The Potential Role of Microorganisms on Enteric Nervous System Development and Disease
Abstract
:1. Introduction
2. ENS Development in Prenatal, Postnatal and Childhood Periods
3. The Microbiota and ENS Development and Disease
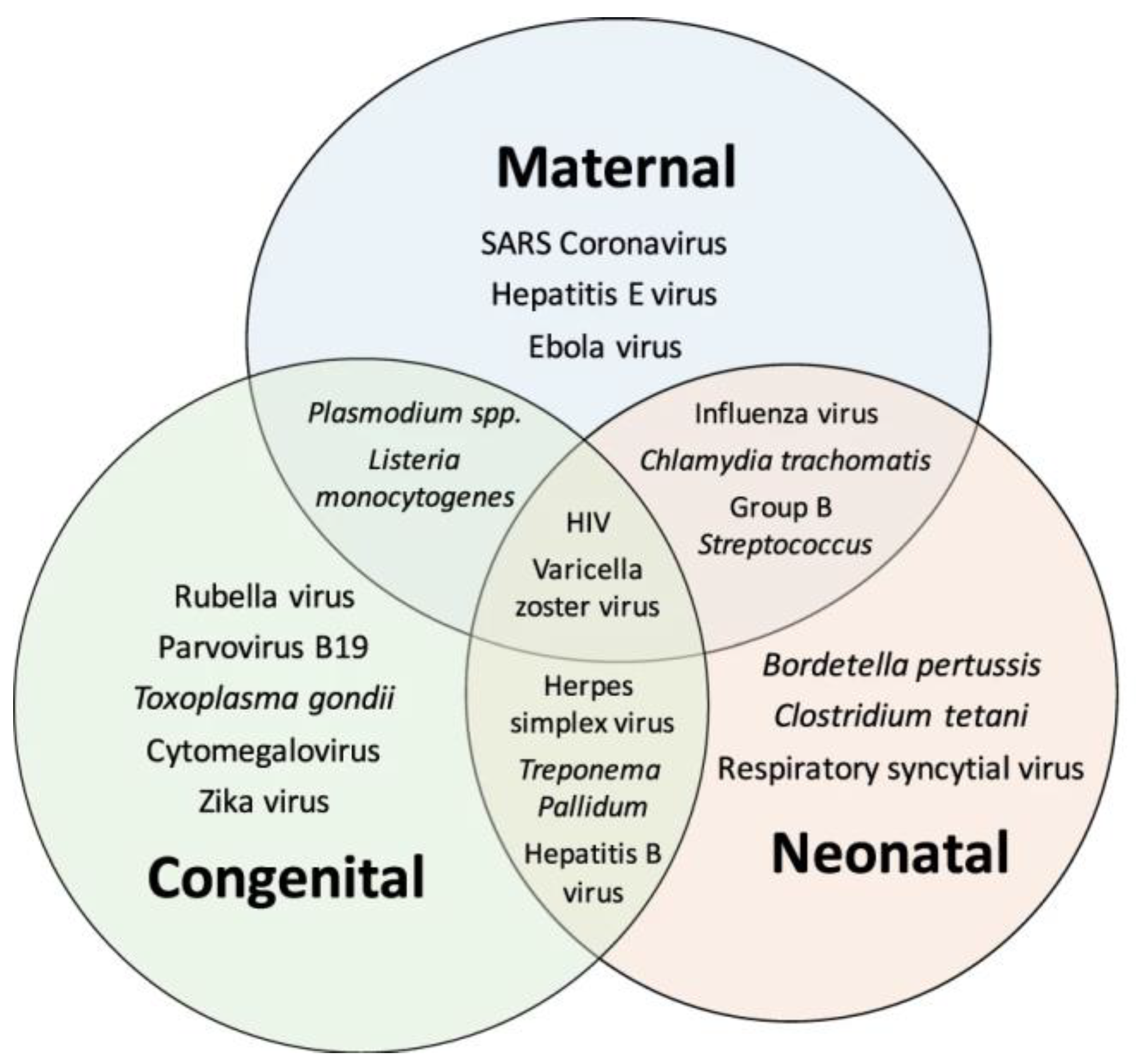
4. Pathogens and ENS Development and Disease
4.1. Bacteria
4.2. Viruses
4.2.1. Herpes simplex Virus
4.2.2. Varicella zoster Virus
4.2.3. Cytomegalovirus
4.2.4. Rotavirus
4.2.5. Coronavirus SARS-CoV-2
4.2.6. Bacteriophages
4.3. Parasites
4.4. Fungi
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rao, M.; Gershon, M.D. Enteric nervous system development: What could possibly go wrong? Nat. Rev. Neurosci. 2018, 19, 552–565. [Google Scholar] [CrossRef]
- Furness, J.B. The enteric nervous system and neurogastroenterology. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 286–294. [Google Scholar] [CrossRef]
- Fung, C.; Vanden Berghe, P. Functional circuits and signal processing in the enteric nervous system. Cell Mol. Life Sci. 2020, 77, 4505–4522. [Google Scholar] [CrossRef]
- Chanpong, A.; Borrelli, O.; Thapar, N. Recent advances in understanding the roles of the enteric nervous system. Fac. Rev. 2022, 11, 7. [Google Scholar] [CrossRef]
- Giuffrè, M.; Moretti, R.; Campisciano, G.; da Silveira, A.B.; Monda, V.M.; Comar, M.; Bella, S.D.; Antonello, R.M.; Luzzati, R.; Crocè, L.S. You Talking to Me? Says the Enteric Nervous System (ENS) to the Microbe. How Intestinal Microbes Interact with the ENS. J. Clin. Med. 2020, 9, 3705. [Google Scholar] [CrossRef]
- Chelakkot, C.; Ghim, J.; Ryu, S.H. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp. Mol. Med. 2018, 50, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Kinashi, Y.; Hase, K. Partners in Leaky Gut Syndrome: Intestinal Dysbiosis and Autoimmunity. Front. Immunol. 2021, 12, 673708. [Google Scholar] [CrossRef] [PubMed]
- Seguella, L.; Sarnelli, G.; Esposito, G. Leaky gut, dysbiosis, and enteric glia activation: The trilogy behind the intestinal origin of Parkinson’s disease. Neural. Regen. Res. 2020, 15, 1037–1038. [Google Scholar]
- Hao, M.M.; Foong, J.P.P.; Bornstein, J.C.; Li, Z.L.; Vanden Berghe, P.; Boesmans, W. Enteric nervous system assembly: Functional integration within the developing gut. Dev. Biol. 2016, 417, 168–181. [Google Scholar] [CrossRef]
- Burns, A.J.; Roberts, R.R.; Bornstein, J.C.; Young, H.M. Development of the enteric nervous system and its role in intestinal motility during fetal and early postnatal stages. Semin. Pediatr. Surg. 2009, 18, 196–205. [Google Scholar] [CrossRef]
- McCann, C.J.; Alves, M.M.; Brosens, E.; Natarajan, D.; Perin, S.; Chapman, C.; Hofstra, R.M.; Burns, A.J.; Thapar, N. Neuronal Development and Onset of Electrical Activity in the Human Enteric Nervous System. Gastroenterology 2019, 156, 1483–1495.e6. [Google Scholar] [CrossRef] [Green Version]
- Foong, J.P.P.; Hung, L.Y.; Poon, S.; Savidge, T.C.; Bornstein, J.C. Early life interaction between the microbiota and the enteric nervous system. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 319, G541–G548. [Google Scholar] [CrossRef]
- Uesaka, T.; Young, H.M.; Pachnis, V.; Enomoto, H. Development of the intrinsic and extrinsic innervation of the gut. Dev. Biol. 2016, 417, 158–167. [Google Scholar] [CrossRef]
- Kulkarni, S.; Micci, M.A.; Leser, J.; Shin, C.; Tang, S.C.; Fu, Y.Y.; Liu, L.S.; Li, Q.; Saha, M.; Li, C.P.; et al. Adult enteric nervous system in health is maintained by a dynamic balance between neuronal apoptosis and neurogenesis. Proc. Natl. Acad. Sci. USA 2017, 114, E3709–E3718. [Google Scholar] [CrossRef] [Green Version]
- Wallace, A.S.; Burns, A.J. Development of the enteric nervous system, smooth muscle and interstitial cells of Cajal in the human gastrointestinal tract. Cell Tissue Res. 2005, 319, 367–382. [Google Scholar] [CrossRef]
- Funkhouser, L.J.; Bordenstein, S.R. Mom Knows Best: The Universality of Maternal Microbial Transmission. PLoS Biol. 2013, 11, e1001631. [Google Scholar] [CrossRef] [Green Version]
- Perez-Muñoz, M.E.; Arrieta, M.-C.; Ramer-Tait, A.E.; Walter, J. A critical assessment of the “sterile womb” and “in utero colonization” hypotheses: Implications for research on the pioneer infant microbiome. Microbiome 2017, 5, 48. [Google Scholar] [CrossRef] [Green Version]
- Senn, V.; Bassler, D.; Choudhury, R.; Scholkmann, F.; Righini-Grunder, F.; Vuille-dit-Bille, R.N.; Restin, T. Microbial Colonization From the Fetus to Early Childhood—A Comprehensive Review. Front. Cell. Infect. Microbiol. 2020, 10, 573735. [Google Scholar] [CrossRef]
- Aagaard, K.; Ma, J.; Antony, K.M.; Ganu, R.; Petrosino, J.; Versalovic, J. The placenta harbors a unique microbiome. Sci. Transl. Med. 2014, 6, 237ra65. [Google Scholar] [CrossRef] [Green Version]
- DiGiulio, D.B. Diversity of microbes in amniotic fluid. Semin. Fetal Neonatal Med. 2012, 17, 2–11. [Google Scholar] [CrossRef]
- Stout, M.J.; Conlon, B.; Landeau, M.; Lee, I.; Bower, C.; Zhao, Q.; Roehl, K.A.; Nelson, D.M.; Macones, G.A.; Mysorekar, I.U. Identification of intracellular bacteria in the basal plate of the human placenta in term and preterm gestations. Am. J. Obstet. Gynecol. 2013, 208, e1–e7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gulavi, E.; Mwendwa, F.; Atandi, D.O.; Okiro, P.O.; Hall, M.; Beiko, R.G.; Adam, R.D. Vaginal microbiota in women with spontaneous preterm labor versus those with term labor in Kenya: A case control study. BMC Microbiol. 2022, 22, 270. [Google Scholar] [CrossRef] [PubMed]
- Gil, A.; Rueda, R.; Ozanne, S.E.; van der Beek, E.M.; van Loo-Bouwman, C.; Schoemaker, M.; Marinello, V.; Venema, K.; Stanton, C.; Schelkle, B. Is there evidence for bacterial transfer via the placenta and any role in the colonization of the infant gut?—A systematic review. Crit. Rev. Microbiol. 2020, 46, 493–507. [Google Scholar] [CrossRef] [PubMed]
- Collado, M.C.; Rautava, S.; Aakko, J.; Isolauri, E.; Salminen, S. Human gut colonisation may be initiated in utero by distinct microbial communities in the placenta and amniotic fluid. Sci. Rep. 2016, 6, 2312. [Google Scholar] [CrossRef] [Green Version]
- Jiménez, E.; Fernández, L.; Marín, M.L.; Martín, R.; Odriozola, J.M.; Nueno-Palop, C.; Narbad, A.; Olivares, M.; Xaus, J.; Rodríguez, J.M. Isolation of commensal bacteria from umbilical cord blood of healthy neonates born by cesarean section. Curr. Microbiol. 2005, 51, 270–274. [Google Scholar] [CrossRef]
- Jiménez, E.; Marín, M.L.; Martín, R.; Odriozola, J.M.; Olivares, M.; Xaus, J.; Fernández, L.; Rodríguez, J.M. Is meconium from healthy newborns actually sterile? Res. Microbiol. 2008, 159, 187–193. [Google Scholar] [CrossRef]
- Steel, J.H.; Malatos, S.; Kennea, N.; Edwards, A.D.; Miles, L.; Duggan, P.; Reynolds, P.R.; Feldman, R.G.; Sullivan, M.H.F. Bacteria and inflammatory cells in fetal membranes do not always cause preterm labor. Pediatr. Res. 2005, 57, 404–411. [Google Scholar] [CrossRef] [Green Version]
- Gomez de Agüero, M.; Ganal-Vonarburg, S.C.; Fuhrer, T.; Rupp, S.; Uchimura, Y.; Li, H.; Steinert, A.; Heikenwalder, M.; Hapfelmeier, S.; Sauer, U.; et al. The maternal microbiota drives early postnatal innate immune development. Science 2016, 351, 1296–1302. [Google Scholar] [CrossRef]
- Obata, Y.; Pachnis, V. The Effect of Microbiota and the Immune System on the Development and Organization of the Enteric Nervous System. Gastroenterology 2016, 151, 836–844. [Google Scholar] [CrossRef] [Green Version]
- Hou, K.; Wu, Z.-X.; Chen, X.-Y.; Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.Y.; Li, J.L.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef]
- Sarkar, A.; Yoo, J.Y.; Valeria Ozorio Dutra, S.; Morgan, K.H.; Groer, M. The Association between Early-Life Gut Microbiota and Long-Term Health and Diseases. J. Clin. Med. 2021, 10, 459. [Google Scholar] [CrossRef] [PubMed]
- Heiss, C.N.; Olofsson, L.E. The role of the gut microbiota in development, function and disorders of the central nervous system and the enteric nervous system. J. Neuroendocrinol. 2019, 31, e12684. [Google Scholar] [CrossRef] [PubMed]
- Shin, A.; Preidis, G.A.; Shulman, R.; Kashyap, P.C. The Gut Microbiome in Adult and Pediatric Functional Gastrointestinal Disorders. Clin. Gastroenterol. Hepatol. 2019, 17, 256–274. [Google Scholar] [CrossRef] [PubMed]
- Guaraldi, F.; Salvatori, G. Effect of breast and formula feeding on gut microbiota shaping in newborns. Front. Cell Infect. Microbiol. 2012, 2, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gopalakrishna, K.P.; Hand, T.W. Influence of Maternal Milk on the Neonatal Intestinal Microbiome. Nutrients 2020, 12, 823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demmelmair, H.; Jiménez, E.; Collado, M.C.; Salminen, S.; McGuire, M.K. Maternal and Perinatal Factors Associated with the Human Milk Microbiome. Curr. Dev. Nutr. 2020, 4, nzaa027. [Google Scholar] [CrossRef] [Green Version]
- Notarbartolo, V.; Giuffrè, M.; Montante, C.; Corsello, G.; Carta, M. Composition of Human Breast Milk Microbiota and Its Role in Children’s Health. Pediatr. Gastroenterol. Hepatol. Nutr. 2022, 25, 194–210. [Google Scholar] [CrossRef]
- Collins, J.; Borojevic, R.; Verdu, E.F.; Huizinga, J.D.; Ratcliffe, E.M. Intestinal microbiota influence the early postnatal development of the enteric nervous system. Neurogastroenterol. Motil. 2014, 26, 98–107. [Google Scholar] [CrossRef]
- De Vadder, F.; Grasset, E.; Mannerås Holm, L.; Karsenty, G.; Macpherson, A.J.; Olofsson, L.E.; Bäckhed, F. Gut microbiota regulates maturation of the adult enteric nervous system via enteric serotonin networks. Proc. Natl. Acad. Sci. USA 2018, 115, 6458–6463. [Google Scholar] [CrossRef] [Green Version]
- Kabouridis, P.S.; Lasrado, R.; McCallum, S.; Chng, S.H.; Snippert, H.J.; Clevers, H.; Pettersson, S.; Pachnis, V. Microbiota controls the homeostasis of glial cells in the gut lamina propria. Neuron 2015, 85, 289–295. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, B.; Mulder, I.E.; Musk, C.C.; Aminov, R.I.; Lewis, M.; Stokes, C.R.; Bailey, M.; Prosser, J.I.; Gill, B.P.; Pluske, J.R.; et al. Establishment of Normal Gut Microbiota Is Compromised under Excessive Hygiene Conditions. PLoS ONE 2011, 6, e28284. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.-M.; Chou, H.-C.; Yang, Y.-C.S.H. Maternal Antibiotic Treatment Disrupts the Intestinal Microbiota and Intestinal Development in Neonatal Mice. Front. Microbiol. 2021, 12, 684233. [Google Scholar] [CrossRef] [PubMed]
- Dierikx, T.H.; Visser, D.H.; Benninga, M.A.; van Kaam, A.H.L.C.; de Boer, N.K.H.; de Vries, R.; Limbergen, J.V.; de Meij, T.G.J. The influence of prenatal and intrapartum antibiotics on intestinal microbiota colonisation in infants: A systematic review. J. Infect. 2020, 81, 190–204. [Google Scholar] [CrossRef] [PubMed]
- Teshome, A.; Yitayeh, A. Relationship between periodontal disease and preterm low birth weight: Systematic review. Pan Afr. Med. J. 2016, 24, 215. [Google Scholar] [CrossRef] [PubMed]
- Bayar, E.; Bennett, P.R.; Chan, D.; Sykes, L.; MacIntyre, D.A. The pregnancy microbiome and preterm birth. Semin. Immunopathol. 2020, 42, 487–499. [Google Scholar] [CrossRef] [PubMed]
- Jain, V.G.; Willis, K.A.; Jobe, A.; Ambalavanan, N. Chorioamnionitis and neonatal outcomes. Pediatr. Res. 2022, 91, 289–296. [Google Scholar] [CrossRef]
- Been, J.V.; Lievense, S.; Zimmermann, L.J.; Kramer, B.W.; Wolfs, T.G. Chorioamnionitis as a risk factor for necrotizing enterocolitis: A systematic review and meta-analysis. J. Pediatr. 2013, 162, 236–242.e2. [Google Scholar] [CrossRef] [Green Version]
- Garzoni, L.; Faure, C.; Frasch, M. Fetal cholinergic anti-inflammatory pathway and necrotizing enterocolitis: The brain-gut connection begins in utero. Front. Integr. Neurosci. 2013, 7, 57. [Google Scholar] [CrossRef] [Green Version]
- Heymans, C.; de Lange, I.H.; Lenaerts, K.; Kessels, L.C.G.A.; Hadfoune, M.; Rademakers, G.; Melotte, V.; Boesmans, W.; Kramer, B.W.; Jobe, A.H.; et al. Chorioamnionitis induces enteric nervous system injury: Effects of timing and inflammation in the ovine fetus. Mol. Med. 2020, 26, 82. [Google Scholar] [CrossRef]
- Sigge, W.; Wedel, T.; Kühnel, W.; Krammer, H.J. Morphologic alterations of the enteric nervous system and deficiency of non-adrenergic non-cholinergic inhibitory innervation in neonatal necrotizing enterocolitis. Eur. J. Pediatr. Surg. 1998, 8, 87–94. [Google Scholar] [CrossRef]
- Wedel, T.; Krammer, H.J.; Kühnel, W.; Sigge, W. Alterations of the enteric nervous system in neonatal necrotizing enterocolitis revealed by whole-mount immunohistochemistry. Pediatr. Pathol. Lab. Med. 1998, 18, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yang, J.; Watkins, D.J.; Boomer, L.A.; Matthews, M.A.; Su, Y.; Besner, G.E. Enteric nervous system abnormalities are present in human necrotizing enterocolitis: Potential neurotransplantation therapy. Stem. Cell Res. Ther. 2013, 4, 157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, S.; Sun, Y.; Shao, X.; Zhou, Y.; Yu, Y.; Kuai, X.; Zhou, C.L. Leaky Gut in IBD: Intestinal Barrier-Gut Microbiota Interaction. J. Microbiol. Biotechnol. 2022, 32, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Cornet, A.; Savidge, T.C.; Cabarrocas, J.; Deng, W.L.; Colombel, J.F.; Lassmann, H.; Desreumaux, P.; Liblau, R.S. Enterocolitis induced by autoimmune targeting of enteric glial cells: A possible mechanism in Crohn’s disease? Proc. Natl. Acad. Sci. USA 2001, 98, 13306–13311. [Google Scholar] [CrossRef] [Green Version]
- Vermillion, M.S.; Klein, S.L. Pregnancy and infection: Using disease pathogenesis to inform vaccine strategy. Npj Vaccines 2018, 3, 6. [Google Scholar] [CrossRef] [Green Version]
- Caputi, V.; Marsilio, I.; Filpa, V.; Cerantola, S.; Orso, G.; Bistoletti, M.; Paccagnella, N.; Martin, S.D.; Montopoli, M.; Acqua, S.D.; et al. Antibiotic-induced dysbiosis of the microbiota impairs gut neuromuscular function in juvenile mice. Br. J. Pharmacol. 2017, 174, 3623–3639. [Google Scholar] [CrossRef] [Green Version]
- Al-Nedawi, K.; Mian, M.F.; Hossain, N.; Karimi, K.; Mao, Y.K.; Forsythe, P.; Min, K.K.; Stanisz, A.M.; Kunze, W.A.; Bienenstock, J. Gut commensal microvesicles reproduce parent bacterial signals to host immune and enteric nervous systems. FASEB J. 2015, 29, 684–695. [Google Scholar] [CrossRef]
- Obata, Y.; Castaño, Á.; Boeing, S.; Bon-Frauches, A.C.; Fung, C.; Fallesen, T.; de Agüero, M.G.; Yilmaz, B.; Lopes, R.; Huseynova, A.; et al. Neuronal programming by microbiota regulates intestinal physiology. Nature 2020, 578, 284–289. [Google Scholar] [CrossRef]
- Cremon, C.; Stanghellini, V.; Pallotti, F.; Fogacci, E.; Bellacosa, L.; Morselli-Labate, A.M.; Paccapelo, A.; Nardo, G.D.; Cogliandro, R.F.; Giorgio, R.D.; et al. Salmonella Gastroenteritis During Childhood Is a Risk Factor for Irritable Bowel Syndrome in Adulthood. Gastroenterology 2014, 147, 69–77. [Google Scholar] [CrossRef] [Green Version]
- Saps, M.; Pensabene, L.; Di Martino, L.; Staiano, A.; Wechsler, J.; Zheng, X.; Lorenzo, C.D. Post-infectious functional gastrointestinal disorders in children. J. Pediatr. 2008, 152, 812–816.e1. [Google Scholar] [CrossRef]
- Di Nardo, G.; Cremon, C.; Staiano, A.; Stanghellini, V.; Borrelli, O.; Strisciuglio, C.; Romano, C.; Mallardo, S.; Scarpato, E.; Marasco, G.; et al. Role of inflammation in pediatric irritable bowel syndrome. Neurogastroenterol. Motil. 2022, e14365. [Google Scholar] [CrossRef] [PubMed]
- Shaidullov, I.F.; Sorokina, D.M.; Sitdikov, F.G.; Hermann, A.; Abdulkhakov, S.R.; Sitdikova, G.F. Short chain fatty acids and colon motility in a mouse model of irritable bowel syndrome. BMC Gastroenterol. 2021, 21, 37. [Google Scholar] [CrossRef] [PubMed]
- Ostertag, D.; Buhner, S.; Michel, K.; Pehl, C.; Kurjak, M.; Götzberger, M.; Schulte-Frohlinde, E.; Frieling, T.; Enck, P.; Phillip, J.; et al. Reduced Responses of Submucous Neurons from Irritable Bowel Syndrome Patients to a Cocktail Containing Histamine, Serotonin, TNFα, and Tryptase (IBS-Cocktail). Front. Neurosci. 2015, 9, 465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DiGiulio, D.B.; Romero, R.; Amogan, H.P.; Kusanovic, J.P.; Bik, E.M.; Gotsch, F.; Kim, C.J.; Erez, O.; Edwin, S.; Relman, D.A. Microbial prevalence, diversity and abundance in amniotic fluid during preterm labor: A molecular and culture-based investigation. PLoS ONE 2008, 3, e3056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Contini, C.; Rotondo, J.C.; Magagnoli, F.; Maritati, M.; Seraceni, S.; Graziano, A.; Poggi, A.; Capucci, R.; Vesce, F.; Tognon, M.; et al. Investigation on silent bacterial infections in specimens from pregnant women affected by spontaneous miscarriage. J. Cell Physiol. 2018, 234, 100–107. [Google Scholar] [CrossRef] [Green Version]
- Doyle, R.M.; Alber, D.G.; Jones, H.E.; Harris, K.; Fitzgerald, F.; Peebles, D.; Klein, N. Term and preterm labour are associated with distinct microbial community structures in placental membranes which are independent of mode of delivery. Placenta 2014, 35, 1099–1101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zakis, D.R.; Paulissen, E.; Kornete, L.; Kaan, A.M.; Nicu, E.A.; Zaura, E. The evidence for placental microbiome and its composition in healthy pregnancies: A systematic review. J. Reprod. Immunol. 2022, 149, 103455. [Google Scholar] [CrossRef] [PubMed]
- Neu, J. The microbiome during pregnancy and early postnatal life. Semin. Fetal Neonatal. Med. 2016, 21, 373–379. [Google Scholar] [CrossRef]
- Baker, J.M.; Chase, D.M.; Herbst-Kralovetz, M.M. Uterine Microbiota: Residents, Tourists, or Invaders? Front. Immunol. 2018, 9, 208. [Google Scholar] [CrossRef] [Green Version]
- Benner, M.; Ferwerda, G.; Joosten, I.; van der Molen, R.G. How uterine microbiota might be responsible for a receptive, fertile endometrium. Hum. Reprod. Update 2018, 24, 393–415. [Google Scholar] [CrossRef] [Green Version]
- Heymans, C.; de Lange, I.H.; Hütten, M.C.; Lenaerts, K.; de Ruijter, N.J.E.; Kessels, L.C.G.A.; Rademakers, G.; Melotte, V.; Boesmans, W.; Saito, M.; et al. Chronic Intra-Uterine Ureaplasma parvum Infection Induces Injury of the Enteric Nervous System in Ovine Fetuses. Front. Immunol. 2020, 11, 189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, A.; Harker, N.; Moreira-Santos, L.; Ferreira, M.; Alden, K.; Timmis, J.; Foster, K.; Garefalaki, A.; Pachnis, P.; Andrews, P.; et al. Differential RET signaling pathways drive development of the enteric lymphoid and nervous systems. Sci. Signal 2012, 5, ra55. [Google Scholar] [CrossRef] [PubMed]
- Virgin, H.W.; Wherry, E.J.; Ahmed, R. Redefining Chronic Viral Infection. Cell 2009, 138, 30–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brun, P.; Giron, M.C.; Zoppellaro, C.; Bin, A.; Porzionato, A.; De Caro, R.; Barbara, G.; Stanghellini, V.; Corinaldesi, R.; Zaninotto, G.; et al. Herpes simplex virus type 1 infection of the rat enteric nervous system evokes small-bowel neuromuscular abnormalities. Gastroenterology 2010, 138, 1790–1801. [Google Scholar] [CrossRef]
- Brun, P.; Qesari, M.; Marconi, P.C.; Kotsafti, A.; Porzionato, A.; Macchi, V.; Schwendener, R.A.; Scarpa, M.; Giron, M.C.; Palù, G.; et al. Herpes Simplex Virus Type 1 Infects Enteric Neurons and Triggers Gut Dysfunction via Macrophage Recruitment. Front. Cell. Infect. Microbiol. 2018, 8. [Google Scholar] [CrossRef] [Green Version]
- Brun, P.; Scarpa, M.; Marchiori, C.; Conti, J.; Kotsafti, A.; Porzionato, A.; Caro, R.D.; Scarpa, M.; Calistri, A.; Castagliuolo, I. Herpes Simplex Virus Type 1 Engages Toll Like Receptor 2 to Recruit Macrophages During Infection of Enteric Neurons. Front. Microbiol. 2018, 9, 2148. [Google Scholar] [CrossRef] [Green Version]
- Ikebuchi, Y.; Kanda, T.; Ikeda, H.; Yoshida, A.; Sakaguchi, T.; Urabe, S.; Minami, H.; Nakao, K.; Kuwamoto, S.; Inoue, H.; et al. Identification of human herpes virus 1 encoded microRNAs in biopsy samples of lower esophageal sphincter muscle during peroral endoscopic myotomy for esophageal achalasia. Dig. Endosc. 2020, 32, 136–142. [Google Scholar] [CrossRef] [Green Version]
- Becker, J.; Niebisch, S.; Ricchiuto, A.; Schaich, E.J.; Lehmann, G.; Waltgenbach, T.; Schafft, A.; Hess, T.; Lenze, F.; Venerito, M.; et al. Comprehensive epidemiological and genotype-phenotype analyses in a large European sample with idiopathic achalasia. Eur. J. Gastroenterol. Hepatol. 2016, 28, 689–695. [Google Scholar] [CrossRef]
- Chaudhury, A.; Dendi, V.S.R.; Chaudhury, M.; Jain, A.; Kasarla, M.R.; Panuganti, K.; Jain, G.; Ramanujam, A.; Rena, B.; Koyagura, S.R.; et al. HSV1/2 Genital Infection in Mice Cause Reversible Delayed Gastrointestinal Transit: A Model for Enteric Myopathy. Front. Med. 2018, 5, 176. [Google Scholar] [CrossRef]
- Khoury-Hanold, W.; Yordy, B.; Kong, P.; Kong, Y.; Ge, W.; Szigeti-Buck, K.; Ralevski, A.; Horvath, T.L.; Iwasaki, A. Viral Spread to Enteric Neurons Links Genital HSV-1 Infection to Toxic Megacolon and Lethality. Cell Host Microbe. 2016, 19, 788–799. [Google Scholar] [CrossRef] [Green Version]
- Gershon, M.; Gershon, A. Varicella-Zoster Virus and the Enteric Nervous System. J. Infect. Dis. 2018, 218 (Suppl. S2), S113–S119. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.J.; Gershon, A.A.; Li, Z.; Cowles, R.A.; Gershon, M.D. Varicella zoster virus (VZV) infects and establishes latency in enteric neurons. J. Neurovirol. 2011, 17, 578–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gershon, A.A.; Chen, J.; Davis, L.; Krinsky, C.; Cowles, R.; Reichard, R.; Gershon, M. Latency of varicella zoster virus in dorsal root, cranial, and enteric ganglia in vaccinated children. Trans. Am. Clin. Climatol. Assoc. 2012, 123, 17–33, discussion 5. [Google Scholar] [PubMed]
- Pui, J.C.; Furth, E.E.; Minda, J.; Montone, K.T. Demonstration of varicella-zoster virus infection in the muscularis propria and myenteric plexi of the colon in an HIV-positive patient with herpes zoster and small bowel pseudo-obstruction (Ogilvie’s syndrome). Am. J. Gastroenterol. 2001, 96, 1627–1630. [Google Scholar] [CrossRef] [PubMed]
- Edelman, D.A.; Antaki, F.; Basson, M.D.; Salwen, W.A.; Gruber, S.A.; Losanoff, J.E. Ogilvie syndrome and herpes zoster: Case report and review of the literature. J. Emerg. Med. 2010, 39, 696–700. [Google Scholar] [CrossRef]
- Windster, J.D.; Ouwendijk, W.J.D.; Sloots, C.E.J.; Verjans, G.; Verdijk, R.M. Ileocolic Intussusception as the Presenting Symptom of Primary Enteric Varicella-Zoster Virus Infection in a 7-Month-Old Infant. J. Infect. Dis. 2020, 222, 305–308. [Google Scholar] [CrossRef] [Green Version]
- Sauve, R.S.; Leung, A.K. Congenital varicella syndrome with colonic atresias. Clin. Pediatr. 2003, 42, 451–453. [Google Scholar] [CrossRef]
- Hendriks, G.; McPartland, J.; El-Matary, W. Gastrointestinal presentation and outcome of perinatal cytomegalovirus infection. BMJ Case Rep. 2013, 2013, bcr2012007671. [Google Scholar] [CrossRef] [Green Version]
- Déchelotte, P.J.; Mulliez, N.M.; Bouvier, R.J.; Vanlieféringhen, P.C.; Lémery, D.J. Pseudo-meconium ileus due to cytomegalovirus infection: A report of three cases. Pediatr. Pathol. 1992, 12, 73–82. [Google Scholar] [CrossRef]
- Bonnard, A.; Le Huidoux, P.; Carricaburu, E.; Farnoux, C.; Berrebi, D.; Aigrain, Y.; de Lagausie, P. Cytomegalovirus infection as a possible underlying factor in neonatal surgical conditions. J. Pediatr. Surg. 2006, 41, 1826–1829. [Google Scholar] [CrossRef]
- Yeung, F.; Chung, P.H.Y.; Wong, K.K.Y.; Tam, P.K.H. Cytomegalovirus-associated colitis mimicking necrotizing enterocolitis—A near miss diagnosis of neonatal colonic stricture. J. Pediatr. Surg. Case Rep. 2014, 2, 459–461. [Google Scholar] [CrossRef] [Green Version]
- Colomba, C.; Giuffrè, M.; La Placa, S.; Cascio, A.; Trizzino, M.; De Grazia, S.; Corsello, G. Congenital cytomegalovirus related intestinal malrotation: A case report. Ital. J. Pediatr. 2016, 42, 105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thapar, N.; Saliakellis, E.; Benninga, M.A.; Borrelli, O.; Curry, J.; Faure, C.; Giorgio, R.D.; Gupte, G.; Knowles, C.H.; Staiano, A.; et al. Paediatric Intestinal Pseudo-obstruction: Evidence and Consensus-based Recommendations From an ESPGHAN-Led Expert Group. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 991–1019. [Google Scholar] [CrossRef]
- Chanpong, A.; Borrelli, O.; Thapar, N. Hirschsprung disease and Paediatric Intestinal Pseudo-obstruction. Best Pract. Res. Clin. Gastroenterol. 2022, 56–57, 101765. [Google Scholar] [CrossRef] [PubMed]
- Sinagra, E.; Pellegatta, G.; Maida, M.; Rossi, F.; Conoscenti, G.; Pallio, S.; Alloro, R.; Raimondo, D.; Anderloni, A. Could Chronic Idiopatic Intestinal Pseudo-Obstruction Be Related to Viral Infections? J. Clin. Med. 2021, 10, 268. [Google Scholar] [CrossRef] [PubMed]
- Besnard, M.; Faure, C.; Fromont-Hankard, G.; Ansart-Pirenne, H.; Peuchmaur, M.; Cezard, J.P.; Navarro, J. Intestinal pseudo-obstruction and acute pandysautonomia associated with Epstein-Barr virus infection. Am. J. Gastroenterol. 2000, 95, 280–284. [Google Scholar] [CrossRef] [PubMed]
- White, J.P.; Xiong, S.; Malvin, N.P.; Khoury-Hanold, W.; Heuckeroth, R.O.; Stappenbeck, T.S.; Diamond, M.S. Intestinal Dysmotility Syndromes following Systemic Infection by Flaviviruses. Cell 2018, 175, 1198–1212.e12. [Google Scholar] [CrossRef] [Green Version]
- Lu, W.; Xiao, Y.; Huang, J.; Lu, L.; Tao, Y.; Yan, W.; Cao, Y.; Cai, W. Causes and prognosis of chronic intestinal pseudo-obstruction in 48 subjects: A 10-year retrospective case series. Medicine 2018, 97, e12150. [Google Scholar] [CrossRef]
- Ko, D.; Yang, H.-B.; Youn, J.; Kim, H.-Y. Clinical Outcomes of Pediatric Chronic Intestinal Pseudo-Obstruction. J. Clin. Med. 2021, 10, 2376. [Google Scholar] [CrossRef]
- Sonsino, E.; Mouy, R.; Foucaud, P.; Cezard, J.P.; Aigrain, Y.; Bocquet, L.; Navarro, J. Intestinal pseudoobstruction related to cytomegalovirus infection of myenteric plexus. N. Engl. J. Med. 1984, 311, 196–197. [Google Scholar]
- Ategbo, S.; Turck, D.; Gottrand, F.; Bonnevalle, M.; Wattre, P.; Lecomte-Houcke, M.; Farriaux, J.P. Chronic intestinal pseudo-obstruction associated with cytomegalovirus infection in an infant. J. Pediatr. Gastroenterol. Nutr. 1996, 23, 457–460. [Google Scholar] [CrossRef]
- Asabe, K.; Nagasaki, A.; Sato, K.; Nakayama, M. Intestinal obstruction caused by congenital cytomegalovirus infection: Report of a case. Surg. Today. 2003, 33, 764–767. [Google Scholar] [CrossRef] [PubMed]
- Crawford, S.E.; Ramani, S.; Tate, J.E.; Parashar, U.D.; Svensson, L.; Hagbom, M.; Franco, M.A.; Greenberg, H.B.; Ryan, M.O.; Kang, G.; et al. Rotavirus infection. Nat. Rev. Dis. Prim. 2017, 3, 17083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ball, J.M.; Tian, P.; Zeng, C.Q.; Morris, A.P.; Estes, M.K. Age-dependent diarrhea induced by a rotaviral nonstructural glycoprotein. Science 1996, 272, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Bialowas, S.; Hagbom, M.; Nordgren, J.; Karlsson, T.; Sharma, S.; Magnusson, K.-E.; Svensson, L. Rotavirus and Serotonin Cross-Talk in Diarrhoea. PLoS ONE 2016, 11, e0159660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Istrate, C.; Hagbom, M.; Vikström, E.; Magnusson, K.E.; Svensson, L. Rotavirus infection increases intestinal motility but not permeability at the onset of diarrhea. J. Virol. 2014, 88, 3161–3169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hellysaz, A.; Svensson, L.; Hagbom, M. Rotavirus pre-symptomatically downregulates ileum-innervating sympathetic nerves concomitant with increased intestinal transit and altered brain activity. bioRxiv 2021. [Google Scholar] [CrossRef]
- Bardhan, P.K.; Salam, M.A.; Molla, A.M. Gastric emptying of liquid in children suffering from acute rotaviral gastroenteritis. Gut 1992, 33, 26–29. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.L.; Kim, Y.H.; Kang, K.S. A Case of Gastroparesis Followed after Rotavirus Gastroenteritis. Korean J. Pediatr. Gastroenterol. Nutr. 2006, 9, 65–69. [Google Scholar] [CrossRef]
- Hung, C.-W.; Wu, W.-F.; Wu, C.-L. Rotavirus gastroenteritis complicated with toxic megacolon. Acta Paediatr. 2009, 98, 1850–1852. [Google Scholar] [CrossRef]
- Borghan, M.A.; Mori, Y.; El-Mahmoudy, A.B.; Ito, N.; Sugiyama, M.; Takewaki, T.; Minamoto, N. Induction of nitric oxide synthase by rotavirus enterotoxin NSP4: Implication for rotavirus pathogenicity. J. Gen Virol. 2007, 88, 2064–2072. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Yu, M.L.; Tao, X.; Cheng, M.H.; Liu, C.C.; Liu, Y.; Li, Y.G. Analysis of the intestinal microbial community altered during rotavirus infection in suckling mice. Virol. J. 2021, 18, 254. [Google Scholar] [CrossRef] [PubMed]
- Puoti, M.G.; Rybak, A.; Kiparissi, F.; Gaynor, E.; Borrelli, O. SARS-CoV-2 and the Gastrointestinal Tract in Children. Front. Pediatr. 2021, 9, 617980. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef]
- Giobbe, G.G.; Bonfante, F.; Jones, B.C.; Gagliano, O.; Luni, C.; Zambaiti, E.; Perin, S.; Laterza, C.; Busslinger, G.; Stuart, H.; et al. SARS-CoV-2 infection and replication in human gastric organoids. Nat. Commun. 2021, 12, 6610. [Google Scholar] [CrossRef]
- Giobbe, G.G.; Bonfante, F.; Zambaiti, E.; Gagliano, O.; Jones, B.C.; Luni, C.; Laterza, C.; Perin, S.; Stuart, H.T.; Pagliari, M.; et al. SARS-CoV-2 infection and replication in human fetal and pediatric gastric organoids. bioRxiv 2020. [Google Scholar] [CrossRef]
- Zhang, H.; Li, H.-B.; Lyu, J.-R.; Lei, X.-M.; Li, W.; Wu, G.; Lyu, J.; Dai, Z.M. Specific ACE2 expression in small intestinal enterocytes may cause gastrointestinal symptoms and injury after 2019-nCoV infection. Int. J. Infect. Dis. 2020, 96, 19–24. [Google Scholar] [CrossRef]
- Deffner, F.; Scharr, M.; Klingenstein, S.; Klingenstein, M.; Milazzo, A.; Scherer, S.; Wagner, A.; Hirt, B.; Mack, A.F.; Neckel, P.H. Histological Evidence for the Enteric Nervous System and the Choroid Plexus as Alternative Routes of Neuroinvasion by SARS-CoVFrontiers in Neuroanatomy. Front. Neuroanat. 2020, 14, 596439. [Google Scholar] [CrossRef]
- Coles, M.J.; Masood, M.; Crowley, M.M.; Hudgi, A.; Okereke, C.; Klein, J. It Ain’t Over Til It’s Over: SARS CoV-2 and Post-infectious Gastrointestinal Dysmotility. Dig. Dis. Sci. 2022, 67, 5407–5415. [Google Scholar] [CrossRef]
- Furuzawa-Carballeda, J.; Icaza-Chávez, M.E.; Aguilar-León, D.; Uribe-Uribe, N.; Nuñez-Pompa, M.C.; Trigos-Díaz, A.; Areán-Sanz, R.; Fernández-Camargo, D.A.; Coss-Adame, E.; Valdovinos, M.A.; et al. Is the Sars-CoV-2 virus a possible trigger agent for the development of achalasia? Neurogastroenterol. Motil. 2023, 35, e14502. [Google Scholar] [CrossRef]
- Scottoni, F.; Giobbe, G.G.; Zambaiti, E.; Khalaf, S.; Sebire, N.J.; Curry, J.; Coppi, P.D.; Gennari, F. Intussusception and COVID-19 in Infants: Evidence for an Etiopathologic Correlation. Pediatrics 2022, 149, e2021054644. [Google Scholar] [CrossRef] [PubMed]
- Beesley, M.A.; Davidson, J.R.; Panariello, F.; Shibuya, S.; Scaglioni, D.; Jones, B.C.; Maksym, K.; Ogunbiyi, O.; Sebire, N.J.; Cacchiarelli, D.; et al. COVID-19 and vertical transmission: Assessing the expression of ACE2/TMPRSS2 in the human fetus and placenta to assess the risk of SARS-CoV-2 infection. Bjog 2022, 129, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Romano-Keeler, J.; Zhang, J.; Sun, J. COVID-19 and the neonatal microbiome: Will the pandemic cost infants their microbes? Gut Microbes 2021, 13, 1912562. [Google Scholar] [CrossRef]
- Tetz, G.; Tetz, V. Bacteriophage infections of microbiota can lead to leaky gut in an experimental rodent model. Gut Pathog. 2016, 8, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Foong, J.P.P.; Harris, N.L.; Bornstein, J.C. Enteric neuroimmune interactions coordinate intestinal responses in health and disease. Mucosal Immunol. 2022, 15, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Vacca, F.; Le Gros, G. Tissue-specific immunity in helminth infections. Mucosal Immunol. 2022, 15, 1212–1223. [Google Scholar] [CrossRef] [PubMed]
- Viola, M.F.; Boeckxstaens, G. Muscularis macrophages: Trained guardians of enteric neurons. Cell Res. 2022, 32, 229–230. [Google Scholar] [CrossRef]
- Naveed, A.; Abdullah, S. Impact of parasitic infection on human gut ecology and immune regulations. Transl. Med. Commun. 2021, 6, 11. [Google Scholar] [CrossRef]
- Khan, A.A.; Langston, H.C.; Costa, F.C.; Olmo, F.; Taylor, M.C.; McCann, C.J.; Kelly, J.M.; Lewis, M.D. Local association of Trypanosoma cruzi chronic infection foci and enteric neuropathic lesions at the tissue micro-domain scale. PLoS Pathog. 2021, 17, e1009864. [Google Scholar] [CrossRef]
- Góis, M.B.; Hermes-Uliana, C.; Barreto Zago, M.C.; Zanoni, J.N.; da Silva, A.V.; de Miranda-Neto, M.H.; de Almeida Araújo, E.J.; de Mello Gonçales Sant’Ana, D. Chronic infection with Toxoplasma gondii induces death of submucosal enteric neurons and damage in the colonic mucosa of rats. Exp. Parasitol. 2016, 164, 56–63. [Google Scholar] [CrossRef]
- van Tilburg Bernardes, E.; Pettersen, V.K.; Gutierrez, M.W.; Laforest-Lapointe, I.; Jendzjowsky, N.G.; Cavin, J.-B.; Vicentini, F.A.; Keenan, C.M.; Ramay, H.R.; Samara, J.; et al. Intestinal fungi are causally implicated in microbiome assembly and immune development in mice. Nat. Commun. 2020, 11, 2577. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chanpong, A.; Borrelli, O.; Thapar, N. The Potential Role of Microorganisms on Enteric Nervous System Development and Disease. Biomolecules 2023, 13, 447. https://doi.org/10.3390/biom13030447
Chanpong A, Borrelli O, Thapar N. The Potential Role of Microorganisms on Enteric Nervous System Development and Disease. Biomolecules. 2023; 13(3):447. https://doi.org/10.3390/biom13030447
Chicago/Turabian StyleChanpong, Atchariya, Osvaldo Borrelli, and Nikhil Thapar. 2023. "The Potential Role of Microorganisms on Enteric Nervous System Development and Disease" Biomolecules 13, no. 3: 447. https://doi.org/10.3390/biom13030447
APA StyleChanpong, A., Borrelli, O., & Thapar, N. (2023). The Potential Role of Microorganisms on Enteric Nervous System Development and Disease. Biomolecules, 13(3), 447. https://doi.org/10.3390/biom13030447





