Stalling the Course of Neurodegenerative Diseases: Could Cyanobacteria Constitute a New Approach toward Therapy?
Abstract
:1. Introduction
2. Cyanobacteria
3. Neurodegeneration
4. Cyanobacteria Potential against Neurodegenerative Diseases
4.1. Cyanobacteria against Alzheimer’s Disease
| Strain | Compound/Extract | Effect | In Vitro Assays | In Vivo Assays | Reference |
|---|---|---|---|---|---|
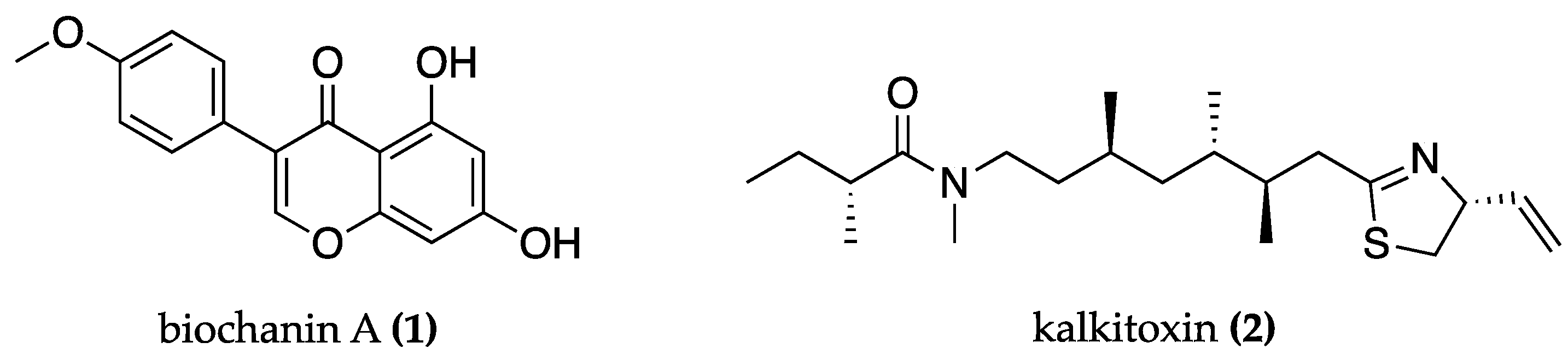
| Anabaena flos-aquae NRC-525-17 | Anatoxin-a(s) (3) | AChE and BChE inhibition | AChE and BChE inhibition assay | [55] | |
| Nostoc 78-12A | Nostocarboline (4) | BChE inhibition | AChE and BChE inhibition assay | [57] | |
| Phormidium autumnale | SFE-EtOH extract | AChE and LOX inhibition. Antioxidant. | AChE inhibition assay. LOX inhibition assay. ORAC assay. | [60] | |
| Anabaena variabilis | Methylene chloride/ methanol extract (Fraction 7) | AChE inhibition | AChE inhibition assay | [61] | |
| Oscillatoria sancta | Methylene chloride/ methanol (1:1) extract | AChE inhibition | AChE inhibition assay | [62] | |
| Nostoc sp. | Ethanolic Extract | AChE and BChE inhibition. Antioxidant. | AChE and BChE inhibition assay. DPPH assay. | [63] | |
| Spirulina sp. | Phycocyanin | Inhibition of Aβ formation | Fluorimetric assay. Kinetic analysis. Circular dichroism analysis. | [65] | |
| Spirulina sp. | Phycocyanin | Inhibition of Aβ40/42 amyloid fibrillation | Fibrillar and amorphous aggregation assays. Transmission electron microscopy imaging. | [66] | |
| Symploca sp. | Tasiamide B (8) | BACE-1 inhibition | BACE-1 inhibition assay | [67] | |
| Lyngbya sp. | Tasiamide F (9) | BACE-1 inhibition | BACE-1 inhibition assay | [69] | |
| Leptolyngbya sp. N62DM | Phycocyanin | BACE-1 inhibition | Protein-complex interface identification | Caenorhabditis elegans CL4176 transgenic AD-model: Paralysis assay | [70] |
| Lyngbya sp. A09DM | Phycoerythrin | BACE-1 inhibition | Surface plasmon resonance. Isothermal titration calorimetry. Enzyme activity by kinetic parameters. | Caenorhabditis elegans CL4176 transgenic AD-model: Thioflavin-T staining assay | [71] |
| Spirulina platensis | Lipopolysaccharide | Downregulation of p-tau expression. Antioxidant. Anti-inflammatory. | Wistar albino rats exposed to nicotine: Biochemical assessments (Oxidative and inflammatory markers). RT-PCR. Western Blot (p-tau). | [78] | |
| Spirulina maxima | 70% ethanol extract | AChE inhibition. Reduced Aβ, APP, and BACE-1 levels. BDNF/PI3K/Akt pathway activation. Antioxidant. Improved cognition. | ICR mice injected with Aβ1–42: Passive Avoidance Test. Morris WaterMaze Test. Biochemical Analysis (Aβ1–42, GSH, BDNF, AChE). Western Blot. | [72] | |
| Aphanizomenon flos-aquae | KlamExtra® | Reduced Aβ, APP and BACE-1 levels. Anti-inflammatory and anti-gliosis. Improved metabolic parameters. Protection of neuronal morphology and synapses. | High-Fat Diet C57BL/6J mice: Metabolic parameters analysis. Western Blot (IR, Akt, PSEN-1, BACE-1, PSD-95, synaptophysin, TNF-α, GFAP, IL-10, TREM-2). Histopathology and Immunohistochemistry (GFAP, TREM-2, Aβ). Thioflavin T staining. TUNEL assay. | [73] | |
| Spirulina platensis | Diet supplementation | Decreased Aβ1–42, APP, BACE-1, p-tau, and p-GSK levels. Anti-inflammatory. Improved microbiota dysbiosis. Improved metabolic parameters. Improved locomotor and cognitive function. | High-Fat Diet C57BL/6J mice: Barnes Maze test. Morris Water Maze test. ELISA (Aβ1–42, TNF-α, IL-1β, IL-6, LPS). RT-PCR. Western Blot (APP, BACE-1, p-tau, p-GSK, IBA-1). Microbial diversity analysis. GC (SCFAs). | [79] | |
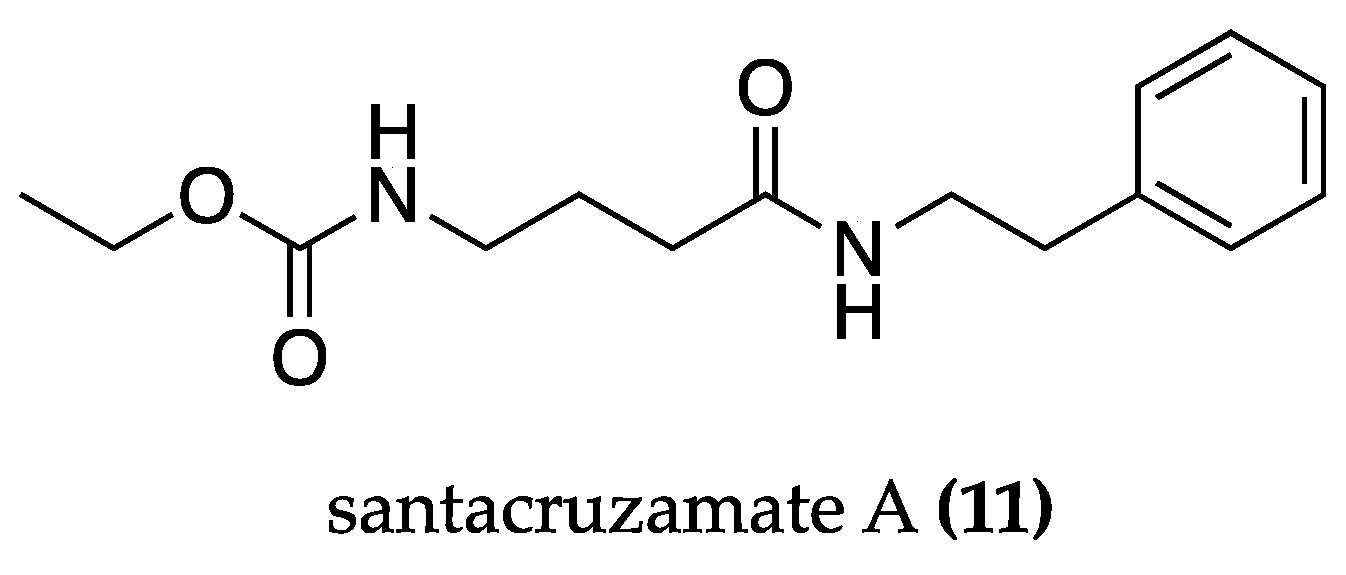
| cf. Symploca sp. | Santacruzamate A (11) | Anti-apoptotic. Anti-UPR and ER stress. Improvement of the mitochondrial fission pathway. Modulation of KDELR and Mia40-ALR. Memory improvement. | PC12 cells: Cell viability and apoptosis assays. Electrophysiological recordings. Immunoblot analyses. Measurement of mitochondrial permeability transition pore. Opening and mitochondrial membrane potentials. | APPswe/PS1dE9 mice: Open-Field test. Morris Water Maze test. RT-PCR (Mia40, KDEL). | [80] |
| Spirulina platensis | Diet supplementation (tablets) | Protection of neuronal morphology. Reduction in Aβ accumulation. Improvement of metabolic parameters. Antioxidant. Anti-inflammatory. | Wistar rats treated with AlCl3: TBARS assay. GSH content assay. Total thiol content assay. TAC assay. GPx, GST, SOD activity assay. Lipid profile determination. ELISA (TNF-α). Histology. Immunofluorescence (Aβ). | [82] | |
| Spirulina platensis | S. platensis- loaded niosome | Protection of neuronal morphology. Restored levels of AChE and ACh. Gene modulation. Recognition and working memory improvement. | Wistar rats treated with AlCl3: Novel object recognition test. Y-maze test. TAC assay. MDA assay. AChE assay. Histology. HPLC (ACh, NE, 5HT, DA, DOPAC). qPCR (Bax, Bcl-2, AChE, MAO). | [83] | |
| Spirulina platensis | Enzyme Digested Phycocyanin (EDPC) | Cognitive function improvement. Gene modulation. | Male Slc:ddY SPF mice injected with Aβ25–35: Y Maze test. DNA microarray. | [85] | |
| Spirulina platensis | Phycocyanin | Gene and miRNA modulation. Anti-inflammatory. Anti-apoptotic. Memory improvement. | Male C57BL/6 mice injected with oligomeric Aβ1–42: Eight-arm radial maze. RT-PCR (caspase-3, caspase-9, miR-335). Western Blot (HDAC3, Bcl-2, Bax, IL-6, IL-1β). Immunohistochemistry (Bcl-2, Bax). Immunofluorescence (BDNF, HDAC3). | [86] | |
| Spirulina platensis | Phycocyanin | AChE inhibition. ChAT activity increase. Gene modulation. Increased PI3K/Akt pathway. Anti-inflammatory. Memory improvement. | Female Wistar Rats injected with STZ: Morris Water Maze. Memory consolidation test. Novel object recognition test. Open field test. AChE and ChAT activity assays. ELISA (TNF-α, NF-kB p56, Bcl-2, Bax, BDNF, IGF-1). qRT-PCR (IRS-1, INS, PI3K, Akt, PTEN). | [87] | |
| Spirulina maxima | 70% ethanolic extract (SM70EE) pills | Memory and vocabulary improvement. | Randomized, double-blind, and placebo- controlled clinical trial. Visual learning, visual working memory, and verbal learning tests. | [88] | |
| Spirulina platensis | Dietary supplementation | Improved cognitive function. Improved metabolic status. | Randomized, double-blind, and placebo. -controlled clinical trial. Mini-mental state exam. ELISA (hs-CRP, Insulin). Biochemical analysis (NO, TAC, GSH, MDA, FPG, lipid profile). | [89] |
4.2. Cyanobacteria against Parkinson’s Disease
| Strain | Compound/Extract | Effect | In Vitro Assays | In Vivo Assays | Reference |
|---|---|---|---|---|---|
| Spirulina sp. | Phycocyanin | Inhibition of A53Tα-synuclein amyloid fibrillation | Fibrillar and amorphous aggregation assays. Transmission electron microscopy imaging. | [66] | |
| Spirulina platensis | Phycocyanin | Reduction in α-synuclein inclusions. Gene modulation. Antioxidant. Improved proteostasis. | BY4741 Yeast transformed with p42FAL-αsyn-GFP: Spot assay. Fluorescence microscopy. Western Blot (α-syn). Flow cytometry. TBARS assay. CAT activity. Total thiols assay. qRT-PCR (SOD1, SOD2, HAP4, LHS1, HRD1, GSH1, GLR1, RPN4, ATG8). | [93] | |
| Spirulina platensis | Phycocyanin derived peptides (MHLWAAK, MAQAAEYYR, MDYYFEER) | Improved locomotion. Neuronal protection. Antioxidant. Anti-apoptosis. Gene modulation. | MPTP-induced parkinsonism in transgenic zebrafish: Fluorescence Microscopy. Behavioral tests. Fluorescence ROS determination. Biochemical analysis (SOD, CAT, GSH-Px, CO, AChE). Acridine orange staining. qRT-PCR. | [98] | |
| Spirulina maxima | Diet supplementation | Protection of DA and HVA content. Blockage of lipid peroxidation. | MPTP-induced parkinsonism in male C-57 rats: HPLC (DA, HVA, 5-HIAA, 5-HT). TBARS Assay. | [95] | |
| Spirulina maxima | Diet supplementation | Improved locomotion. Recovery of mitochondrial activity. Protection of DA, DOPAC, and HVA levels. Antioxidant. | 6-OHDA-induced parkinsonism in male Wistar rats: Turn-behavior test. Closed-field test. Cylinder test. Fluorescence ROS determination. Griess reaction. TBARS assay. MTT assay. HPLC (DA, DOPAC, HVA). | [96] | |
| Spirulina fusiform | Aqueous freeze-dried extract suspended in olive oil | Improved behavior and locomotion. Protection of DA levels. Antioxidant. | 6-OHDA-induced parkinsonism in male Wistar albino rats: Amphetamine- and Apomorphine -induced rotations. Locomotor activity. Rota rod. TBARS assay. Reduced glutathione content assay. HPLC (DA). | [97] | |
| Spirulina platensis | Methanolic extract | Increased lifespan and locomotion. Antioxidant. Protection of DA content. | Drosophila Melanogaster exposed to FeSO4: Total phenol Content. DPPH radical scavenging activity. Survival rate. Negative Geotaxis assay. Lipid Peroxidation Assay. DA content assay. | [100] | |
| Spirulina platensis | Diet supplementation | Increased lifespan and locomotion. Antioxidant. Reduced cellular stress. | DJ-1βΔ93 Drosophila Melanogaster exposed to paraquat: Survival assay. Locomotor assay. PCR (HSP70). SOD and CAT enzymatic assays. Immunostaining (Hsp70 and JNK). | [101] | |
| Spirulina platensis | Polysaccharide | Increased TH and DAT expression. Antioxidant. | MPTP-induced parkinsonism in male C57BL/6J mice: Immunohistochemistry and RT-PCR (TH, DAT). SOD and GSH-Px assays. | [103] | |
| Spirulina platensis | Protein-rich fraction (SPF) | Improved behavior. Protection of DA and DOPAC levels. Increased TH and DAT expression. Reduced iNOS, COX-2, and GFAP expression. Antioxidant. | 6-OHDA-induced hemiparkinsonism in male Wistar rats: Apomorphine-induced rotational test. Open-field test. Forced swim test. HPLC (DA, DOPAC). Griess Reaction. TBARS assay. Immunohistochemistry (TH, DAT, iNOS, GFAP, COX-2) | [104] | |
| Spirulina platensis | 10% (w/v) aqueous extract | Improved behavior. Protection of DA and DOPAC levels. Protection of TH and DAT expression. Decreased iNOS and COX-2. Antioxidant. | 6-OHDA-induced parkinsonism in male Wistar rats: Apomorphine-induced rotational test. HPLC (DA, DOPAC). Griess Reaction. TBARS assay. Immunohistochemistry (TH, DAT, iNOS, COX-2). | [105] | |
| Spirulina | Diet supplementation | Increase in TH+ and NeuN+ neurons. Anti-inflammatory. | F344 rats treated with AAV9α-synuclein: Immunohistochemistry (TH, α-synuclein, OX-6, NeuN). Stereology. Western Blot (CX3CR1). | [106] | |
| Spirulina | Diet supplementation | Recovery of striatal dopamine innervation. Increased TH+ fibers. Anti-inflammatory. | 6-OHDA-induced parkinsonism in F344 male rats: Immunohistochemistry (TH, OX-6, Iba1, GFAP). Cell counting. | [107] |
4.3. Cyanobacteria against Multiple Sclerosis
| Strain | Compound/Extract | Effect | In Vitro Assays | In Vivo Assays | Reference |
|---|---|---|---|---|---|
| Spirulina platensis | Phycocyanin | Decreased the mean cumulative score. Neuronal Morphology Protection. Antioxidant. Anti-inflammatory. Treg induction. | PBMCs: RT-PCR (TGF-β, IL-10, CD25, Foxp3). Flow cytometry (CD4, CD25, CD69). | EAE induction in male Lewis rats: MDA assay. PP assay. TOP assay. AOPP assay. FRAP assay. Transmission electron microscopy studies. | [112] |
| Spirulina platensis | Phycocyanin | Improvement in disease onset and locomotion. Neuronal Morphology Protection. Antioxidant. Anti-inflammatory. | EAE induction in male Lewis rats and female C57BL/6 mice: Rotarod test. MDA assay. PP assay. FRA assay. Transmission electron microscopy studies. ELISA (IL-17, IL-6, IFN-γ). | [113] | |
| Spirulina platensis | Phycocyanin | Improvement in disease onset. Antioxidant. Anti-inflammatory. Anti-demyelination. Neuronal Protection. Gene Modulation. | EAE induction in C57BL/6 mice: Immunohistochemistry (CD3, Mac-3, APP). Morphometric Analysis. MDA assay. PP assay. SOD, CAT, and GSH assays. IL-17 quantification. RT-PCR. Microarray Analysis. | [114] | |
| Spirulina platensis | Phycocyanobilin (12) | Improvement in disease onset. Anti-inflammatory. Antioxidant. Anti-apoptosis. Gene modulation. | Human SHSY5Y cells: RT-PCR. Gene expression profile analysis. | EAE induction in C57BL/6 mice: ELISA (IL-17, IL-6, IFN-γ). Transmission electron microscopy. Immunohistochemistry (caspase-3, CD11). | [115] |
| Spirulina | Phycocyanobilin (12) | Improvement in disease onset. Anti-inflammatory. Anti-demyelination. Neuronal protection. Gene modulation. | TMBP-GFP cells: Proliferation assay. Fluoresce microscopy. | EAE induction in C57BL/6 mice: Immunohistochemistry (CD3, Mac-3, APP, TPPP/p25, Olig2). Morphometric analysis. ELISA (IL-17A, IL-6, and IL-10). qPCR. Flow cytometry. | [116] |
4.4. Cyanobacteria against Amyotrophic Lateral Sclerosis
| Strain | Compound/Extract | Effect | In Vitro Assays | In Vivo Assays | Reference |
|---|---|---|---|---|---|
| Oscillatoria planktothrix sp. | LPS-like molecule VB3323 | TLR4 antagonist. Improved motor function tests. Anti-inflammatory and anti-gliosis. Neuroprotection. | Purified microglial cells: Immunocytochemistry (CD11b). Immunoblotting (CD68). Live cell imaging (GFP) Motor neurons/glia co-culture: ELISA (TNF-α, IL-1β and IL-6). Motor neurons/glia coculture and purified motor neurons: Motor Neuron Viability Assay (SMI32). | Wobbler Mice: Paw abnormality and grip strength test. Immunohistochemistry (GFAP, CD11, and TNF-α). | [121] |
| Spirulina | Diet supplementation | Maintenance of extension reflex. Anti-inflammatory. Neuroprotection against motor neuron degeneration. | SOD1G93A mice: Weight and measurement. Extension Reflex test. Ribonuclease Protection Assay (IL-1α, IL-1β, IL-6, TNF-α). Immunohistochemistry (Fluoro-Jade, GFAP) | [124] |
4.5. Cyanobacteria against Huntington’s Disease
| Strain | Compound/Extract | Effect | In Vitro Assays | In Vivo Assays | Reference |
|---|---|---|---|---|---|
| Leptolyngbya sp. N62DM | Phycocyanin | Anti-polyQ aggregation. Antioxidant. Increased lifespan. | DPPH assay. FRAP assay. SRSA assay. R-Power assay. | N2 Caenorhabditis elegans: Life span assay. Pharyngeal pumping and locomotion assays. DCFH-DA fluorescence staining. Stress resistance assay. DAF-16::GFP localization Caenorhabditis elegans AM141: PolyQ aggregation assay. Paraquat sensitivity assay. Life span assay. DAF-16::GFP localization | [30] |
| Nostoc sphaeroides | Chemically derived oligosaccharides (NOS-HCA, NOS-TFA) | Improved chemosensory behavior. Improved lifespan. Antioxidant. Gene modulation. | ABTS assay. DPPH assay. | N2 Caenorhabditis elegans: Oxidative survival assay. Lifespan assay. qPCR. Caenorhabditis elegans HA759: Oxidative survival assay. Chemosensory behavior assay. Lifespan assay. qPCR (gst-4, ctl-2, hsp-6, and hsp-6). | [129] |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilson, D.M.; Cookson, M.R.; Van Den Bosch, L.; Zetterberg, H.; Holtzman, D.M.; Dewachter, I. Hallmarks of neurodegenerative diseases. Cell 2023, 186, 693–714. [Google Scholar] [CrossRef] [PubMed]
- Erkkinen, M.G.; Kim, M.-O.; Geschwind, M.D. Clinical Neurology and Epidemiology of the Major Neurodegenerative Diseases. Cold Spring Harb. Perspect. Biol. 2018, 10, a033118. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 2019, 15, 565–581. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Wu, Y.; Chen, X.; Chen, Y.; Wu, Z.; Lin, Z.; Kang, D.; Fang, W.; Chen, F. Global, regional, and national burden and attributable risk factors of neurological disorders: The Global Burden of Disease study 1990–2019. Front. Public Health 2022, 10, 952161. [Google Scholar] [CrossRef]
- Kumar, D.; Md Ashraf, G.; Bilgrami, A.L.; Imtaiyaz Hassan, M. Emerging therapeutic developments in neurodegenerative diseases: A clinical investigation. Drug Discov. Today 2022, 27, 103305. [Google Scholar] [CrossRef] [PubMed]
- Breijyeh, Z.; Karaman, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef]
- Gouda, N.A.; Elkamhawy, A.; Cho, J. Emerging Therapeutic Strategies for Parkinson’s Disease and Future Prospects: A 2021 Update. Biomedicines 2022, 10, 371. [Google Scholar] [CrossRef]
- Masrori, P.; Van Damme, P. Amyotrophic lateral sclerosis: A clinical review. Eur. J. Neurol. 2020, 27, 1918–1929. [Google Scholar] [CrossRef]
- Tabrizi, S.J.; Flower, M.D.; Ross, C.A.; Wild, E.J. Huntington disease: New insights into molecular pathogenesis and therapeutic opportunities. Nat. Rev. Neurol. 2020, 16, 529–546. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, T.; Lee, T.H. Cellular Mechanisms of Melatonin: Insight from Neurodegenerative Diseases. Biomolecules 2020, 10, 1158. [Google Scholar] [CrossRef]
- Porro, C.; Cianciulli, A.; Panaro, M.A. The Regulatory Role of IL-10 in Neurodegenerative Diseases. Biomolecules 2020, 10, 1017. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Pentón-Rol, G.; Marín-Prida, J.; McCarty, M.F. C-Phycocyanin-derived phycocyanobilin as a potential nutraceutical approach for major neurodegenerative disorders and COVID-19-induced damage to the nervous system. Curr. Neuropharmacol. 2021, 19, 2250. [Google Scholar] [CrossRef] [PubMed]
- Trotta, T.; Porro, C.; Cianciulli, A.; Panaro, M.A. Beneficial effects of spirulina consumption on brain health. Nutrients 2022, 14, 676. [Google Scholar] [CrossRef]
- Calella, P.; Cerullo, G.; Di Dio, M.; Liguori, F.; Di Onofrio, V.; Gallè, F.; Liguori, G. Antioxidant, anti-inflammatory and immunomodulatory effects of spirulina in exercise and sport: A systematic review. Front. Nutr. 2022, 9, 1048258. [Google Scholar] [CrossRef]
- Waditee-Sirisattha, R.; Kageyama, H. Chapter 1—Cyanobacterial cells. In Cyanobacterial Physiology; Kageyama, H., Waditee-Sirisattha, R., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 3–16. [Google Scholar]
- Whitton, B.A.; Potts, M. Introduction to the Cyanobacteria. In Ecology of Cyanobacteria II: Their Diversity in Space and Time; Whitton, B.A., Ed.; Springer Netherlands: Dordrecht, The Netherlands, 2012; pp. 1–13. [Google Scholar]
- Morone, J.; Alfeus, A.; Vasconcelos, V.; Martins, R. Revealing the potential of cyanobacteria in cosmetics and cosmeceuticals—A new bioactive approach. Algal Res. 2019, 41, 101541. [Google Scholar] [CrossRef]
- Tiwari, A.K.; Tiwari, B.S. Cyanotherapeutics: An emerging field for future drug discovery. Appl. Phycol. 2020, 1, 44–57. [Google Scholar] [CrossRef]
- AlFadhly, N.K.Z.; Alhelfi, N.; Altemimi, A.B.; Verma, D.K.; Cacciola, F. Tendencies Affecting the Growth and Cultivation of Genus Spirulina: An Investigative Review on Current Trends. Plants 2022, 11, 3063. [Google Scholar] [CrossRef]
- Khalifa, S.A.M.; Shedid, E.S.; Saied, E.M.; Jassbi, A.R.; Jamebozorgi, F.H.; Rateb, M.E.; Du, M.; Abdel-Daim, M.M.; Kai, G.-Y.; Al-Hammady, M.A.M.; et al. Cyanobacteria—From the Oceans to the Potential Biotechnological and Biomedical Applications. Mar. Drugs 2021, 19, 241. [Google Scholar] [CrossRef]
- Singh, R.K.; Tiwari, S.P.; Rai, A.K.; Mohapatra, T.M. Cyanobacteria: An emerging source for drug discovery. J. Antibiot. 2011, 64, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Castaneda, A.; Ferraz, R.; Vieira, M.; Cardoso, I.; Vasconcelos, V.; Martins, R. Bridging Cyanobacteria to Neurodegenerative Diseases: A New Potential Source of Bioactive Compounds against Alzheimer’s Disease. Mar. Drugs 2021, 19, 343. [Google Scholar] [CrossRef]
- Nugumanova, G.; Ponomarev, E.D.; Askarova, S.; Fasler-Kan, E.; Barteneva, N.S. Freshwater Cyanobacterial Toxins, Cyanopeptides and Neurodegenerative Diseases. Toxins 2023, 15, 233. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, G.; Molzahn, C.; Mayor, T. Protein interaction networks in neurodegenerative diseases: From physiological function to aggregation. J. Biol. Chem. 2022, 298, 102062. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, P.; Park, H.; Baumann, M.; Dunlop, J.; Frydman, J.; Kopito, R.; McCampbell, A.; Leblanc, G.; Venkateswaran, A.; Nurmi, A.; et al. Protein misfolding in neurodegenerative diseases: Implications and strategies. Transl. Neurodegener. 2017, 6, 6. [Google Scholar] [CrossRef]
- Peng, C.; Trojanowski, J.Q.; Lee, V.M.Y. Protein transmission in neurodegenerative disease. Nat. Rev. Neurol. 2020, 16, 199–212. [Google Scholar] [CrossRef]
- Nuzzo, D.; Presti, G.; Picone, P.; Galizzi, G.; Gulotta, E.; Giuliano, S.; Mannino, C.; Gambino, V.; Scoglio, S.; Di Carlo, M. Effects of the Aphanizomenon flos-aquae Extract (Klamin®) on a Neurodegeneration Cellular Model. Oxidative Med. Cell. Longev. 2018, 2018, 9089016. [Google Scholar] [CrossRef]
- Singh, N.K.; Sonani, R.R.; Awasthi, A.; Prasad, B.; Patel, A.R.; Kumar, J.; Madamwar, D. Phycocyanin moderates aging and proteotoxicity in Caenorhabditis elegans. J. Appl. Phycol. 2016, 28, 2407–2417. [Google Scholar] [CrossRef]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef]
- Olufunmilayo, E.O.; Gerke-Duncan, M.B.; Holsinger, R.M.D. Oxidative Stress and Antioxidants in Neurodegenerative Disorders. Antioxidants 2023, 12, 517. [Google Scholar] [CrossRef]
- Ghanbari, A.; Vafaei, A.A.; Naghibi nasab, F.S.; Attarmoghaddam, M.; Bandegi, A.R.; Moradi- Kor, N. Spirulina microalgae improves memory deficit induced by scopolamine in male pup rats: Role of oxidative stress. S. Afr. J. Bot. 2019, 127, 220–225. [Google Scholar] [CrossRef]
- Moradi-Kor, N.; Ghanbari, A.; Rashidipour, H.; Bandegi, A.R.; Yousefi, B.; Barati, M.; Kokhaei, P.; Rashidy-Pour, A. Therapeutic Effects of Spirulina platensis Against Adolescent Stress-Induced Oxidative Stress, Brain-Derived Neurotrophic Factor Alterations and Morphological Remodeling in the Amygdala of Adult Female Rats. J. Exp. Pharmacol. 2020, 12, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Bermejo-Bescós, P.; Piñero-Estrada, E.; Villar del Fresno, Á.M. Neuroprotection by Spirulina platensis protean extract and phycocyanin against iron-induced toxicity in SH-SY5Y neuroblastoma cells. Toxicol. Vitr. 2008, 22, 1496–1502. [Google Scholar] [CrossRef]
- Pérez-Juárez, A.; Chamorro, G.; Alva-Sánchez, C.; Paniagua-Castro, N.; Pacheco-Rosado, J. Neuroprotective effect of Arthrospira (Spirulina) platensis against kainic acid-neuronal death. Pharm. Biol. 2016, 54, 1408–1412. [Google Scholar] [CrossRef]
- Marín-Prida, J.; Pentón-Rol, G.; Rodrigues, F.P.; Alberici, L.C.; Stringhetta, K.; Leopoldino, A.M.; Naal, Z.; Polizello, A.C.M.; Llópiz-Arzuaga, A.; Rosa, M.N.; et al. C-Phycocyanin protects SH-SY5Y cells from oxidative injury, rat retina from transient ischemia and rat brain mitochondria from Ca2+/phosphate-induced impairment. Brain Res. Bull. 2012, 89, 159–167. [Google Scholar] [CrossRef]
- Kwon, H.S.; Koh, S.-H. Neuroinflammation in neurodegenerative disorders: The roles of microglia and astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Badoni, H.; Abu-Izneid, T.; Olatunde, A.; Rahman, M.M.; Painuli, S.; Semwal, P.; Wilairatana, P.; Mubarak, M.S. Neuroinflammatory Markers: Key Indicators in the Pathology of Neurodegenerative Diseases. Molecules 2022, 27, 3194. [Google Scholar] [CrossRef]
- Chen, J.-C.; Liu, K.S.; Yang, T.-J.; Hwang, J.-H.; Chan, Y.-C.; Lee, I.T. Spirulina and C-phycocyanin reduce cytotoxicity and inflammation-related genes expression of microglial cells. Nutr. Neurosci. 2012, 15, 252–256. [Google Scholar] [CrossRef]
- Bigagli, E.; D’Ambrosio, M.; Cinci, L.; Pieraccini, G.; Romoli, R.; Biondi, N.; Niccolai, A.; Rodolfi, L.; Tredici, M.R.; Luceri, C. A comparative study of metabolites profiles, anti-inflammatory and antioxidant activity of methanolic extracts from three Arthrospira strains in RAW 264.7 macrophages. Algal Res. 2023, 73, 103171. [Google Scholar] [CrossRef]
- Chei, S.; Oh, H.-J.; Song, J.-H.; Seo, Y.-J.; Lee, K.; Kim, K.-J.; Lee, B.-Y. Spirulina maxima extract prevents activation of the NLRP3 inflammasome by inhibiting ERK signaling. Sci. Rep. 2020, 10, 2075. [Google Scholar] [CrossRef]
- Piovan, A.; Battaglia, J.; Filippini, R.; Dalla Costa, V.; Facci, L.; Argentini, C.; Pagetta, A.; Giusti, P.; Zusso, M. Pre- and Early Post-treatment With Arthrospira platensis (Spirulina) Extract Impedes Lipopolysaccharide-triggered Neuroinflammation in Microglia. Front. Pharmacol. 2021, 12, 724993. [Google Scholar] [CrossRef] [PubMed]
- Khalil, S.R.; Khalifa, H.A.; Abdel-Motal, S.M.; Mohammed, H.H.; Elewa, Y.H.A.; Mahmoud, H.A. Spirulina platensis attenuates the associated neurobehavioral and inflammatory response impairments in rats exposed to lead acetate. Ecotoxicol. Environ. Saf. 2018, 157, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Armada-Moreira, A.; Gomes, J.I.; Pina, C.C.; Savchak, O.K.; Gonçalves-Ribeiro, J.; Rei, N.; Pinto, S.; Morais, T.P.; Martins, R.S.; Ribeiro, F.F.; et al. Going the Extra (Synaptic) Mile: Excitotoxicity as the Road Toward Neurodegenerative Diseases. Front. Cell. Neurosci. 2020, 14, 90. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Ryu, G.H.; Choi, W.Y.; Yang, W.S.; Lee, H.W.; Ma, C.J. Protective effect of water extracted Spirulina maxima on glutamate-induced neuronal cell death in mouse hippocampal HT22 cell. Pharmacogn. Mag. 2018, 14, 242. [Google Scholar]
- Procházková, T.; Sychrová, E.; Javůrková, B.; Večerková, J.; Kohoutek, J.; Lepšová-Skácelová, O.; Bláha, L.; Hilscherová, K. Phytoestrogens and sterols in waters with cyanobacterial blooms—Analytical methods and estrogenic potencies. Chemosphere 2017, 170, 104–112. [Google Scholar] [CrossRef]
- Tan, J.W.; Kim, M.K. Neuroprotective Effects of Biochanin A against β-Amyloid-Induced Neurotoxicity in PC12 Cells via a Mitochondrial-Dependent Apoptosis Pathway. Molecules 2016, 21, 548. [Google Scholar] [CrossRef]
- LePage, K.T.; Goeger, D.; Yokokawa, F.; Asano, T.; Shioiri, T.; Gerwick, W.H.; Murray, T.F. The neurotoxic lipopeptide kalkitoxin interacts with voltage-sensitive sodium channels in cerebellar granule neurons. Toxicol. Lett. 2005, 158, 133–139. [Google Scholar] [CrossRef]
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef]
- 2023 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2023, 19, 1598–1695. [CrossRef]
- Doroszkiewicz, J.; Mroczko, B. New possibilities in the therapeutic approach to Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 8902. [Google Scholar] [CrossRef]
- Yu, T.-W.; Lane, H.-Y.; Lin, C.-H. Novel therapeutic approaches for Alzheimer’s disease: An updated review. Int. J. Mol. Sci. 2021, 22, 8208. [Google Scholar] [CrossRef] [PubMed]
- Gholami, A.; Minai-Tehrani, D.; Eriksson, L.A. In silico and in vitro studies confirm Ondansetron as a novel acetylcholinesterase and butyrylcholinesterase inhibitor. Sci. Rep. 2023, 13, 643. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, N.A.; Carmichael, W.W. Anatoxin-a (s), an anticholinesterase from the cyanobacterium Anabaena flos-aquae NRC-525-17. Toxicon 1987, 25, 1221–1227. [Google Scholar] [CrossRef] [PubMed]
- Becher, P.G.; Baumann, H.I.; Gademann, K.; Jüttner, F. The cyanobacterial alkaloid nostocarboline: An inhibitor of acetylcholinesterase and trypsin. J. Appl. Phycol. 2009, 21, 103–110. [Google Scholar] [CrossRef]
- Becher, P.G.; Beuchat, J.; Gademann, K.; Jüttner, F. Nostocarboline: Isolation and synthesis of a new cholinesterase inhibitor from Nostoc 78-12A. J. Nat. Prod. 2005, 68, 1793–1795. [Google Scholar] [CrossRef]
- Fiore, M.F.; de Lima, S.T.; Carmichael, W.W.; McKinnie, S.M.K.; Chekan, J.R.; Moore, B.S. Guanitoxin, re-naming a cyanobacterial organophosphate toxin. Harmful Algae 2020, 92, 101737. [Google Scholar] [CrossRef]
- Rodgers, K.J.; Main, B.J.; Samardzic, K. Cyanobacterial Neurotoxins: Their Occurrence and Mechanisms of Toxicity. Neurotox. Res. 2018, 33, 168–177. [Google Scholar] [CrossRef]
- Fagundes, M.B.; Alvarez-Rivera, G.; Mendiola, J.A.; Bueno, M.; Sánchez-Martínez, J.D.; Wagner, R.; Jacob-Lopes, E.; Zepka, L.Q.; Ibañez, E.; Cifuentes, A. Phytosterol-rich compressed fluids extracts from Phormidium autumnale cyanobacteria with neuroprotective potential. Algal Res. 2021, 55, 102264. [Google Scholar] [CrossRef]
- Refaay, D.A.; Abdel-Hamid, M.I.; Alyamani, A.A.; Abdel Mougib, M.; Ahmed, D.M.; Negm, A.; Mowafy, A.M.; Ibrahim, A.A.; Mahmoud, R.M. Growth Optimization and Secondary Metabolites Evaluation of Anabaena variabilis for Acetylcholinesterase Inhibition Activity. Plants 2022, 11, 735. [Google Scholar] [CrossRef]
- Touliabah, H.E.; Refaay, D.A. Enhancement of Anticancer, Antibacterial, and Acetylcholinesterase Inhibition Activities from Oscillatoria sancta under Starvation Conditions. Water 2023, 15, 664. [Google Scholar] [CrossRef]
- Khemiri, S.; Khelifi, N.; Messaoud, C.; Smaali, I. Bioprospecting of microalgae for a potential use as enzyme inhibitors, anti-ageing and prebiotic agents. Biocatal. Agric. Biotechnol. 2023, 51, 102759. [Google Scholar] [CrossRef]
- Hampel, H.; Hardy, J.; Blennow, K.; Chen, C.; Perry, G.; Kim, S.H.; Villemagne, V.L.; Aisen, P.; Vendruscolo, M.; Iwatsubo, T.; et al. The Amyloid-β Pathway in Alzheimer’s Disease. Mol. Psychiatry 2021, 26, 5481–5503. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.-C.; Jing, P. Molecular Interaction of Protein-Pigment C-Phycocyanin with Bovine Serum Albumin in a Gomphosis Structure Inhibiting Amyloid Formation. Int. J. Mol. Sci. 2020, 21, 8207. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jovcevski, B.; Pukala, T.L. C-Phycocyanin from Spirulina Inhibits α-Synuclein and Amyloid-β Fibril Formation but Not Amorphous Aggregation. J. Nat. Prod. 2019, 82, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, W.; Xu, Y.; Ren, S.; Zhang, W.; Li, Y. Design, synthesis and biological evaluation of tasiamide B derivatives as BACE1 inhibitors. Bioorganic Med. Chem. 2015, 23, 1963–1974. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, W.; Li, L.; Salvador, L.A.; Chen, T.; Chen, W.; Felsenstein, K.M.; Ladd, T.B.; Price, A.R.; Golde, T.E.; et al. Cyanobacterial Peptides as a Prototype for the Design of Potent β-Secretase Inhibitors and the Development of Selective Chemical Probes for Other Aspartic Proteases. J. Med. Chem. 2012, 55, 10749–10765. [Google Scholar] [CrossRef]
- Al-Awadhi, F.H.; Ratnayake, R.; Paul, V.J.; Luesch, H. Tasiamide F, a potent inhibitor of cathepsins D and E from a marine cyanobacterium. Bioorganic Med. Chem. 2016, 24, 3276–3282. [Google Scholar] [CrossRef]
- Singh, K.N.; Hasan, S.S.; Kumar, J.; Raj, I.; Pathan, A.A.; Parmar, A.; Shakil, S.; Gourinath, S.; Madamwar, D. Crystal Structure and Interaction of Phycocyanin with β-Secretase: A Putative Therapy for Alzheimer’s Disease. CNS Neurol. Disord. Drug Targets 2014, 13, 691–698. [Google Scholar] [CrossRef]
- Chaubey, M.G.; Patel, S.N.; Rastogi, R.P.; Srivastava, P.L.; Singh, A.K.; Madamwar, D.; Singh, N.K. Therapeutic potential of cyanobacterial pigment protein phycoerythrin: In silico and in vitro study of BACE1 interaction and in vivo Aβ reduction. Int. J. Biol. Macromol. 2019, 134, 368–378. [Google Scholar] [CrossRef]
- Koh, E.-J.; Kim, K.-J.; Song, J.-H.; Choi, J.; Lee, H.Y.; Kang, D.-H.; Heo, H.J.; Lee, B.-Y. Spirulina maxima Extract Ameliorates Learning and Memory Impairments via Inhibiting GSK-3β Phosphorylation Induced by Intracerebroventricular Injection of Amyloid-β 1–42 in Mice. Int. J. Mol. Sci. 2017, 18, 2401. [Google Scholar] [CrossRef]
- Galizzi, G.; Deidda, I.; Amato, A.; Calvi, P.; Terzo, S.; Caruana, L.; Scoglio, S.; Mulè, F.; Di Carlo, M. Aphanizomenon flos-aquae (AFA) Extract Prevents Neurodegeneration in the HFD Mouse Model by Modulating Astrocytes and Microglia Activation. Int. J. Mol. Sci. 2023, 24, 4731. [Google Scholar] [CrossRef] [PubMed]
- Klamin. Available online: https://www.klamathshop.eu/klamin/ (accessed on 18 August 2023).
- AphaMax. Available online: https://www.klamathshop.eu/aphamax/ (accessed on 18 August 2023).
- Chandrasekaran, V.; Hediyal, T.A.; Anand, N.; Kendaganna, P.H.; Gorantla, V.R.; Mahalakshmi, A.M.; Ghanekar, R.K.; Yang, J.; Sakharkar, M.K.; Chidambaram, S.B. Polyphenols, Autophagy and Neurodegenerative Diseases: A Review. Biomolecules 2023, 13, 1196. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Gómez, M.-Á.; Llorens-Álvarez, E.; Alom, J.; del Ser, T.; Avila, J.; Sáez-Valero, J.; García-Ayllón, M.-S. Tau phosphorylation by glycogen synthase kinase 3β modulates enzyme acetylcholinesterase expression. J. Neurochem. 2021, 157, 2091–2105. [Google Scholar] [CrossRef] [PubMed]
- Elsonbaty, S.M.; Ismail, A.F.M. Nicotine encourages oxidative stress and impairment of rats’ brain mitigated by Spirulina platensis lipopolysaccharides and low-dose ionizing radiation. Arch. Biochem. Biophys. 2020, 689, 108382. [Google Scholar] [CrossRef]
- Zhou, T.; Liu, Y.; Wang, Q.; Dou, Q.; Li, X.; Pan, Y.; Meng, L.; Xue, T. Spirulina platensis alleviates high fat diet-induced cognitive impairment in mice via the gut-brain axis. J. Funct. Foods 2021, 86, 104706. [Google Scholar] [CrossRef]
- Chen, L.; Liu, Y.-c.; Tan, H.; Zhang, Y.; Xu, J.; Liu, W.-l.; Li, Z.-y.; Li, W.-p. Santacruzamate A Ameliorates AD-Like Pathology by Enhancing ER Stress Tolerance through Regulating the Functions of KDELR and Mia40-ALR in vivo and in vitro. Front. Cell. Neurosci. 2019, 13, 61. [Google Scholar] [CrossRef]
- Malm, T.; Koistinaho, J.; Kanninen, K. Utilization of APPswe/PS1dE9 Transgenic Mice in Research of Alzheimer’s Disease: Focus on Gene Therapy and Cell-Based Therapy Applications. Int. J. Alzheimer’s Dis. 2011, 2011, 517160. [Google Scholar] [CrossRef]
- Yousef, M.I.; Abdou, H.M.; Abd Elkader, H.-T.A.E.A.; Hussein, H.K.; Abou Samra, W.E.M. Neuroprotective Potential of Spirulina Platensis against Aluminium Chloride-Induced Neural Degeneration. Curr. Top. Nutraceutical Res. 2020, 18, 310–318. [Google Scholar] [CrossRef]
- Abdelghany, A.K.; Gamal, A.; Abdel-Wahab, A.; Abdel-Razik, A.-R.H.; El-Samannoudy, S.I.; Ibrahim, M.A.; Hassan, W.H.; El-Ela, F.I.A. Evaluating the neuroprotective effect of Spirulina platensis–loaded niosomes against Alzheimer’s disease induced in rats. Drug Deliv. Transl. Res. 2023, 12, 2690. [Google Scholar] [CrossRef]
- Cammann, D.; Lu, Y.; Cummings, M.J.; Zhang, M.L.; Cue, J.M.; Do, J.; Ebersole, J.; Chen, X.; Oh, E.C.; Cummings, J.L.; et al. Genetic correlations between Alzheimer’s disease and gut microbiome genera. Sci. Rep. 2023, 13, 5258. [Google Scholar] [CrossRef]
- Imai, Y.; Koseki, Y.; Hirano, M.; Nakamura, S. Nutrigenomic Studies on the Ameliorative Effect of Enzyme-Digested Phycocyanin in Alzheimer’s Disease Model Mice. Nutrients 2021, 13, 4431. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Gan, L.; Yan, S.; Yan, Y.; Huang, W. Effect of C-phycocyanin on HDAC3 and miRNA-335 in Alzheimer’s disease. Transl. Neurosci. 2020, 11, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, M.; Perumal, Y.; Bansal, S.; Arora, S.; Chopra, K. Phycocyanin alleviates ICV-STZ induced cognitive and molecular deficits via PI3-Kinase dependent pathway. Food Chem. Toxicol. 2020, 145, 111684. [Google Scholar] [CrossRef]
- Choi, W.-Y.; Lee, W.-K.; Kim, T.-H.; Ryu, Y.-K.; Park, A.; Lee, Y.-J.; Heo, S.-J.; Oh, C.; Chung, Y.-C.; Kang, D.-H. The Effects of Spirulina maxima Extract on Memory Improvement in Those with Mild Cognitive Impairment: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients 2022, 14, 3714. [Google Scholar] [CrossRef]
- Tamtaji, O.R.; Heidari-soureshjani, R.; Asemi, Z.; Kouchaki, E. The effects of spirulina intake on clinical and metabolic parameters in Alzheimer’s disease: A randomized, double-blind, controlled trial. Phytother. Res. 2023, 37, 2957–2964. [Google Scholar] [CrossRef]
- Bloem, B.R.; Okun, M.S.; Klein, C. Parkinson’s disease. Lancet 2021, 397, 2284–2303. [Google Scholar] [CrossRef] [PubMed]
- Balestrino, R.; Schapira, A.H.V. Parkinson disease. Eur. J. Neurol. 2020, 27, 27–42. [Google Scholar] [CrossRef]
- Zaman, V.; Shields, D.C.; Shams, R.; Drasites, K.P.; Matzelle, D.; Haque, A.; Banik, N.L. Cellular and molecular pathophysiology in the progression of Parkinson’s disease. Metab. Brain Dis. 2021, 36, 815–827. [Google Scholar] [CrossRef]
- Macedo, D.; Bertolin, T.E.; Oro, T.; Backes, L.T.H.; Brás, I.C.; Santos, C.N.; Tenreiro, S.; Outeiro, T.F. Phycocyanin protects against Alpha-Synuclein toxicity in yeast. J. Funct. Foods 2017, 38, 553–560. [Google Scholar] [CrossRef]
- Latif, S.; Jahangeer, M.; Maknoon Razia, D.; Ashiq, M.; Ghaffar, A.; Akram, M.; El Allam, A.; Bouyahya, A.; Garipova, L.; Ali Shariati, M.; et al. Dopamine in Parkinson’s disease. Clin. Chim. Acta 2021, 522, 114–126. [Google Scholar] [CrossRef]
- Chamorro, G.; Pérez-Albiter, M.; Serrano-García, N.; Mares-Sámano, J.J.; Rojas, P. Spirulina maxima pretreatment partially protects against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine neurotoxicity. Nutr. Neurosci. 2006, 9, 207–212. [Google Scholar] [CrossRef]
- Tobón-Velasco, J.C.; Palafox-Sánchez, V.; Mendieta, L.; García, E.; Santamaría, A.; Chamorro-Cevallos, G.; Limón, I.D. Antioxidant effect of Spirulina (Arthrospira) maxima in a neurotoxic model caused by 6-OHDA in the rat striatum. J. Neural Transm. 2013, 120, 1179–1189. [Google Scholar] [CrossRef]
- Chattopadhyaya, I.; Gupta, S.; Mohammed, A.; Mushtaq, N.; Chauhan, S.; Ghosh, S. Neuroprotective effect of Spirulina fusiform and amantadine in the 6-OHDA induced Parkinsonism in rats. BMC Complement. Altern. Med. 2015, 15, 296. [Google Scholar]
- Xu, F.-h.; Qiu, Y.-z.; Zhang, Y.; Yang, F.-h.; Ji, M.-m.; Liu, K.-c.; Jin, M.; Zhang, S.-s.; Li, B. The molecular mechanism of three novel peptides from C-phycocyanin alleviates MPTP-induced Parkinson’s disease-like pathology in zebrafish. Food Funct. 2023, 14, 6157–6171. [Google Scholar] [CrossRef] [PubMed]
- Aryal, B.; Lee, Y. Disease model organism for Parkinson disease: Drosophila melanogaster. BMB Rep. 2019, 52, 250. [Google Scholar] [CrossRef] [PubMed]
- Salim, M.; Subandi, M.; Yuniarti, Y. Neuroprotective Benefits of S. platensis Extract on Drosophila melanogaster Model of Parkinson’s Disease. In Proceedings of the 1st International Conference on Islam, Science and Technology, ICONISTECH 2019, Bandung, Indonesia, 11–12 July 2019. [Google Scholar]
- Kumar, A.; Christian, P.K.; Panchal, K.; Guruprasad, B.R.; Tiwari, A.K. Supplementation of spirulina (Arthrospira platensis) improves lifespan and locomotor activity in paraquat-sensitive DJ-1β Δ93 flies, a Parkinson’s Disease model in Drosophila melanogaster. J. Diet. Suppl. 2017, 14, 573–588. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, A.; Mackie, P.; Hashimi, B.; Buchanan, A.M.; Smith, A.R.; Bouchard, R.; Shaw, G.; Badov, M.; Saadatpour, L.; Gittis, A.; et al. DAT and TH expression marks human Parkinson’s disease in peripheral immune cells. NPJ Park. Dis. 2022, 8, 72. [Google Scholar] [CrossRef]
- Zhang, F.; Lu, J.; Zhang, J.-g.; Xie, J.-x. Protective effects of a polysaccharide from Spirulina platensis on dopaminergic neurons in an MPTP-induced Parkinson’s disease model in C57BL/6J mice. Neural Regen. Res. 2015, 10, 308. [Google Scholar] [CrossRef]
- Lopes, M.J.P.; Delmondes, G.d.A.; Leite, G.M.d.L.; Cavalcante, D.R.A.; Aquino, P.É.A.d.; Lima, F.A.V.d.; Neves, K.R.T.; Costa, A.S.; Oliveira, H.D.d.; Bezerra Felipe, C.F. The protein-rich fraction from Spirulina platensis exerts neuroprotection in hemiparkinsonian rats by decreasing brain inflammatory-related enzymes and glial fibrillary acidic protein expressions. J. Med. Food 2022, 25, 695–709. [Google Scholar] [CrossRef]
- Lima, F.A.V.; Joventino, I.P.; Joventino, F.P.; de Almeida, A.C.; Neves, K.R.T.; do Carmo, M.R.; Leal, L.K.A.M.; de Andrade, G.M.; de Barros Viana, G.S. Neuroprotective activities of Spirulina platensis in the 6-OHDA model of Parkinson’s disease are related to its anti-inflammatory effects. Neurochem. Res. 2017, 42, 3390–3400. [Google Scholar] [CrossRef]
- Pabon, M.M.; Jernberg, J.N.; Morganti, J.; Contreras, J.; Hudson, C.E.; Klein, R.L.; Bickford, P.C. A spirulina-enhanced diet provides neuroprotection in an α-synuclein model of Parkinson’s disease. PLoS ONE 2012, 7, e45256. [Google Scholar] [CrossRef] [PubMed]
- Strömberg, I.; Gemma, C.; Vila, J.; Bickford, P.C. Blueberry-and spirulina-enriched diets enhance striatal dopamine recovery and induce a rapid, transient microglia activation after injury of the rat nigrostriatal dopamine system. Exp. Neurol. 2005, 196, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.H.; Rempe, T.; Whitmire, N.; Dunn-Pirio, A.; Graves, J.S. Therapeutic Advances in Multiple Sclerosis. Front. Neurol. 2022, 13, 824926. [Google Scholar] [CrossRef] [PubMed]
- Dobson, R.; Giovannoni, G. Multiple sclerosis—A review. Eur. J. Neurol. 2019, 26, 27–40. [Google Scholar] [CrossRef]
- Filippi, M.; Bar-Or, A.; Piehl, F.; Preziosa, P.; Solari, A.; Vukusic, S.; Rocca, M.A. Multiple sclerosis. Nat. Rev. Dis. Primers 2018, 4, 43. [Google Scholar] [CrossRef]
- Procaccini, C.; De Rosa, V.; Pucino, V.; Formisano, L.; Matarese, G. Animal models of Multiple Sclerosis. Eur. J. Pharmacol. 2015, 759, 182–191. [Google Scholar] [CrossRef]
- Pentón-Rol, G.; Martínez-Sánchez, G.; Cervantes-Llanos, M.; Lagumersindez-Denis, N.; Acosta-Medina, E.F.; Falcón-Cama, V.; Alonso-Ramírez, R.; Valenzuela-Silva, C.; Rodríguez-Jiménez, E.; Llópiz-Arzuaga, A. C-Phycocyanin ameliorates experimental autoimmune encephalomyelitis and induces regulatory T cells. Int. Immunopharmacol. 2011, 11, 29–38. [Google Scholar] [CrossRef]
- Cervantes-Llanos, M.; Lagumersindez-Denis, N.; Marín-Prida, J.; Pavón-Fuentes, N.; Falcon-Cama, V.; Piniella-Matamoros, B.; Camacho-Rodríguez, H.; Fernández-Massó, J.R.; Valenzuela-Silva, C.; Raíces-Cruz, I.; et al. Beneficial effects of oral administration of C-Phycocyanin and Phycocyanobilin in rodent models of experimental autoimmune encephalomyelitis. Life Sci. 2018, 194, 130–138. [Google Scholar] [CrossRef]
- Pentón-Rol, G.; Lagumersindez-Denis, N.; Muzio, L.; Bergami, A.; Furlan, R.; Fernández-Massó, J.R.; Nazabal-Galvez, M.; Llópiz-Arzuaga, A.; Herrera-Rolo, T.; Veliz-Rodriguez, T. Comparative neuroregenerative effects of C-phycocyanin and IFN-beta in a model of multiple sclerosis in mice. J. Neuroimmune Pharmacol. 2016, 11, 153–167. [Google Scholar] [CrossRef]
- Gardón, D.P.; Cervantes-Llanos, M.; Matamoros, B.P.; Rodríguez, H.C.; Tan, C.-y.; Marín–Prida, J.; Falcón-Cama, V.; Pavón-Fuentes, N.; Lemus, J.G.; Ruiz, L.d.l.C.B. Positive effects of Phycocyanobilin on gene expression in glutamate-induced excitotoxicity in SH-SY5Y cells and animal models of multiple sclerosis and cerebral ischemia. Heliyon 2022, 8, e09769. [Google Scholar] [CrossRef]
- Marín-Prida, J.; Pavón-Fuentes, N.; Lagumersindez-Denis, N.; Camacho-Rodríguez, H.; García-Soca, A.M.; Sarduy-Chávez, R.d.l.C.; Vieira, É.L.M.; Carvalho-Tavares, J.; Falcón-Cama, V.; Fernández-Massó, J.R. Anti-inflammatory mechanisms and pharmacological actions of phycocyanobilin in a mouse model of experimental autoimmune encephalomyelitis: A therapeutic promise for multiple sclerosis. Front. Immunol. 2022, 13, 1036200. [Google Scholar] [CrossRef] [PubMed]
- Longinetti, E.; Fang, F. Epidemiology of amyotrophic lateral sclerosis: An update of recent literature. Curr. Opin. Neurol. 2019, 32, 771. [Google Scholar] [CrossRef] [PubMed]
- Goutman, S.A.; Hardiman, O.; Al-Chalabi, A.; Chió, A.; Savelieff, M.G.; Kiernan, M.C.; Feldman, E.L. Emerging insights into the complex genetics and pathophysiology of amyotrophic lateral sclerosis. Lancet Neurol. 2022, 21, 465–479. [Google Scholar] [CrossRef] [PubMed]
- Mead, R.J.; Shan, N.; Reiser, H.J.; Marshall, F.; Shaw, P.J. Amyotrophic lateral sclerosis: A neurodegenerative disorder poised for successful therapeutic translation. Nat. Rev. Drug Discov. 2023, 22, 185–212. [Google Scholar] [CrossRef]
- Johnson, S.A.; Fang, T.; De Marchi, F.; Neel, D.; Van Weehaeghe, D.; Berry, J.D.; Paganoni, S. Pharmacotherapy for Amyotrophic Lateral Sclerosis: A Review of Approved and Upcoming Agents. Drugs 2022, 82, 1367–1388. [Google Scholar] [CrossRef]
- De Paola, M.; Mariani, A.; Bigini, P.; Peviani, M.; Ferrara, G.; Molteni, M.; Gemma, S.; Veglianese, P.; Castellaneta, V.; Boldrin, V. Neuroprotective effects of toll-like receptor 4 antagonism in spinal cord cultures and in a mouse model of motor neuron degeneration. Mol. Med. 2012, 18, 971–981. [Google Scholar] [CrossRef]
- Krishnaraj, R.N.; Kumari, S.S.S.; Mukhopadhyay, S.S. Antagonistic molecular interactions of photosynthetic pigments with molecular disease targets: A new approach to treat AD and ALS. J. Recept. Signal Transduct. 2016, 36, 67–71. [Google Scholar] [CrossRef]
- Bonifacino, T.; Zerbo, R.A.; Balbi, M.; Torazza, C.; Frumento, G.; Fedele, E.; Bonanno, G.; Milanese, M. Nearly 30 Years of Animal Models to Study Amyotrophic Lateral Sclerosis: A Historical Overview and Future Perspectives. Int. J. Mol. Sci. 2021, 22, 12236. [Google Scholar] [CrossRef]
- Garbuzova-Davis, S.; C Bickford, P. Neuroprotective effect of Spirulina in a mouse model of ALS. Open Tissue Eng. Regen. Med. J. 2010, 3, 36–41. [Google Scholar] [CrossRef]
- McColgan, P.; Tabrizi, S.J. Huntington’s disease: A clinical review. Eur. J. Neurol. 2018, 25, 24–34. [Google Scholar] [CrossRef]
- Bates, G.P.; Dorsey, R.; Gusella, J.F.; Hayden, M.R.; Kay, C.; Leavitt, B.R.; Nance, M.; Ross, C.A.; Scahill, R.I.; Wetzel, R.; et al. Huntington disease. Nat. Rev. Dis. Primers 2015, 1, 15005. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Feigin, A. Huntington’s Disease: New Frontiers in Therapeutics. Curr. Neurol. Neurosci. Rep. 2021, 21, 10. [Google Scholar] [CrossRef] [PubMed]
- Nedosekin, D.A.; Chen, T.; Ayyadevara, S.; Zharov, V.P.; Shmookler Reis, R.J. Label-free photothermal disruption of cytotoxic aggregates rescues pathology in a C. elegans model of Huntington’s disease. Sci. Rep. 2021, 11, 19732. [Google Scholar] [CrossRef]
- Zhong, G.; Pan, W.; Huang, Z.; Guo, K.; Hu, J.; Liu, P.; Chen, S.; Wang, Y.; Ai, L.; Huang, Z. Physicochemical and geroprotective comparison of Nostoc sphaeroides polysaccharides across colony growth stages and with derived oligosaccharides. J. Appl. Phycol. 2021, 33, 939–952. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos, V.; Reis, M.; Ferreira, L.; Silva, A.M.; Ferraz, R.; Vieira, M.; Vasconcelos, V.; Martins, R. Stalling the Course of Neurodegenerative Diseases: Could Cyanobacteria Constitute a New Approach toward Therapy? Biomolecules 2023, 13, 1444. https://doi.org/10.3390/biom13101444
Ramos V, Reis M, Ferreira L, Silva AM, Ferraz R, Vieira M, Vasconcelos V, Martins R. Stalling the Course of Neurodegenerative Diseases: Could Cyanobacteria Constitute a New Approach toward Therapy? Biomolecules. 2023; 13(10):1444. https://doi.org/10.3390/biom13101444
Chicago/Turabian StyleRamos, Vitória, Mariana Reis, Leonor Ferreira, Ana Margarida Silva, Ricardo Ferraz, Mónica Vieira, Vitor Vasconcelos, and Rosário Martins. 2023. "Stalling the Course of Neurodegenerative Diseases: Could Cyanobacteria Constitute a New Approach toward Therapy?" Biomolecules 13, no. 10: 1444. https://doi.org/10.3390/biom13101444
APA StyleRamos, V., Reis, M., Ferreira, L., Silva, A. M., Ferraz, R., Vieira, M., Vasconcelos, V., & Martins, R. (2023). Stalling the Course of Neurodegenerative Diseases: Could Cyanobacteria Constitute a New Approach toward Therapy? Biomolecules, 13(10), 1444. https://doi.org/10.3390/biom13101444









