Protein-Targeting Drug Discovery
Author Contributions
Acknowledgments
Conflicts of Interest
List of Contributions
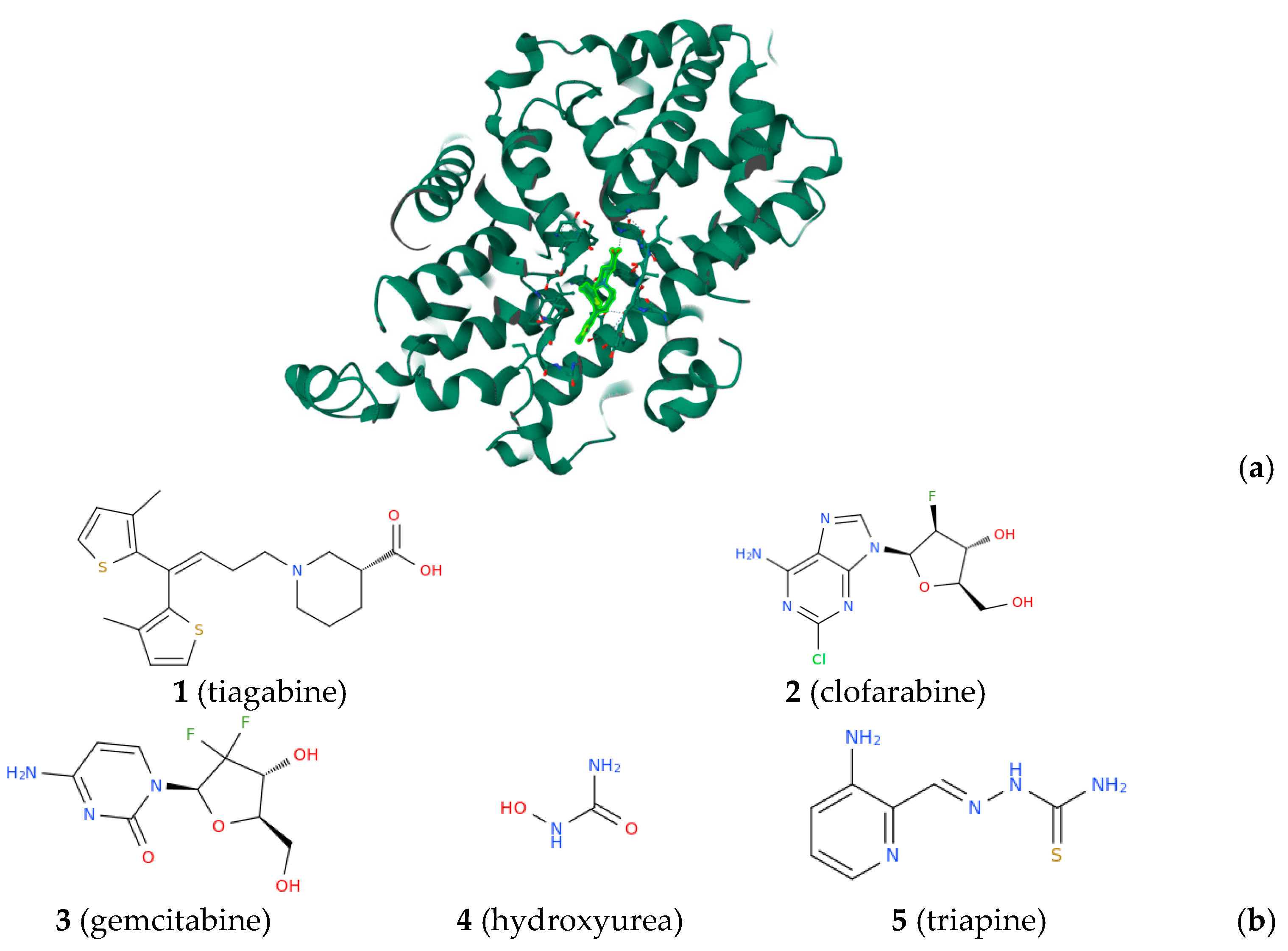
- Łątka, K.; Bajda, M. Analysis of Different Binding Modes for Tiagabine within the GAT-1 Transporter. Biomolecules 2022, 12, 1663.
- Donu, D.; Sharma, C.; Cen, Y. Plasmodium falciparum Nicotinamidase as A Novel Antimalarial Target. Biomolecules 2022, 12, 1109.
- Greco, F.; Falanga, A.P.; Terracciano, M.; D’Ambrosio, C.; Piccialli, G.; Oliviero, G.; Roviello, G.N.; Borbone, N. CD, UV, and In Silico Insights on the Effect of 1, 3-Bis (1′-uracilyl)-2-propanone on Serum Albumin Structure. Biomolecules 2022, 12, 1071.
- Scognamiglio, P.L.; Vicidomini, C.; Fontanella, F.; De Stefano, C.; Palumbo, R.; Roviello, G.N. Protein Binding of Benzofuran Derivatives: A CD Spectroscopic and In Silico Comparative Study of the Effects of 4-Nitrophenyl Functionalized Benzofurans and Benzodifurans on BSA Protein Structure. Biomolecules 2022, 12, 262.
- Szymańska, M.; Pospieszna-Markiewicz, I.; Mańka, M.; Insińska-Rak, M.; Dutkiewicz, G.; Patroniak, V.; Fik-Jaskółka, M.A. Synthesis and spectroscopic investigations of Schiff base ligand and its bimetallic Ag (I) complex as DNA and BSA binders. Biomolecules 2021, 11, 1449.
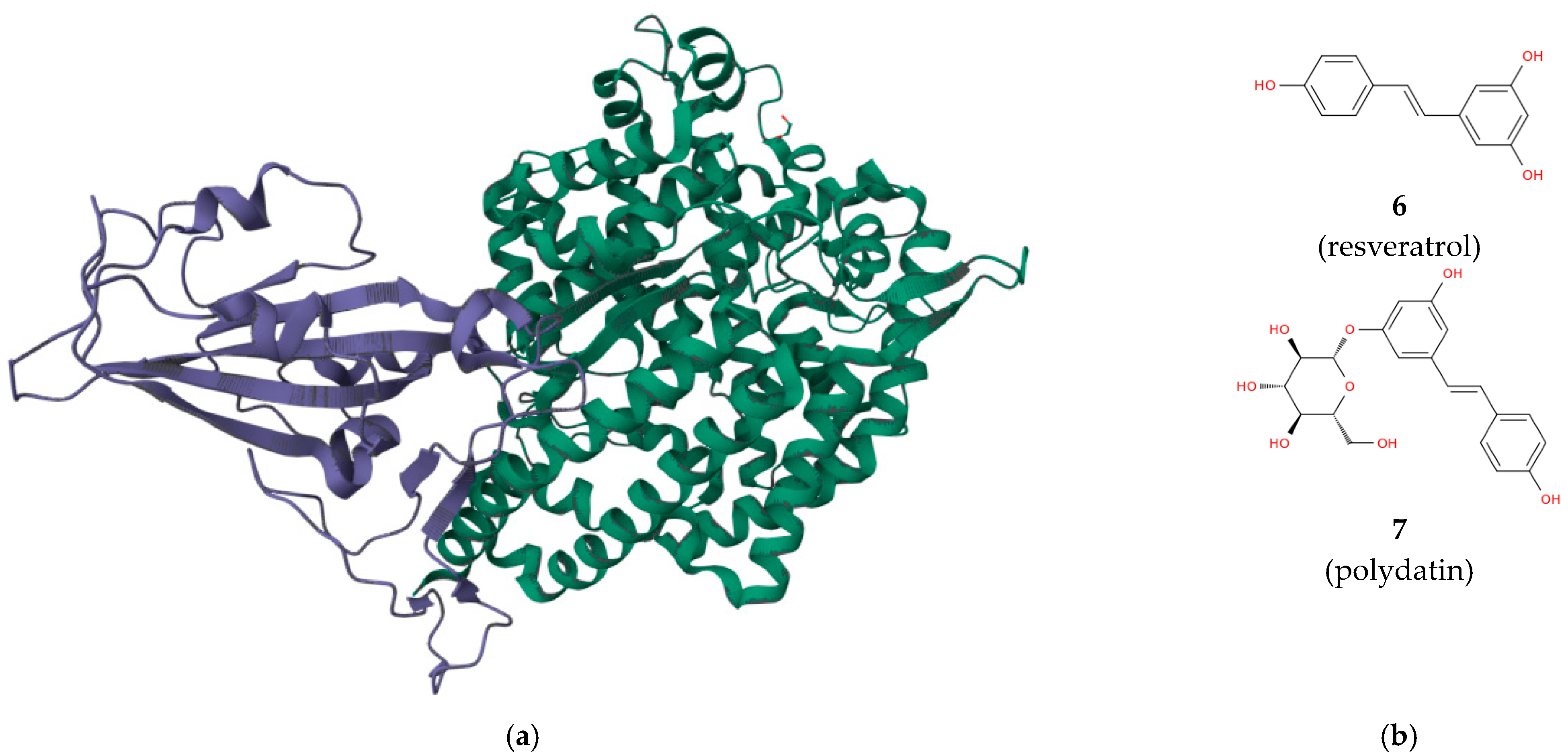
- Perrella, F.; Coppola, F.; Petrone, A.; Platella, C.; Montesarchio, D.; Stringaro, A.; Ravagnan, G.; Fuggetta, M.P.; Rega, N.; Musumeci, D. Interference of polydatin/resveratrol in the ACE2: Spike recognition during COVID-19 infection. A focus on their potential mechanism of action through computational and biochemical assays. Biomolecules 2021, 11, 1048.
- Huff, S.E.; Winter, J.M.; Dealwis, C.G. Inhibitors of the cancer target ribonucleotide reductase, past and present. Biomolecules 2022, 12, 815.
- Manco, G.; Lacerra, G.; Porzio, E.; Catara, G. ADP-Ribosylation Post-translational modification: An overview with a focus on RNA biology and new pharmacological perspectives. Biomolecules 2022, 12, 443.
- Wu, D.; Liu, X.; Mu, J.; Yang, J.; Wu, F.; Zhou, H. Therapeutic approaches targeting proteins in tumor-associated macrophages and their applications in cancers. Biomolecules 2022, 12, 392.
References
- Hanna, R.; Dalvi, S.; Sălăgean, T.; Pop, I.D.; Bordea, I.R.; Benedicenti, S. Understanding COVID-19 pandemic: Molecular mechanisms and potential therapeutic strategies. An evidence-based review. J. Inflamm. Res. 2021, 14, 13. [Google Scholar] [CrossRef] [PubMed]
- Soudijn, W.; van Wijngaarden, I. The GABA transporter and its inhibitors. Curr. Med. Chem. 2000, 7, 1063–1079. [Google Scholar] [CrossRef]
- Madsen, K.K.; White, H.S.; Schousboe, A. Neuronal and non-neuronal GABA transporters as targets for antiepileptic drugs. Pharmacol. Ther. 2010, 125, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Sałat, K.; Podkowa, A.; Mogilski, S.; Zaręba, P.; Kulig, K.; Sałat, R.; Malikowska, N.; Filipek, B. The effect of GABA transporter 1 (GAT1) inhibitor, tiagabine, on scopolamine-induced memory impairments in mice. Pharmacol. Rep. 2015, 67, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, J.K.; Kerwin, L.J.; Cobbold, S.A.; Tai, J.; Bedell, T.A.; Reider, P.J.; Llinás, M. Targeting NAD+ metabolism in the human malaria parasite Plasmodium falciparum. PLoS ONE 2014, 9, e94061. [Google Scholar] [CrossRef]
- Capasso, D.; Marino, P.; Di Gaetano, S.; Borbone, N.; Terracciano, M.; Trani, R.; Longo, C.; Piccialli, V. Synthesis of Brominated Lactones Related to Mycalin A: Selective Antiproliferative Activity on Metastatic Melanoma Cells and Inhibition of the Cell Migration. Mar. Drugs 2023, 21, 349. [Google Scholar] [CrossRef]
- Palumbo, R.; Simonyan, H.; Roviello, G.N. Advances in Amino Acid-Based Chemistry. Pharmaceuticals 2023, 16, 1490. [Google Scholar] [CrossRef]
- Parisi, E.; Capasso, D.; Capobianco, A.; Peluso, A.; Di Gaetano, S.; Fusco, S.; Manfredi, C.; Mozzillo, R.; Pinto, G.; Centore, R. Tautomeric and conformational switching in a new versatile N-rich heterocyclic ligand. Dalton Trans. 2020, 49, 14452–14462. [Google Scholar] [CrossRef] [PubMed]
- Tramontano, C.; Martins, J.P.; De Stefano, L.; Kemell, M.; Correia, A.; Terracciano, M.; Borbone, N.; Rea, I.; Santos, H.A. Microfluidic-Assisted Production of Gastro-Resistant Active-Targeted Diatomite Nanoparticles for the Local Release of Galunisertib in Metastatic Colorectal Cancer Cells. Adv. Healthc. Mater. 2023, 12, 2202672. [Google Scholar] [CrossRef]
- Bothou, C.; Sharma, A.; Oo, A.; Kim, B.; Perge, P.; Igaz, P.; Ronchi, C.L.; Shapiro, I.; Hantel, C. Novel insights into the molecular regulation of ribonucleotide reductase in adrenocortical carcinoma treatment. Cancers 2021, 13, 4200. [Google Scholar] [CrossRef]
- Wijerathna, S.R.; Ahmad, M.F.; Xu, H.; Fairman, J.W.; Zhang, A.; Kaushal, P.S.; Wan, Q.; Kiser, J.; Dealwis, C.G. Targeting the large subunit of human ribonucleotide reductase for cancer chemotherapy. Pharmaceuticals 2011, 4, 1328–1354. [Google Scholar] [CrossRef] [PubMed]
- Greene, B.L.; Kang, G.; Cui, C.; Bennati, M.; Nocera, D.G.; Drennan, C.L.; Stubbe, J. Ribonucleotide reductases: Structure, chemistry, and metabolism suggest new therapeutic targets. Annu. Rev. Biochem. 2020, 89, 45–75. [Google Scholar] [CrossRef] [PubMed]
- Gaur, K.; Pérez Otero, S.C.; Benjamín-Rivera, J.A.; Rodríguez, I.; Loza-Rosas, S.A.; Vázquez Salgado, A.M.; Akam, E.A.; Hernández-Matias, L.; Sharma, R.K.; Alicea, N. Iron chelator transmetalative approach to inhibit human ribonucleotide reductase. JACS Au 2021, 1, 865–878. [Google Scholar] [CrossRef]
- Croushore, E.E.; Koppenhafer, S.L.; Goss, K.L.; Geary, E.L.; Gordon, D.J. Activator Protein-1 (AP-1) Signaling Inhibits the Growth of Ewing Sarcoma Cells in Response to DNA Replication Stress. Cancer Res. Commun. 2023, 3, 1580–1593. [Google Scholar] [CrossRef] [PubMed]
- Rudd, S.G.; Tsesmetzis, N.; Sanjiv, K.; Paulin, C.B.; Sandhow, L.; Kutzner, J.; Hed Myrberg, I.; Bunten, S.S.; Axelsson, H.; Zhang, S.M. Ribonucleotide reductase inhibitors suppress SAMHD 1 ara-CTP ase activity enhancing cytarabine efficacy. EMBO Mol. Med. 2020, 12, e10419. [Google Scholar] [CrossRef]
- Poltronieri, P.; Miwa, M.; Masutani, M. ADP-ribosylation as post-translational modification of proteins: Use of inhibitors in cancer control. Int. J. Mol. Sci. 2021, 22, 10829. [Google Scholar] [CrossRef] [PubMed]
- Horne, J.R.; Vohl, M.-C. Biological plausibility for interactions between dietary fat, resveratrol, ACE2, and SARS-CoV illness severity. Am. J. Physiol.-Endocrinol. Metab. 2020, 318, E830–E833. [Google Scholar] [CrossRef]
- Ahmad, I.; Pawara, R.; Surana, S.; Patel, H. The repurposed ACE2 inhibitors: SARS-CoV-2 entry blockers of COVID-19. Top. Curr. Chem. 2021, 379, 40. [Google Scholar] [CrossRef]
- Agrawal, P.K.; Blunden, G. Phytochemicals Against SARS-CoV-2 Infection; SAGE Publications Sage: Los Angeles, CA, USA, 2023; Volume 18, p. 1934578X231152168. [Google Scholar]
- Wang, M.; Qin, K.; Zhai, X. Combined network pharmacology, molecular docking, and experimental verification approach to investigate the potential mechanisms of polydatin against COVID-19. Nat. Prod. Commun. 2022, 17, 1934578X221095352. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Li, Y.; Xu, G.C.; Li, J.Y.; Luo, H.Y.; Li, J.Y.; Zhang, L.; Jia, D.Z. Synthesis, crystal structure, DNA/bovine serum albumin binding and antitumor activity of two transition metal complexes with 4-acylpyrazolone derivative. Appl. Organomet. Chem. 2019, 33, e4668. [Google Scholar] [CrossRef]
- Van de Sande, L.; Cosyns, S.; Willaert, W.; Ceelen, W. Albumin-based cancer therapeutics for intraperitoneal drug delivery: A review. Drug Deliv. 2020, 27, 40–53. [Google Scholar] [CrossRef]
- Greco, F.; Falanga, A.P.; Terracciano, M.; D’Ambrosio, C.; Piccialli, G.; Oliviero, G.; Roviello, G.N.; Borbone, N. CD, UV, and In Silico Insights on the Effect of 1, 3-Bis (1′-uracilyl)-2-propanone on Serum Albumin Structure. Biomolecules 2022, 12, 1071. [Google Scholar] [CrossRef] [PubMed]
- Scognamiglio, P.L.; Riccardi, C.; Palumbo, R.; Gale, T.F.; Musumeci, D.; Roviello, G.N. Self-assembly of thyminyl l-tryptophanamide (TrpT) building blocks for the potential development of drug delivery nanosystems. J. Nanostruct. Chem. 2023, 1–19. [Google Scholar] [CrossRef]
- Scognamiglio, P.L.; Vicidomini, C.; Fontanella, F.; De Stefano, C.; Palumbo, R.; Roviello, G.N. Protein Binding of Benzofuran Derivatives: A CD Spectroscopic and In Silico Comparative Study of the Effects of 4-Nitrophenyl Functionalized Benzofurans and Benzodifurans on BSA Protein Structure. Biomolecules 2022, 12, 262. [Google Scholar] [CrossRef] [PubMed]
- Cassetta, L.; Pollard, J.W. Tumor-associated macrophages. Curr. Biol. 2020, 30, R246–R248. [Google Scholar] [CrossRef]
- Liu, H.; He, R.; Yang, X.; Huang, B.; Liu, H. Mechanism of TCF21 Downregulation Leading to Immunosuppression of Tumor-Associated Macrophages in Non-Small Cell Lung Cancer. Pharmaceutics 2023, 15, 2295. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.-K.; Huang, B.-R.; Charoensaensuk, V.; Yang, L.-Y.; Tsai, C.-F.; Liu, Y.-S.; Lu, D.-Y.; Yeh, W.-L.; Lin, C. Bradykinin B1 Receptor Affects Tumor-Associated Macrophage Activity and Glioblastoma Progression. Antioxidants 2023, 12, 1533. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vicidomini, C.; Roviello, G.N. Protein-Targeting Drug Discovery. Biomolecules 2023, 13, 1591. https://doi.org/10.3390/biom13111591
Vicidomini C, Roviello GN. Protein-Targeting Drug Discovery. Biomolecules. 2023; 13(11):1591. https://doi.org/10.3390/biom13111591
Chicago/Turabian StyleVicidomini, Caterina, and Giovanni N. Roviello. 2023. "Protein-Targeting Drug Discovery" Biomolecules 13, no. 11: 1591. https://doi.org/10.3390/biom13111591





