Relaxin-like Gonad-Stimulating Peptides in Asteroidea
Abstract
:1. Introduction
2. Identification of Starfish GTH-like Active Hormone
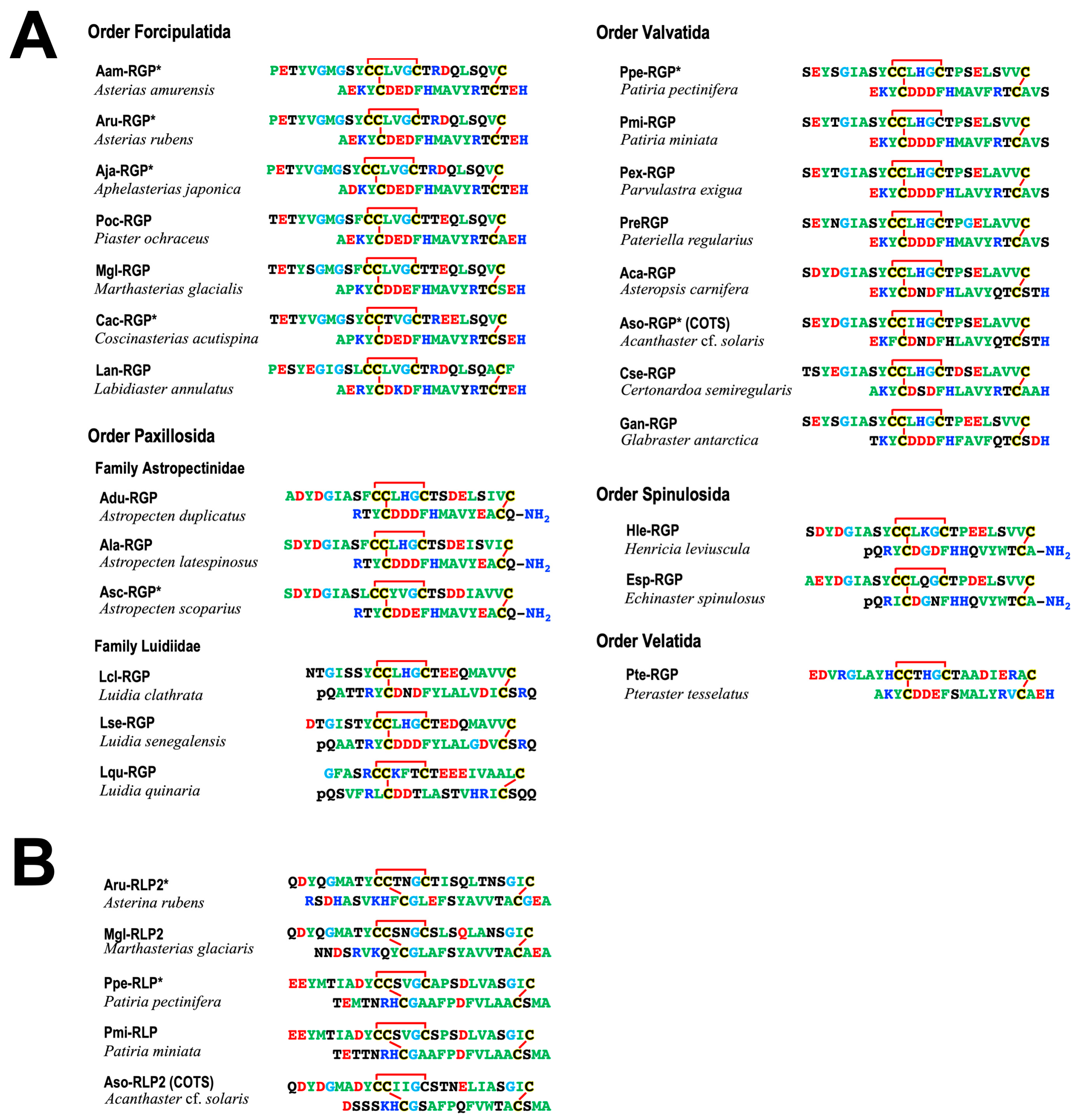
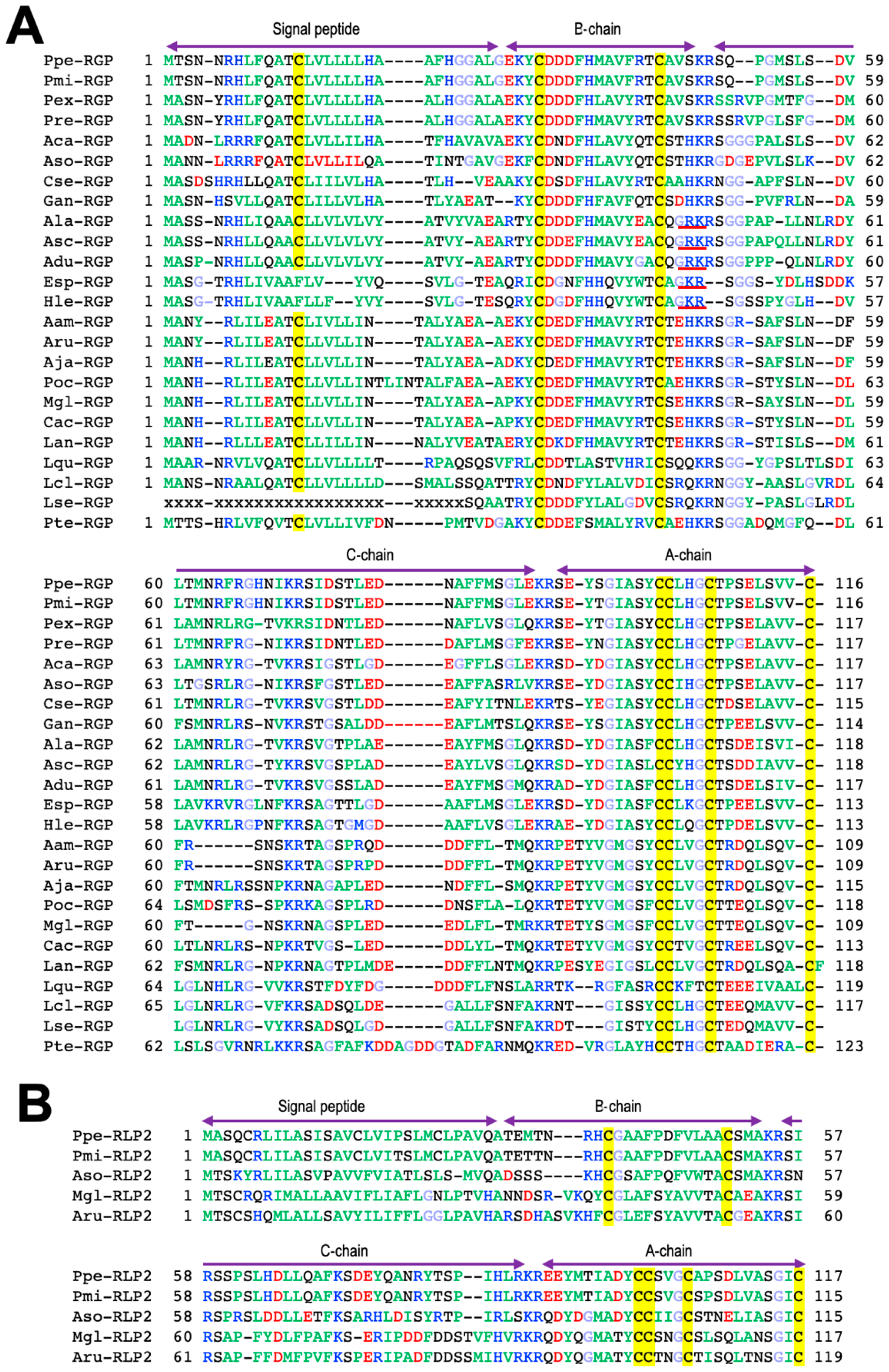
3. Orthologs of RGP
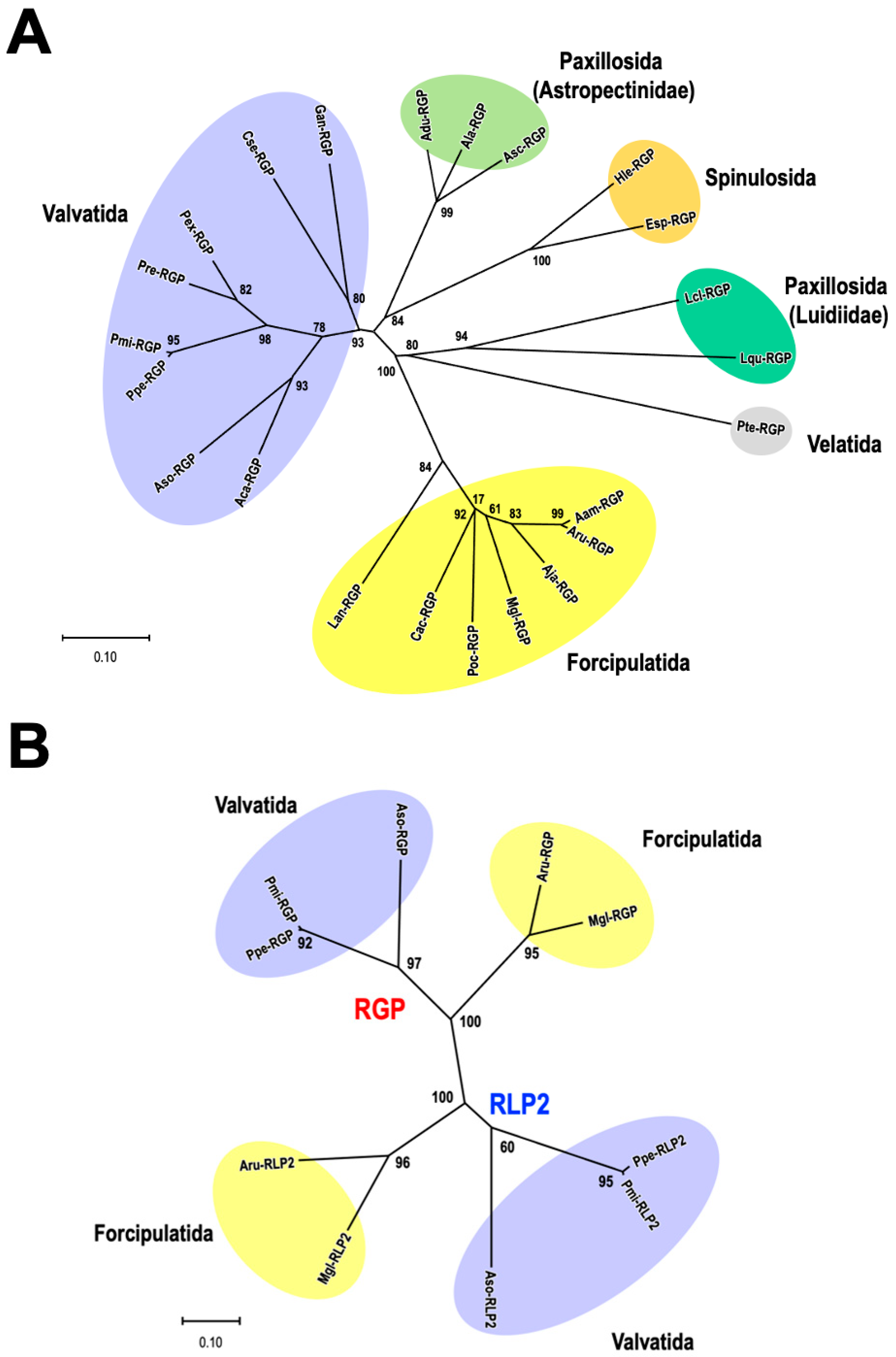
4. Phylogenetic Analysis of RGP
5. Localization of RGP
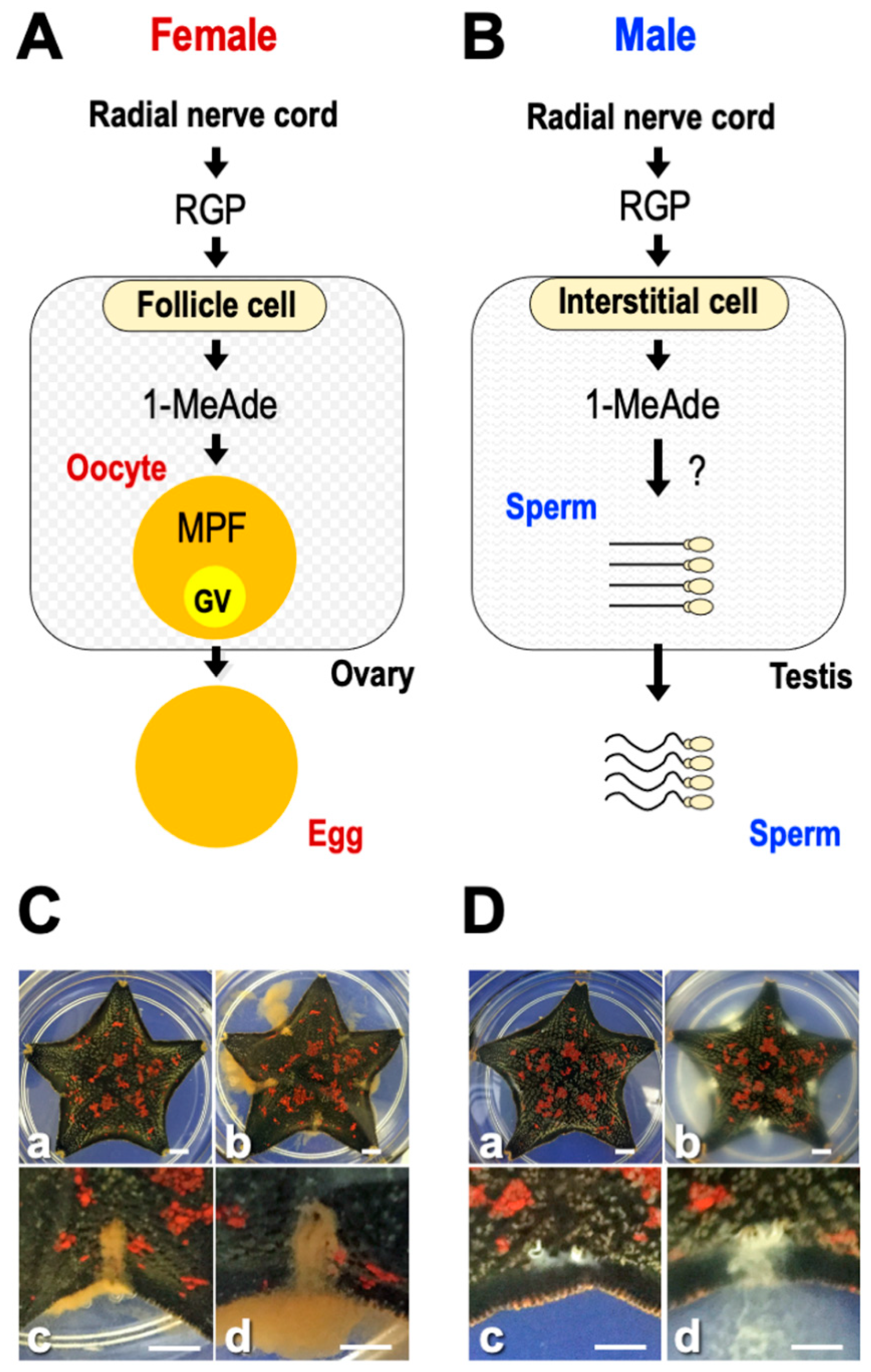
6. Hormonal Action of RGP
7. RGP Receptor
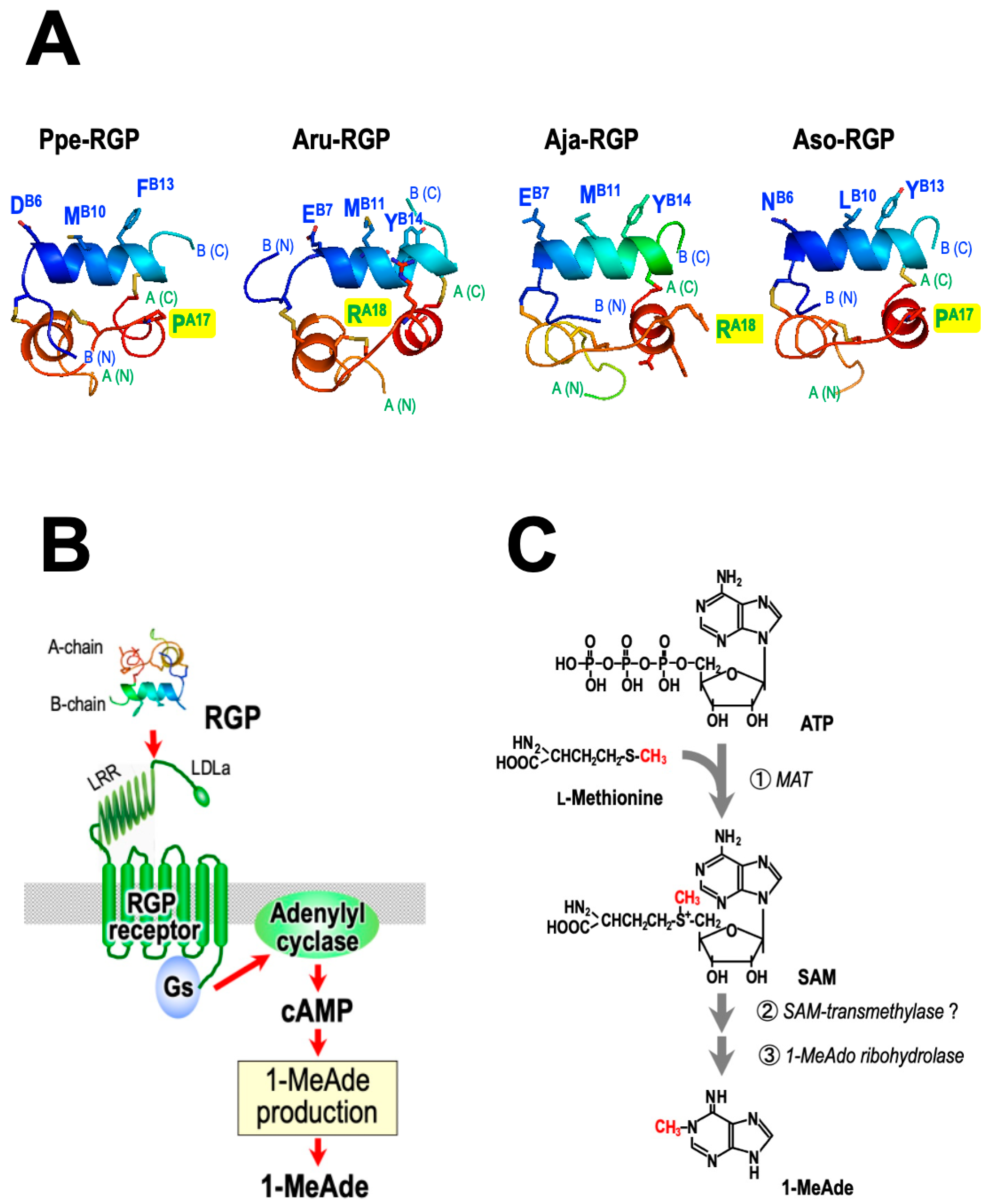
8. Species Specificity of RGP
9. RGP-Induced 1-MeAde Production
10. Gamete Shedding
11. RGP in Larvae
12. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kawauchi, H.; Suzuki, K.; Itoh, H.; Swanson, P.; Naito, N.; Nagahama, Y.; Nozaki, M.; Nakai, Y.; Itoh, S. The duality of teleost gonadotropins. Fish Physiol. Biochem. 1988, 7, 29–38. [Google Scholar] [CrossRef]
- Uchida, K.; Moriyama, S.; Chiba, H.; Shimotani, T.; Honda, K.; Miki, M.; Takahashi, A.; Sower, S.A.; Nozaki, M. Evolutionary origin of a functional gonadotropin in the pituitary of the most primitive vertebrate, hagfish. Proc. Natl. Acad. Sci. USA 2010, 107, 15832–15837. [Google Scholar] [CrossRef] [Green Version]
- Uchida, K.; Moriyama, S.; Sower, S.A.; Nozaki, M. Glycoprotein hormone in the pituitary of hagfish and its evolutionary implications. Fish Physiol. Biochem. 2013, 39, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Kuczer, M.; Rosinski, G.; Konopinska, D. Insect gonadotropic peptide hormones: Some recent developments. J. Pept. Sci. 2007, 13, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Masler, E.P.; Hagedorn, H.H.; Petzel, D.H.; Borkovec, A.B. Partial purification of egg development neurosecretory hormone with reverse-phase liquid chromatographic techniques. Life Sci. 1983, 33, 1925–1931. [Google Scholar] [CrossRef] [PubMed]
- Chiu, A.Y.; Hunkapiller, M.W.; Heller, E.; Stuart, D.K.; Hood, L.E.; Strumwasser, F. Purification and primary structure of the neuropeptide egg-laying hormone of Aplysia californica. Proc. Natl. Acad. Sci. USA 1979, 76, 6656–6660. [Google Scholar] [CrossRef] [Green Version]
- Hasegawa, Y.; Haino-Fukushima, K.; Katakura, Y. Isolation and properties of androgenic gland hormone from the terrestrial isopod, Armadillidium vulgare. Gen. Comp. Endocrinol. 1987, 67, 101–110. [Google Scholar] [CrossRef]
- Brown, M.R.; Clark, K.D.; Gulia, M.; Zhao, Z.; Garczynski, S.F.; Crim, J.W.; Suderman, R.J.; Strand, M.R. An insulin-like peptide regulates egg maturation and metabolism in the mosquito Aedes aegypti. Proc. Natl. Acad. Sci. USA 2008, 105, 5716–5721. [Google Scholar] [CrossRef] [Green Version]
- Liao, S.; Nässel, D.R. Drosophila insulin-like peptide 8 (DILP8) in ovarian follicle cells regulates ovulation and metabolism. Front. Endocrinol. 2020, 11, 461. [Google Scholar] [CrossRef]
- Kanatani, H.; Shirai, H.; Nakanishi, K.; Kurokawa, T. Isolation and identification of meiosis-inducing substance in starfish, Asterias amurensis. Nature 1969, 221, 273–274. [Google Scholar] [CrossRef]
- Kanatani, H. Oocyte Growth and Maturation in Starfish. In Biology of Fertilization; Metz, C.B., Monroy, A., Eds.; Academic Press: New York, NY, USA, 1985; Volume 1, pp. 119–140. ISBN 0-12-492601-0. [Google Scholar]
- Kishimoto, T. Entry into mitosis: A solution to the decades-long enigma of MPF. Chromosoma 2015, 124, 417–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaet, A.B.; McConnaughy, R.A. Physiologic activity of nerve extracts. Biol. Bull. 1959, 117, 407–408. [Google Scholar]
- Kanatani, H.; Ohguri, M. Mechanism of starfish spawning I. Distribution of active substance responsible for maturation of oocytes and shedding of gametes. Biol. Bull. 1966, 131, 104–114. [Google Scholar] [CrossRef]
- Kanatani, H.; Shirai, H. Mechanism of starfish spawning. III. Properties and action of meiosis-inducing substance produced in gonad under influence of gonad-stimulating substance. Dev. Growth Differ. 1970, 12, 119–140. [Google Scholar] [CrossRef] [PubMed]
- Kanatani, H.; Shirai, H. In vitro production of meiosis inducing substance by nerve extract in ovary of starfish. Nature 1967, 216, 284–286. [Google Scholar] [CrossRef]
- Kanatani, H.; Shirai, H. Mechanism of starfish spawning. II. Some aspects of action of a neural substance obtained from radial nerve. Biol. Bull. 1969, 137, 297–311. [Google Scholar] [CrossRef]
- Mita, M. Starfish gonadotropic hormone: From gamete-shedding substance to relaxin-like gonad-stimulating peptide. Front. Endocrinol. 2019, 10, 182. [Google Scholar] [CrossRef] [Green Version]
- Mita, M.; Yoshikuni, M.; Ohno, K.; Shibata, Y.; Paul-Prasanth, B.; Pitchayawasin, S.; Isobe, M.; Nagahama, Y. A relaxin-like peptide purified from radial nerves induces oocyte maturation and ovulation in the starfish, Asterina pectinifera. Proc. Natl. Acad. Sci. USA 2009, 106, 9507–9512. [Google Scholar] [CrossRef] [Green Version]
- Haraguchi, S.; Ikeda, N.; Abe, M.; Tsutsui, K.; Mita, M. Nucleotide sequence and expression of relaxin-like gonad-stimulating peptide gene in starfish Asterine pectinifera. Gen. Comp. Endocrinol. 2016, 227, 115–119. [Google Scholar] [CrossRef]
- Mita, M. Relaxin-like gonad-stimulating substance in an echinoderm, the starfish: A novel relaxin system in reproduction of invertebrates. Gen. Comp. Endocrinol. 2013, 181, 241–245. [Google Scholar] [CrossRef]
- Mita, M. Starfish gonadotropic hormone: Relaxin-like gonad-stimulating peptides. Gen. Comp. Endocrinol. 2016, 230–231, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Mita, M.; Daiya, M.; Haraguchi, S.; Tsutsui, K.; Nagahama, Y. A new relaxin-like gonad-stimulating peptide identified in the starfish Asterias amurensis. Gen. Comp. Endocrinol. 2015, 222, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Semmens, D.C.; Mirabeau, O.; Moghul, I.; Pancholi, M.R.; Wurm, Y.; Elphick, M.R. Transcriptomic identification of starfish neuropeptide precursors yields new insights into neuropeptide evolution. Open Biol. 2016, 6, 150224. [Google Scholar] [CrossRef] [Green Version]
- Lin, M.; Mita, M.; Egertová, M.; Zampronio, C.G.; Jones, A.M.; Elphick, M.R. Cellular localization of relaxin-like gonad-stimulating peptide expression in Asterias rubens: New insights into hormonal control of spawning in starfish. J. Comp. Neurol. 2017, 525, 1599–1617. [Google Scholar] [CrossRef]
- Mita, M.; Katayama, H. A relaxin-like gonad-stimulating peptide from the starfish Aphelasterias japonica. Gen. Comp. Endocrinol. 2016, 229, 56–61. [Google Scholar] [CrossRef]
- Mita, M.; Osugi, T.; Matsubara, S.; Kawada, T.; Satake, H.; Katayama, H. A relaxin-like gonad-stimulating peptide identified from the starfish Astropecten scoparius. Mol. Reprod. Dev. 2021, 88, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Katayama, H.; Mita, M. The C-terminally amidated relaxin-like gonad-stimulating peptide in the starfish Astropecten scoparius. Gen. Comp. Endocrinol. 2023, 334, 114226. [Google Scholar] [CrossRef] [PubMed]
- Mita, M.; Ikeda, N.; Haraguchi, S.; Tsutsui, K.; Nakano, Y.; Nakamura, M. A gonad-stimulating peptide of the crown-of-thorns starfish, Acanthaster planci. Invertebr. Reprod. Dev. 2015, 59, 212–217. [Google Scholar] [CrossRef]
- Smith, M.K.; Wang, T.; Suwansa-ard, S.; Motti, C.A.; Elizur, A.; Zhao, M.; Rowe, M.L.; Hall, M.R.; Elphick, M.R.; Cummins, S.F. The neuropeptidome of the Crown-of-Thorns Starfish, Acanthaster planci. J. Proteom. 2017, 165, 61–68. [Google Scholar] [CrossRef]
- Smith, M.K.; Chieu, H.D.; Aizen, J.; Mos, B.; Motti, C.; Elizur, A.; Cummins, S.F. A Crown-of-Thorns Seastar recombinant relaxin-like gonad-stimulating peptide triggers oocyte maturation and ovulation. Gen. Comp. Endocrinol. 2019, 281, 41–48. [Google Scholar] [CrossRef]
- Mita, M.; Osugi, T.; Kawada, T.; Satake, H.; Katayama, H.; Kitamura, T.; Miura, T.; Miura, C. Characterization and localization of relaxin-like gonad-stimulating peptide in the crown-of-thorns starfish, Acanthaster cf. solaris. Gen. Comp. Endocrinol. 2022, 328, 114107. [Google Scholar] [CrossRef]
- Burke, R.D.; Angerer, L.M.; Elphick, M.R.; Humphrey, G.W.; Yaguchi, S.; Kiyama, T.; Liang, S.; Mu, X.; Agca, C.; Klein, W.H.; et al. A genomic view of the sea urchin nervous system. Dev. Biol. 2006, 300, 434–460. [Google Scholar] [CrossRef] [Green Version]
- Suwansa-ard, S.; Chaiyamoon, A.; Talarovicova, A.; Tinikul, R.; Tinikul, Y.; Poomtong, T.; Elphick, M.R.; Cummins, S.F.; Sobhon, P. Transcriptomic discovery and comparative analysis of neuropeptide precursors in sea cucumbers (Holothuroidea). Ppetides 2018, 99, 231–240. [Google Scholar] [CrossRef]
- Chieu, H.D.; Turner, L.; Smith, M.K.; Wang, T.; Nocillado, J.; Palma, P.; Suwansa-ard, S.; Elizur, A.; Cummins, S.F. Aquaculture breeding enhancement: Maturation and spawning in sea cucumbers using a recombinant relaxin-like gonad-stimulating peptide. Front. Genet. 2019, 10, 77. [Google Scholar] [CrossRef] [Green Version]
- Zandawala, M.; Moghul, I.; Guerra, L.A.Y.; Delroisse, J.; Abylkassimova, N.; Hugall, A.F.; O’Hara, T.D.; Elphick, M.R. Discovery of novel representatives of bilaterian neuropeptide families and reconstruction of neuropeptide precursor evolution in ophiuroid echinoderms. Open Biol. 2017, 7, 170129. [Google Scholar] [CrossRef] [Green Version]
- Aleotti, A.; Wilkie, I.C.; Yañez-Guerra, L.A.; Gattoni, G.; Rahman, T.A.; Wademan, R.F.; Ahmad, Z.; Ivanova, D.A.; Semmens, D.C.; Delroisse, J.; et al. Discovery and functional characterization of neuropeptides in crinoid echinoderms. Front Neurosci. 2022, 16, 1006594. [Google Scholar] [CrossRef]
- Kato, S.; Tsurumaru, S.; Taga, M.; Yamane, T.; Shibata, Y.; Ohno, K.; Fujiwara, A.; Yamano, K.; Yoshikuni, M. Neuronal peptides induce oocyte maturation and gamete spawning of sea cucumber, Apostichopus japonicus. Dev. Biol. 2009, 326, 169–176. [Google Scholar] [CrossRef] [Green Version]
- Takeda, N.; Kon, Y.; Artigas, G.Q.; Lapébie, P.; Barreau, C.; Koizumi, O.; Kishimoto, T.; Tachibana, K.; Houliston, E.; Deguchi, R. Identification of jellyfish neuropeptides that act directly as oocyte maturation-inducing hormones. Development 2018, 145, dev156786. [Google Scholar] [CrossRef] [Green Version]
- Deguchi, R.; Osanai, K. Serotonin-induced meiosis reinitiation from the first prophase and from the first metaphase in oocytes of the marine bivalve Hiatella flaccida: Respective changes in intracellular Ca2+ and pH. Dev. Biol. 1995, 171, 483–496. [Google Scholar] [CrossRef]
- Matsubara, S.; Shiraishi, A.; Osugi, T.; Kawada, T.; Satake, H. The regulation of oocyte maturation and ovulation in the closest sister group of vertebrates. eLife 2019, 8, e49062. [Google Scholar] [CrossRef]
- Osugi, T.; Miyasaka, N.; Shiraishi, A.; Matsubara, S.; Satake, H. Cioni, a vertebrate cholecystokinin/gastrin homolog, induces ovulation in the ascidian Ciona intestinalis type A. Sci. Rep. 2021, 11, 10911. [Google Scholar] [CrossRef] [PubMed]
- Veenstra, J.A. Ambulacrarian insulin-related peptides and their putative receptors suggest how insulin and similar peptides may have evolved from insulin-like growth factor. PeerJ 2021, 9, e11799. [Google Scholar] [CrossRef]
- Mah, C.L.; Foltz, D.W. Molecular physiology of the Valvatacea (Asteroidea, Echinodermata). Zool. J. Linn. Soc. 2011, 161, 769–788. [Google Scholar] [CrossRef] [Green Version]
- Blake, D.B. Two late Ordovician asteroids (Echinodermata) with characters suggestive of early ophiuroids. J. Paleontol. 2007, 81, 1476–1485. [Google Scholar] [CrossRef]
- Blake, D.B. Toward a history of the Paleozoic Asteroidea (Echinodermata). Bull. Am. Paleontol. 2018, 394, 1–96. [Google Scholar]
- Mita, M.; Ito, C.; Kubota, E.; Nagahama, Y.; Shibata, Y. Expression and distribution of gonad-stimulating substance in various organs of the starfish Asterina pectinifera. Ann. N. Y. Acad. Sci. 2009, 1163, 472–474. [Google Scholar] [CrossRef] [PubMed]
- Katayama, H.; Mita, M. A sulfanyl-PEG derivative of relaxin-like peptide utilizable for the conjugation with KLH and the antibody production. Bioorg. Med. Chem. 2016, 24, 3596–3602. [Google Scholar] [CrossRef]
- Katayama, H.; Mizuno, R.; Mita, M. A novel approach for preparing disulfide-rich peptide-KLH conjugate applicable to the antibody production. Biosci. Biotechnol. Biochem. 2019, 2, 1791–1799. [Google Scholar] [CrossRef]
- Mita, M.; Katayama, H. Enzyme-linked immunosorbent assay of relaxin-like gonad-stimulating peptide in the starfish Patiria (Asterina) pectinifera. Gen. Comp. Endocrinol. 2018, 258, 157–162. [Google Scholar] [CrossRef]
- Yamamoto, K.; Kiyomoto, M.; Katayama, H.; Mita, M. Radioimmunoassay of relaxin-like gonad-stimulating peptide in the starfish Patiria (=Asterina) pectinifera. Gen. Comp. Endocrinol. 2017, 243, 84–88. [Google Scholar] [CrossRef]
- Mita, M.; Elphick, M.R.; Katayama, H. A specific and sensitive enzyme-linked immunosorbent assay for the measurement of relaxin-like gonad-stimulating peptide in the starfish Asterias rubens. Gen. Comp. Endocrinol. 2021, 310, 113831. [Google Scholar] [CrossRef] [PubMed]
- Unger, H. Experimentelle und histologisch Untersuchungen über Wirkfactoren aus dem Nervensystem von Asterias (Marthasterias) glacialis (Asteroidea; Echinormata). Zool. Jahrb. Abt. Allg. Zool. Physiol. Tiere. 1962, 69, 481–536. [Google Scholar]
- Beijnink, F.B.; Walker, C.W.; Voogt, P.A. An ultrastructural study of relationships between the ovarian haemal system, follicle cells, and primary oocytes in the sea star, Asterias rubens. Implications for oocyte nutrition. Cell Tissue Res. 1984, 238, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Mita, M. Release of relaxin-like gonad-stimulating substance from starfish radial nerves by ionomycin. Zool. Sci. 2013, 30, 602–606. [Google Scholar] [CrossRef] [PubMed]
- Mita, M.; Oka, H.; Thorndyke, M.C.; Shibata, Y.; Yoshikuni, M.; Nagahama, Y. Inhibitory effect of a SALMFamide neuropeptide on secretion of gonad-stimulating substance from radial nerves in the starfish Asterina pectinifera. Zool. Sci. 2004, 21, 299–303. [Google Scholar] [CrossRef] [Green Version]
- Jönsson, M.; Morin, M.; Wang, C.K.; Craik, D.J.; Degnan, S.M.; Degnan, B.M. Sex-specific expression of pheromones and other signals in gravid starfish. BMC Biol. 2022, 20, 288. [Google Scholar] [CrossRef]
- Feng, Y.; Gonzalez, V.M.P.; Lin, M.; Egertová, M.; Mita, M.; Elphick, M.R. Localization of relaxin-like gonad-stimulating peptide expression in starfish reveals gonoducts as a source for its role as a regular of spawning. J. Comp. Neurol. 2023. submitted. [Google Scholar]
- Hirai, S.; Kanatani, H. Site of production of meiosis-inducing substance in ovary of starfish. Exp Cell Res. 1971, 67, 224–227. [Google Scholar] [CrossRef]
- Hirai, S.; Chida, K.; Kanatani, H. Role of follicle cells in maturation of starfish oocytes. Dev. Growth Differ. 1973, 15, 21–31. [Google Scholar] [CrossRef]
- Kubota, J.; Nakao, K.; Shirai, H.; Kanatani, H. 1-Methyladenine producing cell in starfish testis. Exp. Cell Res. 1977, 106, 63–70. [Google Scholar] [CrossRef]
- Mita, M.; Yamamoto, K.; Yoshikuni, M.; Ohno, K.; Nagahama, Y. Preliminary study on the receptor of gonad-stimulating substance (GSS) as a gonadotropin of starfish. Gen. Comp. Endocrinol. 2007, 153, 299–301. [Google Scholar] [CrossRef] [PubMed]
- Mita, M.; Yamamoto, K.; Nagahama, Y. Interaction of relaxin-like gonad-stimulating substance with ovarian follicle cells of the starfish, Asterina pectinifera. Zool. Sci. 2011, 28, 764–769. [Google Scholar] [CrossRef] [PubMed]
- Mita, M.; Ueta, N.; Nagahama, Y. Regulatory functions of cyclic adenosine 3′,5′-monophosphate in 1-methyladenine production by starfish follicle cells. Biochem. Biophys. Res. Commun. 1987, 147, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Mita, M.; Ueta, N.; Nagahama, Y. Mediation of cyclic adenosine 3’,5’-monophosphate in 1-methyladenine production of starfish ovarian follicle cells. Gen. Comp. Endocrinol. 1989, 76, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Mita, M. Methylation during 1-methyladenine production by starfish ovarian follicle cells. Comp. Biochem. Physiol. Part B 1991, 99, 459–462. [Google Scholar] [CrossRef]
- Mita, M. Absence of 1-methyladenine production in follicle cells obtained from starfish ovaries in the post-spawning season. Dev. Growth Differ. 1991, 33, 509–515. [Google Scholar] [CrossRef]
- Mita, M. Involvement of cyclic adenosine 3′,5′-monophosphate in methylation process of 1-methyladenine production by starfish ovarian follicle cells. Gen. Comp. Endocrinol. 1992, 87, 54–62. [Google Scholar] [CrossRef]
- Mita, M. Involvement of cyclic adenosine 3′,5′-monophosphate in methylation during 1-methyladenine production by starfish ovarian follicle cells. Invertebr. Reprod. Dev. 1992, 22, 11–15. [Google Scholar] [CrossRef]
- Mita, M.; Nagahama, Y. Involvement of G-proteins and adenylate cyclase in the action of gonad-stimulating substance on starfish ovarian follicle cells. Dev. Biol. 1991, 144, 262–268. [Google Scholar] [CrossRef]
- Mita, M.; Yamamoto, K.; Nakamura, M.; Nagahama, Y. Hormonal action of relaxin-like gonad-stimulating substance (GSS) on starfish ovaries at growing and fully grown states. Gen. Comp. Endocrinol. 2011, 172, 85–89. [Google Scholar] [CrossRef]
- Mita, M.; Yamamoto, K.; Nakamura, M.; Takeshige, Y.; Watanabe, M.; Nagahama, Y. Participation of Gs-proteins in the action of relaxin-like gonad-stimulating substance (GSS) for 1-methyladenine production in starfish ovarian follicle cells. Gen. Comp. Endocrinol. 2012, 176, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Mita, M.; Haraguchi, S.; Uzawa, H.; Tsutsui, K. Contribution of de novo synthesis of Gαs-proteins to 1-methyladenine production in starfish ovarian follicle cells stimulated by relaxin-like gonad-stimulating substance. Biochem. Biophys. Res. Commun. 2013, 440, 798–801. [Google Scholar] [CrossRef] [PubMed]
- Mita, M.; Haraguchi, S.; Watanabe, M.; Takeshige, Y.; Yamamoto, K.; Tsutsui, K. Involvement of Gαs-proteins in the action of relaxin-like gonad-stimulating substance on starfish ovarian follicle cells. Gen. Comp. Endocrinol. 2014, 205, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Mita, M.; Matsubara, S.; Osugi, T.; Shirahashi, A.; Wada, A.; Satake, H. A novel G protein-coupled receptor for starfish gonadotropic hormone, relaxin-like gonad-stimulating peptide. PLoS ONE 2020, 15, e0242877. [Google Scholar] [CrossRef]
- Hopkins, E.J.; Bathgate, R.A.; Gooley, P.R. The human LGR7 low-density lipoprotein class A module requires calcium for structure. Ann. N. Y. Acad. Sci. 2005, 1041, 27–34. [Google Scholar] [CrossRef]
- Hsu, S.Y.; Nakabayashi, K.; Nishi, S.; Kumagai, J.; Kudo, M.; Sherwood, O.D.; Hsueh, A.J.W. Activation of orphan receptors by the hormone relaxin. Science 2002, 295, 671–674. [Google Scholar] [CrossRef]
- Wilkinson, T.N.; Speed, T.P.; Tregear, G.W.; Bathgate, R.A. Evolution of the relaxin-like peptide family. BMC Evol. Biol. 2005, 612, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Bathgate, R.A.; Samuel, C.S.; Brazin, T.C.D.; Layfield, S.; Claasz, A.A.; Reytomas, I.G.T.; Dawson, N.F.; Zhao, C.; Bond, C.; Summers, R.J.; et al. Human relaxin gene 3 (H3) and the equivalent mouse relaxin (M3) Gene. Novel members of the relaxin peptide family. J. Biol. Chem. 2002, 277, 1148–1157. [Google Scholar] [CrossRef] [Green Version]
- Sherwood, O.D. Relaxin’s physiological roles and other diverse actions. Endocrinol. Rev. 2004, 25, 205–234. [Google Scholar] [CrossRef] [Green Version]
- Van Der Westhuizen, E.T.; Summers, R.J.; Halls, M.L.; Bathgate, R.A.D.; Sexton, P.M. Relaxin receptors—New drug targets for multiple disease states. Curr. Drug Targets 2007, 8, 91–104. [Google Scholar] [CrossRef]
- Bathgate, R.A.; Ivell, R.; Sanborn, B.M.; Sherwood, O.D.; Summers, R.J. International Union of Pharmacology LVII: Recommendations for the nomenclature of receptors for relaxin family peptides. Pharmacol. Rev. 2006, 58, 7–31. [Google Scholar] [CrossRef]
- Bathgate, R.A.D.; Halls, M.L.; van der Westhuizen, E.T.; Callander, G.E.; Kocan, M.; Summers, R.J. Relaxin family peptides and their receptors. Physiol. Rev. 2013, 93, 405–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halls, M.L.; van der Westhuizen, E.T.; Bathgate, R.E.; Summers, R.J. Relaxin family peptide receptors-former orphans reunite with their parent ligands to activate multiple signaling pathways. Br. J. Pharmacol. 2007, 150, 677–691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, R.C.; Shilling, P.J.; Lobb, D.K.; Gooley, P.R.; Bathgate, R.A.D. Membrane receptors: Structure and function of the relaxin family peptide receptors. Mol. Cell. Endocrinol. 2010, 320, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Patil, N.A.; Rosengren, K.J.; Separovic, F.; Wade, J.D.; Bathgate, R.A.D.; Hossain, M.A. Relaxin family peptides: Structure-activity relationship studies. Br. J. Pharmacol. 2017, 174, 950–961. [Google Scholar] [CrossRef] [Green Version]
- Bathgate, R.A.D.; Kocan, M.; Scott, J.D.; Hossain, M.A.; Good, S.V.; Yegorov, S.; Bogerd, J.; Gooley, P.R. Relaxin family peptides and their receptors. Pharmacol. Ther. 2018, 187, 114–132. [Google Scholar] [CrossRef]
- Büllesbach, E.E.; Schwabe, C. On the receptor binding site of relaxins. Int. J. Pept. Protein Res. 1988, 32, 361–367. [Google Scholar] [CrossRef]
- Büllesbach, E.E.; Schwabe, C. The relaxin receptor-binding site geometry suggests a novel gripping mode of interaction. J. Biol. Chem. 2000, 275, 35276–35280. [Google Scholar] [CrossRef] [Green Version]
- Mita, M.; Nakamura, K.; Tsutsui, K.; Katayama, H. Interaction of starfish gonadotropin with its receptor: Effect of chimeric relaxin-like gonad-stimulating peptides. Gen. Comp. Endocrinol. 2019, 276, 30–36. [Google Scholar] [CrossRef]
- Mita, M.; Elphick, M.R.; Katayama, H. Effect of chimeric relaxin-like gonad-stimulating peptides on oocyte maturation and ovulation in the starfish Asterias rubens and Aphelasterisa japonica. Gen. Comp. Endocrinol. 2020, 287, 113351. [Google Scholar] [CrossRef]
- Garelli, A.; Gontijo, A.M.; Miguela, V.; Caparros, E.; Dominguez, M. Imaginal discs secrete insulin-like peptide 8 to mediate plasticity of growth and maturation. Science 2012, 336, 579–582. [Google Scholar] [CrossRef] [PubMed]
- Noumura, T.; Kanatani, H. Induction of spawning by radial nerve extracts in some starfishes. J. Fac. Sci. Univ. Tokyo Sect. IV 1962, 9, 397–402. [Google Scholar]
- Chaet, A.B. The gamete-shedding substances of starfishes: A physiological-biochemical study. Am. Zool. 1966, 6, 263–271. [Google Scholar] [CrossRef] [Green Version]
- Chaet, A.B. Neurochemical control of gamete release in starfish. Biol. Bull. 1966, 130, 43–58. [Google Scholar] [CrossRef] [PubMed]
- Mita, M. 1-Methyladenine production by ovarian follicle cells responsible for spawning in starfish. Invertebr. Reprod. Dev. 1993, 24, 237–242. [Google Scholar] [CrossRef]
- Mita, M.; Nakamura, M. Influence of gonad-stimulating substance on 1-methyladenine level responsible for germinal vesicle breakdown and spawning in the starfish Asterina pectinifera. J. Exp. Zool. 1994, 269, 140–145. [Google Scholar] [CrossRef]
- Mita, M. Prediction of intracellular amount of 1-methyladenine precursor in ovarian follicle cells of the starfish, Asterina pectinifera. Zool. Sci. 1991, 8, 57–62. [Google Scholar]
- Shirai, H. Neosynthesis of 1-methyladenine in the starfish gonad under the influence of gonad-stimulating hormone. Exp. Cell Res. 1972, 74, 124–130. [Google Scholar] [CrossRef]
- Shirai, H.; Kanatani, H.; Taguchi, S. 1-Methyladenine biosynthesis in starfish ovary: Action of gonad-stimulating hormone in methylation. Science 1972, 172, 1366–1368. [Google Scholar] [CrossRef]
- Shirai, H. Effect of methionine and S-adenosylmethionine on production of 1-methyladenine in starfish follicle cells. Dev. Growth Differ. 1973, 15, 307–313. [Google Scholar] [CrossRef]
- Tarr, H.L.A. Biosynthesis of 1-methyladenine by isolated segments of starfish ovary. Gen. Comp. Endocrinol. 1985, 60, 280–292. [Google Scholar] [CrossRef] [PubMed]
- Mita, M.; Saneyoshi, M.; Yoshikuni, M.; Nagahama, Y. A methyl donor for 1-methyladenine biosynthesis in starfish ovarian follicle cells. Mol. Reprod. Dev. 1999, 54, 63–68. [Google Scholar] [CrossRef]
- Mita, M.; Yasumasu, I.; Nagahama, Y. Cyclic AMP-dependent protein kinase in ovarian follicle cells of starfish Asterina pectinifera. Comp. Biochem. Physiol. Part C 1996, 115, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Mita, M.; Yasumasu, I.; Nagahama, Y.; Saneyoshi, M. Change in the levels of adenine-related compounds in starfish ovarian follicle cells following treatment with gonad-stimulating substance. Dev. Growth Differ. 1996, 38, 413–418. [Google Scholar] [CrossRef]
- Mita, M.; Yoshikuni, M.; Nagahama, Y. 1-Methyladenine production from ATP by starfish ovarian follicle cells. Biochim. Biophys. Acta 1999, 1428, 13–20. [Google Scholar] [CrossRef]
- Kanatani, H.; Shirai, H. Chemical structural requirements for induction of oocyte maturation and spawning in starfish. Dev. Growth Differ. 1971, 13, 53–64. [Google Scholar] [CrossRef]
- Shirai, H.; Kanatani, H. Induction of spawning and oocyte maturation in starfish by 1-methyladenosine monophosphate. Dev. Growth Differ. 1973, 15, 217–224. [Google Scholar] [CrossRef]
- Shirai, H.; Kanatani, H. 1-Methyladenosine ribohydrolase in the starfish ovary and its relation to oocyte maturation. Exp. Cell Res. 1972, 75, 79–88. [Google Scholar] [CrossRef]
- Tarr, H.L.A. 1-Methyladenosine hydrolase of starfish (Pisaster ochraceus). J. Fish. Res. Board Can. 1973, 30, 1862–1866. [Google Scholar] [CrossRef]
- Mita, M.; Yasumasu, I.; Saneyoshi, M.; Yoshikuni, M.; Nagahama, Y. Production of the oocyte maturation-inducing substance of starfish by heat treatment of S-adenosylmethionine. Zool. Sci. 1998, 15, 117–122. [Google Scholar] [CrossRef]
- Shirai, H.; Yoshimoto, Y.; Kanatani, H. Mechanism of starfish spawning. IV. Tenstion generation in the ovarian wall by 1-methyladenine at the time of spawning. Biol. Bull. 1981, 161, 172–179. [Google Scholar] [CrossRef]
- Mita, M.; Osugi, T.; Takahashi, T.; Watanabe, T.; Satake, H. Mechanism of gamete shedding in starfish: Involvement of acetylcholine in extracellular Ca2+-dependent contraction of gonadal walls. Gen. Comp. Endocrinol. 2020, 290, 113401. [Google Scholar] [CrossRef] [PubMed]
- Walker, C.W. Spermatogenic columns, somatic cells, and the microenvironment of germinal cells in the testes of Asteroids. J. Morphol. 1980, 166, 81–107. [Google Scholar] [CrossRef] [PubMed]
- Inoue, C.; Shirai, H. Origin of germ cells and early differentiation of gonads in the starfish, Asterina pectinifera. Dev. Growth Differ. 1991, 33, 217–226. [Google Scholar] [CrossRef]
- Chia, F.S.; Koss, R. Asteroidea. In Microscopic Anatomy of Invertebrates: Echinodermata; Harrison, F.W., Chia, F.S., Eds.; Wiley-Liss: New York, NY, USA, 1994; Volume 14, pp. 168–245. [Google Scholar]
- Chia, F.S.; Xing, J. Echinoderm coelomocytes. Zool. Stud. 1996, 35, 231–254. [Google Scholar]
- Kalachev, A.V.; Reunov, A.A. Resorption of gametes in the testes of the sea star Asterina pectinifera (Mueller et Troeschel, 1842). Russ. J. Mar. Biol. 2005, 31, 119–123. [Google Scholar] [CrossRef]
- Klapproth, H.; Reinheimer, T.; Metzen, J.; Munch, M.; Bittinger, F.; Kirkpatrick, C.J.; Hohle, K.D.; Schemann, M.; Racke, K.; Wessler, I. Non-neuronal acetylcholine, a signaling molecule synthesized by surface cells of rat and man. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1997, 355, 515–523. [Google Scholar] [CrossRef]
- Wessler, I.K.; Gärtner, H.-A.; Michael-Schmidt, R.; Brochhausen, C.; Schmitz, L.; Anspach, L.; Grünewald, B.; Kirkpatrick, C.J. Honeybees produce millimolar concentrations of non-neuronal acetylcholine for breeding: Possible adverse effects of neuronicotinoids. PLoS ONE 2016, 11, e0156886. [Google Scholar] [CrossRef] [Green Version]
- Wessler, I.; Kirkpatrick, C.J. Non-neuronal acetylcholine involved in reproduction in mammals and honeybees. J. Neurochem. 2017, 142 (Suppl. S2), 144–150. [Google Scholar] [CrossRef]
- Buznikov, G.A.; Chudakova, I.V.; Berdysheva, L.V.; Vyazmina, N.M. The role of neurohumors in early embryogenesis II. Acetylclioline and catecholamine content in developing embryos of sea urchin. Development 1968, 20, 119–128. [Google Scholar] [CrossRef]
- Gustafson, T.; Toneby, M. On the role of serotonin and acetylcholine in sea urchin morphogenesis. Exp. Cell Res. 1970, 62, 102–117. [Google Scholar] [CrossRef] [PubMed]
- Smit, A.B.; Syed, N.I.; Schaap, D.; van Minnen, J.; Klumperman, J.; Kits, K.S.; Lodder, H.; van der Schors, R.C.; van Elk, R.; Sorgedrager, B.; et al. A glia-derived acetylcholine-binding protein that modulates synaptic transmission. Nature 2001, 411, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Brejc, K.; van Dijk, W.J.; Klaassen, R.V.; Schuurmans, M.; van Der Oost, J.; Smit, A.B.; Sixma, T.K. Crystal structure of an ACh-binding protein reveals the ligand-binding domain of nicotinic receptors. Nature 2001, 411, 269–276. [Google Scholar] [CrossRef]
- Kishimoto, T.; Usui, N.; Kanatani, H. Breakdown of starfish ovarian follicle induced by maturation-promoting factor. Dev. Biol. 1984, 101, 28–34. [Google Scholar] [CrossRef]
- Mita, M.; Takeshige, Y.; Nakamura, M. Effect of relaxin-like gonad-stimulating substance on gamete shedding and 1-methyladenine production in starfish ovaries. In Sexual reproduction in animals and plants; Sawada, H., Inoue, N., Iwao, M., Eds.; Springer Japan KK: Tokyo, Japan, 2013; pp. 115–122. [Google Scholar] [CrossRef] [Green Version]
- Ikegami, S.; Tamura, S.; Kanatani, H. Starfish gonad: Action and chemical identification of spawning inhibitor. Science 1967, 158, 1052–1053. [Google Scholar] [CrossRef] [PubMed]
- Ikegami, S.; Tamura, S. Spawning inhibitor in gonads of the starfish Asterina pectinifera. Agric. Biol. Chem. 1972, 36, 1899–1902. [Google Scholar] [CrossRef]
- Mita, M. Inhibitory mechanism of l-glutamic acid on spawning of the starfish Patiria (Asterina) pectinifera. Mol. Reprod. Dev. 2017, 84, 246–256. [Google Scholar] [CrossRef]
- Nakajima, Y.; Kaneko, H.; Murray, G.; Burke, R.D. Divergent patterns of neural development in larval echinoids and asteroids. Evol. Dev. 2004, 6, 95–104. [Google Scholar] [CrossRef]
- Carter, H.F.; Thompson, J.R.; Elphick, M.R.; Oliveri, P. The developmental and neuronal complexity of bipinnaria larvae of the sea star Asterias rubes. Int. Comp. Biol. 2021, 61, 337–351. [Google Scholar] [CrossRef]
- Mita, M.; Katayama, H.; Yamamoto, K.; Shibata, Y.; Kiyomoto, M. A relaxin-like gonad-stimulating peptide appears in the early development of the starfish Patiria pectinifera. Zool. Sci. 2023, 40, 7–12. [Google Scholar] [CrossRef]
- Murabe, N.; Hatoyama, H.; Komatsu, M.; Kaneko, H.; Nakajima, Y. Adhesive papillae on the brachiolar arms of brachiolaria larvae in two starfishes, Asterina pectinifera and Asterias amurensis, are sensors for metamorphic inducing factor(s). Dev. Growth Differ. 2007, 49, 647–656. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mita, M. Relaxin-like Gonad-Stimulating Peptides in Asteroidea. Biomolecules 2023, 13, 781. https://doi.org/10.3390/biom13050781
Mita M. Relaxin-like Gonad-Stimulating Peptides in Asteroidea. Biomolecules. 2023; 13(5):781. https://doi.org/10.3390/biom13050781
Chicago/Turabian StyleMita, Masatoshi. 2023. "Relaxin-like Gonad-Stimulating Peptides in Asteroidea" Biomolecules 13, no. 5: 781. https://doi.org/10.3390/biom13050781
APA StyleMita, M. (2023). Relaxin-like Gonad-Stimulating Peptides in Asteroidea. Biomolecules, 13(5), 781. https://doi.org/10.3390/biom13050781



