APOE Peripheral and Brain Impact: APOE4 Carriers Accelerate Their Alzheimer Continuum and Have a High Risk of Suicide in PM2.5 Polluted Cities
Abstract
:1. Introduction
2. Peripheral APOE4 Effects
3. APOE4 Brain Effects
3.1. Mitochondria, Energy Generation, the Ubiquitin Proteasome System, and Proteotoxicity
3.2. Ethnicity and APOE4
4. APOE4 in Children and Young Adults: Air Pollution Plays a Major Role in the Cognitive, Behavioral, and Brain Structural Responses in Pediatric APOE4 Carriers
4.1. Brain MRI in APOE4 Children and Young Adults
4.2. Magnetic Resonance Spectroscopy (MRS) and APOE4
5. APOE, Alzheimer’s Disease, Air Pollution, and Nanoparticles: The Impact of Air Pollution on the Progression of Biological Alzheimer’s in Pediatric and Young Adult Metropolitan Mexico City Residents
6. CSF Alzheimer’s and TDP 43 Pathology Markers in MMC Children and Young Adults
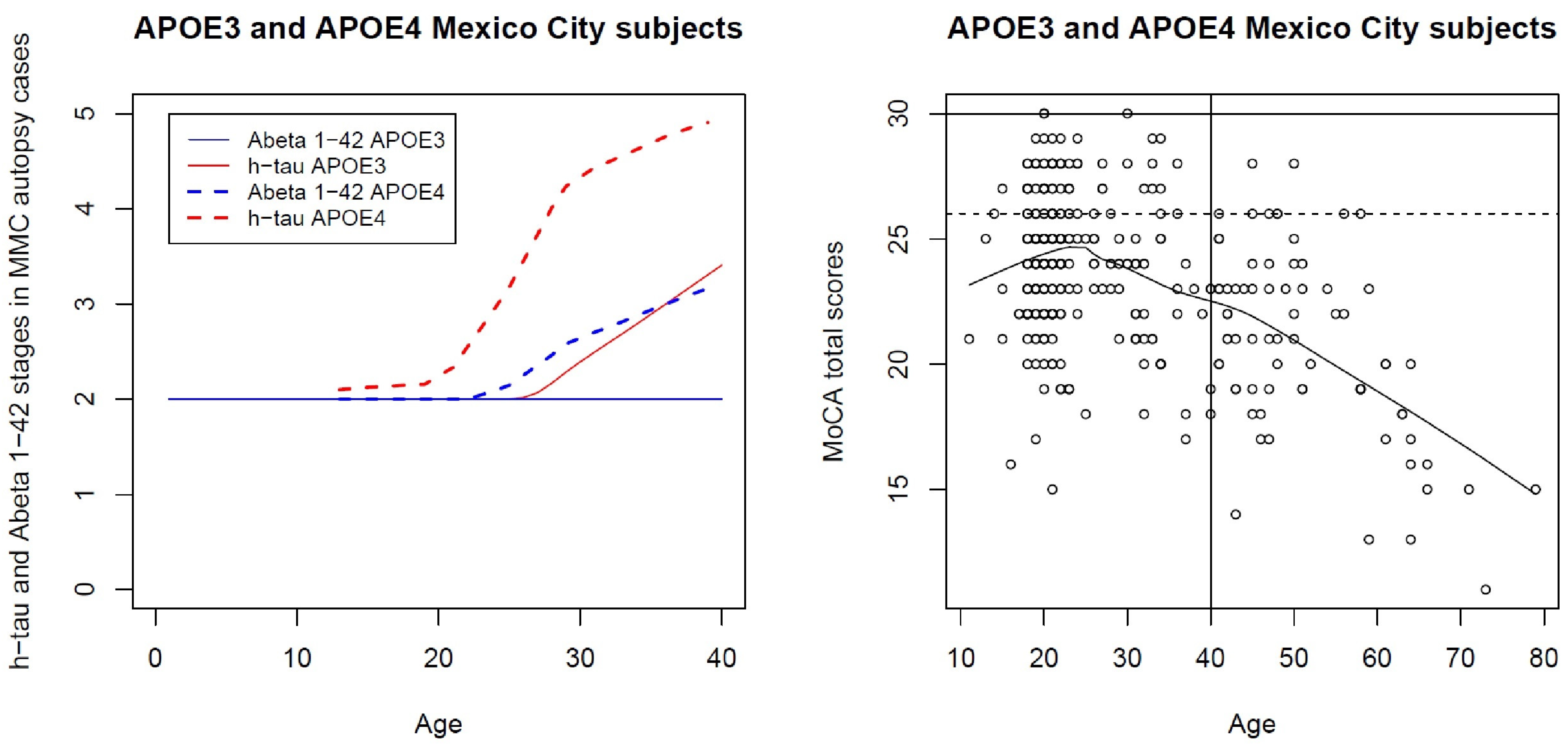
7. Cognition Deficits in MMC APOE4 Children Are Significantly Higher than APOE3
8. Summary
- We need to longitudinally monitor pediatric populations with non-invasive AD biomarkers reflective of the neuropathological changes.
- We have evidence of structural brain changes in young children, and metabolic changes are readily present in APOE4 children and their parents, which offers available non-invasive (N) neurodegeneration/neuronal injury markers. We need to implement MRI and MRS longitudinal studies contrasting children in high- versus low-polluted cities, matching SES, mother’s IQ, educational level, age, gestational duration, breastfeeding history, birth weight, sex, maternal age, education, and socioeconomic status. Cumulative PM2.5 exposures to the complex mixture of urban ambient pollution ought to be included.
- Based on our research findings, APOE4 carriers living in highly polluted environments should be labeled as high risk for AD; thus, all studies should include APOE genotyping. A highly polluted environment can be defined as one with ambient pollution concentrations that exceed the existing air quality standards in the Organization for Economic Cooperation and Development [211].
- We can longitudinally follow BMI, BAEP changes, gait and equilibrium abnormalities, HbA1c, and dyslipidemia profiles in APOE4 subjects. Maintaining normal weight in children is imperative, and in highly polluted cities, only physical education indoor classes should be allowed at elementary, middle, and high school levels.
- Education efforts should be aimed at healthy lifestyle behaviors starting in infancy [214] and included in low-cost government daycares. Access to a healthy breakfast should be available in public elementary schools along with out-of-school time (OST) programs with stimulating environments, including languages, music, arts, theater, and indoor sports. OST programs must provide academic enrichment and tutorial services.
- Education and occupation are proxies for cognitive reserve (CR), defined by Stern [215] as “differences in cognitive processes as a function of lifetime intellectual activities or other environmental factors that explain differential susceptibility to functional impairment in the presence of pathology or other neurological insult”. Given that young adults with higher college and formal education years did significantly better at cognitive testing versus less formal education years [203] and young adulthood is associated with better late-life cognition [216], efforts should be made to keep young adults in school and increase their CR. The 2021 NEET of 18–24 y olds in Mexico must be decreased from the 22% reported [211].
- Good balanced diets implemented in pregnant women and in infancy are a key requirement in our Mexican populations. Two factors are important: i. healthy dietary behavior patterns and ii. monetary resources. In a country with 126.7 million people- including 55.7 million in poverty- [217] with limited access to poor medical assistance and deficient education, we have an increment in chronic non-communicable diseases, as Manderson and Jewett [218] commented, “… the result of poverty and the manipulation of food markets”. Thus, the best way to fight poverty is through the creation of local jobs, improvement in the quality of technical program training, access to education from elementary to college levels, and the retention of students at school.
- Purpose in life promotes resilience against brain changes already observable in middle age [219]. The work of Abellaneda-Pérez and colleagues [219] is highly recommended and emphasizes the fact that “having a purposeful life implies larger functional integration” of the dorsal default-mode network “dDMN, which may potentially reflect greater brain reserve associated with better cognitive function”. Parents and teachers could help children and teens to find purpose in their lives using a combination of experiences, education, social needs, and values.
- The importance of APOE isoforms on the developing brain functions and the development and progression of Alzheimer’s Disease is clear. We need to neuroprotect individuals at high risk, and certainly, APOE4 is a potential therapeutic target, as commented by Ayyubova [220]. Among the 21.8 million MMC residents, we potentially have 4.36 million APOE4 subjects at earlier accelerated AD risk. These individuals are targets for neuroprotective interventions where specific pathways can be targeted in the disease process [220].
- The NIA-AA framework can serve for testing the impact of particulate air pollution, specifically ultrafine PM and nanoparticles, as the key players in the processes of oxidative stress, neuroinflammation, DNA damage, protein aggregation and misfolding, and faulty complex protein quality control. We need noninvasive biomarkers indicative of the P-tau and Aβ42 abnormal protein deposits across the disease continuum starting in childhood. The need for non-invasive biomarkers is urgent and equally applicable to Mexican Americans, a highly vulnerable population, severely underrepresented in research [221]. There is no support for highly trained Mexican American researchers working on AD prevention.
- Fine particulate matter PM2.5, UFPs, and NPs are serious health problems in Metropolitan Mexico City [185,186,187,188,189]. The problem of particle pollution is solvable. We are knowledgeable on the impact of old heavy diesel vehicles and other sources of excessive emissions and the brain effects of combustion-generated NPs [61,62,63]. We have the technological capability and the resources to control this pollution and protect young brains. Unfortunately, we lack the political will and aptitude to acknowledge that doing nothing will be far more costly.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Corder, E.H.; Saunders, A.M.; Strittmatter, W.J.; Schmechel, D.E.; Gaskell, P.C.; Small, G.W.; Roses, A.D.; Haines, J.L.; Pericak-Vance, M.A. Gene Dose of Apolipoprotein E Type 4 Allele and the Risk of Alzheimer’s Disease in Late Onset Families. Science 1993, 261, 921–923. [Google Scholar] [CrossRef] [PubMed]
- Mahley, R.W. Apolipoprotein E: Cholesterol Transport Protein with Expanding Role in Cell Biology. Science 1988, 240, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Verghese, P.B.; Castellano, J.M.; Holtzman, D.M. Apolipoprotein E in Alzheimer’s Disease and Other Neurological Disorders. Lancet Neurol. 2011, 10, 241–252. [Google Scholar] [CrossRef]
- Tai, L.M.; Thomas, R.; Marottoli, F.M.; Koster, K.P.; Kanekiyo, T.; Morris, A.W.J.; Bu, G. The Role of APOE in Cerebrovascular Dysfunction. Acta Neuropathol. 2016, 131, 709–723. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Calle, R.; Konings, S.C.; Frontiñán-Rubio, J.; García-Revilla, J.; Camprubí-Ferrer, L.; Svensson, M.; Martinson, I.; Boza-Serrano, A.; Venero, J.L.; Nielsen, H.M.; et al. APOE in the Bullseye of Neurodegenerative Diseases: Impact of the APOE Genotype in Alzheimer’s Disease Pathology and Brain Diseases. Mol. Neurodegener. 2022, 17, 62. [Google Scholar] [CrossRef]
- Benson, G.S.; Bauer, C.; Hausner, L.; Couturier, S.; Lewczuk, P.; Peters, O.; Hüll, M.; Jahn, H.; Jessen, F.; Pantel, J.; et al. Don’t Forget about Tau: The Effects of ApoE4 Genotype on Alzheimer’s Disease Cerebrospinal Fluid Biomarkers in Subjects with Mild Cognitive Impairment-Data from the Dementia Competence Network. J. Neural Transm. 2022, 129, 477–486. [Google Scholar] [CrossRef]
- Lee, S.E.; Yang, H.; Sung, Y.; Kim, Y.; Park, S.A. Region-Specific Differences in the Apoe4-Dependent Response to Focal Brain Injury. Exp. Neurobiol. 2021, 30, 285–293. [Google Scholar] [CrossRef]
- Wang, C.; Xiong, M.; Gratuze, M.; Bao, X.; Shi, Y.; Andhey, P.S.; Manis, M.; Schroeder, C.; Yin, Z.; Madore, C.; et al. Selective Removal of Astrocytic APOE4 Strongly Protects against Tau-Mediated Neurodegeneration and Decreases Synaptic Phagocytosis by Microglia. Neuron 2021, 109, 1657–1674.e7. [Google Scholar] [CrossRef]
- Gutiérrez-de Pablo, V.; Gómez, C.; Poza, J.; Maturana-Candelas, A.; Martins, S.; Gomes, I.; Lopes, A.M.; Pinto, N.; Hornero, R. Relationship between the Presence of the ApoE Ε4 Allele and EEG Complexity along the Alzheimer’s Disease Continuum. Sensors 2020, 20, 3849. [Google Scholar] [CrossRef]
- Steele, O.G.; Stuart, A.C.; Minkley, L.; Shaw, K.; Bonnar, O.; Anderle, S.; Penn, A.C.; Rusted, J.; Serpell, L.; Hall, C.; et al. A Multi-Hit Hypothesis for an APOE4-Dependent Pathophysiological State. Eur. J. Neurosci. 2022, 56, 5476–5515. [Google Scholar] [CrossRef]
- Jin, Y.; Li, F.; Sonoustoun, B.; Kondru, N.C.; Martens, Y.A.; Qiao, W.; Heckman, M.G.; Ikezu, T.C.; Li, Z.; Burgess, J.D.; et al. APOE4 Exacerbates α-Synuclein Seeding Activity and Contributes to Neurotoxicity in Alzheimer’s Disease with Lewy Body Pathology. Acta Neuropathol. 2022, 143, 641–662. [Google Scholar] [CrossRef] [PubMed]
- Umeh, C.C.; Mahajan, A.; Mihailovic, A.; Pontone, G.M. APOE4 Allele, Sex, and Dementia Risk in Parkinson’s Disease: Lessons from a Longitudinal Cohort. J. Geriatr. Psychiatry Neurol. 2022, 35, 810–815. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Shang, L.; Liu, Q.; Shen, D.; Sun, X.; Cai, Z.; Zhao, X.; Liu, L.; Yang, X.; Liu, M.; et al. Association of Apolipoprotein E Ε4 Allele and Amyotrophic Lateral Sclerosis in Chinese Population. Amyotroph. Lateral Scler. Front. Degener. 2022, 23, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, Y.; Liu, C.-C.; Yamazaki, A.; Shue, F.; Martens, Y.A.; Chen, Y.; Qiao, W.; Kurti, A.; Oue, H.; Ren, Y.; et al. Vascular ApoE4 Impairs Behavior by Modulating Gliovascular Function. Neuron 2021, 109, 438–447.e6. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Garcidueñas, L.; Mora-Tiscareño, A.; Franco-Lira, M.; Zhu, H.; Lu, Z.; Solorio, E.; Torres-Jardón, R.; D’Angiulli, A. Decreases in Short Term Memory, IQ, and Altered Brain Metabolic Ratios in Urban Apolipoprotein Ε4 Children Exposed to Air Pollution. J. Alzheimers Dis. 2015, 45, 757–770. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; Mora-Tiscareño, A.; Melo-Sánchez, G.; Rodríguez-Díaz, J.; Torres-Jardón, R.; Styner, M.; Mukherjee, P.S.; Lin, W.; Jewells, V. A Critical Proton MR Spectroscopy Marker of Alzheimer’s Disease Early Neurodegenerative Change: Low Hippocampal NAA/Cr Ratio Impacts APOE ε4 Mexico City Children and Their Parents. J. Alzheimers Dis. 2015, 48, 1065–1075. [Google Scholar] [CrossRef]
- Alemany, S.; Vilor-Tejedor, N.; García-Esteban, R.; Bustamante, M.; Dadvand, P.; Esnaola, M.; Mortamais, M.; Forns, J.; van Drooge, B.L.; Álvarez-Pedrerol, M.; et al. Traffic-Related Air Pollution, APOEε4 Status, and Neurodevelopmental Outcomes among School Children Enrolled in the BREATHE Project (Catalonia, Spain). Environ. Health Perspect. 2018, 126, 087001. [Google Scholar] [CrossRef]
- Essers, E.; Binter, A.-C.; Neumann, A.; White, T.; Alemany, S.; Guxens, M. Air Pollution Exposure during Pregnancy and Childhood, APOE Ε4 Status and Alzheimer Polygenic Risk Score, and Brain Structural Morphology in Preadolescents. Environ. Res. 2023, 216 Pt 2, 114595. [Google Scholar] [CrossRef]
- Shore, V.G.; Shore, B. Heterogeneity of Human Plasma Very Low Density Lipoproteins. Separation of Species Differing in Protein Components. Biochemistry 1973, 12, 502–507. [Google Scholar] [CrossRef]
- Corbo, R.M.; Scacchi, R. Apolipoprotein E (APOE) Allele Distribution in the World. Is APOE*4 a “thrifty” Allele? Ann. Hum. Genet. 1999, 63 Pt 4, 301–310. [Google Scholar] [CrossRef]
- Heffernan, A.L.; Chidgey, C.; Peng, P.; Masters, C.L.; Roberts, B.R. The Neurobiology and Age-Related Prevalence of the Ε4 Allele of Apolipoprotein E in Alzheimer’s Disease Cohorts. J. Mol. Neurosci. 2016, 60, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Crean, S.; Ward, A.; Mercaldi, C.J.; Collins, J.M.; Cook, M.N.; Baker, N.L.; Arrighi, H.M. Apolipoprotein E Ε4 Prevalence in Alzheimer’s Disease Patients Varies across Global Populations: A Systematic Literature Review and Meta-Analysis. Dement. Geriatr. Cogn. Disord. 2011, 31, 20–30. [Google Scholar] [CrossRef]
- Fujioka, H.; Phelix, C.F.; Friedland, R.P.; Zhu, X.; Perry, E.A.; Castellani, R.J.; Perry, G. Apolipoprotein E4 Prevents Growth of Malaria at the Intraerythrocyte Stage: Implications for Differences in Racial Susceptibility to Alzheimer’s Disease. J. Health Care Poor Underserved 2013, 24 (Suppl. 4), 70–78. [Google Scholar] [CrossRef]
- González, H.M.; Tarraf, W.; Jian, X.; Vásquez, P.M.; Kaplan, R.; Thyagarajan, B.; Daviglus, M.; Lamar, M.; Gallo, L.C.; Zeng, D.; et al. Apolipoprotein E Genotypes among Diverse Middle-Aged and Older Latinos: Study of Latinos-Investigation of Neurocognitive Aging Results (HCHS/SOL). Sci. Rep. 2018, 8, 17578. [Google Scholar] [CrossRef]
- Campos, M.; Edland, S.D.; Peavy, G.M. Exploratory Study of Apolipoprotein E Ε4 Genotype and Risk of Alzheimer’s Disease in Mexican Hispanics. J. Am. Geriatr. Soc. 2013, 61, 1038–1040. [Google Scholar] [CrossRef]
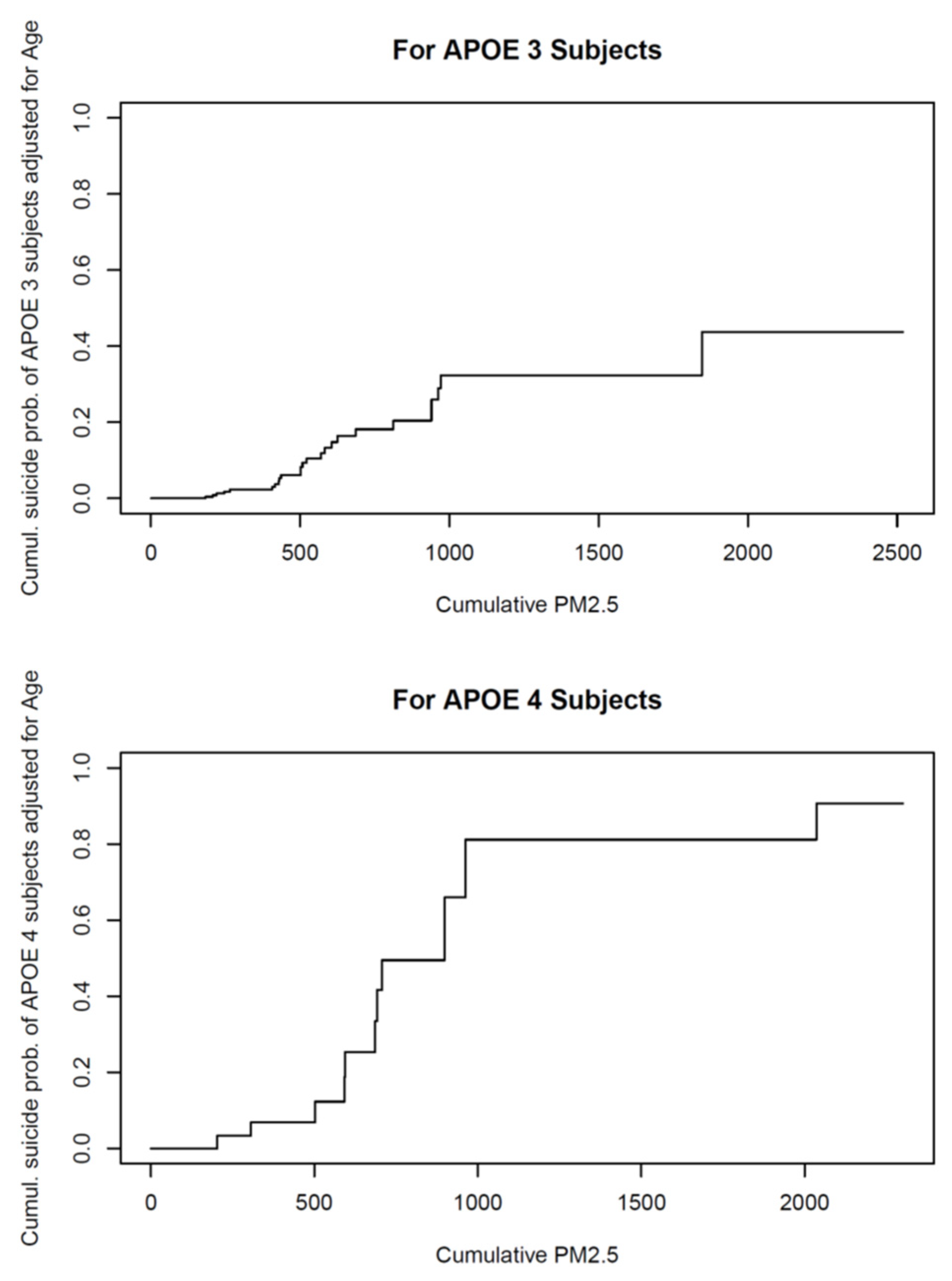
- Calderón-Garcidueñas, L.; Gónzalez-Maciel, A.; Reynoso-Robles, R.; Delgado-Chávez, R.; Mukherjee, P.S.; Kulesza, R.J.; Torres-Jardón, R.; Ávila-Ramírez, J.; Villarreal-Ríos, R. Hallmarks of Alzheimer Disease Are Evolving Relentlessly in Metropolitan Mexico City Infants, Children and Young Adults. APOE4 Carriers Have Higher Suicide Risk and Higher Odds of Reaching NFT Stage V at ≤40 Years of Age. Environ. Res. 2018, 164, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Garcidueñas, L.; González-Maciel, A.; Reynoso-Robles, R.; Hammond, J.; Kulesza, R.; Lachmann, I.; Torres-Jardón, R.; Mukherjee, P.S.; Maher, B.A. Quadruple Abnormal Protein Aggregates in Brainstem Pathology and Exogenous Metal-Rich Magnetic Nanoparticles (and Engineered Ti-Rich Nanorods). The Substantia Nigrae Is a Very Early Target in Young Urbanites and the Gastrointestinal Tract a Key Brainstem Portal. Environ. Res. 2020, 191, 110139. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Garcidueñas, L.; Ayala, A. Air Pollution, Ultrafine Particles, and Your Brain: Are Combustion Nanoparticle Emissions and Engineered Nanoparticles Causing Preventable Fatal Neurodegenerative Diseases and Common Neuropsychiatric Outcomes? Environ. Sci. Technol. 2022, 56, 6847–6856. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; Pérez-Calatayud, Á.A.; González-Maciel, A.; Reynoso-Robles, R.; Silva-Pereyra, H.G.; Ramos-Morales, A.; Torres-Jardón, R.; Soberanes-Cerino, C.D.J.; Carrillo-Esper, R.; Briones-Garduño, J.C.; et al. Environmental Nanoparticles Reach Human Fetal Brains. Biomedicines 2022, 10, 410. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Zhao, N.; Caulfield, T.R.; Liu, C.-C.; Bu, G. Apolipoprotein E and Alzheimer Disease: Pathobiology and Targeting Strategies. Nat. Rev. Neurol. 2019, 15, 501–518. [Google Scholar] [CrossRef]
- Reiman, E.M.; Chen, K.; Liu, X.; Bandy, D.; Yu, M.; Lee, W.; Ayutyanont, N.; Keppler, J.; Reeder, S.A.; Langbaum, J.B.S.; et al. Fibrillar Amyloid-Beta Burden in Cognitively Normal People at 3 Levels of Genetic Risk for Alzheimer’s Disease. Proc. Natl. Acad. Sci. USA 2009, 106, 6820–6825. [Google Scholar] [CrossRef] [PubMed]
- Castellano, J.M.; Kim, J.; Stewart, F.R.; Jiang, H.; DeMattos, R.B.; Patterson, B.W.; Fagan, A.M.; Morris, J.C.; Mawuenyega, K.G.; Cruchaga, C.; et al. Human ApoE Isoforms Differentially Regulate Brain Amyloid-β Peptide Clearance. Sci. Transl. Med. 2011, 3, 89ra57. [Google Scholar] [CrossRef] [PubMed]
- Davies, G.; Harris, S.E.; Reynolds, C.A.; Payton, A.; Knight, H.M.; Liewald, D.C.; Lopez, L.M.; Luciano, M.; Gow, A.J.; Corley, J.; et al. A Genome-Wide Association Study Implicates the APOE Locus in Nonpathological Cognitive Ageing. Mol. Psychiatry 2014, 19, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Funk, Q.; Rockers, E.; Shulman, J.M.; Masdeu, J.C.; Pascual, B.; Alzheimer’s Disease Neuroimaging Initiative. In Alzheimer-Prone Brain Regions, Metabolism and Risk-Gene Expression Are Strongly Correlated. Brain Commun. 2022, 4, fcac216. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-C.; Zhao, J.; Fu, Y.; Inoue, Y.; Ren, Y.; Chen, Y.; Doss, S.V.; Shue, F.; Jeevaratnam, S.; Bastea, L.; et al. Peripheral ApoE4 Enhances Alzheimer’s Pathology and Impairs Cognition by Compromising Cerebrovascular Function. Nat. Neurosci. 2022, 25, 1020–1033. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; Mora-Tiscareño, A.; Ontiveros, E.; Gómez-Garza, G.; Barragán-Mejía, G.; Broadway, J.; Chapman, S.; Valencia-Salazar, G.; Jewells, V.; Maronpot, R.R.; et al. Air Pollution, Cognitive Deficits and Brain Abnormalities: A Pilot Study with Children and Dogs. Brain Cogn. 2008, 68, 117–127. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; Franco-Lira, M.; Henríquez-Roldán, C.; Osnaya, N.; González-Maciel, A.; Reynoso-Robles, R.; Villarreal-Calderon, R.; Herritt, L.; Brooks, D.; Keefe, S.; et al. Urban Air Pollution: Influences on Olfactory Function and Pathology in Exposed Children and Young Adults. Exp. Toxicol. Pathol. 2010, 62, 91–102. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; Reynoso-Robles, R.; González-Maciel, A. Combustion and Friction-Derived Nanoparticles and Industrial-Sourced Nanoparticles: The Culprit of Alzheimer and Parkinson’s Diseases. Environ. Res. 2019, 176, 108574. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; González-Maciel, A.; Reynoso-Robles, R.; Kulesza, R.J.; Mukherjee, P.S.; Torres-Jardón, R.; Rönkkö, T.; Doty, R.L. Alzheimer’s Disease and Alpha-Synuclein Pathology in the Olfactory Bulbs of Infants, Children, Teens and Adults ≤40 Years in Metropolitan Mexico City. APOE4 Carriers at Higher Risk of Suicide Accelerate Their Olfactory Bulb Pathology. Environ. Res. 2018, 166, 348–362. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; Stommel, E.W.; Lachmann, I.; Waniek, K.; Chao, C.-K.; González-Maciel, A.; García-Rojas, E.; Torres-Jardón, R.; Delgado-Chávez, R.; Mukherjee, P.S. TDP-43 CSF Concentrations Increase Exponentially with Age in Metropolitan Mexico City Young Urbanites Highly Exposed to PM2.5 and Ultrafine Particles and Historically Showing Alzheimer and Parkinson’s Hallmarks. Brain TDP-43 Pathology in MMC Residents Is Associated with High Cisternal CSF TDP-43 Concentrations. Toxics 2022, 10, 559. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; Hernández-Luna, J.; Mukherjee, P.S.; Styner, M.; Chávez-Franco, D.A.; Luévano-Castro, S.C.; Crespo-Cortés, C.N.; Stommel, E.W.; Torres-Jardón, R. Hemispheric Cortical, Cerebellar and Caudate Atrophy Associated to Cognitive Impairment in Metropolitan Mexico City Young Adults Exposed to Fine Particulate Matter Air Pollution. Toxics 2022, 10, 156. [Google Scholar] [CrossRef] [PubMed]
- Husain, M.A.; Vachon, A.; Chouirnard-Watkins, R.; Vandal, M.; Calon, F.; Plourde, M. Investigating the plasma-liver-brain axis of omega-3 fatty acid metabolism in mouse knock-in for the human apolipoprotein E epsilon 4 allele. J. Nutr. Biochem. 2023, 111, 109181. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Martínez, A.B.; Torres-Perez, E.; Devanney, N.; Del Moral, R.; Johnson, L.A.; Arbones-Mainar, J.M. Beyond the CNS: The Many Peripheral Roles of APOE. Neurobiol. Dis. 2020, 138, 104809. [Google Scholar] [CrossRef]
- Heeren, J.; Scheja, L. Metabolic-Associated Fatty Liver Disease and Lipoprotein Metabolism. Mol. Metab. 2021, 50, 101238. [Google Scholar] [CrossRef]
- Abulikemu, A.; Zhao, X.; Xu, H.; Li, Y.; Ma, R.; Yao, Q.; Wang, J.; Sun, Z.; Li, Y.; Guo, C. Silica Nanoparticles Aggravated the Metabolic Associated Fatty Liver Disease through Disturbed Amino Acid and Lipid Metabolisms-Mediated Oxidative Stress. Redox Biol. 2023, 59, 102569. [Google Scholar] [CrossRef] [PubMed]
- Chernick, D.; Ortiz-Valle, S.; Jeong, A.; Qu, W.; Li, L. Peripheral versus Central Nervous System APOE in Alzheimer’s Disease: Interplay across the Blood-Brain Barrier. Neurosci. Lett. 2019, 708, 134306. [Google Scholar] [CrossRef]
- Rhea, E.M.; Raber, J.; Banks, W.A. ApoE and Cerebral Insulin: Trafficking, Receptors, and Resistance. Neurobiol. Dis. 2020, 137, 104755. [Google Scholar] [CrossRef]
- Miao, G.; Zhuo, D.; Han, X.; Yao, W.; Liu, C.; Liu, H.; Cao, H.; Sun, Y.; Chen, Z.; Feng, T. From Degenerative Disease to Malignant Tumors: Insight to the Function of ApoE. Biomed. Pharmacother. 2023, 158, 114127. [Google Scholar] [CrossRef]
- Huang, Y.; Mahley, R.W. Apolipoprotein E: Structure and Function in Lipid Metabolism, Neurobiology, and Alzheimer’s Diseases. Neurobiol. Dis. 2014, 72 Pt A, 3–12. [Google Scholar] [CrossRef]
- Sing, C.F.; Davignon, J. Role of the Apolipoprotein E Polymorphism in Determining Normal Plasma Lipid and Lipoprotein Variation. Am. J. Hum. Genet. 1985, 37, 268–285. [Google Scholar]
- Ekblad, L.L.; Tuisku, J.; Koivumäki, M.; Helin, S.; Rinne, J.O.; Snellman, A. Insulin resistance and body mass index are associated with TSPO PET in cognitively unimpaired elderly. J Cereb Blood Flow Metab 2023, 271678X231172519. [Google Scholar] [CrossRef] [PubMed]
- Heeren, J.; Grewal, T.; Laatsch, A.; Becker, N.; Rinninger, F.; Rye, K.-A.; Beisiegel, U. Impaired Recycling of Apolipoprotein E4 Is Associated with Intracellular Cholesterol Accumulation. J. Biol. Chem. 2004, 279, 55483–55492. [Google Scholar] [CrossRef] [PubMed]
- Scheja, L.; Heeren, J. Metabolic Interplay between White, Beige, Brown Adipocytes and the Liver. J. Hepatol. 2016, 64, 1176–1186. [Google Scholar] [CrossRef]
- Golden, L.R.; Johnson, L.A. Liver-Ing in Your Head Rent Free: Peripheral ApoE4 Drives CNS Pathology. Mol. Neurodegener. 2022, 17, 65. [Google Scholar] [CrossRef]
- Haroon, H.B.; Hunter, A.C.; Farhangrazi, Z.S.; Moghimi, S.M. A Brief History of Long Circulating Nanoparticles. Adv. Drug. Deliv. Rev. 2022, 188, 114396. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Sohn, J.; Kirichenko, A. Quantifying Liver Heterogeneity via R2*-MRI with Super-Paramagnetic Iron Oxide Nanoparticles (SPION) to Characterize Liver Function and Tumor. Cancers 2022, 14, 5269. [Google Scholar] [CrossRef] [PubMed]
- Casey, L.M.; Hughes, K.R.; Saunders, M.N.; Miller, S.D.; Pearson, R.M.; Shea, L.D. Mechanistic Contributions of Kupffer Cells and Liver Sinusoidal Endothelial Cells in Nanoparticle-Induced Antigen-Specific Immune Tolerance. Biomaterials 2022, 283, 121457. [Google Scholar] [CrossRef]
- Arsiwala, T.; Vogt, A.-C.S.; Barton, A.E.; Manolova, V.; Funk, F.; Flühmann, B.; Bachmann, M.F. Kupffer Cells and Blood Monocytes Orchestrate the Clearance of Iron-Carbohydrate Nanoparticles from Serum. Int. J. Mol. Sci. 2022, 23, 2666. [Google Scholar] [CrossRef]
- Habenicht, L.K.L.; Wang, Z.; Zhang, X.; Li, Y.; Mogler, C.; Huspenina, J.S.; Schmid, R.M.; Weber, C.; Mohanta, S.K.; Ma, Z.; et al. The C1q-ApoE Complex: A New Hallmark Pathology of Viral Hepatitis and Nonalcoholic Fatty Liver Disease. Front. Immunol. 2022, 13, 970938. [Google Scholar] [CrossRef]
- Yin, F.; Gupta, R.; Vergnes, L.; Driscoll, W.S.; Ricks, J.; Ramanathan, G.; Stewart, J.A.; Shih, D.M.; Faull, K.F.; Beaven, S.W.; et al. Diesel Exhaust Induces Mitochondrial Dysfunction, Hyperlipidemia, and Liver Steatosis. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1776–1786. [Google Scholar] [CrossRef]
- Young, L.-H.; Lai, C.-W.; Lu, J.-H.; Yang, H.-H.; Wang, L.-C.; Chen, Y.-H. Elevated Emissions of Volatile and Nonvolatile Nanoparticles from Heavy-Duty Diesel Engine Running on Diesel-Gas Co-Fuels. Sci. Total. Environ. 2022, 821, 153459. [Google Scholar] [CrossRef] [PubMed]
- Ehsanifar, M.; Montazeri, Z.; Taheri, M.A.; Rafati, M.; Behjati, M.; Karimian, M. Hippocampal Inflammation and Oxidative Stress Following Exposure to Diesel Exhaust Nanoparticles in Male and Female Mice. Neurochem. Int. 2021, 145, 104989. [Google Scholar] [CrossRef] [PubMed]
- Bujak-Pietrek, S.; Mikołajczyk, U. Evaluation of Exposure to Nano-Sized Particles among Transport and Vehicle Service Workers. Med. Pract. 2021, 72, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Milici, A.; Talavera, K. TRP Channels as Cellular Targets of Particulate Matter. Int. J. Mol. Sci. 2021, 22, 2783. [Google Scholar] [CrossRef] [PubMed]
- Meroni, M.; Longo, M.; Paolini, E.; Tria, G.; Ripolone, M.; Napoli, L.; Moggio, M.; Fracanzani, A.L.; Dongiovanni, P. Expanding the Phenotypic Spectrum of Non-Alcoholic Fatty Liver Disease and Hypertriglyceridemia. Front. Nutr. 2022, 9, 967899. [Google Scholar] [CrossRef]
- Liang, Q.; Sun, M.; Ma, Y.; Wang, F.; Sun, Z.; Duan, J. Adverse Effects and Underlying Mechanism of Amorphous Silica Nanoparticles in Liver. Chemosphere 2023, 311 Pt 1, 136955. [Google Scholar] [CrossRef]
- Han, H.-Y.; Yang, M.-J.; Yoon, C.; Lee, G.-H.; Kim, D.-W.; Kim, T.-W.; Kwak, M.; Heo, M.B.; Lee, T.G.; Kim, S.; et al. Toxicity of Orally Administered Food-Grade Titanium Dioxide Nanoparticles. J. Appl. Toxicol. 2021, 41, 1127–1147. [Google Scholar] [CrossRef]
- Dąbrowska-Bouta, B.; Sulkowski, G.; Gewartowska, M.; Strużyńska, L. Endoplasmic Reticulum Stress Underlies Nanosilver-Induced Neurotoxicity in Immature Rat Brain. Int. J. Mol. Sci. 2022, 23, 13013. [Google Scholar] [CrossRef]
- Villarreal-Calderon, R.; Franco-Lira, M.; González-Maciel, A.; Reynoso-Robles, R.; Harritt, L.; Pérez-Guillé, B.; Ferreira-Azevedo, L.; Drecktrah, D.; Zhu, H.; Sun, Q.; et al. Up-Regulation of MRNA Ventricular PRNP Prion Protein Gene Expression in Air Pollution Highly Exposed Young Urbanites: Endoplasmic Reticulum Stress, Glucose Regulated Protein 78, and Nanosized Particles. Int. J. Mol. Sci. 2013, 14, 23471–23491. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; González-Maciel, A.; Mukherjee, P.S.; Reynoso-Robles, R.; Pérez-Guillé, B.; Gayosso-Chávez, C.; Torres-Jardón, R.; Cross, J.V.; Ahmed, I.A.M.; Karloukovski, V.V.; et al. Combustion- and Friction-Derived Magnetic Air Pollution Nanoparticles in Human Hearts. Environ. Res. 2019, 176, 108567. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; González-Maciel, A.; Reynoso-Robles, R.; Rodríguez-López, J.L.; Silva-Pereyra, H.G.; Labrada-Delgado, G.J.; Pérez-Guillé, B.; Soriano-Rosales, R.E.; Jiménez-Bravo Luna, M.A.; Brito-Aguilar, R.; et al. Environmental Fe, Ti, Al, Cu, Hg, Bi, and Si Nanoparticles in the Atrioventricular Conduction Axis and the Associated Ultrastructural Damage in Young Urbanites: Cardiac Arrhythmias Caused by Anthropogenic, Industrial, E-Waste, and Indoor Nanoparticles. Environ. Sci. Technol. 2021, 55, 8203–8214. [Google Scholar] [CrossRef] [PubMed]
- Ozen, E.; Mihaylova, R.G.; Lord, N.J.; Lovegrove, J.A.; Jackson, K.G. Association between APOE Genotype with Body Composition and Cardiovascular Disease Risk Markers Is Modulated by BMI in Healthy Adults: Findings from the BODYCON Study. Int. J. Mol. Sci. 2022, 23, 9766. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-J.; Ma, Y.-H.; Bi, Y.-L.; Shen, X.-N.; Hou, X.-H.; Cao, X.-P.; Ou, Y.-N.; Zhao, B.; Dong, Q.; Tan, L.; et al. Metabolically Healthy Obesity and Lipids May Be Protective Factors for Pathological Changes of Alzheimer’s Disease in Cognitively Normal Adults. J. Neurochem. 2021, 157, 834–845. [Google Scholar] [CrossRef] [PubMed]
- Subramaniapillai, S.; Suri, S.; Barth, C.; Maximov, I.I.; Voldsbekk, I.; van der Meer, D.; Gurholt, T.P.; Beck, D.; Draganski, B.; Andreassen, O.A.; et al. Sex- and Age-Specific Associations between Cardiometabolic Risk and White Matter Brain Age in the UK Biobank Cohort. Hum. Brain Mapp. 2022, 43, 3759–3774. [Google Scholar] [CrossRef] [PubMed]
- Stuchell-Brereton, M.D.; Zimmerman, M.I.; Miller, J.J.; Mallimadugula, U.L.; Incicco, J.J.; Roy, D.; Smith, L.G.; Cubuk, J.; Baban, B.; DeKoster, G.T.; et al. Apolipoprotein E4 Has Extensive Conformational Heterogeneity in Lipid-Free and Lipid-Bound Forms. Proc. Natl. Acad. Sci. USA 2023, 120, e2215371120. [Google Scholar] [CrossRef]
- Lazar, A.-N.; Hanbouch, L.; Boussicaut, L.; Fourmaux, B.; Daira, P.; Millan, M.J.; Bernoud-Hubac, N.; Potier, M.-C. Lipid Dys-Homeostasis Contributes to APOE4-Associated AD Pathology. Cells 2022, 11, 3616. [Google Scholar] [CrossRef]
- Tcw, J.; Qian, L.; Pipalia, N.H.; Chao, M.J.; Liang, S.A.; Shi, Y.; Jain, B.R.; Bertelsen, S.E.; Kapoor, M.; Marcora, E.; et al. Cholesterol and Matrisome Pathways Dysregulated in Astrocytes and Microglia. Cell 2022, 185, 2213–2233.e25. [Google Scholar] [CrossRef]
- Schaeffer, S.; Iadecola, C. Revisiting the Neurovascular Unit. Nat. Neurosci. 2021, 24, 1198–1209. [Google Scholar] [CrossRef]
- Santisteban, M.M.; Iadecola, C.; Carnevale, D. Hypertension, Neurovascular Dysfunction, and Cognitive Impairment. Hypertension 2023, 80, 22–34. [Google Scholar] [CrossRef]
- Ando, T.; Uchida, K.; Sugimoto, T.; Kimura, A.; Saji, N.; Niida, S.; Sakurai, T. ApoE4 Is Associated with Lower Body Mass, Particularly Fat Mass, in Older Women with Cognitive Impairment. Nutrients 2022, 14, 539. [Google Scholar] [CrossRef]
- Saleh, R.N.M.; Hornberger, M.; Ritchie, C.W.; Minihane, A.M. Hormone Replacement Therapy Is Associated with Improved Cognition and Larger Brain Volumes in At-Risk APOE4 Women: Results from the European Prevention of Alzheimer’s Disease (EPAD) Cohort. Alzheimers Res. Ther. 2023, 15, 10. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Braak, E. Staging of Alzheimer’s Disease-Related Neurofibrillary Changes. Neurobiol. Aging 1995, 16, 271–278; discussion 278–284. [Google Scholar] [CrossRef] [PubMed]
- Del Tredici, K.; Braak, H. To Stage, or Not to Stage. Curr. Opin. Neurobiol. 2020, 61, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Hernández, F.; Avila, J. Tauopathies. Cell. Mol. Life Sci. 2007, 64, 2219–2233. [Google Scholar] [CrossRef]
- Braak, H.; Del Tredici, K. Spreading of Tau Pathology in Sporadic Alzheimer’s Disease Along Cortico-Cortical Top-Down Connections. Cereb. Cortex 2018, 28, 3372–3384. [Google Scholar] [CrossRef]
- Arnsten, A.F.T.; Datta, D.; Del Tredici, K.; Braak, H. Hypothesis: Tau Pathology Is an Initiating Factor in Sporadic Alzheimer’s Disease. Alzheimers Dement. 2021, 17, 115–124. [Google Scholar] [CrossRef]
- Stopschinski, B.E.; Del Tredici, K.; Estill-Terpack, S.-J.; Ghebremdehin, E.; Yu, F.F.; Braak, H.; Diamond, M.I. Anatomic Survey of Seeding in Alzheimer’s Disease Brains Reveals Unexpected Patterns. Acta Neuropathol. Commun. 2021, 9, 164. [Google Scholar] [CrossRef]
- Marx, G.A.; Koenigsberg, D.G.; McKenzie, A.T.; Kauffman, J.; Hanson, R.W.; Whitney, K.; Signaevsky, M.; Prastawa, M.; Iida, M.A.; White, C.L.; et al. Artificial Intelligence-Derived Neurofibrillary Tangle Burden Is Associated with Antemortem Cognitive Impairment. Acta Neuropathol. Commun. 2022, 10, 157. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; González-Maciel, A.; Reynoso-Robles, R.; Silva-Pereyra, H.G.; Torres-Jardón, R.; Brito-Aguilar, R.; Ayala, A.; Stommel, E.W.; Delgado-Chávez, R. Environmentally Toxic Solid Nanoparticles in Noradrenergic and Dopaminergic Nuclei and Cerebellum of Metropolitan Mexico City Children and Young Adults with Neural Quadruple Misfolded Protein Pathologies and High Exposures to Nano Particulate Matter. Toxics 2022, 10, 164. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; Kulesza, R.; Greenough, G.P.; García-Rojas, E.; Revueltas-Ficachi, P.; Rico-Villanueva, A.; Flores-Vázquez, J.O.; Brito-Aguilar, R.; Ramírez-Sánchez, S.; Vacaseydel-Aceves, N.; et al. Fall Risk, Sleep Behavior, and Sleep-Related Movement Disorders in Young Urbanites Exposed to Air Pollution. J. Alzheimers Dis. 2023, 91, 847–862. [Google Scholar] [CrossRef]
- Deczkowska, A.; Weiner, A.; Amit, I. The Physiology, Pathology, and Potential Therapeutic Applications of the TREM2 Signaling Pathway. Cell 2020, 181, 1207–1217. [Google Scholar] [CrossRef] [PubMed]
- Gratuze, M.; Schlachetzki, J.C.M.; D’Oliveira Albanus, R.; Jain, N.; Novotny, B.; Brase, L.; Rodriguez, L.; Mansel, C.; Kipnis, M.; O’Brien, S.; et al. TREM2-Independent Microgliosis Promotes Tau-Mediated Neurodegeneration in the Presence of ApoE4. Neuron 2023, 111, 202–219.e7. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T. ApoE4 Makes Microglia Trem2bling. Neuron 2023, 111, 142–144. [Google Scholar] [CrossRef] [PubMed]
- Vontell, R.T.; de Rivero Vaccari, J.P.; Sun, X.; Gultekin, S.H.; Bramlett, H.M.; Dietrich, W.D.; Keane, R.W. Identification of Inflammasome Signaling Proteins in Neurons and Microglia in Early and Intermediate Stages of Alzheimer’s Disease. Brain Pathol. 2022, e13142. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, Y.; Huang, G.; Yang, M.; Zhu, Y.; Jin, C.; Jing, D.; Ji, K.; Shi, Y. LilrB3 Is a Putative Cell Surface Receptor of APOE4. Cell. Res. 2023, 33, 116–130. [Google Scholar] [CrossRef]
- Civeira-Marín, M.; Cenarro, A.; Marco-Benedí, V.; Bea, A.M.; Mateo-Gallego, R.; Moreno-Franco, B.; Ordovás, J.M.; Laclaustra, M.; Civeira, F.; Lamiquiz-Moneo, I. APOE Genotypes Modulate Inflammation Independently of Their Effect on Lipid Metabolism. Int. J. Mol. Sci. 2022, 23, 12947. [Google Scholar] [CrossRef]
- Young, C.B.; Johns, E.; Kennedy, G.; Belloy, M.E.; Insel, P.S.; Greicius, M.D.; Sperling, R.A.; Johnson, K.A.; Poston, K.L.; Mormino, E.C.; et al. APOE Effects on Regional Tau in Preclinical Alzheimer’s Disease. Mol. Neurodegener. 2023, 18, 1. [Google Scholar] [CrossRef]
- Blanchard, J.W.; Akay, L.A.; Davila-Velderrain, J.; von Maydell, D.; Mathys, H.; Davidson, S.M.; Effenberger, A.; Chen, C.-Y.; Maner-Smith, K.; Hajjar, I.; et al. APOE4 Impairs Myelination via Cholesterol Dysregulation in Oligodendrocytes. Nature 2022, 611, 769–779. [Google Scholar] [CrossRef]
- Mok, K.K.-S.; Yeung, S.H.-S.; Cheng, G.W.-Y.; Ma, I.W.-T.; Lee, R.H.-S.; Herrup, K.; Tse, K.-H. Apolipoprotein E Ε4 Disrupts Oligodendrocyte Differentiation by Interfering with Astrocyte-Derived Lipid Transport. J. Neurochem. 2022, 165, 55–75. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; Reynoso-Robles, R.; Vargas-Martínez, J.; Gómez-Maqueo-Chew, A.; Pérez-Guillé, B.; Mukherjee, P.S.; Torres-Jardón, R.; Perry, G.; Gónzalez-Maciel, A. Prefrontal White Matter Pathology in Air Pollution Exposed Mexico City Young Urbanites and Their Potential Impact on Neurovascular Unit Dysfunction and the Development of Alzheimer’s Disease. Environ. Res. 2016, 146, 404–417. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; Torres-Jardón, R.; Kulesza, R.J.; Mansour, Y.; González-González, L.O.; Gónzalez-Maciel, A.; Reynoso-Robles, R.; Mukherjee, P.S. Alzheimer Disease Starts in Childhood in Polluted Metropolitan Mexico City. A Major Health Crisis in Progress. Environ. Res. 2020, 183, 109137. [Google Scholar] [CrossRef] [PubMed]
- den Brave, F.; Gupta, A.; Becker, T. Protein Quality Control at the Mitochondrial Surface. Front. Cell. Dev. Biol. 2021, 9, 795685. [Google Scholar] [CrossRef] [PubMed]
- Karbowski, M.; Oshima, Y.; Verhoeven, N. Mitochondrial Proteotoxicity: Implications and Ubiquitin-Dependent Quality Control Mechanisms. Cell. Mol. Life Sci. 2022, 79, 574. [Google Scholar] [CrossRef] [PubMed]
- Büttiker, P.; Weissenberger, S.; Esch, T.; Anders, M.; Raboch, J.; Ptacek, R.; Kream, R.M.; Stefano, G.B. Dysfunctional Mitochondrial Processes Contribute to Energy Perturbations in the Brain and Neuropsychiatric Symptoms. Front. Pharmacol. 2022, 13, 1095923. [Google Scholar] [CrossRef] [PubMed]
- Desai, S.; Juncker, M.; Kim, C. Regulation of Mitophagy by the Ubiquitin Pathway in Neurodegenerative Diseases. Exp. Biol. Med. 2018, 243, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, L.; Senatore, E.; Iannucci, R.; Chiuso, F.; Feliciello, A. Control of Mitochondrial Activity by the Ubiquitin Code in Health and Cancer. Cells 2023, 12, 234. [Google Scholar] [CrossRef]
- Devall, M.; Soanes, D.M.; Smith, A.R.; Dempster, E.L.; Smith, R.G.; Burrage, J.; Iatrou, A.; Hannon, E.; Troakes, C.; Moore, K.; et al. Genome-Wide Characterization of Mitochondrial DNA Methylation in Human Brain. Front. Endocrinol. 2022, 13, 1059120. [Google Scholar] [CrossRef]
- Gil, M.; Gama, V. Emerging Mitochondrial-Mediated Mechanisms Involved in Oligodendrocyte Development. J. Neurosci. Res. 2023, 101, 354–366. [Google Scholar] [CrossRef]
- Pires, M.; Rego, A.C. Apoe4 and Alzheimer’s Disease Pathogenesis-Mitochondrial Deregulation and Targeted Therapeutic Strategies. Int. J. Mol. Sci. 2023, 24, 778. [Google Scholar] [CrossRef]
- Mahley, R.W. Apolipoprotein E4 Targets Mitochondria and the Mitochondria-Associated Membrane Complex in Neuropathology, Including Alzheimer’s Disease. Curr. Opin. Neurobiol. 2023, 79, 102684. [Google Scholar] [CrossRef]
- Mishra, E.; Thakur, M.K. Mitophagy: A Promising Therapeutic Target for Neuroprotection during Ageing and Age-Related Diseases. Br. J. Pharmacol. 2023, 180, 1542–1561. [Google Scholar] [CrossRef] [PubMed]
- Iwata, R.; Casimir, P.; Erkol, E.; Boubakar, L.; Planque, M.; Gallego López, I.M.; Ditkowska, M.; Gaspariunaite, V.; Beckers, S.; Remans, D.; et al. Mitochondria Metabolism Sets the Species-Specific Tempo of Neuronal Development. Science 2023, 379, eabn4705. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.-C.; Lim, Y.S.; Chu, P.-W.; Chen, S.-K. Inflammatory Milieu Induces Mitochondrial Alterations and Neuronal Activations in Hypothalamic POMC Neurons in a Time-Dependent Manner. Mol. Neurobiol. 2023, 60, 1164–1178. [Google Scholar] [CrossRef] [PubMed]
- Taylor, E.B.; Rutter, J. Mitochondrial Quality Control by the Ubiquitin-Proteasome System. Biochem. Soc. Trans. 2011, 39, 1509–1513. [Google Scholar] [CrossRef]
- Livnat-Levanon, N.; Glickman, M.H. Ubiquitin-Proteasome System and Mitochondria—Reciprocity. Biochim. Biophys. Acta 2011, 1809, 80–87. [Google Scholar] [CrossRef]
- Zhang, Y.; Karmon, O.; Das, K.; Wiener, R.; Lehming, N.; Pines, O. Ubiquitination Occurs in the Mitochondrial Matrix by Eclipsed Targeted Components of the Ubiquitination Machinery. Cells 2022, 11, 4109. [Google Scholar] [CrossRef]
- Strope, T.A.; Wilkins, H.M. Amyloid Precursor Protein and Mitochondria. Curr. Opin. Neurobiol. 2023, 78, 102651. [Google Scholar] [CrossRef]
- Schmitt, L.O.; Gaspar, J.M. Obesity-Induced Brain Neuroinflammatory and Mitochondrial Changes. Metabolites 2023, 13, 86. [Google Scholar] [CrossRef]
- Pradeepkiran, J.A.; Baig, J.; Selman, A.; Reddy, P.H. Mitochondria in Aging and Alzheimer’s Disease: Focus on Mitophagy. Neuroscientist 2023, 10738584221139760. [Google Scholar] [CrossRef]
- Mary, A.; Eysert, F.; Checler, F.; Chami, M. Mitophagy in Alzheimer’s Disease: Molecular Defects and Therapeutic Approaches. Mol. Psychiatry 2023, 28, 202–216. [Google Scholar] [CrossRef]
- Katusic, Z.S.; d’Uscio, L.V.; He, T. Emerging Roles of Endothelial Nitric Oxide in Preservation of Cognitive Health. Stroke 2023, 54, 686–696. [Google Scholar] [CrossRef]
- Ashwood, P.; Thompson, R.P.H.; Powell, J.J. Fine Particles That Adsorb Lipopolysaccharide via Bridging Calcium Cations May Mimic Bacterial Pathogenicity towards Cells. Exp. Biol. Med. 2007, 232, 107–117. [Google Scholar]
- Villarreal-Calderon, R.; Dale, G.; Delgado-Chávez, R.; Torres-Jardón, R.; Zhu, H.; Herritt, L.; Gónzalez-Maciel, A.; Reynoso-Robles, R.; Yuan, Y.; Wang, J.; et al. Intra-City Differences in Cardiac Expression of Inflammatory Genes and Inflammasomes in Young Urbanites: A Pilot Study. J. Toxicol. Pathol. 2012, 25, 163–173. [Google Scholar] [CrossRef]
- Simonovitch, S.; Schmukler, E.; Masliah, E.; Pinkas-Kramarski, R.; Michaelson, D.M. The Effects of APOE4 on Mitochondrial Dynamics and Proteins in Vivo. J. Alzheimers Dis. 2019, 70, 861–875. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Reiman, E.M.; Beach, T.G.; Serrano, G.E.; Sabbagh, M.N.; Nielsen, M.; Caselli, R.J.; Shi, J. Effect of ApoE Isoforms on Mitochondria in Alzheimer Disease. Neurology 2020, 94, e2404–e2411. [Google Scholar] [CrossRef] [PubMed]
- Oliver, D.M.A.; Reddy, P.H. Molecular Basis of Alzheimer’s Disease: Focus on Mitochondria. J. Alzheimers Dis. 2019, 72 (Suppl. S1), S95–S116. [Google Scholar] [CrossRef]
- Orr, A.L.; Kim, C.; Jimenez-Morales, D.; Newton, B.W.; Johnson, J.R.; Krogan, N.J.; Swaney, D.L.; Mahley, R.W. Neuronal Apolipoprotein E4 Expression Results in Proteome-Wide Alterations and Compromises Bioenergetic Capacity by Disrupting Mitochondrial Function. J. Alzheimers Dis. 2019, 68, 991–1011. [Google Scholar] [CrossRef]
- Tambini, M.D.; Pera, M.; Kanter, E.; Yang, H.; Guardia-Laguarta, C.; Holtzman, D.; Sulzer, D.; Area-Gomez, E.; Schon, E.A. ApoE4 Upregulates the Activity of Mitochondria-Associated ER Membranes. EMBO Rep. 2016, 17, 27–36. [Google Scholar] [CrossRef]
- Chen, H.; Chen, F.; Jiang, Y.; Zhang, L.; Hu, G.; Sun, F.; Zhang, M.; Ji, Y.; Chen, Y.; Che, G.; et al. A Review of ApoE4 Interference Targeting Mitophagy Molecular Pathways for Alzheimer’s Disease. Front. Aging Neurosci. 2022, 14, 881239. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Mattson, M.P. Apolipoprotein E and oxidative stress in brain with relevance to Alzheimer’s disease. Neurobiol. Dis. 2020, 138, 104795. [Google Scholar] [CrossRef]
- Zhao, N.; Liu, C.-C.; Van Ingelgom, A.J.; Martens, Y.A.; Linares, C.; Knight, J.A.; Painter, M.M.; Sullivan, P.M.; Bu, G. Apolipoprotein E4 Impairs Neuronal Insulin Signaling by Trapping Insulin Receptor in the Endosomes. Neuron 2017, 96, 115–129.e5. [Google Scholar] [CrossRef] [PubMed]
- Qi, G.; Mi, Y.; Shi, X.; Gu, H.; Brinton, R.D.; Yin, F. ApoE4 Impairs Neuron-Astrocyte Coupling of Fatty Acid Metabolism. Cell Rep. 2021, 34, 108572. [Google Scholar] [CrossRef] [PubMed]
- Gainutdinov, T.; Gizatullina, Z.; Debska-Vielhaber, G.; Vielhaber, S.; Feldmann, R.E.; Orynbayeva, Z.; Gellerich, F.N. Age-Associated Alterations of Brain Mitochondria Energetics. Biochem. Biophys. Res. Commun. 2023, 643, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.L.; Meyer, O.L.; Farias, S.T.; Whitmer, R.A.; Rajan, K.; Olichney, J.; Johnson, D.; Mungas, D. APOE Effects on Late Life Cognitive Trajectories in Diverse Racial/Ethnic Groups. J. Int. Neuropsychol. Soc. 2023, 29, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Llibre-Guerra, J.J.; Li, J.; Qian, Y.; Llibre-Rodriguez, J.D.J.; Jiménez-Velázquez, I.Z.; Acosta, D.; Salas, A.; Llibre-Guerra, J.C.; Valvuerdi, A.; Harrati, A.; et al. Apolipoprotein E (APOE) Genotype, Dementia, and Memory Performance among Caribbean Hispanic versus US Populations. Alzheimers Dement. 2023, 19, 602–610. [Google Scholar] [CrossRef]
- Dhana, K.; Barnes, L.L.; Liu, X.; Agarwal, P.; Desai, P.; Krueger, K.R.; Holland, T.M.; Halloway, S.; Aggarwal, N.T.; Evans, D.A.; et al. Genetic Risk, Adherence to a Healthy Lifestyle, and Cognitive Decline in African Americans and European Americans. Alzheimers Dement. 2022, 18, 572–580. [Google Scholar] [CrossRef]
- O’Bryant, S.E.; Barber, R.C.; Philips, N.; Johnson, L.A.; Hall, J.R.; Subasinghe, K.; Petersen, M.; Toga, A.W.; Yaffe, K.; Rissman, R.A.; et al. The Link between APOE4 Presence and Neuropsychological Test Performance among Mexican Americans and Non-Hispanic Whites of the Multiethnic Health & Aging Brain Study—Health Disparities Cohort. Dement. Geriatr. Cogn. Disord. 2022, 51, 26–31. [Google Scholar] [CrossRef]
- Kallio, M.J.; Salmenperä, L.; Siimes, M.A.; Perheentupa, J.; Gylling, H.; Miettinen, T.A. The Apolipoprotein E Phenotype Has a Strong Influence on Tracking of Serum Cholesterol and Lipoprotein Levels in Children: A Follow-up Study from Birth to the Age of 11 Years. Pediatr. Res. 1998, 43, 381–385. [Google Scholar] [CrossRef]
- Fulton, J.E.; Dai, S.; Grunbaum, J.A.; Boerwinkle, E.; Labarthe, D.R. Effects of Apolipoprotein E Genotype on Blood Cholesterol in Adolescent Girls. Am. J. Prev. Med. 2009, 37 (Suppl. 1), S78–S85. [Google Scholar] [CrossRef]
- Hassan, N.E.; El Ashmawi, A.A.; El-Masry, S.A.; Zarouk, W.A.; Mira, M.F.; El-Saeed, G.S.; Dwidar, O.H. Metabolic Syndrome in a Sample of Egyptian Adolescent Girls and Its Association with Apolipoprotein E. J. Paediatr. Child. Health 2019, 55, 1344–1350. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; de la Monte, S.M. Apolipoprotein E4, Gender, Body Mass Index, Inflammation, Insulin Resistance, and Air Pollution Interactions: Recipe for Alzheimer’s Disease Development in Mexico City Young Females. J. Alzheimers Dis. 2017, 58, 613–630. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Garcidueñas, L.; Jewells, V.; Galaz-Montoya, C.; van Zundert, B.; Pérez-Calatayud, A.; Ascencio-Ferrel, E.; Valencia-Salazar, G.; Sandoval-Cano, M.; Carlos, E.; Solorio, E.; et al. Interactive and Additive Influences of Gender, BMI and Apolipoprotein 4 on Cognition in Children Chronically Exposed to High Concentrations of PM2.5 and Ozone. APOE 4 Females Are at Highest Risk in Mexico City. Environ. Res. 2016, 150, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Dzietko, M.; Schulz, S.; Preuss, M.; Haertel, C.; Stein, A.; Felderhoff-Mueser, U.; Goepel, W. Apolipoprotein E Gene Polymorphisms and Intraventricular Haemorrhage in Infants Born Preterm: A Large Prospective Multicentre Cohort Study. Dev. Med. Child. Neurol. 2019, 61, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Humberg, A.; Dzietko, M.; Schulz, S.; Felderhoff-Müser, U.; Hüning, B.; Stein, A.; Fortmann, M.I.; Marissen, J.; Rausch, T.K.; Herting, E.; et al. Association of ApoE Genotypes and Recovery from Intracerebral Hemorrhage in Very Low Birth Weight Infants. Stroke 2022, 53, 514–522. [Google Scholar] [CrossRef]
- Lien, E.; Andersen, G.L.; Bao, Y.; Gordish-Dressman, H.; Skranes, J.S.; Vik, T.; Blackman, J.A. Apolipoprotein E Polymorphisms and Severity of Cerebral Palsy: A Cross-Sectional Study in 255 Children in Norway. Dev. Med. Child. Neurol. 2013, 55, 372–377. [Google Scholar] [CrossRef]
- Acevedo, S.F.; Piper, B.J.; Craytor, M.J.; Benice, T.S.; Raber, J. Apolipoprotein E4 and Sex Affect Neurobehavioral Performance in Primary School Children. Pediatr. Res. 2010, 67, 293–299. [Google Scholar] [CrossRef]
- Holm, S.M.; Balmes, J.R.; Gunier, R.B.; Kogut, K.; Harley, K.G.; Eskenazi, B. Cognitive Development and Prenatal Air Pollution Exposure in the CHAMACOS Cohort. Environ. Health Perspect. 2023, 131, 37007. [Google Scholar] [CrossRef]
- Luciano, M.; Gow, A.J.; Harris, S.E.; Hayward, C.; Allerhand, M.; Starr, J.M.; Visscher, P.M.; Deary, I.J. Cognitive Ability at Age 11 and 70 Years, Information Processing Speed, and APOE Variation: The Lothian Birth Cohort 1936 Study. Psychol. Aging 2009, 24, 129–138. [Google Scholar] [CrossRef]
- Bloss, C.S.; Delis, D.C.; Salmon, D.P.; Bondi, M.W. Decreased Cognition in Children with Risk Factors for Alzheimer’s Disease. Biol. Psychiatry 2008, 64, 904–906. [Google Scholar] [CrossRef]
- Forte, G.I.; Piccione, M.; Scola, L.; Crivello, A.; Galfano, C.; Corsi, M.M.; Chiappelli, M.; Candore, G.; Giuffrè, M.; Verna, R.; et al. Apolipoprotein E Genotypic Frequencies among Down Syndrome Patients Imply Early Unsuccessful Aging for ApoE4 Carriers. Rejuvenation Res. 2007, 10, 293–299. [Google Scholar] [CrossRef]
- Gozal, D.; Capdevila, O.S.; Kheirandish-Gozal, L.; Crabtree, V.M. APOE Epsilon 4 Allele, Cognitive Dysfunction, and Obstructive Sleep Apnea in Children. Neurology 2007, 69, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Moceri, V.M.; Kukull, W.A.; Emanual, I.; van Belle, G.; Starr, J.R.; Schellenberg, G.D.; McCormick, W.C.; Bowen, J.D.; Teri, L.; Larson, E.B. Using Census Data and Birth Certificates to Reconstruct the Early-Life Socioeconomic Environment and the Relation to the Development of Alzheimer’s Disease. Epidemiology 2001, 12, 383–389. [Google Scholar] [CrossRef]
- Shih, P.; Huang, C.-C.; Pan, S.-C.; Chiang, T.-L.; Guo, Y.L. Hyperactivity Disorder in Children Related to Traffic-Based Air Pollution during Pregnancy. Environ. Res. 2020, 188, 109588. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, E.; Malmqvist, E.; Rittner, R.; Gustafsson, P.; Källén, K.; Oudin, A. Exposure to Local, Source-Specific Ambient Air Pollution during Pregnancy and Autism in Children: A Cohort Study from Southern Sweden. Sci. Rep. 2023, 13, 3848. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.-J.; Cho, J.; Hong, Y.-C.; Lee, D.-W.; Moon, S.; Park, S.J.; Lee, K.-S.; Shin, C.H.; Lee, Y.A.; Kim, B.-N.; et al. DNA Methylation Is Associated with Prenatal Exposure to Sulfur Dioxide and Childhood Attention-Deficit Hyperactivity Disorder Symptoms. Sci. Rep. 2023, 13, 3501. [Google Scholar] [CrossRef] [PubMed]
- US EPA. Criteria Pollutant Standards O. NAAQS Table. Available online: https://www.epa.gov/criteria-air-pollutants/naaqs-table (accessed on 29 March 2023).
- Calderón-Garcidueñas, L.; Engle, R.; Mora-Tiscareño, A.; Styner, M.; Gómez-Garza, G.; Zhu, H.; Jewells, V.; Torres-Jardón, R.; Romero, L.; Monroy-Acosta, M.E.; et al. Exposure to Severe Urban Air Pollution Influences Cognitive Outcomes, Brain Volume and Systemic Inflammation in Clinically Healthy Children. Brain Cogn. 2011, 77, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulos, P.; Richter-Schmidinger, T.; Horn, M.; Maus, S.; Reichel, M.; Sidiropoulos, C.; Rhein, C.; Lewczuk, P.; Doerfler, A.; Kornhuber, J. Hippocampal Volume Differences between Healthy Young Apolipoprotein E Ε2 and Ε4 Carriers. J. Alzheimers Dis. 2011, 26, 207–210. [Google Scholar] [CrossRef]
- Nuremberg Air Quality Index (AQI) and Germany Air Pollution|IQAir. Available online: https://www.iqair.com/us/germany/bavaria/nuremberg (accessed on 29 March 2023).
- O’Dwyer, L.; Lamberton, F.; Matura, S.; Tanner, C.; Scheibe, M.; Miller, J.; Rujescu, D.; Prvulovic, D.; Hampel, H. Reduced Hippocampal Volume in Healthy Young ApoE4 Carriers: An MRI Study. PLoS ONE 2012, 7, e48895. [Google Scholar] [CrossRef]
- Dean, D.C.; Jerskey, B.A.; Chen, K.; Protas, H.; Thiyyagura, P.; Roontiva, A.; O’Muircheartaigh, J.; Dirks, H.; Waskiewicz, N.; Lehman, K.; et al. Brain Differences in Infants at Differential Genetic Risk for Late-Onset Alzheimer Disease: A Cross-Sectional Imaging Study. JAMA Neurol. 2014, 71, 11–22. [Google Scholar] [CrossRef]
- Hollingshead Four-Factor Index of Socioeconomic Status (SES-Child)—Nathan Kline Institute—Rockland Sample Documentation. Available online: http://fcon_1000.projects.nitrc.org/indi/enhanced/assessments/ses-child.html (accessed on 29 March 2023).
- Rhode Island. Air Quality: Department of Health. Available online: https://health.ri.gov/data/airquality/ (accessed on 29 March 2023).
- Khan, W.; Giampietro, V.; Ginestet, C.; Dell’Acqua, F.; Bouls, D.; Newhouse, S.; Dobson, R.; Banaschewski, T.; Barker, G.J.; Bokde, A.L.W.; et al. No Differences in Hippocampal Volume between Carriers and Non-Carriers of the ApoE Ε4 and Ε2 Alleles in Young Healthy Adolescents. J. Alzheimers Dis. 2014, 40, 37–43. [Google Scholar] [CrossRef]
- Chang, L.; Douet, V.; Bloss, C.; Lee, K.; Pritchett, A.; Jernigan, T.L.; Akshoomoff, N.; Murray, S.S.; Frazier, J.; Kennedy, D.N.; et al. Gray Matter Maturation and Cognition in Children with Different APOE ε Genotypes. Neurology 2016, 87, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Remer, J.; Dean, D.C.; Chen, K.; Reiman, R.A.; Huentelman, M.J.; Reiman, E.M.; Deoni, S.C.L. Longitudinal White Matter and Cognitive Development in Pediatric Carriers of the Apolipoprotein Ε4 Allele. Neuroimage 2020, 222, 117243. [Google Scholar] [CrossRef]
- Tuminello, E.R.; Han, S.D. The Apolipoprotein e Antagonistic Pleiotropy Hypothesis: Review and Recommendations. Int. J. Alzheimers Dis. 2011, 2011, 726197. [Google Scholar] [CrossRef] [PubMed]
- Zink, N.; Bensmann, W.; Arning, L.; Beste, C.; Stock, A.-K. Apolipoprotein Ε4 Is Associated with Better Cognitive Control Allocation in Healthy Young Adults. Neuroimage 2019, 185, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Joe, E.; Medina, L.D.; Ringman, J.M.; O’Neill, J. 1H MRS Spectroscopy in Preclinical Autosomal Dominant Alzheimer Disease. Brain Imaging Behav. 2019, 13, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Jett, S.; Dyke, J.P.; Boneu Yepez, C.; Zarate, C.; Carlton, C.; Schelbaum, E.; Jang, G.; Pahlajani, S.; Williams, S.; Diaz Brinton, R.; et al. Effects of Sex and APOE Ε4 Genotype on Brain Mitochondrial High-Energy Phosphates in Midlife Individuals at Risk for Alzheimer’s Disease: A 31Phosphorus MR Spectroscopy Study. PLoS ONE 2023, 18, e0281302. [Google Scholar] [CrossRef] [PubMed]
- Parasoglou, P.; Osorio, R.S.; Khegai, O.; Kovbasyuk, Z.; Miller, M.; Ho, A.; Dehkharghani, S.; Wisniewski, T.; Convit, A.; Mosconi, L.; et al. Phosphorus Metabolism in the Brain of Cognitively Normal Midlife Individuals at Risk for Alzheimer’s Disease. Neuroimage Rep. 2022, 2, 100121. [Google Scholar] [CrossRef]
- Jiménez-Balado, J.; Ycaza Herrera, A.; Igwe, K.; Klem, L.; Buyukturkoglu, K.; Irimia, A.; Chen, L.; Guo, J.; Brickman, A.M.; Eich, T.S. Reduced Hippocampal GABA+ Is Associated with Poorer Episodic Memory in Healthy Older Women: A Pilot Study. Front. Behav. Neurosci. 2021, 15, 695416. [Google Scholar] [CrossRef]
- Nedelska, Z.; Przybelski, S.A.; Lesnick, T.G.; Schwarz, C.G.; Lowe, V.J.; Machulda, M.M.; Kremers, W.K.; Mielke, M.M.; Roberts, R.O.; Boeve, B.F.; et al. 1H-MRS Metabolites and Rate of β-Amyloid Accumulation on Serial PET in Clinically Normal Adults. Neurology 2017, 89, 1391–1399. [Google Scholar] [CrossRef]
- Voevodskaya, O.; Sundgren, P.C.; Strandberg, O.; Zetterberg, H.; Minthon, L.; Blennow, K.; Wahlund, L.-O.; Westman, E.; Hansson, O.; Swedish BioFINDER study group. Myo-Inositol Changes Precede Amyloid Pathology and Relate to APOE Genotype in Alzheimer Disease. Neurology 2016, 86, 1754–1761. [Google Scholar] [CrossRef]
- Laakso, M.P.; Hiltunen, Y.; Könönen, M.; Kivipelto, M.; Koivisto, A.; Hallikainen, M.; Soininen, H. Decreased Brain Creatine Levels in Elderly Apolipoprotein E Epsilon 4 Carriers. J. Neural Transm. 2003, 110, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Abrigo, J.; Liu, W.; Han, E.Y.; Yeung, D.K.W.; Shi, L.; Au, L.W.C.; Deng, M.; Chen, S.; Leung, E.Y.L.; et al. Lower Posterior Cingulate N-Acetylaspartate to Creatine Level in Early Detection of Biologically Defined Alzheimer’s Disease. Brain Sci. 2022, 12, 722. [Google Scholar] [CrossRef] [PubMed]
- Kara, F.; Joers, J.M.; Deelchand, D.K.; Park, Y.W.; Przybelski, S.A.; Lesnick, T.G.; Senjem, M.L.; Zeydan, B.; Knopman, D.S.; Lowe, V.J.; et al. 1H MR Spectroscopy Biomarkers of Neuronal and Synaptic Function Are Associated with Tau Deposition in Cognitively Unimpaired Older Adults. Neurobiol. Aging 2022, 112, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Piersson, A.D.; Mohamad, M.; Rajab, F.; Suppiah, S. Cerebrospinal Fluid Amyloid Beta, Tau Levels, Apolipoprotein, and 1H-MRS Brain Metabolites in Alzheimer’s Disease: A Systematic Review. Acad. Radiol. 2021, 28, 1447–1463. [Google Scholar] [CrossRef] [PubMed]
- Hone-Blanchet, A.; Bohsali, A.; Krishnamurthy, L.C.; Shahid, S.; Lin, Q.; Zhao, L.; Loring, D.; Goldstein, F.; John, S.E.; Fleischer, C.C.; et al. Relationships between Frontal Metabolites and Alzheimer’s Disease Biomarkers in Cognitively Normal Older Adults. Neurobiol. Aging 2022, 109, 22–30. [Google Scholar] [CrossRef]
- Jellinger, K.A. Recent Update on the Heterogeneity of the Alzheimer’s Disease Spectrum. J. Neural Transm. 2022, 129, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a Biological Definition of Alzheimer’s Disease. Alzheimers Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef]
- Dubois, B.; Villain, N.; Frisoni, G.B.; Rabinovici, G.D.; Sabbagh, M.; Cappa, S.; Bejanin, A.; Bombois, S.; Epelbaum, S.; Teichmann, M.; et al. Clinical Diagnosis of Alzheimer’s Disease: Recommendations of the International Working Group. Lancet Neurol. 2021, 20, 484–496. [Google Scholar] [CrossRef]
- Frisoni, G.B.; Altomare, D.; Thal, D.R.; Ribaldi, F.; van der Kant, R.; Ossenkoppele, R.; Blennow, K.; Cummings, J.; van Duijn, C.; Nilsson, P.M.; et al. The Probabilistic Model of Alzheimer Disease: The Amyloid Hypothesis Revised. Nat. Rev. Neurosci. 2022, 23, 53–66. [Google Scholar] [CrossRef]
- Karanth, S.; Nelson, P.T.; Katsumata, Y.; Kryscio, R.J.; Schmitt, F.A.; Fardo, D.W.; Cykowski, M.D.; Jicha, G.A.; Van Eldik, L.J.; Abner, E.L. Prevalence and Clinical Phenotype of Quadruple Misfolded Proteins in Older Adults. JAMA Neurol. 2020, 77, 1299–1307. [Google Scholar] [CrossRef]
- Mugica-Álvarez, V.; Figueroa-Lara, J.; Romero-Romo, M.; Sepúlveda-Sánchez, J.; López-Moreno, T. Concentrations and Properties of Airborne Particles in the Mexico City Subway System. Atmos. Environ. 2012, 49, 284–293. [Google Scholar] [CrossRef]
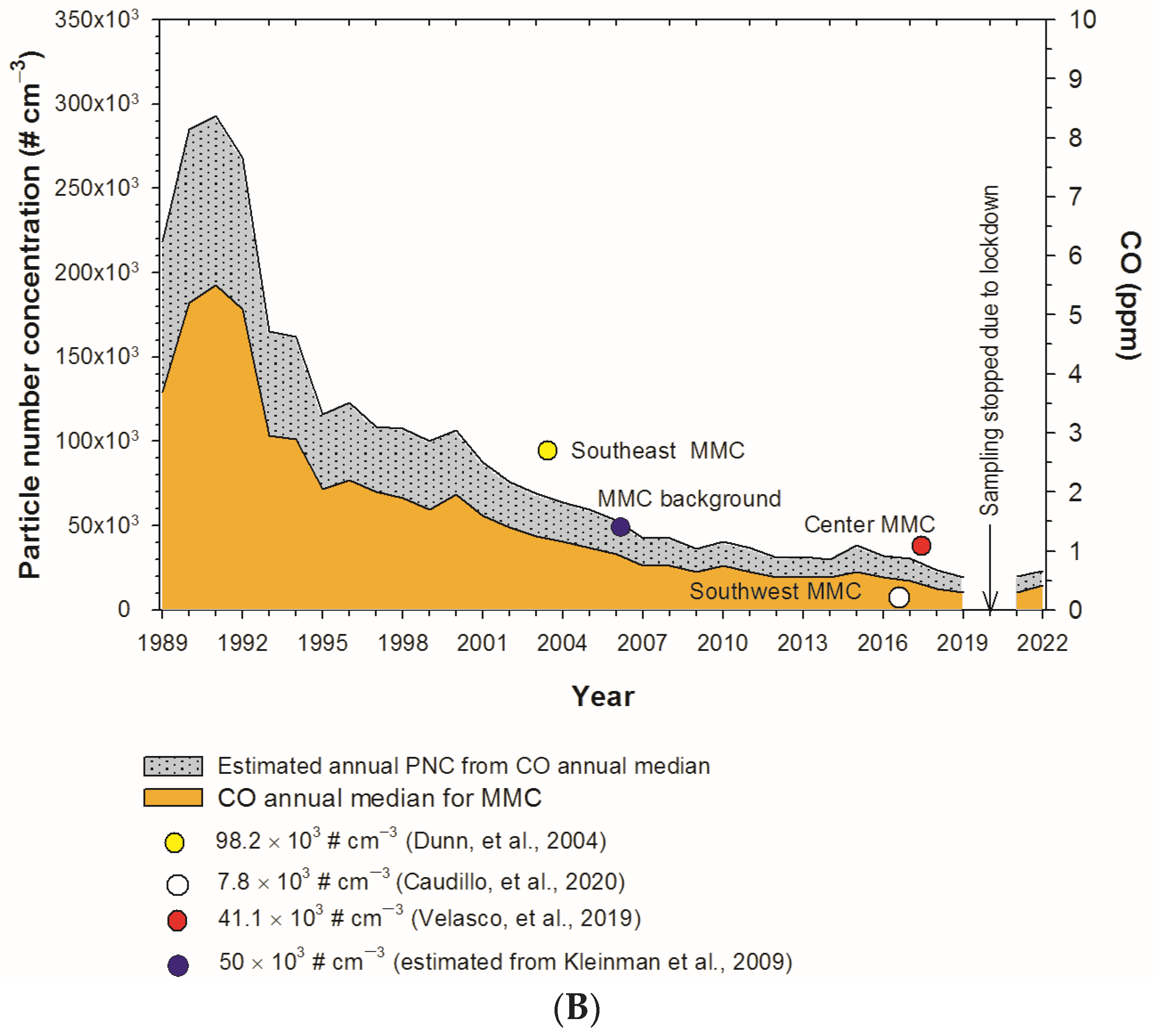
- Dunn, M.J.; Jiménez, J.L.; Baumgardner, D.; Castro, T.; McMurry, P.H.; Smith, J.N. Measurements of Mexico City nanoparticle size distributions: Observations of new particle formation and growth. Geophys. Res. Lett. 2004, 31. [Google Scholar] [CrossRef]
- Caudillo, L.; Salcedo, D.; Peralta, O.; Castro, T.; Alvarez-Ospina, H. Nanoparticle Size Distributions in Mexico City. Atmos. Pollut. Res. 2020, 11, 78–84. [Google Scholar] [CrossRef]
- Velasco, E.; Retama, A.; Segovia, E.; Ramos, R. Particle Exposure and Inhaled Dose While Commuting by Public Transport in Mexico City. Atmos. Environ. 2019, 219, 117044. [Google Scholar] [CrossRef]
- Kleinman, L.I.; Springston, S.R.; Wang, J.; Daum, P.H.; Lee, Y.-N.; Nunnermacker, L.J.; Senum, G.I.; Weinstein-Lloyd, J.; Alexander, M.L.; Hubbe, J.; et al. The Time Evolution of Aerosol Size Distribution over the Mexico City Plateau. Atmos. Chem. Phys. 2009, 9, 4261–4278. [Google Scholar] [CrossRef]
- Erickson, M.A.; Shulyatnikova, T.; Banks, W.A.; Hayden, M.R. Ultrastructural Remodeling of the Blood-Brain Barrier and Neurovascular Unit by Lipopolysaccharide-Induced Neuroinflammation. Int. J. Mol. Sci. 2023, 24, 1640. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Del Tredici, K. The Preclinical Phase of the Pathological Process Underlying Sporadic Alzheimer’s Disease. Brain 2015, 138 Pt 10, 2814–2833. [Google Scholar] [CrossRef]
- James, B.D.; Wilson, R.S.; Boyle, P.A.; Trojanowski, J.Q.; Bennett, D.A.; Schneider, J.A. TDP-43 Stage, Mixed Pathologies, and Clinical Alzheimer’s-Type Dementia. Brain 2016, 139, 2983–2993. [Google Scholar] [CrossRef]
- Scarioni, M.; Gami-Patel, P.; Peeters, C.F.W.; de Koning, F.; Seelaar, H.; Mol, M.O.; van Swieten, J.C.; Netherlands Brain Bank; Rozemuller, A.J.M.; Hoozemans, J.J.M.; et al. Psychiatric Symptoms of Frontotemporal Dementia and Subcortical (Co-)Pathology Burden: New Insights. Brain 2023, 146, 307–320. [Google Scholar] [CrossRef]
- Bayram, E.; Shan, G.; Cummings, J.L. Associations between Comorbid TDP-43, Lewy Body Pathology, and Neuropsychiatric Symptoms in Alzheimer’s Disease. J. Alzheimers Dis. 2019, 69, 953–961. [Google Scholar] [CrossRef]
- Ferman, T.J.; Aoki, N.; Boeve, B.F.; Aakre, J.A.; Kantarci, K.; Graff-Radford, J.; Parisi, J.E.; Van Gerpen, J.A.; Graff-Radford, N.R.; Uitti, R.J.; et al. Subtypes of Dementia with Lewy Bodies Are Associated with α-Synuclein and Tau Distribution. Neurology 2020, 95, e155–e165. [Google Scholar] [CrossRef] [PubMed]
- Virgilio, E.; De Marchi, F.; Contaldi, E.; Dianzani, U.; Cantello, R.; Mazzini, L.; Comi, C. The Role of Tau beyond Alzheimer’s Disease: A Narrative Review. Biomedicines 2022, 10, 760. [Google Scholar] [CrossRef] [PubMed]
- Mercan, D.; Heneka, M.T. The Contribution of the Locus Coeruleus-Noradrenaline System Degeneration during the Progression of Alzheimer’s Disease. Biology 2022, 11, 1822. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Garcidueñas, L.; Torres-Solorio, A.K.; Kulesza, R.J.; Torres-Jardón, R.; González-González, L.O.; García-Arreola, B.; Chávez-Franco, D.A.; Luévano-Castro, S.C.; Hernández-Castillo, A.; Carlos-Hernández, E.; et al. Gait and Balance Disturbances Are Common in Young Urbanites and Associated with Cognitive Impairment. Air Pollution and the Historical Development of Alzheimer’s Disease in the Young. Environ. Res. 2020, 191, 110087. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Garcidueñas, L.; Avila-Ramírez, J.; Calderón-Garcidueñas, A.; González-Heredia, T.; Acuña-Ayala, H.; Chao, C.-K.; Thompson, C.; Ruiz-Ramos, R.; Cortés-González, V.; Martínez-Martínez, L.; et al. Cerebrospinal Fluid Biomarkers in Highly Exposed PM2.5 Urbanites: The Risk of Alzheimer’s and Parkinson’s Diseases in Young Mexico City Residents. J. Alzheimers Dis. 2016, 54, 597–613. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; Cross, J.V.; Franco-Lira, M.; Aragón-Flores, M.; Kavanaugh, M.; Torres-Jardón, R.; Chao, C.-K.; Thompson, C.; Chang, J.; Zhu, H.; et al. Brain Immune Interactions and Air Pollution: Macrophage Inhibitory Factor (MIF), Prion Cellular Protein (PrP(C)), Interleukin-6 (IL-6), Interleukin 1 Receptor Antagonist (IL-1Ra), and Interleukin-2 (IL-2) in Cerebrospinal Fluid and MIF in Serum Differentiate Urban Children Exposed to Severe vs. Low Air Pollution. Front. Neurosci. 2013, 7, 183. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; Mukherjee, P.S.; Waniek, K.; Holzer, M.; Chao, C.-K.; Thompson, C.; Ruiz-Ramos, R.; Calderón-Garcidueñas, A.; Franco-Lira, M.; Reynoso-Robles, R.; et al. Non-Phosphorylated Tau in Cerebrospinal Fluid Is a Marker of Alzheimer’s Disease Continuum in Young Urbanites Exposed to Air Pollution. J. Alzheimers Dis. 2018, 66, 1437–1451. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; Mukherjee, P.S.; Kulesza, R.J.; Torres-Jardón, R.; Hernández-Luna, J.; Ávila-Cervantes, R.; Macías-Escobedo, E.; González-González, O.; González-Maciel, A.; García-Hernández, K.; et al. Mild Cognitive Impairment and Dementia Involving Multiple Cognitive Domains in Mexican Urbanites. J. Alzheimers Dis. 2019, 68, 1113–1123. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; Chávez-Franco, D.A.; Luévano-Castro, S.C.; Macías-Escobedo, E.; Hernández-Castillo, A.; Carlos-Hernández, E.; Franco-Ortíz, A.; Castro-Romero, S.P.; Cortés-Flores, M.; Crespo-Cortés, C.N.; et al. Metals, Nanoparticles, Particulate Matter, and Cognitive Decline. Front. Neurol. 2021, 12, 794071. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; Kulesza, R.J.; Mansour, Y.; Aiello-Mora, M.; Mukherjee, P.S.; González-González, L.O. Increased Gain in the Auditory Pathway, Alzheimer’s Disease Continuum, and Air Pollution: Peripheral and Central Auditory System Dysfunction Evolves Across Pediatric and Adult Urbanites. J. Alzheimers Dis. 2019, 70, 1275–1286. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; González-González, L.O.; Kulesza, R.J.; Fech, T.M.; Pérez-Guillé, G.; Luna, M.A.J.-B.; Soriano-Rosales, R.E.; Solorio, E.; Miramontes-Higuera, J.D.J.; Gómez-Maqueo Chew, A.; et al. Exposures to Fine Particulate Matter (PM2.5) and Ozone above USA Standards Are Associated with Auditory Brainstem Dysmorphology and Abnormal Auditory Brainstem Evoked Potentials in Healthy Young Dogs. Environ. Res. 2017, 158, 324–332. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; D’Angiulli, A.; Kulesza, R.J.; Torres-Jardón, R.; Osnaya, N.; Romero, L.; Keefe, S.; Herritt, L.; Brooks, D.M.; Avila-Ramirez, J.; et al. Air Pollution Is Associated with Brainstem Auditory Nuclei Pathology and Delayed Brainstem Auditory Evoked Potentials. Int. J. Dev. Neurosci. 2011, 29, 365–375. [Google Scholar] [CrossRef]
- Alipour-Haris, G.; Armstrong, M.J.; Sullivan, J.L.; Survadevara, U.; Rouhizadeh, M.; Brown, J.D. Suicidal Ideation and Suicide-Attempt-Related Hospitalizations among People with Alzheimer’s Disease (AD) and AD-Related Dementias in the United States during 2016-2018. J. Clin. Med. 2022, 11, 943. [Google Scholar] [CrossRef]
- Lozupone, M.; Donghia, R.; Sardone, R.; Mollica, A.; Barardino, G.; Lampignano, L.; Griseta, C.; Zupo, R.; Castellana, F.; Bortone, I.; et al. Apolipoprotein E genotype, inflammatory biomarkers, and non-psychiatric multimorbidity contribute to the suicidal ideation phenotype in older age. The Salus in Apulia Study. J. Affect. Disord. 2022, 319, 202–212. [Google Scholar] [CrossRef]
- Abramova, O.; Soloveva, K.; Zorkina, Y.; Gryadunov, D.; Ikonnikova, A.; Fedoseeva, E.; Emelyanova, M.; Ochneva, A.; Andriushchenko, N.; Pavlov, K.; et al. Suicide-Related Single Nucleotide Polymorphisms, rs4918918 and rs10903034: Association with Dementia in Older Adults. Genes 2022, 13, 2174. [Google Scholar] [CrossRef]
- Education in Mexico. Available online: https://www.planeacion.sep.gob.mx/Doc/estadistica_e_indicadores/principales_cifras/principales_cifras_2020_2021_bolsillo.pdf (accessed on 22 March 2023).
- OECD Mexico. Available online: https://gpseducation.oecd.org/Content/EAGCountryNotes/EAG2022_Mexico.pdf (accessed on 29 March 2023).
- Thal, D.R.; Rüb, U.; Orantes, M.; Braak, H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology 2002, 58, 1791–1800. [Google Scholar] [CrossRef] [PubMed]
- Gustavsson, A.; Norton, N.; Fast, T.; Frölich, L.; Georges, J.; Holzapfel, D.; Kirabali, T.; Krolak-Salmon, P.; Rossini, P.M.; Ferretti, M.T.; et al. Global estimates on the number of persons across the Alzheimer’s disease continuum. Alzheimers Dement. 2023, 19, 658–670. [Google Scholar] [CrossRef] [PubMed]
- Krijger, A.; Steenbergen, E.; Schiphof-Godart, L.; van Rossum, C.; Verkaik-Kloosterman, J.; Elstgeest, L.; Ter Borg, S.; Raat, H.; Joosten, K. Clusters of Lifestyle Behaviours and Their Associations with Socio-Demographic Characteristics in Dutch Toddlers. Eur. J. Nutr. 2023, 62, 1143–1151. [Google Scholar] [CrossRef] [PubMed]
- Stern, Y. Cognitive Reserve. Neuropsychologia 2009, 47, 2015–2028. [Google Scholar] [CrossRef]
- Litkouhi, P.N.; Numbers, K.; Valenzuela, M.; Crawford, J.D.; Lam, B.C.P.; Litkouhi, P.N.; Sachdev, P.S.; Kochan, N.A.; Brodaty, H. Critical Periods for Cognitive Reserve Building Activities for Late Life Global Cognition and Cognitive Decline: The Sydney Memory and Aging Cohort Study. Neuropsychol. Dev. Cogn. B Aging Neuropsychol. Cogn. 2023, 1–17. [Google Scholar] [CrossRef]
- Poverty in Mexico|CONEVAL. Available online: https://www.coneval.org.mx/Medicion/Paginas/PobrezaInicio.aspx (accessed on 29 March 2023).
- Manderson, L.; Jewett, S. Risk, Lifestyle and Non-Communicable Diseases of Poverty. Glob. Health 2023, 19, 13. [Google Scholar] [CrossRef] [PubMed]
- Abellaneda-Pérez, K.; Cattaneo, G.; Cabello-Toscano, M.; Solana-Sánchez, J.; Mulet-Pons, L.; Vaqué-Alcázar, L.; Perellón-Alfonso, R.; Solé-Padullés, C.; Bargalló, N.; Tormos, J.M.; et al. Purpose in Life Promotes Resilience to Age-Related Brain Burden in Middle-Aged Adults. Alzheimers Res. Ther. 2023, 15, 49. [Google Scholar] [CrossRef] [PubMed]
- Ayyubova, G. Apoe4 Is A Risk Factor and Potential Therapeutic Target for Alzheimer’s Disease. CNS Neurol. Disord. Drug. Targets 2023. [Google Scholar] [CrossRef] [PubMed]
- O’Bryant, S.E.; Petersen, M.; Hall, J.; Johnson, L.A. Depression Is Differentially Related to Cognitive and Biomarker Outcomes among Mexican Americans. Front. Psychiatry 2022, 13, 901403. [Google Scholar] [CrossRef] [PubMed]


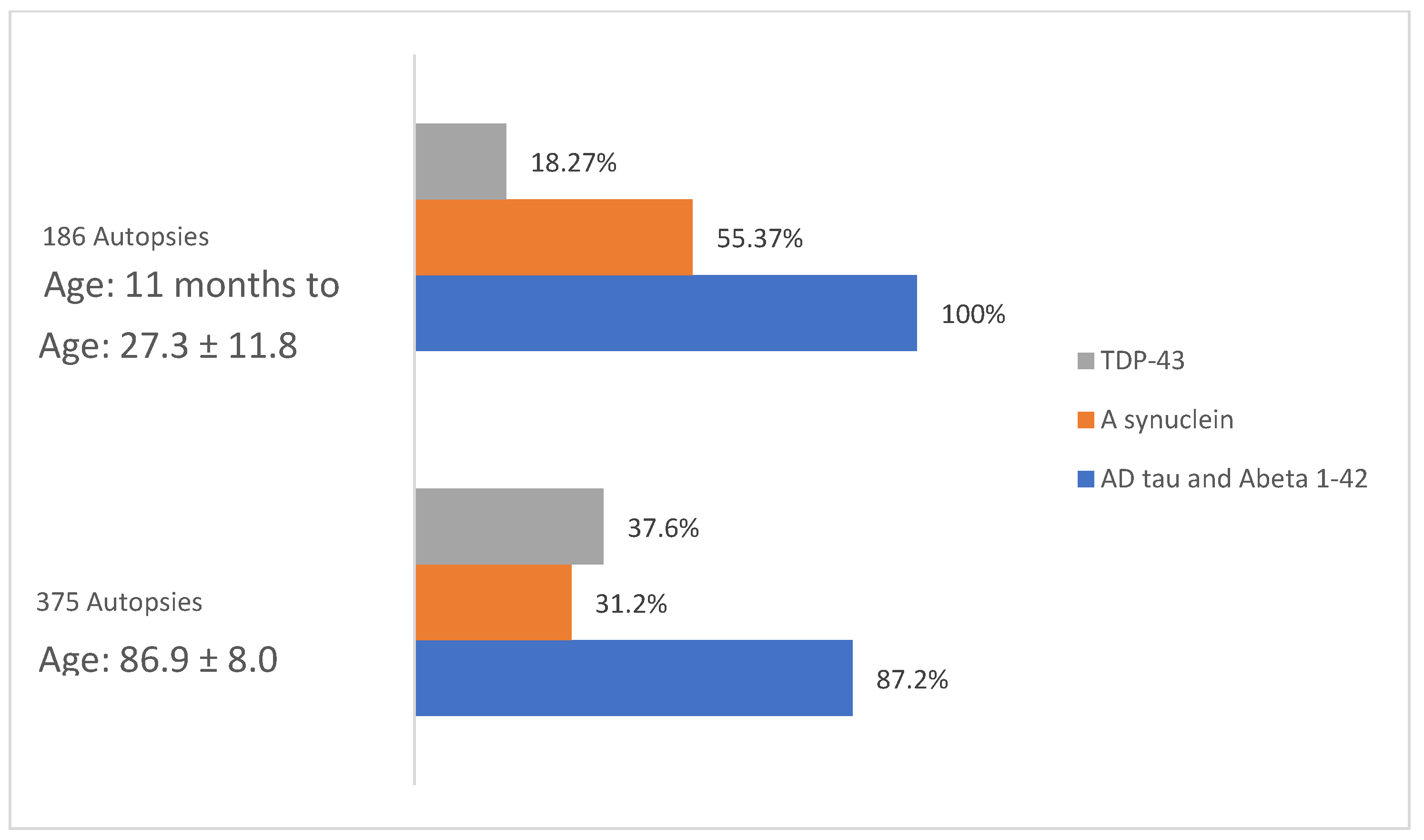
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calderón-Garcidueñas, L.; Hernández-Luna, J.; Aiello-Mora, M.; Brito-Aguilar, R.; Evelson, P.A.; Villarreal-Ríos, R.; Torres-Jardón, R.; Ayala, A.; Mukherjee, P.S. APOE Peripheral and Brain Impact: APOE4 Carriers Accelerate Their Alzheimer Continuum and Have a High Risk of Suicide in PM2.5 Polluted Cities. Biomolecules 2023, 13, 927. https://doi.org/10.3390/biom13060927
Calderón-Garcidueñas L, Hernández-Luna J, Aiello-Mora M, Brito-Aguilar R, Evelson PA, Villarreal-Ríos R, Torres-Jardón R, Ayala A, Mukherjee PS. APOE Peripheral and Brain Impact: APOE4 Carriers Accelerate Their Alzheimer Continuum and Have a High Risk of Suicide in PM2.5 Polluted Cities. Biomolecules. 2023; 13(6):927. https://doi.org/10.3390/biom13060927
Chicago/Turabian StyleCalderón-Garcidueñas, Lilian, Jacqueline Hernández-Luna, Mario Aiello-Mora, Rafael Brito-Aguilar, Pablo A. Evelson, Rodolfo Villarreal-Ríos, Ricardo Torres-Jardón, Alberto Ayala, and Partha S. Mukherjee. 2023. "APOE Peripheral and Brain Impact: APOE4 Carriers Accelerate Their Alzheimer Continuum and Have a High Risk of Suicide in PM2.5 Polluted Cities" Biomolecules 13, no. 6: 927. https://doi.org/10.3390/biom13060927
APA StyleCalderón-Garcidueñas, L., Hernández-Luna, J., Aiello-Mora, M., Brito-Aguilar, R., Evelson, P. A., Villarreal-Ríos, R., Torres-Jardón, R., Ayala, A., & Mukherjee, P. S. (2023). APOE Peripheral and Brain Impact: APOE4 Carriers Accelerate Their Alzheimer Continuum and Have a High Risk of Suicide in PM2.5 Polluted Cities. Biomolecules, 13(6), 927. https://doi.org/10.3390/biom13060927





