Mitochondrial Modulators: The Defender
Abstract
1. Introduction
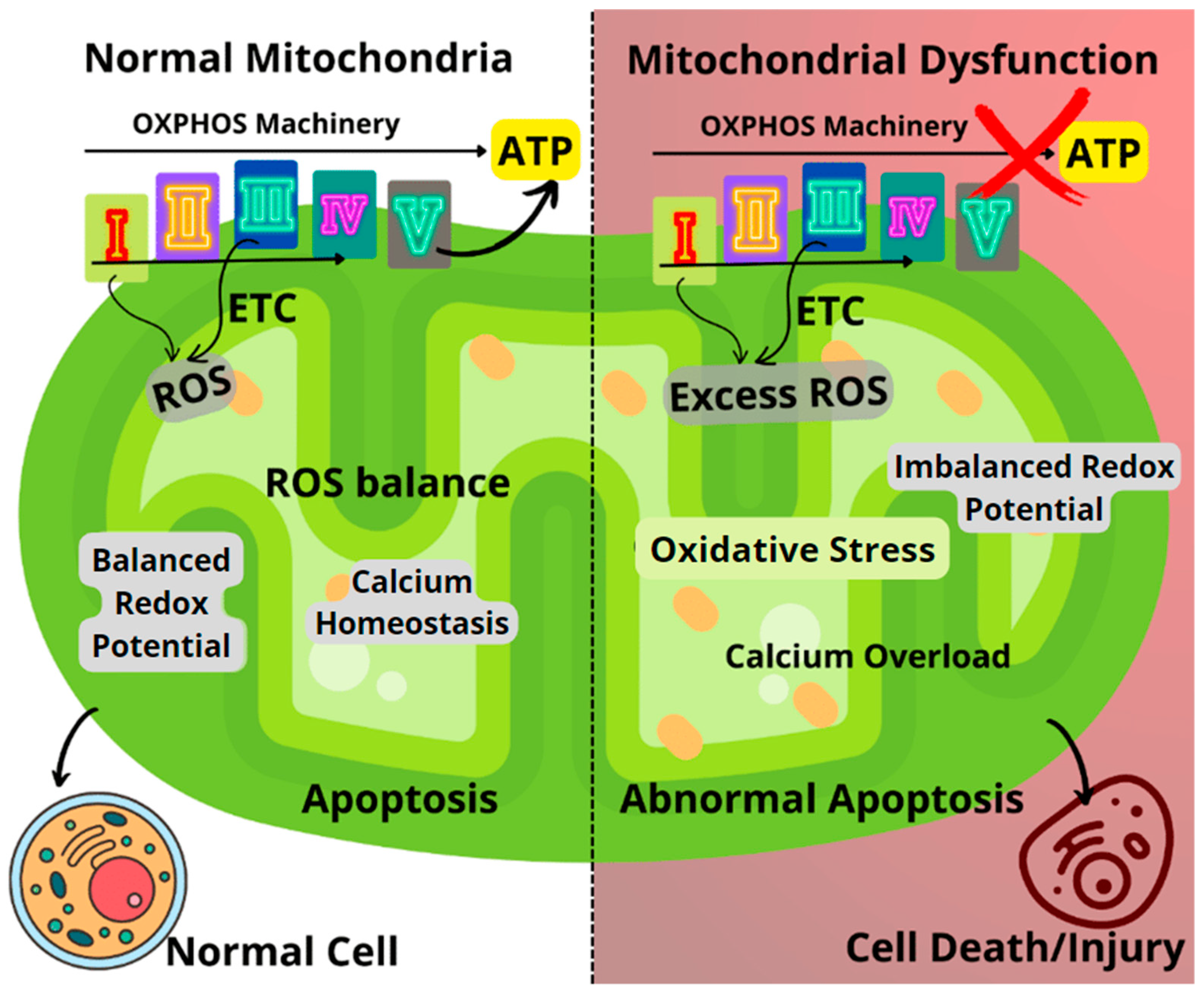
1.1. Mitochondria in Human Diseases
1.1.1. Mitochondria in Neurodegenerative Diseases and Ageing
1.1.2. Mitochondria in Metabolic Diseases
1.1.3. Mitochondria in Cancer
1.1.4. Mitochondria and infectious diseases
1.2. Materials and methods
2. Mitochondrial Modulators, Mechanisms, and Targets
2.1. Antioxidative Mechanisms of Mitochondrial Modulators
2.2. Inhibition of Apoptosis
2.3. Mitochondrial Biogenesis and Mitophagy
2.4. Other Effects of Mitochondrial Modulators
2.5. The Standouts
3. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, L.K.; Lu, J.; Bai, Y. Mitochondrial Respiratory Complex I: Structure, Function and Implication in Human Diseases. Curr. Med. Chem. 2009, 16, 1266–1277. [Google Scholar] [PubMed]
- Annesley, S.J.; Fisher, P.R. Mitochondria in Health and Disease. Cells 2019, 8, 680. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, J.B.; Haigis, M.C. The Multifaceted Contributions of Mitochondria to Cellular Metabolism. Nat. Cell Biol. 2018, 20, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Nowinski, S.M.; Solmonson, A.; Rusin, S.F.; Maschek, J.A.; Bensard, C.L.; Fogarty, S.; Jeong, M.-Y.; Lettlova, S.; Berg, J.A.; Morgan, J.T.; et al. Mitochondrial Fatty Acid Synthesis Coordinates Oxidative Metabolism in Mammalian Mitochondria. eLife 2020, 9, e58041. [Google Scholar] [CrossRef] [PubMed]
- Janssen, R.J.R.J.; Nijtmans, L.G.; van den Heuvel, L.P.; Smeitink, J.A.M. Mitochondrial Complex I: Structure, Function and Pathology. J. Inherit. Metab. Dis. 2006, 29, 499–515. [Google Scholar] [CrossRef]
- Mimaki, M.; Wang, X.; McKenzie, M.; Thorburn, D.R.; Ryan, M.T. Understanding Mitochondrial Complex I Assembly in Health and Disease. Biochim. Biophys. Acta BBA-Bioenerg. 2012, 1817, 851–862. [Google Scholar] [CrossRef]
- Lenaz, G.; Fato, R.; Genova, M.L.; Bergamini, C.; Bianchi, C.; Biondi, A. Mitochondrial Complex I: Structural and Functional Aspects. Biochim. Biophys. Acta BBA-Bioenerg. 2006, 1757, 1406–1420. [Google Scholar] [CrossRef]
- Nunnari, J.; Suomalainen, A. Mitochondria: In Sickness and in Health. Cell 2012, 148, 1145–1159. [Google Scholar] [CrossRef]
- Kausar, S.; Wang, F.; Cui, H. The Role of Mitochondria in Reactive Oxygen Species Generation and Its Implications for Neurodegenerative Diseases. Cells 2018, 7, 274. [Google Scholar] [CrossRef]
- Lenaz, G.; Bovina, C.; D’aurelio, M.; Fato, R.; Formiggini, G.; Genova, M.L.; Giuliano, G.; Pich, M.M.; Paolucci, U.; Castelli, G.P.; et al. Role of Mitochondria in Oxidative Stress and Aging. Ann. N. Y. Acad. Sci. 2002, 959, 199–213. [Google Scholar] [CrossRef]
- Trushina, E.; Trushin, S.; Hasan, M.F. Mitochondrial Complex I as a Therapeutic Target for Alzheimer’s Disease. Acta Pharm. Sin. B 2022, 12, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Zambrano, K.; Barba, D.; Castillo, K.; Noboa, L.; Argueta-Zamora, D.; Robayo, P.; Arizaga, E.; Caicedo, A.; Gavilanes, A.W.D. Fighting Parkinson’s Disease: The Return of the Mitochondria. Mitochondrion 2022, 64, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Grünewald, A.; Kumar, K.R.; Sue, C.M. New Insights into the Complex Role of Mitochondria in Parkinson’s Disease. Prog. Neurobiol. 2019, 177, 73–93. [Google Scholar] [CrossRef] [PubMed]
- Vona, R.; Ascione, B.; Malorni, W.; Straface, E. Mitochondria and Sex-Specific Cardiac Function. In Sex-Specific Analysis of Cardiovascular Function; Kerkhof, P.L.M., Miller, V.M., Eds.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2018; pp. 241–256. ISBN 978-3-319-77932-4. [Google Scholar]
- Matigian, N.; Abrahamsen, G.; Sutharsan, R.; Cook, A.L.; Vitale, A.M.; Nouwens, A.; Bellette, B.; An, J.; Anderson, M.; Beckhouse, A.G.; et al. Disease-Specific, Neurosphere-Derived Cells as Models for Brain Disorders. Dis. Model. Mech. 2010, 3, 785–798. [Google Scholar] [CrossRef] [PubMed]
- Bandara, A.B.; Drake, J.C.; Brown, D.A. Complex II Subunit SDHD Is Critical for Cell Growth and Metabolism, Which Can Be Partially Restored with a Synthetic Ubiquinone Analog. BMC Mol. Cell Biol. 2021, 22, 35. [Google Scholar] [CrossRef]
- Rango, M.; Bresolin, N. Brain Mitochondria, Aging, and Parkinson’s Disease. Genes 2018, 9, 250. [Google Scholar] [CrossRef]
- Rangaraju, V.; Lewis, T.L.; Hirabayashi, Y.; Bergami, M.; Motori, E.; Cartoni, R.; Kwon, S.-K.; Courchet, J. Pleiotropic Mitochondria: The Influence of Mitochondria on Neuronal Development and Disease. J. Neurosci. 2019, 39, 8200–8208. [Google Scholar] [CrossRef]
- Faris, R.; Moore, R.A.; Ward, A.; Sturdevant, D.E.; Priola, S.A. Mitochondrial Respiration Is Impaired during Late-Stage Hamster Prion Infection. J. Virol. 2017, 91, e00524-17. [Google Scholar] [CrossRef]
- Kann, O.; Kovács, R. Mitochondria and Neuronal Activity. Am. J. Physiol.-Cell Physiol. 2007, 292, C641–C657. [Google Scholar] [CrossRef]
- Picone, P.; Nuzzo, D.; Caruana, L.; Scafidi, V.; Di Carlo, M. Mitochondrial Dysfunction: Different Routes to Alzheimer’s Disease Therapy. Oxid. Med. Cell. Longev. 2014, 2014, e780179. [Google Scholar] [CrossRef]
- Murtaza, M.; Shan, J.; Matigian, N.; Todorovic, M.; Cook, A.L.; Ravishankar, S.; Dong, L.F.; Neuzil, J.; Silburn, P.; Mackay-Sim, A.; et al. Rotenone Susceptibility Phenotype in Olfactory Derived Patient Cells as a Model of Idiopathic Parkinson’s Disease. PLoS ONE 2016, 11, e0154544. [Google Scholar] [CrossRef]
- Cook, A.L.; Vitale, A.M.; Ravishankar, S.; Matigian, N.; Sutherland, G.T.; Shan, J.; Sutharsan, R.; Perry, C.; Silburn, P.A.; Mellick, G.D.; et al. NRF2 Activation Restores Disease Related Metabolic Deficiencies in Olfactory Neurosphere-Derived Cells from Patients with Sporadic Parkinson’s Disease. PLoS ONE 2011, 6, e21907. [Google Scholar] [CrossRef]
- Mohamed, T.M.; Youssef, M.A.M.; Bakry, A.A.; El-Keiy, M.M. Alzheimer’s Disease Improved through the Activity of Mitochondrial Chain Complexes and Their Gene Expression in Rats by Boswellic Acid. Metab. Brain Dis. 2021, 36, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.-H.; Zhao, X.-Y.; Yao, P.-P.; Xu, D.-E.; Ma, Q.-H. The Mitochondrion: A Potential Therapeutic Target for Alzheimer’s Disease. Neurosci. Bull. 2018, 34, 1127–1130. [Google Scholar] [CrossRef]
- Wada, J.; Nakatsuka, A. Mitochondrial Dynamics and Mitochondrial Dysfunction in Diabetes. Acta Med. Okayama 2016, 70, 151–158. [Google Scholar] [CrossRef]
- Henriksen, E.J.; Diamond-Stanic, M.K.; Marchionne, E.M. Oxidative Stress and the Etiology of Insulin Resistance and Type 2 Diabetes. Free Radic. Biol. Med. 2011, 51, 993–999. [Google Scholar] [CrossRef]
- Kujoth, G.C.; Hiona, A.; Pugh, T.D.; Someya, S.; Panzer, K.; Wohlgemuth, S.E.; Hofer, T.; Seo, A.Y.; Sullivan, R.; Jobling, W.A.; et al. Mitochondrial DNA Mutations, Oxidative Stress, and Apoptosis in Mammalian Aging. Science 2005, 309, 481–484. [Google Scholar] [CrossRef]
- Vona, R.; Gambardella, L.; Cittadini, C.; Straface, E.; Pietraforte, D. Biomarkers of Oxidative Stress in Metabolic Syndrome and Associated Diseases. Oxid. Med. Cell. Longev. 2019, 2019, e8267234. [Google Scholar] [CrossRef]
- Chiu, H.Y.; Tay, E.X.Y.; Ong, D.S.T.; Taneja, R. Mitochondrial Dysfunction at the Center of Cancer Therapy. Antioxid. Redox Signal. 2020, 32, 309–330. [Google Scholar] [CrossRef]
- Warburg, O. On the Origin of Cancer Cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef]
- Vaupel, P.; Multhoff, G. Revisiting the Warburg Effect: Historical Dogma versus Current Understanding. J. Physiol. 2021, 599, 1745–1757. [Google Scholar] [CrossRef] [PubMed]
- Vaupel, P.; Schmidberger, H.; Mayer, A. The Warburg Effect: Essential Part of Metabolic Reprogramming and Central Contributor to Cancer Progression. Int. J. Radiat. Biol. 2019, 95, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does It Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Sharma, L.K.; Fang, H.; Liu, J.; Vartak, R.; Deng, J.; Bai, Y. Mitochondrial Respiratory Complex I Dysfunction Promotes Tumorigenesis through ROS Alteration and AKT Activation. Hum. Mol. Genet. 2011, 20, 4605–4616. [Google Scholar] [CrossRef]
- Chatterjee, A.; Mambo, E.; Sidransky, D. Mitochondrial DNA Mutations in Human Cancer. Oncogene 2006, 25, 4663–4674. [Google Scholar] [CrossRef]
- Srinivasan, S.; Guha, M.; Kashina, A.; Avadhani, N.G. Mitochondrial Dysfunction and Mitochondrial Dynamics-The Cancer Connection. Biochim. Biophys. Acta BBA-Bioenerg. 2017, 1858, 602–614. [Google Scholar] [CrossRef]
- Sharma, P.; Sampath, H. Mitochondrial DNA Integrity: Role in Health and Disease. Cells 2019, 8, 100. [Google Scholar] [CrossRef]
- Guaragnella, N.; Giannattasio, S.; Moro, L. Mitochondrial Dysfunction in Cancer Chemoresistance. Biochem. Pharmacol. 2014, 92, 62–72. [Google Scholar] [CrossRef]
- Banoth, B.; Cassel, S.L. Mitochondria in Innate Immune Signaling. Transl. Res. 2018, 202, 52–68. [Google Scholar] [CrossRef]
- Tiku, V.; Tan, M.-W.; Dikic, I. Mitochondrial Functions in Infection and Immunity. Trends Cell Biol. 2020, 30, 263–275. [Google Scholar] [CrossRef]
- Andrieux, P.; Chevillard, C.; Cunha-Neto, E.; Nunes, J.P.S. Mitochondria as a Cellular Hub in Infection and Inflammation. Int. J. Mol. Sci. 2021, 22, 11338. [Google Scholar] [CrossRef] [PubMed]
- Brokatzky, D.; Häcker, G. Mitochondria: Intracellular Sentinels of Infections. Med. Microbiol. Immunol. (Berl.) 2022, 211, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Nakahira, K.; Hisata, S.; Choi, A.M.K. The Roles of Mitochondrial Damage-Associated Molecular Patterns in Diseases. Antioxid. Redox Signal. 2015, 23, 1329–1350. [Google Scholar] [CrossRef] [PubMed]
- Koch, R.E.; Josefson, C.C.; Hill, G.E. Mitochondrial Function, Ornamentation, and Immunocompetence. Biol. Rev. 2017, 92, 1459–1474. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-W.; Han, R.; He, H.-J.; Li, J.; Chen, S.-Y.; Gu, Y.; Xie, C. Administration of Quercetin Improves Mitochondria Quality Control and Protects the Neurons in 6-OHDA-Lesioned Parkinson’s Disease Models. Aging 2021, 13, 11738–11751. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.K.; Garabadu, D. Quercetin Exhibits A7nAChR/Nrf2/HO-1-Mediated Neuroprotection Against STZ-Induced Mitochondrial Toxicity and Cognitive Impairments in Experimental Rodents. Neurotox. Res. 2021, 39, 1859–1879. [Google Scholar] [CrossRef]
- Vanani, A.R.; Mahdavinia, M.; Shirani, M.; Alizadeh, S.; Dehghani, M.A. Protective Effects of Quercetin against Oxidative Stress Induced by Bisphenol-A in Rat Cardiac Mitochondria. Environ. Sci. Pollut. Res. 2020, 27, 15093–15102. [Google Scholar] [CrossRef]
- Zhang, Q.; Song, W.; Zhao, B.; Xie, J.; Sun, Q.; Shi, X.; Yan, B.; Tian, G.; Liang, X. Quercetin Attenuates Diabetic Peripheral Neuropathy by Correcting Mitochondrial Abnormality via Activation of AMPK/PGC-1α Pathway in vivo and in vitro. Front. Neurosci. 2021, 15, 636172. [Google Scholar]
- Kuang, L.; Cao, X.; Lu, Z. Baicalein Protects against Rotenone-Induced Neurotoxicity through Induction of Autophagy. Biol. Pharm. Bull. 2017, 40, 1537–1543. [Google Scholar] [CrossRef]
- Lee, I.K.; Kang, K.A.; Zhang, R.; Kim, B.J.; Kang, S.S.; Hyun, J.W. Mitochondria Protection of Baicalein against Oxidative Damage via Induction of Manganese Superoxide Dismutase. Environ. Toxicol. Pharmacol. 2011, 31, 233–241. [Google Scholar] [CrossRef]
- Wang, S.-F.; Liu, L.-F.; Wu, M.-Y.; Cai, C.-Z.; Su, H.; Tan, J.; Lu, J.-H.; Li, M. Baicalein Prevents 6-OHDA/Ascorbic Acid-Induced Calcium-Dependent Dopaminergic Neuronal Cell Death. Sci. Rep. 2017, 7, 8398. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Yang, B.; Qiao, Y.; Zhou, Q.; He, H.; He, M. Kaempferol Protects Mitochondria and Alleviates Damages against Endotheliotoxicity Induced by Doxorubicin. Biomed. Pharmacother. 2020, 126, 110040. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, K.; Prem, P.N.; Boovarahan, S.R.; Sivakumar, B.; Kurian, G.A. FIsetin Preserves Interfibrillar Mitochondria to Protect Against Myocardial Ischemia-Reperfusion Injury. Cell Biochem. Biophys. 2022, 80, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Alikatte, K.; Palle, S.; Rajendra Kumar, J.; Pathakala, N. Fisetin Improved Rotenone-Induced Behavioral Deficits, Oxidative Changes, and Mitochondrial Dysfunctions in Rat Model of Parkinson’s Disease. J. Diet. Suppl. 2021, 18, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Singh, A.K.; Garg, G.; Rizvi, S.I. Fisetin as a Caloric Restriction Mimetic Protects Rat Brain against Aging Induced Oxidative Stress, Apoptosis and Neurodegeneration. Life Sci. 2018, 193, 171–179. [Google Scholar] [CrossRef]
- Wu, B.; Song, H.; Fan, M.; You, F.; Zhang, L.; Luo, J.; Li, J.; Wang, L.; Li, C.; Yuan, M. Luteolin Attenuates Sepsis-induced Myocardial Injury by Enhancing Autophagy in Mice. Int. J. Mol. Med. 2020, 45, 1477–1487. [Google Scholar] [CrossRef]
- Akhtar, A.; Dhaliwal, J.; Sah, S.P. 7,8-Dihydroxyflavone Improves Cognitive Functions in ICV-STZ Rat Model of Sporadic Alzheimer’s Disease by Reversing Oxidative Stress, Mitochondrial Dysfunction, and Insulin Resistance. Psychopharmacology 2021, 238, 1991–2009. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, S.; Shao, Q.; Li, P.; Sun, Y.; Luo, L.; Yan, X.; Fan, Z.; Hu, J.; Zhao, J.; et al. Brain-Derived Neurotrophic Factor Mimetic, 7,8-Dihydroxyflavone, Protects against Myocardial Ischemia by Rebalancing Optic Atrophy 1 Processing. Free Radic. Biol. Med. 2019, 145, 187–197. [Google Scholar] [CrossRef]
- Kesh, S.; Kannan, R.R.; Balakrishnan, A. Naringenin Alleviates 6-Hydroxydopamine Induced Parkinsonism in SHSY5Y Cells and Zebrafish Model. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 239, 108893. [Google Scholar] [CrossRef]
- de Oliveira, M.R.; Custódio de Souza, I.C.; Fürstenau, C.R. Promotion of Mitochondrial Protection by Naringenin in Methylglyoxal-Treated SH-SY5Y Cells: Involvement of the Nrf2/GSH Axis. Chem. Biol. Interact. 2019, 310, 108728. [Google Scholar] [CrossRef]
- de Oliveira, M.R.; Brasil, F.B.; Andrade, C.M.B. Naringenin Attenuates H2O2-Induced Mitochondrial Dysfunction by an Nrf2-Dependent Mechanism in SH-SY5Y Cells. Neurochem. Res. 2017, 42, 3341–3350. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Wang, H. Naringenin Inhibit the Hydrogen Peroxide-Induced SH-SY5Y Cells Injury Through Nrf2/HO-1 Pathway. Neurotox. Res. 2019, 36, 796–805. [Google Scholar] [CrossRef]
- Luo, M.; Zheng, L.-W.; Wang, Y.-S.; Huang, J.-C.; Yang, Z.-Q.; Yue, Z.-P.; Guo, B. Genistein Exhibits Therapeutic Potential for PCOS Mice via the ER-Nrf2-Foxo1-ROS Pathway. Food Funct. 2021, 12, 8800–8811. [Google Scholar] [CrossRef]
- Qian, Y.; Guan, T.; Huang, M.; Cao, L.; Li, Y.; Cheng, H.; Jin, H.; Yu, D. Neuroprotection by the Soy Isoflavone, Genistein, via Inhibition of Mitochondria-Dependent Apoptosis Pathways and Reactive Oxygen Induced-NF-ΚB Activation in a Cerebral Ischemia Mouse Model. Neurochem. Int. 2012, 60, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Farruggio, S.; Raina, G.; Cocomazzi, G.; Librasi, C.; Mary, D.; Gentilli, S.; Grossini, E. Genistein Improves Viability, Proliferation and Mitochondrial Function of Cardiomyoblasts Cultured in Physiologic and Peroxidative Conditions. Int. J. Mol. Med. 2019, 44, 2298–2310. [Google Scholar] [CrossRef]
- Hua, W.; Li, S.; Luo, R.; Wu, X.; Zhang, Y.; Liao, Z.; Song, Y.; Wang, K.; Zhao, K.; Yang, S.; et al. Icariin Protects Human Nucleus Pulposus Cells from Hydrogen Peroxide-Induced Mitochondria-Mediated Apoptosis by Activating Nuclear Factor Erythroid 2-Related Factor 2. Biochim. Biophys. Acta BBA-Mol. Basis Dis. 2020, 1866, 165575. [Google Scholar] [CrossRef]
- Zhang, T.; Mu, Y.; Yang, M.; Al Maruf, A.; Li, P.; Li, C.; Dai, S.; Lu, J.; Dong, Q. (+)-Catechin Prevents Methylglyoxal-Induced Mitochondrial Dysfunction and Apoptosis in EA.Hy926 Cells. Arch. Physiol. Biochem. 2017, 123, 121–127. [Google Scholar] [CrossRef]
- Rafiei, H.; Omidian, K.; Bandy, B. Dietary Polyphenols Protect Against Oleic Acid-Induced Steatosis in an in vitro Model of NAFLD by Modulating Lipid Metabolism and Improving Mitochondrial Function. Nutrients 2019, 11, 541. [Google Scholar] [CrossRef]
- Silva Santos, L.F.; Stolfo, A.; Calloni, C.; Salvador, M. Catechin and Epicatechin Reduce Mitochondrial Dysfunction and Oxidative Stress Induced by Amiodarone in Human Lung Fibroblasts. J. Arrhythmia 2017, 33, 220–225. [Google Scholar] [CrossRef]
- Ling, J.; Wu, Y.; Zou, X.; Chang, Y.; Li, G.; Fang, M. (−)-Epicatechin Reduces Neuroinflammation, Protects Mitochondria Function, and Prevents Cognitive Impairment in Sepsis-Associated Encephalopathy. Oxid. Med. Cell. Longev. 2022, 2022, 2657713. [Google Scholar] [CrossRef]
- Wu, Q.; Li, Z.; Lu, X.; Song, J.; Wang, H.; Liu, D.; Guo, D.; Bi, H. Epigallocatechin Gallate Protects the Human Lens Epithelial Cell Survival against UVB Irradiation through AIF/Endo G Signalling Pathways in vitro. Cutan. Ocul. Toxicol. 2021, 40, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Song, J.; Gao, Y.; Zou, Y.; Guo, J.; Zhang, X.; Liu, D.; Guo, D.; Bi, H. Epigallocatechin Gallate Enhances Human Lens Epithelial Cell Survival after UVB Irradiation via the Mitochondrial Signaling Pathway. Mol. Med. Rep. 2022, 25, 87. [Google Scholar] [CrossRef] [PubMed]
- Antunes, M.S.; Ladd, F.V.L.; Ladd, A.A.B.L.; Moreira, A.L.; Boeira, S.P.; Cattelan Souza, L. Hesperidin Protects against Behavioral Alterations and Loss of Dopaminergic Neurons in 6-OHDA-Lesioned Mice: The Role of Mitochondrial Dysfunction and Apoptosis. Metab. Brain Dis. 2021, 36, 153–167. [Google Scholar] [CrossRef] [PubMed]
- Kamaraj, S.; Anandakumar, P.; Jagan, S.; Ramakrishnan, G.; Devaki, T. Hesperidin Attenuates Mitochondrial Dysfunction during Benzo(a)Pyrene-Induced Lung Carcinogenesis in Mice. Fundam. Clin. Pharmacol. 2011, 25, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.N.; Lim, J.H.; Kim, M.Y.; Ban, T.H.; Jang, I.-A.; Yoon, H.E.; Park, C.W.; Chang, Y.S.; Choi, B.S. Resveratrol, an Nrf2 Activator, Ameliorates Aging-Related Progressive Renal Injury. Aging 2018, 10, 83–99. [Google Scholar] [CrossRef]
- Chen, J.; Liu, Q.; Wang, Y.; Guo, Y.; Xu, X.; Huang, P.; Lian, B.; Zhang, R.; Chen, Y.; Ha, Y. Protective Effects of Resveratrol Liposomes on Mitochondria in Substantia Nigra Cells of Parkinsonized Rats. Ann. Palliat. Med. 2021, 10, 2458468. [Google Scholar] [CrossRef]
- Ma, J.; Wang, Z.; Zhao, J.; Miao, W.; Ye, T.; Chen, A. Resveratrol Attenuates Lipopolysaccharides (LPS)-Induced Inhibition of Osteoblast Differentiation in MC3T3-E1 Cells. Med. Sci. Monit. 2018, 24, 2045–2052. [Google Scholar] [CrossRef]
- Dewapriya, P.; Himaya, S.W.A.; Li, Y.-X.; Kim, S.-K. Tyrosol Exerts a Protective Effect against Dopaminergic Neuronal Cell Death in in vitro Model of Parkinson’s Disease. Food Chem. 2013, 141, 1147–1157. [Google Scholar] [CrossRef]
- Hsu, S.-S.; Lin, Y.-S.; Liang, W.-Z. Inhibition of the Pesticide Rotenone-Induced Ca2+ Signaling, Cytotoxicity and Oxidative Stress in HCN-2 Neuronal Cells by the Phenolic Compound Hydroxytyrosol. Pestic. Biochem. Physiol. 2021, 179, 104979. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, L.; Zhu, L.; Jia, X.; Li, X.; Jia, H.; Wang, Y.; Weber, P.; Long, J.; Liu, J. Hydroxytyrosol Protects Retinal Pigment Epithelial Cells from Acrolein-Induced Oxidative Stress and Mitochondrial Dysfunction. J. Neurochem. 2007, 103, 2690–2700. [Google Scholar]
- Naserzadeh, P.; Mehr, S.N.; Sadabadi, Z.; Seydi, E.; Salimi, A.; Pourahmad, J. Curcumin Protects Mitochondria and Cardiomyocytes from Oxidative Damage and Apoptosis Induced by Hemiscorpius Lepturus Venom. Drug Res. 2018, 68, 113–120. [Google Scholar] [CrossRef]
- Uğuz, A.C.; Öz, A.; Nazıroğlu, M. Curcumin Inhibits Apoptosis by Regulating Intracellular Calcium Release, Reactive Oxygen Species and Mitochondrial Depolarization Levels in SH-SY5Y Neuronal Cells. J. Recept. Signal Transduct. 2016, 36, 395–401. [Google Scholar] [CrossRef]
- Han, M.H.; Park, C.; Lee, D.-S.; Hong, S.-H.; Choi, I.-W.; Kim, G.-Y.; Choi, S.H.; Shim, J.-H.; Chae, J.-I.; Yoo, Y.H.; et al. Cytoprotective Effects of Esculetin against Oxidative Stress Are Associated with the Upregulation of Nrf2-Mediated NQO1 Expression via the Activation of the ERK Pathway. Int. J. Mol. Med. 2017, 39, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Guan, S.; Jiang, B.; Bao, Y.M.; An, L.J. Protocatechuic Acid Suppresses MPP+-Induced Mitochondrial Dysfunction and Apoptotic Cell Death in PC12 Cells. Food Chem. Toxicol. 2006, 44, 1659–1666. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-M.; Jiang, B.; Bao, Y.-M.; An, L.-J. Protocatechuic Acid Inhibits Apoptosis by Mitochondrial Dysfunction in Rotenone-Induced PC12 Cells. Toxicol. In Vitro 2008, 22, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Ya, F.; Li, K.; Chen, H.; Tian, Z.; Fan, D.; Shi, Y.; Song, F.; Xu, X.; Ling, W.; Adili, R.; et al. Protocatechuic Acid Protects Platelets from Apoptosis via Inhibiting Oxidative Stress-Mediated PI3K/Akt/GSK3β Signaling. Thromb. Haemost. 2021, 121, 931–943. [Google Scholar] [CrossRef]
- Wang, X.-J.; Wang, Z.-B.; Xu, J.-X. Effect of Salvianic Acid A on Lipid Peroxidation and Membrane Permeability in Mitochondria. J. Ethnopharmacol. 2005, 97, 441–445. [Google Scholar] [CrossRef]
- Wang, X.-J.; Xu, J.-X. Salvianic Acid A Protects Human Neuroblastoma SH-SY5Y Cells against MPP+-Induced Cytotoxicity. Neurosci. Res. 2005, 51, 129–138. [Google Scholar] [CrossRef]
- Bai, Q.; Wang, Z.; Piao, Y.; Zhou, X.; Piao, Q.; Jiang, J.; Liu, H.; Piao, H.; Li, L.; Song, Y.; et al. Sesamin Alleviates Asthma Airway Inflammation by Regulating Mitophagy and Mitochondrial Apoptosis. J. Agric. Food Chem. 2022, 70, 4921–4933. [Google Scholar] [CrossRef]
- Ding, Y.; Kong, D.; Zhou, T.; Yang, N.; Xin, C.; Xu, J.; Wang, Q.; Zhang, H.; Wu, Q.; Lu, X.; et al. α-Arbutin Protects Against Parkinson’s Disease-Associated Mitochondrial Dysfunction in vitro and in vivo. NeuroMolecular Med. 2020, 22, 56–67. [Google Scholar] [CrossRef]
- Mohammad Khanlou, E.; Atashbar, S.; Kahrizi, F.; Shokouhi Sabet, N.; Salimi, A. Bevacizumab as a Monoclonal Antibody Inhibits Mitochondrial Complex II in Isolated Rat Heart Mitochondria: Ameliorative Effect of Ellagic Acid. Drug Chem. Toxicol. 2022, 45, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Cuevas-Magaña, M.Y.; Vega-García, C.C.; León-Contreras, J.C.; Hernández-Pando, R.; Zazueta, C.; García-Niño, W.R. Ellagic Acid Ameliorates Hexavalent Chromium-Induced Renal Toxicity by Attenuating Oxidative Stress, Suppressing TNF-α and Protecting Mitochondria. Toxicol. Appl. Pharmacol. 2022, 454, 116242. [Google Scholar] [CrossRef] [PubMed]
- Firdaus, F.; Zafeer, M.F.; Waseem, M.; Anis, E.; Hossain, M.M.; Afzal, M. Ellagic Acid Mitigates Arsenic-Trioxide-Induced Mitochondrial Dysfunction and Cytotoxicity in SH-SY5Y Cells. J. Biochem. Mol. Toxicol. 2018, 32, e22024. [Google Scholar] [CrossRef]
- Khodaei, F.; Rashedinia, M.; Heidari, R.; Rezaei, M.; Khoshnoud, M.J. Ellagic Acid Improves Muscle Dysfunction in Cuprizone-Induced Demyelinated Mice via Mitochondrial Sirt3 Regulation. Life Sci. 2019, 237, 116954. [Google Scholar] [CrossRef]
- Ebrahimi, R.; Sepand, M.R.; Seyednejad, S.A.; Omidi, A.; Akbariani, M.; Gholami, M.; Sabzevari, O. Ellagic Acid Reduces Methotrexate-Induced Apoptosis and Mitochondrial Dysfunction via up-Regulating Nrf2 Expression and Inhibiting the IĸBα/NFĸB in Rats. DARU J. Pharm. Sci. 2019, 27, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, M.; Manivasagam, T.; Essa, M.M.; Tamilselvam, K.; Selvakumar, G.P.; Karthikeyan, S.; Thenmozhi, J.A.; Subash, S. Mangiferin Antagonizes Rotenone: Induced Apoptosis Through Attenuating Mitochondrial Dysfunction and Oxidative Stress in SK-N-SH Neuroblastoma Cells. Neurochem. Res. 2014, 39, 668–676. [Google Scholar] [CrossRef]
- Tang, Z.; Lai, C.-C.; Luo, J.; Ding, Y.-T.; Chen, Q.; Guan, Z.-Z. Mangiferin Prevents the Impairment of Mitochondrial Dynamics and an Increase in Oxidative Stress Caused by Excessive Fluoride in SH-SY5Y Cells. J. Biochem. Mol. Toxicol. 2021, 35, e22705. [Google Scholar] [CrossRef]
- Wang, X.-L.; Feng, S.-T.; Wang, Y.-T.; Zhang, N.-N.; Guo, Z.-Y.; Yan, X.; Yuan, Y.-H.; Wang, Z.-Z.; Chen, N.-H.; Zhang, Y. Mangiferin, a Natural Glucoxilxanthone, Inhibits Mitochondrial Dynamin-Related Protein 1 and Relieves Aberrant Mitophagic Proteins in Mice Model of Parkinson’s Disease. Phytomedicine 2022, 104, 154281. [Google Scholar] [CrossRef] [PubMed]
- Worakajit, N.; Thipboonchoo, N.; Chaturongakul, S.; Jutabha, P.; Soontornniyomkij, V.; Tuchinda, P.; Soodvilai, S. Nephroprotective Potential of Panduratin A against Colistin-Induced Renal Injury via Attenuating Mitochondrial Dysfunction and Cell Apoptosis. Biomed. Pharmacother. 2022, 148, 112732. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.-M.; Li, L.-D.; Duan, C.-L.; Li, Y.-J. Neuroprotective Effect of α-Mangostin on Mitochondrial Dysfunction and α-Synuclein Aggregation in Rotenone-Induced Model of Parkinson’s Disease in Differentiated SH-SY5Y Cells. J. Asian Nat. Prod. Res. 2017, 19, 833–845. [Google Scholar] [CrossRef]
- Cai, G.; Lin, F.; Wu, D.; Lin, C.; Chen, H.; Wei, Y.; Weng, H.; Chen, Z.; Wu, M.; Huang, E.; et al. Rosmarinic Acid Inhibits Mitochondrial Damage by Alleviating Unfolded Protein Response. Front. Pharmacol. 2022, 13, 859978. [Google Scholar] [PubMed]
- Diao, J.; Zhao, H.; You, P.; You, H.; Wu, H.; Shou, X.; Cheng, G. Rosmarinic Acid Ameliorated Cardiac Dysfunction and Mitochondrial Injury in Diabetic Cardiomyopathy Mice via Activation of the SIRT1/PGC-1α Pathway. Biochem. Biophys. Res. Commun. 2021, 546, 29–34. [Google Scholar] [CrossRef]
- Han, X.; Han, B.; Zhao, Y.; Li, G.; Wang, T.; He, J.; Du, W.; Cao, X.; Gan, J.; Wang, Z.; et al. Rosmarinic Acid Attenuates Rotenone-Induced Neurotoxicity in SH-SY5Y Parkinson’s Disease Cell Model through Abl Inhibition. Nutrients 2022, 14, 3508. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Han, Y.; Wang, Z.; Chen, T.; Qian, H.; He, J.; Li, J.; Han, B.; Wang, T. Rosmarinic Acid Protects against 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine-Induced Dopaminergic Neurotoxicity in Zebrafish Embryos. Toxicol. In Vitro 2020, 65, 104823. [Google Scholar] [CrossRef]
- Singh, S.S.; Rai, S.N.; Birla, H.; Zahra, W.; Rathore, A.S.; Dilnashin, H.; Singh, R.; Singh, S.P. Neuroprotective Effect of Chlorogenic Acid on Mitochondrial Dysfunction-Mediated Apoptotic Death of DA Neurons in a Parkinsonian Mouse Model. Oxid. Med. Cell. Longev. 2020, 2020, e6571484. [Google Scholar] [CrossRef] [PubMed]
- Tsai, K.-L.; Hung, C.-H.; Chan, S.-H.; Hsieh, P.-L.; Ou, H.-C.; Cheng, Y.-H.; Chu, P.-M. Chlorogenic Acid Protects Against OxLDL-Induced Oxidative Damage and Mitochondrial Dysfunction by Modulating SIRT1 in Endothelial Cells. Mol. Nutr. Food Res. 2018, 62, 1700928. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, L.; Ruan, Z.; Mi, S.; Jiang, M.; Li, X.; Wu, X.; Deng, Z.; Yin, Y. Chlorogenic Acid Ameliorates Intestinal Mitochondrial Injury by Increasing Antioxidant Effects and Activity of Respiratory Complexes. Biosci. Biotechnol. Biochem. 2016, 80, 962–971. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, J.; Wan, Z.; Li, G.; Chen, L.; Guo, Y. Theaflavin Ameliorates Renal Ischemia/Reperfusion Injury by Activating the Nrf2 Signalling Pathway in vivo and in vitro. Biomed. Pharmacother. 2021, 134, 111097. [Google Scholar] [CrossRef]
- Sun, J.; Leng, P.; Li, X.; Guo, Q.; Zhao, J.; Liang, Y.; Zhang, X.; Yang, X.; Li, J. Salvianolic Acid A Promotes Mitochondrial Biogenesis and Mitochondrial Function in 3T3-L1 Adipocytes through Regulation of the AMPK-PGC1α Signalling Pathway. Adipocyte 2022, 11, 562–571. [Google Scholar] [CrossRef]
- Li, X.; Fan, J.; Liu, J.; Liang, L. Salvianolic Acid A Protects Neonatal Cardiomyocytes Against Hypoxia/Reoxygenation-Induced Injury by Preserving Mitochondrial Function and Activating Akt/GSK-3β Signals. Chin. J. Integr. Med. 2019, 25, 23–30. [Google Scholar] [CrossRef]
- Wang, D.; Lu, X.; Wang, E.; Shi, L.; Ma, C.; Tan, X. Salvianolic Acid B Attenuates Oxidative Stress-Induced Injuries in Enterocytes by Activating Akt/GSK3β Signaling and Preserving Mitochondrial Function. Eur. J. Pharmacol. 2021, 909, 174408. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, Y.; Zhang, J.; Yang, G. Salvianolic Acid B Protects against MPP+-Induced Neuronal Injury via Repressing Oxidative Stress and Restoring Mitochondrial Function. NeuroReport 2021, 32, 815–823. [Google Scholar] [CrossRef]
- Alvariño, R.; Alonso, E.; Tribalat, M.-A.; Gegunde, S.; Thomas, O.P.; Botana, L.M. Evaluation of the Protective Effects of Sarains on H2O2-Induced Mitochondrial Dysfunction and Oxidative Stress in SH-SY5Y Neuroblastoma Cells. Neurotox. Res. 2017, 32, 368–380. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, P.; Vaibhav, K.; Tabassum, R.; Khan, A.; Ishrat, T.; Khan, M.M.; Ahmad, A.; Islam, F.; Safhi, M.M.; Islam, F. Anti-Apoptotic and Anti-Inflammatory Effect of Piperine on 6-OHDA Induced Parkinson’s Rat Model. J. Nutr. Biochem. 2013, 24, 680–687. [Google Scholar] [CrossRef] [PubMed]
- Dutta, M.; Ghosh, A.K.; Mishra, P.; Jain, G.; Rangari, V.; Chattopadhyay, A.; Das, T.; Bhowmick, D.; Bandyopadhyay, D. Protective Effects of Piperine against Copper-Ascorbate Induced Toxic Injury to Goat Cardiac Mitochondria in vitro. Food Funct. 2014, 5, 2252–2267. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, P.; Ali, M.; Salman, M.; Tabassum, H.; Parvez, S. Harnessing the Mitochondrial Integrity for Neuroprotection: Therapeutic Role of Piperine against Experimental Ischemic Stroke. Neurochem. Int. 2021, 149, 105138. [Google Scholar] [CrossRef]
- Nakaso, K.; Ito, S.; Nakashima, K. Caffeine Activates the PI3K/Akt Pathway and Prevents Apoptotic Cell Death in a Parkinson’s Disease Model of SH-SY5Y Cells. Neurosci. Lett. 2008, 432, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Dragicevic, N.; Delic, V.; Cao, C.; Copes, N.; Lin, X.; Mamcarz, M.; Wang, L.; Arendash, G.W.; Bradshaw, P.C. Caffeine Increases Mitochondrial Function and Blocks Melatonin Signaling to Mitochondria in Alzheimer’s Mice and Cells. Neuropharmacology 2012, 63, 1368–1379. [Google Scholar] [CrossRef]
- Kolahdouzan, M.; Hamadeh, M.J. The Neuroprotective Effects of Caffeine in Neurodegenerative Diseases. CNS Neurosci. Ther. 2017, 23, 272–290. [Google Scholar] [CrossRef]
- Mishra, J.; Kumar, A. Improvement of Mitochondrial NAD+/FAD+-Linked State-3 Respiration by Caffeine Attenuates Quinolinic Acid Induced Motor Impairment in Rats: Implications in Huntington’s Disease. Pharmacol. Rep. 2014, 66, 1148–1155. [Google Scholar] [CrossRef]
- Abu Bakar, M.H.; Nor Shahril, N.S.; Mohamad Khalid, M.S.F.; Mohammad, S.; Shariff, K.A.; Karunakaran, T.; Mohd Salleh, R.; Mohamad Rosdi, M.N. Celastrol Alleviates High-Fat Diet-Induced Obesity via Enhanced Muscle Glucose Utilization and Mitochondrial Oxidative Metabolism-Mediated Upregulation of Pyruvate Dehydrogenase Complex. Toxicol. Appl. Pharmacol. 2022, 449, 116099. [Google Scholar] [CrossRef] [PubMed]
- Abu Bakar, M.H.; Shariff, K.A.; Tan, J.S.; Lee, L.K. Celastrol Attenuates Inflammatory Responses in Adipose Tissues and Improves Skeletal Muscle Mitochondrial Functions in High Fat Diet-Induced Obese Rats via Upregulation of AMPK/SIRT1 Signaling Pathways. Eur. J. Pharmacol. 2020, 883, 173371. [Google Scholar] [CrossRef]
- Barakat, B.M.; Ahmed, H.I.; Bahr, H.I.; Elbahaie, A.M. Protective Effect of Boswellic Acids against Doxorubicin-Induced Hepatotoxicity: Impact on Nrf2/HO-1 Defense Pathway. Oxid. Med. Cell. Longev. 2018, 2018, e8296451. [Google Scholar] [CrossRef]
- Nataraj, J.; Manivasagam, T.; Justin Thenmozhi, A.; Essa, M.M. Neuroprotective Effect of Asiatic Acid on Rotenone-Induced Mitochondrial Dysfunction and Oxidative Stress-Mediated Apoptosis in Differentiated SH-SYS5Y Cells. Nutr. Neurosci. 2017, 20, 351–359. [Google Scholar] [CrossRef]
- Cong, C.; Kluwe, L.; Li, S.; Liu, X.; Liu, Y.; Liu, H.; Gui, W.; Liu, T.; Xu, L. Paeoniflorin Inhibits Tributyltin Chloride-Induced Apoptosis in Hypothalamic Neurons via Inhibition of MKK4-JNK Signaling Pathway. J. Ethnopharmacol. 2019, 237, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Liu, J.; Yu, M.; Xia, M.; Zhang, Y.; Sun, X.; Xu, Y.; Cui, X. Paeoniflorin Ameliorates BiPN by Reducing IL6 Levels and Regulating PARKIN-Mediated Mitochondrial Autophagy. Drug Des. Devel. Ther. 2022, 16, 2241–2259. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhu, L.; Zhu, X.; Zhang, K.; Huang, B.; Zhang, J.; Zhang, Y.; Zhu, L.; Zhou, B.; Zhou, F. Protective Effect of Paeoniflorin on Aβ25–35-Induced SH-SY5Y Cell Injury by Preventing Mitochondrial Dysfunction. Cell. Mol. Neurobiol. 2014, 34, 227–234. [Google Scholar] [CrossRef]
- Balakrishnan, R.; Elangovan, N.; Mohankumar, T.; Nataraj, J.; Manivasagam, T.; Thenmozhi, A.J.; Essa, M.M.; Akbar, M.; Khan, M.A.S. Isolongifolene Attenuates Rotenone-Induced Mitochondrial Dysfunction, Oxidative Stress and Apoptosis. Front. Biosci.-Sch. 2018, 10, 248–261. [Google Scholar] [CrossRef]
- Balakrishnan, R.; Vijayraja, D.; Mohankumar, T.; Manimaran, D.; Ganesan, P.; Choi, D.-K.; Elangovan, N. Isolongifolene Mitigates Rotenone-Induced Dopamine Depletion and Motor Deficits through Anti-Oxidative and Anti-Apoptotic Effects in a Rat Model of Parkinson’s Disease. J. Chem. Neuroanat. 2021, 112, 101890. [Google Scholar] [CrossRef]
- Qu, M.; Ni, Y.; Guo, B.; Feng, X.; Jiang, Z. Lycopene Antagonizes Lead Toxicity by Reducing Mitochondrial Oxidative Damage and Mitochondria-mediated Apoptosis in Cultured Hippocampal Neurons. MedComm 2020, 1, 228–239. [Google Scholar] [CrossRef]
- Jang, Y.; Choo, H.; Lee, M.J.; Han, J.; Kim, S.J.; Ju, X.; Cui, J.; Lee, Y.L.; Ryu, M.J.; Oh, E.S.; et al. Auraptene Mitigates Parkinson’s Disease-Like Behavior by Protecting Inhibition of Mitochondrial Respiration and Scavenging Reactive Oxygen Species. Int. J. Mol. Sci. 2019, 20, 3409. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Jang, Y.; Zhu, J.; Namgung, E.; Go, D.; Seo, C.; Ju, X.; Cui, J.; Lee, Y.L.; Kang, H.; et al. Auraptene Enhances Junction Assembly in Cerebrovascular Endothelial Cells by Promoting Resilience to Mitochondrial Stress through Activation of Antioxidant Enzymes and MtUPR. Antioxidants 2021, 10, 475. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Mo, W.; Feng, J.; Li, J.; Yu, Q.; Li, S.; Zhang, J.; Chen, K.; Ji, J.; Dai, W.; et al. Astaxanthin Attenuates Hepatic Damage and Mitochondrial Dysfunction in Non-alcoholic Fatty Liver Disease by Up-regulating the FGF21/PGC-1α Pathway. Br. J. Pharmacol. 2020, 177, 3760–3777. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Yang, P.; Gao, T.; Liu, M.; Li, X. Allicin Attenuates Myocardial Apoptosis, Inflammation and Mitochondrial Injury during Hypoxia-Reoxygenation: An in vitro Study. BMC Cardiovasc. Disord. 2021, 21, 200. [Google Scholar] [CrossRef]
- Lv, R.; Du, L.; Lu, C.; Wu, J.; Ding, M.; Wang, C.; Mao, N.; Shi, Z. Allicin Protects against H2O2-Induced Apoptosis of PC12 Cells via the Mitochondrial Pathway. Exp. Ther. Med. 2017, 14, 2053–2059. [Google Scholar] [CrossRef]
- Han, Y.-S.; Lee, J.H.; Lee, S.H. Fucoidan Suppresses Mitochondrial Dysfunction and Cell Death against 1-Methyl-4-Phenylpyridinum-Induced Neuronal Cytotoxicity via Regulation of PGC-1α Expression. Mar. Drugs 2019, 17, 518. [Google Scholar] [CrossRef]
- Díaz-Resendiz, K.J.G.; Covantes-Rosales, C.E.; Benítez-Trinidad, A.B.; Navidad-Murrieta, M.S.; Razura-Carmona, F.F.; Carrillo-Cruz, C.D.; Frias-Delgadillo, E.J.; Pérez-Díaz, D.A.; Díaz-Benavides, M.V.; Zambrano-Soria, M.; et al. Effect of Fucoidan on the Mitochondrial Membrane Potential (ΔΨm) of Leukocytes from Patients with Active COVID-19 and Subjects That Recovered from SARS-CoV-2 Infection. Mar. Drugs 2022, 20, 99. [Google Scholar] [CrossRef]
- Lei, P.; Tian, S.; Teng, C.; Huang, L.; Liu, X.; Wang, J.; Zhang, Y.; Li, B.; Shan, Y. Sulforaphane Improves Lipid Metabolism by Enhancing Mitochondrial Function and Biogenesis in vivo and in vitro. Mol. Nutr. Food Res. 2019, 63, 1800795. [Google Scholar] [CrossRef]
- Tian, S.; Lei, P.; Zhang, J.; Sun, Y.; Li, B.; Shan, Y. Sulforaphane Balances Ca2+ Homeostasis Injured by Excessive Fat via Mitochondria-Associated Membrane (MAM). Mol. Nutr. Food Res. 2021, 65, 2001076. [Google Scholar] [CrossRef]
- Jeong, M.H.; Kim, J.H.; Seo, K.; Kwak, T.H.; Park, W.J. β-Lapachone Attenuates Mitochondrial Dysfunction in MELAS Cybrid Cells. Biochem. Biophys. Res. Commun. 2014, 454, 417–422. [Google Scholar] [CrossRef]
- Niu, Y.-J.; Zhou, W.; Nie, Z.-W.; Shin, K.-T.; Cui, X.-S. Melatonin Enhances Mitochondrial Biogenesis and Protects against Rotenone-Induced Mitochondrial Deficiency in Early Porcine Embryos. J. Pineal Res. 2020, 68, e12627. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.; Tian, L.; Liu, J.; Wu, Q.; Wang, N.; Wang, G.; Wang, Y.; Seto, S. Ligustilide Ameliorates Hippocampal Neuronal Injury after Cerebral Ischemia Reperfusion through Activating PINK1/Parkin-Dependent Mitophagy. Phytomedicine 2022, 101, 154111. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Yao, X.; Liu, Z.; Zhang, H.; Li, W.; Li, Z.; Wang, G.-L.; Pang, J.; Lin, Y.; Xu, Z.; et al. Protective Effects of Xyloketal B against MPP+-Induced Neurotoxicity in Caenorhabditis Elegans and PC12 Cells. Brain Res. 2010, 1332, 110–119. [Google Scholar] [CrossRef]
- Zhao, J.; Li, L.; Ling, C.; Li, J.; Pang, J.-Y.; Lin, Y.-C.; Liu, J.; Huang, R.; Wang, G.-L.; Pei, Z.; et al. Marine Compound Xyloketal B Protects PC12 Cells against OGD-Induced Cell Damage. Brain Res. 2009, 1302, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Shokoohinia, Y.; Hosseinzadeh, L.; Moieni-Arya, M.; Mostafaie, A.; Mohammadi-Motlagh, H.-R. Osthole Attenuates Doxorubicin-Induced Apoptosis in PC12 Cells through Inhibition of Mitochondrial Dysfunction and ROS Production. BioMed Res. Int. 2014, 2014, 156848. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, L.; Feng, F.; Yuan, H.; Gao, D.; Fu, L.; Fei, Z. Osthole Attenuates Spinal Cord Ischemia–Reperfusion Injury through Mitochondrial Biogenesis–Independent Inhibition of Mitochondrial Dysfunction in Rats. J. Surg. Res. 2013, 185, 805–814. [Google Scholar] [CrossRef]
- Anupama, N.; Preetha Rani, M.R.; Shyni, G.L.; Raghu, K.G. Glucotoxicity Results in Apoptosis in H9c2 Cells via Alteration in Redox Homeostasis Linked Mitochondrial Dynamics and Polyol Pathway and Possible Reversal with Cinnamic Acid. Toxicol. In Vitro 2018, 53, 178–192. [Google Scholar] [CrossRef]
- Zhang, B.; Zhao, J.; Wang, Z.; Xu, L.; Liu, A.; Du, G. DL0410 Attenuates Oxidative Stress and Neuroinflammation via BDNF/TrkB/ERK/CREB and Nrf2/HO-1 Activation. Int. Immunopharmacol. 2020, 86, 106729. [Google Scholar] [CrossRef]
- Kang, L.; Liu, S.; Li, J.; Tian, Y.; Xue, Y.; Liu, X. The Mitochondria-Targeted Anti-Oxidant MitoQ Protects against Intervertebral Disc Degeneration by Ameliorating Mitochondrial Dysfunction and Redox Imbalance. Cell Prolif. 2020, 53, e12779. [Google Scholar] [CrossRef]
- Fang, F.; Liu, G. Protective Effects of Compound FLZ, a Novel Synthetic Analogue of Squamosamide, on β-Amyloid-Induced Rat Brain Mitochondrial Dysfunction in vitro. Acta Pharmacol. Sin. 2009, 30, 522–529. [Google Scholar] [CrossRef]
- Yang, H.; Wang, L.; Zang, C.; Yang, X.; Bao, X.; Shang, J.; Zhang, Z.; Liu, H.; Ju, C.; Li, F.; et al. Squamosamide Derivative FLZ Diminishes Aberrant Mitochondrial Fission by Inhibiting Dynamin-Related Protein 1. Front. Pharmacol. 2021, 12, 588003. [Google Scholar] [PubMed]
- Jang, W.B.; Park, J.H.; Ji, S.T.; Lee, N.K.; Kim, D.Y.; Kim, Y.J.; Jung, S.Y.; Kang, S.; Lamichane, S.; Lamichane, B.D.; et al. Cytoprotective Roles of a Novel Compound, MHY-1684, against Hyperglycemia-Induced Oxidative Stress and Mitochondrial Dysfunction in Human Cardiac Progenitor Cells. Oxid. Med. Cell. Longev. 2018, 2018, e4528184. [Google Scholar] [CrossRef]
- de Oliveira, J.; Moreira, E.L.G.; Mancini, G.; Hort, M.A.; Latini, A.; Ribeiro-do-Valle, R.M.; Farina, M.; da Rocha, J.B.T.; de Bem, A.F. Diphenyl Diselenide Prevents Cortico-Cerebral Mitochondrial Dysfunction and Oxidative Stress Induced by Hypercholesterolemia in LDL Receptor Knockout Mice. Neurochem. Res. 2013, 38, 2028–2036. [Google Scholar] [CrossRef] [PubMed]
- Dobrachinski, F.; da Silva, M.H.; Tassi, C.L.C.; de Carvalho, N.R.; Dias, G.R.M.; Golombieski, R.M.; da Silva Loreto, É.L.; da Rocha, J.B.T.; Fighera, M.R.; Soares, F.A.A. Neuroprotective Effect of Diphenyl Diselenide in a Experimental Stroke Model: Maintenance of Redox System in Mitochondria of Brain Regions. Neurotox. Res. 2014, 26, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Quispe, R.L.; Jaramillo, M.L.; Galant, L.S.; Engel, D.; Dafre, A.L.; Teixeira da Rocha, J.B.; Radi, R.; Farina, M.; de Bem, A.F. Diphenyl Diselenide Protects Neuronal Cells against Oxidative Stress and Mitochondrial Dysfunction: Involvement of the Glutathione-Dependent Antioxidant System. Redox Biol. 2019, 20, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Straliotto, M.R.; Hort, M.A.; Fiuza, B.; Rocha, J.B.T.; Farina, M.; Chiabrando, G.; de Bem, A.F. Diphenyl Diselenide Modulates OxLDL-Induced Cytotoxicity in Macrophage by Improving the Redox Signaling. Biochimie 2013, 95, 1544–1551. [Google Scholar] [CrossRef]
- Akao, Y.; Maruyama, W.; Shimizu, S.; Yi, H.; Nakagawa, Y.; Shamoto-Nagai, M.; Youdim, M.B.H.; Tsujimoto, Y.; Naoi, M. Mitochondrial Permeability Transition Mediates Apoptosis Induced by N-Methyl(R)Salsolinol, an Endogenous Neurotoxin, and Is Inhibited by Bcl-2 and Rasagiline, N-Propargyl-1(R)-Aminoindan. J. Neurochem. 2002, 82, 913–923. [Google Scholar] [CrossRef]
- Naoi, M.; Maruyama, W.; Yi, H. Rasagiline Prevents Apoptosis Induced by PK11195, a Ligand of the Outer Membrane Translocator Protein (18 KDa), in SH-SY5Y Cells through Suppression of Cytochrome c Release from Mitochondria. J. Neural Transm. 2013, 120, 1539–1551. [Google Scholar] [CrossRef]
- Youdim, M.B.H.; Bar Am, O.; Yogev-Falach, M.; Weinreb, O.; Maruyama, W.; Naoi, M.; Amit, T. Rasagiline: Neurodegeneration, Neuroprotection, and Mitochondrial Permeability Transition. J. Neurosci. Res. 2005, 79, 172–179. [Google Scholar] [CrossRef]
- Chau, K.Y.; Cooper, J.M.; Schapira, A.H.V. Rasagiline Protects against Alpha-Synuclein Induced Sensitivity to Oxidative Stress in Dopaminergic Cells. Neurochem. Int. 2010, 57, 525–529. [Google Scholar] [CrossRef]
- Colle, D.; Santos, D.B.; Hartwig, J.M.; Godoi, M.; Engel, D.F.; de Bem, A.F.; Braga, A.L.; Farina, M. Succinobucol, a Lipid-Lowering Drug, Protects Against 3-Nitropropionic Acid-Induced Mitochondrial Dysfunction and Oxidative Stress in SH-SY5Y Cells via Upregulation of Glutathione Levels and Glutamate Cysteine Ligase Activity. Mol. Neurobiol. 2016, 53, 1280–1295. [Google Scholar] [CrossRef]
- Rehfeldt, S.C.H.; Laufer, S.; Goettert, M.I. A Highly Selective in vitro JNK3 Inhibitor, FMU200, Restores Mitochondrial Membrane Potential and Reduces Oxidative Stress and Apoptosis in SH-SY5Y Cells. Int. J. Mol. Sci. 2021, 22, 3701. [Google Scholar] [CrossRef]
- Jayaraj, R.L.; Tamilselvam, K.; Manivasagam, T.; Elangovan, N. Neuroprotective Effect of CNB-001, a Novel Pyrazole Derivative of Curcumin on Biochemical and Apoptotic Markers Against Rotenone-Induced SK-N-SH Cellular Model of Parkinson’s Disease. J. Mol. Neurosci. 2013, 51, 863–870. [Google Scholar] [CrossRef]
- Jayaraj, R.L.; Elangovan, N.; Manigandan, K.; Singh, S.; Shukla, S. CNB-001 a Novel Curcumin Derivative, Guards Dopamine Neurons in MPTP Model of Parkinson’s Disease. BioMed Res. Int. 2014, 2014, e236182. [Google Scholar] [CrossRef]
- Fernandez-Panchon, M.S.; Villano, D.; Troncoso, A.M.; Garcia-Parrilla, M.C. Antioxidant Activity of Phenolic Compounds: From in vitro Results to in vivo Evidence. Crit. Rev. Food Sci. Nutr. 2008, 48, 649–671. [Google Scholar] [CrossRef]
- Rahman, M.M.; Rahaman, M.S.; Islam, M.R.; Rahman, F.; Mithi, F.M.; Alqahtani, T.; Almikhlafi, M.A.; Alghamdi, S.Q.; Alruwaili, A.S.; Hossain, M.S.; et al. Role of Phenolic Compounds in Human Disease: Current Knowledge and Future Prospects. Molecules 2021, 27, 233. [Google Scholar] [CrossRef]
- Xicoy, H.; Wieringa, B.; Martens, G.J.M. The SH-SY5Y Cell Line in Parkinson’s Disease Research: A Systematic Review. Mol. Neurodegener. 2017, 12, 10. [Google Scholar] [CrossRef]
- Xie, H.; Hu, L.; Li, G. SH-SY5Y Human Neuroblastoma Cell Line: In vitro Cell Model of Dopaminergic Neurons in Parkinson’s Disease. Chin. Med. J. 2010, 123, 1086–1092. [Google Scholar] [CrossRef]
- Lopes, F.M.; Schröder, R.; da Frota, M.L.C., Jr.; Zanotto-Filho, A.; Müller, C.B.; Pires, A.S.; Meurer, R.T.; Colpo, G.D.; Gelain, D.P.; Kapczinski, F.; et al. Comparison between Proliferative and Neuron-like SH-SY5Y Cells as an in vitro Model for Parkinson Disease Studies. Brain Res. 2010, 1337, 85–94. [Google Scholar] [CrossRef]
- Yarmohammadi, F.; Wallace Hayes, A.; Najafi, N.; Karimi, G. The Protective Effect of Natural Compounds against Rotenone-Induced Neurotoxicity. J. Biochem. Mol. Toxicol. 2020, 34, e22605. [Google Scholar] [CrossRef]
- Copeland, W.C.; Longley, M.J. Mitochondrial Genome Maintenance in Health and Disease. DNA Repair 2014, 19, 190–198. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxid. Med. Cell Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Bagheri, H.; Ghasemi, F.; Barreto, G.E.; Rafiee, R.; Sathyapalan, T.; Sahebkar, A. Effects of Curcumin on Mitochondria in Neurodegenerative Diseases. BioFactors 2020, 46, 5–20. [Google Scholar] [CrossRef]
- Holmström, K.M.; Baird, L.; Zhang, Y.; Hargreaves, I.; Chalasani, A.; Land, J.M.; Stanyer, L.; Yamamoto, M.; Dinkova-Kostova, A.T.; Abramov, A.Y. Nrf2 Impacts Cellular Bioenergetics by Controlling Substrate Availability for Mitochondrial Respiration. Biol. Open 2013, 2, 761–770. [Google Scholar] [CrossRef]
- Itoh, K.; Ye, P.; Matsumiya, T.; Tanji, K.; Ozaki, T. Emerging Functional Cross-Talk between the Keap1-Nrf2 System and Mitochondria. J. Clin. Biochem. Nutr. 2015, 56, 91–97. [Google Scholar] [CrossRef]
- Brand, M.D.; Nicholls, D.G. Assessing Mitochondrial Dysfunction in Cells. Biochem. J. 2011, 435, 297–312. [Google Scholar] [CrossRef]
- Katwal, G.; Baral, D.; Fan, X.; Weiyang, H.; Zhang, X.; Ling, L.; Xiong, Y.; Ye, Q.; Wang, Y. SIRT3 a Major Player in Attenuation of Hepatic Ischemia-Reperfusion Injury by Reducing ROS via Its Downstream Mediators: SOD2, CYP-D, and HIF-1α. Oxid. Med. Cell. Longev. 2018, 2018, e2976957. [Google Scholar] [CrossRef]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial Reactive Oxygen Species (ROS) and ROS-Induced ROS Release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef]
- Corona, J.C.; Duchen, M.R. Impaired Mitochondrial Homeostasis and Neurodegeneration: Towards New Therapeutic Targets? J. Bioenerg. Biomembr. 2015, 47, 89–99. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular Mechanisms of Cell Death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef]
- Izzo, V.; Bravo-San Pedro, J.M.; Sica, V.; Kroemer, G.; Galluzzi, L. Mitochondrial Permeability Transition: New Findings and Persisting Uncertainties. Trends Cell Biol. 2016, 26, 655–667. [Google Scholar] [CrossRef]
- Friberg, H.; Ferrand-Drake, M.; Bengtsson, F.; Halestrap, A.P.; Wieloch, T. Cyclosporin A, but Not FK 506, Protects Mitochondria and Neurons against Hypoglycemic Damage and Implicates the Mitochondrial Permeability Transition in Cell Death. J. Neurosci. Off. J. Soc. Neurosci. 1998, 18, 5151–5159. [Google Scholar] [CrossRef]
- Seaton, T.A.; Cooper, J.M.; Schapira, A.H.V. Cyclosporin Inhibition of Apoptosis Induced by Mitochondrial Complex I Toxins. Brain Res. 1998, 809, 12–17. [Google Scholar] [CrossRef]
- Abate, M.; Festa, A.; Falco, M.; Lombardi, A.; Luce, A.; Grimaldi, A.; Zappavigna, S.; Sperlongano, P.; Irace, C.; Caraglia, M.; et al. Mitochondria as Playmakers of Apoptosis, Autophagy and Senescence. Semin. Cell Dev. Biol. 2020, 98, 139–153. [Google Scholar] [CrossRef]
- Chamcheu, J.C.; Roy, T.; Uddin, M.B.; Banang-Mbeumi, S.; Chamcheu, R.-C.N.; Walker, A.L.; Liu, Y.-Y.; Huang, S. Role and Therapeutic Targeting of the PI3K/Akt/MTOR Signaling Pathway in Skin Cancer: A Review of Current Status and Future Trends on Natural and Synthetic Agents Therapy. Cells 2019, 8, 803. [Google Scholar] [CrossRef]
- Kitagishi, Y.; Nakanishi, A.; Ogura, Y.; Matsuda, S. Dietary Regulation of PI3K/AKT/GSK-3β Pathway in Alzheimer’s Disease. Alzheimers Res. Ther. 2014, 6, 35. [Google Scholar] [CrossRef]
- Biasutto, L.; Szabo’, I.; Zoratti, M. Mitochondrial Effects of Plant-Made Compounds. Antioxid. Redox Signal. 2011, 15, 3039–3059. [Google Scholar] [CrossRef]
- Yoon, J.C.; Ng, A.; Kim, B.H.; Bianco, A.; Xavier, R.J.; Elledge, S.J. Wnt Signaling Regulates Mitochondrial Physiology and Insulin Sensitivity. Genes Dev. 2010, 24, 1507–1518. [Google Scholar] [CrossRef]
- Scarpulla, R.C. Metabolic Control of Mitochondrial Biogenesis through the PGC-1 Family Regulatory Network. Biochim. Biophys. Acta BBA-Mol. Cell Res. 2011, 1813, 1269–1278. [Google Scholar] [CrossRef]
- Viña, J.; Gomez-Cabrera, M.C.; Borras, C.; Froio, T.; Sanchis-Gomar, F.; Martinez-Bello, V.E.; Pallardo, F.V. Mitochondrial Biogenesis in Exercise and in Ageing. Adv. Drug Deliv. Rev. 2009, 61, 1369–1374. [Google Scholar] [CrossRef]
- Chen, M.; Cui, Y.; Li, H.; Luan, J.; Zhou, X.; Han, J. Icariin Promotes the Osteogenic Action of BMP2 by Activating the CAMP Signaling Pathway. Molecules 2019, 24, 3875. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-E.; Chen, J.; Lou, Z. DBC1 Is a Negative Regulator of SIRT1. Nature 2008, 451, 583–586. [Google Scholar] [CrossRef] [PubMed]
- Pelosse, M.; Cottet-Rousselle, C.; Bidan, C.M.; Dupont, A.; Gupta, K.; Berger, I.; Schlattner, U. Synthetic Energy Sensor AMPfret Deciphers Adenylate-Dependent AMPK Activation Mechanism. Nat. Commun. 2019, 10, 1038. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.-M.; Jung, Y.-K. A Molecular Approach to Mitophagy and Mitochondrial Dynamics. Mol. Cells 2018, 41, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Liu, Q.; Gao, W.; Sehgal, S.A.; Wu, H. The Multifaceted Regulation of Mitophagy by Endogenous Metabolites. Autophagy 2022, 18, 1216–1239. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Klionsky, D.J. Eaten Alive: A History of Macroautophagy. Nat. Cell Biol. 2010, 12, 814–822. [Google Scholar] [CrossRef] [PubMed]
- Ge, P.; Dawson, V.L.; Dawson, T.M. PINK1 and Parkin Mitochondrial Quality Control: A Source of Regional Vulnerability in Parkinson’s Disease. Mol. Neurodegener. 2020, 15, 20. [Google Scholar] [CrossRef]
- Salazar, C.; Ruiz-Hincapie, P.; Ruiz, L.M. The Interplay among PINK1/PARKIN/Dj-1 Network during Mitochondrial Quality Control in Cancer Biology: Protein Interaction Analysis. Cells 2018, 7, 154. [Google Scholar] [CrossRef]
- Merkwirth, C.; Langer, T. Prohibitin Function within Mitochondria: Essential Roles for Cell Proliferation and Cristae Morphogenesis. Biochim. Biophys. Acta BBA-Mol. Cell Res. 2009, 1793, 27–32. [Google Scholar] [CrossRef]
- Signorile, A.; Sgaramella, G.; Bellomo, F.; De Rasmo, D. Prohibitins: A Critical Role in Mitochondrial Functions and Implication in Diseases. Cells 2019, 8, 71. [Google Scholar] [CrossRef]
- Thuaud, F.; Ribeiro, N.; Nebigil, C.G.; Désaubry, L. Prohibitin Ligands in Cell Death and Survival: Mode of Action and Therapeutic Potential. Chem. Biol. 2013, 20, 316–331. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.-T.; Chen, P.; Ouyang, R.-Y.; Song, L. Multifaceted Role of Prohibitin in Cell Survival and Apoptosis. Apoptosis 2015, 20, 1135–1149. [Google Scholar] [CrossRef]
- Núñez-Vázquez, S.; Sánchez-Vera, I.; Saura-Esteller, J.; Cosialls, A.M.; Noisier, A.F.M.; Albericio, F.; Lavilla, R.; Pons, G.; Iglesias-Serret, D.; Gil, J. NOXA Upregulation by the Prohibitin-Binding Compound Fluorizoline Is Transcriptionally Regulated by Integrated Stress Response-Induced ATF3 and ATF4. FEBS J. 2021, 288, 1271–1285. [Google Scholar] [CrossRef]
- Sato, S.; Murata, A.; Orihara, T.; Shirakawa, T.; Suenaga, K.; Kigoshi, H.; Uesugi, M. Marine Natural Product Aurilide Activates the OPA1-Mediated Apoptosis by Binding to Prohibitin. Chem. Biol. 2011, 18, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Stocchi, F.; Fossati, C.; Torti, M. Rasagiline for the Treatment of Parkinson’s Disease: An Update. Expert Opin. Pharmacother. 2015, 16, 2231–2241. [Google Scholar] [CrossRef] [PubMed]
- Nayak, L.; Henchcliffe, C. Rasagiline in Treatment of Parkinson’s Disease. Neuropsychiatr. Dis. Treat. 2008, 4, 23–32. [Google Scholar]
- Naoi, M.; Maruyama, W.; Shamoto-Nagai, M. Rasagiline and Selegiline Modulate Mitochondrial Homeostasis, Intervene Apoptosis System and Mitigate α-Synuclein Cytotoxicity in Disease-Modifying Therapy for Parkinson’s Disease. J. Neural Transm. 2020, 127, 131–147. [Google Scholar] [CrossRef]
- Matthews, D.C.; Ritter, A.; Thomas, R.G.; Andrews, R.D.; Lukic, A.S.; Revta, C.; Kinney, J.W.; Tousi, B.; Leverenz, J.B.; Fillit, H.; et al. Rasagiline Effects on Glucose Metabolism, Cognition, and Tau in Alzheimer’s Dementia. Alzheimers Dement. Transl. Res. Clin. Interv. 2021, 7, e12106. [Google Scholar] [CrossRef]
- Mayer, G.; Happe, S.; Evers, S.; Hermann, W.; Jansen, S.; Kallweit, U.; Muntean, M.-L.; Pöhlau, D.; Riemann, D.; Saletu, M.; et al. Insomnia in Neurological Diseases. Neurol. Res. Pract. 2021, 3, 15. [Google Scholar] [CrossRef]
- Quera-Salva, M.-A.; Claustrat, B. Mélatonine: Aspects physiologiques et pharmacologiques en relation avec le sommeil, intérêt d’une forme galénique à libération prolongée (Circadin®) dans l’insomnie. L’Encéphale 2018, 44, 548–557. [Google Scholar] [CrossRef]
- Wade, A.G.; Farmer, M.; Harari, G.; Fund, N.; Laudon, M.; Nir, T.; Frydman-Marom, A.; Zisapel, N. Add-on Prolonged-Release Melatonin for Cognitive Function and Sleep in Mild to Moderate Alzheimer’s Disease: A 6-Month, Randomized, Placebo-Controlled, Multicenter Trial. Clin. Interv. Aging 2014, 9, 947–961. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.L.; Edrada-Ebel, R.; Quinn, R.J. The Re-Emergence of Natural Products for Drug Discovery in the Genomics Era. Nat. Rev. Drug Discov. 2015, 14, 111–129. [Google Scholar] [CrossRef] [PubMed]
- Howes, M.-J.R.; Quave, C.L.; Collemare, J.; Tatsis, E.C.; Twilley, D.; Lulekal, E.; Farlow, A.; Li, L.; Cazar, M.-E.; Leaman, D.J.; et al. Molecules from Nature: Reconciling Biodiversity Conservation and Global Healthcare Imperatives for Sustainable Use of Medicinal Plants and Fungi. Plants People Planet 2020, 2, 463–481. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural Products in Drug Discovery: Advances and Opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Rasouli, H.; Farzaei, M.H.; Khodarahmi, R. Polyphenols and Their Benefits: A Review. Int. J. Food Prop. 2017, 20, 1700–1741. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef]
- Muller, A.G.; Sarker, S.D.; Saleem, I.Y.; Hutcheon, G.A. Delivery of Natural Phenolic Compounds for the Potential Treatment of Lung Cancer. DARU J. Pharm. Sci. 2019, 27, 433–449. [Google Scholar] [CrossRef]
- Chu, K.O.; Pang, C.C.P. Pharmacokinetics and Disposition of Green Tea Catechins. In Pharmacokinetics and Adverse Effects of Drugs; Malangu, N., Ed.; IntechOpen: Rijeka, Croatia, 2018; ISBN 978-1-78923-139-7. [Google Scholar]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of Curcumin: Problems and Promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Yang, K.-Y.; Lin, L.-C.; Tseng, T.-Y.; Wang, S.-C.; Tsai, T.-H. Oral Bioavailability of Curcumin in Rat and the Herbal Analysis from Curcuma Longa by LC–MS/MS. J. Chromatogr. B 2007, 853, 183–189. [Google Scholar] [CrossRef]
- Walle, T.; Hsieh, F.; DeLegge, M.H.; Oatis, J.E.; Walle, U.K. High Absorption but Very Low Bioavailability of Oral Resveratrol in Humans. Drug Metab. Dispos. 2004, 32, 1377–1382. [Google Scholar] [CrossRef]
- Berman, A.Y.; Motechin, R.A.; Wiesenfeld, M.Y.; Holz, M.K. The Therapeutic Potential of Resveratrol: A Review of Clinical Trials. Npj Precis. Oncol. 2017, 1, 1–9. [Google Scholar] [CrossRef]
- Herbers, E.; Kekäläinen, N.J.; Hangas, A.; Pohjoismäki, J.L.; Goffart, S. Tissue Specific Differences in Mitochondrial DNA Maintenance and Expression. Mitochondrion 2019, 44, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsov, A.V.; Margreiter, R. Heterogeneity of Mitochondria and Mitochondrial Function within Cells as Another Level of Mitochondrial Complexity. Int. J. Mol. Sci. 2009, 10, 1911–1929. [Google Scholar] [CrossRef]
- Kuznetsov, A.V.; Hermann, M.; Saks, V.; Hengster, P.; Margreiter, R. The Cell-Type Specificity of Mitochondrial Dynamics. Int. J. Biochem. Cell Biol. 2009, 41, 1928–1939. [Google Scholar] [CrossRef] [PubMed]





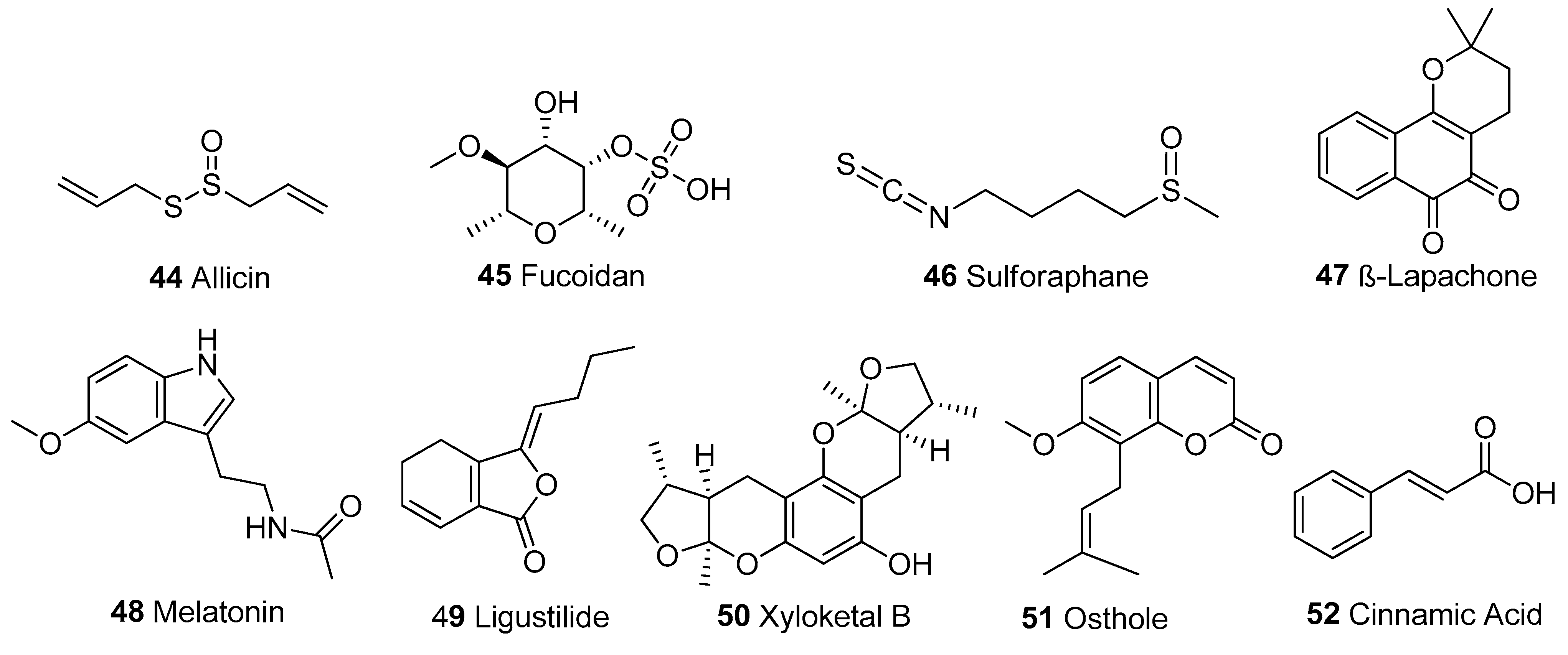
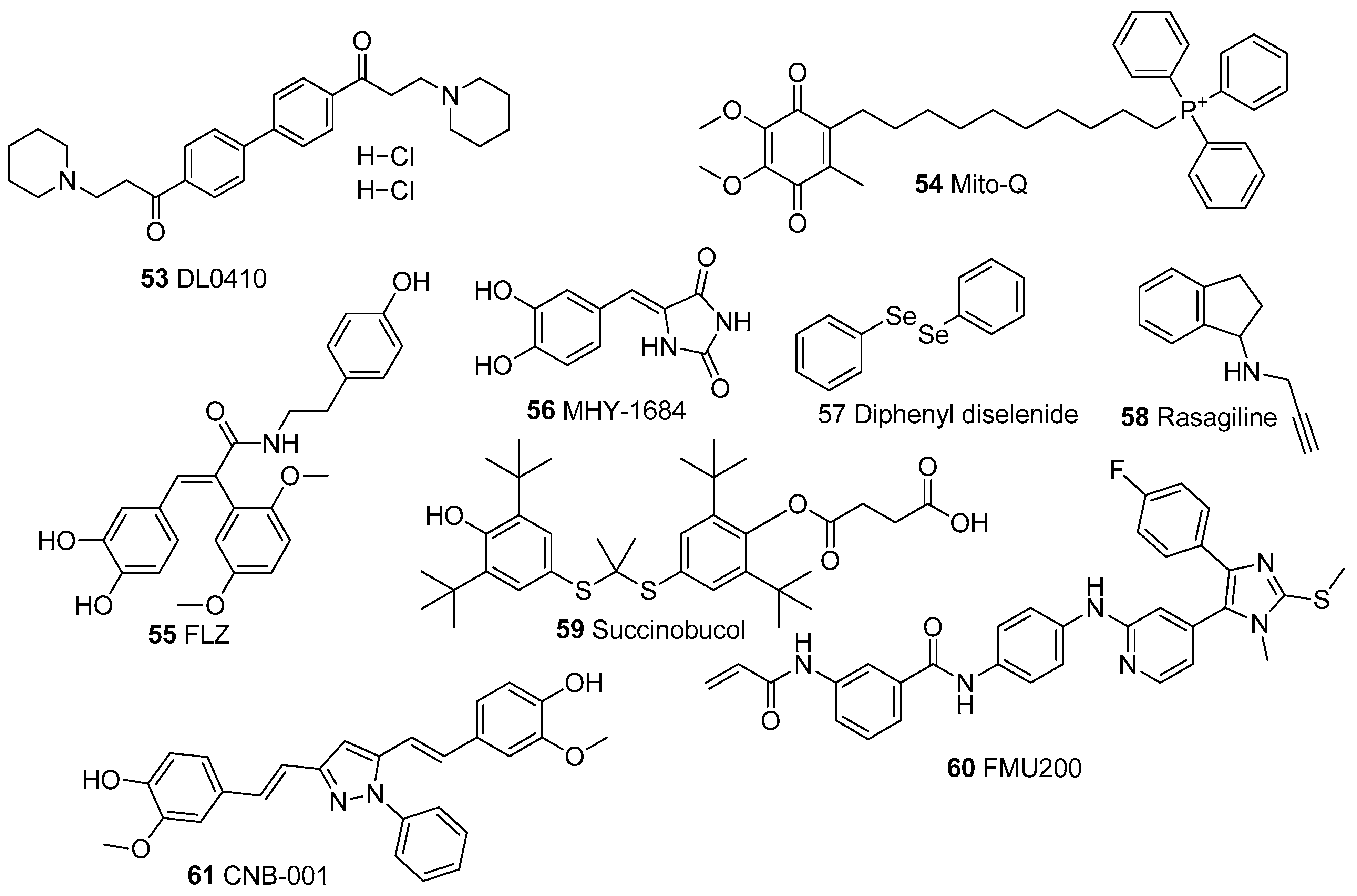

| Compounds | Model | Dose | Mechanisms | References | |
|---|---|---|---|---|---|
| 1. | Quercetin | PC12 cells Wistar rats | 1–100 μM 2–100 mg/kg | ↓ROS, ↓LPO, ↑SOD, ↑GSH, ↑CAT, ↓Apoptosis, ↑MMP, ↑ATP, ↑Nrf2 ↑AMPK, ↑PGC-1α, ↑SIRT1, Restored Mt morphology, ↑PINK1 and ↑Parkin | [46,47,48,49] |
| 2. | Baicalein | SH-SY5Y cells V79-4 cells | 10–25 μM 10 μg/mL | ↓ROS, ↓LPO, ↑SOD, ↓Bax, ↑Bcl-2, ↓Cyt c, ↓Caspase-3 ↓Ca2+, ↑Nrf2, ↑MMP | [50,51,52] |
| 3. | Kaempferol | HUVECs cells L2 cells | 10–40 μM | ↓ROS, ↓LPO, ↑SOD, ↑GSH, ↑GPx, ↓Bax, ↑Bcl-2, ↓Cyt c, ↓Caspase-3 ↑MMP, mPTP blockage, ↑ATP, ↑SIRT1 | [53] |
| 4. | Fisetin | Wistar rats | 15 and 20 mg/kg | ↓ROS, ↓LPO, ↑SOD, ↑GSH, ↑CAT, ↑MMP, ↓Caspase-3, ↑ATP, ↑Complex I | [54,55,56] |
| 5. | Luteolin | C57BL/6 mice | 10 μg/kg | ↓ROS, ↓LPO, ↑MMP, ↓Caspase-3, -9, ↑ATP, ↑AMPK, ↑Complexes I, II, III, IV, and V | [57] |
| 6. | 7,8-dihydroxyflavone | Wistar Rat H92c cells | 5–20 mg/kg 100 μM | ↓ROS, ↓LPO, ↑SOD, GSH, CAT, GPx ↑MMP, ↑Complexes I, II, III, IV | [58,59] |
| 7. | Naringenin | SH-SY5Y cells | 10–80 μM | ↓ROS, ↓LPO, ↑SOD, ↑GSH, ↑CAT, ↑PI3K/Akt/GSK-3β, ↑MMP, ↑ATP, ↑Nrf2, ↑Complexes I, V | [60,61,62,63] |
| 8. | Genistein | C57/BL6J mice H9c2 cells | 2.5–10 mg/kg 10 pM–1 μM | ↓ROS, ↓LPO, ↑SOD, ↑GSH, ↑CAT ↑GPx ↑MMP, ↓Cyt c, ↓Caspase-3, ↑Nrf2 | [64,65,66] |
| 9. | Icariin | Human NP cells | 10 μM | ↓ROS, ↓Bax, ↑Bcl-2, ↓Cyt c, ↓Caspase-3, ↑MMP, ↑Nrf2 | [67] |
| 10. | Catechin | EA.hy926 cells HepG2 cells | 4 mM 10 μM | ↓ROS, ↓LPO, ↑SOD, ↑CAT, ↑MMP, Restored Mt morphology, ↑SIRT1, ↑Complex I, ↑ATP | [68,69,70] |
| 11. | Epicatechin | MRC-5 cells BV2 cells | 10 μM 100 μM | ↓ROS, ↓LPO, ↑SOD, ↑CAT, ↑MMP Restored Mt morphology, ↑AMPK, ↑SIRT1, ↑Complex I, ↑ATP | [68,71] |
| 12. | Epigallocatechin gallate | HLE B-3 cells | 50 μM | ↓ROS, ↓LPO, ↑SOD, ↑GSH, ↑CAT ↑GPx, ↓Bax, ↑Bcl-2, ↓Cyt c, ↓Caspase-3, -9, ↑MMP, ↑ATP | [72,73] |
| 13. | Hesperidin | Mice | 25–50 mg/kg | ↑SOD, GSH, CAT, GPx, ↓Caspase-3, -9, ↑MMP, ↑ATP, ↑Complexes I, II, IV, V | [74,75] |
| 14. | Resveratrol | Wistar rats C57BL/6 mice MC3T3-E1 cells | 20 mg/kg 40 mg/kg 25 μM | ↓ROS, ↑SOD,↑Bcl-2, ↓Cyt c, ↑SIRT1-AMPK-PGC-1α, ↑PINK1 ↑MMP, ↑Nrf2, ↑ATP, ↑Complex I, CypD | [76,77,78] |
| 15. | Tyrosol | CATH.a cells SH-SY5Y cells | 50–200 μM | ↓ROS, ↑MMP ↓Bax, ↑Bcl-2, ↓Cyt c, ↓Caspase-3, -9, ↑ATP | [79] |
| 16. | Hydroxytyrosol | ARPE cells HCN-2 cells | 100 μM 30 μM | ↓ROS ↓LPO, ↑SOD, GSH, CAT, GPx ↓Ca2+, ↑Nrf2, ↑MMP, ↑Complexes I, II, V | [80,81] |
| 17. | Curcumin | SH-SY5Y cells | 5 μM | ↓ROS, ↓LPO, ↑GSH, ↑GPx, ↑MMP, ↑ATP, ↓Ca2+, ↓Caspase-3, -9, ↑Complexes II, IV | [82,83] |
| 18. | Esculetin Mito-esculetin | C2C12 cells HAEC cells | 5 μM 2.5 μM | ↓ROS, ↑GSH, ↑MMP, ↑Nrf2 ↓Caspase-3, -8, ↑AMPK/SIRT3/ PGC1α | [84] |
| 19. | Protocatechuic Acid | PC12 cells Human Platelets | 0.1–1.2 mM | ↓ROS, ↓LPO, ↑GSH, ↑GPx, ↑MMP ↓Bax, ↑Bcl-2, ↓Cyt c, ↓Caspase-3, -9, ↓PI3K/Akt/ GSK-3β | [85,86,87] |
| 20. | Salvianic acid A | SH-SY5Y cells SD Rat | 1–100 μg/mL 52 μg/mL | ↓ROS, ↓LPO, ↑MMP ↓Bax, ↑Bcl-2, ↓Cyt c, ↓Caspase-3 | [88,89] |
| 21. | Sesamin | BEAS-2B cells | 40 μM | ↓ROS, ↓LPO, ↑SOD, ↑CAT, ↓Bax, ↑Bcl-2, ↓Caspase-3 ↑MMP, ↑Nrf2, ↓PINK1, Parkin | [90] |
| 22. | α-Arbutin | SH-SY5Y cells | 1–100 μM | ↓ROS, ↑SOD, ↑GSH, ↑MMP, ↑ATP, ↓AMPK | [91] |
| 23. | Ellagic acid | Wistar rats C57BL/6 mice SH-SY5Y cells | 10–100 mg/kg 20 μM | ↓ROS, ↑SOD↑, MMP, ↑Nrf2 mPTP blockage, ↓Cyt c, ↑ATP, ↑Sirt3 ↑Complexes I, II, III, and IV | [92,93,94,95,96] |
| 24. | Mangiferin | SH-SY5Y cells C57BL/6 mice | 10–50 μM 10–50 mg/kg | ↓ROS, ↓LPO, ↑SOD, ↑GSH ↑CAT, ↑GPx, ↓Bax, ↑Bcl-2, ↓Cyt c, ↓Caspase-3, -9, ↑Nrf2, ↑MMP, ↑ATP, ↑Complex I | [97,98,99] |
| 25. | Panduratin A | RPTEC/TERT1 | 5 μM | ↓ROS, ↑MMP, ↑Bcl-2, ↓Cyt c, ↓Caspase-3 | [100] |
| 26. | α-Mangostin | SH-SY5Y cells | 0.03–0.3 μM | ↓ROS, ↑MMP, ↑ATP, ↓Caspase-3, -8 | [101] |
| 27. | Rosmarinic acid | H9c2 cells SH-SY5Y cells Zebra fish C57BL/6 Mice | 1–200 μM 20–80 mg/kg | ↓ROS ↑GSH, ↑Nrf2, ↑MMP, ↑SIRT1/PGC-1a ↑PI3K/Akt Restored Mt Morphology, ↑ATP | [102,103,104,105] |
| 28. | Chlorogenic acid | HUVECs cell Albino mice | 25–160 μM 50 mg/kg | ↑SOD, ↑GSH, ↑MMP, ↑ATP, ↑SIRT1 ↓Caspase-3, ↑Complexes I, II, III, IV, and V | [106,107,108] |
| 29. | Theaflavin | TCMK-1 cells | 2–10 μM | ↓ROS, ↓LPO, ↑SOD, ↑MMP, ↑Nrf2, ↓Bax, ↑Bcl-2, ↓Caspase-3 ↑ATP, Restored Mt morphology | [109] |
| 30. | Salvianolic acid A | Cardiomyocyte 3T3-L1 cells | 12.5–50 μg/mL 1–100 nM | ↓ROS, ↓Bax/Bcl-2 ratio, ↓Caspase-3, ↑Akt/GSK-3β, ↑MMP mPTP blockage, ↑ATP, ↑PGC-1α, ↑Complexes III and IV | [110,111] |
| 31. | Salvianolic acid B | HL-7702 cells IEC-6 cells | 50–200 μM 2.5–40 μM | ↓ROS, ↓LPO, ↑SOD, ↑CAT, ↑MMP, ↑PI3K/Akt/GSK-3β, ↑ATP Restored Mt morphology, ↑AMPK/Sirt3 | [112,113] |
| 32. | Sarain A | SH-SY5Y cells | 0.01–10 μM | ↓ROS, ↑SOD, ↑Nrf2, ↑MMP, mPTP blockage, ↓Cyp D, ↑ATP | [114] |
| 33. | Sarain 2 | SH-SY5Y cells | 10 μM | ↓ROS, ↑MMP | [114] |
| 34. | Piperine | Wistar rats | 10 mg/kg | ↓ROS, ↓LPO, ↑GSH, ↑MMP, ↑Bax/Bcl-2, ↓Cyt c, ↓Caspase-3, -9 ↑Complexes I, II, and II | [115,116,117] |
| 35. | Caffeine | SH-SY5Y cells SD rats APPsw rats | 1–100 μM 40 mg/kg 120 mg/L | ↓ROS, ↑MMP, ↑ATP ↓Bax/Bcl-2, ↓Cyt c, ↓Caspase-3, -6 ↑PI3K/Akt, ↑Complexes I, II, and III | [118,119,120,121] |
| 36. | Celastrol | C57BL6/J mice SD rats | 100 μg/kg 1–3 mg/kg | ↓ROS, ↑GSH, ↑MMP, ↑ATP ↑AMPK/SIRT1/ PGC1α, ↑Complexes I, and III | [122,123] |
| 37. | Boswellic acid | Albino rats | 100–250 mg/kg | ↓ROS, ↓LPO, ↑SOD, ↑GPx, ↑CAT, ↑Nrf2, ↓Caspase-3 ↑Complexes I, II, III, and IV | [24,124] |
| 38. | Asiatic acid | SH-SY5Y cells | 10 nM | ↓ROS↑, MMP, ↓Bax, ↑Bcl-2, ↓Cyt c, ↓Caspase-3, -8, -8, -9 | [125] |
| 39. | Paeoniflorin | SD rats, PC12 cells | 25–100 μM | ↓ROS, ↑MMP, ↑ATP, ↓Bax, ↑Bcl-2, ↓Cyt c, ↓Caspase-3, -9 | [126,127,128] |
| 40. | Isolongifolene | Rats SH-SY5Y cells | 10 mg/kg 10 μM | ↓ROS, ↓LPO, ↑SOD, GSH, CAT, GPx, ↑Bax, Bcl-2, ↓Cyt c, ↓Caspase-3, -6, -8, -9, ↑PI3K/Akt/ GSK-3β, ↑MMP | [129,130] |
| 41. | Lycopene | SD rats | 5 μM | ↓ROS, ↑MMP, mPTP blockage, ↑ATP, ↓Bax, ↑Bcl-2, ↓Bax/Bcl-2 ratio, ↓Cyt c, ↓Caspase-3, -9, ↑PGC1α, ↑Complexes I, II, III, IV | [131] |
| 42. | Auraptene | SN4741 cells bEnd.3 cells | 10 μM 1 μM | ↓ROS, ↑MMP, ↑Nrf2 | [132,133] |
| 43. | Astaxanthin | LO2 cells | 30–90 μM | ↓ROS, ↑MMP, ↑ATP, ↓Bax, ↓Caspase-3, ↑PGC1α, Restored Mt morphology | [134] |
| 44. | Allicin | PC12 cells | 0.01–1 μg/mL | ↓ROS, ↑MMP, ↑PGC1α, ↓Bax, ↑Bcl-2, ↓Cyt c, ↓Caspase-3 | [135,136] |
| 45. | Fucoidan | SH-SY5Y cells HPBM cells | 50 μg/mL 20 and 50 μM | ↓ROS, ↑MMP, ↓Bax, ↑Bcl-2, ↓Caspase-3 ↑Complexes I and IV, ↑AMPK/PGC1α | [137,138] |
| 46. | Sulforaphane | HHL-5 cells | 10 and 250 μM | ↓ROS, ↓LPO, ↑SOD, ↑GSH, ↑Nrf2, ↑MMP, ↑ATP, ↓Apoptosis, ↑PGC1α, ↓Ca2+, ↑Complexes I and IV | [139,140] |
| 47. | β-Lapachone | MELAS cells | 1 μM | ↓ROS, ↑MMP, ↑ATP | [141] |
| 48. | Melatonin | Porcine oocytes | 500 nM | ↓ROS, ↑MMP, ↑ATP,↓Caspase-3 ↑PGC1α/SIRT1 | [142] |
| 49. | Ligustilide | HT-22 cells, SD rats | 20 μM, 10, 20 mg/kg | ↓ROS, ↑MMP, ↑PINK1/Parkin | [143] |
| 50. | Xyloketal B | PC12 cells | 100–250 μM | ↓ROS, ↑GSH, and ↑MMP Restored Mt morphology | [144,145] |
| 51. | Osthole | PC12 cells SD rats | 7 μg/cc 50 mg/kg | ↓ROS, ↑MMP, ↑ATP, ↓Bax, ↑Bcl-2, ↓Bax/Bcl-2 ratio, ↓Cyt c, ↓Caspase-3, -9, ↑Complexes I, II, III and IV | [146,147] |
| 52. | Cinnamic Acid | H9c2 cells | 100–500 nM | ↓ROS, ↓LPO, ↑SOD, ↑GSH, ↑MMP, ↓Bax, ↑Bcl-2, ↓Caspase-3 | [148] |
| 53. | DL0410 | SH-SY5Y cells | 1–10 μM | ↓ROS, ↓LPO, ↑Nrf2, ↑MMP, ↓Bax, ↑Bcl-2, ↓Cyt c, ↓Caspase-3 | [149] |
| 54. | Mito-Q | NP cells | 500 nM | ↓ROS, ↓LPO, ↑SOD, ↑GSH, ↑Nrf2, ↑MMP, ↓PINK1/Parkin | [150] |
| 55. | FLZ | SH-SY5Y cell | 100 μM | ↓ROS, ↑GSH, ↑MMP, mPTP blockage, ↑Complexes IV | [151,152] |
| 56. | MHY-1684 | hCPCsc-kit+ | 1 μM | ↓ROS, ↑AKT signaling, ↓Apoptosis | [153] |
| 57. | Diphenyl diselenide | HT22 cells LDLr−/− mice | 2 μM 1 mg/kg | ↓ROS, ↓LPO, ↑SOD, ↑GSH, ↑GPx, ↑MMP ↑Complexes I and II | [154,155,156,157] |
| 58. | Rasagiline | SH-SY5Y cells Rat mitochondria | 100 nM 1–10 μM | ↓ROS, ↑SOD, ↑GSH, ↑MMP, ↓Cyt c, ↑ATP | [158,159,160,161] |
| 59. | Succinnobucol | SH-SY5Y cells | 3 μM | ↓ROS, ↑GSH, ↑MMP, ↓Cyt c, ↑ATP | [162] |
| 60. | FMU200 | SH-SY5Y cells | 0.1 and 1 μM | ↓ROS, ↑MMP | [163] |
| 61. | CNB-001 | C57BL/6 mice SK-N-SH cells | 6–48 mg/kg 2 μM | ↓ROS, ↓LPO, ↑SOD, ↑GSH, ↑GPx,↑CAT, ↑MMP ↓Bax, ↑Bcl-2, ↓Cyt c, ↓Caspase-3 | [164,165] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makinde, E.; Ma, L.; Mellick, G.D.; Feng, Y. Mitochondrial Modulators: The Defender. Biomolecules 2023, 13, 226. https://doi.org/10.3390/biom13020226
Makinde E, Ma L, Mellick GD, Feng Y. Mitochondrial Modulators: The Defender. Biomolecules. 2023; 13(2):226. https://doi.org/10.3390/biom13020226
Chicago/Turabian StyleMakinde, Emmanuel, Linlin Ma, George D. Mellick, and Yunjiang Feng. 2023. "Mitochondrial Modulators: The Defender" Biomolecules 13, no. 2: 226. https://doi.org/10.3390/biom13020226
APA StyleMakinde, E., Ma, L., Mellick, G. D., & Feng, Y. (2023). Mitochondrial Modulators: The Defender. Biomolecules, 13(2), 226. https://doi.org/10.3390/biom13020226





