An Overview of SARS-CoV-2 Etiopathogenesis and Recent Developments in COVID-19 Vaccines
Abstract
:1. Introduction
2. SARS-CoV-2 Pathogenesis and Treatment of COVID-19
2.1. Clinical Features and Etiopathogenesis of SARS-CoV-2
2.2. Variants of SARS-CoV-2
2.3. Treatment of COVID-19
3. COVID-19 Vaccine Development
3.1. Characteristics of a COVID-19 Vaccine
3.2. Types of COVID-19 Vaccine
3.2.1. Whole Virus Vaccines
3.2.2. Component Viral Vaccines
Protein Subunit Vaccines
DNA Vaccines
mRNA Vaccines
Virus-Like Particles (VLPs)
Viral Vectors
3.3. Mix and Match Concept
3.4. Routes of Vaccination
4. Summary and Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yuki, K.; Fujiogi, M.; Koutsogiannaki, S. COVID-19 pathophysiology: A review. Clin. Immunol. 2020, 215, 108427. [Google Scholar] [CrossRef] [PubMed]
- Pititto, B.A.; Ferreira, S.R.G. Diabetes and COVID-19: More than the sum of two morbidities. Rev. Saude Publica 2020, 54, 54. [Google Scholar] [CrossRef] [PubMed]
- Ouassou, H.; Kharchoufa, L.; Bouhrim, M.; Daoudi, N.E.; Imtara, H.; Bencheikh, N.; Amine, E.L.; Bnouham, M. The Pathogenesis of Coronavirus Disease 2019 (COVID-19): Evaluation and Prevention. J. Immunol. Res. 2020, 2020, 1357983. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.I.; Hu, Y.L.; Chen, P.Y.; Huang, Y.C.; Hsueh, P.R. Are children less susceptible to COVID-19? J. Microbiol. Immunol. Infect. 2020, 53, 371–372. [Google Scholar] [CrossRef] [PubMed]
- Weiss, S.R.; Leibowitz, J.L. Coronavirus pathogenesis. Adv. Virus Res. 2011, 81, 85–164. [Google Scholar] [CrossRef]
- Zeng, Z.Q.; Chen, D.H.; Tan, W.P.; Qiu, S.Y.; Xu, D.; Liang, H.X.; Chen, M.X.; Li, X.; Lin, Z.S.; Liu, W.K.; et al. Epidemiology and clinical characteristics of human coronaviruses OC43, 229E, NL63, and HKU1: A study of hospitalized children with acute respiratory tract infection in Guangzhou, China. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 363–369. [Google Scholar] [CrossRef]
- Cascella, M.; Rajnik, M.; Aleem, A.; Dulebohn, S.C.; Di Napoli, R. Features, Evaluation, and Treatment of Coronavirus (COVID-19). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.M.; Wang, W.; Song, Z.G.; Hu, Y.; Tao, Z.W.; Tian, J.H.; Pei, Y.Y.; et al. A new coronavirus associated with human respiratory disease in China. Nature 2020, 579, 265–269. [Google Scholar] [CrossRef]
- Harrison, A.G.; Lin, T.; Wang, P. Mechanisms of SARS-CoV-2 Transmission and Pathogenesis. Trends Immunol. 2020, 41, 1100–1115. [Google Scholar] [CrossRef]
- Medina-Enriquez, M.M.; Lopez-Leon, S.; Carlos-Escalante, J.A.; Aponte-Torres, Z.; Cuapio, A.; Wegman-Ostrosky, T. ACE2: The molecular doorway to SARS-CoV-2. Cell Biosci. 2020, 10, 148. [Google Scholar] [CrossRef]
- Ni, W.; Yang, X.; Yang, D.; Bao, J.; Li, R.; Xiao, Y.; Hou, C.; Wang, H.; Liu, J.; Yang, D.; et al. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Crit. Care 2020, 24, 422. [Google Scholar] [CrossRef]
- Lim, S.; Zhang, M.; Chang, T.L. ACE2-Independent Alternative Receptors for SARS-CoV-2. Viruses 2022, 14, 2535. [Google Scholar] [CrossRef] [PubMed]
- Masre, S.F.; Jufri, N.F.; Ibrahim, F.W.; Abdul Raub, S.H. Classical and alternative receptors for SARS-CoV-2 therapeutic strategy. Rev. Med. Virol. 2021, 31, 1–9. [Google Scholar] [CrossRef]
- Zhu, S.; Liu, Y.; Zhou, Z.; Zhang, Z.; Xiao, X.; Liu, Z.; Chen, A.; Dong, X.; Tian, F.; Chen, S.; et al. Genome-wide CRISPR activation screen identifies candidate receptors for SARS-CoV-2 entry. Sci. China Life Sci. 2022, 65, 701–717. [Google Scholar] [CrossRef] [PubMed]
- Gavriatopoulou, M.; Korompoki, E.; Fotiou, D.; Ntanasis-Stathopoulos, I.; Psaltopoulou, T.; Kastritis, E.; Terpos, E.; Dimopoulos, M.A. Organ-specific manifestations of COVID-19 infection. Clin. Exp. Med. 2020, 20, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, S.; Subbian, S. Critical Determinants of Cytokine Storm and Type I Interferon Response in COVID-19 Pathogenesis. Clin. Microbiol. Rev. 2021, 34, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.D.; Sumeh, A.S.; Sheraz, M.; Kavitha, M.S.; Venmathi Maran, B.A.; Rodrigues, K.F. A mini-review on the impact of COVID-19 on vital organs. Biomed. Pharmacother. 2021, 143, 112158. [Google Scholar] [CrossRef]
- Boson, B.; Legros, V.; Zhou, B.; Siret, E.; Mathieu, C.; Cosset, F.L.; Lavillette, D.; Denolly, S. The SARS-CoV-2 envelope and membrane proteins modulate maturation and retention of the spike protein, allowing assembly of virus-like particles. J. Biol. Chem. 2021, 296, 100111. [Google Scholar] [CrossRef]
- Bourgonje, A.R.; Abdulle, A.E.; Timens, W.; Hillebrands, J.L.; Navis, G.J.; Gordijn, S.J.; Bolling, M.C.; Dijkstra, G.; Voors, A.A.; Osterhaus, A.D.; et al. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19). J. Pathol. 2020, 251, 228–248. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, C.; Xu, X.F.; Xu, W.; Liu, S.W. Structural and functional properties of SARS-CoV-2 spike protein: Potential antivirus drug development for COVID-19. Acta Pharmacol. Sin. 2020, 41, 1141–1149. [Google Scholar] [CrossRef]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef]
- Rauf, A.; Abu-Izneid, T.; Olatunde, A.; Ahmed Khalil, A.; Alhumaydhi, F.A.; Tufail, T.; Shariati, M.A.; Rebezov, M.; Almarhoon, Z.M.; Mabkhot, Y.N.; et al. COVID-19 Pandemic: Epidemiology, Etiology, Conventional and Non-Conventional Therapies. Int. J. Environ. Res. Public. Health 2020, 17, 8155. [Google Scholar] [CrossRef] [PubMed]
- Beyer, D.K.; Forero, A. Mechanisms of Antiviral Immune Evasion of SARS-CoV-2. J. Mol. Biol. 2022, 434, 167265. [Google Scholar] [CrossRef] [PubMed]
- Diao, B.; Wang, C.; Tan, Y.; Chen, X.; Liu, Y.; Ning, L.; Chen, L.; Li, M.; Liu, Y.; Wang, G.; et al. Reduction and Functional Exhaustion of T Cells in Patients with Coronavirus Disease 2019 (COVID-19). Front. Immunol. 2020, 11, 827. [Google Scholar] [CrossRef] [PubMed]
- Vabret, N.; Britton, G.J.; Gruber, C.; Hegde, S.; Kim, J.; Kuksin, M.; Levantovsky, R.; Malle, L.; Moreira, A.; Park, M.D.; et al. Immunology of COVID-19: Current State of the Science. Immunity 2020, 52, 910–941. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.S.; Cox, M.A.; Zajac, A.J. T-cell exhaustion: Characteristics, causes and conversion. Immunology 2010, 129, 474–481. [Google Scholar] [CrossRef]
- Siddiqi, H.K.; Mehra, M.R. COVID-19 illness in native and immunosuppressed states: A clinical-therapeutic staging proposal. J. Heart Lung Transplant. 2020, 39, 405–407. [Google Scholar] [CrossRef]
- Zhou, Y.; Fu, B.; Zheng, X.; Wang, D.; Zhao, C.; Qi, Y.; Sun, R.; Tian, Z.; Xu, X.; Wei, H. Pathogenic T-cells and inflammatory monocytes incite inflammatory storms in severe COVID-19 patients. Natl. Sci. Rev. 2020, 7, 998–1002. [Google Scholar] [CrossRef]
- Fernandes, Q.; Inchakalody, V.P.; Merhi, M.; Mestiri, S.; Taib, N.; Moustafa Abo El-Ella, D.; Bedhiafi, T.; Raza, A.; Al-Zaidan, L.; Mohsen, M.O.; et al. Emerging COVID-19 variants and their impact on SARS-CoV-2 diagnosis, therapeutics and vaccines. Ann. Med. 2022, 54, 524–540. [Google Scholar] [CrossRef]
- Chavda, V.P.; Patel, A.B.; Vaghasiya, D.D. SARS-CoV-2 variants and vulnerability at the global level. J. Med. Virol. 2022, 94, 2986–3005. [Google Scholar] [CrossRef]
- Gong, W.; Parkkila, S.; Wu, X.; Aspatwar, A. SARS-CoV-2 variants and COVID-19 vaccines: Current challenges and future strategies. Int. Rev. Immunol. 2022, 28, 1–22. [Google Scholar] [CrossRef]
- Gunl, F.; Mecate-Zambrano, A.; Rehlander, S.; Hinse, S.; Ludwig, S.; Brunotte, L. Shooting at a Moving Target-Effectiveness and Emerging Challenges for SARS-CoV-2 Vaccine Development. Vaccines 2021, 9, 1052. [Google Scholar] [CrossRef] [PubMed]
- WHO. Tracking SARS-CoV-2 Variants. Available online: https://www.who.int/activities/tracking-SARS-CoV-2-variants (accessed on 22 September 2023).
- Parums, D.V. Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Patients. Med. Sci. Monit. 2022, 28, e935952. [Google Scholar] [CrossRef] [PubMed]
- Islam, T.; Hasan, M.; Rahman, M.S.; Islam, M.R. Comparative evaluation of authorized drugs for treating COVID-19 patients. Health Sci. Rep. 2022, 5, e671. [Google Scholar] [CrossRef] [PubMed]
- Chary, M.; Barbuto, A.F.; Izadmehr, S.; Tarsillo, M.; Fleischer, E.; Burns, M.M. COVID-19 Therapeutics: Use, Mechanism of Action, and Toxicity (Vaccines, Monoclonal Antibodies, and Immunotherapeutics). J. Med. Toxicol. 2023, 19, 205–218. [Google Scholar] [CrossRef]
- Corti, D.; Purcell, L.A.; Snell, G.; Veesler, D. Tackling COVID-19 with neutralizing monoclonal antibodies. Cell 2021, 184, 3086–3108. [Google Scholar] [CrossRef]
- Aleem, A.; Vaqar, S. Monoclonal Antibody Therapy For High-Risk Coronavirus (COVID 19) Patients With Mild To Moderate Disease Presentations. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Kaur, S.P.; Gupta, V. COVID-19 Vaccine: A comprehensive status report. Virus Res. 2020, 288, 198114. [Google Scholar] [CrossRef]
- WHO. WHO Target Product Profiles for COVID-19 Vaccines. Available online: https://cdn.who.int/media/docs/default-source/blue-print/tpp-6apr-2022-final.pdf?sfvrsn=4f8cede5_4&download=true (accessed on 22 September 2023).
- Shahzamani, K.; Mahmoudian, F.; Ahangarzadeh, S.; Ranjbar, M.M.; Beikmohammadi, L.; Bahrami, S.; Mohammadi, E.; Esfandyari, S.; Alibakhshi, A.; Javanmard, S.H. Vaccine design and delivery approaches for COVID-19. Int. Immunopharmacol. 2021, 100, 108086. [Google Scholar] [CrossRef]
- Soraci, L.; Lattanzio, F.; Soraci, G.; Gambuzza, M.E.; Pulvirenti, C.; Cozza, A.; Corsonello, A.; Luciani, F.; Rezza, G. COVID-19 Vaccines: Current and Future Perspectives. Vaccines 2022, 10, 608. [Google Scholar] [CrossRef]
- Ahmed, T.I.; Rishi, S.; Irshad, S.; Aggarwal, J.; Happa, K.; Mansoor, S. Inactivated vaccine Covaxin/BBV152: A systematic review. Front. Immunol. 2022, 13, 863162. [Google Scholar] [CrossRef]
- Behera, P.; Singh, A.K.; Subba, S.H.; Mc, A.; Sahu, D.P.; Chandanshive, P.D.; Pradhan, S.K.; Parida, S.P.; Mishra, A.; Patro, B.K.; et al. Effectiveness of COVID-19 vaccine (Covaxin) against breakthrough SARS-CoV-2 infection in India. Hum. Vaccin. Immunother. 2022, 18, 2034456. [Google Scholar] [CrossRef]
- Das, S.; Kar, S.S.; Samanta, S.; Banerjee, J.; Giri, B.; Dash, S.K. Immunogenic and reactogenic efficacy of Covaxin and Covishield: A comparative review. Immunol. Res. 2022, 70, 289–315. [Google Scholar] [CrossRef] [PubMed]
- Vikkurthi, R.; Ansari, A.; Pai, A.R.; Jha, S.N.; Sachan, S.; Pandit, S.; Nikam, B.; Kalia, A.; Jit, B.P.; Parray, H.A.; et al. Inactivated whole-virion vaccine BBV152/Covaxin elicits robust cellular immune memory to SARS-CoV-2 and variants of concern. Nat. Microbiol. 2022, 7, 974–985. [Google Scholar] [CrossRef] [PubMed]
- Hua, Q.; Zhang, H.; Yao, P.; Xu, N.; Sun, Y.; Lu, H.; Xu, F.; Liao, Y.; Yang, J.; Mao, H.; et al. Immunogenicity and immune-persistence of the CoronaVac or Covilo inactivated COVID-19 Vaccine: A 6-month population-based cohort study. Front. Immunol. 2022, 13, 939311. [Google Scholar] [CrossRef]
- Palacios, R.; Patino, E.G.; de Oliveira Piorelli, R.; Conde, M.; Batista, A.P.; Zeng, G.; Xin, Q.; Kallas, E.G.; Flores, J.; Ockenhouse, C.F.; et al. Double-Blind, Randomized, Placebo-Controlled Phase III Clinical Trial to Evaluate the Efficacy and Safety of treating Healthcare Professionals with the Adsorbed COVID-19 (Inactivated) Vaccine Manufactured by Sinovac—PROFISCOV: A structured summary of a study protocol for a randomised controlled trial. Trials 2020, 21, 853. [Google Scholar] [CrossRef] [PubMed]
- Tanriover, M.D.; Doganay, H.L.; Akova, M.; Guner, H.R.; Azap, A.; Akhan, S.; Kose, S.; Erdinc, F.S.; Akalin, E.H.; Tabak, O.F.; et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): Interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet 2021, 398, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Chantasrisawad, N.; Puthanakit, T.; Kornsitthikul, K.; Jaru-Ampornpan, P.; Tawan, M.; Matapituk, P.; Sophonphan, J.; Anugulruengkitt, S.; Tangsathapornpong, A.; Katanyutanon, A.; et al. Immunogenicity to SARS-CoV-2 Omicron variant among school-aged children with 2-dose of inactivated SARS-CoV-2 vaccines followed by BNT162b2 booster. Vaccine X 2022, 12, 100221. [Google Scholar] [CrossRef]
- WHO. COVID-19 Vaccine Tracker and Landscape. Available online: https://www.who.int/publications/m/item/draft-landscape-of-COVID-19-candidate-vaccines (accessed on 22 September 2023).
- Anez, G.; Dunkle, L.M.; Gay, C.L.; Kotloff, K.L.; Adelglass, J.M.; Essink, B.; Campbell, J.D.; Cloney-Clark, S.; Zhu, M.; Plested, J.S.; et al. Safety, Immunogenicity, and Efficacy of the NVX-CoV2373 COVID-19 Vaccine in Adolescents: A Randomized Clinical Trial. JAMA Netw. Open 2023, 6, e239135. [Google Scholar] [CrossRef]
- Bhiman, J.N.; Richardson, S.I.; Lambson, B.E.; Kgagudi, P.; Mzindle, N.; Kaldine, H.; Crowther, C.; Gray, G.; Bekker, L.G.; Novavax Trial Clinical Lead Author Group; et al. Novavax NVX-COV2373 triggers neutralization of Omicron sub-lineages. Sci. Rep. 2023, 13, 1222. [Google Scholar] [CrossRef]
- Heath, P.T.; Galiza, E.P.; Baxter, D.N.; Boffito, M.; Browne, D.; Burns, F.; Chadwick, D.R.; Clark, R.; Cosgrove, C.; Galloway, J.; et al. Safety and Efficacy of NVX-CoV2373 COVID-19 Vaccine. N. Eng. J. Med. 2021, 385, 1172–1183. [Google Scholar] [CrossRef]
- Parums, D.V. Editorial: First Approval of the Protein-Based Adjuvanted Nuvaxovid (NVX-CoV2373) Novavax Vaccine for SARS-CoV-2 Could Increase Vaccine Uptake and Provide Immune Protection from Viral Variants. Med. Sci. Monit. 2022, 28, e936523. [Google Scholar] [CrossRef]
- Dayan, G.H.; Rouphael, N.; Walsh, S.R.; Chen, A.; Grunenberg, N.; Allen, M.; Antony, J.; Asante, K.P.; Suresh Bhate, A.; Beresnev, T.; et al. Efficacy of a bivalent (D614 + B.1.351) SARS-CoV-2 Protein Vaccine. medRxiv 2023, 2022.12.05.22282933. [Google Scholar] [CrossRef]
- Heidary, M.; Kaviar, V.H.; Shirani, M.; Ghanavati, R.; Motahar, M.; Sholeh, M.; Ghahramanpour, H.; Khoshnood, S. A Comprehensive Review of the Protein Subunit Vaccines Against COVID-19. Front. Microbiol. 2022, 13, 927306. [Google Scholar] [CrossRef] [PubMed]
- Grana, C.; Ghosn, L.; Evrenoglou, T.; Jarde, A.; Minozzi, S.; Bergman, H.; Buckley, B.S.; Probyn, K.; Villanueva, G.; Henschke, N.; et al. Efficacy and safety of COVID-19 vaccines. Cochrane Database Syst. Rev. 2022, 12, CD015477. [Google Scholar] [CrossRef]
- Chavda, V.P.; Balar, P.; Vaghela, D.; Solanki, H.K.; Vaishnav, A.; Hala, V.; Vora, L. Omicron Variant of SARS-CoV-2: An Indian Perspective of Vaccination and Management. Vaccines 2023, 11, 160. [Google Scholar] [CrossRef] [PubMed]
- Khobragade, A.; Bhate, S.; Ramaiah, V.; Deshpande, S.; Giri, K.; Phophle, H.; Supe, P.; Godara, I.; Revanna, R.; Nagarkar, R.; et al. Efficacy, safety, and immunogenicity of the DNA SARS-CoV-2 vaccine (ZyCoV-D): The interim efficacy results of a phase 3, randomised, double-blind, placebo-controlled study in India. Lancet 2022, 399, 1313–1321. [Google Scholar] [CrossRef]
- Momin, T.; Kansagra, K.; Patel, H.; Sharma, S.; Sharma, B.; Patel, J.; Mittal, R.; Sanmukhani, J.; Maithal, K.; Dey, A.; et al. Safety and Immunogenicity of a DNA SARS-CoV-2 vaccine (ZyCoV-D): Results of an open-label, non-randomized phase I part of phase I/II clinical study by intradermal route in healthy subjects in India. EClinicalMedicine 2021, 38, 101020. [Google Scholar] [CrossRef]
- Yadav, P.D.; Kumar, S.; Agarwal, K.; Jain, M.; Patil, D.R.; Maithal, K.; Mathapati, B.; Giri, S.; Mohandas, S.; Shete, A.; et al. Needle-free injection system delivery of ZyCoV-D DNA vaccine demonstrated improved immunogenicity and protective efficacy in rhesus macaques against SARS-CoV-2. J. Med. Virol. 2023, 95, e28484. [Google Scholar] [CrossRef]
- Fang, E.; Liu, X.; Li, M.; Zhang, Z.; Song, L.; Zhu, B.; Wu, X.; Liu, J.; Zhao, D.; Li, Y. Advances in COVID-19 mRNA vaccine development. Signal Transduct. Target. Ther. 2022, 7, 94. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Eng. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Kralickova, P.; Jankovicova, K.; Sejkorova, I.; Soucek, O.; Koprivova, K.; Drahosova, M.; Andrys, C.; Krejsek, J. Immunogenicity and Safety of the Spikevax(R) (Moderna) mRNA SARS-CoV-2 Vaccine in Patients with Primary Humoral Immunodeficiency. Int. Arch. Allergy Immunol. 2022, 183, 1297–1310. [Google Scholar] [CrossRef]
- Fedele, G.; Trentini, F.; Schiavoni, I.; Abrignani, S.; Antonelli, G.; Baldo, V.; Baldovin, T.; Bandera, A.; Bonura, F.; Clerici, P.; et al. Evaluation of humoral and cellular response to four vaccines against COVID-19 in different age groups: A longitudinal study. Front. Immunol. 2022, 13, 1021396. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.M.; Mok, C.K.P.; Chan, K.C.; Ng, S.S.; Lam, B.H.; Luk, L.L.; Ko, F.W.; Chen, C.; Yiu, K.; Li, J.K.; et al. SARS-CoV-2 Omicron variant BA.2 neutralisation in sera of people with Comirnaty or CoronaVac vaccination, infection or breakthrough infection, Hong Kong, 2020 to 2022. Eurosurveillance 2022, 27, 2200178. [Google Scholar] [CrossRef] [PubMed]
- Fabiani, M.; Ramigni, M.; Gobbetto, V.; Mateo-Urdiales, A.; Pezzotti, P.; Piovesan, C. Effectiveness of the Comirnaty (BNT162b2, BioNTech/Pfizer) vaccine in preventing SARS-CoV-2 infection among healthcare workers, Treviso province, Veneto region, Italy, 27 December 2020 to 24 March 2021. Eurosurveillance 2021, 26, 2100420. [Google Scholar] [CrossRef] [PubMed]
- Goldin, S.; Adler, L.; Azuri, J.; Mendel, L.; Haviv, S.; Maimon, N. BNT162b2 mRNA COVID-19 (Comirnaty) Vaccine Effectiveness in Elderly Patients Who Live in Long-Term Care Facilities: A Nationwide Cohort. Gerontology 2022, 68, 1350–1357. [Google Scholar] [CrossRef] [PubMed]
- Sharifzadeh, M.; Mottaghi-Dastjerdi, N.; Soltany Rezae Raad, M. A Review of Virus-Like Particle-Based SARS-CoV-2 Vaccines in Clinical Trial Phases. Iran. J. Pharm. Res. 2022, 21, e127042. [Google Scholar] [CrossRef]
- Ward, B.J.; Gobeil, P.; Seguin, A.; Atkins, J.; Boulay, I.; Charbonneau, P.Y.; Couture, M.; D’Aoust, M.A.; Dhaliwall, J.; Finkle, C.; et al. Phase 1 randomized trial of a plant-derived virus-like particle vaccine for COVID-19. Nat. Med. 2021, 27, 1071–1078. [Google Scholar] [CrossRef]
- Hager, K.J.; Perez Marc, G.; Gobeil, P.; Diaz, R.S.; Heizer, G.; Llapur, C.; Makarkov, A.I.; Vasconcellos, E.; Pillet, S.; Riera, F.; et al. Efficacy and Safety of a Recombinant Plant-Based Adjuvanted COVID-19 Vaccine. N. Eng. J. Med. 2022, 386, 2084–2096. [Google Scholar] [CrossRef]
- Hofmeyer, K.A.; Bianchi, K.M.; Wolfe, D.N. Utilization of Viral Vector Vaccines in Preparing for Future Pandemics. Vaccines 2022, 10, 436. [Google Scholar] [CrossRef]
- Matic, Z.; Santak, M. Current view on novel vaccine technologies to combat human infectious diseases. Appl. Microbiol. Biotechnol. 2022, 106, 25–56. [Google Scholar] [CrossRef]
- Sunagar, R.; Prasad, S.D.; Ella, R.; Vadrevu, K.M. Preclinical evaluation of safety and immunogenicity of a primary series intranasal COVID-19 vaccine candidate (BBV154) and humoral immunogenicity evaluation of a heterologous prime-boost strategy with COVAXIN (BBV152). Front. Immunol. 2022, 13, 1063679. [Google Scholar] [CrossRef]
- Halperin, S.A.; Ye, L.; MacKinnon-Cameron, D.; Smith, B.; Cahn, P.E.; Ruiz-Palacios, G.M.; Ikram, A.; Lanas, F.; Lourdes Guerrero, M.; Munoz Navarro, S.R.; et al. Final efficacy analysis, interim safety analysis, and immunogenicity of a single dose of recombinant novel coronavirus vaccine (adenovirus type 5 vector) in adults 18 years and older: An international, multicentre, randomised, double-blinded, placebo-controlled phase 3 trial. Lancet 2022, 399, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Knisely, J.M.; Buyon, L.E.; Mandt, R.; Farkas, R.; Balasingam, S.; Bok, K.; Buchholz, U.J.; D’Souza, M.P.; Gordon, J.L.; King, D.F.L.; et al. Mucosal vaccines for SARS-CoV-2: Scientific gaps and opportunities-workshop report. NPJ Vaccines 2023, 8, 53. [Google Scholar] [CrossRef] [PubMed]
- Jones, I.; Roy, P. Sputnik V COVID-19 vaccine candidate appears safe and effective. Lancet 2021, 397, 642–643. [Google Scholar] [CrossRef] [PubMed]
- Logunov, D.Y.; Dolzhikova, I.V.; Zubkova, O.V.; Tukhvatullin, A.I.; Shcheblyakov, D.V.; Dzharullaeva, A.S.; Grousova, D.M.; Erokhova, A.S.; Kovyrshina, A.V.; Botikov, A.G.; et al. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: Two open, non-randomised phase 1/2 studies from Russia. Lancet 2020, 396, 887–897. [Google Scholar] [CrossRef]
- Shkoda, A.S.; Gushchin, V.A.; Ogarkova, D.A.; Stavitskaya, S.V.; Orlova, O.E.; Kuznetsova, N.A.; Keruntu, E.N.; Pochtovyi, A.A.; Pukhov, A.V.; Kleymenov, D.A.; et al. Sputnik V Effectiveness against Hospitalization with COVID-19 during Omicron Dominance. Vaccines 2022, 10, 938. [Google Scholar] [CrossRef]
- Sukhikh, G.T.; Priputnevich, T.V.; Ogarkova, D.A.; Pochtovyi, A.A.; Kustova, D.D.; Zlobin, V.I.; Logunov, D.Y.; Gushchin, V.A.; Gintsburg, A.L. Sputnik Light and Sputnik V Vaccination Is Effective at Protecting Medical Personnel from COVID-19 during the Period of Delta Variant Dominance. Vaccines 2022, 10, 1804. [Google Scholar] [CrossRef]
- Tukhvatulin, A.I.; Dolzhikova, I.V.; Shcheblyakov, D.V.; Zubkova, O.V.; Dzharullaeva, A.S.; Kovyrshina, A.V.; Lubenets, N.L.; Grousova, D.M.; Erokhova, A.S.; Botikov, A.G.; et al. An open, non-randomised, phase 1/2 trial on the safety, tolerability, and immunogenicity of single-dose vaccine “Sputnik Light” for prevention of coronavirus infection in healthy adults. Lancet Reg. Health Eur. 2021, 11, 100241. [Google Scholar] [CrossRef]
- Vanaparthy, R.; Mohan, G.; Vasireddy, D.; Atluri, P. Review of COVID-19 viral vector-based vaccines and COVID-19 variants. Infez. Med. 2021, 29, 328–338. [Google Scholar] [CrossRef]
- Bos, R.; Rutten, L.; van der Lubbe, J.E.M.; Bakkers, M.J.G.; Hardenberg, G.; Wegmann, F.; Zuijdgeest, D.; de Wilde, A.H.; Koornneef, A.; Verwilligen, A.; et al. Ad26 vector-based COVID-19 vaccine encoding a prefusion-stabilized SARS-CoV-2 Spike immunogen induces potent humoral and cellular immune responses. NPJ Vaccines 2020, 5, 91. [Google Scholar] [CrossRef]
- Sadoff, J.; Gray, G.; Vandebosch, A.; Cardenas, V.; Shukarev, G.; Grinsztejn, B.; Goepfert, P.A.; Truyers, C.; Fennema, H.; Spiessens, B.; et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against COVID-19. N. Eng. J. Med. 2021, 384, 2187–2201. [Google Scholar] [CrossRef]
- Asano, M.; Okada, H.; Itoh, Y.; Hirata, H.; Ishikawa, K.; Yoshida, E.; Matsui, A.; Kelly, E.J.; Shoemaker, K.; Olsson, U.; et al. Immunogenicity and safety of AZD1222 (ChAdOx1 nCoV-19) against SARS-CoV-2 in Japan: A double-blind, randomized controlled phase 1/2 trial. Int. J. Infect. Dis. 2022, 114, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Ewer, K.J.; Barrett, J.R.; Belij-Rammerstorfer, S.; Sharpe, H.; Makinson, R.; Morter, R.; Flaxman, A.; Wright, D.; Bellamy, D.; Bittaye, M.; et al. T cell and antibody responses induced by a single dose of ChAdOx1 nCoV-19 (AZD1222) vaccine in a phase 1/2 clinical trial. Nat. Med. 2021, 27, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Falsey, A.R.; Sobieszczyk, M.E.; Hirsch, I.; Sproule, S.; Robb, M.L.; Corey, L.; Neuzil, K.M.; Hahn, W.; Hunt, J.; Mulligan, M.J.; et al. Phase 3 Safety and Efficacy of AZD1222 (ChAdOx1 nCoV-19) COVID-19 Vaccine. N. Eng. J. Med. 2021, 385, 2348–2360. [Google Scholar] [CrossRef] [PubMed]
- Madhi, S.A.; Kwatra, G.; Richardson, S.I.; Koen, A.L.; Baillie, V.; Cutland, C.L.; Fairlie, L.; Padayachee, S.D.; Dheda, K.; Barnabas, S.L.; et al. Durability of ChAdOx1 nCoV-19 (AZD1222) vaccine and hybrid humoral immunity against variants including omicron BA.1 and BA.4 6 months after vaccination (COV005): A post-hoc analysis of a randomised, phase 1b-2a trial. Lancet Infect. Dis. 2023, 23, 295–306. [Google Scholar] [CrossRef]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Lv, J.; Wu, H.; Xu, J.; Liu, J. Immunogenicity and safety of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine: A systematic review. Infect. Dis. Poverty 2022, 11, 53. [Google Scholar] [CrossRef]
- Borobia, A.M.; Carcas, A.J.; Perez-Olmeda, M.; Castano, L.; Bertran, M.J.; Garcia-Perez, J.; Campins, M.; Portoles, A.; Gonzalez-Perez, M.; Garcia Morales, M.T.; et al. Immunogenicity and reactogenicity of BNT162b2 booster in ChAdOx1-S-primed participants (CombiVacS): A multicentre, open-label, randomised, controlled, phase 2 trial. Lancet 2021, 398, 121–130. [Google Scholar] [CrossRef]
- Hillus, D.; Schwarz, T.; Tober-Lau, P.; Vanshylla, K.; Hastor, H.; Thibeault, C.; Jentzsch, S.; Helbig, E.T.; Lippert, L.J.; Tscheak, P.; et al. Safety, reactogenicity, and immunogenicity of homologous and heterologous prime-boost immunisation with ChAdOx1 nCoV-19 and BNT162b2: A prospective cohort study. Lancet Respir. Med. 2021, 9, 1255–1265. [Google Scholar] [CrossRef]
- Liu, X.; Shaw, R.H.; Stuart, A.S.V.; Greenland, M.; Aley, P.K.; Andrews, N.J.; Cameron, J.C.; Charlton, S.; Clutterbuck, E.A.; Collins, A.M.; et al. Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): A single-blind, randomised, non-inferiority trial. Lancet 2021, 398, 856–869. [Google Scholar] [CrossRef]
- Pereson, M.J.; Amaya, L.; Neukam, K.; Bare, P.; Echegoyen, N.; Badano, M.N.; Lucero, A.; Martelli, A.; Garcia, G.H.; Videla, C.; et al. Heterologous gam-COVID-vac (sputnik V)/mRNA-1273 (moderna) vaccination induces a stronger humoral response than homologous sputnik V in a real-world data analysis. Clin. Microbiol. Infect. 2022, 28, 1382–1388. [Google Scholar] [CrossRef]
- Rose, W.; Raju, R.; Babji, S.; George, A.; Madhavan, R.; Leander Xavier, J.V.; David Chelladurai, J.S.; Nikitha, O.S.; Deborah, A.A.; Vijayakumar, S.; et al. Immunogenicity and safety of homologous and heterologous booster vaccination of ChAdOx1 nCoV-19 (COVISHIELD) and BBV152 (COVAXIN(R)): A non-inferiority phase 4, participant and observer-blinded, randomised study. Lancet Reg. Health Southeast Asia 2023, 12, 100141. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Chozhavel Rajanathan, T.M.; Chandra, H.; Pericherla, H.P.R.; Kumar, S.; Choonia, H.S.; Bajpai, M.; Singh, A.K.; Sinha, A.; Saini, G.; et al. Immunogenic potential of DNA vaccine candidate, ZyCoV-D against SARS-CoV-2 in animal models. Vaccine 2021, 39, 4108–4116. [Google Scholar] [CrossRef] [PubMed]
- Jin, P.; Guo, X.; Chen, W.; Ma, S.; Pan, H.; Dai, L.; Du, P.; Wang, L.; Jin, L.; Chen, Y.; et al. Safety and immunogenicity of heterologous boost immunization with an adenovirus type-5-vectored and protein-subunit-based COVID-19 vaccine (Convidecia/ZF2001): A randomized, observer-blinded, placebo-controlled trial. PLoS Med. 2022, 19, e1003953. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hou, L.; Guo, X.; Jin, P.; Wu, S.; Zhu, J.; Pan, H.; Wang, X.; Song, Z.; Wan, J.; et al. Heterologous AD5-nCOV plus CoronaVac versus homologous CoronaVac vaccination: A randomized phase 4 trial. Nat. Med. 2022, 28, 401–409. [Google Scholar] [CrossRef]
- Singh, C.; Verma, S.; Reddy, P.; Diamond, M.S.; Curiel, D.T.; Patel, C.; Jain, M.K.; Redkar, S.V.; Bhate, A.S.; Gundappa, V.; et al. Phase III Pivotal comparative clinical trial of intranasal (iNCOVACC) and intramuscular COVID-19 vaccine (Covaxin((R))). NPJ Vaccines 2023, 8, 125. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Coronavirus (COVID-19) Dashboard. Available online: https://COVID19.who.int/ (accessed on 22 September 2023).
- CDC. COVID-19 Treatments and Medications. Available online: https://www.cdc.gov/coronavirus/2019-ncov/your-health/treatments-for-severe-illness.html (accessed on 14 October 2023).
- CMS. COVID-19 Monoclonal Antibodies. Available online: https://www.cms.gov/monoclonal#:~:text=COVID%2D19%20Monoclonal%20Antibody%20Products&text=The%20FDA%20authorized%20the%20following,January%2026%2C%202023 (accessed on 14 October 2023).


| Characteristics | Vaccine for Long-Term Protection | Vaccine for Reactive Use |
|---|---|---|
| Indications | Use in long-term protection of persons at high ongoing risk of COVID-19; Potential for administration with other vaccines. | Reactive use in outbreak settings with rapid onset of immunity; Stand lone administration is acceptable. |
| Target population | Adult and children | Adult |
| Contraindications | Minor | Contraindications accepted in some conditions |
| Safety | Substantial evidence required | Acceptable if it outweighs potential risk |
| Dose regimen | Single or double dose along with mix and match options. | Double dose is preferred. |
| Durability | At least 1 year before use of another booster dose | Until protection from severe disease. |
| Route of administration | Preferably nasal or oral with no use of needle/syringe | Any suitable mode |
| Storage | Higher thermostability is preferred with shelf life of preferably 2 months at 2–8 °C. | Stability in deep freezer with shelf life of at least a month at 2–8 °C. |
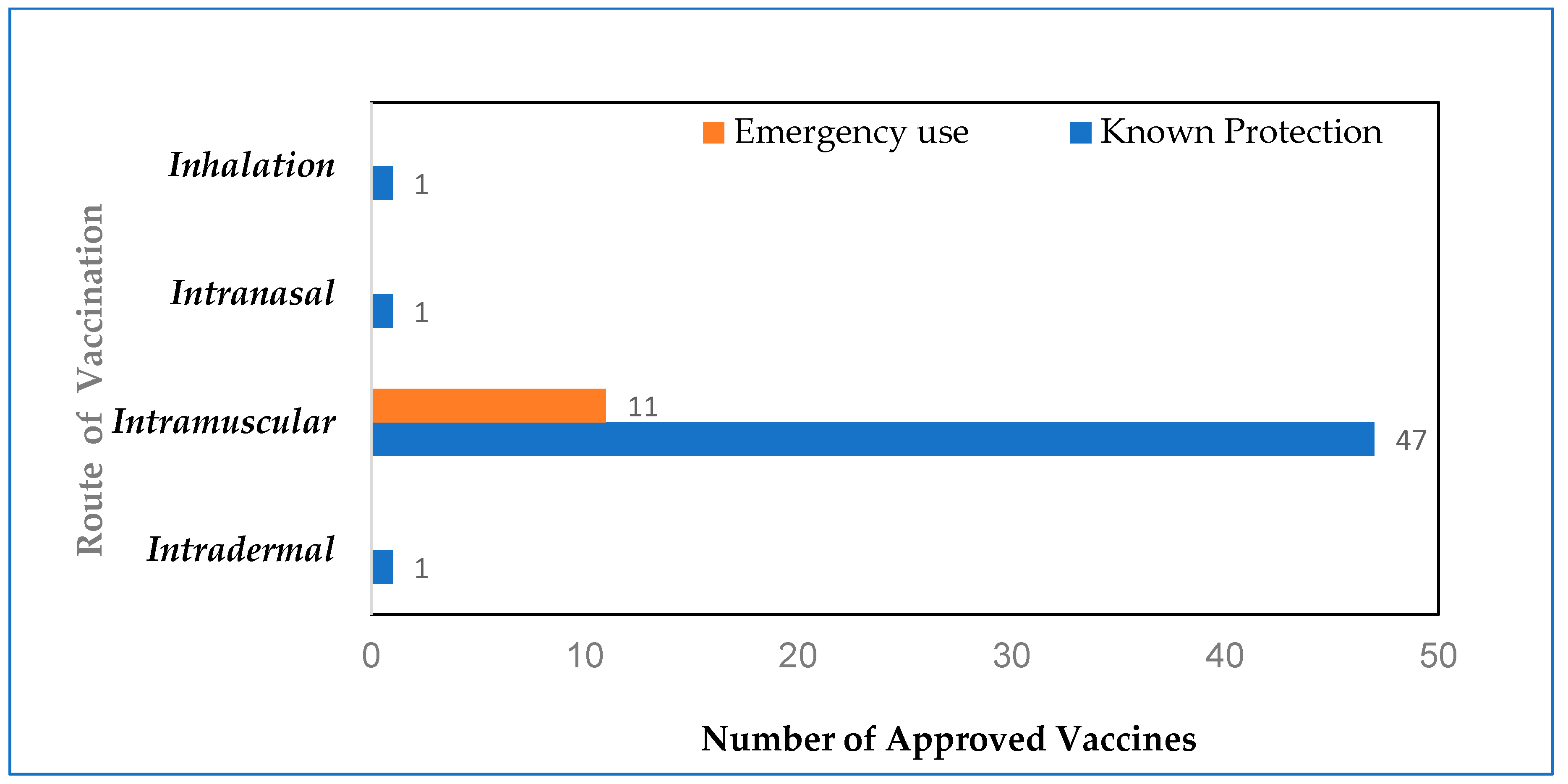
| Number of vaccines approved by WHO (as of August 2023) | 50 (47 administered intramuscularly) | 11 (all administered intramuscularly) |
| Type of Vaccine | Clinical Trials | Phase 4 | Phase 3 |
|---|---|---|---|
| Inactivated | 22 | 3 | 10 |
| Live attenuated | 2 | NIL | 1 |
| Protein subunit | 59 | 1 | 23 |
| DNA Vaccines | 17 | NIL | 2 |
| mRNA vaccines | 43 | 3 | 7 |
| Virus-like particle | 7 | NIL | 3 |
| Non-replicating viral vector | 26 | 4 | 3 |
| Replicating viral vector | 6 | NIL | 1 |
| S. No. | Type of Vaccine | Pros | Cons |
|---|---|---|---|
| 1 | Inactivated virus |
|
|
| 2 | Live attenuated virus |
|
|
| 3 | Protein subunit vaccine |
|
|
| 4 | DNA vaccine |
|
|
| 5 | mRNA vaccine |
|
|
| 6 | Virus-like particles (VLPs) |
|
|
| 7 | Viral vectors |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mathew, D.S.; Pandya, T.; Pandya, H.; Vaghela, Y.; Subbian, S. An Overview of SARS-CoV-2 Etiopathogenesis and Recent Developments in COVID-19 Vaccines. Biomolecules 2023, 13, 1565. https://doi.org/10.3390/biom13111565
Mathew DS, Pandya T, Pandya H, Vaghela Y, Subbian S. An Overview of SARS-CoV-2 Etiopathogenesis and Recent Developments in COVID-19 Vaccines. Biomolecules. 2023; 13(11):1565. https://doi.org/10.3390/biom13111565
Chicago/Turabian StyleMathew, Dona Susan, Tirtha Pandya, Het Pandya, Yuzen Vaghela, and Selvakumar Subbian. 2023. "An Overview of SARS-CoV-2 Etiopathogenesis and Recent Developments in COVID-19 Vaccines" Biomolecules 13, no. 11: 1565. https://doi.org/10.3390/biom13111565
APA StyleMathew, D. S., Pandya, T., Pandya, H., Vaghela, Y., & Subbian, S. (2023). An Overview of SARS-CoV-2 Etiopathogenesis and Recent Developments in COVID-19 Vaccines. Biomolecules, 13(11), 1565. https://doi.org/10.3390/biom13111565








