Abstract
Cisplatin is an FDA approved anti-cancer drug that is widely used for the treatment of a variety of solid tumors. However, the severe adverse effects of cisplatin, particularly kidney toxicity, restrict its clinical and medication applications. The major mechanisms of cisplatin-induced renal toxicity involve oxidative stress, inflammation, and renal fibrosis, which are covered in this short review. In particular, we review the underlying mechanisms of cisplatin kidney injury in the context of NAD+-dependent redox enzymes including mitochondrial complex I, NAD kinase, CD38, sirtuins, poly-ADP ribosylase polymerase, and nicotinamide nucleotide transhydrogenase (NNT) and their potential contributing roles in the amelioration of cisplatin-induced kidney injury conferred by natural products derived from plants. We also cover general procedures used to create animal models of cisplatin-induced kidney injury involving mice and rats. We highlight the fact that more studies will be needed to dissect the role of each NAD+-dependent redox enzyme and its involvement in modulating cisplatin-induced kidney injury, in conjunction with intensive research in NAD+ redox biology and the protective effects of natural products against cisplatin-induced kidney injury.
1. Introduction
Cisplatin is a widely used anti-solid tumor drug that can target a variety of cancers including those of the breast, ovary, lung, testis, head, and neck [1,2,3,4,5]. However, cisplatin’s clinical application and efficacy is highly limited due to its severe adverse effects, in particular, its nephrotoxicity [6,7,8,9,10,11]. It has been estimated that nearly 30% of cancer patients receiving cisplatin treatment could exhibit acute kidney injury (AKI) after the ingestion of a single high dose of cisplatin [12]. If cisplatin-induced AKI is left unmanaged, patients can develop chronic kidney disease (CKD) that can progress to end-stage kidney failure and may also increase the risk of death [13,14,15].
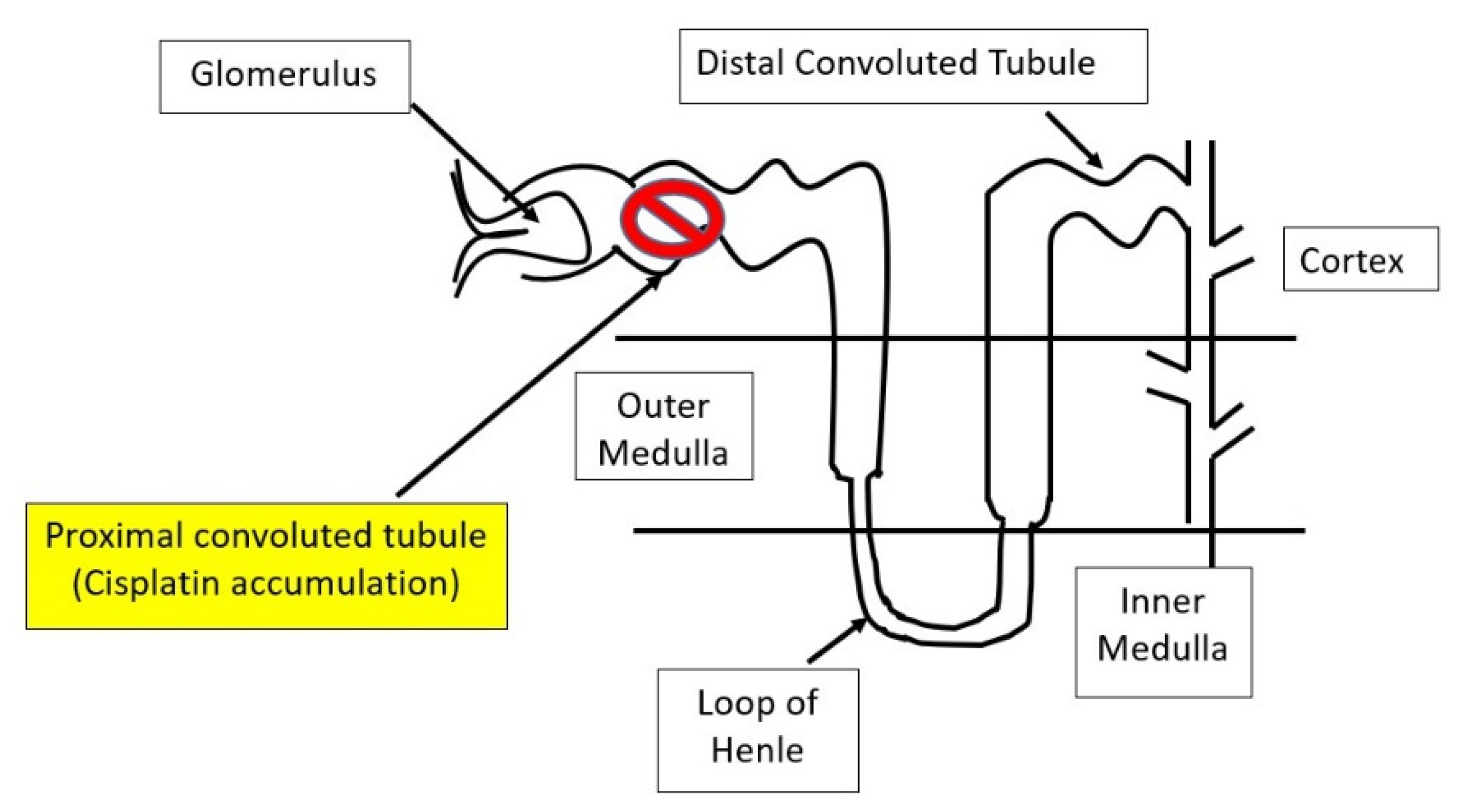
As a vital organ responsible for removal and elimination of cisplatin and its metabolites from the body, the kidney can sustain major damaging effects of cisplatin [16,17]. It is believed that cisplatin accumulates in the proximal tubular region of a nephron [18] (Figure 1) and the proximal tubular epithelial mitochondria are major intracellular sites of cisplatin accumulation [19,20]. Therefore, cisplatin can disrupt mitochondrial function including mitochondrial membrane potential, electron transport chain, the Krebs cycle, and oxidative phosphorylation [20]. Moreover, as the major underlying mechanism of action of cisplatin is its binding to DNA, thus interfering with DNA replication and cancer cell survival, mitochondrial DNA replication in the nephron can also be impaired [20,21], leading to abnormal mitochondrial genesis and kidney dysfunction [21]. Given that the disruption of numerous mitochondrial pathways can eventually converge on the increased mitochondrial generation of reactive oxygen species (ROS), antioxidants, in particular those derived from natural plants, have been widely used to counteract cisplatin kidney toxicity in preclinical and clinical settings [22,23].

Figure 1.
Diagram showing the proximal convoluted tubule (PCT) as the major site of cisplatin accumulation and toxicity in the nephrons.
In this article, we review the major existing mechanisms of cisplatin-induced kidney injury, the role of major NAD+-dependent redox enzymes in cisplatin-induced nephrotoxicity, and the protective effects of natural products that are derived from plants, with particular reference to their anti-oxidative stress roles and redox maintenance capacity. We also briefly cover rodent models used for studying cisplatin-induced kidney injury and highlight the need for dissecting the role of redox-dependent enzymes in treating cisplatin-induced kidney injury.
2. Methods
We conducted searches using PubMed, Google, and Science Direct. Search terms included “cisplatin”, “kidney injury” or “renal toxicity” and “nephroprotection” or “nephroprotective”. Among the search results, we only chose articles that involve plant extracts or compounds derived from plants including herbs and vegetables. If a compound or a plant extract has been studied by many authors, we selected only the most recent studies, as these studies likely cited previous published articles on the same compound or extract.
3. Major Molecular Mechanisms of Cisplatin-Induced Kidney Injury
As mentioned above, the major site of cisplatin accumulation in the nephron is the proximal tubules [24,25,26]. The entry of cisplatin into the tubular epithelial cells is thought to be mediated by two receptors: Crt1 and OCT2 (copper transporter 1 and organic cation transporter 2) [17]. Once inside the cells, cisplatin can rapidly accumulate in the mitochondria and damage mitochondrial components such as metabolic enzymes and mitochondrial DNA (mtDNA) [20]. It has been reported that mtDNA cisplatin adducts are more abundant than that of nuclear DNA [20], demonstrating that mitochondria are the major site of intracellular cisplatin accumulation. This would also indicate that mitochondria are the major organelle that receives cisplatin attack. As damage to mitochondria often culminates in an elevated level of ROS production, mitochondrial oxidative stress has been thought to be a major underlying mechanism of cisplatin-induced kidney injury [20,21,27].
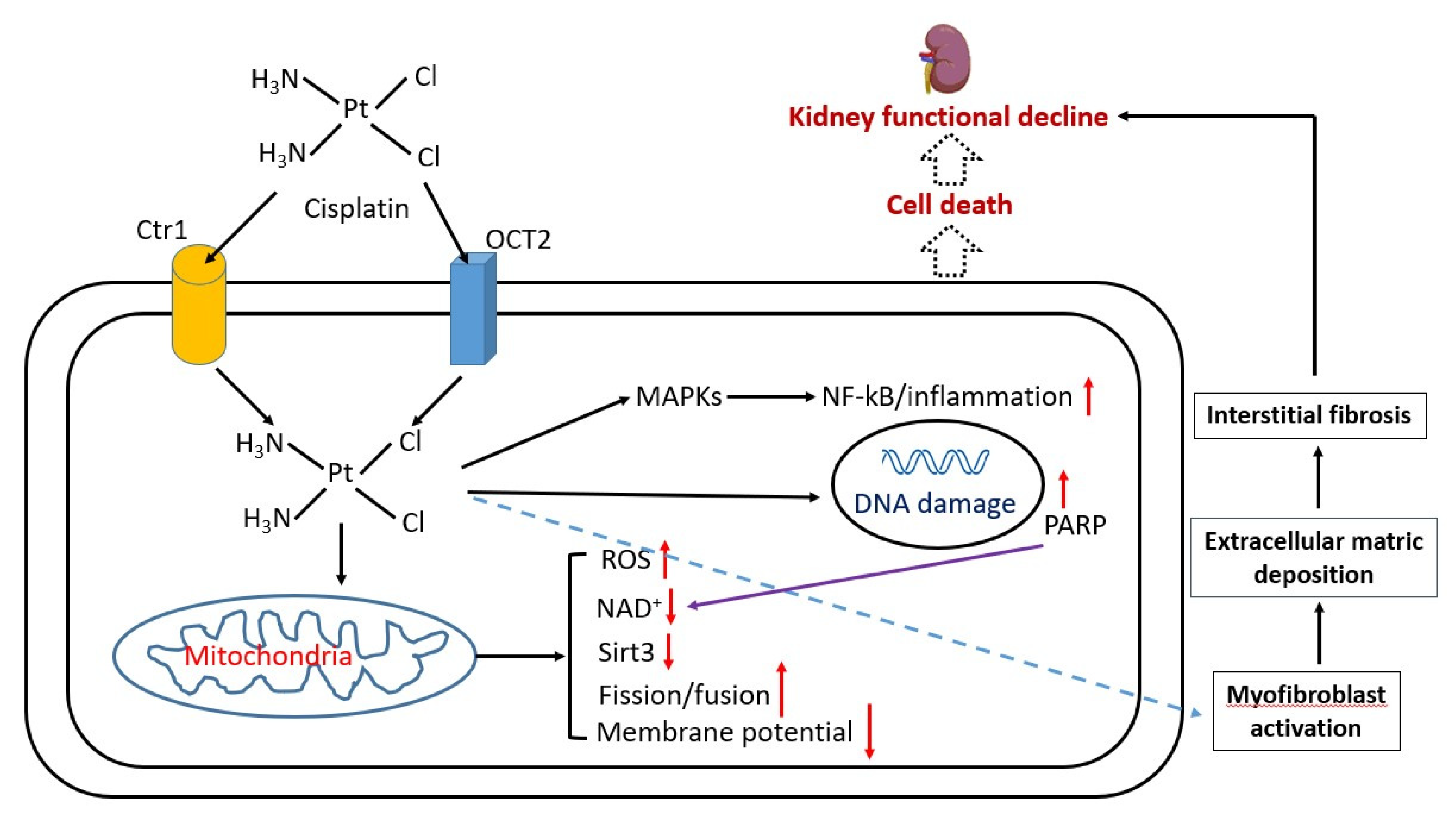
In addition to the oxidative stress implicated in the pathogenesis of cisplatin kidney injury [28,29,30,31,32,33], inflammation and renal fibrosis have also been postulated to be involved in cisplatin-induced kidney injury [34,35,36,37,38,39]. Cisplatin-induced kidney injury may also involve NAD+ redox signaling pathways such as sirt3 [40], poly-ADP ribosylase (PARP) [41], and mitochondrial dynamics [18] including mitochondrial fission and fusion [42,43,44]. The eventual outcome of these cisplatin-impaired pathways converges toward cell death, renal fibrosis, and functional decline of the kidney [45]. Figure 2 summarizes the major molecular mechanisms that underlie cisplatin-induced kidney injury.

Figure 2.
Major pathological mechanisms of cisplatin-induced kidney injury. Cisplatin enters into cell via copper transporter 1 (Ctr1) or organic anion transporter 2 (OCT2) receptors on the cell surface. Once inside the cell, cisplatin can go on to elicit a variety of actions or cellular responses such as nuclear and mitochondrial DNA damage, perturbation of mitochondrial function that can elevate ROS production, and decrease in NAD content and decrease in activity of NAD-dependent enzymes such as sirtuins. DNA damage could activate PARP, which consumes NAD, and in turn could further lower the NAD content, leading to NAD redox imbalance. Cisplatin can also activate inflammation-signaling pathways such as NF-kB activation via MAPKs. These events can result in interstitial fibrosis and eventual kidney failure.
4. Rodent Models of Cisplatin-Induced Kidney Injury
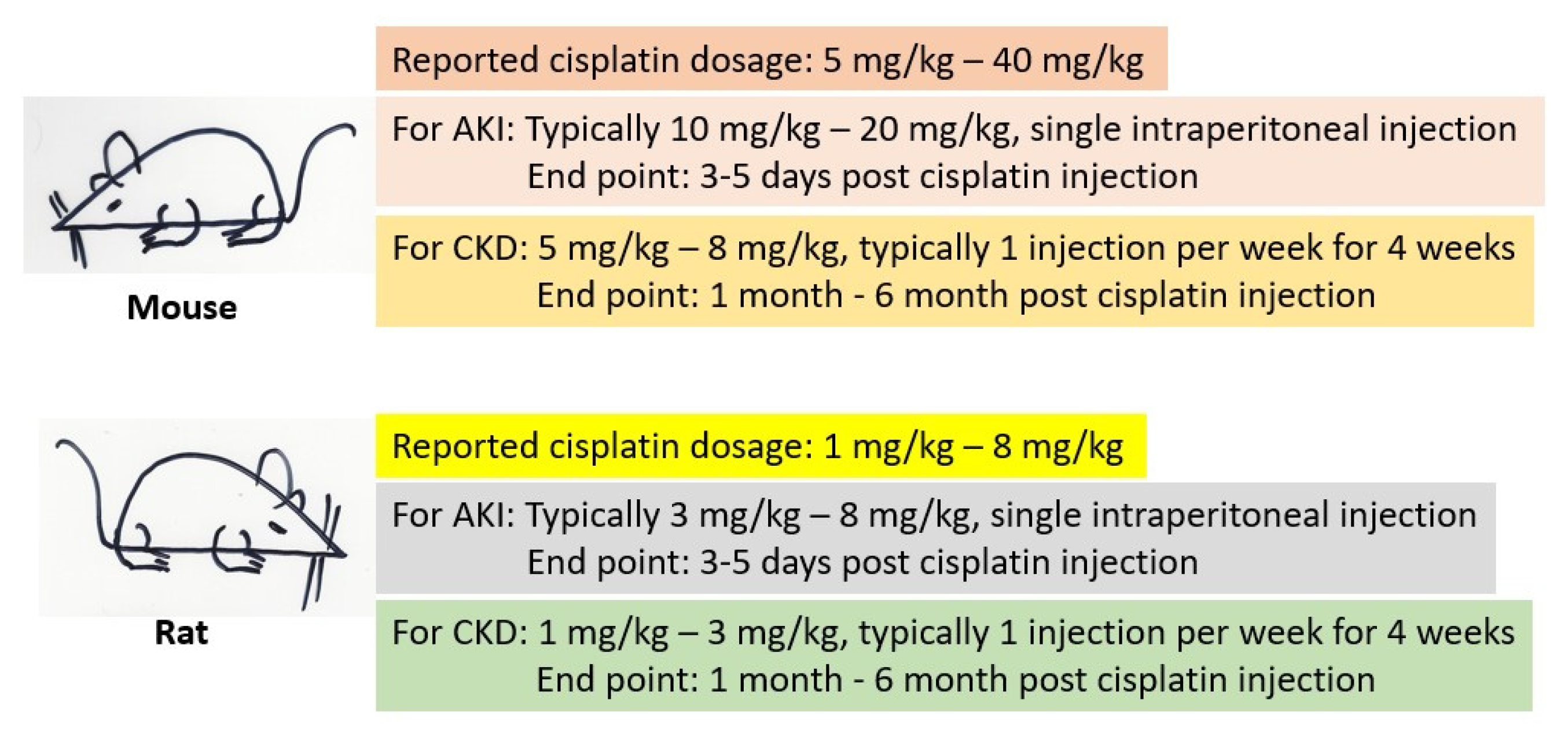
When it comes to animal models, mice and rats are the widely used species for investigating the mechanisms of cisplatin kidney injury and evaluating the antioxidant properties of a variety of natural products [46,47,48,49]. Both AKI and CKD models can be created, depending on the objective of the studies, and there are no standard operation procedures that can be followed [48,49]. A general scheme for the use of mice and rats as cisplatin-induced kidney injury models is shown in Figure 3. For AKI, a single high dose of cisplatin is often applied to either the mouse or the rat. For CKD, multiple low dose cisplatin administration is often used and the treatment frequency and duration can also vary depending on the purpose of the studies. It has been reported that weekly intraperitoneal injection of low-dose cisplatin for 4 weeks can create a robust model of CKD [50,51]. However, the end point analysis of kidney function and measurement of kidney functional parameters after low-dose cisplatin induction of CKD can range from weeks to months [51,52]. It should be pointed out that many cisplatin studies using rodent models only involve healthy animals, instead of animals bearing cancers or tumors. Therefore, data obtained from healthy animals may not be comparable to those from cancer animals, given that cancer itself can affect kidney function and the vulnerability of a kidney to cisplatin toxicity [49]. Additionally, differences in genetic background of mouse strains can also affect the susceptibility of the kidneys to cisplatin challenges [53]. For example, the C57BL/J mouse lacks nicotinamide nucleotide transhydrogenase (NNT) [54,55,56,57,58,59] and should be used with extreme caution when the focus is studying NAD+ redox biochemistry in kidney disease.

Figure 3.
Outlines of rodent models used for studying cisplatin-induced kidney injury. Shown are the dose ranges for either mice or rats involving either AKI or CKD. It should be noted that these are just general guidelines for designing an experiment and should be modified for specific experimental objectives if needed.
5. Effects of Cisplatin on Major Individual NAD+-Dependent Redox Enzymes
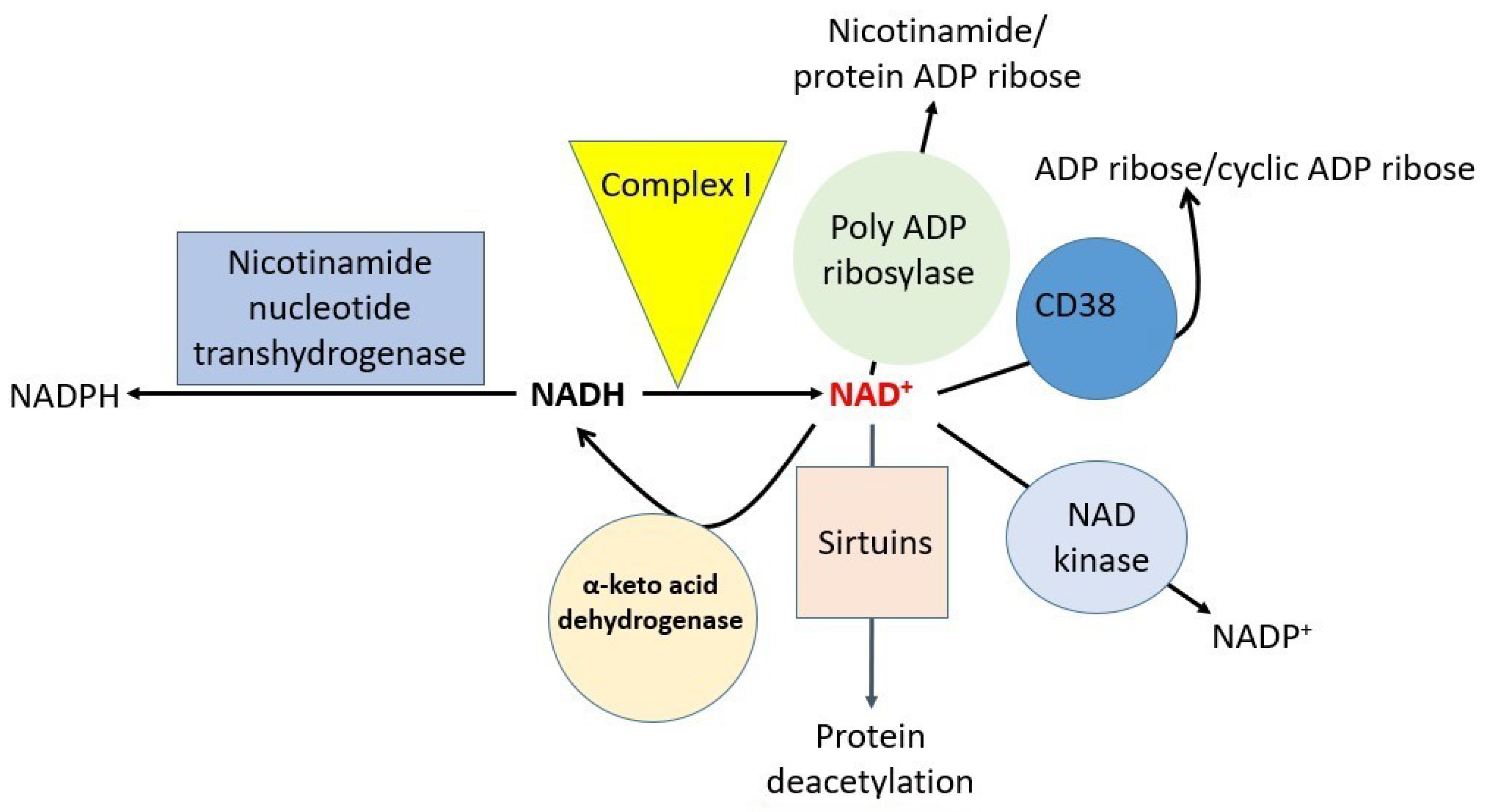
Targeting NAD+ redox balance has been suggested as a strategy for fighting cisplatin-induced kidney injury [18,60]. Therefore, there has been an increasing interest in studying redox biochemistry and NAD+ redox signaling in the pathogenesis of cisplatin renal toxicity [32,61,62,63,64]. The major NAD+-dependent redox enzymes that may be involved in cisplatin-induced kidney injury are shown in Figure 4. These include mitochondrial complex I [65,66], sirtuins [40,67,68], alpha-keto acid dehydrogenases involving dihydrolipoamide dehydrogenase [69], NAD kinase (NADK) [70,71,72,73], CD38 [74,75,76], poly-ADP ribosylase [77,78,79], and nicotinamide nucleotide transhydrogenase (NNT) [57,80,81]. Numerous natural products that possess antioxidant activities have been shown to display antioxidant properties such as the inhibition of lipid peroxidation, DNA damage, and protein oxidation, which are collectively the popular parameters used to assess oxidative stress and antioxidant natural products [69,82,83,84]. However, many investigations did not analyze further to pinpoint the NAD+-implicated molecular mechanisms of natural plant products that are being tested. For example, with respect to NAD+-dependent oxidative stress and amelioration, the determination of the exact NAD+-involved redox enzymes that are involved or are modulated by the tested natural products has largely been unaddressed. Another example is mitochondrial complex I. Although it has been reported that complex I-generated superoxide anion is involved in cisplatin-induced kidney injury [19,85,86], exactly which subunits of complex I are responsible for the eventual superoxide production upon cisplatin stimulation have not been explored. In particular, future studies should focus on dissecting these potential NAD+-dependent redox enzymes involved in cisplatin-induced kidney injury. Nevertheless, a limited number of studies have shed light on these NAD+-dependent redox enzymes. For example, it has been reported that CD38 can mediate calcium mobilization in cisplatin-induced kidney injury [87]. Likewise, poly-ADP ribosylase has been found to be activated in cisplatin-induced kidney injury [88] and enhancement of sirtuin protein function can attenuate cisplatin-induced kidney injury [89]. Of note, studies on the potential modulation of NNT, complex I, NADK, and alpha keto acid dehydrogenase by plant-derived natural products are extremely lacking. Future studies may also need to be conducted on other cellular components, such as electron transport chain components complex II to IV, mitochondrial dynamics and biogenesis, and TCA cycle components as well as the fatty acid oxidation pathways. Elucidating the mechanisms of cisplatin damage to these components not only can explain the mechanisms of action of cisplatin, but can also provide novel insights into further strategies designed to counteract cisplatin kidney toxicity. It is conceivable that damage to the redox enzymes shown in Figure 4 would impair NAD+-associated redox balance, thereby accentuating kidney injury by cisplatin. Moreover, it is also conceivable that approaches elevating NAD+ content may lead to therapies [18,60,90].

Figure 4.
Major NAD-dependent redox enzymes that are potentially involved in cisplatin-induced kidney toxicity.
It should be noted that among the major enzymes shown in Figure 4, poly-ADP ribosylase (PARP), in particular, PARP1, has been demonstrated to exert regulatory effects on the expression of many inflammatory proteins including IL-1-beta, TNF-alpha, IL-6, and toll-like receptor 4 (TLR4) [91]. PARP1 is upstream of TLR4, as pharmacological inhibition of PARP1 can attenuate the deleterious effect of cisplatin-induced inflammation in the kidney [92]. Likewise, knockout of TLR4 is protective against cisplatin-induced kidney injury [93,94] indicating that TLR4, among other TLRs [95], is required for cisplatin-induced renal toxicity. The downstream pathways of TLR4 such as the JNK and p38 pathways are likely involved in TLR4 knockout nephroprotection, as activation of each pathway by cisplatin was mitigated in the TLR4 knockout animals [91]. Nonetheless, how this signaling cascade from PARP1 to TLR4 to JNK and p38 is implicated in NAD+/NADH redox imbalance involved in cisplatin-induced kidney injury remains to be investigated in detail. Additionally, whether the other enzymes in Figure 4 could have a similar signaling role to that of PARP1 in regulating TLRs-mediated inflammation response in cisplatin-induced kidney injury also remain to be studied.
6. Counteracting Effects of Natural Products Derived from Plants
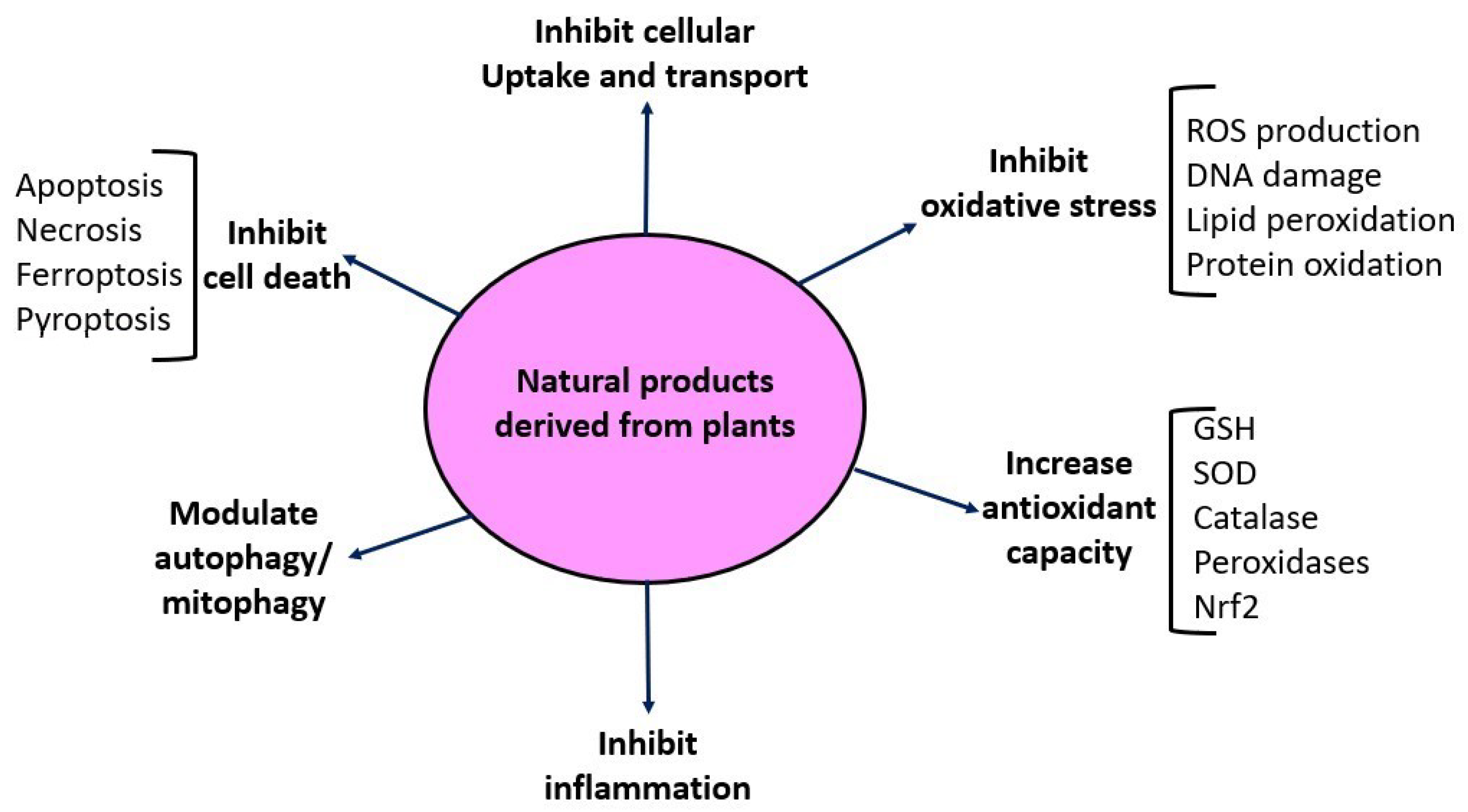
As shown in Table 1, where references are also provided, numerous natural products derived from plants, whether as a purified single compound or in an extract, have been tested for their counteracting effects on cisplatin-induced kidney injury. The general mechanisms of these natural products are summarized in Figure 5. These include blockage of cisplatin renal uptake and transportation [22], inhibition of oxidative stress [96,97], inhibition of inflammation [98,99], inhibition of P53 signaling pathways [52], inhibition of mitogen-activated protein kinases [100], attenuation of cell death, and enhancement of cellular antioxidant defense systems such as SOD, catalase, and the Nrf2 pathway [101,102]. Autophagy and mitophagy are also involved in cisplatin-induced kidney injury [51,103] and can be modulated by natural products for protective purposes [104,105,106,107]. It should be pointed out that administration of these natural products can be achieved either before cisplatin ingestion or after cisplatin ingestion, reflecting heterogeneous approaches to evaluating the ameliorating effects of a given natural product on cisplatin-induced kidney injury [49].

Table 1.
Counteracting effects of plant-derived natural products on cisplatin- induced renal toxicity *.

Figure 5.
Schematic diagram depicting the protective mechanisms of natural products against cisplatin-induced kidney toxicity listed in Table 1.
Among the numerous nephroprotective mechanisms shown in Figure 5, one particular mechanism needs to be highlighted: the Nrf2 signaling pathway [195,196,197]. It has been demonstrated that cisplatin induces the downregulation of the Nrf2 signaling pathway, leading to downregulation of Nrf2 target genes such as HO-1 and NQO1, the two major molecules executing Nrf2′s cytoprotective effects via anti-oxidation and anti-apoptosis [198]. Many studies have shown that cisplatin-induced downregulation of Nrf2 [199,200] can be reversed by natural products derived from plants [201,202,203]. Under normal conditions, Nrf2 is kept inactive in the cytosol through Keap 1 binding [204,205,206,207]. Upon exogenous stress stimulation, Keap 1 releases Nrf2, so that Nrf2 is able to translocate to the nucleus, where it binds to the antioxidant response element (ARP) to induce the expression of many cytoprotective molecules including HO-1 and NQO1 [204,205,206,207]. Accordingly, as shown in Table 1, numerous natural products have been demonstrated to be able to stimulate the release of Nrf2 from the Nrf2–Keap 1 complex, leading to an increased Nrf2 content in the nucleus and enhanced expression of cytoprotective molecules. Further evidence that supports these findings is that an Nrf2 knockout abolishes the nephroprotective effects of a given natural product [208]. It should be pointed out that while the downstream gene expression is well elucidated, the upstream events in the cytosol are less clear and remain to be defined. For example, how a natural product stimulates the release of Nrf2 from the Nrf2–Keap 1 complex is yet to be studied in detail.
7. Other Factors That Can Modulate Cisplatin-Induced Kidney Injury
It is worth noting that in addition to the natural products shown in Table 1, other approaches have been tested to counteract cisplatin-induced injury. These approaches include caloric restriction [209,210] and ketone body ingestion [211]. Both have been demonstrated to ameliorate and modulate cisplatin-induced kidney injury [209,210,211]. These approaches may be applied together with prescribed therapies to enhance the efficacy of given drugs for cisplatin-induced nephrotoxicity. It should also be noted that aging and obesity are prominent risk factors in cisplatin-induced renal toxicity [212,213,214] and such risk factors can be modulated by NAD+ precursors [215] and natural products [216,217].
8. Summary
While the pathogenesis of cisplatin-induced kidney injury is complex, the major underlying mechanisms converge on oxidative stress, inflammation, and renal fibrosis [11,17], which may involve major NAD+-dependent redox enzymes, such as mitochondrial complex I, CD38, NNT, NADK, PARP, alpha-keto acid dehydrogenases, and sirtuins. We also showed that numerous natural products tabulated in this review may directly or indirectly exert their renoprotective effects on cisplatin kidney toxicity via these NAD+ redox enzymes. A further understanding of the molecular mechanisms underlying cisplatin kidney toxicity may provide insights into design of novel strategies for counteracting cisplatin renal toxicity and increasing the clinical applications of cisplatin in cancer patients. In this context, animal models of cisplatin-induced kidney injury will continue to serve as invaluable tools.
Author Contributions
Conceptulization, A.I. and L.-J.Y. original draft preparation; A.I. and L-J.Y; review and editing, L.-J.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhang, J.; Ye, Z.W.; Tew, K.D.; Townsend, D.M. Cisplatin chemotherapy and renal function. Adv. Cancer Res. 2021, 152, 305–327. [Google Scholar] [PubMed]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef] [PubMed]
- Ashrafi, F.; Mortazavi, M.; Nematbakhsh, M. The prevention of cisplatin-induced nephrotoxicity: A general consensus statement of a group of oncologist-hematologists, adult and pediatric nephrologists, radiation oncologists, clinical pathologists, clinical pharmacologists, and renal physiologists on cisplatin therapy in cancer patients. Int. J. Prev. Med. 2022, 13, 21. [Google Scholar] [PubMed]
- Wang, D.; Lippard, S.J. Cellular processing of platinum anticancer drugs. Nat. Rev. Drug Discov. 2005, 4, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.M.; Lippard, S.J. Cisplatin: From DNA damage to cancer chemotherapy. Prog. Nucleic Acid Res. Mol. Biol. 2001, 67, 93–130. [Google Scholar]
- Zazuli, Z.; Op’t Hoog, C.J.P.; Vijverberg, S.J.H.; Masereeuw, R.; Rassekh, S.R.; Medeiros, M.; Rivas-Ruiz, R.; Maitland-van der Zee, A.H.; Carleton, B.C. Cisplatin-induced nephrotoxicity in childhood cancer: Comparison between two countries. Pediatr. Nephrol. 2022. [Google Scholar] [CrossRef]
- Huang, G.; Zhang, Q.; Xu, C.; Chen, L.; Zhang, H. Mechanism of kidney injury induced by cisplatin. Toxicol. Res. 2022, 11, 385–390. [Google Scholar] [CrossRef]
- Pabla, N.; Dong, Z. Cisplatin nephrotoxicity: Mechanisms and renoprotective strategies. Kidney Int. 2008, 73, 994–1007. [Google Scholar] [CrossRef]
- Dong, G.; Luo, J.; Kumar, V.; Dong, Z. Inhibitors of histone deacetylases suppress cisplatin-induced p53 activation and apoptosis in renal tubular cells. Am. J. Physiol. Ren. Physiol. 2010, 298, F293–F300. [Google Scholar] [CrossRef]
- Nematbakhsh, M.; Ashrafi, F.; Nasri, H.; Talebi, A.; Pezeshki, Z.; Eshraghi, F.; Haghighi, M. A model for prediction of cisplatin induced nephrotoxicity by kidney weight in experimental rats. J. Res. Med. Sci. 2013, 18, 370–373. [Google Scholar]
- dos Santos, N.A.; Carvalho Rodrigues, M.A.; Martins, N.M.; dos Santos, A.C. Cisplatin-induced nephrotoxicity and targets of nephroprotection: An update. Arch. Toxicol. 2012, 86, 1233–1250. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.P.; Tadagavadi, R.K.; Ramesh, G.; Reeves, W.B. Mechanisms of cisplatin nephrotoxicity. Toxins 2010, 2, 2490–2518. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Shen, Y.; Fan, J.; Zeng, X.; Zhang, X.; Luan, J.; Wang, Y.; Zhang, J.; Fang, S.; Mei, X.; et al. Il-22-mediated renal metabolic reprogramming via pfkfb3 to treat kidney injury. Clin. Transl. Med. 2021, 11, e324. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Xiao, C.; Zheng, S.; Wang, Q.; Zhu, H.; Zhang, Y.; Wang, R. Microrna-214-3p aggravates ferroptosis by targeting gpx4 in cisplatin-induced acute kidney injury. Cell Stress Chaperones 2022, 27, 325–336. [Google Scholar] [CrossRef]
- Skinner, R.; Parry, A.; Price, L.; Cole, M.; Craft, A.W.; Pearson, A.D. Persistent nephrotoxicity during 10-year follow-up after cisplatin or carboplatin treatment in childhood: Relevance of age and dose as risk factors. Eur. J. Cancer 2009, 45, 3213–3219. [Google Scholar] [CrossRef] [PubMed]
- Sears, S.; Siskind, L. Potential therapeutic targets for cisplatin-induced kidney injury: Lessons from other models of aki and fibrosis. J. Am. Soc. Nephrol. 2021, 32, 1559–1567. [Google Scholar] [CrossRef]
- McSweeney, K.R.; Gadanec, L.K.; Qaradakhi, T.; Ali, B.A.; Zulli, A.; Apostolopoulos, V. Mechanisms of cisplatin-induced acute kidney injury: Pathological mechanisms, pharmacological interventions, and genetic mitigations. Cancers 2021, 13, 1572. [Google Scholar] [CrossRef] [PubMed]
- Oh, G.S.; Kim, H.J.; Shen, A.; Lee, S.B.; Yang, S.H.; Shim, H.; Cho, E.Y.; Kwon, K.B.; Kwak, T.H.; So, H.S. New therapeutic concept of nad redox balance for cisplatin nephrotoxicity. Biomed Res. Int. 2016, 2016, 4048390. [Google Scholar] [CrossRef] [PubMed]
- Mapuskar, K.A.; Wen, H.; Holanda, D.G.; Rastogi, P.; Steinbach, E.; Han, R.; Coleman, M.C.; Attanasio, M.; Riley, D.P.; Spitz, D.R.; et al. Persistent increase in mitochondrial superoxide mediates cisplatin-induced chronic kidney disease. Redox Biol. 2019, 20, 98–106. [Google Scholar] [CrossRef]
- Mapuskar, K.A.; Steinbach, E.J.; Zaher, A.; Riley, D.P.; Beardsley, R.A.; Keene, J.L.; Holmlund, J.T.; Anderson, C.M.; Zepeda-Orozco, D.; Buatti, J.M.; et al. Mitochondrial superoxide dismutase in cisplatin-induced kidney injury. Antioxidants 2021, 10, 1329. [Google Scholar] [CrossRef]
- Santos, N.A.; Catao, C.S.; Martins, N.M.; Curti, C.; Bianchi, M.L.; Santos, A.C. Cisplatin-induced nephrotoxicity is associated with oxidative stress, redox state unbalance, impairment of energetic metabolism and apoptosis in rat kidney mitochondria. Arch. Toxicol. 2007, 81, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Hajian, S.; Rafieian-Kopaei, M.; Nasri, H. Renoprotective effects of antioxidants against cisplatin nephrotoxicity. J. Nephropharmacol. 2014, 3, 39–42. [Google Scholar] [PubMed]
- Fang, C.Y.; Lou, D.Y.; Zhou, L.Q.; Wang, J.C.; Yang, B.; He, Q.J.; Wang, J.J.; Weng, Q.J. Natural products: Potential treatments for cisplatin-induced nephrotoxicity. Acta Pharmacol. Sin. 2021, 42, 1951–1969. [Google Scholar] [CrossRef]
- Eslamifar, Z.; Moridnia, A.; Sabbagh, S.; Ghaffaripour, R.; Jafaripour, L.; Behzadifard, M. Ameliorative effects of gallic acid on cisplatin-induced nephrotoxicity in rat variations of biochemistry, histopathology, and gene expression. Biomed Res. Int. 2021, 2021, 2195238. [Google Scholar] [CrossRef]
- Shalby, A.B.; Assaf, N.; Ahmed, H.H. Possible mechanisms for n-acetyl cysteine and taurine in ameliorating acute renal failure induced by cisplatin in rats. Toxicol. Mech. Methods 2011, 21, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Rattanavich, R.; Plagov, A.; Kumar, D.; Rai, P.; Lederman, R.; Salhan, D.; Vashistha, H.; Malhotra, A.; Meggs, L.G.; Singhal, P.C. Deficit of p66shca restores redox-sensitive stress response program in cisplatin-induced acute kidney injury. Exp. Mol. Pathol. 2013, 94, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Hosohata, K. Role of oxidative stress in drug-induced kidney injury. Int. J. Mol. Sci. 2016, 17, 1826. [Google Scholar] [CrossRef]
- Kim, D.H.; Choi, H.I.; Park, J.S.; Kim, C.S.; Bae, E.H.; Ma, S.K.; Kim, S.W. Farnesoid x receptor protects against cisplatin-induced acute kidney injury by regulating the transcription of ferroptosis-related genes. Redox Biol. 2022, 54, 102382. [Google Scholar] [CrossRef]
- Yin, M.; Li, N.; Makinde, E.A.; Olatunji, O.J.; Ni, Z. N6-2-hydroxyethyl-adenosine ameliorate cisplatin induced acute kidney injury in mice. All Life 2020, 13, 244–251. [Google Scholar] [CrossRef]
- Guo, P.; Zhang, N.; Li, J.; Liu, Y.; Li, Y.; Wang, X.; Wang, J.; Wang, Y.; Wang, A. Protective effects of low-temperature plasma on cisplatin-induced nephrotoxicity. Life Sci. 2022, 289, 120230. [Google Scholar] [CrossRef]
- Wang, X.L.; Wang, L.; Lin, F.L.; Li, S.S.; Lin, T.X.; Jiang, R.W. Protective effect of penetratin analogue-tagged sod1 on cisplatin-induced nephrotoxicity through inhibiting oxidative stress and jnk/p38 mapk signaling pathway. Oxid. Med. Cell. Longev. 2021, 2021, 5526053. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wen, X.; Huang, Q.; Zhu, M.; Lu, J. Selenium status in diet affects nephrotoxicity induced by cisplatin in mice. Antioxidants 2022, 11, 1141. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.M.; Shah, J.; Mercer, E.; Tian, H.S.; Thompson, V.; Cheung, J.M.; Dorso, M.; Kubala, J.M.; Gudas, L.J.; de Stanchina, E.; et al. Kidney-targeted redox scavenger therapy prevents cisplatin-induced acute kidney injury. Front. Pharmacol. 2021, 12, 790913. [Google Scholar] [CrossRef] [PubMed]
- Black, L.M.; Lever, J.M.; Traylor, A.M.; Chen, B.; Yang, Z.; Esman, S.K.; Jiang, Y.; Cutter, G.R.; Boddu, R.; George, J.F.; et al. Divergent effects of aki to ckd models on inflammation and fibrosis. Am. J. Physiol. Ren. Physiol. 2018, 315, F1107–F1118. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Li, K.Y.; Wang, P.J.; Huang, H.W.; Chen, M.J. Alleviating chronic kidney disease progression through modulating the critical genus of gut microbiota in a cisplatin-induced lanyu pig model. J. Food Drug Anal. 2020, 28, 103–114. [Google Scholar] [CrossRef]
- Younis, N.N.; Elsherbiny, N.M.; Shaheen, M.A.; Elseweidy, M.M. Modulation of nadph oxidase and nrf2/ho-1 pathway by vanillin in cisplatin-induced nephrotoxicity in rats. J. Pharm. Pharmacol. 2020, 72, 1546–1555. [Google Scholar] [CrossRef]
- Al Za’abi, M.; Al Salam, S.; Al Suleimani, Y.; Ashique, M.; Manoj, P.; Nemmar, A.; Ali, B.H. Effects of repeated increasing doses of cisplatin as models of acute kidney injury and chronic kidney disease in rats. Naunyn Schmiedeberg’s Arch. Pharmacol. 2021, 394, 249–259. [Google Scholar] [CrossRef]
- Hsiao, Y.P.; Chen, H.L.; Tsai, J.N.; Lin, M.Y.; Liao, J.W.; Wei, M.S.; Ko, J.L.; Ou, C.C. Administration of lactobacillus reuteri combined with clostridium butyricum attenuates cisplatin-induced renal damage by gut microbiota reconstitution, increasing butyric acid production, and suppressing renal inflammation. Nutrients 2021, 13, 2792. [Google Scholar] [CrossRef]
- Tsai, Y.S.; Chen, Y.P.; Lin, S.W.; Chen, Y.L.; Chen, C.C.; Huang, G.J. Lactobacillus rhamnosus gklc1 ameliorates cisplatin-induced chronic nephrotoxicity by inhibiting cell inflammation and apoptosis. Biomed. Pharmacother. 2022, 147, 112701. [Google Scholar] [CrossRef]
- Li, M.; Li, C.M.; Ye, Z.C.; Huang, J.; Li, Y.; Lai, W.; Peng, H.; Lou, T.Q. Sirt3 modulates fatty acid oxidation and attenuates cisplatin-induced aki in mice. J. Cell. Mol. Med. 2020, 24, 5109–5121. [Google Scholar] [CrossRef]
- Singh, M.P.; Chauhan, A.K.; Kang, S.C. Morin hydrate ameliorates cisplatin-induced er stress, inflammation and autophagy in hek-293 cells and mice kidney via parp-1 regulation. Int. Immunopharmacol. 2018, 56, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lu, M.; Xiong, L.; Fan, J.; Zhou, Y.; Li, H.; Peng, X.; Zhong, Z.; Wang, Y.; Huang, F.; et al. Drp1-mediated mitochondrial fission promotes renal fibroblast activation and fibrogenesis. Cell Death Dis. 2020, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Ayanga, B.A.; Badal, S.S.; Wang, Y.; Galvan, D.L.; Chang, B.H.; Schumacker, P.T.; Danesh, F.R. Dynamin-related protein 1 deficiency improves mitochondrial fitness and protects against progression of diabetic nephropathy. J. Am. Soc. Nephrol. 2016, 27, 2733–2747. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, C.; Cai, J.; Chen, G.; Zhang, D.; Zhang, Z.; Dong, Z. Pink1/parkin-mediated mitophagy is activated in cisplatin nephrotoxicity to protect against kidney injury. Cell Death Dis. 2018, 9, 1113. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Li, J.; Tu, G.; Su, Y.; Zhang, X.; Luo, Z.; Rong, R.; Zhang, Y. Comprehensive molecular and cellular characterization of acute kidney injury progression to renal fibrosis. Front. Immunol. 2021, 12, 699192. [Google Scholar] [CrossRef]
- Sears, S.M.; Orwick, A.; Siskind, L.J. Modeling cisplatin-induced kidney injury to increase translational potential. Nephron 2022, 1–4. [Google Scholar] [CrossRef]
- Yan, L.J. Folic acid-induced animal model of kidney disease. Anim. Models Exp. Med. 2021, 4, 329–342. [Google Scholar] [CrossRef]
- Shi, M.; McMillan, K.L.; Wu, J.; Gillings, N.; Flores, B.; Moe, O.W.; Hu, M.C. Cisplatin nephrotoxicity as a model of chronic kidney disease. Lab. Investig. 2018, 98, 1105–1121. [Google Scholar] [CrossRef]
- Perse, M. Cisplatin mouse models: Treatment, toxicity and translatability. Biomedicines 2021, 9, 1406. [Google Scholar] [CrossRef]
- Fu, Y.; Tang, C.; Cai, J.; Chen, G.; Zhang, D.; Dong, Z. Rodent models of aki-ckd transition. Am. J. Physiol. Ren. Physiol. 2018, 315, F1098–F1106. [Google Scholar] [CrossRef]
- Fu, Y.; Xiang, Y.; Wu, W.; Cai, J.; Tang, C.; Dong, Z. Persistent activation of autophagy after cisplatin nephrotoxicity promotes renal fibrosis and chronic kidney disease. Front. Pharmacol. 2022, 13, 918732. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Hu, X.; Ma, Z.; Wei, Q.; Xiang, X.; Li, S.; Wen, L.; Liang, Y.; Dong, Z. P53 in proximal tubules mediates chronic kidney problems after cisplatin treatment. Cells 2022, 11, 712. [Google Scholar] [CrossRef] [PubMed]
- Bufi, R.; Korstanje, R. The impact of genetic background on mouse models of kidney disease. Kidney Int. 2022, 102, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Usami, M.; Okada, A.; Taguchi, K.; Hamamoto, S.; Kohri, K.; Yasui, T. Genetic differences in c57bl/6 mouse substrains affect kidney crystal deposition. Urolithiasis 2018, 46, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Navarro, C.D.C.; Figueira, T.R.; Francisco, A.; Dal’Bo, G.A.; Ronchi, J.A.; Rovani, J.C.; Escanhoela, C.A.F.; Oliveira, H.C.F.; Castilho, R.F.; Vercesi, A.E. Redox imbalance due to the loss of mitochondrial nad(p)-transhydrogenase markedly aggravates high fat diet-induced fatty liver disease in mice. Free Radic. Biol. Med. 2017, 113, 190–202. [Google Scholar] [CrossRef]
- Dogar, I.; Dixon, S.; Gill, R.; Young, A.; Mallay, S.; Oldford, C.; Mailloux, R.J. C57bl/6j mice upregulate catalase to maintain the hydrogen peroxide buffering capacity of liver mitochondria. Free Radic. Biol. Med. 2020, 146, 59–69. [Google Scholar] [CrossRef]
- Ronchi, J.A.; Figueira, T.R.; Ravagnani, F.G.; Oliveira, H.C.; Vercesi, A.E.; Castilho, R.F. A spontaneous mutation in the nicotinamide nucleotide transhydrogenase gene of c57bl/6j mice results in mitochondrial redox abnormalities. Free Radic. Biol. Med. 2013, 63, 446–456. [Google Scholar] [CrossRef]
- Kunath, A.; Heiker, J.T.; Kern, M.; Kosacka, J.; Flehmig, G.; Stumvoll, M.; Kovacs, P.; Bluher, M.; Kloting, N. Nicotinamide nucleotide transhydrogenase (nnt) is related to obesity in mice. Horm. Metab. Res. 2020, 52, 877–881. [Google Scholar] [CrossRef]
- Close, A.F.; Chae, H.; Jonas, J.C. The lack of functional nicotinamide nucleotide transhydrogenase only moderately contributes to the impairment of glucose tolerance and glucose-stimulated insulin secretion in c57bl/6j vs c57bl/6n mice. Diabetologia 2021, 64, 2550–2561. [Google Scholar] [CrossRef]
- Oh, G.S.; Kim, H.J.; Choi, J.H.; Shen, A.; Choe, S.K.; Karna, A.; Lee, S.H.; Jo, H.J.; Yang, S.H.; Kwak, T.H.; et al. Pharmacological activation of nqo1 increases nad(+) levels and attenuates cisplatin-mediated acute kidney injury in mice. Kidney Int. 2014, 85, 547–560. [Google Scholar] [CrossRef]
- Adhikari, A.; Mondal, S.; Chatterjee, T.; Das, M.; Biswas, P.; Ghosh, R.; Darbar, S.; Alessa, H.; Althakafy, J.T.; Sayqal, A.; et al. Redox nanomedicine ameliorates chronic kidney disease (ckd) by mitochondrial reconditioning in mice. Commun. Biol. 2021, 4, 1013. [Google Scholar] [CrossRef] [PubMed]
- Zager, R.A.; Johnson, A.C.M. Early loss of glutathione-s-transferase (gst) activity during diverse forms of acute renal tubular injury. Physiol. Rep. 2022, 10, e15352. [Google Scholar] [CrossRef] [PubMed]
- Gang, G.T.; Kim, Y.H.; Noh, J.R.; Kim, K.S.; Jung, J.Y.; Shong, M.; Hwang, J.H.; Lee, C.H. Protective role of nad(p)h:Quinone oxidoreductase 1 (nqo1) in cisplatin-induced nephrotoxicity. Toxicol. Lett. 2013, 221, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Nazari Soltan Ahmad, S.; Rashtchizadeh, N.; Argani, H.; Roshangar, L.; Ghorbani Haghjo, A.; Sanajou, D.; Panah, F.; Ashrafi Jigheh, Z.; Dastmalchi, S.; Mesgari-Abbasi, M. Dunnione protects against experimental cisplatin-induced nephrotoxicity by modulating nqo1 and nad(+) levels. Free Radic. Res. 2018, 52, 808–817. [Google Scholar] [CrossRef] [PubMed]
- Hirst, J.; King, M.S.; Pryde, K.R. The production of reactive oxygen species by complex i. Biochem. Soc. Trans. 2008, 36, 976–980. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.M.; Mann, V.M.; Krige, D.; Schapira, A.H. Human mitochondrial complex i dysfunction. Biochim. Biophys. Acta 1992, 1101, 198–203. [Google Scholar] [CrossRef]
- Morris, B.J. Seven sirtuins for seven deadly diseases of aging. Free Radic. Biol. Med. 2013, 56, 133–171. [Google Scholar] [CrossRef]
- Sauve, A.A. Sirtuin chemical mechanisms. Biochim. Biophys. Acta 2010, 1804, 1591–1603. [Google Scholar] [CrossRef]
- Yan, L.J.; Sumien, N.; Thangthaeng, N.; Forster, M.J. Reversible inactivation of dihydrolipoamide dehydrogenase by mitochondrial hydrogen peroxide. Free Radic. Res. 2013, 47, 123–133. [Google Scholar] [CrossRef]
- Hoxhaj, G.; Ben-Sahra, I.; Lockwood, S.E.; Timson, R.C.; Byles, V.; Henning, G.T.; Gao, P.; Selfors, L.M.; Asara, J.M.; Manning, B.D. Direct stimulation of nadp(+) synthesis through akt-mediated phosphorylation of nad kinase. Science 2019, 363, 1088–1092. [Google Scholar] [CrossRef]
- Tedeschi, P.M.; Bansal, N.; Kerrigan, J.E.; Abali, E.E.; Scotto, K.W.; Bertino, J.R. Nad+ kinase as a therapeutic target in cancer. Clin. Cancer Res. 2016, 22, 5189–5195. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Kim, H.; Fu, Z.; Qiu, Y.; Yang, Z.; Wang, J.; Zhang, D.; Tong, X.; Yin, L.; Li, J.; et al. Deficiency of the mitochondrial nad kinase causes stress-induced hepatic steatosis in mice. Gastroenterology 2018, 154, 224–237. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R. Mnadk, a long-awaited human mitochondrion-localized nad kinase. J. Cell. Physiol. 2015, 230, 1697–1701. [Google Scholar] [CrossRef] [PubMed]
- Benzi, A.; Sturla, L.; Heine, M.; Fischer, A.W.; Spinelli, S.; Magnone, M.; Sociali, G.; Parodi, A.; Fenoglio, D.; Emionite, L.; et al. Cd38 downregulation modulates nad(+) and nadp(h) levels in thermogenic adipose tissues. Biochim. Biophys. Acta Mol. Cell. Biol. Lipids 2021, 1866, 158819. [Google Scholar] [CrossRef]
- Braidy, N.; Berg, J.; Clement, J.; Khorshidi, F.; Poljak, A.; Jayasena, T.; Grant, R.; Sachdev, P. Role of nicotinamide adenine dinucleotide and related precursors as therapeutic targets for age-related degenerative diseases: Rationale, biochemistry, pharmacokinetics, and outcomes. Antioxid. Redox Signal. 2019, 30, 251–294. [Google Scholar] [CrossRef]
- Camacho-Pereira, J.; Tarrago, M.G.; Chini, C.C.; Nin, V.; Escande, C.; Warner, G.M.; Puranik, A.S.; Schoon, R.A.; Reid, J.M.; Galina, A.; et al. Cd38 dictates age-related nad decline and mitochondrial dysfunction through an sirt3-dependent mechanism. Cell Metab. 2016, 23, 1127–1139. [Google Scholar] [CrossRef]
- Devalaraja-Narashimha, K.; Singaravelu, K.; Padanilam, B.J. Poly(adp-ribose) polymerase-mediated cell injury in acute renal failure. Pharmacol. Res. 2005, 52, 44–59. [Google Scholar] [CrossRef]
- Alemasova, E.E.; Lavrik, O.I. Poly(adp-ribosyl)ation by parp1: Reaction mechanism and regulatory proteins. Nucleic Acids Res. 2019, 47, 3811–3827. [Google Scholar] [CrossRef]
- Butepage, M.; Eckei, L.; Verheugd, P.; Luscher, B. Intracellular mono-adp-ribosylation in signaling and disease. Cells 2015, 4, 569–595. [Google Scholar] [CrossRef]
- Ronchi, J.A.; Francisco, A.; Passos, L.A.; Figueira, T.R.; Castilho, R.F. The contribution of nicotinamide nucleotide transhydrogenase to peroxide detoxification is dependent on the respiratory state and counterbalanced by other sources of nadph in liver mitochondria. J. Biol. Chem. 2016, 291, 20173–20187. [Google Scholar] [CrossRef]
- Santos, L.R.B.; Muller, C.; de Souza, A.H.; Takahashi, H.K.; Spegel, P.; Sweet, I.R.; Chae, H.; Mulder, H.; Jonas, J.C. Nnt reverse mode of operation mediates glucose control of mitochondrial nadph and glutathione redox state in mouse pancreatic beta-cells. Mol. Metab. 2017, 6, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Yang, C.; Amorim, T.; Maqbool, M.; Lin, J.; Li, C.; Fang, C.; Xue, L.; Kwart, A.; Fang, H.; et al. Cisplatin-mediated upregulation of ape2 binding to myh9 provokes mitochondrial fragmentation and acute kidney injury. Cancer Res. 2021, 81, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Hama, T.; Nagesh, P.K.; Chowdhury, P.; Moore, B.M., 2nd; Yallapu, M.M.; Regner, K.R.; Park, F. DNA damage is overcome by trip13 overexpression during cisplatin nephrotoxicity. JCI Insight 2021, 6, e139092. [Google Scholar] [CrossRef]
- Yan, L.J.; Lodge, J.K.; Traber, M.G.; Matsugo, S.; Packer, L. Comparison between copper-mediated and hypochlorite-mediated modifications of human low density lipoproteins evaluated by protein carbonyl formation. J. Lipid Res. 1997, 38, 992–1001. [Google Scholar] [CrossRef]
- Yang, Y.; Fu, Y.; Wang, P.; Liu, S.; Sha, Y.; Zhang, Y.; Zhang, A.; Jia, Z.; Ding, G.; Huang, S. Intervention of mitochondrial activity attenuates cisplatin-induced acute kidney injury. Int. Urol. Nephrol. 2019, 51, 1207–1218. [Google Scholar] [CrossRef] [PubMed]
- Kruidering, M.; Van de Water, B.; de Heer, E.; Mulder, G.J.; Nagelkerke, J.F. Cisplatin-induced nephrotoxicity in porcine proximal tubular cells: Mitochondrial dysfunction by inhibition of complexes i to iv of the respiratory chain. J. Pharmacol. Exp. Ther. 1997, 280, 638–649. [Google Scholar]
- El-Hamoly, T.; El-Sharawy, D.M.; El Refaye, M.S.; Abd El-Rahman, S.S. L-thyroxine modifies nephrotoxicity by regulating the apoptotic pathway: The possible role of cd38/adp-ribosyl cyclase-mediated calcium mobilization. PLoS ONE 2017, 12, e0184157. [Google Scholar] [CrossRef]
- Kim, M.J.; Moon, D.; Jung, S.; Lee, J.; Kim, J. Cisplatin nephrotoxicity is induced via poly(adp-ribose) polymerase activation in adult zebrafish and mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2020, 318, R843–R854. [Google Scholar] [CrossRef]
- Zhang, Y.; Tao, X.; Yin, L.; Xu, L.; Xu, Y.; Qi, Y.; Han, X.; Song, S.; Zhao, Y.; Lin, Y.; et al. Protective effects of dioscin against cisplatin-induced nephrotoxicity via the microrna-34a/sirtuin 1 signalling pathway. Br. J. Pharmacol. 2017, 174, 2512–2527. [Google Scholar] [CrossRef]
- Lu, C.Y. Beta-lapachone ameliorates murine cisplatin nephrotoxicity: Nad(+), nqo1, and sirt1 at the crossroads of metabolism, injury, and inflammation. Kidney Int. 2014, 85, 496–498. [Google Scholar] [CrossRef]
- Ozkok, A.; Edelstein, C.L. Pathophysiology of cisplatin-induced acute kidney injury. Biomed Res. Int. 2014, 2014, 967826. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Long, K.E.; Tang, K.; Padanilam, B.J. Poly(adp-ribose) polymerase 1 activation is required for cisplatin nephrotoxicity. Kidney Int. 2012, 82, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Ramesh, G.; Uematsu, S.; Akira, S.; Reeves, W.B. Tlr4 signaling mediates inflammation and tissue injury in nephrotoxicity. J. Am. Soc. Nephrol. 2008, 19, 923–932. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, G.; Zhang, B.; Uematsu, S.; Akira, S.; Reeves, W.B. Endotoxin and cisplatin synergistically induce renal dysfunction and cytokine production in mice. Am. J. Physiol. Ren. Physiol. 2007, 293, F325–F332. [Google Scholar] [CrossRef] [PubMed]
- Habib, R. Multifaceted roles of toll-like receptors in acute kidney injury. Heliyon 2021, 7, e06441. [Google Scholar] [CrossRef]
- Santos, N.A.; Bezerra, C.S.; Martins, N.M.; Curti, C.; Bianchi, M.L.; Santos, A.C. Hydroxyl radical scavenger ameliorates cisplatin-induced nephrotoxicity by preventing oxidative stress, redox state unbalance, impairment of energetic metabolism and apoptosis in rat kidney mitochondria. Cancer Chemother. Pharmacol. 2008, 61, 145–155. [Google Scholar] [CrossRef]
- Fernandez-Rojas, B.; Rodriguez-Rangel, D.S.; Granados-Castro, L.F.; Negrette-Guzman, M.; Leon-Contreras, J.C.; Hernandez-Pando, R.; Molina-Jijon, E.; Reyes, J.L.; Zazueta, C.; Pedraza-Chaverri, J. C-phycocyanin prevents cisplatin-induced mitochondrial dysfunction and oxidative stress. Mol. Cell. Biochem. 2015, 406, 183–197. [Google Scholar] [CrossRef]
- Shen, J.; Wang, W.; Shao, X.; Wu, J.; Li, S.; Che, X.; Ni, Z. Integrated analysis of m6a methylome in cisplatin-induced acute kidney injury and berberine alleviation in mouse. Front. Genet. 2020, 11, 584460. [Google Scholar] [CrossRef]
- Kuo, H.L.; Mong, M.C.; Chen, H.C.; Wang, Z.H.; Yin, M.C. S-ethyl cysteine, an amino acid derivative, attenuated cisplatin induced nephrotoxicity. Amino Acids 2020, 52, 1181–1190. [Google Scholar] [CrossRef]
- Yang, F.; Jia, M.; Deng, C.; Xiao, B.; Dai, R.; Xiang, Y. Silibinin ameliorates cisplatin-induced acute kidney injury via activating nfe2l1-mediated antioxidative response to suppress the ros/mapk signaling pathway. J. Mol. Histol. 2022, 1–12. [Google Scholar] [CrossRef]
- Mohamed, M.E.; Abduldaium, Y.S.; Younis, N.S. Ameliorative effect of linalool in cisplatin-induced nephrotoxicity: The role of hmgb1/tlr4/nf-kappab and nrf2/ho1 pathways. Biomolecules 2020, 10, 1488. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Dong, H.; Wang, Q.; Bai, J.; Li, Y.N.; Zhao, J.J.; Li, J.Z. Danshensu attenuates cisplatin-induced nephrotoxicity through activation of nrf2 pathway and inhibition of nf-kappab. Biomed. Pharmacother. 2021, 142, 111995. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Ma, Z.; Wen, L.; Li, S.; Dong, Z. Autophagy in cisplatin nephrotoxicity during cancer therapy. Cancers 2021, 13, 5618. [Google Scholar] [CrossRef]
- Bhatia, D.; Choi, M.E. Autophagy in kidney disease: Advances and therapeutic potential. Prog. Mol. Biol. Transl. Sci. 2020, 172, 107–133. [Google Scholar] [PubMed]
- Tang, Y.; Luo, H.; Xiao, Q.; Li, L.; Zhong, X.; Zhang, J.; Wang, F.; Li, G.; Wang, L.; Li, Y. Isoliquiritigenin attenuates septic acute kidney injury by regulating ferritinophagy-mediated ferroptosis. Ren. Fail. 2021, 43, 1551–1560. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Xue, Q.; Kuang, L.; Xie, L.; Luo, R.; Nie, X. Berberine alleviates cisplatin-induced acute kidney injury by regulating mitophagy via pink 1/parkin pathway. Transl. Androl. Urol. 2020, 9, 1712–1724. [Google Scholar] [CrossRef]
- Ma, N.; Wei, Z.; Hu, J.; Gu, W.; Ci, X. Farrerol ameliorated cisplatin-induced chronic kidney disease through mitophagy induction via nrf2/pink1 pathway. Front. Pharmacol. 2021, 12, 768700. [Google Scholar] [CrossRef]
- Nazari, A.; Mirian, M.; Aghaei, M.; Aliomrani, M. 4-hydroxyhalcone effects on cisplatin-induced genotoxicity model. Toxicol. Res. 2021, 10, 11–17. [Google Scholar] [CrossRef]
- Gwon, M.G.; Gu, H.; Leem, J.; Park, K.K. Protective effects of 6-shogaol, an active compound of ginger, in a murine model of cisplatin-induced acute kidney injury. Molecules 2021, 26, 5931. [Google Scholar] [CrossRef]
- Elseweidy, M.M.; Zaghloul, M.S.; Younis, N.N. 10-dhgd ameliorates cisplatin-induced nephrotoxicity in rats. Biomed. Pharmacother. 2016, 83, 241–246. [Google Scholar] [CrossRef]
- Afsar, T.; Razak, S.; Aldisi, D.; Shabbir, M.; Almajwal, A.; Al Kheraif, A.A.; Arshad, M. Acacia hydaspica r. Parker ethyl-acetate extract abrogates cisplatin-induced nephrotoxicity by targeting ros and inflammatory cytokines. Sci. Rep. 2021, 11, 17248. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.O.; Ahmed, M.M.; Arshad, S.; Javaid, U.; Khan, I.A.; Manzoor, M.; Andleeb, S.; Riaz, R.; Munawar, S.H.; Manzoor, Z.; et al. Nephroprotective effects of alhagi camelorum against cisplatin-induced nephrotoxicity in albino wistar rats. Molecules 2022, 27, 941. [Google Scholar] [CrossRef] [PubMed]
- Adeoye, B.O.; Asenuga, E.R.; Oyagbemi, A.A.; Omobowale, T.O.; Adedapo, A.A. The protective effect of the ethanol leaf extract of andrographis paniculata on cisplatin-induced acute kidney injury in rats through nrf2/kim-1 signalling pathway. Drug Res. 2018, 68, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Lee, D.; Jang, H.J.; Jang, D.S.; Kwon, H.C.; Kim, K.H.; Kim, S.N.; Hwang, G.S.; Kang, K.S.; Eom, D.W. Protective effect of artemisia asiatica extract and its active compound eupatilin against cisplatin-induced renal damage. Evid. Based Complement. Alternat. Med. 2015, 2015, 483980. [Google Scholar] [CrossRef]
- Ibrahim, A.; Al-Hizab, F.A.; Abushouk, A.I.; Abdel-Daim, M.M. Nephroprotective effects of benzyl isothiocyanate and resveratrol against cisplatin-induced oxidative stress and inflammation. Front. Pharmacol. 2018, 9, 1268. [Google Scholar] [CrossRef]
- Gholampour, F.; Masoudi, R.; Khaledi, M.; Rooyeh, M.M.; Farzad, S.H.; Ataellahi, F.; Abtahi, S.L.; Owji, S.M. Berberis integerrima hydro-alcoholic root extract and its constituent berberine protect against cisplatin-induced nephro- and hepato-toxicity. Am. J. Med. Sci. 2022, 364, 76–87. [Google Scholar] [CrossRef]
- Zaaba, N.E.; Beegam, S.; Elzaki, O.; Yasin, J.; Nemmar, B.M.; Ali, B.H.; Adeghate, E.; Nemmar, A. The nephroprotective effects of alpha-bisabolol in cisplatin-induced acute kidney injury in mice. Biomedicines 2022, 10, 842. [Google Scholar] [CrossRef]
- Widowati, W.; Prahastuti, S.; Hidayat, M.; Hasianna, S.T.; Wahyudianingsih, R.; Eltania, T.F.; Azizah, A.M.; Aviani, J.K.; Subangkit, M.; Handayani, R.A.S.; et al. Detam 1 black soybean against cisplatin-induced acute ren failure on rat model via antioxidant, antiinflammatory and antiapoptosis potential. J. Tradit. Complement. Med. 2022, 12, 426–435. [Google Scholar] [CrossRef]
- Eltamany, E.E.; Elhady, S.S.; Nafie, M.S.; Ahmed, H.A.; Abo-Elmatty, D.M.; Ahmed, S.A.; Badr, J.M.; Abdel-Hamed, A.R. The antioxidant carrichtera annua dc. Ethanolic extract counteracts cisplatin triggered hepatic and renal toxicities. Antioxidants 2021, 10, 825. [Google Scholar] [CrossRef]
- Ragab, T.I.M.; Zoheir, K.M.A.; Mohamed, N.A.; El Gendy, A.E.G.; Abd-ElGawad, A.M.; Abdelhameed, M.F.; Farrag, A.R.H.; Elshamy, A.I. Cytoprotective potentialities of carvacrol and its nanoemulsion against cisplatin-induced nephrotoxicity in rats: Development of nano-encasulation form. Heliyon 2022, 8, e09198. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, T.; Wang, C.; Meng, Q.; Huo, X.; Wang, C.; Sun, P.; Sun, H.; Ma, X.; Wu, J.; et al. Catalpol-induced ampk activation alleviates cisplatin-induced nephrotoxicity through the mitochondrial-dependent pathway without compromising its anticancer properties. Oxid. Med. Cell. Longev. 2021, 2021, 7467156. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Chen, Q.Y.; He, Y.; Liu, Y.H.; Meng, X.M.; Liu, M.M. Discovery of a chalcone derivative as potent necroptosis inhibitor for the treatment of acute kidney injury. Clin. Exp. Pharmacol. Physiol. 2022, 49, 824–835. [Google Scholar] [CrossRef] [PubMed]
- Adeyemi, O.O.; Ishola, I.O.; Ajani, I.D. Citrullus colocynthis linn. Fruit extract ameliorates cisplatin-induced hepato-renal toxicity in rats. J. Complement. Integr. Med. 2017, 15. [Google Scholar] [CrossRef]
- Wang, R.; Hassan, W.; Ahmad, F.U.D.; Jabeen, Q.; Ahmed, H.; Iqbal, O. Citrus aurantium ameliorates cisplatin-induced nephrotoxicity. Biomed Res. Int. 2019, 2019, 3960908. [Google Scholar] [CrossRef] [PubMed]
- Mahmod, I.I.; Ismail, I.S.; Alitheen, N.B.; Normi, Y.M.; Abas, F.; Khatib, A.; Rudiyanto; Latip, J. Nmr and lcms analytical platforms exhibited the nephroprotective effect of clinacanthus nutans in cisplatin-induced nephrotoxicity in the in vitro condition. BMC Complement. Med. Ther. 2020, 20, 320. [Google Scholar] [CrossRef] [PubMed]
- Sahrial, I.; Solfaine, R. Coleus amboinicus extract increases transforming growth factor-1beta expression in wistar rats with cisplatin-induced nephropathy. Vet. World 2019, 12, 1346–1351. [Google Scholar] [CrossRef]
- Ueki, M.; Ueno, M.; Morishita, J.; Maekawa, N. Curcumin ameliorates cisplatin-induced nephrotoxicity by inhibiting renal inflammation in mice. J. Biosci. Bioeng. 2013, 115, 547–551. [Google Scholar] [CrossRef]
- Sen, Z.; Jie, M.; Jingzhi, Y.; Dongjie, W.; Dongming, Z.; Xiaoguang, C. Total coumarins from hydrangea paniculata protect against cisplatin-induced acute kidney damage in mice by suppressing renal inflammation and apoptosis. Evid. Based Complement. Alternat. Med. 2017, 2017, 5350161. [Google Scholar] [CrossRef]
- Miyawaki, Y.; Ueki, M.; Ueno, M.; Asaga, T.; Tokuda, M.; Shirakami, G. D-allose ameliorates cisplatin-induced nephrotoxicity in mice. Tohoku J. Exp. Med. 2012, 228, 215–221. [Google Scholar] [CrossRef]
- Meng, H.; Fu, G.; Shen, J.; Shen, K.; Xu, Z.; Wang, Y.; Jin, B.; Pan, H. Ameliorative effect of daidzein on cisplatin-induced nephrotoxicity in mice via modulation of inflammation, oxidative stress, and cell death. Oxid. Med. Cell. Longev. 2017, 2017, 3140680. [Google Scholar] [CrossRef]
- Cao, S.S.; Yan, M.; Hou, Z.Y.; Chen, Y.; Jiang, Y.S.; Fan, X.R.; Fang, P.F.; Zhang, B.K. Danshen modulates nrf2-mediated signaling pathway in cisplatin-induced renal injury. J. Huazhong Univ. Sci. Technol. Med. Sci. 2017, 37, 761–765. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.O.; Sial, A.S.; Akhtar, I.; Naeem, M.; Hazafa, A.; Ansari, R.A.; Rizvi, S.A.A. The nephroprotective effects of daucus carota and eclipta prostrata against cisplatin-induced nephrotoxicity in rats. Bioengineered 2021, 12, 12702–12721. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.J.; Kim, K.S.; Park, J.H.; Lee, S.H.; Kim, H.R.; Lee, S.H.; Choi, H.B.; Cao, S.; Kumar, V.; Kwak, J.H.; et al. Protective effects of dendropanoxide isolated from dendropanax morbifera against cisplatin-induced acute kidney injury via the ampk/mtor signaling pathway. Food Chem. Toxicol. 2020, 145, 111605. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Gu, L.B.; Tu, Y.; Hu, H.; Huang, Y.R.; Sun, W. Emodin ameliorates cisplatin-induced apoptosis of rat renal tubular cells in vitro by activating autophagy. Acta Pharmacol. Sin. 2016, 37, 235–245. [Google Scholar] [CrossRef]
- Sioud, F.; Ben Toumia, I.; Lahmer, A.; Khlifi, R.; Dhaouefi, Z.; Maatouk, M.; Ghedira, K.; Chekir-Ghedira, L. Methanolic extract of ephedra alata ameliorates cisplatin-induced nephrotoxicity and hepatotoxicity through reducing oxidative stress and genotoxicity. Environ. Sci. Pollut. Res. Int. 2020, 27, 12792–12801. [Google Scholar] [CrossRef]
- Sharma, S.; Modi, A.; Narayan, G.; Hemalatha, S. Protective effect of exacum lawii on cisplatin-induced oxidative renal damage in rats. Pharmacogn. Mag. 2018, 13, S807–S816. [Google Scholar]
- El-Sayed, S.M.; El-Naggar, M.E.; Hussein, J.; Medhat, D.; El-Banna, M. Effect of ficus carica l. Leaves extract loaded gold nanoparticles against cisplatin-induced acute kidney injury. Colloids Surf. B Biointerfaces 2019, 184, 110465. [Google Scholar] [CrossRef]
- Katanic, J.; Matic, S.; Pferschy-Wenzig, E.M.; Kretschmer, N.; Boroja, T.; Mihailovic, V.; Stankovic, V.; Stankovic, N.; Mladenovic, M.; Stanic, S.; et al. Filipendula ulmaria extracts attenuate cisplatin-induced liver and kidney oxidative stress in rats: In vivo investigation and lc-ms analysis. Food Chem. Toxicol. 2017, 99, 86–102. [Google Scholar] [CrossRef]
- Hao, Y.; Miao, J.; Liu, W.; Peng, L.; Chen, Y.; Zhong, Q. Formononetin protects against cisplatininduced acute kidney injury through activation of the pparalpha/nrf2/ho1/nqo1 pathway. Int. J. Mol. Med. 2021, 47, 511–522. [Google Scholar] [CrossRef]
- El-Naga, R.N. Forskolin alleviates cisplatin-induced acute renal toxicity in rats. J. Pharm. Pharmacol. 2022, 74, 1051–1060. [Google Scholar] [CrossRef]
- Huang, Y.C.; Tsai, M.S.; Hsieh, P.C.; Shih, J.H.; Wang, T.S.; Wang, Y.C.; Lin, T.H.; Wang, S.H. Galangin ameliorates cisplatin-induced nephrotoxicity by attenuating oxidative stress, inflammation and cell death in mice through inhibition of erk and nf-kappab signaling. Toxicol. Appl. Pharmacol. 2017, 329, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Geng, X.; Zhong, D.; Su, L.; Lin, Z.; Yang, B. Preventive and therapeutic effect of ganoderma lucidum on kidney injuries and diseases. Adv. Pharmacol. 2020, 87, 257–276. [Google Scholar] [PubMed]
- Nasr, A.Y. Protective effect of aged garlic extract against the oxidative stress induced by cisplatin on blood cells parameters and hepatic antioxidant enzymes in rats. Toxicol. Rep. 2014, 1, 682–691. [Google Scholar] [CrossRef] [PubMed]
- Sung, M.J.; Kim, D.H.; Jung, Y.J.; Kang, K.P.; Lee, A.S.; Lee, S.; Kim, W.; Davaatseren, M.; Hwang, J.T.; Kim, H.J.; et al. Genistein protects the kidney from cisplatin-induced injury. Kidney Int. 2008, 74, 1538–1547. [Google Scholar] [CrossRef]
- Wei, C.; Zhang, Y.; Zhong, X.; Lu, S.; Zou, X.; Yang, Y.; Huang, S.; Huang, Z. Ginkgo biloba leaf extract mitigates cisplatin-induced chronic renal interstitial fibrosis by inhibiting the epithelial-mesenchymal transition of renal tubular epithelial cells mediated by the smad3/tgf-beta1 and smad3/p38 mapk pathways. Chin. Med. 2022, 17, 25. [Google Scholar] [CrossRef]
- Zhai, J.; Gao, H.; Wang, S.; Zhang, S.; Qu, X.; Zhang, Y.; Tao, L.; Sun, J.; Song, Y.; Fu, L. Ginsenoside rg3 attenuates cisplatin-induced kidney injury through inhibition of apoptosis and autophagy-inhibited nlrp3. J. Biochem. Mol. Toxicol. 2021, 35, e22896. [Google Scholar] [CrossRef]
- Leta, B.; Kenenisa, C.; Wondimnew, T.; Sime, T. Evaluation of renoprotective effects of our locally grown green coffee beans against cisplatin-induced nephrotoxicity in swiss albino mice. Int. J. Nephrol. 2021, 2021, 2805068. [Google Scholar] [CrossRef]
- Fang, L.; Zhang, Y.; Wang, Q.; Zang, Y.; Li, Z.; Duan, Z.; Ren, J.; Xu, Z. A polysaccharide from huaier ameliorates cisplatin nephrotoxicity by decreasing oxidative stress and apoptosis via pi3k/akt signaling. Int. J. Biol. Macromol. 2019, 139, 932–943. [Google Scholar] [CrossRef]
- Sen, S.; De, B.; Devanna, N.; Chakraborty, R. Cisplatin-induced nephrotoxicity in mice: Protective role of leea asiatica leaves. Ren. Fail. 2013, 35, 1412–1417. [Google Scholar] [CrossRef]
- Mao, R.W.; He, S.P.; Lan, J.G.; Zhu, W.Z. Honokiol ameliorates cisplatin-induced acute kidney injury via inhibition of mitochondrial fission. Br. J. Pharmacol. 2022, 179, 3886–3904. [Google Scholar] [CrossRef]
- Ju, S.M.; Kim, M.S.; Jo, Y.S.; Jeon, Y.M.; Bae, J.S.; Pae, H.O.; Jeon, B.H. Licorice and its active compound glycyrrhizic acid ameliorates cisplatin-induced nephrotoxicity through inactivation of p53 by scavenging ros and overexpression of p21 in human renal proximal tubular epithelial cells. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 890–899. [Google Scholar] [PubMed]
- Zhou, M.; Dai, Y.; Ma, Y.; Yan, Y.; Hua, M.; Gao, Q.; Geng, X.; Zhou, Q. Protective effects of liquiritigenin against cisplatin-induced nephrotoxicity via nrf2/sirt3-mediated improvement of mitochondrial function. Molecules 2022, 27, 3823. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Sierra, T.; Medina-Campos, O.N.; Solano, J.D.; Ibarra-Rubio, M.E.; Pedraza-Chaverri, J. Isoliquiritigenin pretreatment induces endoplasmic reticulum stress-mediated hormesis and attenuates cisplatin-induced oxidative stress and damage in llc-pk1 cells. Molecules 2020, 25, 4442. [Google Scholar] [CrossRef] [PubMed]
- Omer Iqbal, M.; Bashir Yahya, E.; Andleeb, S.; Masood Ahmed, M.; Umar Javaid, M.; Shakeel, W.; Iqbal, I. In vivo assessment of reversing cisplatin-induced nephrotoxicity using jatropha mollissima crude extract and its potential cytotoxicity. Saudi J. Biol. Sci. 2021, 28, 7373–7378. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Jo, J.; Leem, J.; Park, K.K. Kahweol ameliorates cisplatin-induced acute kidney injury through pleiotropic effects in mice. Biomedicines 2020, 8, 572. [Google Scholar] [CrossRef] [PubMed]
- Masuda, Y.; Inoue, M.; Miyata, A.; Mizuno, S.; Nanba, H. Maitake beta-glucan enhances therapeutic effect and reduces myelosupression and nephrotoxicity of cisplatin in mice. Int. Immunopharmacol. 2009, 9, 620–626. [Google Scholar] [CrossRef]
- Yuan, L.; Yang, J.; Li, Y.; Yuan, L.; Liu, F.; Yuan, Y.; Tang, X. Matrine alleviates cisplatin-induced acute kidney injury by inhibiting mitochondrial dysfunction and inflammation via sirt3/opa1 pathway. J. Cell. Mol. Med. 2022, 26, 3702–3715. [Google Scholar] [CrossRef]
- Jain, A.; Singhai, A.K. Nephroprotective activity of momordica dioica roxb. In cisplatin-induced nephrotoxicity. Nat. Prod. Res. 2010, 24, 846–854. [Google Scholar] [CrossRef]
- Nematbakhsh, M.; Hajhashemi, V.; Ghannadi, A.; Talebi, A.; Nikahd, M. Protective effects of the morus alba l. Leaf extracts on cisplatin-induced nephrotoxicity in rat. Res. Pharm. Sci. 2013, 8, 71–77. [Google Scholar]
- el Daly, E.S. Protective effect of cysteine and vitamin e, crocus sativus and nigella sativa extracts on cisplatin-induced toxicity in rats. J. Pharm. Belg. 1998, 53, 87–93. [Google Scholar]
- Okur, M.E.; Ayla, S.; Karadag, A.E.; Cicek Polat, D.; Demirci, S.; Seckin, I. Opuntia ficus indica fruits ameliorate cisplatin-induced nephrotoxicity in mice. Biol. Pharm. Bull. 2020, 43, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.R.; Lee, D.; Lee, H.J.; Noh, H.J.; Jung, K.; Kang, K.S.; Kim, K.H. Renoprotective chemical constituents from an edible mushroom, pleurotus cornucopiae in cisplatin-induced nephrotoxicity. Bioorg. Chem. 2017, 71, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Rajakrishnan, R.; Lekshmi, R.; Benil, P.B.; Thomas, J.; AlFarhan, A.H.; Rakesh, V.; Khalaf, S. Phytochemical evaluation of roots of plumbago zeylanica l. And assessment of its potential as a nephroprotective agent. Saudi J. Biol. Sci. 2017, 24, 760–766. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Yu, P.; Wang, T.T.; Du, Y.W.; Chen, Y.; Li, Z.; He, M.L.; Feng, L.; Li, H.R.; Han, X.; et al. Polydatin attenuates cisplatin-induced acute kidney injury by inhibiting ferroptosis. Oxid. Med. Cell. Longev. 2022, 2022, 9947191. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.J.; Leng, B.; Wu, Z.Y.; Bian, J.S. Polysulfide and hydrogen sulfide ameliorate cisplatin-induced nephrotoxicity and renal inflammation through persulfidating stat3 and ikkbeta. Int. J. Mol. Sci. 2020, 21, 7805. [Google Scholar] [CrossRef]
- Karwasra, R.; Kalra, P.; Gupta, Y.K.; Saini, D.; Kumar, A.; Singh, S. Antioxidant and anti-inflammatory potential of pomegranate rind extract to ameliorate cisplatin-induced acute kidney injury. Food Funct. 2016, 7, 3091–3101. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Li, C.; Li, Q.; Li, J.; Lu, X. Puerarin alleviates cisplatin-induced acute renal damage and upregulates microrna-31-related signaling. Exp. Ther. Med. 2020, 20, 3122–3129. [Google Scholar] [CrossRef]
- Aladaileh, S.H.; Al-Swailmi, F.K.; Abukhalil, M.H.; Ahmeda, A.F.; Mahmoud, A.M. Punicalagin prevents cisplatin-induced nephrotoxicity by attenuating oxidative stress, inflammatory response, and apoptosis in rats. Life Sci. 2021, 286, 120071. [Google Scholar] [CrossRef]
- Hasan, M.M.; Tasmin, M.S.; El-Shehawi, A.M.; Elseehy, M.M.; Reza, M.A.; Haque, A.R. Vesicarius l. Exerts nephroprotective effect against cisplatin-induced oxidative stress. BMC Complement. Med. Ther. 2021, 21, 225. [Google Scholar] [CrossRef]
- Kim, Y.J.; Lee, M.Y.; Son, H.Y.; Park, B.K.; Ryu, S.Y.; Jung, J.Y. Red ginseng ameliorates acute cisplatin-induced nephropathy. Planta Med. 2014, 80, 645–654. [Google Scholar] [CrossRef]
- Osman, A.M.; Telity, S.A.; Telity, S.A.; Damanhouri, Z.A.; Al-Harthy, S.E.; Al-Kreathy, H.M.; Ramadan, W.S.; Elshal, M.F.; Khan, L.M.; Kamel, F. Chemosensitizing and nephroprotective effect of resveratrol in cisplatin -treated animals. Cancer Cell Int. 2015, 15, 6. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, A.; Fanoudi, S.; Mollazadeh, H.; Aghaei, A.; Boroushaki, M.T. Protective effect of rheum turkestanicum against cisplatin by reducing oxidative stress in kidney tissue. J. Pharm. Bioallied Sci. 2018, 10, 66–71. [Google Scholar] [PubMed]
- Tlili, N.; Feriani, A.; Allagui, M.S.; Saadoui, E.; Khaldi, A.; Nasri, N. Effects of rhus tripartitum fruit extract on ccl4-induced hepatotoxicity and cisplatin-induced nephrotoxicity in rats. Can. J. Physiol. Pharmacol. 2016, 94, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Tilyek, A.; Chai, C.; Hou, X.; Zhou, B.; Zhang, C.; Cao, Z.; Yu, B. The protective effects of ribes diacanthum pall on cisplatin-induced nephrotoxicity in mice. J. Ethnopharmacol. 2016, 178, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Radwan, R.R.; Abdel Fattah, S.M. Mechanisms involved in the possible nephroprotective effect of rutin and low dose gamma irradiation against cisplatin-induced nephropathy in rats. J. Photochem. Photobiol. B 2017, 169, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Lee, A.Y.; Kim, J.H.; Seong, S.H.; Jang, G.Y.; Cho, E.J.; Choi, J.S.; Kwon, J.; Kim, Y.O.; Lee, S.W.; et al. Protective effect of safflower seed on cisplatin-induced renal damage in mice via oxidative stress and apoptosis-mediated pathways. Am. J. Chin. Med. 2018, 46, 157–174. [Google Scholar] [CrossRef]
- Park, C.H.; Kim, M.J.; Yang, C.Y.; Yokozawa, T.; Shin, Y.S. Safflower seed extract synergizes the therapeutic effect of cisplatin and reduces cisplatin-induced nephrotoxicity in human colorectal carcinoma rko cells and rko-transplanted mice. Drug Discov. Ther. 2019, 13, 328–334. [Google Scholar] [CrossRef]
- Sohail, N.; Hira, K.; Kori, J.A.; Farhat, H.; Urooj, F.; Khan, W.; Sultana, V.; Ali, M.S.; Ehteshamul-Haque, S. Nephroprotective effect of ethanol extract and fractions of a sea lettuce, ulva fasciata against cisplatin-induced kidney injury in rats. Environ. Sci. Pollut. Res. Int. 2021, 28, 9448–9461. [Google Scholar] [CrossRef]
- Ali, B.H.; Al Salam, S.; Al Suleimani, Y.; Al Za’abi, M.; Ashique, M.; Manoj, P.; Sudhadevi, M.; Al Tobi, M.; Nemmar, A. Ameliorative effect of sesamin in cisplatin-induced nephrotoxicity in rats by suppressing inflammation, oxidative/nitrosative stress, and cellular damage. Physiol. Res. 2020, 69, 61–72. [Google Scholar] [CrossRef]
- Ikeda, Y.; Funamoto, M.; Kishi, S.; Imanishi, M.; Aihara, K.I.; Kashiwada, Y.; Tsuchiya, K. The novel preventive effect of a japanese ethical kampo extract formulation tj-90 (seihaito) against cisplatin-induced nephrotoxicity. Phytomedicine 2022, 103, 154213. [Google Scholar] [CrossRef]
- Elhady, S.S.; Abdelhameed, R.F.A.; Mehanna, E.T.; Wahba, A.S.; Elfaky, M.A.; Koshak, A.E.; Noor, A.O.; Bogari, H.A.; Malatani, R.T.; Goda, M.S. Metabolic profiling, chemical composition, antioxidant capacity, and in vivo hepato- and nephroprotective effects of sonchus cornutus in mice exposed to cisplatin. Antioxidants 2022, 11, 819. [Google Scholar] [CrossRef] [PubMed]
- Ademiluyi, A.O.; Oboh, G.; Agbebi, O.J.; Oyeleye, S.I. Dietary inclusion of sorghum (sorghum bicolour) straw dye protects against cisplatin-induced nephrotoxicity and oxidative stress in rats. Pharm. Biol. 2014, 52, 829–834. [Google Scholar] [CrossRef]
- Sadeghi, H.; Mansourian, M.; Panahi Kokhdan, E.; Salehpour, Z.; Sadati, I.; Abbaszadeh-Goudarzi, K.; Asfaram, A.; Doustimotlagh, A.H. Antioxidant and protective effect of stachys pilifera benth against nephrotoxicity induced by cisplatin in rats. J. Food Biochem. 2020, 44, e13190. [Google Scholar] [CrossRef] [PubMed]
- Potocnjak, I.; Broznic, D.; Kindl, M.; Kropek, M.; Vladimir-Knezevic, S.; Domitrovic, R. Stevia and stevioside protect against cisplatin nephrotoxicity through inhibition of erk1/2, stat3, and nf-kappab activation. Food Chem. Toxicol. 2017, 107, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Kim, Y.J.; Han, I.H.; Lee, D.; Ham, J.; Kang, K.S.; Lee, J.W. The synthesis of sulforaphane analogues and their protection effect against cisplatin induced cytotoxicity in kidney cells. Bioorg. Med. Chem. Lett. 2015, 25, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Vu-Huynh, K.L.; Le, T.H.V.; Nguyen, H.T.; Kim, H.M.; Kang, K.S.; Park, J.H.; Nguyen, M.D. Increase in protective effect of panax vietnamensis by heat processing on cisplatin-induced kidney cell toxicity. Molecules 2019, 24, 4627. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.H.; Tang, Y.R.; Lan, J.; Huang, Z.Y.; Tian, W.; Huang, Q.; Peng, Y.; Gao, Y.; Hu, Y.Q.; et al. Protective effects of tanshinone against cisplatin-induced nephrotoxicity in mice. Iran. J. Basic Med. Sci. 2022, 25, 414–418. [Google Scholar]
- Kalra, P.; Karwasra, R.; Gupta, Y.K.; Ray, S.B.; Singh, S. Terminalia chebula supplementation attenuates cisplatin-induced nephrotoxicity in wistar rats through modulation of apoptotic pathway. Nat. Prod. Res. 2019, 33, 1641–1645. [Google Scholar] [CrossRef]
- Song, K.I.; Park, J.Y.; Lee, S.; Lee, D.; Jang, H.J.; Kim, S.N.; Ko, H.; Kim, H.Y.; Lee, J.W.; Hwang, G.S.; et al. Protective effect of tetrahydrocurcumin against cisplatin-induced renal damage: In vitro and in vivo studies. Planta Med. 2015, 81, 286–291. [Google Scholar] [CrossRef]
- Guan, T.; Zheng, Y.; Jin, S.; Wang, S.; Hu, M.; Liu, X.; Huang, S.; Liu, Y. Troxerutin alleviates kidney injury in rats via pi3k/akt pathway by enhancing map4 expression. Food Nutr. Res. 2022, 66. [Google Scholar] [CrossRef]
- Naushad, M.; Urooj, M.; Ahmad, T.; Husain, G.M.; Kazmi, M.H.; Zakir, M. Nephroprotective effect of apium graveolens l. Against cisplatin-induced nephrotoxicity. J. Ayurveda Integr. Med. 2021, 12, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Eren, H.; Aydin, H.R.; Tumkaya, L.; Kazaz, I.O.; Kalkan, Y.; Kazaz, S.N.; Mercantepe, T.; Horsanali, M.O.; Yilmaz, A. Whortleberry protects kidney against the cisplatin-induced nephrotoxicity: An experimental study. Ren. Fail. 2018, 40, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Joshi, A.; Hemalatha, S. Protective effect of withania coagulans fruit extract on cisplatin-induced nephrotoxicity in rats. Pharmacogn. Res. 2017, 9, 354–361. [Google Scholar]
- Kandemir, F.M.; Yildirim, S.; Caglayan, C.; Kucukler, S.; Eser, G. Protective effects of zingerone on cisplatin-induced nephrotoxicity in female rats. Environ. Sci. Pollut. Res. Int. 2019, 26, 22562–22574. [Google Scholar] [CrossRef] [PubMed]
- Kensler, T.W.; Wakabayashi, N.; Biswal, S. Cell survival responses to environmental stresses via the keap1-nrf2-are pathway. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 89–116. [Google Scholar] [CrossRef]
- Jaiswal, A.K. Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radic. Biol. Med. 2004, 36, 1199–1207. [Google Scholar] [CrossRef]
- Guerrero-Hue, M.; Rayego-Mateos, S.; Vazquez-Carballo, C.; Palomino-Antolin, A.; Garcia-Caballero, C.; Opazo-Rios, L.; Morgado-Pascual, J.L.; Herencia, C.; Mas, S.; Ortiz, A.; et al. Protective role of nrf2 in renal disease. Antioxidants 2020, 10, 39. [Google Scholar] [CrossRef]
- Wu, J.; Li, R.; Li, W.; Ren, M.; Thangthaeng, N.; Sumien, N.; Liu, R.; Yang, S.; Simpkins, J.W.; Forster, M.J.; et al. Administration of 5-methoxyindole-2-carboxylic acid that potentially targets mitochondrial dihydrolipoamide dehydrogenase confers cerebral preconditioning against ischemic stroke injury. Free Radic. Biol. Med. 2017, 113, 244–254. [Google Scholar] [CrossRef]
- Ali, F.E.M.; Hassanein, E.H.M.; El-Bahrawy, A.H.; Omar, Z.M.M.; Rashwan, E.K.; Abdel-Wahab, B.A.; Abd-Elhamid, T.H. Nephroprotective effect of umbelliferone against cisplatin-induced kidney damage is mediated by regulation of nrf2, cytoglobin, sirt1/foxo-3, and nf- kb-p65 signaling pathways. J. Biochem. Mol. Toxicol. 2021, 35, e22738. [Google Scholar] [CrossRef]
- Mahran, Y.F. New insights into the protection of growth hormone in cisplatin-induced nephrotoxicity: The impact of igf-1 on the keap1-nrf2/ho-1 signaling. Life Sci. 2020, 253, 117581. [Google Scholar] [CrossRef]
- El-Sayed, R.M.; Abo El Gheit, R.E.; Badawi, G.A. Vincamine protects against cisplatin induced nephrotoxicity via activation of nrf2/ho-1 and hindering tlr4/ ifn-gamma/cd44 cells inflammatory cascade. Life Sci. 2021, 272, 119224. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Cai, F.; Zhou, N.; Liu, S.; Wang, P.; Zhang, S.; Zhang, Y.; Zhang, A.; Jia, Z.; Huang, S. Dimethyl fumarate prevents ferroptosis to attenuate acute kidney injury by acting on nrf2. Clin. Transl. Med. 2021, 11, e382. [Google Scholar] [CrossRef] [PubMed]
- Barakat, N.; Barakat, L.A.A.; Zakaria, M.M.; Khirallah, S.M. Diacerein ameliorates kidney injury induced by cisplatin in rats by activation of nrf2/ho-1 pathway and bax down-regulation. Saudi J. Biol. Sci. 2021, 28, 7219–7226. [Google Scholar] [CrossRef] [PubMed]
- Hosoya, T.; Maruyama, A.; Kang, M.I.; Kawatani, Y.; Shibata, T.; Uchida, K.; Warabi, E.; Noguchi, N.; Itoh, K.; Yamamoto, M. Differential responses of the nrf2-keap1 system to laminar and oscillatory shear stresses in endothelial cells. J. Biol. Chem. 2005, 280, 27244–27250. [Google Scholar] [CrossRef]
- Motohashi, H.; Yamamoto, M. Nrf2-keap1 defines a physiologically important stress response mechanism. Trends Mol. Med. 2004, 10, 549–557. [Google Scholar] [CrossRef]
- Villeneuve, N.F.; Lau, A.; Zhang, D.D. Regulation of the nrf2-keap1 antioxidant response by the ubiquitin proteasome system: An insight into cullin-ring ubiquitin ligases. Antioxid. Redox Signal. 2011, 13, 1699–1712. [Google Scholar] [CrossRef]
- Wang, X.Y.; Wang, Z.Y.; Zhu, Y.S.; Zhu, S.M.; Fan, R.F.; Wang, L. Alleviation of cadmium-induced oxidative stress by trehalose via inhibiting the nrf2-keap1 signaling pathway in primary rat proximal tubular cells. J. Biochem. Mol. Toxicol. 2018, 32, e22011. [Google Scholar] [CrossRef]
- Yan, W.; Xu, Y.; Yuan, Y.; Tian, L.; Wang, Q.; Xie, Y.; Shao, X.; Zhang, M.; Ni, Z.; Mou, S. Renoprotective mechanisms of astragaloside iv in cisplatin-induced acute kidney injury. Free Radic. Res. 2017, 51, 669–683. [Google Scholar] [CrossRef]
- Ning, Y.C.; Cai, G.Y.; Zhuo, L.; Gao, J.J.; Dong, D.; Cui, S.Y.; Shi, S.Z.; Feng, Z.; Zhang, L.; Sun, X.F.; et al. Beneficial effects of short-term calorie restriction against cisplatin-induced acute renal injury in aged rats. Nephron Exp. Nephrol. 2013, 124, 19–27. [Google Scholar] [CrossRef]
- Estrela, G.R.; Wasinski, F.; Batista, R.O.; Hiyane, M.I.; Felizardo, R.J.; Cunha, F.; de Almeida, D.C.; Malheiros, D.M.; Camara, N.O.; Barros, C.C.; et al. Caloric restriction is more efficient than physical exercise to protect from cisplatin nephrotoxicity via ppar-alpha activation. Front. Physiol. 2017, 8, 116. [Google Scholar] [CrossRef]
- Mikami, D.; Kobayashi, M.; Uwada, J.; Yazawa, T.; Kamiyama, K.; Nishimori, K.; Nishikawa, Y.; Morikawa, Y.; Yokoi, S.; Takahashi, N.; et al. Beta-hydroxybutyrate, a ketone body, reduces the cytotoxic effect of cisplatin via activation of hdac5 in human renal cortical epithelial cells. Life Sci. 2019, 222, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Duan, Z.Y.; Liu, J.Q.; Yin, P.; Li, J.J.; Cai, G.Y.; Chen, X.M. Impact of aging on the risk of platinum-related renal toxicity: A systematic review and meta-analysis. Cancer Treat. Rev. 2018, 69, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Han, Y.K.; Kong, M.J.; Park, K.M. Short-term control of diet affects cisplatin-induced acute kidney injury through modulation of mitochondrial dynamics and mitochondrial gsh. Physiol. Rep. 2022, 10, e15348. [Google Scholar] [CrossRef] [PubMed]
- Duan, Z.; Cai, G.; Li, J.; Chen, X. Cisplatin-induced renal toxicity in elderly people. Ther. Adv. Med. Oncol. 2020, 12, 1758835920923430. [Google Scholar] [CrossRef]
- Guan, Y.; Wang, S.R.; Huang, X.Z.; Xie, Q.H.; Xu, Y.Y.; Shang, D.; Hao, C.M. Nicotinamide mononucleotide, an nad(+) precursor, rescues age-associated susceptibility to aki in a sirtuin 1-dependent manner. J. Am. Soc. Nephrol. 2017, 28, 2337–2352. [Google Scholar] [CrossRef]
- Barinda, A.J.; Arozal, W.; Sandhiutami, N.M.D.; Louisa, M.; Arfian, N.; Sandora, N.; Yusuf, M. Curcumin prevents epithelial-to mesenchymal transition-mediated ovarian cancer progression through nrf2/etbr/et-1 axis and preserves mitochondria biogenesis in kidney after cisplatin administration. Adv. Pharm. Bull. 2022, 12, 128–141. [Google Scholar] [CrossRef]
- Ko, J.W.; Shin, N.R.; Jung, T.Y.; Shin, I.S.; Moon, C.; Kim, S.H.; Lee, I.C.; Kim, S.H.; Yun, W.K.; Kim, H.C.; et al. Melatonin attenuates cisplatin-induced acute kidney injury in rats via induction of anti-aging protein, klotho. Food Chem. Toxicol. 2019, 129, 201–210. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).