New Insights on Sperm Function in Male Infertility of Unknown Origin: A Multimodal Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
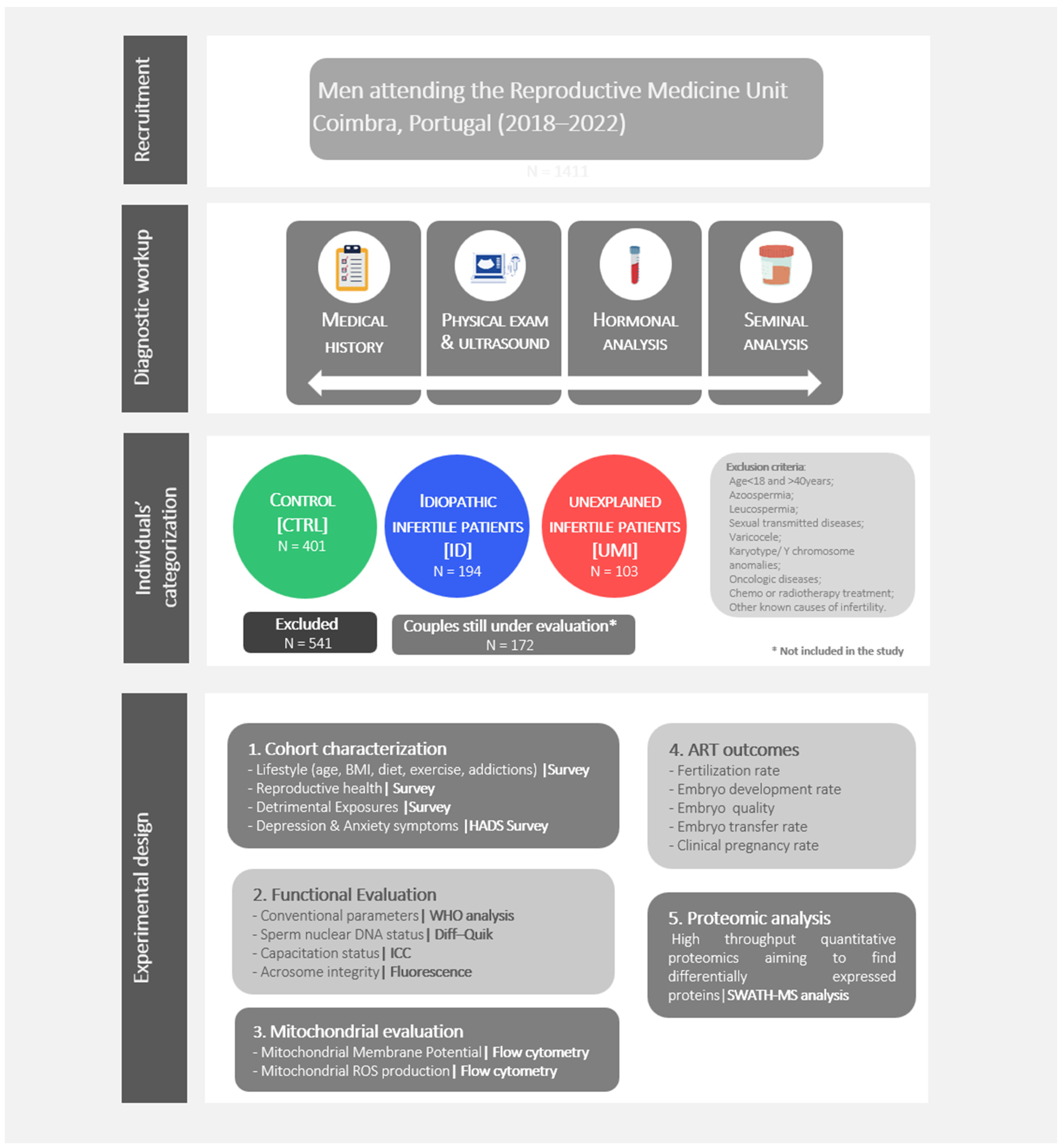
2.2. Individuals’ Recruitment, Diagnostic Work up and Categorization
Semen Samples Collection and Processing
2.3. Cohort Characterization: Socio-Demographic Data, Lifestyle, Reproductive Health, Exposures to Detrimental Agents and Psychological Health
2.3.1. Sperm Function Evaluation
Motility and Viability
Sperm Morphology and Chromatin Status
Capacitation Status: Assessment of Tyrosine Phosphorylation
Acrosomal Integrity
2.3.2. Sperm Mitochondrial Functionality Evaluation
Sperm Mitochondrial Membrane Potential
Sperm Mitochondrial Superoxide Production
2.3.3. Fertility Outcomes
2.3.4. Sperm Proteomics
Protein Solubilization and Digestion
Protein Identification and Quantification
2.3.5. Statistical Analysis
3. Results
3.1. Samples Categorization in the 3 Study Groups
3.2. Cohort Characterization—Lifestyle, Reproductive Health, Occupational Exposures, and Psychological Health
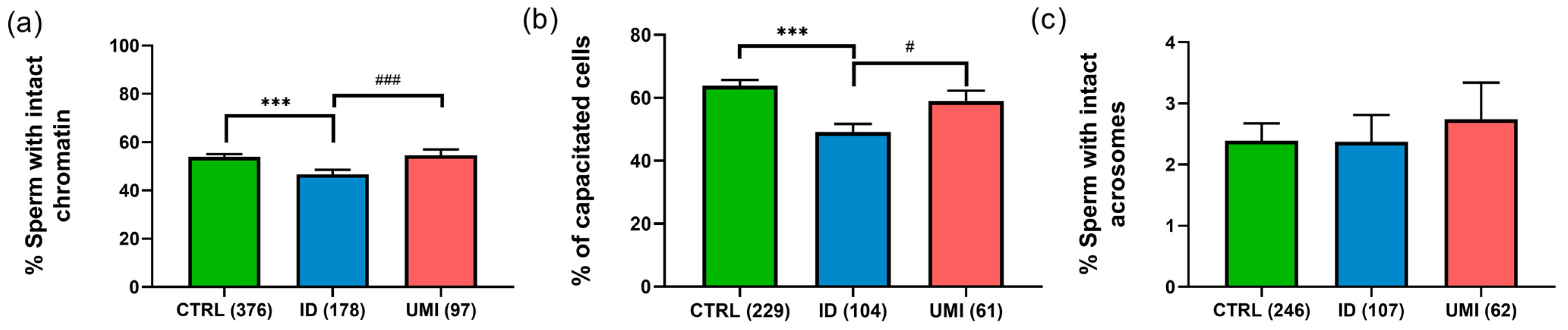
3.3. Functional Sperm Parameters
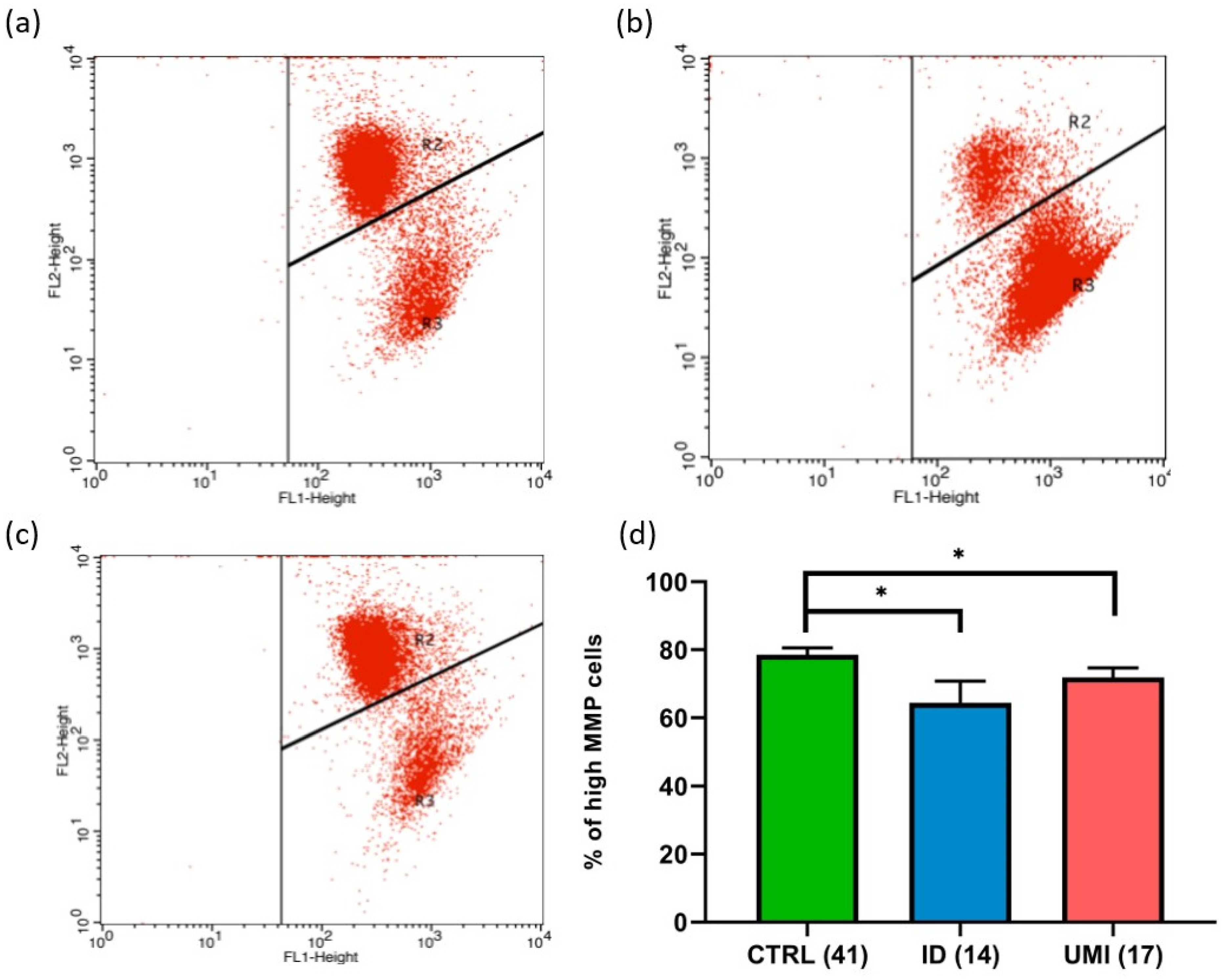
3.4. Sperm Mitochondrial Functionality
3.5. Fertility Outcomes
3.6. Correlations
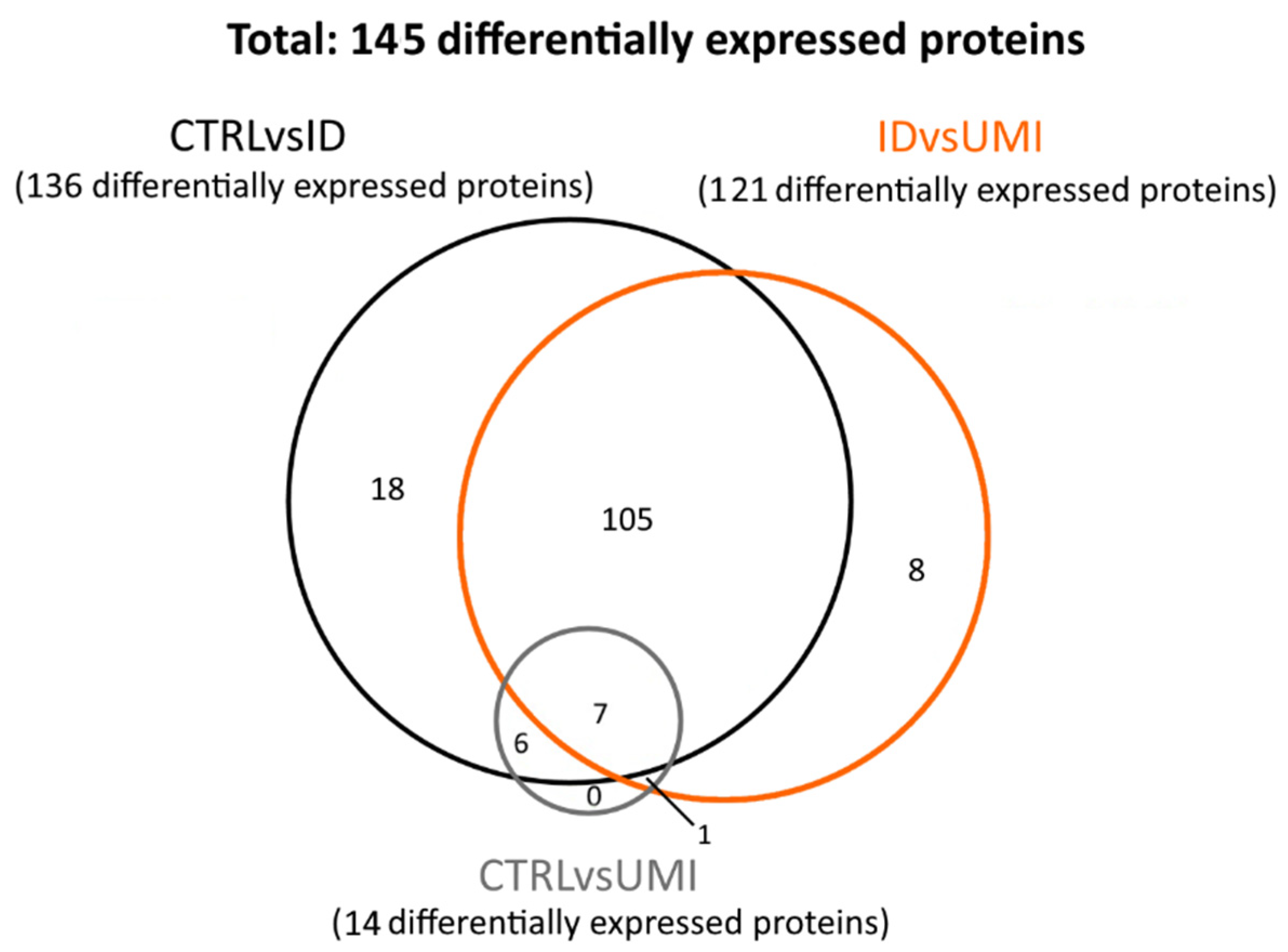
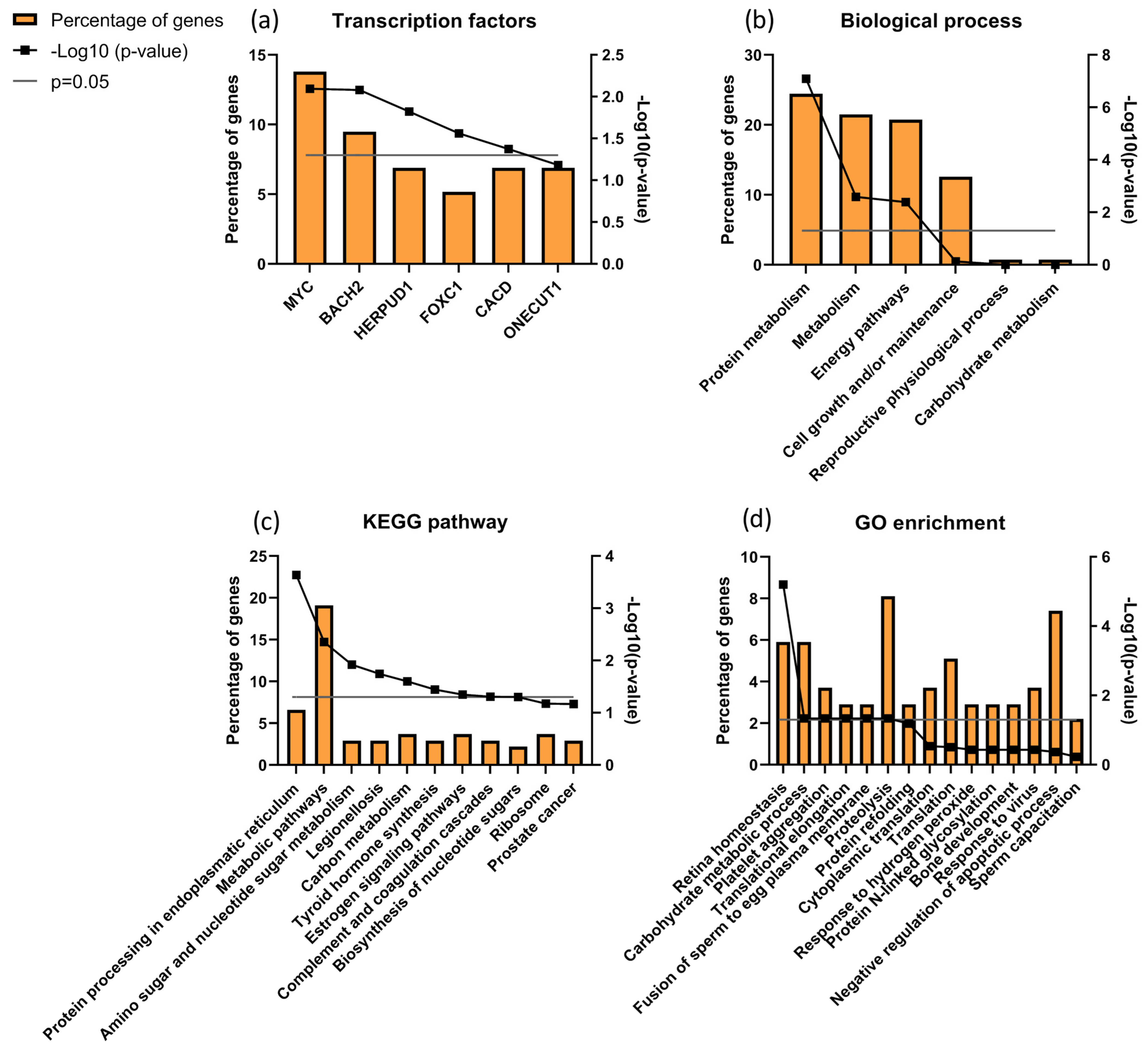
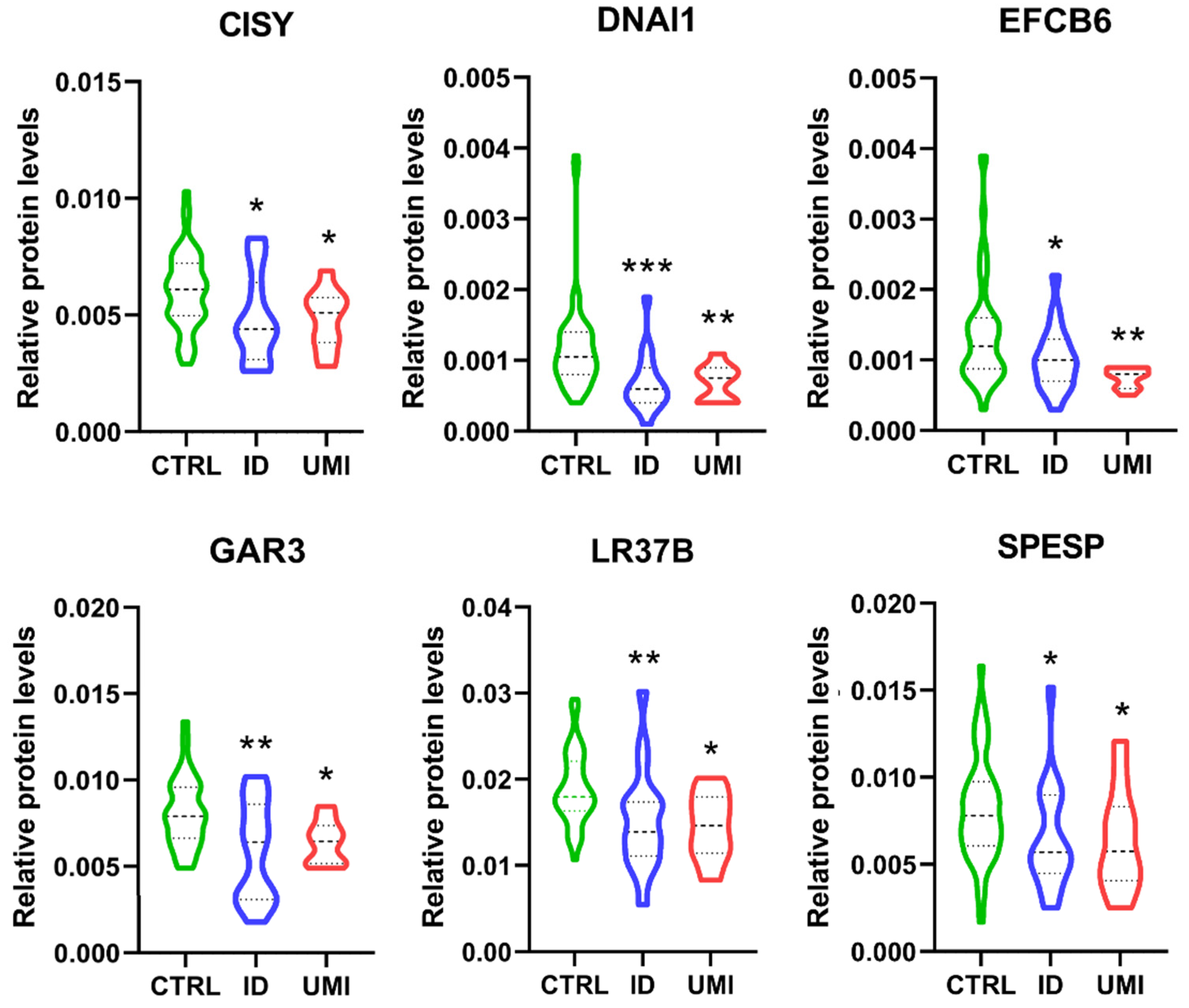
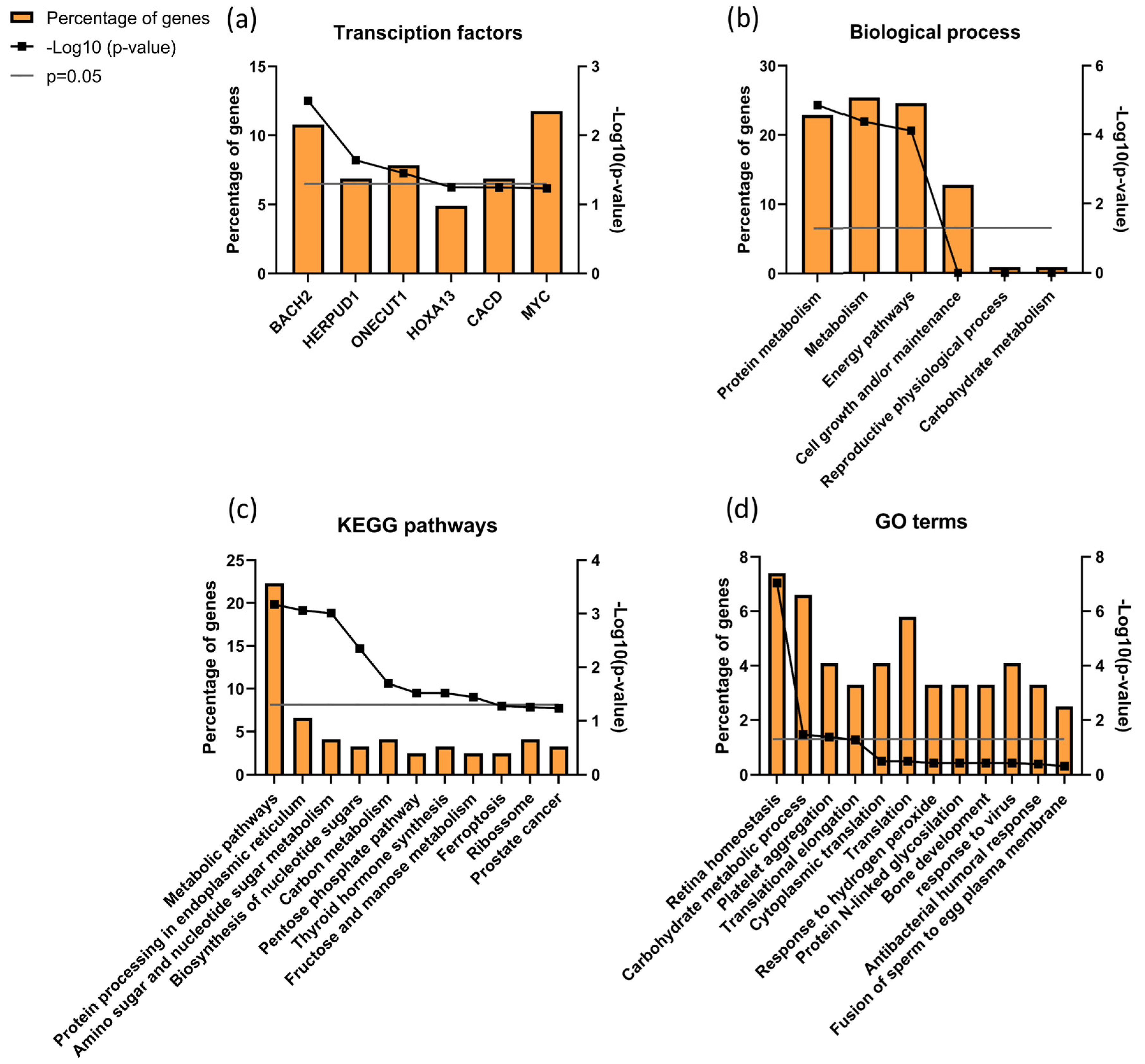
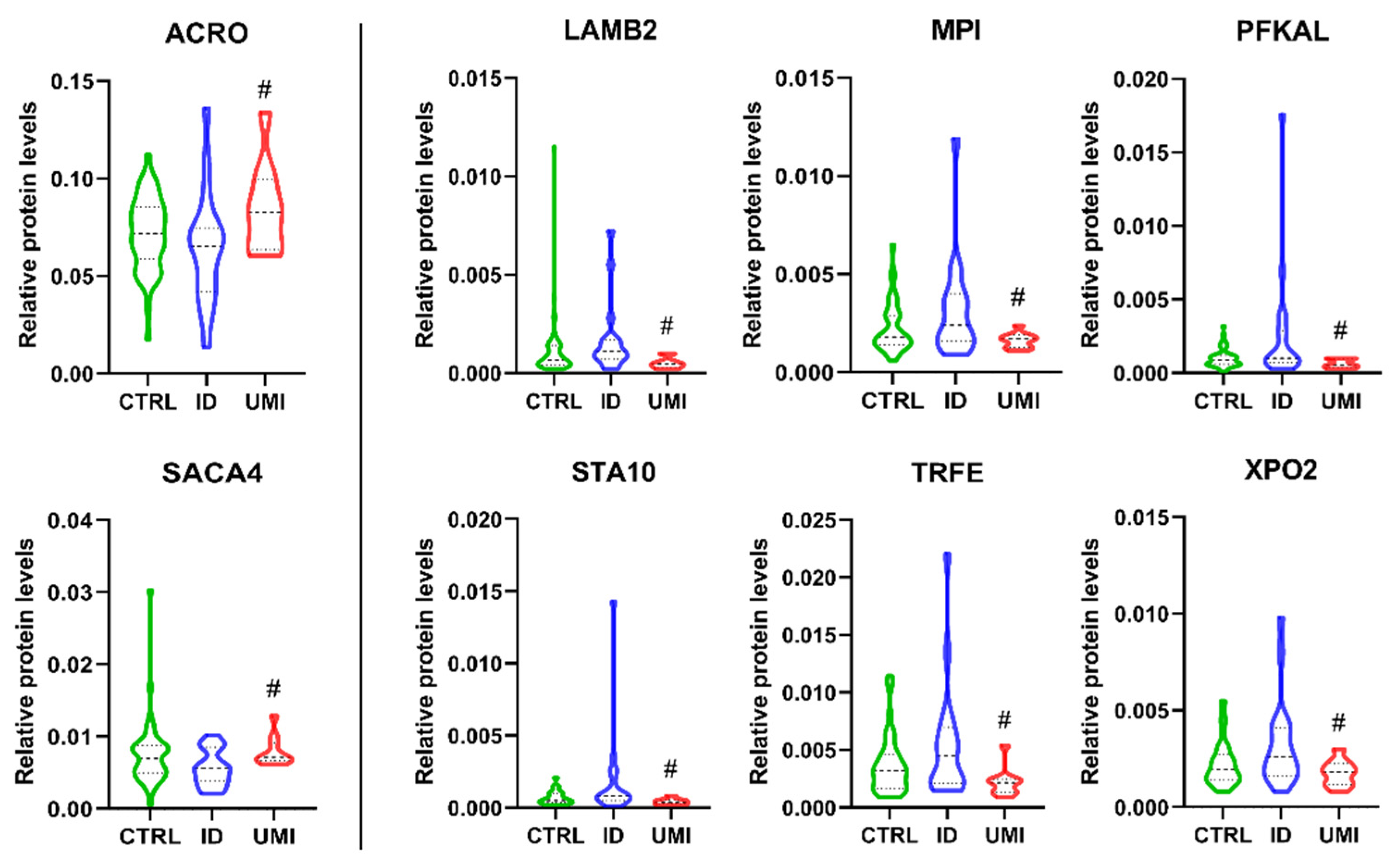
3.7. Sperm Proteomics
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salonia, A.; Bettocchi, C.; Capogrosso, P.; Carvalho, J.; Corona, G.; Hatzichristodoulou, G.; Jones, T.H.; Kadioglu, A.; Martinez-Salamanca, J.I.; Minhas, S.; et al. EAU Guidelines. In Proceedings of the EAU Annual Congress, Milan, Italy, 10–13 March 2023. [Google Scholar]
- Dyer, S.J.; Patel, M. The economic impact of infertility on women in developing countries—A systematic review. Facts Views Vis. Obgyn. 2012, 4, 102–109. [Google Scholar]
- Kawwass, J.F.; Penzias, A.S.; Adashi, E.Y. Fertility-a human right worthy of mandated insurance coverage: The evolution, limitations, and future of access to care. Fertil. Steril. 2021, 115, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Dooley, M.; Dineen, T.; Sarma, K.; Nolan, A. The psychological impact of infertility and fertility treatment on the male partner. Hum. Fertil. 2014, 17, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Mulgund, A.; Hamada, A.; Chyatte, M.R. A unique view on male infertility around the globe. Reprod. Biol. Endocrinol. 2015, 13, 37. [Google Scholar] [CrossRef] [PubMed]
- Rusz, A.; Pilatz, A.; Wagenlehner, F.; Linn, T.; Diemer, T.; Schuppe, H.; Lohmeyer, J.; Hossain, H.; Weidner, W. Influence of urogenital infections and inflammation on semen quality and male fertility. World J. Urol. 2012, 30, 23–30. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E.; Bourguignon, J.-P.; Giudice, L.C.; Hauser, R.; Prins, G.S.; Soto, A.M.; Zoeller, R.T.; Gore, A.C. Endocrine-disrupting chemicals: An Endocrine Society scientific statement. Endocr. Rev. 2009, 30, 293–342. [Google Scholar] [CrossRef]
- Minhas, S.; Bettocchi, C.; Boeri, L.; Capogrosso, P.; Carvalho, J.; Cilesiz, N.C.; Cocci, A.; Corona, G.; Dimitropoulos, K.; Gül, M. European Association of Urology Guidelines on Male Sexual and Reproductive Health: 2021 Update on Male Infertility. Eur. Urol. 2021, 80, 603–620. [Google Scholar] [CrossRef]
- Pierik, F.H.; Van Ginneken, A.M.; Dohle, G.R.; Vreeburg, J.T.; Weber, R.F. The advantages of standardized evaluation of male infertility. Int. J. Androl. 2000, 23, 340–346. [Google Scholar] [CrossRef]
- Hamada, A.; Esteves, S.C.; Agarwal, A. Unexplained male infertility: Potential causes and management. Hum. Androl. 2011, 1, 2–16. [Google Scholar] [CrossRef]
- Arafa, M.; Agarwal, A.; Majzoub, A.; Panner Selvam, M.K.; Baskaran, S.; Henkel, R.; Elbardisi, H. Efficacy of antioxidant supplementation on conventional and advanced sperm function tests in patients with idiopathic male infertility. Antioxidants 2020, 9, 219. [Google Scholar] [CrossRef]
- Hamada, A.; Esteves, S.C.; Nizza, M.; Agarwal, A. Unexplained male infertility: Diagnosis and management. Int. Braz. J. Urol. 2012, 38, 576–594. [Google Scholar] [CrossRef]
- Collins, J.A.; Crosignani, P.G. Unexplained infertility: A review of diagnosis, prognosis, treatment efficacy and management. Int. J. Gynaecol. Obs. 1992, 39, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Hamada, A.; Esteves, S.C.; Agarwal, A. The role of contemporary andrology in unraveling the mystery of unexplained male infertility. Open Reprod. Sci. J. 2011, 3, 27–41. [Google Scholar]
- Amaral, A.; Castillo, J.; Ramalho-Santos, J.; Oliva, R. The combined human sperm proteome: Cellular pathways and implications for basic and clinical science. Hum. Reprod. Update 2014, 20, 40–62. [Google Scholar] [CrossRef] [PubMed]
- Moscatelli, N.; Lunetti, P.; Braccia, C.; Armirotti, A.; Pisanello, F.; De Vittorio, M.; Zara, V.; Ferramosca, A. Comparative proteomic analysis of proteins involved in bioenergetics pathways associated with human sperm motility. Int. J. Mol. Sci. 2019, 20, 3000. [Google Scholar] [CrossRef]
- Shen, S.; Wang, J.; Liang, J.; He, D. Comparative proteomic study between human normal motility sperm and idiopathic asthenozoospermia. World J. Urol. 2013, 31, 1395–1401. [Google Scholar] [CrossRef]
- Xu, W.; Hu, H.; Wang, Z.; Chen, X.; Yang, F.; Zhu, Z.; Fang, P.; Dai, J.; Wang, L.; Shi, H. Proteomic characteristics of spermatozoa in normozoospermic patients with infertility. J. Proteom. 2012, 75, 5426–5436. [Google Scholar] [CrossRef]
- Panner Selvam, M.K.; Agarwal, A.; Pushparaj, P.N.; Baskaran, S.; Bendou, H. Sperm proteome analysis and identification of fertility-associated biomarkers in unexplained male infertility. Genes 2019, 10, 522. [Google Scholar] [CrossRef]
- Panner Selvam, M.K.; Agarwal, A.; Baskaran, S. Proteomic analysis of seminal plasma from bilateral varicocele patients indicates an oxidative state and increased inflammatory response. Asian J. Androl. 2019, 21, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Kliesch, S. Diagnosis of male infertility: Diagnostic work-up of the infertile man. Eur. Urol. Suppl. 2014, 13, 73–82. [Google Scholar] [CrossRef]
- WHO Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen; WHO: Geneva, Switzerland, 2010. [Google Scholar]
- Portela, J.; Tavares, R.; Mota, P.; Ramalho-Santos, J.; Amaral, S. High glucose concentrations per se do not adversely affect human sperm function in vitro. Reproduction 2015, 150, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Pais-Ribeiro, J.; Silva, I.; Ferreira, T.; Martins, A.; Meneses, R.; Baltar, M. Validation study of a Portuguese version of the Hospital Anxiety and Depression Scale. Psychol. Health Med. 2007, 12, 225–235. [Google Scholar] [CrossRef]
- Chaves, C.; Canavarro, M.C.; Moura-Ramos, M. The Role of Dyadic Coping on the Marital and Emotional Adjustment of Couples With Infertility. Fam. Process 2019, 58, 509–523. [Google Scholar] [CrossRef] [PubMed]
- Djukanovic, I.; Carlsson, J.; Årestedt, K. Is the Hospital Anxiety and Depression Scale (HADS) a valid measure in a general population 65–80 years old? A psychometric evaluation study. Health Qual. Life Outcomes 2017, 15, 193. [Google Scholar] [CrossRef]
- Sousa, A.P.M.; Tavares, R.S.; Velez de la Calle, J.F.; Figueiredo, H.; Almeida, V.; Almeida-Santos, T.; Ramalho-Santos, J. Dual use of Diff-Quik-like stains for the simultaneous evaluation of human sperm morphology and chromatin status. Hum. Reprod. 2009, 24, 28–36. [Google Scholar] [CrossRef]
- Ramalho-Santos, J.; Amaral, A.; Sousa, A.P.; Rodrigues, A.S.; Martins, L.; Baptista, M.; Mota, P.C.; Tavares, R.; Amaral, S.; Gamboa, S. Probing the structure and function of mammalian sperm using optical and fluorescence microscopy. Mod. Res. Educ. Top. Microsc. 2007, 1, 394–402. [Google Scholar]
- Amaral, S.; Redmann, K.; Sanchez, V.; Mallidis, C.; Ramalho-Santos, J.; Schlatt, S. UVB irradiation as a tool to assess ROS-induced damage in human spermatozoa. Andrology 2013, 1, 707–714. [Google Scholar] [CrossRef]
- Sousa, M.I.; Amaral, S.; Tavares, R.S.; Paiva, C.; Ramalho-Santos, J. Concentration-dependent Sildenafil citrate (Viagra) effects on ROS production, energy status, and human sperm function. Syst. Biol. Reprod. Med. 2014, 60, 72–79. [Google Scholar] [CrossRef]
- Tavares, R.; Silva, A.; Lourenço, B.; Almeida-Santos, T.; Sousa, A.; Ramalho-Santos, J. Evaluation of human sperm chromatin status after selection using a modified Diff-Quik stain indicates embryo quality and pregnancy outcomes following in vitro fertilization. Andrology 2013, 1, 830–837. [Google Scholar] [CrossRef]
- Anjo, S.I.; Santa, C.; Manadas, B. Short GeLC-SWATH: A fast and reliable quantitative approach for proteomic screenings. Proteomics 2015, 15, 757–762. [Google Scholar] [CrossRef]
- Collins, B.C.; Gillet, L.C.; Rosenberger, G.; Röst, H.L.; Vichalkovski, A.; Gstaiger, M.; Aebersold, R. Quantifying protein interaction dynamics by SWATH mass spectrometry: Application to the 14-3-3 system. Nat. Methods 2013, 10, 1246–1253. [Google Scholar] [CrossRef] [PubMed]
- Gillet, L.C.; Navarro, P.; Tate, S.; Röst, H.; Selevsek, N.; Reiter, L.; Bonner, R.; Aebersold, R. Targeted data extraction of the MS/MS spectra generated by data-independent acquisition: A new concept for consistent and accurate proteome analysis. Mol. Cell. Proteom. 2012, 11, 016717. [Google Scholar] [CrossRef]
- Sennels, L.; Bukowski-Wills, J.-C.; Rappsilber, J. Improved results in proteomics by use of local and peptide-class specific false discovery rates. BMC Bioinform. 2009, 10, 179. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.; Shilov, I.V.; Seymour, S.L. Nonlinear fitting method for determining local false discovery rates from decoy database searches. J. Proteome Res. 2008, 7, 3661–3667. [Google Scholar] [CrossRef]
- Lambert, J.-P.; Ivosev, G.; Couzens, A.L.; Larsen, B.; Taipale, M.; Lin, Z.-Y.; Zhong, Q.; Lindquist, S.; Vidal, M.; Aebersold, R. Mapping differential interactomes by affinity purification coupled with data-independent mass spectrometry acquisition. Nat. Methods 2013, 10, 1239–1245. [Google Scholar] [CrossRef] [PubMed]
- Anjo, S.I.; Simões, I.; Castanheira, P.; Grãos, M.; Manadas, B. Use of recombinant proteins as a simple and robust normalization method for untargeted proteomics screening: Exhaustive performance assessment. Talanta 2019, 205, 120163. [Google Scholar] [CrossRef]
- Perez-Riverol, Y.; Bai, J.; Bandla, C.; García-Seisdedos, D.; Hewapathirana, S.; Kamatchinathan, S.; Kundu, D.J.; Prakash, A.; Frericks-Zipper, A.; Eisenacher, M.; et al. The PRIDE database resources in 2022: A hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res 2022, 50, D543–D552. [Google Scholar] [CrossRef]
- Mi, H.; Muruganujan, A.; Huang, X.; Ebert, D.; Mills, C.; Guo, X.; Thomas, P.D. Protocol Update for large-scale genome and gene function analysis with the PANTHER classification system (v.14.0). Nat. Protoc. 2019, 14, 703–721. [Google Scholar] [CrossRef]
- Huang, d.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]
- Pathan, M.; Keerthikumar, S.; Ang, C.S.; Gangoda, L.; Quek, C.Y.; Williamson, N.A.; Mouradov, D.; Sieber, O.M.; Simpson, R.J.; Salim, A. FunRich: An open access standalone functional enrichment and interaction network analysis tool. Proteomics 2015, 15, 2597–2601. [Google Scholar] [CrossRef]
- Chong, J.; Wishart, D.S.; Xia, J. Using MetaboAnalyst 4.0 for Comprehensive and Integrative Metabolomics Data Analysis. Curr. Protoc. Bioinform. 2019, 68, e86. [Google Scholar] [CrossRef] [PubMed]
- Meizel, S.; Mukerji, S.K. Proacrosin from rabbit epididymal spermatozoa: Partial purification and initial biochemical characterization. Biol. Reprod. 1975, 13, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Hirose, M.; Honda, A.; Fulka, H.; Tamura-Nakano, M.; Matoba, S.; Tomishima, T.; Mochida, K.; Hasegawa, A.; Nagashima, K.; Inoue, K.; et al. Acrosin is essential for sperm penetration through the zona pellucida in hamsters. Proc. Natl. Acad. Sci. USA 2020, 117, 2513–2518. [Google Scholar] [CrossRef]
- Urch, U.A.; Wardrip, N.J.; Hedrick, J.L. Proteolysis of the zona pellucida by acrosin: The nature of the hydrolysis products. J. Exp. Zool. 1985, 236, 239–243. [Google Scholar] [CrossRef]
- Baba, T.; Azuma, S.; Kashiwabara, S.; Toyoda, Y. Sperm from mice carrying a targeted mutation of the acrosin gene can penetrate the oocyte zona pellucida and effect fertilization. J. Biol. Chem. 1994, 269, 31845–31849. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.H.; Zhao, R.L.; Wang, Q.; Zhang, Z.Y. Determination of sperm acrosin activity for evaluation of male fertility. Asian J. Androl. 2000, 2, 229–232. [Google Scholar]
- Zhang, G.; Yang, W.; Zou, P.; Jiang, F.; Zeng, Y.; Chen, Q.; Sun, L.; Yang, H.; Zhou, N.; Wang, X.; et al. Mitochondrial functionality modifies human sperm acrosin activity, acrosome reaction capability and chromatin integrity. Hum. Reprod. 2019, 34, 3–11. [Google Scholar] [CrossRef]
- Zalata, A.; El-Samanoudy, A.Z.; Shaalan, D.; El-Baiomy, Y.; Mostafa, T. In vitro effect of cell phone radiation on motility, DNA fragmentation and clusterin gene expression in human sperm. Int. J. Fertil. Steril. 2015, 9, 129–136. [Google Scholar] [CrossRef]
- Guo, Y.; Jiang, W.; Yu, W.; Niu, X.; Liu, F.; Zhou, T.; Zhang, H.; Li, Y.; Zhu, H.; Zhou, Z.; et al. Proteomics analysis of asthenozoospermia and identification of glucose-6-phosphate isomerase as an important enzyme for sperm motility. J. Proteom. 2019, 208, 103478. [Google Scholar] [CrossRef]
- Mohsenian, M.; Syner, F.N.; Moghissi, K.S. A study of sperm acrosin in patients with unexplained infertility. Fertil. Steril. 1982, 37, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Nixon, B.; Mitchell, L.A.; Anderson, A.L.; McLaughlin, E.A.; O’bryan, M.K.; Aitken, R.J. Proteomic and functional analysis of human sperm detergent resistant membranes. J. Cell Physiol. 2011, 226, 2651–2665. [Google Scholar] [CrossRef]
- Shetty, J.; Wolkowicz, M.J.; Digilio, L.C.; Klotz, K.L.; Jayes, F.L.; Diekman, A.B.; Westbrook, V.A.; Farris, E.M.; Hao, Z.; Coonrod, S.A.; et al. SAMP14, a novel, acrosomal membrane-associated, glycosylphosphatidylinositol-anchored member of the Ly-6/urokinase-type plasminogen activator receptor superfamily with a role in sperm-egg interaction. J. Biol. Chem. 2003, 278, 30506–30515. [Google Scholar] [CrossRef]
- Tapia, S.; Rojas, M.; Morales, P.; Ramirez, M.A.; Diaz, E.S. The laminin-induced acrosome reaction in human sperm is mediated by Src kinases and the proteasome. Biol. Reprod. 2011, 85, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.A.; Wu, X.H.; Wang, L.; DeRossi, C.; Westphal, V.; Wu, R.; Alton, G.; Srikrishna, G.; Freeze, H.H. Molecular cloning, gene organization, and expression of mouse Mpi encoding phosphomannose isomerase. Glycobiology 2002, 12, 435–442. [Google Scholar] [CrossRef]
- DeRossi, C.; Bode, L.; Eklund, E.A.; Zhang, F.; Davis, J.A.; Westphal, V.; Wang, L.; Borowsky, A.D.; Freeze, H.H. Ablation of mouse phosphomannose isomerase (Mpi) causes mannose 6-phosphate accumulation, toxicity, and embryonic lethality. J. Biol. Chem. 2006, 281, 5916–5927. [Google Scholar] [CrossRef]
- Martin-Hidalgo, D.; Serrano, R.; Zaragoza, C.; Garcia-Marin, L.J.; Bragado, M.J. Human sperm phosphoproteome reveals differential phosphoprotein signatures that regulate human sperm motility. J. Proteom. 2020, 215, 103654. [Google Scholar] [CrossRef]
- Yi, W.; Clark, P.M.; Mason, D.E.; Keenan, M.C.; Hill, C.; Goddard III, W.A.; Peters, E.C.; Driggers, E.M.; Hsieh-Wilson, L.C. PFK1 glycosylation is a key regulator of cancer cell growth and central metabolic pathways. Science 2012, 337, 975. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.-J.; Pang, M.-G. Mitochondrial functionality in male fertility: From spermatogenesis to fertilization. Antioxidants 2021, 10, 98. [Google Scholar] [CrossRef]
- Bajpai, M.; Gupta, G.; Setty, B.S. Changes in carbohydrate metabolism of testicular germ cells during meiosis in the rat. Eur. J. Endocrinol. 1998, 138, 322–327. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, Y.; Jin, K.; Lu, H.; Liu, F.; Guo, Y.; Yan, F.; Shi, W.; Liu, Y.; Cao, X. Differential proteomic profiling in human spermatozoa that did or did not result in pregnancy via IVF and AID. Proteom.–Clin. Appl. 2013, 7, 850–858. [Google Scholar] [CrossRef]
- Olayioye, M.A.; Hoffmann, P.; Pomorski, T.; Armes, J.; Simpson, R.J.; Kemp, B.E.; Lindeman, G.J.; Visvader, J.E. The phosphoprotein StarD10 is overexpressed in breast cancer and cooperates with ErbB receptors in cellular transformation. Cancer Res. 2004, 64, 3538–3544. [Google Scholar] [CrossRef]
- Olayioye, M.A.; Vehring, S.; Müller, P.; Herrmann, A.; Schiller, J.; Thiele, C.; Lindeman, G.J.; Visvader, J.E.; Pomorski, T. StarD10, a START domain protein overexpressed in breast cancer, functions as a phospholipid transfer protein. J. Biol. Chem. 2005, 280, 27436–27442. [Google Scholar] [CrossRef]
- Collodel, G.; Signorini, C.; Nerucci, F.; Gambera, L.; Iacoponi, F.; Moretti, E. Semen Biochemical Components in Varicocele, Leukocytospermia, and Idiopathic Infertility. Reprod. Sci. 2021, 28, 91–101. [Google Scholar] [CrossRef] [PubMed]
- González-Cadavid, V.; Martins, J.A.; Moreno, F.B.; Andrade, T.S.; Santos, A.C.; Monteiro-Moreira, A.C.; Moreira, R.A.; Moura, A.A. Seminal plasma proteins of adult boars and correlations with sperm parameters. Theriogenology 2014, 82, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Skinner, M.K.; Griswold, M.D. Sertoli cells synthesize and secrete transferrin-like protein. J. Biol. Chem. 1980, 255, 9523–9525. [Google Scholar] [CrossRef]
- Skinner, M.K.; Griswold, M.D. Secretion of testicular transferrin by cultured Sertoli cells is regulated by hormones and retinoids. Biol. Reprod. 1982, 27, 211–221. [Google Scholar] [CrossRef]
- Holzer, K.; Drucker, E.; Oliver, S.; Winkler, J.; Eiteneuer, E.; Herpel, E.; Breuhahn, K.; Singer, S. Cellular apoptosis susceptibility (CAS) is overexpressed in thyroid carcinoma and maintains tumor cell growth: A potential link to the BRAFV600E mutation. Int. J. Oncol. 2016, 48, 1679–1687. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.C. CAS (CSE1L) signaling pathway in tumor progression and its potential as a biomarker and target for targeted therapy. Tumour Biol. 2016, 37, 13077–13090. [Google Scholar] [CrossRef]
- Liu, C.; Wei, J.; Xu, K.; Sun, X.; Zhang, H.; Xiong, C. CSE1L participates in regulating cell mitosis in human seminoma. Cell Prolif. 2019, 52, e12549. [Google Scholar] [CrossRef]
- Lorenzato, A.; Biolatti, M.; Delogu, G.; Capobianco, G.; Farace, C.; Dessole, S.; Cossu, A.; Tanda, F.; Madeddu, R.; Olivero, M. AKT activation drives the nuclear localization of CSE1L and a pro-oncogenic transcriptional activation in ovarian cancer cells. Exp. Cell Res. 2013, 319, 2627–2636. [Google Scholar] [CrossRef] [PubMed]
- Lorenzato, A.; Martino, C.; Dani, N.; Oligschläger, Y.; Ferrero, A.M.; Biglia, N.; Calogero, R.; Olivero, M.; Di Renzo, M.F. The cellular apoptosis susceptibility CAS/CSE1L gene protects ovarian cancer cells from death by suppressing RASSF1C. FASEB J. 2012, 26, 2446–2456. [Google Scholar] [CrossRef] [PubMed]
- Amaral, A.; Paiva, C.; Attardo Parrinello, C.; Estanyol, J.M.; Ballescà, J.L.; Ramalho-Santos, J.; Oliva, R. Identification of proteins involved in human sperm motility using high-throughput differential proteomics. J. Proteome Res. 2014, 13, 5670–5684. [Google Scholar] [CrossRef] [PubMed]
- Hamada, A.; Esteves, S.; Agarwal, A. Unexplained male infertility—Looking beyond routine semen analysis. Eur. Urol. Rev. 2012, 7, 90–96. [Google Scholar]
- Wang, C.; Swerdloff, R.S. Limitations of semen analysis as a test of male fertility and anticipated needs from newer tests. Fertil. Steril. 2014, 102, 1502–1507. [Google Scholar] [CrossRef]
- Barazani, Y.; Agarwal, A.; Sabanegh Jr, E.S. Functional sperm testing and the role of proteomics in the evaluation of male infertility. Urology 2014, 84, 255–261. [Google Scholar] [CrossRef]
- Schattman, G.L.; Esteves, S.C.; Agarwal, A. Unexplained Infertility: Pathophysiology, Evaluation and Treatment; Springer: New York, NY, USA, 2015. [Google Scholar]
- Esteves, S.C.; Miyaoka, R.; Agarwal, A. An update on the clinical assessment of the infertile male. Clinics 2011, 66, 691–700. [Google Scholar] [CrossRef]
- Arcaniolo, D.; Favilla, V.; Tiscione, D.; Pisano, F.; Bozzini, G.; Creta, M.; Gentile, G.; Fabris, F.M.; Pavan, N.; Veneziano, I.A. Is there a place for nutritional supplements in the treatment of idiopathic male infertility? Arch. Ital. Urol. Androl. 2014, 86, 164–170. [Google Scholar] [CrossRef]
- Kanannejad, Z.; Gharesi-Fard, B. Difference in the seminal plasma protein expression in unexplained infertile men with successful and unsuccessful in vitro fertilisation outcome. Andrologia 2019, 51, e13158. [Google Scholar] [CrossRef]
- Corsini, C.; Boeri, L.; Candela, L.; Pozzi, E.; Belladelli, F.; Capogrosso, P.; Fallara, G.; Schifano, N.; Cignoli, D.; Ventimiglia, E. Is There a Relevant Clinical Impact in Differentiating Idiopathic versus Unexplained Male Infertility? World J. Men’s Health 2021, 40, 354. [Google Scholar] [CrossRef]
- Damsgaard, J.; Joensen, U.N.; Carlsen, E.; Erenpreiss, J.; Jensen, M.B.; Matulevicius, V.; Zilaitiene, B.; Olesen, I.A.; Perheentupa, A.; Punab, M. Varicocele is associated with impaired semen quality and reproductive hormone levels: A study of 7035 healthy young men from six European countries. Eur. Urol. 2016, 70, 1019–1029. [Google Scholar] [CrossRef]
- Santana, V.P.; James, E.R.; Miranda-Furtado, C.L.; de Souza, M.F.; Pompeu, C.P.; Esteves, S.C.; Carrell, D.T.; Aston, K.I.; Jenkins, T.G.; Dos Reis, R.M. Differential DNA methylation pattern and sperm quality in men with varicocele. Fertil. Steril. 2020, 114, 770–778. [Google Scholar] [CrossRef]
- Tahamtan, S.; Tavalaee, M.; Izadi, T.; Barikrow, N.; Zakeri, Z.; Lockshin, R.A.; Abbasi, H.; Nasr-Esfahani, M.H. Reduced sperm telomere length in individuals with varicocele is associated with reduced genomic integrity. Sci. Rep. 2019, 9, 4336. [Google Scholar] [CrossRef]
- Wdowiak, A.; Skrzypek, M.; Stec, M.; Panasiuk, L. Effect of ionizing radiation on the male reproductive system. Ann. Agric. Environ. Med. 2019, 26, 2. [Google Scholar] [CrossRef] [PubMed]
- Ashiru, O.A.; Odusanya, O.O. Fertility and occupational hazards: Review of the literature. Afr. J. Reprod. Health 2009, 13, 160–166. [Google Scholar]
- Yucra, S.; Rubio, J.; Gasco, M.; Gonzales, C.; Steenland, K.; Gonzales, G.F. Semen quality and reproductive sex hormone levels in Peruvian pesticide sprayers. Int. J. Occup. Environ. Health 2006, 12, 355–361. [Google Scholar] [CrossRef]
- Bonde, J.P. Semen quality in welders exposed to radiant heat. Occup. Environ. Med. 1992, 49, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Pozza, A.; Dèttore, D.; Coccia, M.E. Depression and anxiety in pathways of medically assisted reproduction: The role of infertility stress dimensions. Clin. Pract. Epidemiol. Ment. Health CP EMH 2019, 15, 101. [Google Scholar] [CrossRef]
- De Gennaro, L.; Balistreri, S.; Lenzi, A.; Lombardo, F.; Ferrara, M.; Gandini, L. Psychosocial factors discriminate oligozoospermic from normozoospermic men. Fertil. Steril. 2003, 79, 1571–1576. [Google Scholar] [CrossRef]
- Kumar, R.; Venkatesh, S.; Kumar, M.; Tanwar, M.; Shasmsi, M.; Gupta, N.; Sharma, R.; Talwar, P.; Dada, R. Oxidative Stress and Sperm Mitochondrial Dna Mutation in Idiopathic Oligoasthenozoospermic Men. Indian J. Biochem. Biophys. 2009, 46, 172–177. [Google Scholar]
- Mayorga-Torres, B.J.; Cardona-Maya, W.; Cadavid, Á.; Camargo, M. Evaluation of sperm functional parameters in normozoospermic infertile individuals. Actas Urol. Esp. 2013, 37, 221–227. [Google Scholar] [CrossRef]
- Mayorga-Torres, B.J.M.; Camargo, M.; Cadavid, Á.; du Plessis, S.S.; Cardona Maya, W.D. Are oxidative stress markers associated with unexplained male infertility? Andrologia 2017, 49, 12659. [Google Scholar] [CrossRef]
- Lewis, S.E. Is sperm evaluation useful in predicting human fertility? Reproduction 2007, 134, 31–40. [Google Scholar] [CrossRef]
- Agarwal, A.; Said, T.M. Role of sperm chromatin abnormalities and DNA damage in male infertility. Hum. Reprod. Update 2003, 9, 331–345. [Google Scholar] [CrossRef] [PubMed]
- Abou-haila, A.; Tulsiani, D.R. Signal transduction pathways that regulate sperm capacitation and the acrosome reaction. Arch. Biochem. Biophys. 2009, 485, 72–81. [Google Scholar] [CrossRef]
- De Jonge, C. Biological basis for human capacitation—Revisited. Hum. Reprod. Update 2017, 23, 289–299. [Google Scholar] [CrossRef]
- Amaral, S.; Ramalho-Santos, J. Aging, mitochondria and male reproductive function. Curr. Aging Sci. 2009, 2, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.P.; Amaral, A.; Baptista, M.; Tavares, R.; Caballero Campo, P.; Caballero Peregrín, P.; Freitas, A.; Paiva, A.; Almeida-Santos, T.; Ramalho-Santos, J. Not all sperm are equal: Functional mitochondria characterize a subpopulation of human sperm with better fertilization potential. PLoS ONE 2011, 6, e18112. [Google Scholar] [CrossRef]
- Marchetti, P.; Ballot, C.; Jouy, N.; Thomas, P.; Marchetti, C. Influence of mitochondrial membrane potential of spermatozoa on in vitro fertilisation outcome. Andrologia 2012, 44, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Amaral, A.; Lourenço, B.; Marques, M.; Ramalho-Santos, J. Mitochondria functionality and sperm quality. Reproduction 2013, 146, R163–R174. [Google Scholar] [CrossRef]
- Salsabili, N.; Mehrsai, A.; Jalalizadeh, B.; Pourmand, G.; Jalaie, S. Correlation of sperm nuclear chromatin condensation staining method with semen parameters and sperm functional tests in patients with spinal cord injury, varicocele, and idiopathic infertility. Urol. J. 2006, 3, 32–37. [Google Scholar]
- Wu, W.; Shen, O.; Qin, Y.; Niu, X.; Lu, C.; Xia, Y.; Song, L.; Wang, S.; Wang, X. Idiopathic male infertility is strongly associated with aberrant promoter methylation of methylenetetrahydrofolate reductase (MTHFR). PLoS ONE 2010, 5, e13884. [Google Scholar] [CrossRef]
- Palomba, S.; Falbo, A.; Espinola, S.; Rocca, M.; Capasso, S.; Cappiello, F.; Zullo, F. Effects of highly purified follicle-stimulating hormone on sperm DNA damage in men with male idiopathic subfertility: A pilot study. J. Endocrinol. Investig. 2011, 34, 747–752. [Google Scholar] [CrossRef]
- Pelliccione, F.; d’Angeli, A.; Cinque, B.; Falone, S.; Micillo, A.; Francavilla, F.; Amicarelli, F.; Gandini, L.; Francavilla, S. Activation of the immune system and sperm DNA fragmentation are associated with idiopathic oligoasthenoteratospermia in men with couple subfertility. Fertil. Steril. 2011, 95, 2676–2679. [Google Scholar] [CrossRef]
- Saleh, R.A.; Agarwal, A.; Nelson, D.R.; Nada, E.A.; El-Tonsy, M.H.; Alvarez, J.G.; Thomas, A.J.; Sharma, R.K. Increased sperm nuclear DNA damage in normozoospermic infertile men: A prospective study. Fertil. Steril. 2002, 78, 313–318. [Google Scholar] [CrossRef]
- Urdinguio, R.G.; Bayón, G.F.; Dmitrijeva, M.; Toraño, E.G.; Bravo, C.; Fraga, M.F.; Bassas, L.; Larriba, S.; Fernández, A.F. Aberrant DNA methylation patterns of spermatozoa in men with unexplained infertility. Hum. Reprod. 2015, 30, 1014–1028. [Google Scholar] [CrossRef] [PubMed]
- Faduola, P.; Kolade, C.O. Sperm chromatin structure assay results in Nigerian men with unexplained infertility. Clin. Exp. Reprod. Med. 2015, 42, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Peedicayil, J.; Deendayal, M.; Sadasivan, G.; Shivaji, S. Assessment of hyperactivation, acrosome reaction and motility characteristics of spermatozoa from semen of men of proven fertility and unexplained infertility. Andrologia 1997, 29, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Cocuzza, M.; Sikka, S.C.; Athayde, K.S.; Agarwal, A. Clinical relevance of oxidative stress and sperm chromatin damage in male infertility: An evidence based analysis. Int. Braz. J. Urol. 2007, 33, 603–621. [Google Scholar] [CrossRef]
- Tremellen, K. Oxidative stress and male infertility--a clinical perspective. Hum. Reprod. Update 2008, 14, 243–258. [Google Scholar] [CrossRef]
- Agarwal, A.; Allamaneni, S.S. Free radicals and male reproduction. J. Indian Med. Assoc. 2011, 109, 184–187. [Google Scholar]
- Zorova, L.D.; Popkov, V.A.; Plotnikov, E.Y.; Silachev, D.N.; Pevzner, I.B.; Jankauskas, S.S.; Babenko, V.A.; Zorov, S.D.; Balakireva, A.V.; Juhaszova, M.; et al. Mitochondrial membrane potential. Anal. Biochem. 2018, 552, 50–59. [Google Scholar] [CrossRef]
- Pasqualotto, F.F.; Sharma, R.K.; Kobayashi, H.; Nelson, D.R.; Thomas, A.J.; Agarwal, A. Oxidative stress in normospermic men undergoing infertility evaluation. J. Androl. 2001, 22, 316–322. [Google Scholar]
- Aydemir, B.; Onaran, I.; Kiziler, A.R.; Alici, B.; Akyolcu, M.C. Increased oxidative damage of sperm and seminal plasma in men with idiopathic infertility is higher in patients with glutathione S-transferase Mu-1 null genotype. Asian J. Androl. 2007, 9, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Aktan, G.; Doğru-Abbasoğlu, S.; Küçükgergin, C.; Kadıoğlu, A.; Ozdemirler-Erata, G.; Koçak-Toker, N. Mystery of idiopathic male infertility: Is oxidative stress an actual risk? Fertil. Steril. 2013, 99, 1211–1215. [Google Scholar] [CrossRef] [PubMed]
- Soni, K.K.; Zhang, L.T.; Choi, B.R.; Karna, K.K.; You, J.H.; Shin, Y.S.; Lee, S.W.; Kim, C.Y.; Zhao, C.; Chae, H.J.; et al. Protective effect of MOTILIPERM in varicocele-induced oxidative injury in rat testis by activating phosphorylated inositol requiring kinase 1α (p-IRE1α) and phosphorylated c-Jun N-terminal kinase (p-JNK) pathways. Pharm. Biol. 2018, 56, 94–103. [Google Scholar] [CrossRef]
- Amaral, A.; Castillo, J.; Estanyol, J.M.; Ballescà, J.L.; Ramalho-Santos, J.; Oliva, R. Human sperm tail proteome suggests new endogenous metabolic pathways. Mol. Cell. Proteom. 2013, 12, 330–342. [Google Scholar] [CrossRef]
- Petit, F.G.; Kervarrec, C.; Jamin, S.P.; Smagulova, F.; Hao, C.; Becker, E.; Jégou, B.; Chalmel, F.; Primig, M. Combining RNA and protein profiling data with network interactions identifies genes associated with spermatogenesis in mouse and human. Biol. Reprod. 2015, 92, 71. [Google Scholar] [CrossRef]
- de Mateo, S.; Castillo, J.; Estanyol, J.M.; Ballescà, J.L.; Oliva, R. Proteomic characterization of the human sperm nucleus. Proteomics 2011, 11, 2714–2726. [Google Scholar] [CrossRef] [PubMed]
- Asano, A.; Selvaraj, V.; Buttke, D.E.; Nelson, J.L.; Green, K.M.; Evans, J.E.; Travis, A.J. Biochemical characterization of membrane fractions in murine sperm: Identification of three distinct sub-types of membrane rafts. J. Cell Physiol. 2009, 218, 537–548. [Google Scholar] [CrossRef]
- López-Salguero, J.B.; Fierro, R.; Michalski, J.C.; Jiménez-Morales, I.; Lefebvre, T.; Mondragón-Payne, O.; Baldini, S.F.; Vercoutter-Edouart, A.S.; González-Márquez, H. Identification of lipid raft glycoproteins obtained from boar spermatozoa. Glycoconj. J. 2020, 37, 499–509. [Google Scholar] [CrossRef]
- Chen, L.; Wen, C.W.; Deng, M.J.; Li, P.; Zhang, Z.D.; Zhou, Z.H.; Wang, X. Metabolic and transcriptional changes in seminal plasma of asthenozoospermia patients. Biomed. Chromatogr. 2020, 34, e4769. [Google Scholar] [CrossRef]
- Pereira, R.; Oliveira, J.; Ferraz, L.; Barros, A.; Santos, R.; Sousa, M. Mutation analysis in patients with total sperm immotility. J. Assist. Reprod. Genet. 2015, 32, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.Y.; Mannowetz, N.; Zhang, Y.; Everley, R.A.; Gygi, S.P.; Bewersdorf, J.; Lishko, P.V.; Chung, J.J. Dual Sensing of Physiologic pH and Calcium by EFCAB9 Regulates Sperm Motility. Cell 2019, 177, 1480–1494.e19. [Google Scholar] [CrossRef]
- Wolkowicz, M.J.; Shetty, J.; Westbrook, A.; Klotz, K.; Jayes, F.; Mandal, A.; Flickinger, C.J.; Herr, J.C. Equatorial segment protein defines a discrete acrosomal subcompartment persisting throughout acrosomal biogenesis. Biol. Reprod. 2003, 69, 735–745. [Google Scholar] [CrossRef] [PubMed]
- Wolkowicz, M.J.; Digilio, L.; Klotz, K.; Shetty, J.; Flickinger, C.J.; Herr, J.C. Equatorial segment protein (ESP) is a human alloantigen involved in sperm-egg binding and fusion. J. Androl. 2008, 29, 272–282. [Google Scholar] [CrossRef]
- Novak, S.; Smith, T.A.; Paradis, F.; Burwash, L.; Dyck, M.K.; Foxcroft, G.R.; Dixon, W.T. Biomarkers of in vivo fertility in sperm and seminal plasma of fertile stallions. Theriogenology 2010, 74, 956–967. [Google Scholar] [CrossRef] [PubMed]
- Xue, D.; Zhang, Y.; Wang, Y.; Wang, J.; An, F.; Sun, X.; Yu, Z. Quantitative proteomic analysis of sperm in unexplained recurrent pregnancy loss. Reprod. Biol. Endocrinol. 2019, 17, 52. [Google Scholar] [CrossRef]
- Enoiu, S.I.; Nygaard, M.B.; Bungum, M.; Ziebe, S.; Petersen, M.R.; Almstrup, K. Expression of membrane fusion proteins in spermatozoa and total fertilisation failure during in vitro fertilisation. Andrology 2022, 10, 1317–1327. [Google Scholar] [CrossRef]
- Ariga, H.; Takahashi-Niki, K.; Kato, I.; Maita, H.; Niki, T.; Iguchi-Ariga, S.M. Neuroprotective function of DJ-1 in Parkinson’s disease. Oxid. Med. Cell Longev. 2013, 2013, 683920. [Google Scholar] [CrossRef]
- Strobbe, D.; Robinson, A.A.; Harvey, K.; Rossi, L.; Ferraina, C.; de Biase, V.; Rodolfo, C.; Harvey, R.J.; Campanella, M. Distinct Mechanisms of Pathogenic DJ-1 Mutations in Mitochondrial Quality Control. Front. Mol. Neurosci. 2018, 11, 68. [Google Scholar] [CrossRef]
- Wu, Y.Q.; Rao, M.; Hu, S.F.; Ke, D.D.; Zhu, C.H.; Xia, W. Effect of transient scrotal hyperthermia on human sperm: An iTRAQ-based proteomic analysis. Reprod. Biol. Endocrinol. 2020, 18, 83. [Google Scholar] [CrossRef]
- Agarwal, A.; Panner Selvam, M.K.; Samanta, L.; Vij, S.C.; Parekh, N.; Sabanegh, E.; Tadros, N.N.; Arafa, M.; Sharma, R. Effect of Antioxidant Supplementation on the Sperm Proteome of Idiopathic Infertile Men. Antioxidants 2019, 8, 488. [Google Scholar] [CrossRef] [PubMed]
- Kanatsu-Shinohara, M.; Onoyama, I.; Nakayama, K.I.; Shinohara, T. Skp1-Cullin-F-box (SCF)-type ubiquitin ligase FBXW7 negatively regulates spermatogonial stem cell self-renewal. Proc. Natl. Acad. Sci. USA 2014, 111, 8826–8831. [Google Scholar] [CrossRef] [PubMed]
- Kanatsu-Shinohara, M.; Tanaka, T.; Ogonuki, N.; Ogura, A.; Morimoto, H.; Cheng, P.F.; Eisenman, R.N.; Trumpp, A.; Shinohara, T. Myc/Mycn-mediated glycolysis enhances mouse spermatogonial stem cell self-renewal. Genes. Dev. 2016, 30, 2637–2648. [Google Scholar] [CrossRef] [PubMed]
- Meroni, S.B.; Galardo, M.N.; Rindone, G.; Gorga, A.; Riera, M.F.; Cigorraga, S.B. Molecular Mechanisms and Signaling Pathways Involved in Sertoli Cell Proliferation. Front. Endocrinol. 2019, 10, 224. [Google Scholar] [CrossRef]
- Lim, K.; Hwang, B.D. Follicle-stimulating hormone transiently induces expression of protooncogene c-myc in primary Sertoli cell cultures of early pubertal and prepubertal rat. Mol. Cell Endocrinol. 1995, 111, 51–56. [Google Scholar] [CrossRef]
- Riera, M.F.; Regueira, M.; Galardo, M.N.; Pellizzari, E.H.; Meroni, S.B.; Cigorraga, S.B. Signal transduction pathways in FSH regulation of rat Sertoli cell proliferation. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E914–E923. [Google Scholar] [CrossRef]
- Naz, R.K.; Ahmad, K.; Kumar, G. Presence and role of c-myc proto-oncogene product in mammalian sperm cell function. Biol. Reprod. 1991, 44, 842–850. [Google Scholar] [CrossRef]
- Afsari, M.; Fesahat, F.; Talebi, A.R.; Agarwal, A.; Henkel, R.; Zare, F.; Gül, M.; Iraci, N.; Cannarella, R.; Makki, M.; et al. ANXA2, SP17, SERPINA5, PRDX2 genes, and sperm DNA fragmentation differentially represented in male partners of infertile couples with normal and abnormal sperm parameters. Andrologia 2022, 54, e14556. [Google Scholar] [CrossRef]
- Odet, F.; Verot, A.; Le Magueresse-Battistoni, B. The mouse testis is the source of various serine proteases and serine proteinase inhibitors (SERPINs): Serine proteases and SERPINs identified in Leydig cells are under gonadotropin regulation. Endocrinology 2006, 147, 4374–4383. [Google Scholar] [CrossRef] [PubMed]
- Odet, F.; Guyot, R.; Leduque, P.; Le Magueresse-Battistoni, B. Evidence for similar expression of protein C inhibitor and the urokinase-type plasminogen activator system during mouse testis development. Endocrinology 2004, 145, 1481–1489. [Google Scholar] [CrossRef] [PubMed]
- Karna, K.K.; Shin, Y.S.; Choi, B.R.; Kim, H.K.; Park, J.K. The Role of Endoplasmic Reticulum Stress Response in Male Reproductive Physiology and Pathology: A Review. World J. Mens. Health 2020, 38, 484–494. [Google Scholar] [CrossRef]
- Niehues, R.; Hasilik, M.; Alton, G.; Körner, C.; Schiebe-Sukumar, M.; Koch, H.G.; Zimmer, K.P.; Wu, R.; Harms, E.; Reiter, K.; et al. Carbohydrate-deficient glycoprotein syndrome type Ib. Phosphomannose isomerase deficiency and mannose therapy. J. Clin. Investig. 1998, 101, 1414–1420. [Google Scholar] [CrossRef] [PubMed]
- Kamio, T.; Toki, T.; Kanezaki, R.; Sasaki, S.; Tandai, S.; Terui, K.; Ikebe, D.; Igarashi, K.; Ito, E. B-cell-specific transcription factor BACH2 modifies the cytotoxic effects of anticancer drugs. Blood 2003, 102, 3317–3322. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, C.; Yoshida, F.; Sears, D.E.; Hart, S.M.; Ikebe, D.; Muto, A.; Basu, S.; Igarashi, K.; Melo, J.V. Bcr-Abl signaling through the PI-3/S6 kinase pathway inhibits nuclear translocation of the transcription factor Bach2, which represses the antiapoptotic factor heme oxygenase-1. Blood 2007, 109, 1211–1219. [Google Scholar] [CrossRef]
- Dacheux, J.L.; Gatti, J.L.; Dacheux, F. Contribution of epididymal secretory proteins for spermatozoa maturation. Microsc. Res. Tech. 2003, 61, 7–17. [Google Scholar] [CrossRef]
- Watanabe, M.; Roussev, R.; Ahlering, P.; Sauer, R.; Coulam, C.; Jeyendran, R.S. Correlation between neutral alpha-glucosidase activity and sperm DNA fragmentation. Andrologia 2009, 41, 316–318. [Google Scholar] [CrossRef]
- Schmid, N.; Flenkenthaler, F.; Stöckl, J.B.; Dietrich, K.G.; Köhn, F.M.; Schwarzer, J.U.; Kunz, L.; Luckner, M.; Wanner, G.; Arnold, G.J.; et al. Insights into replicative senescence of human testicular peritubular cells. Sci. Rep. 2019, 9, 15052. [Google Scholar] [CrossRef]

| Parameter | CTRL | ID | UMI | p Value |
|---|---|---|---|---|
| Total motility (%) | 79.95 ± 0.73 (401) | 52.13 ± 1.98 *** (194) | 79.83 ± 1.41 ### (103) | <0.001 |
| Viability (%) | 86.09 ± 0.61 (401) | 66.31 ± 1.66 *** (194) | 84.71 ± 1.22 ### (103) | <0.001 |
| Normal morphology (%) | 2.30 ± 0.11 (376) | 0.99 ± 0.12 *** (178) | 2.47 ± 0.22 ### (97) | <0.001 |
| ART Outcomes | CTRL | ID | UMI | p Value |
|---|---|---|---|---|
| Fertilization rate (123) | 0.62 ± 0.04 | 0.57 ± 0.05 | 0.61 ± 0.07 | 0.786 |
| Embryonic development rate (123) | 0.71 ± 0.04 | 0.76 ± 0.06 | 0.79 ± 0.06 | 0.110 |
| Embryonic transfer rate (123) | 0.18 ± 0.03 | 0.21 ± 0.05 | 0.29 ± 0.09 | 0.917 |
| Biochemical pregnancy (60) | 16 (47.1%) | 5 (35.7%) | 3 (25%) | 0.379 |
| Protein ID (Uniprot) | Name | ID/UMI | Biological Process Source UniProt | Function (Sperm or Male Reproductive Tract) |
|---|---|---|---|---|
| P10323 | Acrosin (ACRO) | 0.79 | Not applicable |
|
| Q8TDM5 | Sperm acrosome membrane-associated protein 4 (SACA4) | 0.79 | Not applicable | |
| P55268 | Laminin subunit beta-2 (LAMB2) | 2.46 | Mediate the attachment, migration and organization of cells into tissues during embryonic development by interacting with other extracellular matrix components. | |
| P34949 | Mannose-6-phosphate isomerase (MPI) | 1.45 | Catalyzes the interconversion of fructose-6-phosphate and mannose-6-phosphate and is critical for the supply of D-mannose derivatives |
|
| P17858 | ATP-dependent 6-phosphofructokinase liver type (PFKAL) | 1.99 | Catalyses the first committing step of glycolysis, the phosphorylation of D-fructose 6-phosphate [60] |
|
| Q9Y365 | START domain-containing protein 10 (STA10) | 1.85 | Phospholipid transfer protein known to bind specifically to phosphatidylcholine and phosphatidylethmine, it can interact with the plasm membrane both in vitro and in vivo [64,65]. |
|
| P02787 | Serotransferrin (TRFE) | 2.10 | Iron binding transport proteins responsible for the transport of iron from sites of absorption and heme degradation to those of storage and utilization. Serum transferrin may also have a further role in stimulating cell proliferation. This protein has antimicrobial activity. |
|
| P55060 | Exportin-2 (XPO2) | 1.48 | This protein is expressed in several types of cancers including ovarian, thyroid and testicular germ cells tumours and correlated with poor cancer outcomes [70,71,72]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pacheco, R.I.; Cristo, M.I.; Anjo, S.I.; Silva, A.F.; Sousa, M.I.; Tavares, R.S.; Sousa, A.P.; Almeida Santos, T.; Moura-Ramos, M.; Caramelo, F.; et al. New Insights on Sperm Function in Male Infertility of Unknown Origin: A Multimodal Approach. Biomolecules 2023, 13, 1462. https://doi.org/10.3390/biom13101462
Pacheco RI, Cristo MI, Anjo SI, Silva AF, Sousa MI, Tavares RS, Sousa AP, Almeida Santos T, Moura-Ramos M, Caramelo F, et al. New Insights on Sperm Function in Male Infertility of Unknown Origin: A Multimodal Approach. Biomolecules. 2023; 13(10):1462. https://doi.org/10.3390/biom13101462
Chicago/Turabian StylePacheco, Rita I., Maria I. Cristo, Sandra I. Anjo, Andreia F. Silva, Maria Inês Sousa, Renata S. Tavares, Ana Paula Sousa, Teresa Almeida Santos, Mariana Moura-Ramos, Francisco Caramelo, and et al. 2023. "New Insights on Sperm Function in Male Infertility of Unknown Origin: A Multimodal Approach" Biomolecules 13, no. 10: 1462. https://doi.org/10.3390/biom13101462
APA StylePacheco, R. I., Cristo, M. I., Anjo, S. I., Silva, A. F., Sousa, M. I., Tavares, R. S., Sousa, A. P., Almeida Santos, T., Moura-Ramos, M., Caramelo, F., Manadas, B., Ramalho-Santos, J., & Amaral, S. G. (2023). New Insights on Sperm Function in Male Infertility of Unknown Origin: A Multimodal Approach. Biomolecules, 13(10), 1462. https://doi.org/10.3390/biom13101462









