D. Brassinosteroids regulate root meristem development by mediating BIN2-UPB1 module in Arabidopsis. PLoS Genet. 2020, 16, e1008883. [Google Scholar] [CrossRef] [PubMed]
- Bajguz, A.; Chmur, M.; Gruszka, D. Comprehensive overview of the brassinosteroid biosynthesis pathways: Substrates, products, inhibitors, connections. Front. Plant Sci. 2020, 11, 1034. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.L.; Li, J. Regulation of brassinosteroid biosynthesis inactivation. J. Integr. Plant Biol. 2012, 54, 746–759. [Google Scholar] [CrossRef]
- Wang, X.; Kota, U.; He, K.; Blackburn, K.; Li, J.; Goshe, M.B.; Clouse, S.D. Sequential transphosphorylation of the BRI1/BAK1 receptor kinase complex impacts early events in brassinosteroid signaling. Dev. Cell 2008, 15, 220–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Lu, Z.; Su, J.; Li, Y.; Cao, M.; Gao, H. 24-Epibrassinolide delays senescence in harvested kiwifruit through effects on mitochondrial membrane antioxidant activity. Lwt Food. Sci. Technol. 2020, 118, 108833. [Google Scholar] [CrossRef]
- Zhao, X.; Dou, L.; Gong, Z.; Wang, X.; Mao, T. BES1 hinders Abscisic Acid Insensitive5 promotes seed germination in Arabidopsis. New Phytol. 2019, 221, 908–991. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Li, J.; Ban, Q.; Han, S.; Rao, J. Role of brassinosteroids in Persimmon (Diospyros kaki L.) Fruit Ripening. J. Agric. Food Chem. 2018, 66, 2637–2644. [Google Scholar] [CrossRef]
- Hu, S.; Liu, L.; Li, S.; Shao, Z.; Meng, F.; Liu, H.; Duan, W.; Liang, D.; Zhu, C.; Xu, T.; et al. Regulation of fruit ripening by the brassinosteroid biosynthetic gene SlCYP90B3 via an ethylene-dependent pathway in tomato. Hortic. Res. 2020, 7, 163. [Google Scholar] [CrossRef]
- Yao, Y.; Zhao, N.; Xian, T.; Tu, S.; Pan, L.; Tu, K. Effect of 2,4-epibrassinolide treatment on the postharvest quality physiological metabolism of fresh daylily flower buds during storage. Sci. Hortic. 2017, 226, 110–116. [Google Scholar] [CrossRef]
- Lu, Z.; Wang, X.; Cao, M.; Li, Y.; Su, J.; Gao, H. Effect of 24-epibrassinolide on sugar metabolism delaying postharvest senescence of kiwifruit during ambient storage. Sci. Hortic. 2019, 253, 1–7. [Google Scholar] [CrossRef]
- Jan, S.; Noman, A.; Kaya, C.; Ashraf, M.; Alyemeni, M.N.; Ahmad, P. 24-epibrassinolide alleviates the injurious effects of Cr (VI) toxicity in tomato plants: Insights into growth, physio-biochemical attributes, antioxidant activity regulation of ascorbate–glutathione glyoxalase cycles. J. Plant Growth Regul. 2020, 39, 1587–1604. [Google Scholar] [CrossRef]
- Rattan, A.; Kapoor, D.; Kapoor, N.; Bhardwaj, R.; Sharma, A. Brassinosteroids regulate functional components of antioxidative defense system in salt stressed maize seedlings. J. Plant Growth Regul. 2020, 39, 1465–1475. [Google Scholar] [CrossRef]
- Fang, P.; Yan, M.; Chi, C.; Wang, M.Q.; Zhou, Y.H.; Zhou, J.; Shi, K.; Xia, X.J.; Foyer, C.H.; Yu, J. Brassinosteroids act as a positive regulator of photoprotection in response to chilling stress. Plant Physiol. 2019, 180, 2061–2076. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Sun, P.; Luo, Y.; Zhou, H.; Gao, J.; Zhao, D.; Pubu, Z.; Liu, J.; Hu, T. Brassinosteroids enhance cold tolerance in Elymus nutans via mediating redox homeostasis proline biosynthesis. Environ. Exp. Bot. 2019, 167, 103831. [Google Scholar] [CrossRef]
- Soliman, M.; Elkelish, A.; Souad, T.; Alhaithloul, H.; Farooq, M. Brassinosteroid seed priming with nitrogen supplementation improves salt tolerance in soybean. Physiol. Mol. Biol. Plants 2020, 26, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Nazir, F.; Fariduddin, Q.; Hussain, A.; Khan, T.A. Brassinosteroid hydrogen peroxide improve photosynthetic machinery, stomatal movement, root morphology cell viability reduce Cu-triggered oxidative burst in tomato. Ecotoxicol. Environ. Saf. 2020, 207, 111081. [Google Scholar] [CrossRef]
- Kolomeichuk, L.V.; Efimova, M.V.; Zlobin, I.E.; Kreslavski, V.D.; Allakhverdiev, S.I. 24-epibrassinolide alleviates the toxic effects of nacl on photosynthetic processes in potato plants. Photosynth. Res. 2020, 146, 151–163. [Google Scholar] [CrossRef]
- Junior, U.O.B.; Lima, M.D.R.; Alsahli, A.A.; Lobato, A.K.S. Unraveling the roles of brassinosteroids in alleviating drought stress in young Eucalyptus urophylla plants: Implications on redox homeostasis photosynthetic apparatus. Physiol. Plant. 2020, 172, 2. [Google Scholar]
- Yang, P.; Wang, Y.; Li, J.; Bian, Z. Effects of brassinosteroids on photosynthetic performance nitrogen metabolism in pepper seedlings under chilling stress. Agronomy 2019, 9, 839. [Google Scholar] [CrossRef] [Green Version]
- Maghsoudi, K.; Arvin, M.J.; Ashraf, M. Mitigation of arsenic toxicity in wheat by the exogenously applied salicylic acid, 24-epi-brassinolide silicon. J. Soil Sci. Plant Nut. 2019, 20, 577–588. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Q.; Zhang, Y.; Zhang, P.; Jiang, M. BIP130 enhances salt tolerance through modulation of aba synthesis scavenging ros in rice (Oryza sativa L.). Plant Growth Regul. 2020, 93, 163–173. [Google Scholar] [CrossRef]
- Nie, S.; Huang, S.; Wang, S.; Mao, Y.; Liu, J.; Ma, R.; Wang, X.F. Enhanced brassinosteroid signaling intensity via SLBRI1 overexpression negatively regulates drought resistance in a manner opposite of that via exogenous br application in tomato. Plant Physiol. Biochem. 2019, 138, 36–47. [Google Scholar] [CrossRef]
- Choudhary, S.P.; Oral, H.V.; Bhardwaj, R.; Yu, J.Q.; Tran, L.S.P. Interaction of brassinosteroids polyamines enhances copper stress tolerance in Raphanus sativus. J. Exp. Bot. 2012, 63, 5659–5675. [Google Scholar] [CrossRef] [Green Version]
- Kong, Q.; Mostafa, H.; Yang, W.; Wang, J.; Nuerawuti, M.; Wang, Y.; Song, J.; Zhang, X.; Ma, L.; Wang, H.; et al. Comparative transcriptome profiling reveals that brassinosteroid-mediated lignification plays an important role in garlic adaption to salt stress. Plant Physiol. Biochem. 2021, 158, 34–42. [Google Scholar] [CrossRef]
- Hussain, M.; Khan, T.A.; Yusuf, M.; Fariduddin, Q. Silicon-mediated role of 24-epibrassinolide in wheat under high-temperature stress. Environ. Sci. Pollut. Res. 2019, 26, 17163–17172. [Google Scholar] [CrossRef]
- Li, X.; Wei, J.P.; Ahammed, G.J. Brassinosteroids attenuate moderate high temperature-caused decline in tea quality by enhancing theanine biosynthesis in Camellia sinensis L. Front. Plant Sci. 2018, 9, 1016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, L.J.; Deng, X.G.; Zhang, L.E.; Zhu, T.; Lin, H.H. Nitric oxide as a signaling molecule in brassinosteroid-mediated virus resistance to cucumber mosaic virus in Arabidopsis thaliana. Physiol. Plant. 2017, 163, 196–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bibi, N.; Ahmed, I.M.; Fan, K.; Dawood, M.; Wang, X. Role of brassinosteroids in alleviating toxin-induced stress of Verticillium dahliae on cotton callus growth. Environ. Sci. Pollut. Res. 2017, 24, 12281–12292. [Google Scholar] [CrossRef] [PubMed]
- Hajihashemi, S.; Skalicky, M.; Brestic, M.; Pavla, V. Cross-talk between nitric oxide, hydrogen peroxide calcium in salt-stressed Chenopodium quinoa Willd. At seed germination stage. Plant Physiol. Biochem. 2020, 154, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.Z.; Wang, C.L.; Huo, J.Q.; Hu, W.L.; Liao, W.B. The involvement of NO in ABA-delayed the senescence of cut roses by maintaining water content antioxidant enzymes activity. Sci. Hortic. 2019, 247, 35–41. [Google Scholar] [CrossRef]
- Liao, W.B.; Huang, G.B.; Yu, J.H.; Zhang, M.L. Nitric oxide hydrogen peroxide alleviate drought stress in marigold explants promote its adventitious root development. Plant Physiol. Biochem. 2012, 58, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Ahammed, G.J.; Li, X.; Mao, Q.; Wan, H.; Zhou, G.; Cheng, Y. The SlWRKY81 transcription factor inhibits stomatal closure by attenuating nitric oxide accumulation in the guard cells of tomato under drought. Physiol. Plant 2020, 172, 885–895. [Google Scholar] [CrossRef]
- Asgher, M.; Per, T.S.; Masood, A.; Fatma, M.; Freschi, L.; Corpas, F.J.; Khan, N. Nitric oxide signaling its crosstalk with other plant growth regulators in plant responses to abiotic stress. Environ. Sci. Pollut. Res. 2017, 24, 2273–2285. [Google Scholar] [CrossRef]
- Li, S.W.; Yi, L.; Leng, Y.; Zeng, X.Y.; Ma, Y.H. Nitric oxide donor improves adventitious rooting in mung bean hypocotyl cuttings exposed to cadmium osmotic stresses. Environ. Exp. Bot. 2019, 164, 114–123. [Google Scholar] [CrossRef]
- Rezayian, M.; Ebrahimzadeh, H.; Niknam, V. Nitric oxide stimulates antioxidant system osmotic adjustment in soybean under drought stress. J. Soil Sci. Plant Nut. 2020, 20, 1122–1132. [Google Scholar] [CrossRef]
- Kong, W.W.; Huang, C.Y.; Chen, Q.; Zou, Y.J.; Zhao, M.R.; Zhang, J.X. Nitric oxide is involved in the regulation of trehalose accumulation under heat stress in Pleurotus eryngii var. tuoliensis. Biotechnol. Lett. 2012, 34, 1915–1919. [Google Scholar] [CrossRef]
- Qian, C.; Ji, Z.; Zhu, Q.; Qi, X.; Xiao, L. Effects of 1-mcp on proline, polyamine, nitric oxide metabolism in postharvest peach fruit under chilling stress. Hortic. Plant J. 2021, 7, 188–196. [Google Scholar] [CrossRef]
- Elkelish, A.; Ibrahim, M.; Ashour, H.; Bondok, A.; El-Gawad, H. Exogenous application of nitric oxide mitigates water stress reduces natural viral disease incidence of tomato plants subjected to deficit irrigation. Agronomy 2021, 11, 87. [Google Scholar] [CrossRef]
- Khan, M.N.; Siddiqui, M.H.; Mohammad, F.; Naeem, M. Interactive role of nitric oxide calcium chloride in enhancing tolerance to salt stress. Nitric Oxide Biol. Chem. 2012, 27, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Karpets, Y.; Shkliarevskyi, M.; Khripach, V.A.; Kolupaev, Y.E. State of enzymatic antioxidative system heat resistance of wheat plantlets treated by combination of 24-epibrassinolide no donor. Cereal Res. Commun. 2020, 49, 207–216. [Google Scholar] [CrossRef]
- Zhu, T.; Deng, X.G.; Tan, W.R.; Zhou, X.; Luo, S.S.; Han, X.Y.; Zhang, D.W.; Lin, H.H. Nitric oxide is involved in brassinosteroid-induced alternative respiratory pathway in Nicotiana benthamiana seedlings’ response to salt stress. Physiol. Plant. 2016, 156, 150–163. [Google Scholar] [CrossRef]
- Hahm, M.S.; Son, J.S.; Hwang, Y.J.; Kwon, D.K.; Ghim, S.Y. Alleviation of salt stress in pepper (Capsicum annum L.) plants by plant growth-promoting rhizobacteria. World J. Microb. Biot. 2017, 27, 1790–1797. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.T.; Wu, Y.; Liao, W.B.; Hu, L.L.; Yu, J.H. Nitric oxide is involved in the brassinolide-induced adventitious root development in cucumber. BMC Plant Biol. 2020, 20, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tossi, V.; Lorenzo, L.; Raúl, C. Pharmacological genetical evidence supporting nitric oxide requirement for 2,4-epibrassinolide regulation of root architecture in Arabidopsis thaliana. Plant Signal. Behav. 2013, 8, e24712. [Google Scholar] [CrossRef] [Green Version]
- Shi, C.; Qi, C.; Ren, H.; Huang, A.; Hei, S.; She, X. Ethylene mediates brassinosteroid-induced stomatal closure via Gα protein-activated hydrogen peroxide nitric oxide production in Arabidopsis. Plant J. 2015, 82, 280–301. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, L.; Ahammed, G.J.; Li, Z.X.; Wei, J.P.; Shen, C.; Han, W.Y. Nitric oxide mediates brassinosteroid-induced flavonoid biosynthesis in Camellia sinensis L. J. Plant Physiol. 2017, 214, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.G.; Dai, C.C. Nitric oxide brassinosteroids mediated fungal endophyte-induced volatile oil production through protein phosphorylation pathways in Atractylodes lancea Plantlets. J. Integr. Plant Biol. 2013, 55, 1136–1146. [Google Scholar] [CrossRef]
- Kaya, A.; Ma, B.; Mna, C.; Pac, D. Nitrate reductase rather than nitric oxide synthase activity is involved in 24-epibrassinolide-induced nitric oxide synthesis to improve tolerance to iron deficiency in strawberry (Fragaria × annassa) by up-regulating the ascorbate-glutathione cycle. Plant Physiol. Biochem. 2020, 151, 486–499. [Google Scholar] [CrossRef]
- Kaya, A.; Ma, B.; Mna, C.; Pac, D. The role of nitrate reductase in brassinosteroid-induced endogenous nitric oxide generation to improve cadmium stress tolerance of pepper plants by upregulating the ascorbate-glutathione cycle. Ecotoxicol. Environ. Safe 2020, 196, 110483. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.J.; Deng, X.G.; Zhu, T.; Zheng, T.; Li, P.X.; Wu, J.Q. Ethylene is involved in brassinosteroids induced alternative respiratory pathway in cucumber (Cucumis sativus L.) seedlings response to abiotic stress. Front. Plant Sci. 2015, 6, 982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, J.W.; He, R.; Yang, F.; Zou, L.; Zhang, D. Brassinosteroids is involved in ethylene-induced Pst DC3000 resistance in Nicotiana benthamiana. Plant Biol. 2019, 22, 309–316. [Google Scholar] [CrossRef]
- Khan, T.A.; Yusuf, M.; Ahmad, A.; Bashir, Z.; Saeed, T.; Fariduddin, Q.; Shamsul, H.; Mock, H.P.; Wu, T.Q. Proteomic physiological assessment of stress sensitive tolerant variety of tomato treated with brassinosteroids hydrogen peroxide under low-temperature stress. Food Chem. 2019, 289, 500–511. [Google Scholar] [CrossRef]
- Deng, X.G.; Zhu, T.; Zou, L.J.; Han, X.Y.; Zhou, X.; Xi, D.H.; Zhang, D.W.; Lin, H.H. Orchestration of hydrogen peroxide nitric oxide in brassinosteroid-mediated systemic virus resistance in Nicotiana benthamiana. Plant J. 2016, 85, 478–493. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.; Kim, S.Y.; Park, C.H.; Kim, S.K. BES1 negatively regulates the expression of ACC oxidase 2 to control the endogenous level of ethylene in Arabidopsis thaliana. Plant Signal. Behav. 2020, 16, 1850625. [Google Scholar]
- Jiang, Y.P.; Cheng, F.; Zhou, Y.H.; Yu, J.Q. Hydrogen peroxide functions as a secondary messenger for brassinosteroids-induced CO2 assimilation carbohydrate metabolism in Cucumis sativus. J. Zhejiang Univ. Sci. B 2012, 13, 811–823. [Google Scholar] [CrossRef]
- Corbacho, J.; Inês, C.; Paredes, M.A.; Labrador, J.; Cordeiro, A.M.; Gallardo, M.; Gomez-Jimenez, M.C. Modulation of sphingolipid long-chain base composition gene expression during early olive-fruit development, putative role of brassinosteroid. J. Plant Physiol. 2018, 231, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, J.Q.; Crawford, N.M. Identification characterization of a chlorate-resistant mutant of Arabidopsis thaliana with mutations in both nitrate reductase structural genes NIA1 NIA2. Mol. Gen. Genet. 1993, 239, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.G.; Zhu, T.; Zhang, D.W.; Lin, H.H. The alternative respiratory pathway is involved in brassinosteroid-induced environmental stress tolerance in Nicotiana benthamiana. J. Exp. Bot. 2015, 66, 6219–6232. [Google Scholar] [CrossRef] [Green Version]
- Salama, A.A.E.; Elgawad, H.G.A.; Ibrahim, M.F.M.; Mukherjee, S.; Elkelish, A.; Azab, E. Hydrogen peroxide supplementation in irrigation water alleviates drought stress boosts growth productivity of potato plants. Sustainability 2021, 13, 899. [Google Scholar]
- Ye, X.F.; Xue, Y.; Ling, T.; Wang, Y.; Chen, J. Cinnamaldehyde ameliorates cadmium-inhibited root elongation in tobacco seedlings via decreasing endogenous hydrogen sulfide production. Molecules 2017, 22, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alamri, S.; Kushwaha, B.K.; Singh, V.P.; Siddiqui, M.H. Dose dependent differential effects of toxic metal cadmium in tomato roots: Role of endogenous hydrogen sulfide. Ecotoxicol. Environ. Saf. 2020, 203, 110978. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.; Zhou, L.; Wang, Y.; Zhang, G.; Chen, X. Hydrogen sulfide acts downstream of jasmonic acid to inhibit stomatal development in Arabidopsis. Planta 2020, 251, 42. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.Y.; Fu, X.; Zhang, X.W.; Liu, F.J.; Ai, X.Z. Hydrogen sulfide is required for salicylic acid–induced chilling tolerance of cucumber seedlings. Protoplasma 2020, 5, 1543–1557. [Google Scholar] [CrossRef] [PubMed]
- Alden, K.P.; Dhondt-Cordelier, S.; Mcdonald, K.L.; Reape, T.J.; Ng, K.Y.; Mccabe, P.F.; Leaver, C.J. Sphingolipid long chain base phosphates can regulate apoptotic-like programmed cell death in plants. Biochem. Biophys. Res. Commun. 2011, 410, 574–580. [Google Scholar] [CrossRef]
- Montefusco, D.J.; Matmati, N.; Hannun, Y.A. The yeast sphingolipid signaling landscape. Chem. Phys. Lipids 2014, 177, 26–40. [Google Scholar] [CrossRef] [Green Version]
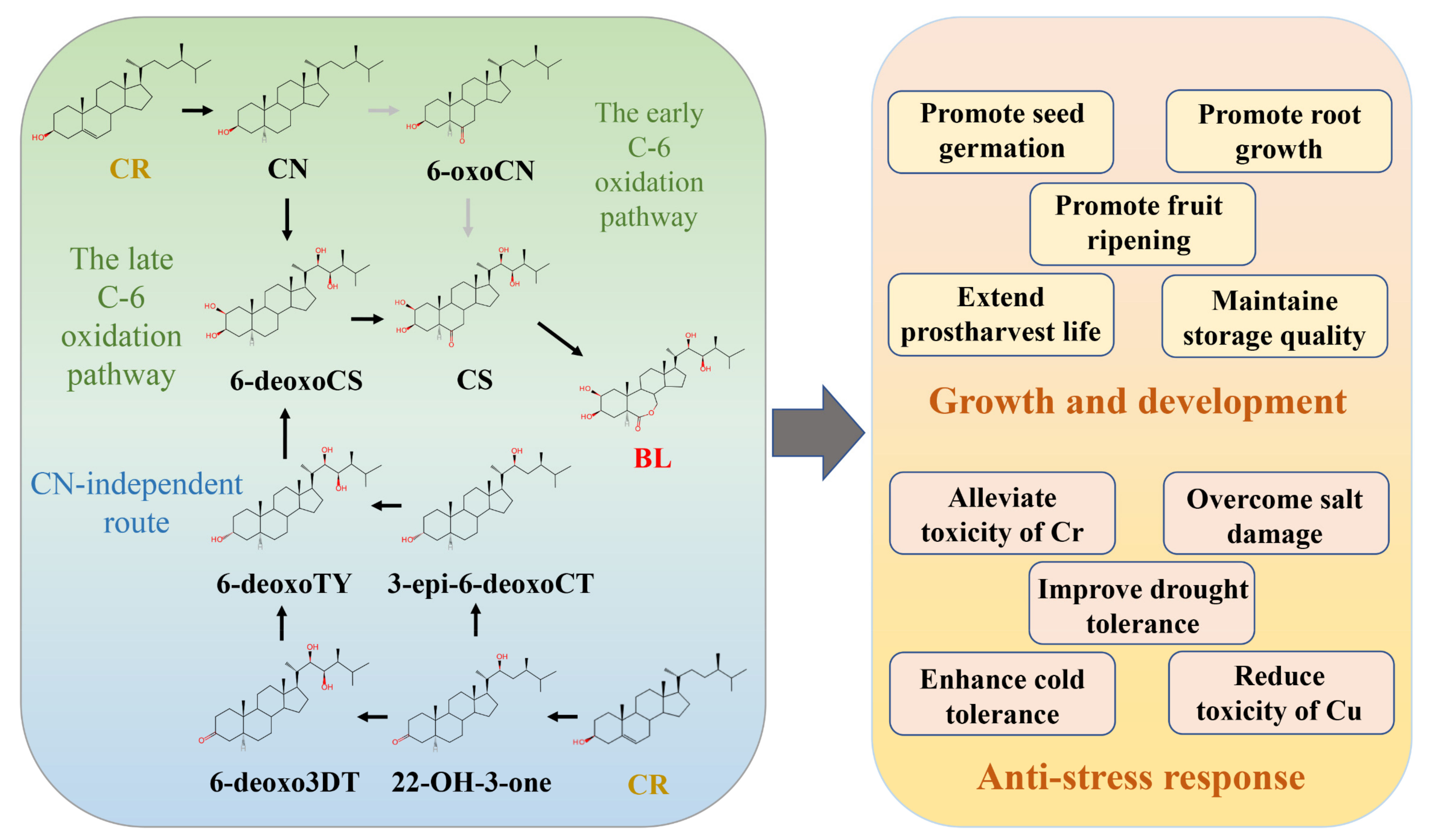
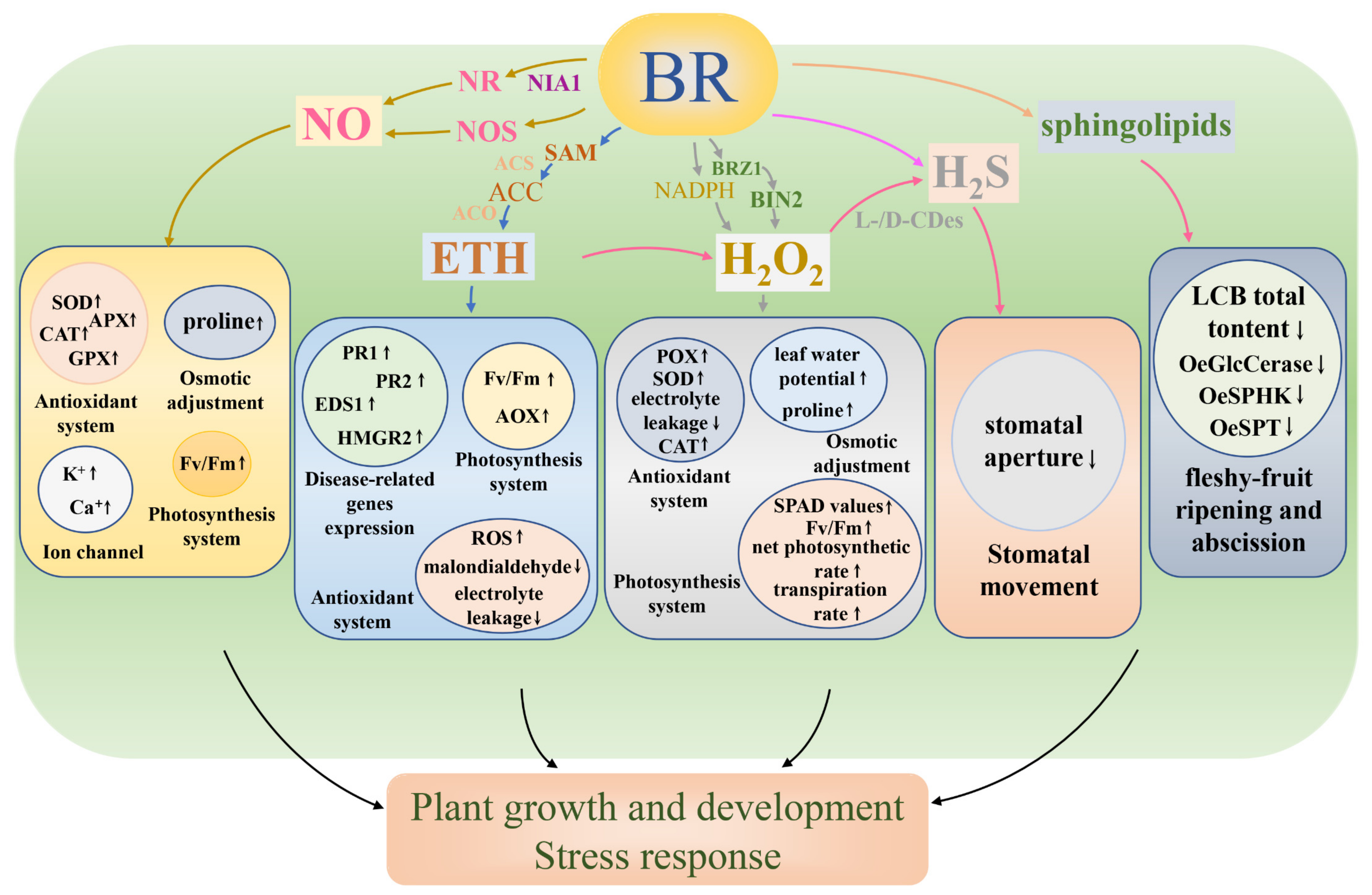
| Small-Molecule Compound | Type of BRs | Type of Stress | Plant Species | Plant Tissue | Effect | Reference |
|---|---|---|---|---|---|---|
| NO | EBR | Heat | Triticum aestivum L. | Seedlings | Improves the antioxidant system’s ability to enhance tolerance | [64] |
| EBR | Iron deficiency | Fragaria × annassa Duch. | Leaves | Improves the antioxidant system’s ability to enhance tolerance | [72] | |
| BL | Salt | Nicotiana benthamiana L. | Seedlings | Enhances tolerance by playing a role in the photosystem | [65] | |
| EBR | Cd | Capsicum annuum L. | Leaves | Improves the antioxidant system’s ability and the ASA-GSH cycle | [73] | |
| Ethylene | BL | Drought, salt, cold | Cucumis sativus L. | Seedlings | Increases the AOX activity to enhance photo-oxidative resistance | [74] |
| BL | Pst DC3000 | Nicotiana benthamiana L. | leaves | Improves the antioxidant system’s ability and activates the expression of disease-related genes | [75] | |
| H2O2 | EBR | Cold | Lycopersicon esculentum | Seedlings | Enhances the antioxidant system’s ability and the photosynthetic system | [76] |
| EBR | Cu | Solanum lycopersicum | Seedlings | Enhances the antioxidant system’s ability and the photosynthetic system as well as the total protein content | [40] | |
| BL | TMV | Nicotiana benthamiana L. | Leaves | Enhances the systemic virus resistance | [77] |
| Small-Molecule Compound | Type of BRs | Plant Species | Plant Tissue | Effect | Reference |
|---|---|---|---|---|---|
| NO | BL | Cucumis sativus L. | Roots | BL-induced NO generation promotes adventitious root formation | [67] |
| EBR | Arabidopsis thaliana L. | Roots | NO participates in EBR-induced changes in root architecture | [68] | |
| EBR | Arabidopsis thaliana L. | Roots | EBR-induced NO affects the stomatal closure of the root system | [69] | |
| BL | Atractylodes lancea | Plantlets | BL and NO activate secondary metabolites and improve the medicinal value | [71] | |
| Ethylene | EBR | Pisum sativum L. | Seedlings | EBR-induced ethylene inhibits seedling growth | [19] |
| - | Solanum lycopersicum | Fruits | The BR biosynthetic gene SICYP90B3-OE enhances ethylene generation to promote fruit ripening | [32] | |
| BL | Arabidopsis thaliana L. | Seedlings | The BR transcriptional factor BES1 regulates the expression of ACO2 to maintain the level of endogenous ethylene | [78] | |
| H2O2 | EBR | Cucumis sativus L. | Seedlings | EBR and H2O2 co-regulate the sugar metabolism and Calvin cycle through the redox signaling pathway | [79] |
| BL | Arabidopsis thaliana L. | Seedlings | BL-induced H2O2 promotes hypocotyl elongation | [20] | |
| H2S | EBR | Arabidopsis thaliana L. | Leaves | H2S participates in EBR-induced stomatal closure | [21] |
| Sphingolipids | EBR | Olea europaea L. | Fruits | BRs negatively regulate sphingolipid content in fruit | [80] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, D.; Wei, L.; Liao, W. Brassinosteroids in Plants: Crosstalk with Small-Molecule Compounds. Biomolecules 2021, 11, 1800. https://doi.org/10.3390/biom11121800
Hu D, Wei L, Liao W. Brassinosteroids in Plants: Crosstalk with Small-Molecule Compounds. Biomolecules. 2021; 11(12):1800. https://doi.org/10.3390/biom11121800
Chicago/Turabian StyleHu, Dongliang, Lijuan Wei, and Weibiao Liao. 2021. "Brassinosteroids in Plants: Crosstalk with Small-Molecule Compounds" Biomolecules 11, no. 12: 1800. https://doi.org/10.3390/biom11121800







