Decanoic Acid Rescues Differences in AMPA-Mediated Calcium Rises in Hippocampal CA1 Astrocytes and Neurons in the 5xFAD Mouse Model of Alzheimer’s Disease
Abstract
:1. Introduction
2. Methods
2.1. Animals
2.2. Brain Slices
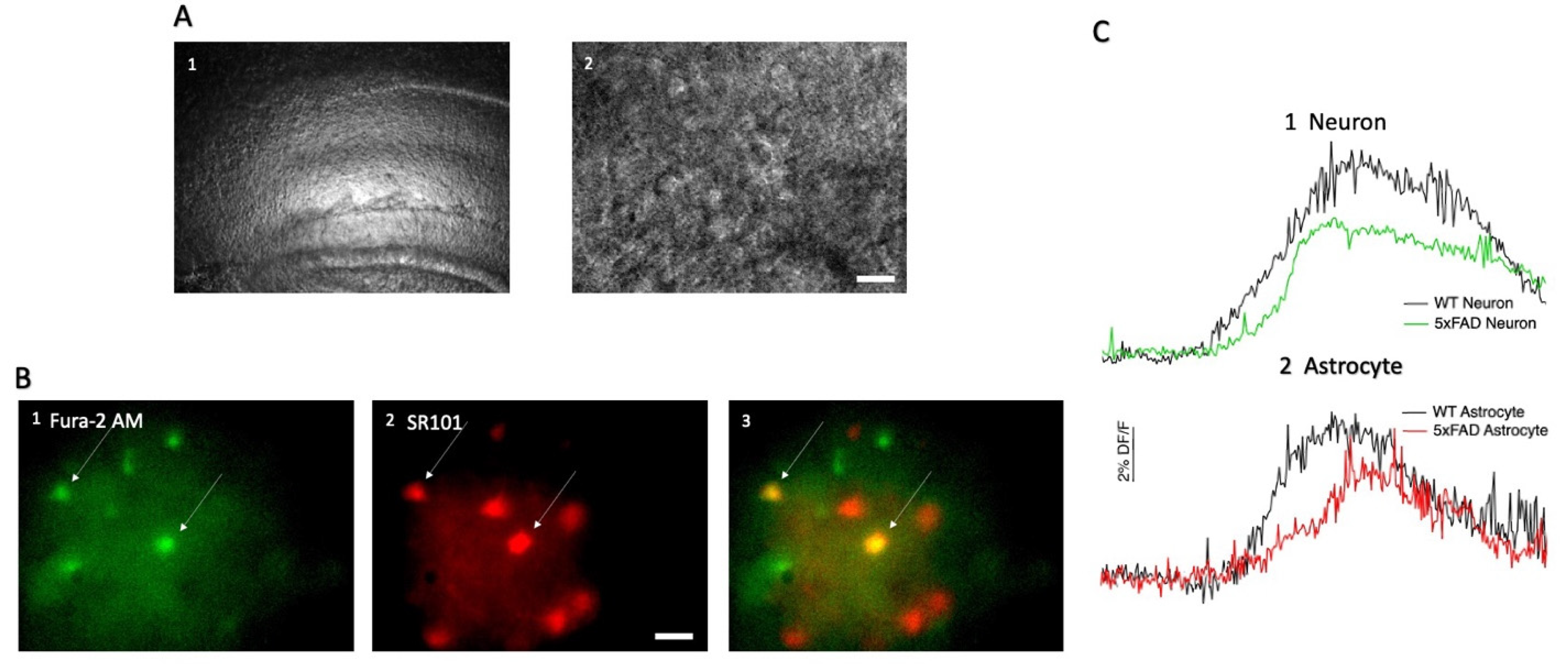
2.3. Hippocampal CA1 Cell Loading with SR101 and Fura-2 AM
2.4. Decanoic Acid Pre-Incubation and Acute AMPA Exposures
2.5. Calcium Imaging
2.6. Statistical Analysis and Data Presentation
3. Results
3.1. AMPA-Induced Changes in Fluorescence in Hippocampal CA1 Neurons and Astrocytes
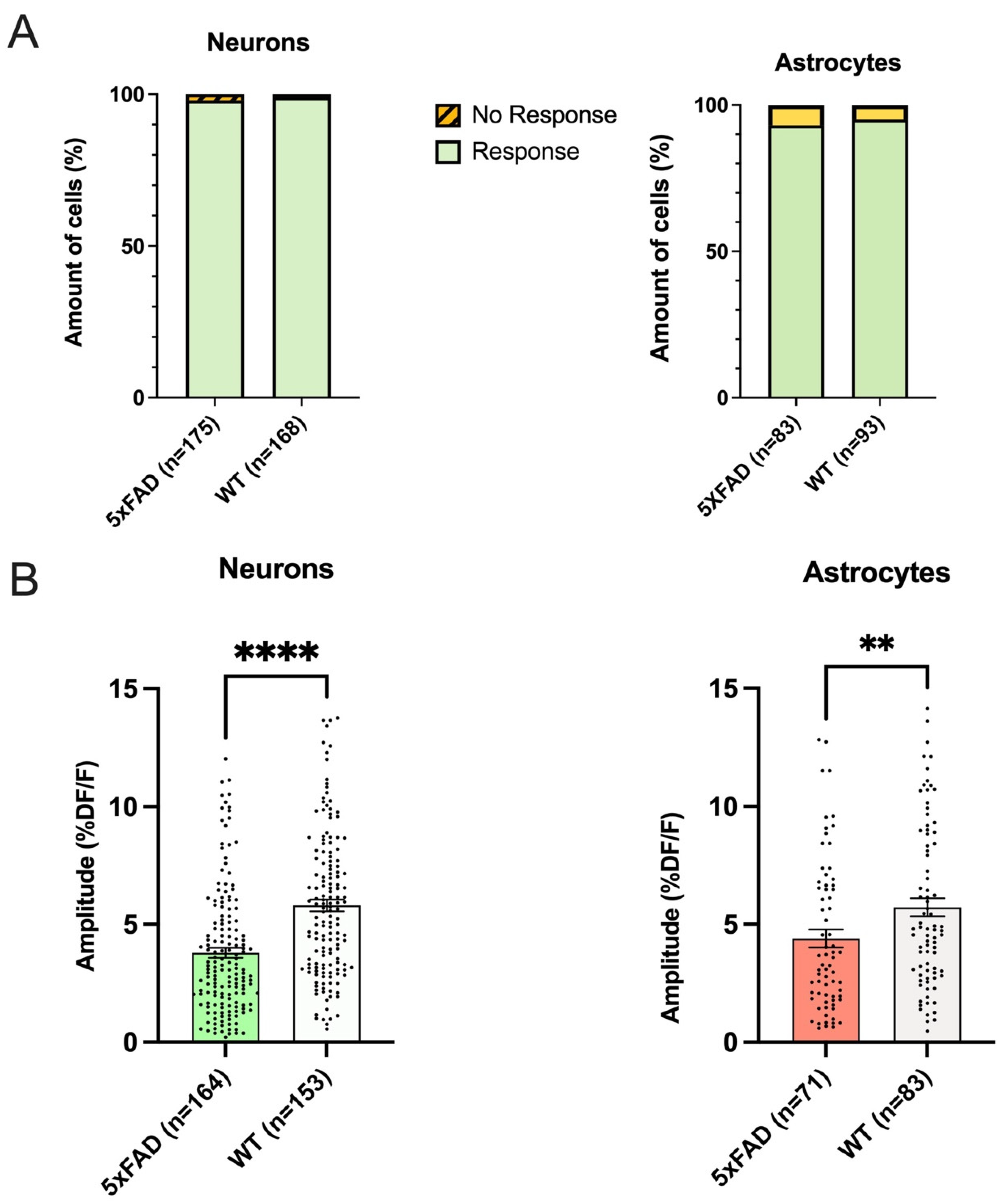
3.2. The Proportion of Responding Neurons and Astrocytes in Hippocampal CA1 Did Not Differ between 5xFAD and WT
3.3. Both Astrocytes and Neurons in 5xFAD Mice Exhibited Smaller AMPA-Induced Calcium Responses Than WT
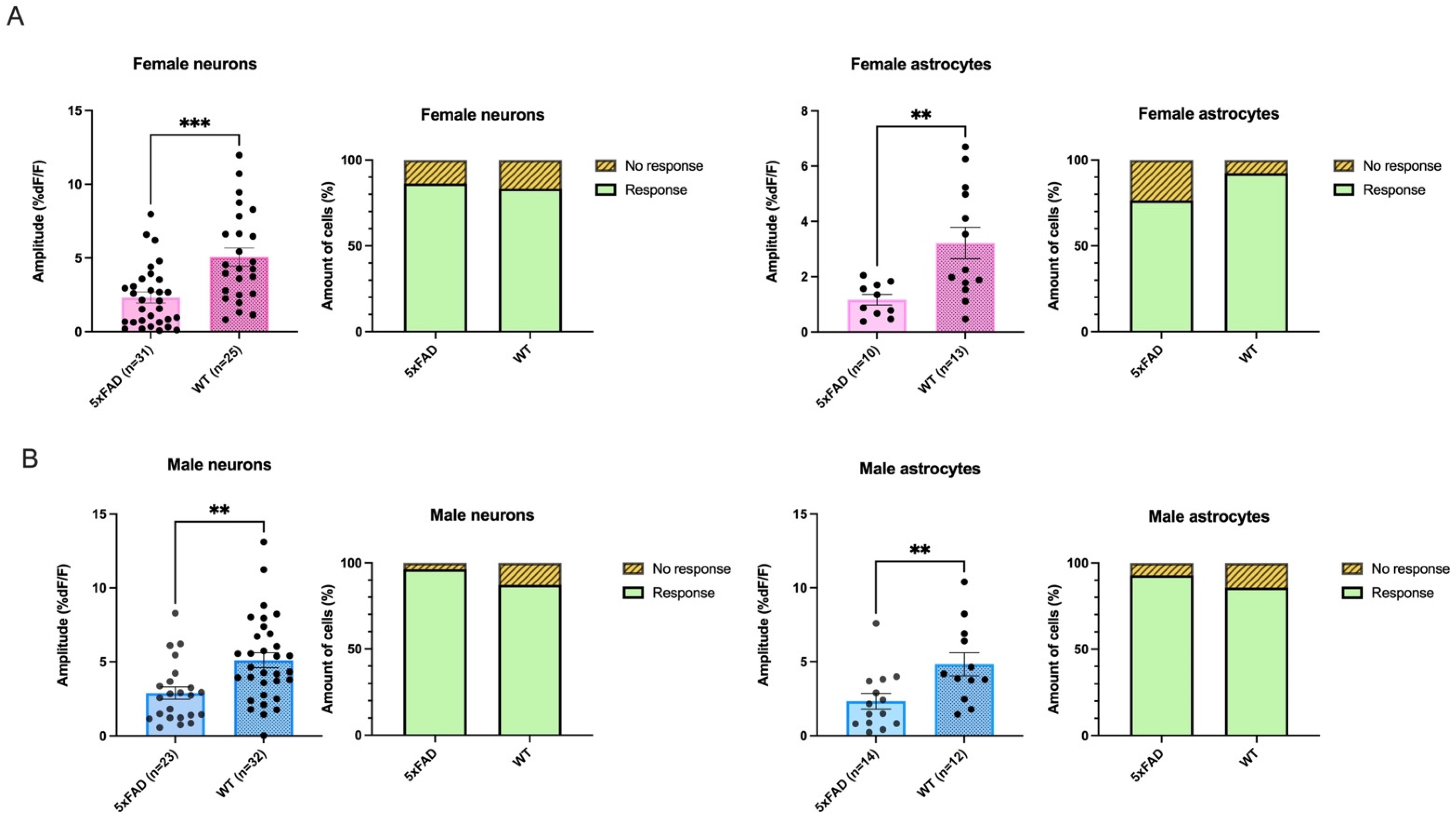
3.4. Smaller AMPA-Mediated Calcium Rises in 5XFAD Neurons and Astrocytes Were Seen in Both Sexes
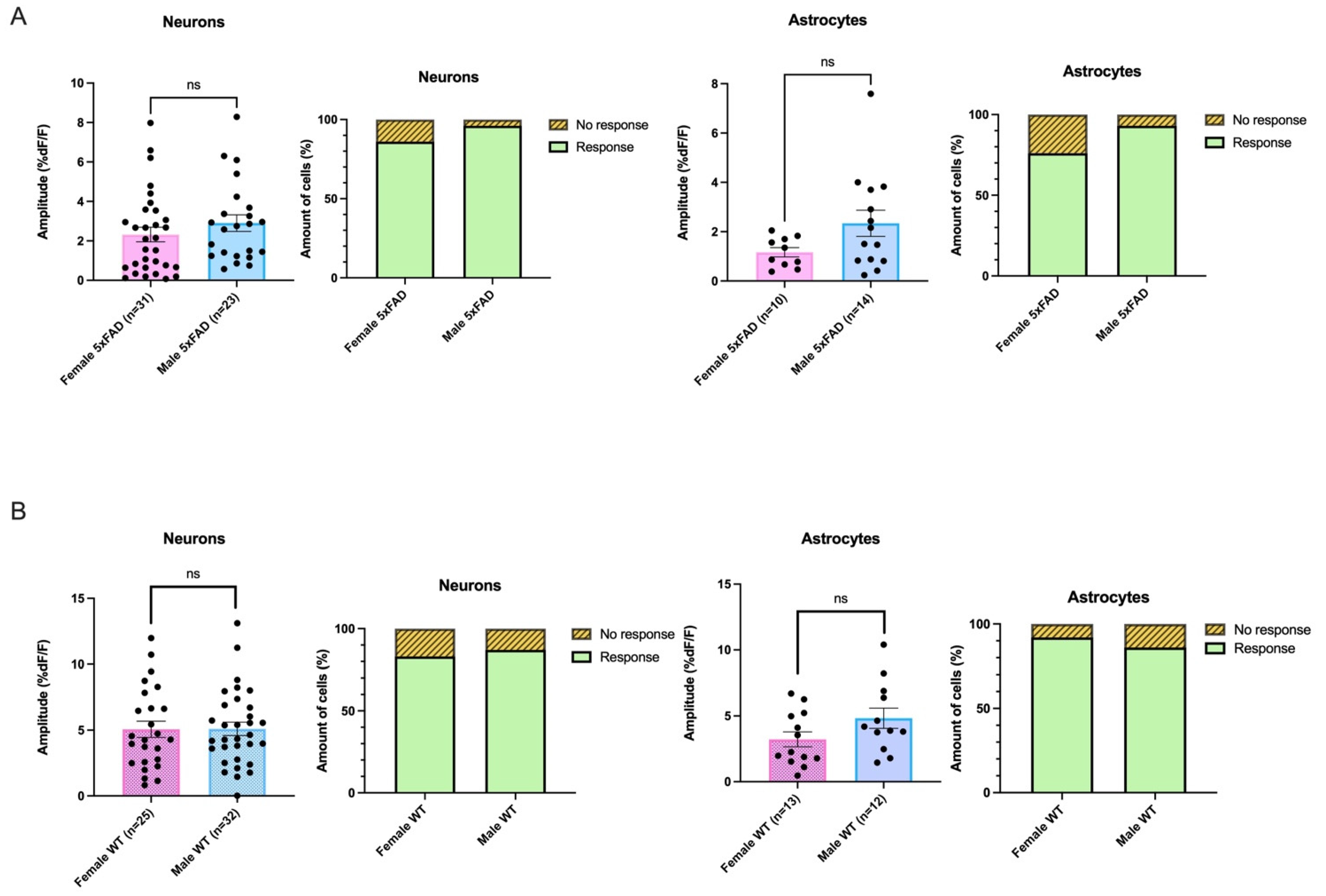
3.5. AMPA-Mediated Calcium Transients Do Not Exhibit a Sex-Based Difference in 5xFAD or WT CA1 Neurons
3.6. Decanoic Acid Pre-Treatment Results in Significantly Heightened Calcium Responses in 5xFAD Astrocytes and Neurons in Male and Females
3.7. Decanoic Acid Pre-Treatment Does Not Result in a Significantly Different Average Amplitude of AMPA-Mediated Transients between Males and Females in the 5xFAD Hippocampus
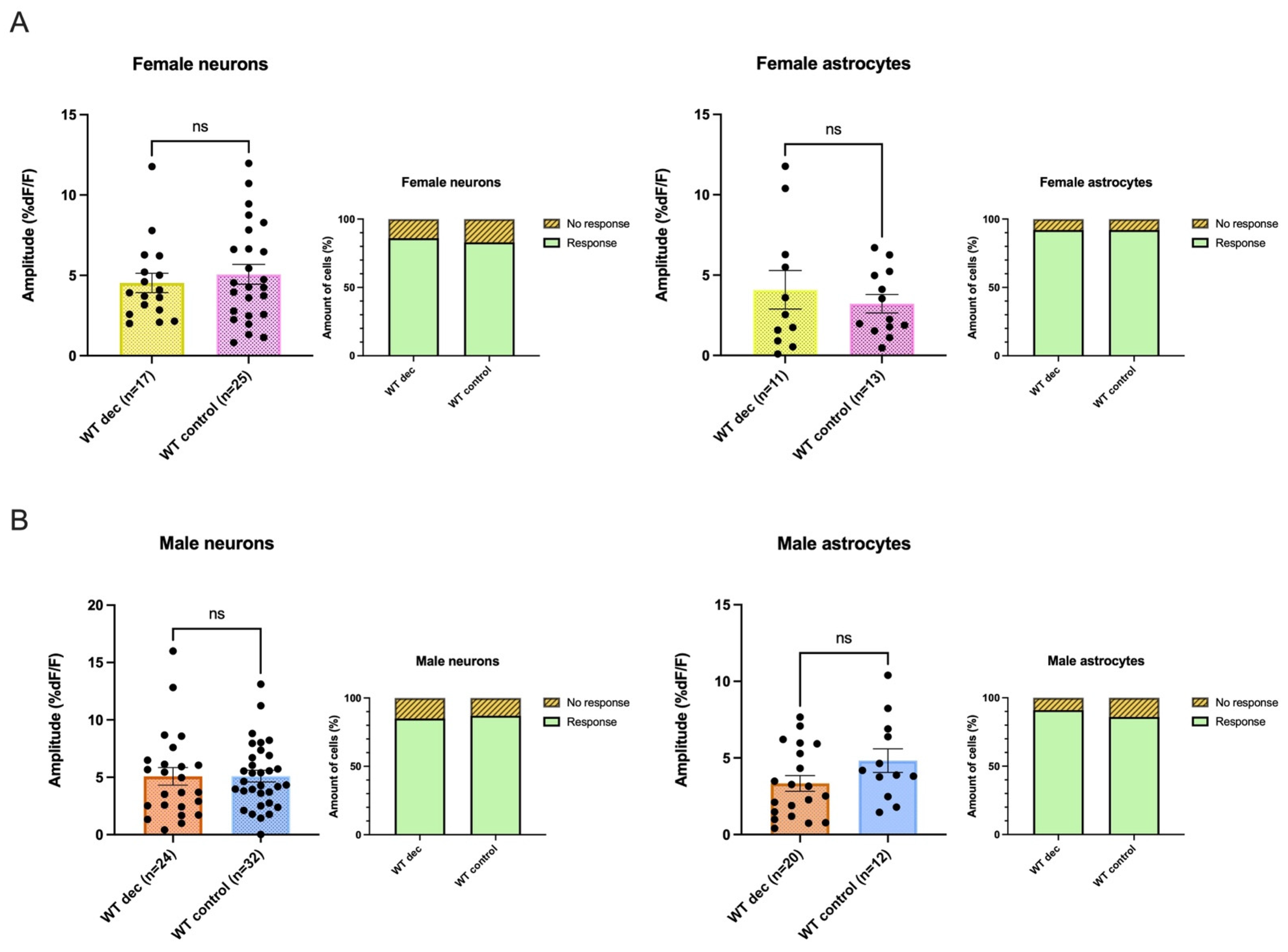
3.8. Decanoic Acid Pre-Treatment Does Not Result in Significantly Heightened Calcium Responses in WT Astrocytes and Neurons in Male and Females
3.9. There Was No Significant Difference between 5xFAD AMPA Responses following Decanoic Acid Exposure When Compared to WT in Females or in Males, Suggesting That Decanoic Acid Rescues Altered Calcium Responses in 5xFAD Neurons and Astrocytes in Both Females and Males
4. Discussion
4.1. Summary of Data
4.2. AMPA Mediated Transients Are Lower in Both Female and Male 5xFAD
4.3. Decanoic Acid Restores the Amplitude of AMPA-Mediated Transients to WT Levels
4.4. There Were No Sex-Differences in AMPA-Mediated Transients in the 5xFAD Mouse, and Dec Showed a Sex-Independent Effect on AMPA-Transients
4.5. Caveats
4.6. Conclusions and Significance
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guan, R.; Wen, X.; Liang, Y.; Xu, D.; He, B.; Feng, X. Trends in Alzheimer’s Disease Research Based upon Machine Learning Analysis of PubMed Abstracts. Int. J. Biol. Sci. 2019, 15, 2065–2074. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.L.; Rivara, C.B.; Rocher, A.B.; Hof, P.R. The nature and effects of cortical microvascular pathology in aging and Alzheimer’s disease. Neurol. Res. 2004, 26, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Barnes, L.L.; Wilson, R.S.; Bienias, J.L.; Schneider, J.A.; Evans, D.A.; Bennett, D.A. Sex differences in the clinical manifestations of Alzheimer disease pathology. Arch. Gen. Psychiatry 2005, 62, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Dumitrescu, L.; Barnes, L.L.; Thambisetty, M.; Beecham, G.; Kunkle, B.; Bush, W.S.; Gifford, K.A.; Chibnik, L.B.; Mukherjee, S.; De Jager, P.L.; et al. Sex differences in the genetic predictors of Alzheimer’s pathology. Brain 2019, 142, 2581–2589. [Google Scholar] [CrossRef]
- Chene, G.; Beiser, A.; Au, R.; Preis, S.R.; Wolf, P.A.; Dufouil, C.; Seshadri, S. Gender and incidence of dementia in the Framingham Heart Study from mid-adult life. Alzheimers Dement. 2015, 11, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Bird, T.D. Alzheimer Disease Overview [Internet]. Available online: https://www.ncbi.nlm.nih.gov/books/NBK1161/-Alzheimer.Clinical_Characteristics_of_Al (accessed on 23 September 2023).
- Tanzi, R.E.; Bertram, L. Twenty years of the Alzheimer’s disease amyloid hypothesis: A genetic perspective. Cell 2005, 120, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Oakley, H.; Cole, S.L.; Logan, S.; Maus, E.; Shao, P.; Craft, J.; Guillozet-Bongaarts, A.; Ohno, M.; Disterhoft, J.; Van Eldik, L.; et al. Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: Potential factors in amyloid plaque formation. J. Neurosci. 2006, 26, 10129–10140. [Google Scholar] [CrossRef] [PubMed]
- Glenner, G.G.; Wong, C.W. Alzheimer’s disease and Down’s syndrome: Sharing of a unique cerebrovascular amyloid fibril protein. Biochem. Biophys. Res. Commun. 1984, 122, 1131–1135. [Google Scholar] [CrossRef] [PubMed]
- Glenner, G.G.; Wong, C.W.; Quaranta, V.; Eanes, E.D. The amyloid deposits in Alzheimer’s disease: Their nature and pathogenesis. Appl. Pathol. 1984, 2, 357–369. [Google Scholar]
- Stahl, S.M. Dementia and It’s Treatment; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- De Leon, M.J.; Ferris, S.H.; George, A.E.; Christman, D.R.; Fowler, J.S.; Gentes, C.; Reisberg, B.; Gee, B.; Emmerich, M.; Yonekura, Y.; et al. Positron emission tomographic studies of aging and Alzheimer disease. Am. J. Neuroradiol. 1983, 4, 568–571. [Google Scholar]
- Cunnane, S.C.; Courchesne-Loyer, A.; Vandenberghe, C.; St-Pierre, V.; Fortier, M.; Hennebelle, M.; Croteau, E.; Bocti, C.; Fulop, T.; Castellano, C.A. Can Ketones Help Rescue Brain Fuel Supply in Later Life? Implications for Cognitive Health during Aging and the Treatment of Alzheimer’s Disease. Front. Mol. Neurosci. 2016, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Mosconi, L.; Brys, M.; Glodzik-Sobanska, L.; De Santi, S.; Rusinek, H.; de Leon, M.J. Early detection of Alzheimer’s disease using neuroimaging. Exp. Gerontol. 2007, 42, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Mosconi, L.; De Santi, S.; Rusinek, H.; Convit, A.; de Leon, M.J. Magnetic resonance and PET studies in the early diagnosis of Alzheimer’s disease. Expert Rev. Neurother. 2004, 4, 831–849. [Google Scholar] [CrossRef] [PubMed]
- Small, G.W.; Ercoli, L.M.; Silverman, D.H.; Huang, S.C.; Komo, S.; Bookheimer, S.Y.; Lavretsky, H.; Miller, K.; Siddarth, P.; Rasgon, N.L.; et al. Cerebral metabolic and cognitive decline in persons at genetic risk for Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2000, 97, 6037–6042. [Google Scholar] [CrossRef] [PubMed]
- Gordon, B.A.; Blazey, T.M.; Su, Y.; Hari-Raj, A.; Dincer, A.; Flores, S.; Christensen, J.; McDade, E.; Wang, G.; Xiong, C.; et al. Spatial patterns of neuroimaging biomarker change in individuals from families with autosomal dominant Alzheimer’s disease: A longitudinal study. Lancet Neurol. 2018, 17, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Kyrtata, N.; Emsley, H.C.A.; Sparasci, O.; Parkes, L.M.; Dickie, B.R. A Systematic Review of Glucose Transport Alterations in Alzheimer’s Disease. Front. Neurosci. 2021, 15, 626636. [Google Scholar] [CrossRef] [PubMed]
- Kashiwaya, Y.; Bergman, C.; Lee, J.H.; Wan, R.; King, M.T.; Mughal, M.R.; Okun, E.; Clarke, K.; Mattson, M.P.; Veech, R.L. A ketone ester diet exhibits anxiolytic and cognition-sparing properties, and lessens amyloid and tau pathologies in a mouse model of Alzheimer’s disease. Neurobiol. Aging 2013, 34, 1530–1539. [Google Scholar] [CrossRef]
- Mamelak, M. Energy and the Alzheimer brain. Neurosci. Biobehav. Rev. 2017, 75, 297–313. [Google Scholar] [CrossRef]
- Cunnane, S.C.; Trushina, E.; Morland, C.; Prigione, A.; Casadesus, G.; Andrews, Z.B.; Beal, M.F.; Bergersen, L.H.; Brinton, R.D.; de la Monte, S.; et al. Brain energy rescue: An emerging therapeutic concept for neurodegenerative disorders of ageing. Nat. Rev. Drug Discov. 2020, 19, 609–633. [Google Scholar] [CrossRef]
- Solon-Biet, S.M.; McMahon, A.C.; Ballard, J.W.; Ruohonen, K.; Wu, L.E.; Cogger, V.C.; Warren, A.; Huang, X.; Pichaud, N.; Melvin, R.G.; et al. The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-fed mice. Cell Metab. 2014, 19, 418–430. [Google Scholar] [CrossRef]
- Wahl, D.; Cogger, V.C.; Solon-Biet, S.M.; Waern, R.V.; Gokarn, R.; Pulpitel, T.; Cabo, R.; Mattson, M.P.; Raubenheimer, D.; Simpson, S.J.; et al. Nutritional strategies to optimise cognitive function in the aging brain. Ageing Res. Rev. 2016, 31, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Rusek, M.; Pluta, R.; Ulamek-Koziol, M.; Czuczwar, S.J. Ketogenic Diet in Alzheimer’s Disease. Int. J. Mol. Sci. 2019, 20, 3892. [Google Scholar] [CrossRef] [PubMed]
- St-Pierre, V.; Vandenberghe, C.; Lowry, C.M.; Fortier, M.; Castellano, C.A.; Wagner, R.; Cunnane, S.C. Plasma Ketone and Medium Chain Fatty Acid Response in Humans Consuming Different Medium Chain Triglycerides During a Metabolic Study Day. Front. Nutr. 2019, 6, 46. [Google Scholar] [CrossRef] [PubMed]
- Augustin, K.; Khabbush, A.; Williams, S.; Eaton, S.; Orford, M.; Cross, J.H.; Heales, S.J.R.; Walker, M.C.; Williams, R.S.B. Mechanisms of action for the medium-chain triglyceride ketogenic diet in neurological and metabolic disorders. Lancet Neurol. 2018, 17, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.V.; Westi, E.W.; Jakobsen, E.; Urruticoechea, N.; Borges, K.; Aldana, B.I. Astrocyte metabolism of the medium-chain fatty acids octanoic acid and decanoic acid promotes GABA synthesis in neurons via elevated glutamine supply. Mol. Brain 2021, 14, 132. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Rai, R.; Singh, D.; Vohora, D. Octanoic acid a major component of widely consumed medium-chain triglyceride ketogenic diet is detrimental to bone. Sci. Rep. 2021, 11, 7003. [Google Scholar] [CrossRef] [PubMed]
- Mett, J. The Impact of Medium Chain and Polyunsaturated omega-3-Fatty Acids on Amyloid-beta Deposition, Oxidative Stress and Metabolic Dysfunction Associated with Alzheimer’s Disease. Antioxidants 2021, 10, 1991. [Google Scholar] [CrossRef]
- Manji, Z.; Rojas, A.; Wang, W.; Dingledine, R.; Varvel, N.H.; Ganesh, T. 5xFAD Mice Display Sex-Dependent Inflammatory Gene Induction During the Prodromal Stage of Alzheimer’s Disease. J. Alzheimers Dis. 2019, 70, 1259–1274. [Google Scholar] [CrossRef]
- Kafitz, K.W.; Meier, S.D.; Stephan, J.; Rose, C.R. Developmental profile and properties of sulforhodamine 101--Labeled glial cells in acute brain slices of rat hippocampus. J. Neurosci. Methods 2008, 169, 84–92. [Google Scholar] [CrossRef]
- Hauberg, K.; Kohlmeier, K.A. The appetite-inducing peptide, ghrelin, induces intracellular store-mediated rises in calcium in addiction and arousal-related laterodorsal tegmental neurons in mouse brain slices. Peptides 2015, 65, 34–45. [Google Scholar] [CrossRef]
- Franklin, K.B.J.; Paxinos, G. Paxinos and Franklin’s the Mouse Brain in Sterotaxic Coordinates, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Andersen, J.V.; Skotte, N.H.; Christensen, S.K.; Polli, F.S.; Shabani, M.; Markussen, K.H.; Haukedal, H.; Westi, E.W.; Diaz-delCastillo, M.; Sun, R.C.; et al. Hippocampal disruptions of synaptic and astrocyte metabolism are primary events of early amyloid pathology in the 5xFAD mouse model of Alzheimer’s disease. Cell Death Dis. 2021, 12, 954. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J.; Lipp, P.; Bootman, M.D. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 2000, 1, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J.; Lipp, P.; Bootman, M.D. Signal transduction. The calcium entry pas de deux. Science 2000, 287, 1604–1605. [Google Scholar] [CrossRef] [PubMed]
- Babaei, P. NMDA and AMPA receptors dysregulation in Alzheimer’s disease. Eur. J. Pharmacol. 2021, 908, 174310. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.; Mehrian Shai, R.; Wu, Y.; Hsu, Y.H.; Sitzer, T.; Spann, B.; McCleary, C.; Mo, Y.; Miller, C.A. Transcriptome analysis of synaptoneurosomes identifies neuroplasticity genes overexpressed in incipient Alzheimer’s disease. PLoS ONE 2009, 4, e4936. [Google Scholar] [CrossRef] [PubMed]
- Tu, S.; Okamoto, S.; Lipton, S.A.; Xu, H. Oligomeric Abeta-induced synaptic dysfunction in Alzheimer’s disease. Mol. Neurodegener. 2014, 9, 48. [Google Scholar] [CrossRef]
- Liu, S.J.; Gasperini, R.; Foa, L.; Small, D.H. Amyloid-beta decreases cell-surface AMPA receptors by increasing intracellular calcium and phosphorylation of GluR2. J. Alzheimers. Dis. 2010, 21, 655–666. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, J.; Sun, S.; Pchitskaya, E.; Popugaeva, E.; Bezprozvanny, I. Calcium signaling, excitability, and synaptic plasticity defects in a mouse model of Alzheimer’s disease. J. Alzheimers. Dis. 2015, 45, 561–580. [Google Scholar] [CrossRef]
- Keifer, J.; Zheng, Z. AMPA receptor trafficking and learning. Eur. J. Neurosci. 2010, 32, 269–277. [Google Scholar] [CrossRef]
- Malenka, R.C.; Bear, M.F. LTP and LTD: An embarrassment of riches. Neuron 2004, 44, 5–21. [Google Scholar] [CrossRef]
- Tanaka, H.; Sakaguchi, D.; Hirano, T. Amyloid-beta oligomers suppress subunit-specific glutamate receptor increase during LTP. Alz Dement. 2019, 5, 797–808. [Google Scholar] [CrossRef]
- Terashima, A.; Suh, Y.H.; Isaac, J.T.R. The AMPA Receptor Subunit GluA1 is Required for CA1 Hippocampal Long-Term Potentiation but is not Essential for Synaptic Transmission. Neurochem. Res. 2019, 44, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Gavello, D.; Calorio, C.; Franchino, C.; Cesano, F.; Carabelli, V.; Carbone, E.; Marcantoni, A. Early Alterations of Hippocampal Neuronal Firing Induced by Abeta42. Cereb. Cortex 2018, 28, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Marcantoni, A.; Cerullo, M.S.; Buxeda, P.; Tomagra, G.; Giustetto, M.; Chiantia, G.; Carabelli, V.; Carbone, E. Amyloid Beta42 oligomers up-regulate the excitatory synapses by potentiating presynaptic release while impairing postsynaptic NMDA receptors. J. Physiol. 2020, 598, 2183–2197. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, J.; Sidoryk-Wegrzynowicz, M.; Zielinska, M.; Aschner, M. Roles of glutamine in neurotransmission. Neuron Glia Biol. 2010, 6, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, E.; Hansen, S.V.; Urban, C.; Hudson, B.D.; Wargent, E.T.; Grundmann, M.; Jenkins, L.; Zaibi, M.; Stocker, C.J.; Ullrich, S.; et al. Discovery of TUG-770: A Highly Potent Free Fatty Acid Receptor 1 (FFA1/GPR40) Agonist for Treatment of Type 2 Diabetes. ACS Med. Chem. Lett. 2013, 4, 441–445. [Google Scholar] [CrossRef]
- Ma, D.; Lu, L.; Boneva, N.B.; Warashina, S.; Kaplamadzhiev, D.B.; Mori, Y.; Nakaya, M.A.; Kikuchi, M.; Tonchev, A.B.; Okano, H.; et al. Expression of free fatty acid receptor GPR40 in the neurogenic niche of adult monkey hippocampus. Hippocampus 2008, 18, 326–333. [Google Scholar] [CrossRef]
- Warren, E.C.; Dooves, S.; Lugara, E.; Damstra-Oddy, J.; Schaf, J.; Heine, V.M.; Walker, M.C.; Williams, R.S.B. Decanoic acid inhibits mTORC1 activity independent of glucose and insulin signaling. Proc. Natl. Acad. Sci. USA 2020, 117, 23617–23625. [Google Scholar] [CrossRef]
- Chen, K.T.; Tsai, M.H.; Wu, C.H.; Jou, M.J.; Wei, I.H.; Huang, C.C. AMPA Receptor-mTOR Activation is Required for the Antidepressant-Like Effects of Sarcosine during the Forced Swim Test in Rats: Insertion of AMPA Receptor may Play a Role. Front. Behav. Neurosci. 2015, 9, 162. [Google Scholar] [CrossRef]
- Jahrling, J.B.; Laberge, R.M. Age-Related Neurodegeneration Prevention Through mTOR Inhibition: Potential Mechanisms and Remaining Questions. Curr. Top. Med. Chem. 2015, 15, 2139–2151. [Google Scholar] [CrossRef]
- Wei, Z.; Lin, B.J.; Chen, T.W.; Daie, K.; Svoboda, K.; Druckmann, S. A comparison of neuronal population dynamics measured with calcium imaging and electrophysiology. PLoS Comput. Biol. 2020, 16, e1008198. [Google Scholar] [CrossRef] [PubMed]
- Garland, E.F.; Hartnell, I.J.; Boche, D. Microglia and Astrocyte Function and Communication: What Do We Know in Humans? Front. Neurosci. 2022, 16, 824888. [Google Scholar] [CrossRef] [PubMed]
- Dore, K.; Carrico, Z.; Alfonso, S.; Marino, M.; Koymans, K.; Kessels, H.W.; Malinow, R. PSD-95 protects synapses from β-amyloid. Cell Rep. 2021, 35, 109194. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.; Augustin, K.; Boddum, K.; Williams, S.; Sun, M.; Terschak, J.A.; Hardege, J.D.; Chen, P.E.; Walker, M.C.; Williams, R.S. Seizure control by decanoic acid through direct AMPA receptor inhibition. Brain 2016, 139, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Sada, N.; Inoue, T. Electrical Control in Neurons by the Ketogenic Diet. Front. Cell. Neurosci. 2018, 12, 208. [Google Scholar] [CrossRef]
- Launer, L.J.; Andersen, K.; Dewey, M.E.; Letenneur, L.; Ott, A.; Amaducci, L.A.; Brayne, C.; Copeland, J.R.; Dartigues, J.F.; Kragh-Sorensen, P.; et al. Rates and risk factors for dementia and Alzheimer’s disease: Results from EURODEM pooled analyses. EURODEM Incidence Research Group and Work Groups. European Studies of Dementia. Neurology 1999, 52, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Mielke, M.M.; Vemuri, P.; Rocca, W.A. Clinical epidemiology of Alzheimer’s disease: Assessing sex and gender differences. Clin. Epidemiol. 2014, 6, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Rocca, W.A.; Cha, R.H.; Waring, S.C.; Kokmen, E. Incidence of dementia and Alzheimer’s disease: A reanalysis of data from Rochester, Minnesota, 1975-1984. Am. J. Epidemiol. 1998, 148, 51–62. [Google Scholar] [CrossRef]
- Wickens, M.M.; Bangasser, D.A.; Briand, L.A. Sex Differences in Psychiatric Disease: A Focus on the Glutamate System. Front. Mol. Neurosci. 2018, 11, 197. [Google Scholar] [CrossRef]
- Santos-Galindo, M.; Acaz-Fonseca, E.; Bellini, M.J.; Garcia-Segura, L.M. Sex differences in the inflammatory response of primary astrocytes to lipopolysaccharide. Biol. Sex Differ. 2011, 2, 7. [Google Scholar] [CrossRef]
- Morizawa, Y.; Sato, K.; Takaki, J.; Kawasaki, A.; Shibata, K.; Suzuki, T.; Ohta, S.; Koizumi, S. Cell-autonomous enhancement of glutamate-uptake by female astrocytes. Cell. Mol. Neurobiol. 2012, 32, 953–956. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Singh, M. Sex differences in cognitive impairment and Alzheimer’s disease. Front. Neuroendocrinol. 2014, 35, 385–403. [Google Scholar] [CrossRef] [PubMed]
- Merlo, S.; Spampinato, S.F.; Sortino, M.A. Early compensatory responses against neuronal injury: A new therapeutic window of opportunity for Alzheimer’s Disease? CNS Neurosci. Ther. 2019, 25, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Maarouf, C.L.; Kokjohn, T.A.; Whiteside, C.M.; Macias, M.P.; Kalback, W.M.; Sabbagh, M.N.; Beach, T.G.; Vassar, R.; Roher, A.E. Molecular Differences and Similarities Between Alzheimer’s Disease and the 5XFAD Transgenic Mouse Model of Amyloidosis. Biochem. Insights 2013, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Placanica, L.; Zhu, L.; Li, Y.M. Gender- and age-dependent gamma-secretase activity in mouse brain and its implication in sporadic Alzheimer disease. PLoS ONE 2009, 4, e5088. [Google Scholar] [CrossRef] [PubMed]
- Eckman, C.B.; Mehta, N.D.; Crook, R.; Perez-tur, J.; Prihar, G.; Pfeiffer, E.; Graff-Radford, N.; Hinder, P.; Yager, D.; Zenk, B.; et al. A new pathogenic mutation in the APP gene (I716V) increases the relative proportion of A beta 42(43). Hum. Mol. Genet. 1997, 6, 2087–2089. [Google Scholar] [CrossRef] [PubMed]
- Citron, M.; Eckman, C.B.; Diehl, T.S.; Corcoran, C.; Ostaszewski, B.L.; Xia, W.; Levesque, G.; St George Hyslop, P.; Younkin, S.G.; Selkoe, D.J. Additive effects of PS1 and APP mutations on secretion of the 42-residue amyloid beta-protein. Neurobiol. Dis. 1998, 5, 107–116. [Google Scholar] [CrossRef]
- Drummond, E.; Wisniewski, T. Alzheimer’s disease: Experimental models and reality. Acta Neuropathol. 2017, 133, 155–175. [Google Scholar] [CrossRef]
- Jack, C.R., Jr.; Wiste, H.J.; Lesnick, T.G.; Weigand, S.D.; Knopman, D.S.; Vemuri, P.; Pankratz, V.S.; Senjem, M.L.; Gunter, J.L.; Mielke, M.M.; et al. Brain beta-amyloid load approaches a plateau. Neurology 2013, 80, 890–896. [Google Scholar] [CrossRef]
- Tada, H.; Koide, M.; Ara, W.; Shibata, Y.; Funabashi, T.; Suyama, K.; Goto, T.; Takahashi, T. Estrous Cycle-Dependent Phasic Changes in the Stoichiometry of Hippocampal Synaptic AMPA Receptors in Rats. PLoS ONE 2015, 10, e0131359. [Google Scholar] [CrossRef]
- Palomero-Gallagher, N.; Bidmon, H.J.; Zilles, K. AMPA, kainate, and NMDA receptor densities in the hippocampus of untreated male rats and females in estrus and diestrus. J. Comp. Neurol. 2003, 459, 468–474. [Google Scholar] [CrossRef]
- Warren, S.G.; Humphreys, A.G.; Juraska, J.M.; Greenough, W.T. LTP varies across the estrous cycle: Enhanced synaptic plasticity in proestrus rats. Brain Res. 1995, 703, 26–30. [Google Scholar] [CrossRef]
- Yagi, S.; Galea, L.A.M. Sex differences in hippocampal cognition and neurogenesis. Neuropsychopharmacology 2019, 44, 200–213. [Google Scholar] [CrossRef]


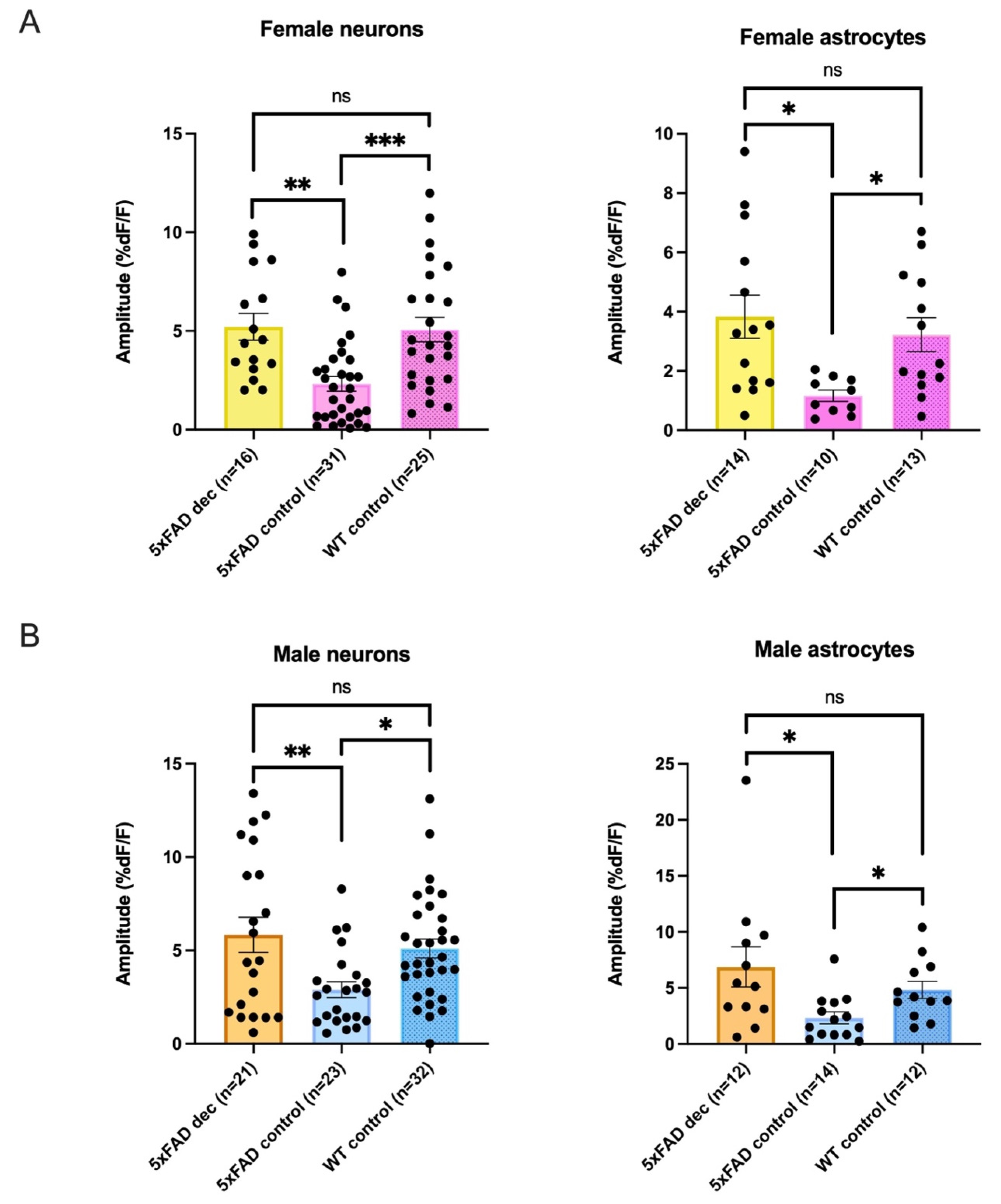
| Female Average Amplitudes %DF/F | ||||
|---|---|---|---|---|
| Neurons | 5xFADDec | 5xFADControl: | WTControl | Kruskal-Wallis |
| 5.21 ± 0.7 %DF/F n = 16 | 2.32 ± 0.4 %DF/F n = 31 | 5.07 ± 0.6 %DF/F n = 25 | p = 0.0001 | |
| Astrocytes * | 3.83 ± 0.7 %DF/F n = 14 | 1.17 ± 0.2 %DF/F n = 10 | 3.22 ± 0.6 %DF/F n = 13 | p = 0.0071 |
| Male Average Amplitudes %DF/F | ||||
| Neurons | 5xFADDec | 5xFADControl: | WTControl | Kruskal-Wallis |
| 5.84 ± 0.9 %DF/F n = 21 | 2.90 ± 0.4 %DF/F n = 23 | 5.10 ± 0.5 %DF/F n = 32 | p = 0.0066 | |
| Astrocytes * | 6.87 ± 1.8 %DF/F n = 12 | 2.34 ± 0.5 %DF/F n = 14 | 4.83 ± 0.8 %DF/F n = 12 | p = 0.0108 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abghari, M.; Vu, J.T.C.M.; Eckberg, N.; Aldana, B.I.; Kohlmeier, K.A. Decanoic Acid Rescues Differences in AMPA-Mediated Calcium Rises in Hippocampal CA1 Astrocytes and Neurons in the 5xFAD Mouse Model of Alzheimer’s Disease. Biomolecules 2023, 13, 1461. https://doi.org/10.3390/biom13101461
Abghari M, Vu JTCM, Eckberg N, Aldana BI, Kohlmeier KA. Decanoic Acid Rescues Differences in AMPA-Mediated Calcium Rises in Hippocampal CA1 Astrocytes and Neurons in the 5xFAD Mouse Model of Alzheimer’s Disease. Biomolecules. 2023; 13(10):1461. https://doi.org/10.3390/biom13101461
Chicago/Turabian StyleAbghari, Mina, Jenny Thythy Cecilia Mai Vu, Ninna Eckberg, Blanca I. Aldana, and Kristi A. Kohlmeier. 2023. "Decanoic Acid Rescues Differences in AMPA-Mediated Calcium Rises in Hippocampal CA1 Astrocytes and Neurons in the 5xFAD Mouse Model of Alzheimer’s Disease" Biomolecules 13, no. 10: 1461. https://doi.org/10.3390/biom13101461
APA StyleAbghari, M., Vu, J. T. C. M., Eckberg, N., Aldana, B. I., & Kohlmeier, K. A. (2023). Decanoic Acid Rescues Differences in AMPA-Mediated Calcium Rises in Hippocampal CA1 Astrocytes and Neurons in the 5xFAD Mouse Model of Alzheimer’s Disease. Biomolecules, 13(10), 1461. https://doi.org/10.3390/biom13101461







