The Role of Doxycycline and IL-17 in Regenerative Potential of Periodontal Ligament Stem Cells: Implications in Periodontitis
Abstract
:1. Introduction
2. Materials and Methods
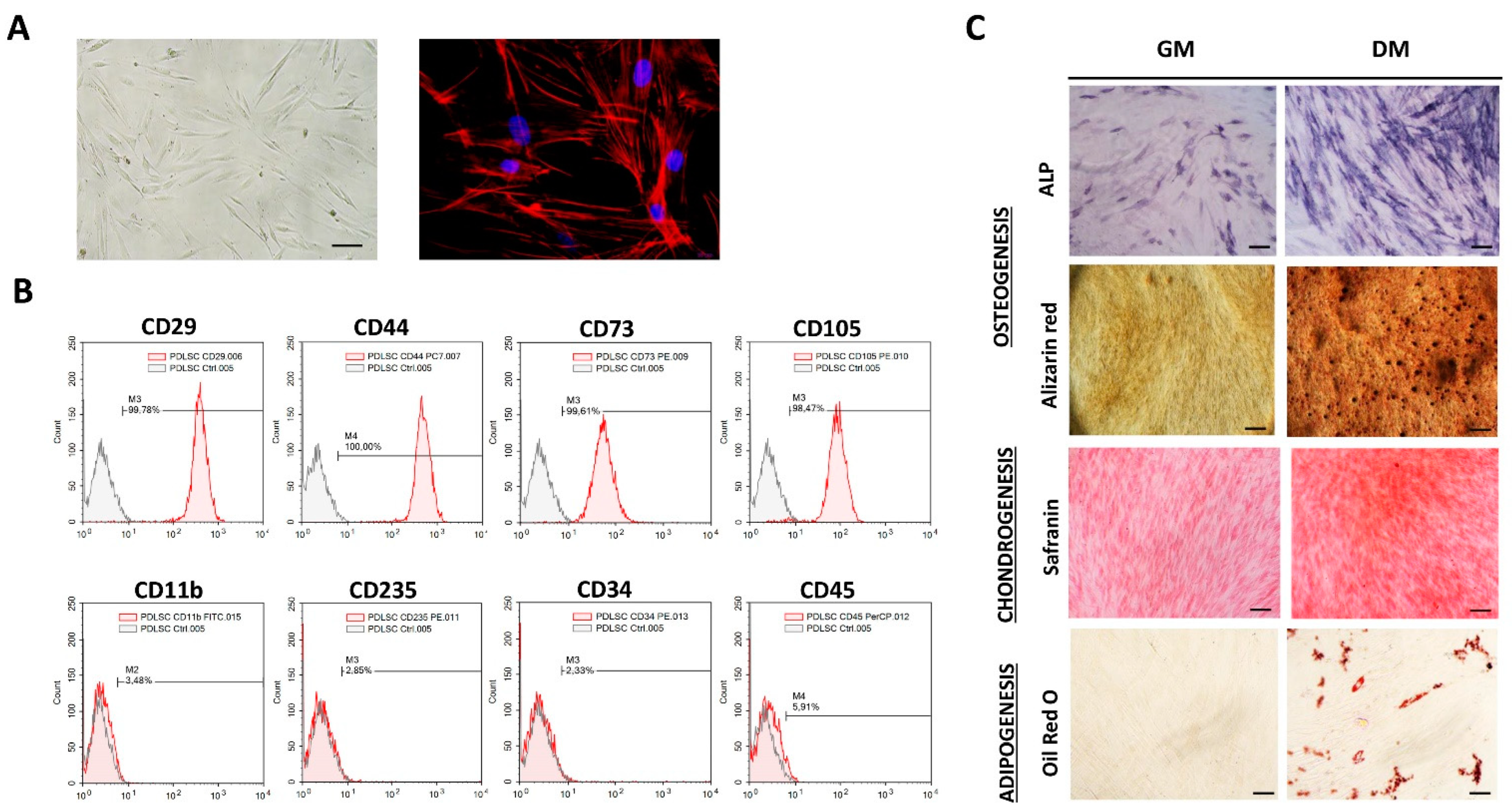
2.1. Isolation and Characterization of PDLSCs
2.2. Flow Cytometry Analyses
2.3. Viability Assay (MTT)
2.4. Migration
2.5. Immunofluorescence
2.6. Zymography
2.7. Differentiation
2.8. Quantitative Real-Time PCR
2.9. Analyses of Cellular Bioenergetics—Mito Stress Assay
2.10. Statistical Analyses
3. Results
3.1. Phenotypic Properties and Multilineage Differentiation of PDLSCs
3.2. Dox Inhibits Cell Migration of PDLSCs Treated with IL-17
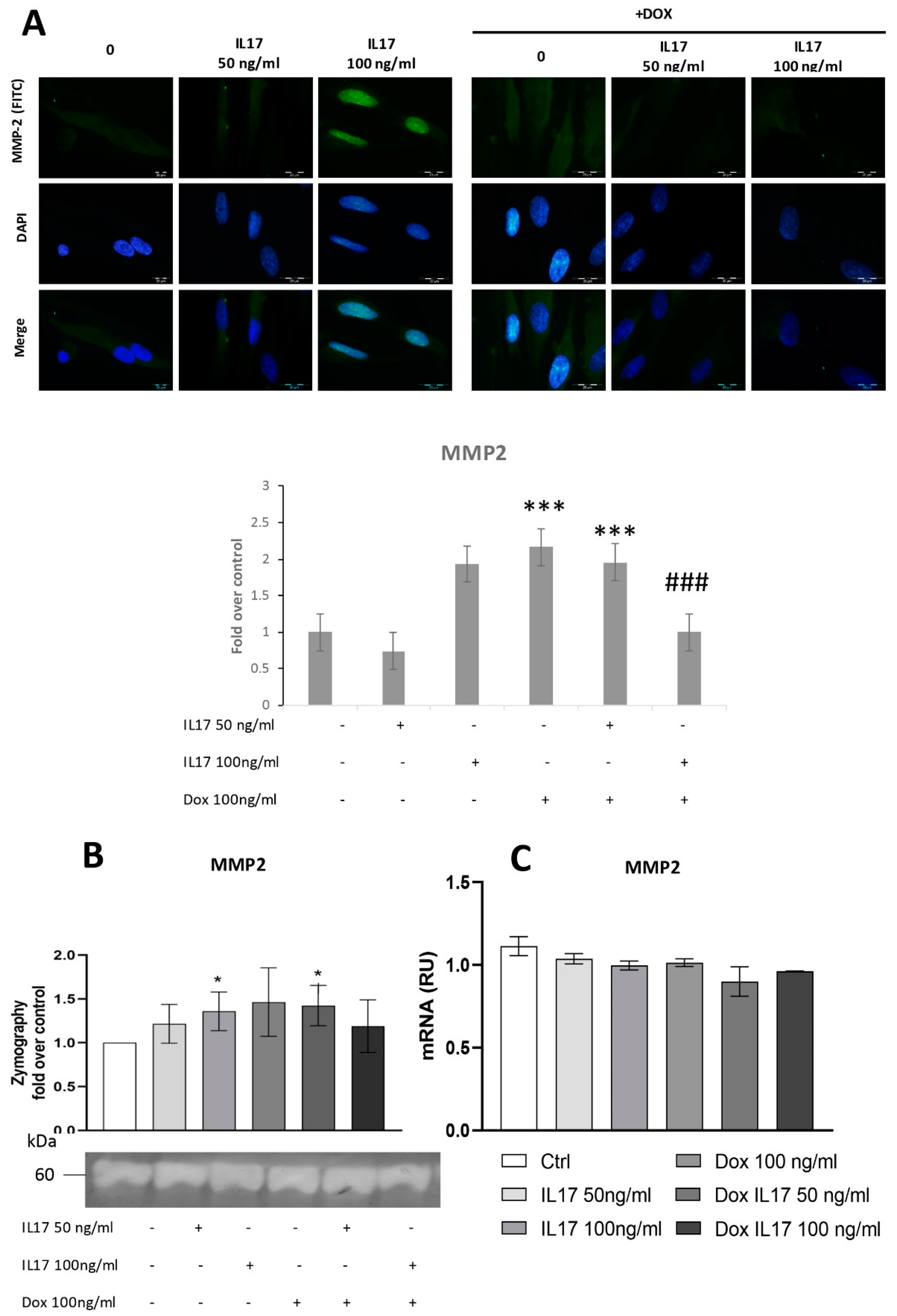
3.3. Dox Inhibits MMP2 Expression of PDLSCs Abrogating the Effect of IL-17
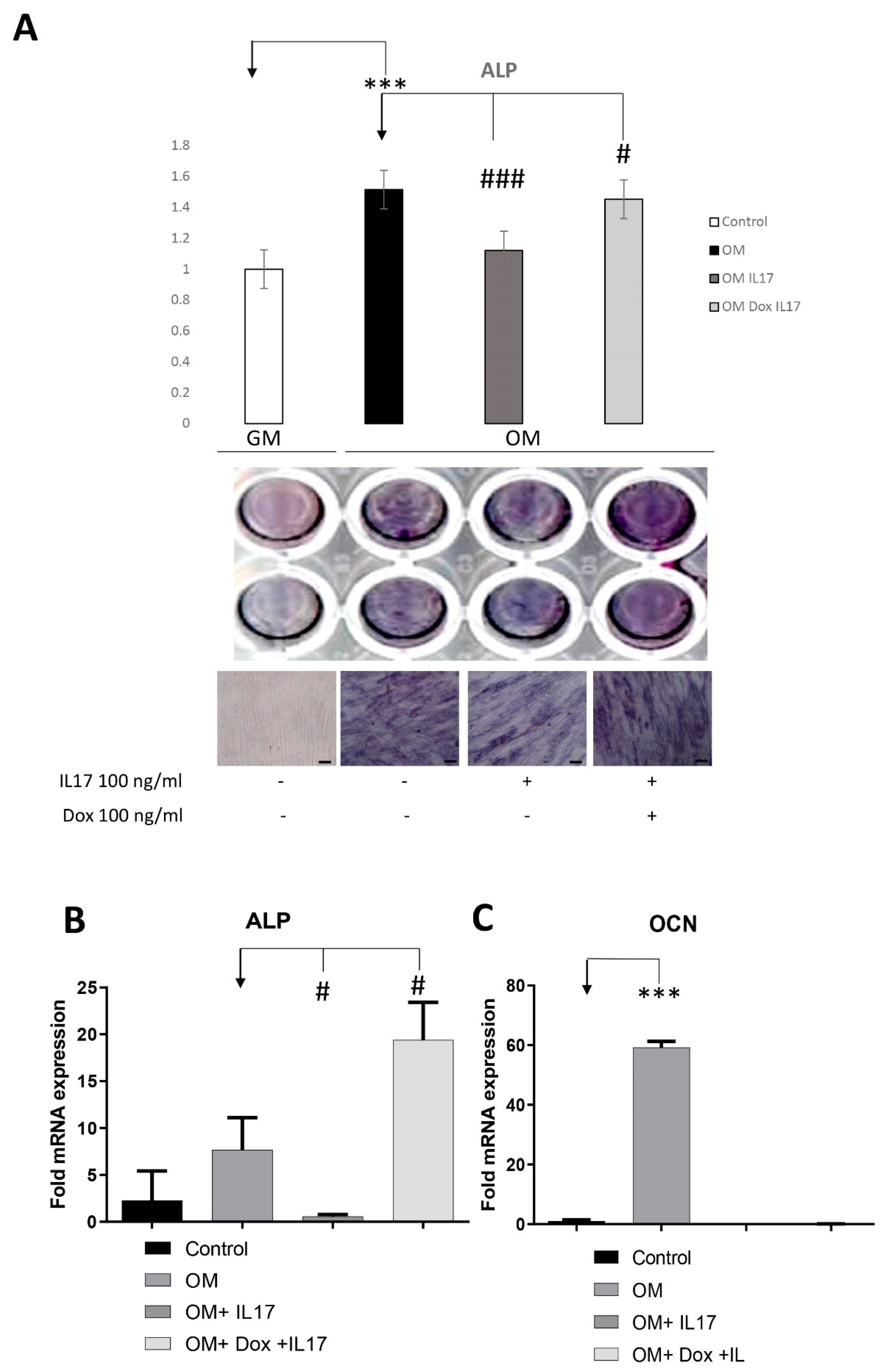
3.4. Dox Stimulates Osteogenic Differentiation of PDLSCs Annulling the Inhibitory Effect of IL-17 on PDLSCs Osteogenesis
3.5. Impact of IL-17 and Dox on PDLSC Mitochondrial Bioenergetics
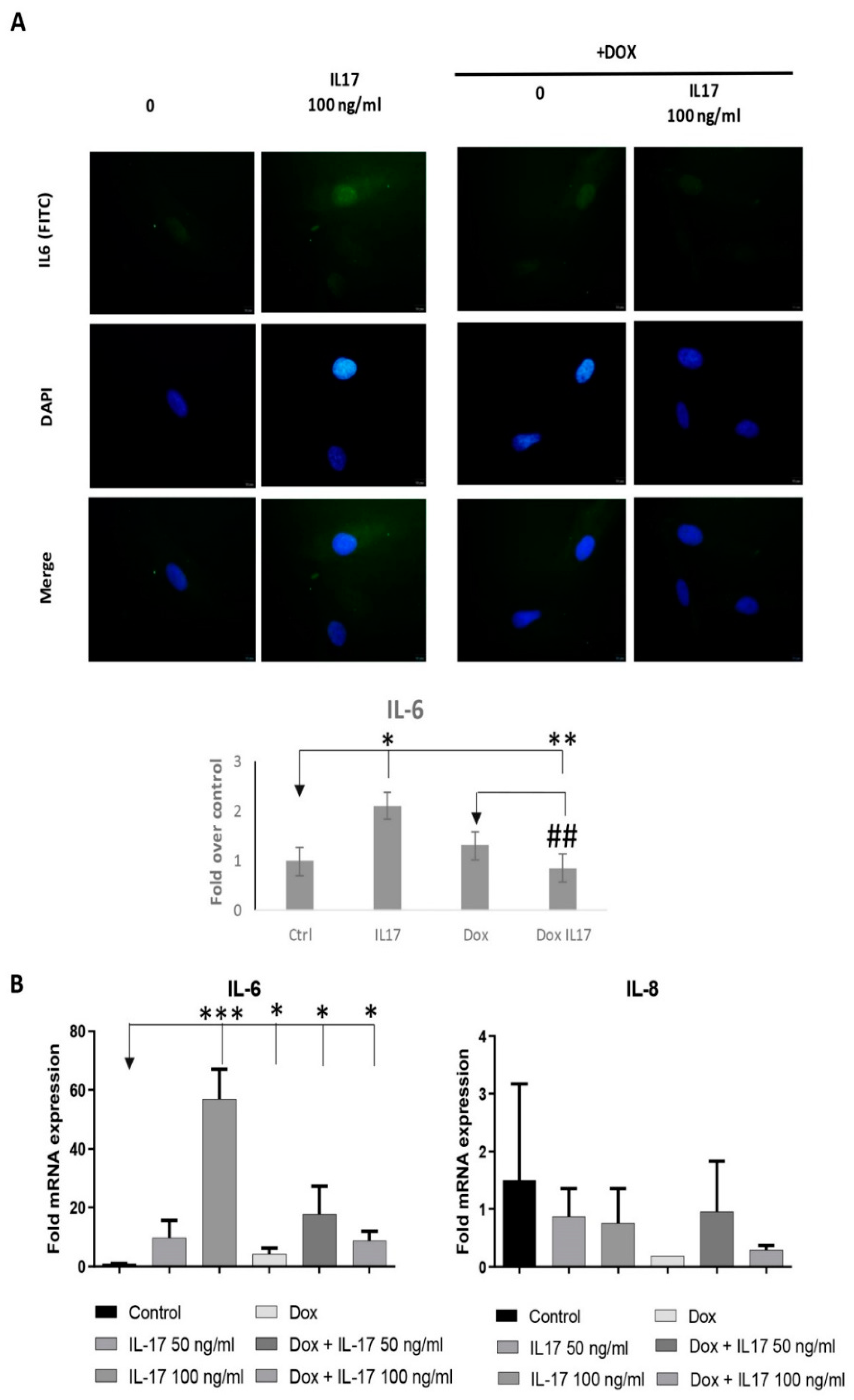
3.6. Dox Alters the Expression of Inflammatory Factors of PDLSCs Treated with IL-17
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cecoro, G.; Annunziata, M.; Iuorio, M.T.; Nastri, L.; Guida, L. Periodontitis, Low-Grade Inflammation and Systemic Health: A Scoping Review. Medicina 2020, 56, 272. [Google Scholar] [CrossRef]
- Gupta, N.; Gupta, N.D.; Gupta, A.; Khan, S.; Bansal, N. Role of salivary matrix metalloproteinase-8 (MMP-8) in chronic periodontitisdiagnosis. Front. Med. 2015, 9, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Sundararaj, S.C.; Thomas, M.V.; Peyyala, R.; Dziubla, T.D.; Puleo, D.A. Design of a multiple drug delivery system directed at periodontitis. Biomaterials 2013, 34, 8835–8842. [Google Scholar] [CrossRef] [PubMed]
- Spasovski, S.; Belazelkoska, Z.; Popovska, M.; Atanasovska-Stojanovska, A.; Radojkova-Nikolovska, V.; Muratovska, I.; Toseska-Spasova, N.; Dzipunova, B.; Nikolovski, B. Clinical Therapeutic Effects of the Application of Doxycycline in the Treatment of Periodontal Disease. J. Med. Sci. 2016, 4, 152–157. [Google Scholar] [CrossRef]
- Stechmiller, J.; Cowan, L.; Schultz, G. The role of doxycycline as a matrix metalloproteinase inhibitor for the treatment of chronic wounds. Biol. Res. Nurs. 2010, 11, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Checchi, V.; Maravic, T.; Bellini, P.; Generali, L.; Consolo, U.; Breschi, L.; Mazzoni, A. The Role of Matrix Metalloproteinases in Periodontal Disease. Int. J. Environ. Res. Public Health 2020, 17, 4923. [Google Scholar] [CrossRef]
- Tatullo, M.; Codispoti, B.; Paduano, F.; Nuzzolese, M.; Makeeva, I. Strategic Tools in Regenerative and Translational Dentistry. Int. J. Mol. Sci. 2019, 20, 1879. [Google Scholar] [CrossRef]
- Okić-Đorđević, I.; Obradović, H.; Kukolj, T.; Petrović, A.; Mojsilović, S.; Bugarski, D.; Jauković, A. Dental mesenchymal stromal/stem cells in different microenvironments—Implications in regenerative therapy. World J. Stem Cells 2021, 13, 1863–1880. [Google Scholar] [CrossRef]
- Queiroz, A.; Albuquerque-Souza, E.; Gasparoni, L.M.; de França, B.N.; Pelissari, C.; Trierveiler, M.; Holzhausen, M. Therapeutic potential of periodontal ligament stem cells. World J. Stem Cells 2021, 13, 605–618. [Google Scholar] [CrossRef]
- Huang, N.; Dong, H.; Luo, Y.; Shao, B. Th17 Cells in Periodontitis and Its Regulation by A20. Front. Immunol. 2021, 12, 742925. [Google Scholar] [CrossRef]
- Kotake, S.; Yago, T.; Kawamoto, M.; Nanke, Y. Role of osteoclasts and interleukin-17 in the pathogenesis ofrheumatoid arthritis: Crucial “human osteoclastology”. J. Bone Miner. Metab. 2012, 30, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhou, Y.; Xu, Y.; Sun, Y.; Li, L.; Chen, W. Effect of non-surgical periodontal therapy on the levels ofTh17/Th1/Th2 cytokines and their transcription factors in Chinese chronic periodontitis patients. J. Clin. Periodontol. 2011, 38, 509–516. [Google Scholar] [CrossRef]
- Lin, H.; Chen, H.; Zhao, X.; Chen, Z.; Zhang, P.; Tian, Y.; Wang, Y.; Ding, T.; Wang, L.; Shen, Y. Advances in mesenchymal stem cell conditioned medium-mediated periodontal tissue regeneration. J. Transl. Med. 2021, 19, 456. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Kim, H.J.; Chang, E.J.; Lee, Z.H.; Hwang, S.J.; Kim, H.M.; Lee, Y.; Kim, H.-H. IL-17 stimulates the proliferation and differentiation of human mesenchymal stem cells: Implications for bone remodeling. Cell Death Differ. 2009, 16, 1332–1343. [Google Scholar] [CrossRef] [PubMed]
- Mills, K.H.G. IL-17 and IL-17-producing cells in protection versus pathology. Nat. Rev. Immunol. 2022, 23, 38–54. [Google Scholar] [CrossRef] [PubMed]
- Okić Đorđević, I.; Kukolj, T.; Obradović, H.; Trivanović, D.; Petrović, A.; Mojsilović, S.; Miletić, M.; Bugarski, D.; Jauković, A. Interleukin-17 modulates uPA and MMP2 expression in human periodontal ligament mesenchymal stem cells: Involvement of the ERK1/2 MAPK pathway. Arch. Biol. Sci. 2022, 74, 15–24. [Google Scholar] [CrossRef]
- Okić Đorđević, I.; Kukolj, T.; Krstić, J.; Trivanović, D.; Obradović, H.; Santibañez, J.F.; Mojsilović, S.; Ilić, V.; Bugarski, D.; Jauković, A. The inhibition of periodontal ligament stem cells osteogenic differentiation by IL-17 is mediated via MAPKs. Int. J. Biochem. Cell Biol. 2016, 71, 92–101. [Google Scholar] [CrossRef]
- Miletic, M.; Mojsilovic, S.; Okic Dordevic, I.; Kukolj, T.; Jaukovic, A.; Santibanez, J.F.; Jovcic, G.; Bugarski, D. Mesenchymal stem cells isolated from human periodontal ligament. Arch. Biol. Sci. 2014, 66, 261–271. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.J.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Villar, V.; Kocic, J.; Santibanez, J.F. Skip Regulates TGF-β1-Induced Extracellular Matrix Degrading Proteases Expression in Human PC-3 Prostate Cancer Cells. Prostate Cancer 2013, 2013, 398253. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Corotti, M.V.; Zambuzzi, W.F.; Paiva, K.B.S.; Menezes, R.; Pinto, L.C.; Lara, V.S.; Granjeiro, J.M. Immunolocalization of matrix metalloproteinases-2 and -9 during apical periodontitis development. Arch. Oral Biol. 2009, 54, 764–771. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.M.; Strasberg-Rieber, M.; Rieber, M. Invasion-associated MMP-2 and MMP-9 are up-regulated intracellularly in concert with apoptosis linked to melanoma cell detachment. Clin. Exp. Metastasis 2005, 22, 285–295. [Google Scholar] [CrossRef]
- Wurm, C.A.; Neumann, D.; Lauterbach, M.A.; Harke, B.; Egner, A.; Hell, S.W.; Jakobs, S. Nanoscale distribution of mitochondrial import receptor Tom20 is adjusted to cellular conditions and exhibits an inner-cellular gradient. Proc. Natl. Acad. Sci. USA 2011, 108, 13546–13551. [Google Scholar] [CrossRef] [PubMed]
- Patergnani, S.; Bouhamida, E.; Leo, S.; Pinton, P.; Rimessi, A. Mitochondrial Oxidative Stress and “Mito-Inflammation”: Actors in the Diseases. Biomedicines 2021, 9, 216. [Google Scholar] [CrossRef]
- Shi, Y.; Xiong, Y.; Jiang, Y.; Zhang, Z.; Zhou, G.; Zhang, W.; Cao, Y.; He, J.; Liu, W. Conditional tenomodulin overexpression favors tenogenic lineage differentiation of transgenic mouse derived cells. Gene 2017, 598, 9–19. [Google Scholar] [CrossRef]
- Gomes, P.S.; Resende, M.; Fernandes, M.H. Doxycycline restores the impaired osteogenic commitment of diabetic-derived bone marrow mesenchymal stromal cells by increasing the canonical WNT signaling. Mol. Cell Endocrinol. 2020, 518, 110975. [Google Scholar] [CrossRef]
- Chernoivanenko, I.S.; Minin, A.A.; Minin, A.A. Role of vimentin in cell migration. Ontogenez 2013, 44, 186–202. (In Russian) [Google Scholar] [CrossRef]
- Sang, Q.-X.A.; Jin, Y.; Newcomer, R.G.; Monroe, S.C.; Fang, X.; Hurst, D.R.; Lee, S.; Cao, Q.; Schwartz, M.A. Matrix metalloproteinase inhibitors as prospective agents for the prevention and treatment of cardiovascular and neoplastic diseases. Curr. Top. Med. Chem. 2006, 6, 289–316. [Google Scholar] [CrossRef]
- Caley, M.P.; Martins, V.L.; O’Toole, E.A. Metalloproteinases and Wound Healing. Adv. Wound Care 2015, 4, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S.M.; Eisen, A.Z.; Teter, M.; Clark, S.D.; Kronenberger, A.; Goldberg, G. Human fibroblast collagenase. Glycosylation and tissue-specific level of enzyme synthesis. Proc. Natl. Acad. Sci. USA 1986, 83, 3756–3760. [Google Scholar] [CrossRef]
- Corcoran, M.L.; Hewitt, R.E.; Kleiner, D.E., Jr.; Stetler-Stevenson, W.G. MMP-2: Expression, activation and inhibition. Enzyme Protein 1996, 49, 7–19. [Google Scholar] [CrossRef]
- Zhang, C.; Tang, T.T.; Ren, W.P.; Zhang, X.L.; Dai, K.R. Inhibiting wear particles-induced osteolysis with doxycycline. Acta Pharmacol. Sin. 2007, 28, 1603–1610. [Google Scholar] [CrossRef] [PubMed]
- Ramamurthy, N.S.; Rifkin, B.R.; Greenwald, R.A.; Xu, J.W.; Liu, Y.; Turner, G.; Golub, L.M.; Vernillo, A.T. Inhibition of matrix metalloproteinasemediated periodontal bone loss in rats: A comparison of 6 chemically modified tetracyclines. J. Periodontol. 2002, 73, 726–734. [Google Scholar] [CrossRef]
- Williams, S.; Wakisaka, A.; Zeng, Q.Q.; Barnes, J.; Martin, G.; Wechter, W.J.; Liang, C.T. Minocycline prevents the decrease in bone mineral density and trabecular bone in ovariectomized aged rats. Bone 1996, 19, 637–644. [Google Scholar] [CrossRef]
- Gomes, P.S.; Fernandes, M.H. Effect of therapeutic levels of doxycycline and minocycline in the proliferation and differentiation of human bone marrow osteoblastic cells. Arch. Oral Biol. 2007, 52, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Kinugawa, S.; Koide, M.; Kobayashi, Y.; Mizoguchi, T.; Ninomiya, T.; Muto, A.; Kawahara, I.; Nakamura, M.; Yasuda, H.; Takahashi, N.; et al. Tetracyclines convert the osteoclastic-differentiation pathway of progenitor cells to produce dendritic cell-like cells. J. Immunol. 2012, 188, 1772–1781. [Google Scholar] [CrossRef] [PubMed]
- Gomes, K.D.N.; Alves, A.P.N.N.; Dutra, P.G.P.; Viana, G.S.B. Doxycycline induces bone repair and changes in Wnt signalling. Int. J. Oral Sci. 2017, 9, 158–166. [Google Scholar] [CrossRef]
- Inchingolo, F.; Ballini, A.; Cagiano, R.; Inchingolo, A.D.; Serafini, M.; De Benedittis, M.; Cortelazzi, R.; Tatullo, M.; Marrelli, M.; Inchingolo, A.M.; et al. Immediately loaded dental implants bioactivated with platelet-rich plasma (PRP) placed in maxillary and mandibular region. Clin. Ter. 2015, 166, e146–e152. [Google Scholar]
- Chen, C.T.; Shih, Y.R.; Kuo, T.K.; Lee, O.K.; Wei, Y.H. Coordinated changes of mitochondrial biogenesis and antioxidant enzymes during osteogenic differentiation of human mesenchymal stem cells. Stem Cells 2008, 26, 960–968. [Google Scholar] [CrossRef]
- Nuschke, A.; Rodrigues, M.; Wells, A.W.; Sylakowski, K.; Wells, A. Mesenchymal stem cells/multipotent stromal cells (MSCs) are glycolytic and thus glucose is a limiting factor of in vitro models of MSC starvation. Stem Cell Res. Ther. 2016, 7, 179. [Google Scholar] [CrossRef]
- Lee, I.; Hüttemann, M. Energy crisis: The role of oxidative phosphorylation in acute inflammation and sepsis. Biochim. Biophys. Acta. 2014, 1842, 1579–1586. [Google Scholar] [CrossRef] [PubMed]
- Chatzispyrou, I.A.; Held, N.M.; Mouchiroud, L.; Auwerx, J.; Houtkooper, R.H. Tetracycline antibiotics impair mitochondrial function and its experimental use confounds research. Cancer Res. 2015, 75, 4446–4449. [Google Scholar] [CrossRef]
- Padhi, A.; Thomson, A.H.; Perry, J.B.; Davis, G.N.; McMillan, R.P.; Loesgen, S.; Kaweesa, E.N.; Kapania, R.; Nain, A.S.; Brown, D.A. Bioenergetics underlying single-cell migration on aligned nanofiber scaffolds. Am. J. Physiol. Cell Physiol. 2020, 318, C476–C485. [Google Scholar] [CrossRef]
- Chen, X.L.; Wang, Y.; Peng, W.W.; Zheng, Y.J.; Zhang, T.N.; Wang, P.J.; Huang, J.D.; Zeng, Q.Y. Effects of interleukin-6 and IL-6/AMPK signaling pathway on mitochondrial biogenesis and astrocytes viability under experimental septic condition. Int. Immunopharmacol. 2018, 59, 287–294. [Google Scholar] [CrossRef]
- Trubiani, O.; Pizzicannella, J.; Caputi, S.; Marchisio, M.; Mazzon, E.; Paganelli, R.; Paganelli, A.; Diomede, F. Periodontal Ligament Stem Cells: Current Knowledge and Future Perspectives. Stem Cells Dev. 2019, 28, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Li, S.H.; Wu, J.; Zang, W.F.; Dhingra, S.; Sun, L.; Weisel, R.D.; Li, R.K. Interleukin-6 downregulation with mesenchymal stem cell differentiation results in loss of immunoprivilege. J. Cell Mol. Med. 2013, 17, 1136–1145. [Google Scholar] [CrossRef]
- Panduwawala, C.P.; Zhan, X.; Dissanayaka, W.L.; Samaranayake, L.P.; Jin, L.; Zhang, C. In vivo periodontal tissue regeneration by periodontal ligament stem cells and endothelial cells in three-dimensional cell sheet constructs. J. Periodontal. Res. 2017, 52, 408–418. [Google Scholar] [CrossRef] [PubMed]


| Gene | Sequence (5′–3′) |
|---|---|
| GAPDH | F: GAAGGTGAAGGTCGGAGT |
| R: GAAGATGGTGATGGGATTTC | |
| MMP-2 | F: GCCAAGCGTCTAGCAATACC |
| R: TCTGGGGCAGTCCAAAGAAC | |
| IL6 | F: TACCCCCAGGAGAAGATTCC |
| R: TTTTCTGCCAGTGCCTCTTT | |
| IL8 | F: GTG CAG TTTTGC CAA GGA GT |
| R: CTC TGC ACC CAG TTT TCC TT | |
| Osteocalcin | F: TCACACTCCTCGCCCTATTGG |
| R: GGGCAAGGGGAAGAGGAAAGA | |
| ALP | F: CACCCACGTCGATTGCATCT |
| R: TAGCCACGTTGGTGTTGAGC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okić Đorđević, I.; Kukolj, T.; Živanović, M.; Momčilović, S.; Obradović, H.; Petrović, A.; Mojsilović, S.; Trivanović, D.; Jauković, A. The Role of Doxycycline and IL-17 in Regenerative Potential of Periodontal Ligament Stem Cells: Implications in Periodontitis. Biomolecules 2023, 13, 1437. https://doi.org/10.3390/biom13101437
Okić Đorđević I, Kukolj T, Živanović M, Momčilović S, Obradović H, Petrović A, Mojsilović S, Trivanović D, Jauković A. The Role of Doxycycline and IL-17 in Regenerative Potential of Periodontal Ligament Stem Cells: Implications in Periodontitis. Biomolecules. 2023; 13(10):1437. https://doi.org/10.3390/biom13101437
Chicago/Turabian StyleOkić Đorđević, Ivana, Tamara Kukolj, Milena Živanović, Sanja Momčilović, Hristina Obradović, Anđelija Petrović, Slavko Mojsilović, Drenka Trivanović, and Aleksandra Jauković. 2023. "The Role of Doxycycline and IL-17 in Regenerative Potential of Periodontal Ligament Stem Cells: Implications in Periodontitis" Biomolecules 13, no. 10: 1437. https://doi.org/10.3390/biom13101437





