Fibrotic Response of Human Trabecular Meshwork Cells to Transforming Growth Factor-Beta 3 and Autotaxin in Aqueous Humor
Abstract
:1. Introduction
2. Materials and Methods
2.1. AH Samples from Patients
2.2. Measurement of ATX, ATX Isoforms, TGF-β1, TGF-β2, and TGF-β3 in the AH
2.3. Cell Culture and Passage
2.4. WST-1 Assay
2.5. Immunocytochemistry
2.6. Real Time Quantitative Polymerase Chain Reaction (qPCR)
2.7. Western Blotting
2.8. Statistical Analysis
3. Results
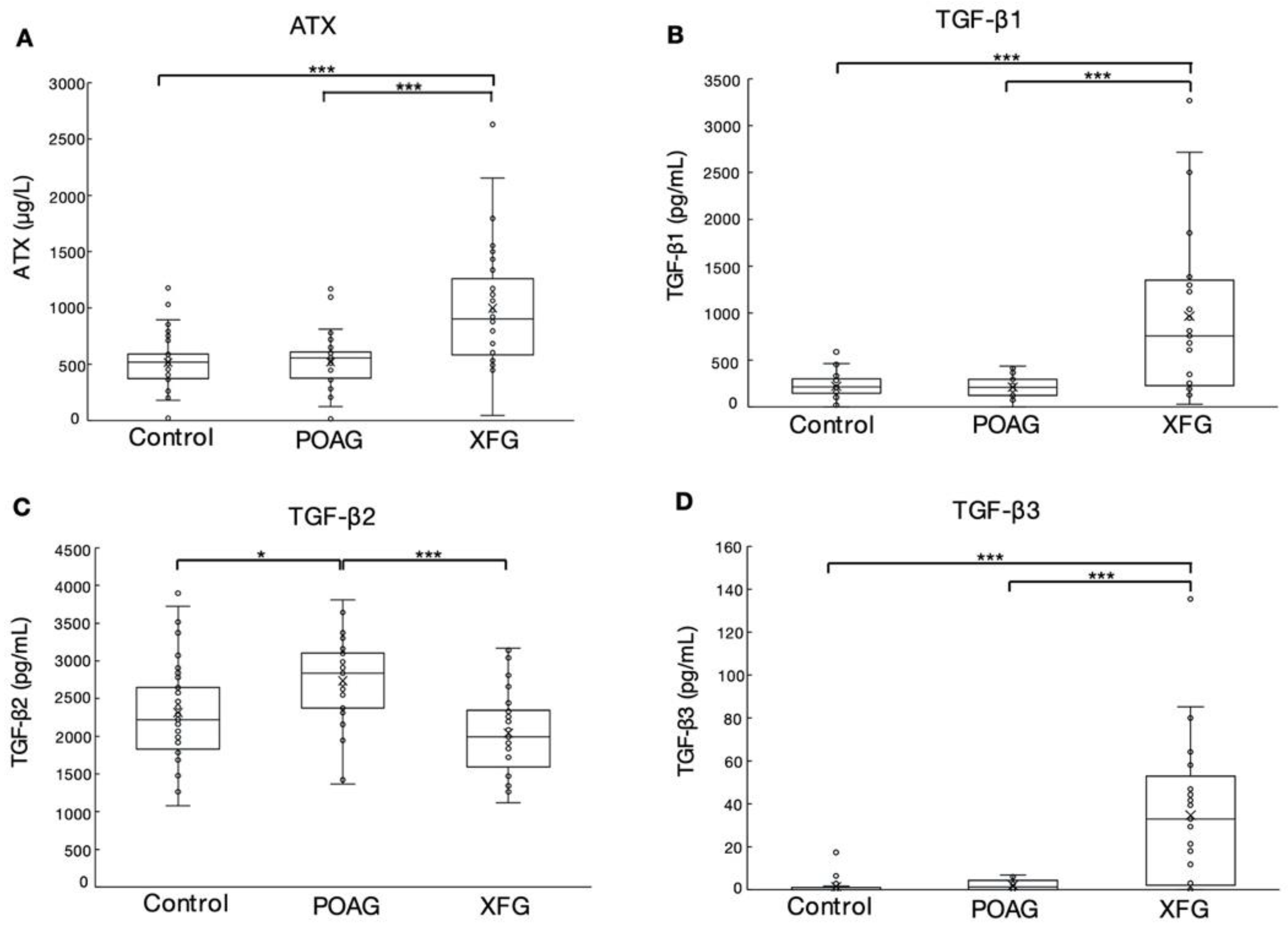
3.1. The Levels of ATX, TGF-β1, TGF-β2, and TGF-β3 in the AH of POAG and XFG Patients
3.2. Effects of TGF-β3 in HTM Cell Viability
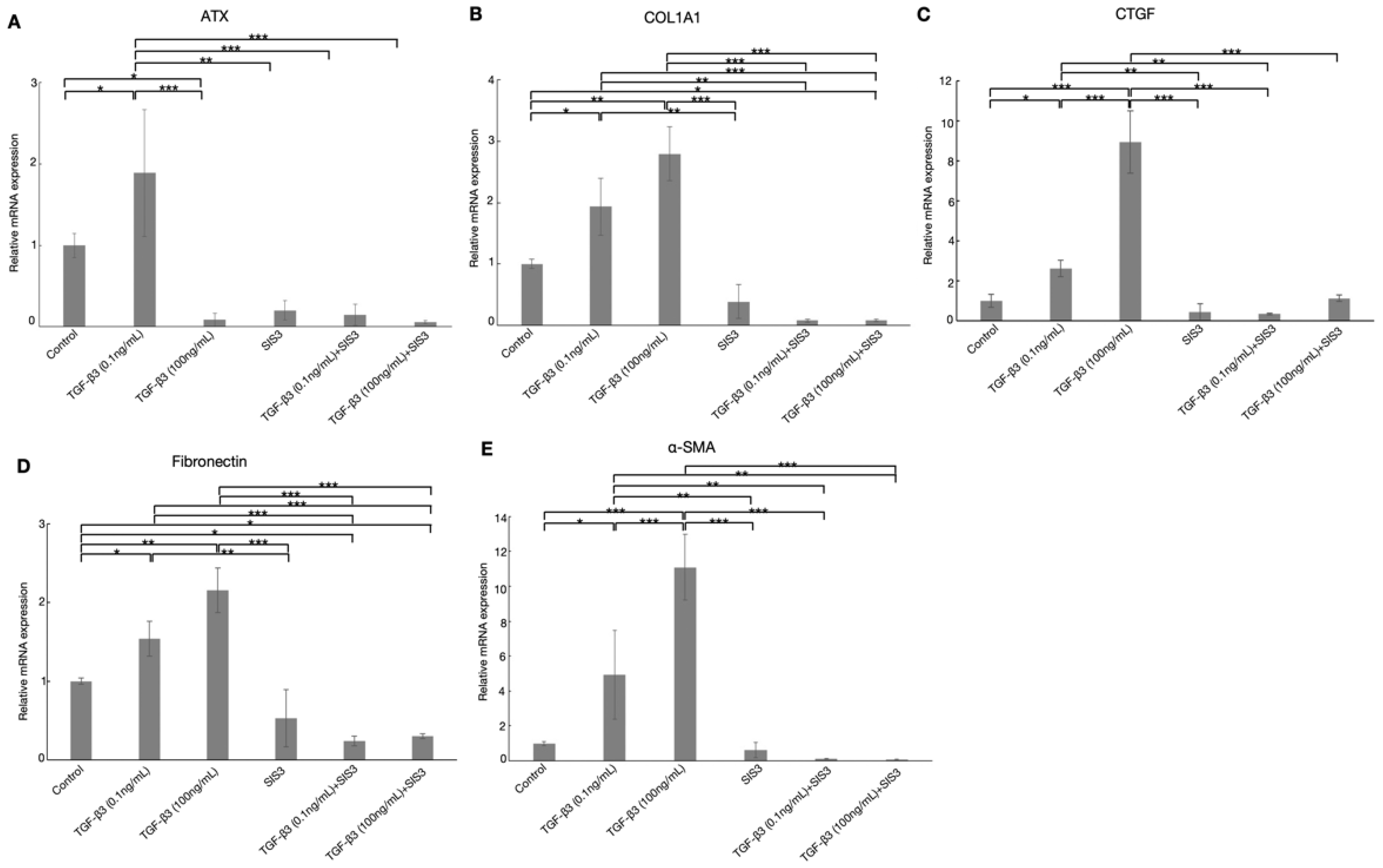
3.3. Effects of TGF-β3 on COL1A1, CTGF, Fibronectin, and α-SMA in HTM Cells
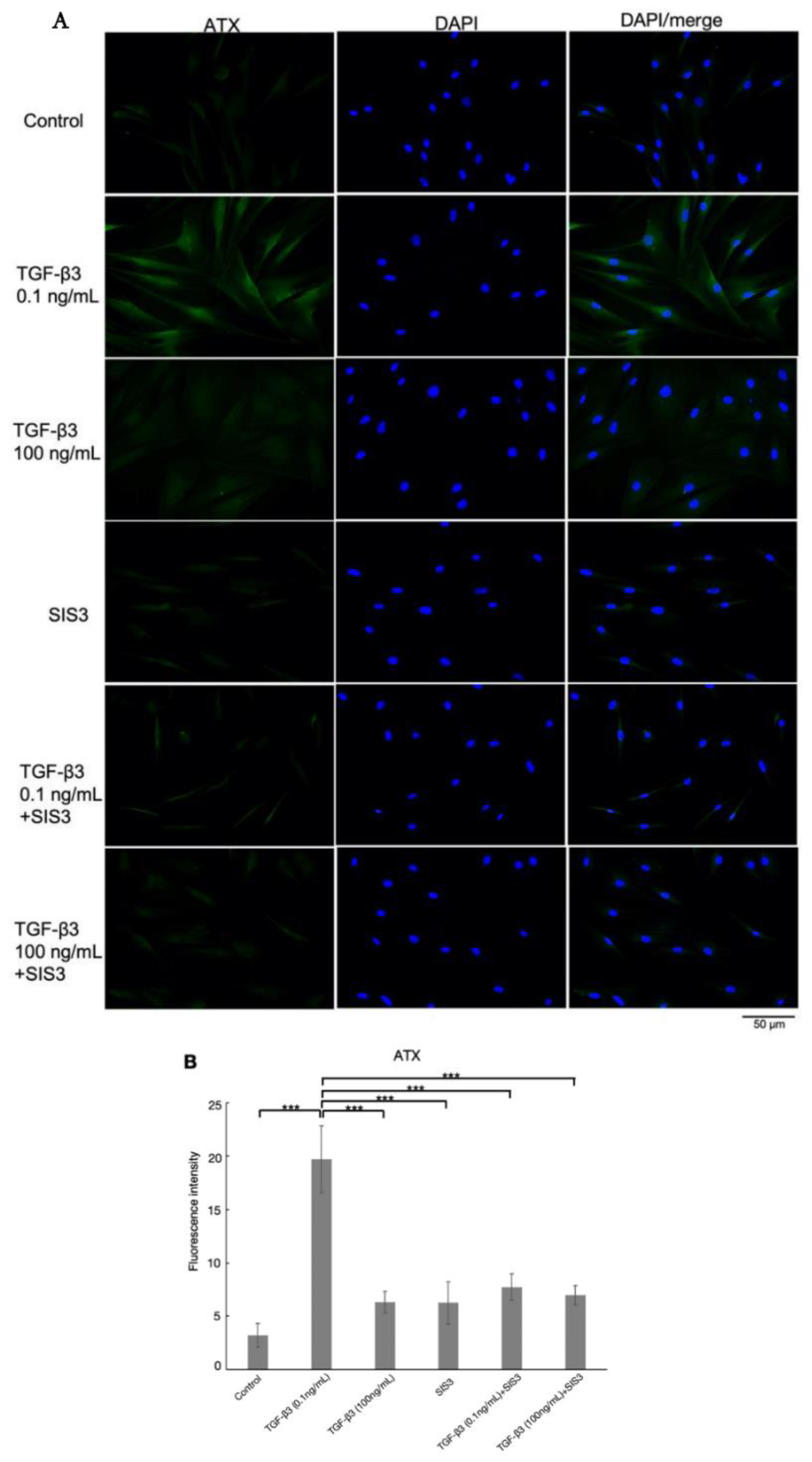
3.4. Effects of TGF-β3 on ATX, TGF-β1, TGF-β2, and TGF-β-Induced Protein in HTM Cells
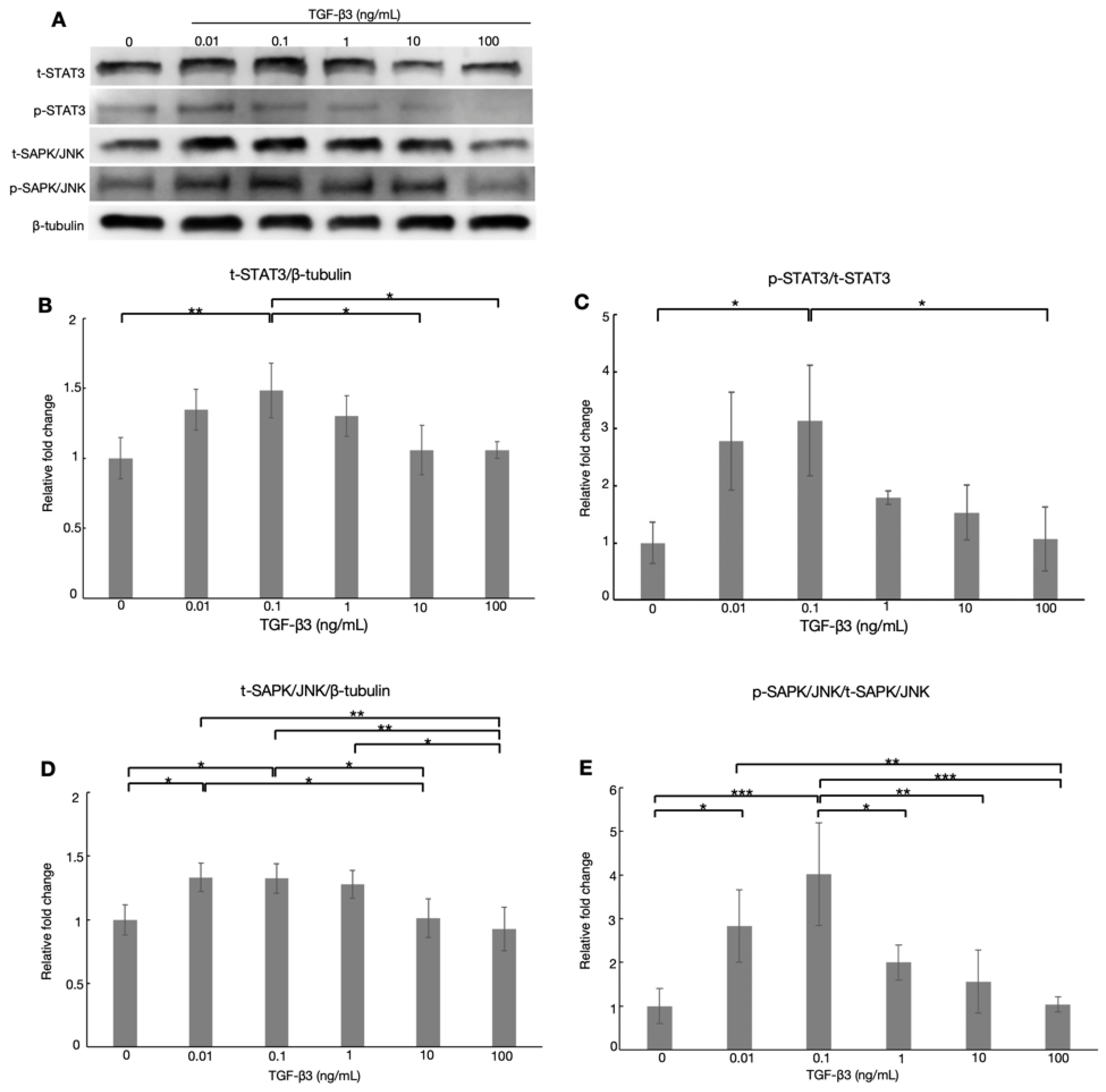
3.5. Effects of TGF-β3 on the ATX-Related Transcription Factors via TGF-β Noncanonical Pathway in HTM Cells
3.6. Effects of TGF-β3 on the Expression of ATX via TGF-β Canonical Pathway in HTM Cells
3.7. TGF-β3 Affects Cytoskeletal Proteins and Fibrotic Changes via TGF-β Canonical Pathway in HTM Cells
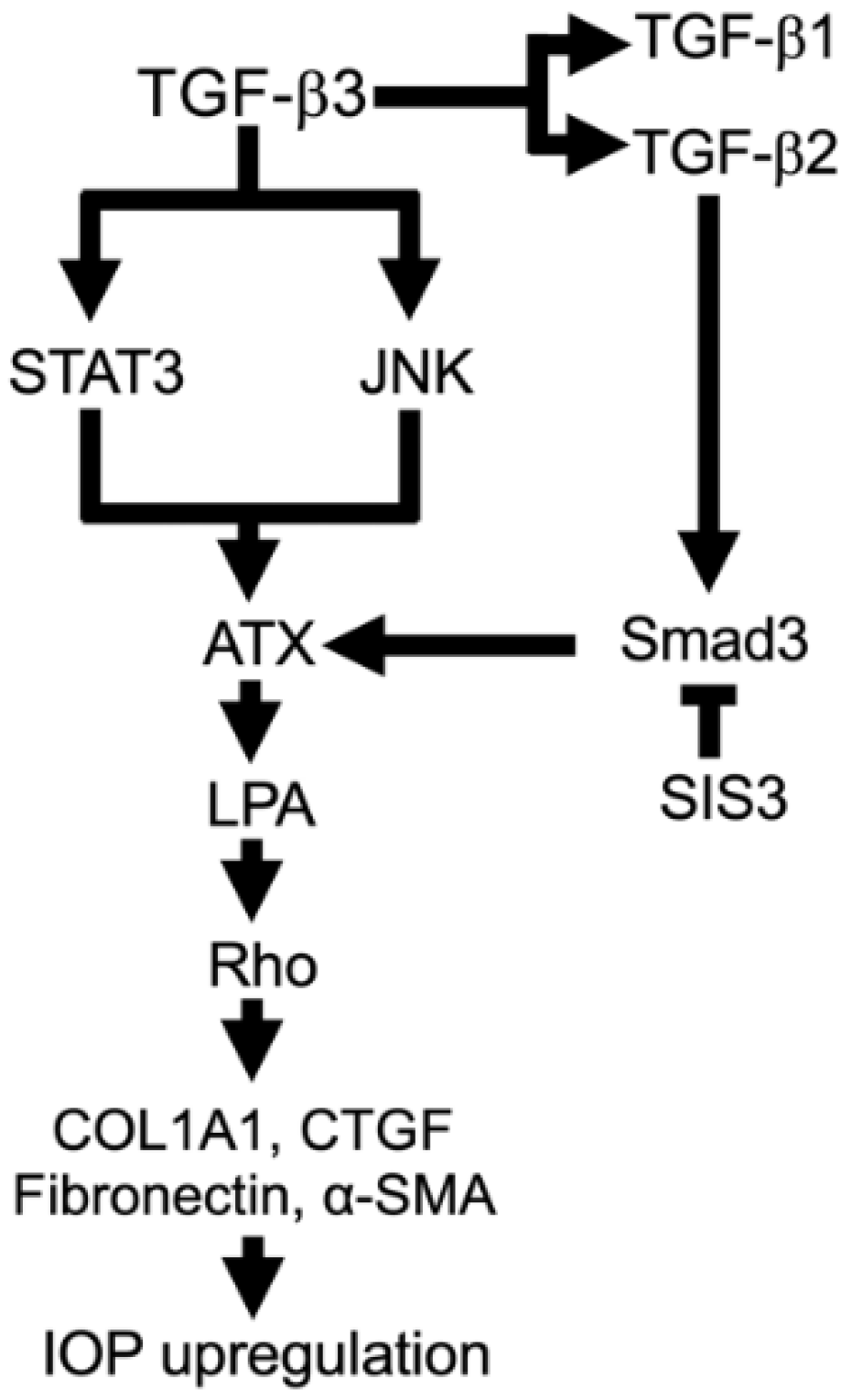
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saito, Y.; Kizaki, J.; Wada, Y.; Shibasaki, Y.; Kishimoto, N.; Aihara, M. Comparison of the 24-h Efficacy and Safety of Fixed Combination Carteolol/Latanoprost and Timolol/Latanoprost in Patients with Primary Open-Angle Glaucoma and Ocular Hypertension: A Prospective Crossover Study. Jpn. J. Ophthalmol. 2021, 65, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Omoto, T.; Fujishiro, T.; Asano-Shimizu, K.; Sugimoto, K.; Sakata, R.; Murata, H.; Asaoka, R.; Honjo, M.; Aihara, M. Comparison of the Short-Term Effectiveness and Safety Profile of Ab Interno Combined Trabeculotomy Using 2 Types of Trabecular Hooks. Jpn. J. Ophthalmol. 2020, 64, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Fujita, A.; Sakata, R.; Ueda, K.; Nakajima, K.; Fujishiro, T.; Honjo, M.; Shirato, S.; Aihara, M. Evaluation of Fornix-Based Trabeculectomy Outcomes in Japanese Glaucoma Patients Based on Concrete Long-Term Preoperative Data. Jpn. J. Ophthalmol. 2021, 65, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Montecchi-Palmer, M.; Bermudez, J.Y.; Webber, H.C.; Patel, G.C.; Clark, A.F.; Mao, W. TGFβ2 Induces the Formation of Cross-Linked Actin Networks (CLANs) in Human Trabecular Meshwork Cells Through the Smad and Non-Smad Dependent Pathways. Investig. Ophthalmol. Vis. Sci. 2017, 58, 1288. [Google Scholar] [CrossRef]
- Igarashi, N.; Honjo, M.; Yamagishi, R.; Kurano, M.; Yatomi, Y.; Igarashi, K.; Kaburaki, T.; Aihara, M. Crosstalk between Transforming Growth Factor β-2 and Autotaxin in Trabecular Meshwork and Different Subtypes of Glaucoma. J. Biomed. Sci. 2021, 28, 47. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Zhong, Y. Rho/Rho-Associated Kinase Pathway in Glaucoma. Int. J. Oncol. 2013, 43, 1357–1367. [Google Scholar] [CrossRef]
- Aihara, M.; Ropo, A.; Lu, F.; Kawata, H.; Iwata, A.; Odani-Kawabata, N.; Shams, N. Intraocular Pressure-Lowering Effect of Omidenepag Isopropyl in Latanoprost Non-/Low-Responder Patients with Primary Open-Angle Glaucoma or Ocular Hypertension: The FUJI Study. Jpn. J. Ophthalmol. 2020, 64, 398–406. [Google Scholar] [CrossRef]
- Wang, X.; Huai, G.; Wang, H.; Liu, Y.; Qi, P.; Shi, W.; Peng, J.; Yang, H.; Deng, S.; Wang, Y. Mutual Regulation of the Hippo/Wnt/LPA/TGF-β Signaling Pathways and Their Roles in Glaucoma (Review). Int. J. Mol. Med. 2017, 41, 1201–1212. [Google Scholar] [CrossRef]
- Iyer, P.; Lalane, R.; Morris, C.; Challa, P.; Vann, R.; Rao, P.V. Autotaxin-Lysophosphatidic Acid Axis Is a Novel Molecular Target for Lowering Intraocular Pressure. PLoS ONE 2012, 7, e42627. [Google Scholar] [CrossRef]
- Angelilli, A.; Ritch, R. Directed Therapy for Exfoliation Syndrome. TOOPHTJ 2009, 3, 70–74. [Google Scholar] [CrossRef]
- Raghunathan, V.K.; Morgan, J.T.; Chang, Y.-R.; Weber, D.; Phinney, B.; Murphy, C.J.; Russell, P. Transforming Growth Factor Beta 3 Modifies Mechanics and Composition of Extracellular Matrix Deposited by Human Trabecular Meshwork Cells. ACS Biomater. Sci. Eng. 2015, 1, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xia, X.; Yang, E.; Wang, Y.; Marra, K.G.; Ethier, C.R.; Schuman, J.S.; Du, Y. Adipose-derived Stem Cells Integrate into Trabecular Meshwork with Glaucoma Treatment Potential. FASEB J. 2020, 34, 7160–7177. [Google Scholar] [CrossRef] [PubMed]
- Konstas, A.G.P.; Hollo, G.; Irkec, M.; Tsironi, S.; Durukan, I.; Goldenfeld, M.; Melamed, S. Diurnal IOP Control with Bimatoprost versus Latanoprost in Exfoliative Glaucoma: A Crossover, Observer-Masked, Three-Centre Study. Br. J. Ophthalmol. 2007, 91, 757–760. [Google Scholar] [CrossRef] [PubMed]
- Haque, S.; Morris, J.C. Transforming Growth Factor-β: A Therapeutic Target for Cancer. Hum. Vaccines Immunother. 2017, 13, 1741–1750. [Google Scholar] [CrossRef] [PubMed]
- Halder, S.K.; Goodwin, J.S.; Al-Hendy, A. 1,25-Dihydroxyvitamin D3 Reduces TGF-Β3-Induced Fibrosis-Related Gene Expression in Human Uterine Leiomyoma Cells. J. Clin. Endocrinol. Metab. 2011, 96, E754–E762. [Google Scholar] [CrossRef]
- Massague, J. Transcriptional Control by the TGF-Beta/Smad Signaling System. EMBO J. 2000, 19, 1745–1754. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, X.; Liang, J.; Wu, J.; Tan, D.; Hu, W. Lefty-1 Inhibits Renal Epithelial–Mesenchymal Transition by Antagonizing the TGF-β/Smad Signaling Pathway. J. Mol. Hist. 2020, 51, 77–87. [Google Scholar] [CrossRef]
- Karamichos, D.; Hutcheon, A.E.K.; Zieske, J.D. Transforming Growth Factor-Β3 Regulates Assembly of a Non-Fibrotic Matrix in a 3D Corneal Model. J. Tissue Eng. Regen. Med. 2011, 5, e228–e238. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, X.-F.; Wang, Z.-C.; Lou, D.; Fang, Q.-Q.; Hu, Y.-Y.; Zhao, W.-Y.; Zhang, L.-Y.; Wu, L.-H.; Tan, W.-Q. Current Potential Therapeutic Strategies Targeting the TGF-β/Smad Signaling Pathway to Attenuate Keloid and Hypertrophic Scar Formation. Biomed. Pharmacother. 2020, 129, 110287. [Google Scholar] [CrossRef]
- Wilson, S.E. TGF Beta −1, −2 and −3 in the Modulation of Fibrosis in the Cornea and Other Organs. Exp. Eye Res. 2021, 207, 108594. [Google Scholar] [CrossRef]
- Yoneda, K.; Nakano, M.; Mori, K.; Kinoshita, S.; Tashiro, K. Disease-Related Quantitation of TGF-Beta3 in Human Aqueous Humor. Growth Factors 2007, 25, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Garweg, J.G.; Zandi, S.; Gerhardt, C.; Pfister, I.B. Isoforms of TGF-β in the Aqueous Humor of Patients with Pseudoexfoliation Syndrome and a Possible Association with the Long-Term Stability of the Capsular Bag after Cataract Surgery. Graefe’s Arch. Clin. Exp. Ophthalmol. 2017, 255, 1763–1769. [Google Scholar] [CrossRef]
- Inatani, M.; Tanihara, H.; Katsuta, H.; Honjo, M.; Kido, N.; Honda, Y. Transforming Growth Factor-Β2 Levels in Aqueous Humor of Glaucomatous Eyes. Graefe’s Arch. Clin. Exp. Ophthalmol. 2001, 239, 109–113. [Google Scholar] [CrossRef]
- Igarashi, N.; Honjo, M.; Kurano, M.; Yatomi, Y.; Igarashi, K.; Kano, K.; Aoki, J.; Aihara, M. Increased Aqueous Autotaxin and Lysophosphatidic Acid Levels Are Potential Prognostic Factors after Trabeculectomy in Different Types of Glaucoma. Sci. Rep. 2018, 8, 11304. [Google Scholar] [CrossRef]
- Rao, P.V. Bioactive Lysophospholipids: Role in Regulation of Aqueous Humor Outflow and Intraocular Pressure in the Context of Pathobiology and Therapy of Glaucoma. J. Ocul. Pharmacol. Ther. 2014, 30, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tseng, S.C.G.; Zhu, Y.-T. Suppression of TGF-Β1 Signaling by Matrigel via FAK Signaling in Cultured Human Trabecular Meshwork Cells. Sci. Rep. 2021, 11, 7319. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, N.; Yamagishi, R.; Honjo, M.; Igarashi, N.; Shimizu, S.; Aihara, M. Effects of Topical TGF-Β1, TGF-Β2, ATX, and LPA on IOP Elevation and Regulation of the Conventional Aqueous Humor Outflow Pathway. Mol. Vis. 2021, 17, 61–77. [Google Scholar]
- Honjo, M.; Igarashi, N.; Kurano, M.; Yatomi, Y.; Igarashi, K.; Kano, K.; Aoki, J.; Weinreb, R.N.; Aihara, M. Autotaxin–Lysophosphatidic Acid Pathway in Intraocular Pressure Regulation and Glaucoma Subtypes. Investig. Ophthalmol. Vis. Sci. 2018, 59, 693. [Google Scholar] [CrossRef]
- Igarashi, N.; Honjo, M.; Yamagishi, R.; Kurano, M.; Yatomi, Y.; Igarashi, K.; Kaburaki, T.; Aihara, M. Involvement of Autotaxin in the Pathophysiology of Elevated Intraocular Pressure in Posner-Schlossman Syndrome. Sci. Rep. 2020, 10, 6265. [Google Scholar] [CrossRef]
- Igarashi, N.; Honjo, M.; Aihara, M. MTOR Inhibitors Potentially Reduce TGF-Β2-Induced Fibrogenic Changes in Trabecular Meshwork Cells. Sci. Rep. 2021, 11, 14111. [Google Scholar] [CrossRef]
- Keller, K.E.; Bhattacharya, S.K.; Borrás, T.; Brunner, T.M.; Chansangpetch, S.; Clark, A.F.; Dismuke, W.M.; Du, Y.; Elliott, M.H.; Ethier, C.R.; et al. Consensus Recommendations for Trabecular Meshwork Cell Isolation, Characterization and Culture. Exp. Eye Res. 2018, 171, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Jensen, E.C. Quantitative Analysis of Histological Staining and Fluorescence Using ImageJ: Histological Staining/Fluorescence Using ImageJ. Anat. Rec. 2013, 296, 378–381. [Google Scholar] [CrossRef] [PubMed]
- Honjo, M.; Igarashi, N.; Nishida, J.; Kurano, M.; Yatomi, Y.; Igarashi, K.; Kano, K.; Aoki, J.; Aihara, M. Role of the Autotaxin-LPA Pathway in Dexamethasone-Induced Fibrotic Responses and Extracellular Matrix Production in Human Trabecular Meshwork Cells. Investig. Ophthalmol. Vis. Sci. 2018, 59, 21. [Google Scholar] [CrossRef]
- Yang, L.; Yu, X.; Yang, Y. Autotaxin Upregulated by STAT3 Activation Contributes to Invasion in Pancreatic Neuroendocrine Neoplasms. Endocr. Connect. 2018, 7, 1299–1307. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Daher, A.M.; Agarwal, R. Aqueous Humor TGF-Β2 Levels in Patients with Open-Angle. Mol. Vis. 2015, 21, 612–620. [Google Scholar] [PubMed]
- Kottler, U.B.; Jünemann, A.G.M.; Aigner, T.; Zenkel, M.; Rummelt, C.; Schlötzer-Schrehardt, U. Comparative Effects of TGF-Β1 and TGF-Β2 on Extracellular Matrix Production, Proliferation, Migration, and Collagen Contraction of Human Tenon’s Capsule Fibroblasts in Pseudoexfoliation and Primary Open-Angle Glaucoma. Exp. Eye Res. 2005, 80, 121–134. [Google Scholar] [CrossRef]
- Khan, S.A.; Joyce, J.; Tsuda, T. Quantification of Active and Total Transforming Growth Factor-β Levels in Serum and Solid Organ Tissues by Bioassay. BMC Res. Notes 2012, 5, 636. [Google Scholar] [CrossRef]
- Massague, J. Transforming Growth Factor-/3 Modulates the High-Affinity Receptors for Epidermal Growth Factor and Transforming Growth Factor-c. 7. J. Cell Biol. 1985, 100, 1508–1514. [Google Scholar] [CrossRef]
- Tamm, E.R.; Siegner, A.; Baur, A.; Lütjen-Drecoll, E. Transforming Growth Factor-Β1 Induces α-Smooth Muscle-Actin Expression in Cultured Human and Monkey Trabecular Meshwork. Exp. Eye Res. 1996, 62, 389–398. [Google Scholar] [CrossRef]
- Nakamura, Y.; Hirano, S.; Suzuki, K.; Seki, K.; Sagara, T.; Nishida, T. Signaling Mechanism of TGF-β1–Induced Collagen Contraction Mediated by Bovine Trabecular Meshwork Cells. Investig. Ophthalmol. Vis. Sci. 2002, 43, 8. [Google Scholar]
- Schlötzer-Schrehardt, U.; Zenkel, M.; Küchle, M.; Sakai, L.Y.; Naumann, G.O.H. Role of Transforming Growth Factor-Β1 and Its Latent Form Binding Protein in Pseudoexfoliation Syndrome. Exp. Eye Res. 2001, 73, 765–780. [Google Scholar] [CrossRef] [PubMed]
- Kirwan, R.P.; Crean, J.K.; Fenerty, C.H.; Clark, A.F.; O’Brien, C.J. Effect of Cyclical Mechanical Stretch and Exogenous Transforming Growth Factor-β1 on Matrix Metalloproteinase-2 Activity in Lamina Cribrosa Cells from the Human Optic Nerve Head. J. Glaucoma 2004, 13, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Yemanyi, F.; Raghunathan, V. Lysophosphatidic Acid and IL-6 Trans-Signaling Interact via YAP/TAZ and STAT3 Signaling Pathways in Human Trabecular Meshwork Cells. Investig. Ophthalmol. Vis. Sci. 2020, 61, 29. [Google Scholar] [CrossRef] [PubMed]
- Ho, L.T.Y.; Osterwald, A.; Ruf, I.; Hunziker, D.; Mattei, P.; Challa, P.; Vann, R.; Ullmer, C.; Rao, P.V. Role of the Autotaxin-Lysophosphatidic Acid Axis in Glaucoma, Aqueous Humor Drainage and Fibrogenic Activity. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2020, 1866, 165560. [Google Scholar] [CrossRef]
- Tripathi, H.; Al-Darraji, A.; Abo-Aly, M.; Peng, H.; Shokri, E.; Chelvarajan, L.; Donahue, R.R.; Levitan, B.M.; Gao, E.; Hernandez, G.; et al. Autotaxin Inhibition Reduces Cardiac Inflammation and Mitigates Adverse Cardiac Remodeling after Myocardial Infarction. J. Mol. Cell. Cardiol. 2020, 149, 95–114. [Google Scholar] [CrossRef]
- Oikonomou, N.; Mouratis, M.-A.; Tzouvelekis, A.; Kaffe, E.; Valavanis, C.; Vilaras, G.; Karameris, A.; Prestwich, G.D.; Bouros, D.; Aidinis, V. Pulmonary Autotaxin Expression Contributes to the Pathogenesis of Pulmonary Fibrosis. Am. J. Respir. Cell Mol. Biol. 2012, 47, 566–574. [Google Scholar] [CrossRef]
- Trovato, F.M.; Zia, R.; Napoli, S.; Wolfer, K.; Huang, X.; Morgan, P.E.; Husbyn, H.; Elgosbi, M.; Lucangeli, M.; Miquel, R.; et al. Dysregulation of the Lysophosphatidylcholine/Autotaxin/Lysophosphatidic Acid Axis in Acute-on-Chronic Liver Failure Is Associated With Mortality and Systemic Inflammation by Lysophosphatidic Acid–Dependent Monocyte Activation. Hepatology 2021, 74, 907–925. [Google Scholar] [CrossRef]
- Wang, Z.; Shi, W.; Tian, D.; Qin, H.; Vallance, B.A.; Yang, H.; Yu, H.B.; Yu, Q. Autotaxin Stimulates LPA2 Receptor in Macrophages and Exacerbates Dextran Sulfate Sodium-Induced Acute Colitis. J. Mol. Med. 2020, 98, 1781–1794. [Google Scholar] [CrossRef]
- Kostadinova, L.; Shive, C.L.; Anthony, D.D. Elevated Autotaxin and LPA Levels during Chronic Viral Hepatitis and Hepatocellular Carcinoma Associate with Systemic Immune Activation. Cancers 2019, 11, 1867. [Google Scholar] [CrossRef]
- Ninou, I.; Magkrioti, C.; Aidinis, V. Autotaxin in Pathophysiology and Pulmonary Fibrosis. Front. Med. 2018, 5, 180. [Google Scholar] [CrossRef]
- Bissey, P.-A.; Law, J.H.; Bruce, J.P.; Shi, W.; Renoult, A.; Chua, M.L.K.; Yip, K.W.; Liu, F.-F. Dysregulation of the MiR-449b Target TGFBI Alters the TGFβ Pathway to Induce Cisplatin Resistance in Nasopharyngeal Carcinoma. Oncogenesis 2018, 7, 40. [Google Scholar] [CrossRef] [PubMed]
- Xue, K.; Zhang, J.; Li, C.; Li, J.; Wang, C.; Zhang, Q.; Chen, X.; Yu, X.; Sun, L.; Yu, X. The Role and Mechanism of Transforming Growth Factor Beta 3 in Human Myocardial Infarction-induced Myocardial Fibrosis. J. Cell. Mol. Med. 2019, 23, 4229–4243. [Google Scholar] [CrossRef] [PubMed]
- Karamichos, D.; Rich, C.B.; Zareian, R.; Hutcheon, A.E.K.; Ruberti, J.W.; Trinkaus-Randall, V.; Zieske, J.D. TGF-Β3 Stimulates Stromal Matrix Assembly by Human Corneal Keratocyte-Like Cells. Investig. Ophthalmol. Vis. Sci. 2013, 54, 6612. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Variables | Normal | POAG | XFG | p Value |
|---|---|---|---|---|
| Patients (n) | 39 | 27 | 29 | |
| Number of Eyes (n) | 39 | 27 | 29 | |
| Gender Ratio (male:female) | 18:21 | 16:11 | 19:10 | NS * |
| Ages (years) | 77.1 ± 3.9 | 76.1 ± 3.9 | 75.5 ± 7.8 | NS ** |
| IOP (mmHg) | 13.2 ± 3.2 | 16.4 ± 5.0 | 20.7 ± 7.7 | †, †† ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; Honjo, M.; Yamagishi, R.; Igarashi, N.; Nakamura, N.; Kurano, M.; Yatomi, Y.; Igarashi, K.; Aihara, M. Fibrotic Response of Human Trabecular Meshwork Cells to Transforming Growth Factor-Beta 3 and Autotaxin in Aqueous Humor. Biomolecules 2022, 12, 1231. https://doi.org/10.3390/biom12091231
Liu M, Honjo M, Yamagishi R, Igarashi N, Nakamura N, Kurano M, Yatomi Y, Igarashi K, Aihara M. Fibrotic Response of Human Trabecular Meshwork Cells to Transforming Growth Factor-Beta 3 and Autotaxin in Aqueous Humor. Biomolecules. 2022; 12(9):1231. https://doi.org/10.3390/biom12091231
Chicago/Turabian StyleLiu, Mengxuan, Megumi Honjo, Reiko Yamagishi, Nozomi Igarashi, Natsuko Nakamura, Makoto Kurano, Yutaka Yatomi, Koji Igarashi, and Makoto Aihara. 2022. "Fibrotic Response of Human Trabecular Meshwork Cells to Transforming Growth Factor-Beta 3 and Autotaxin in Aqueous Humor" Biomolecules 12, no. 9: 1231. https://doi.org/10.3390/biom12091231





