Melatonin: Facts, Extrapolations and Clinical Trials
Abstract
:1. Foreword
2. Background, Facts
3. Background, History
4. Melatonin Goes to Clinic Why? For What?
5. Clinical Studies
6. Melatonin in Kids
7. Some Proposed Mechanisms behind the Claimed Beneficial Effects of Melatonin
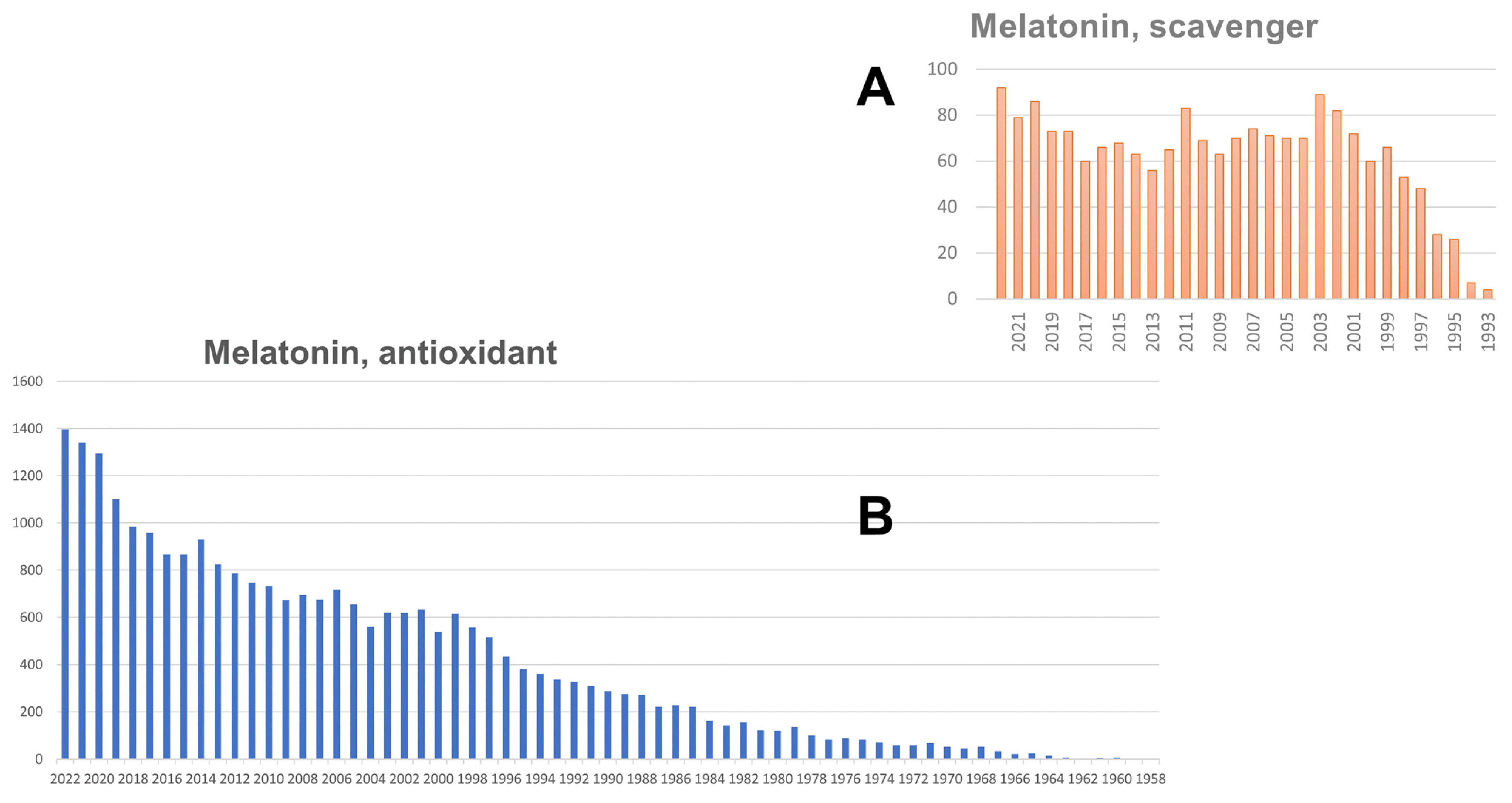
7.1. Melatonin as an Antioxidant
7.1.1. Melatonin as a Scavenger
7.1.2. If Not a Scavenger, Then What?
7.2. Melatonin Nuclear Receptor
7.3. Melatonin in Bacteria and Mitochondria
7.3.1. Melatonin in Bacteria
- In an article by Byeon and Back on the cloning of E. coli using the necessary enzymatic machinery for this bacterium to produce melatonin, the only mention of two bacterial melatonin productions is in the following sentence: “Melatonin is predicted to have evolved from the precursor bacteria of mitochondria and chloroplasts, such as Rhodospirillum rubrum and Cyanobacteria, respectively, via an endosymbiotic event with their ancestral eukaryotic host” [95]. This is not evidence of these bacteria producing melatonin.
- Manchester et al. reported a melatonin immunoreactivity in this Rhodospirillum rubrum [96]; however, this observation has never been reported independently, nor has melatonin been directly measured in the bacterium. Given the capital importance of Rhodospirillum rubrum and other purple non-sulfur bacteria, predicted to be at the origin of mitochondria according to the endosymbiotic theory of mitochondria [100], it is surprising that no more effort has been spent to clarify this point.
- Jiao et al. [97] reported a thorough study on eight strains of bacteria and found melatonin in only four: Agrobacterium tumefaciens, Bacillus amyloliquefaciens, Bacillus thuringiensis and barely in Pseudomonas sp.
- A review by Tan et al., while not providing any experimental data on bacteria melatonin production [98], stated that a series of Lactobacillus had been patented for their production of melatonin. Furthermore, they wrote: “If these speculations are valid, the beneficial effects in consumption of these products may, at least partially, be explained by the presence of melatonin and its isomers” [98]. This statement was later used as evidence that several Lactobacillus produce melatonin. For example, in a trial on abdominal pain in children, Lactobacillus and exogenous melatonin were used for the health of the patient [101], as if melatonin produced by Lactobacillus was not enough to obtain the desired effect. In another study, melatonin was shown to increase the amount of Lactobacillus previously decreased by sleep deprivation as if the production of melatonin by Lactobacillus was not enough to counterbalance the decrease in circulating melatonin [102]. Finally, in an earlier study on the possible relationship between several “antioxidant” molecules and their capacity to inhibit bacterial growth in the presence of mycotoxins, melatonin did not show any significant effect on the growth of Lactobacillus [103]; however, if this bacterium produces melatonin, those authors should have seen an effect of the addition of melatonin to the bacteria. In conclusion, the situation of Lactobacillus as a source of melatonin production remains, at the very least, unclear.
7.3.2. Melatonin in Mitochondria
7.3.3. Conclusions
7.4. Melatonin as a Co-Substrate of NQO2
8. Melatonin Has Many Effects and Targets
- (i)
- The concentration of melatonin needed for most of the abovementioned effects is high, e.g., in the range of 1µM and up to several mMs. This leads to two remarks: (1) whether these effects are specific for melatonin or would they also be observed in structurally related molecules; (2) related to this first point is the question of the molecular target(s) or mechanism(s) behind it. Most studies lack the appropriate experimental conditions to—or at least start to—address these questions appropriately. For the first point, the most obvious structurally related candidate class of molecules is indoles (see below, Section 9); however, other classes of chemical compounds should also be considered, such as primary amines, etc. The second remark on molecular targets and mechanisms is a crucial step toward a better understanding of the observed effects [158]. The most obvious experiments, in this respect, are the establishment of concentration (dose)–response curves to determine an EC50 value. Low EC50 values generally hint at specific molecular targets, whereas a high EC50 value hints at targets with lower specificity. The absence of EC50 values (no saturation) may hint at a general property, such as membrane fluidity or intactness. Unfortunately, many of the articles describing the effects of melatonin use only a single (often high) melatonin concentration/dose.
- (ii)
- The reported effects of melatonin tend to be over-interpreted. The effects are not only system-dependent but, on their own, do not necessarily allow for conclusions on the precise mechanism or targets involved. These ‘descriptive’ data should therefore be interpreted with caution, not only in terms of the molecular mechanism and specificity but also in terms of translatability into another cell type, tissue and its relevance for pathologies. Unfortunately, the often-used perspective phrase “… these findings open new avenues in therapeutics” should be used with more precaution, considering the high melatonin concentrations used and the fact that almost all experimental protocols use melatonin in a preventive paradigm instead of a treatment paradigm.
9. The Indole Hypothesis
10. What to do?
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lerner, A.B.; Case, J.D.; Takahashi, Y.; Lee, T.H.; Mori, W. Isolation of melatonin, the pineal gland factor that lightens melanocytes. J. Am. Chem. Soc. 1958, 80, 2587. [Google Scholar] [CrossRef]
- Kennaway, D.J. Measuring melatonin by immunoassay. J. Pineal Res. 2020, 69, e12657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luboshitzky, R.; Yanai, D.; Shen-Orr, Z.; Israeli, E.; Herer, P.; Lavie, P. Daily and seasonal variations in the concentration of melatonin in the human pineal gland. Brain Res. Bull. 1998, 47, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Kennaway, D.J. The Dim Light Melatonin Onset (DLMO) across ages, methodologies and sex and its relationship with Morningness/Eveningness. Sleep 2023, 46, zsad033. [Google Scholar] [CrossRef] [PubMed]
- Klein, D.C. Arylalkylamine N-acetyltransferase: “The Timezyme”. J. Biol. Chem. 2007, 282, 4233–4237. [Google Scholar] [CrossRef] [Green Version]
- Falcón, J.; Coon, S.L.; Besseau, L.; Cazaméa-Catalan, D.; Fuentès, M.; Magnanou, E.; Paulin, C.-H.; Boeuf, G.; Sauzet, S.; Jørgensen, E.H.; et al. Drastic neofunctionalization associated with evolution of the timezyme AANAT 500 Mya. Proc. Natl. Acad. Sci. USA 2014, 111, 314–319. [Google Scholar] [CrossRef] [Green Version]
- Cassone, V.M. Effects of melatonin on vertebrate circadian systems. Trends Neurosci. 1990, 13, 457–464. [Google Scholar] [CrossRef]
- Hastings, M.H.; Herbert, J.; Martensz, N.D.; Roberts, A.C. Annual reproductive rhythms in mammals: Mechanisms of light synchronization. Ann. N. Y. Acad. Sci. 1985, 453, 182–204. [Google Scholar] [CrossRef]
- Cecon, E.; Boutin, J.A.; Jockers, R. Molecular Characterization and Pharmacology of Melatonin receptors in Animals. Receptors 2023, 2, 127–147. [Google Scholar] [CrossRef]
- Dijk, D.J.; Cajochen, C. Melatonin and the circadian regulation of sleep initiation, consolidation, structure, and the sleep EEG. J. Biol. Rhythms 1997, 12, 627–635. [Google Scholar] [CrossRef]
- Pevet, P.; Challet, E.; Felder-Schmittbuhl, M.-P. Melatonin and the circadian system: Keys for health with a focus on sleep. Handb. Clin. Neurol. 2021, 179, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.-L.; Chang, X.-W.; Zheng, J.-W.; Shi, L.; Xiang, Y.-J.; Que, J.-Y.; Yuan, K.; Deng, J.-H.; Teng, T.; Li, Y.-Y.; et al. Efficacy and tolerability of pharmacological treatments for insomnia in adults: A systematic review and network meta-analysis. Sleep Med. Rev. 2023, 68, 101746. [Google Scholar] [CrossRef] [PubMed]
- Hickie, I.B.; Rogers, N.L. Novel melatonin-based therapies: Potential advances in the treatment of major depression. Lancet 2011, 378, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Kasper, S.; Corruble, E.; Hale, A.; Lemoine, P.; Montgomery, S.A.; Quera-Salva, M.-A. Antidepressant efficacy of agomelatine versus SSRI/SNRI: Results from a pooled analysis of head-to-head studies without a placebo control. Int. Clin. Psychopharmacol. 2013, 28, 12–19. [Google Scholar] [CrossRef]
- Cipriani, A.; Furukawa, T.A.; Salanti, G.; Chaimani, A.; Atkinson, L.Z.; Ogawa, Y.; Leucht, S.; Ruhe, H.G.; Turner, E.H.; Higgins, J.P.T.; et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: A systematic review and network meta-analysis. Lancet 2018, 391, 1357–1366. [Google Scholar] [CrossRef] [Green Version]
- Boutin, J.A.; Jockers, R. Melatonin controversies, an update. J. Pineal Res. 2021, 70, e12702. [Google Scholar] [CrossRef]
- Boutin, J.A. Quinone reductase 2 as a promising target of melatonin therapeutic actions. Expert Opin. Ther. Targets 2016, 20, 303–317. [Google Scholar] [CrossRef]
- Boutin, J.A. How Can Molecular Pharmacology Help Understand the Multiple Actions of Melatonin: 20 Years of Research and Trends. In Melatonin-Molecular Biology, Clinical and Pharmaceutical Approaches; Manuela Drăgoi, C., Crenguţa Nicolae, A., Eds.; IntechOpen: London, UK, 2018; ISBN 978-1-78984-504-4. [Google Scholar]
- Arendt, J. Melatonin: Countering Chaotic Time Cues. Front. Endocrinol. 2019, 10, 391. [Google Scholar] [CrossRef] [Green Version]
- Martín Giménez, V.M.; de Las Heras, N.; Lahera, V.; Tresguerres, J.A.F.; Reiter, R.J.; Manucha, W. Melatonin as an Anti-Aging Therapy for Age-Related Cardiovascular and Neurodegenerative Diseases. Front. Aging Neurosci. 2022, 14, 888292. [Google Scholar] [CrossRef]
- Bocheva, G.; Slominski, R.M.; Janjetovic, Z.; Kim, T.-K.; Böhm, M.; Steinbrink, K.; Reiter, R.J.; Kleszczyński, K.; Slominski, A.T. Protective Role of Melatonin and Its Metabolites in Skin Aging. Int. J. Mol. Sci. 2022, 23, 1238. [Google Scholar] [CrossRef]
- Shukla, M.; Govitrapong, P.; Boontem, P.; Reiter, R.J.; Satayavivad, J. Mechanisms of Melatonin in Alleviating Alzheimer’s Disease. Curr. Neuropharmacol. 2017, 15, 1010–1031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Repova, K.; Baka, T.; Krajcirovicova, K.; Stanko, P.; Aziriova, S.; Reiter, R.J.; Simko, F. Melatonin as a Potential Approach to Anxiety Treatment. Int. J. Mol. Sci. 2022, 23, 6187. [Google Scholar] [CrossRef] [PubMed]
- Ajoolabady, A.; Bi, Y.; McClements, D.J.; Lip, G.Y.H.; Des Richardson, R.; Reiter, R.J.; Klionsky, D.J.; Ren, J. Melatonin-based therapeutics for atherosclerotic lesions and beyond: Focusing on macrophage mitophagy. Pharmacol. Res. 2022, 176, 106072. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Liu, Y.; Li, P.; Wu, X.; Xia, Y.; Zhang, D.; Li, N.; Peng, Y.; Zhu, G.; Hardeland, R.; et al. Melatonin inhibits Gram-negative pathogens by targeting citrate synthase. Sci. China Life Sci. 2022, 65, 1430–1444. [Google Scholar] [CrossRef]
- Davoodvandi, A.; Nikfar, B.; Reiter, R.J.; Asemi, Z. Melatonin and cancer suppression: Insights into its effects on DNA methylation. Cell. Mol. Biol. Lett. 2022, 27, 73. [Google Scholar] [CrossRef]
- Cucielo, M.S.; Cesário, R.C.; Silveira, H.S.; Gaiotte, L.B.; Dos Santos, S.A.A.; de Campos Zuccari, D.A.P.; Seiva, F.R.F.; Reiter, R.J.; de Almeida Chuffa, L.G. Melatonin Reverses the Warburg-Type Metabolism and Reduces Mitochondrial Membrane Potential of Ovarian Cancer Cells Independent of MT1 Receptor Activation. Molecules 2022, 27, 4350. [Google Scholar] [CrossRef]
- Targhazeh, N.; Reiter, R.J.; Rahimi, M.; Qujeq, D.; Yousefi, T.; Shahavi, M.H.; Mir, S.M. Oncostatic activities of melatonin: Roles in cell cycle, apoptosis, and autophagy. Biochimie 2022, 202, 34–48. [Google Scholar] [CrossRef]
- Tobeiha, M.; Jafari, A.; Fadaei, S.; Mirazimi, S.M.A.; Dashti, F.; Amiri, A.; Khan, H.; Asemi, Z.; Reiter, R.J.; Hamblin, M.R.; et al. Evidence for the Benefits of Melatonin in Cardiovascular Disease. Front. Cardiovasc. Med. 2022, 9, 888319. [Google Scholar] [CrossRef]
- Zhai, X.; Wang, N.; Jiao, H.; Zhang, J.; Li, C.; Ren, W.; Reiter, R.J.; Su, S. Melatonin and other indoles show antiviral activities against swine coronaviruses in vitro at pharmacological concentrations. J. Pineal Res. 2021, 71, e12754. [Google Scholar] [CrossRef]
- Hajam, Y.A.; Rai, S.; Pandi-Perumal, S.R.; Brown, G.M.; Reiter, R.J.; Cardinali, D.P. Coadministration of Melatonin and Insulin Improves Diabetes-Induced Impairment of Rat Kidney Function. Neuroendocrinology 2022, 112, 807–822. [Google Scholar] [CrossRef]
- Rong, B.; Wu, Q.; Reiter, R.J.; Sun, C. The Mechanism of Oral Melatonin Ameliorates Intestinal and Adipose Lipid Dysmetabolism Through Reducing Escherichia Coli-Derived Lipopolysaccharide. Cell. Mol. Gastroenterol. Hepatol. 2021, 12, 1643–1667. [Google Scholar] [CrossRef] [PubMed]
- Luchetti, F.; Nasoni, M.G.; Burattini, S.; Mohammadi, A.; Pagliarini, M.; Canonico, B.; Ambrogini, P.; Balduini, W.; Reiter, R.J.; Carloni, S. Melatonin Attenuates Ischemic-like Cell Injury by Promoting Autophagosome Maturation via the Sirt1/FoxO1/Rab7 Axis in Hippocampal HT22 Cells and in Organotypic Cultures. Cells 2022, 11, 3701. [Google Scholar] [CrossRef] [PubMed]
- Luchetti, F.; Carloni, S.; Nasoni, M.G.; Reiter, R.J.; Balduini, W. Tunneling nanotubes and mesenchymal stem cells: New insights into the role of melatonin in neuronal recovery. J. Pineal Res. 2022, 73, e12800. [Google Scholar] [CrossRef]
- Loh, D.; Reiter, R.J. Melatonin: Regulation of Biomolecular Condensates in Neurodegenerative Disorders. Antioxidants 2021, 10, 1483. [Google Scholar] [CrossRef]
- Tamtaji, O.R.; Reiter, R.J.; Alipoor, R.; Dadgostar, E.; Kouchaki, E.; Asemi, Z. Melatonin and Parkinson Disease: Current Status and Future Perspectives for Molecular Mechanisms. Cell. Mol. Neurobiol. 2020, 40, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Sharma, R.; Tan, D.-X.; Neel, R.L.; Simko, F.; Manucha, W.; Rosales-Corral, S.; Cardinali, D.P. Melatonin use for SARS-CoV-2 infection: Time to diversify the treatment portfolio. J. Med. Virol. 2022, 94, 2928–2930. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Sharma, R.; Simko, F.; Dominguez-Rodriguez, A.; Tesarik, J.; Neel, R.L.; Slominski, A.T.; Kleszczynski, K.; Martin-Gimenez, V.M.; Manucha, W.; et al. Melatonin: Highlighting its use as a potential treatment for SARS-CoV-2 infection. Cell. Mol. Life Sci. 2022, 79, 143. [Google Scholar] [CrossRef]
- Andersen, L.P.H.; Gögenur, I.; Rosenberg, J.; Reiter, R.J. Pharmacokinetics of Melatonin: The Missing Link in Clinical Efficacy? Clin. Pharmacokinet. 2016, 55, 1027–1030. [Google Scholar] [CrossRef]
- Vakkuri, O.; Leppäluoto, J.; Kauppila, A. Oral administration and distribution of melatonin in human serum, saliva and urine. Life Sci. 1985, 37, 489–495. [Google Scholar] [CrossRef]
- Harpsøe, N.G.; Andersen, L.P.H.; Gögenur, I.; Rosenberg, J. Clinical pharmacokinetics of melatonin: A systematic review. Eur. J. Clin. Pharmacol. 2015, 71, 901–909. [Google Scholar] [CrossRef]
- Rolling, J.; Rabot, J.; Schroder, C.M. Melatonin Treatment for Pediatric Patients with Insomnia: Is There a Place for It? Nat. Sci. Sleep 2022, 14, 1927–1944. [Google Scholar] [CrossRef] [PubMed]
- Kennaway, D.J. What do we really know about the safety and efficacy of melatonin for sleep disorders? Curr. Med. Res. Opin. 2022, 38, 211–227. [Google Scholar] [CrossRef] [PubMed]
- Skrzelowski, M.; Brookhaus, A.; Shea, L.A.; Berlau, D.J. Melatonin Use in Pediatrics: Evaluating the Discrepancy in Evidence Based on Country and Regulations Regarding Production. J. Pediatr. Pharmacol. Ther. 2021, 26, 4–20. [Google Scholar] [CrossRef]
- Zisapel, N. Assessing the potential for drug interactions and long term safety of melatonin for the treatment of insomnia in children with autism spectrum disorder. Expert Rev. Clin. Pharmacol. 2022, 15, 175–185. [Google Scholar] [CrossRef]
- Kuehn, B.M. Climbing Melatonin Use for Insomnia Raises Safety Concerns. JAMA 2022, 328, 605–607. [Google Scholar] [CrossRef] [PubMed]
- Vine, T.; Brown, G.M.; Frey, B.N. Melatonin use during pregnancy and lactation: A scoping review of human studies. Braz. J. Psychiatry. 2022, 44, 342–348. [Google Scholar] [CrossRef]
- Seabra, M.L.; Bignotto, M.; Pinto, L.R.; Tufik, S. Randomized, double-blind clinical trial, controlled with placebo, of the toxicology of chronic melatonin treatment. J. Pineal Res. 2000, 29, 193–200. [Google Scholar] [CrossRef] [Green Version]
- ANSE. Available online: https://www.anses.fr/fr/system/files/NUT2016SA0209.pdf (accessed on 22 February 2023).
- NCI. Available online: www.nccih.nih.gov/health/melatonin-what-you-need-to-know (accessed on 21 March 2023).
- Bishop-Freeman, S.C.; Young, K.A.; Labay, L.M.; Beuhler, M.C.; Hudson, J.S. Melatonin Supplementation in Undetermined Pediatric Deaths. J. Anal. Toxicol. 2022, 46, 808–816. [Google Scholar] [CrossRef]
- Rishi, M.A.; Khosla, S.; Sullivan, S.S. Health advisory: Melatonin use in children. J. Clin. Sleep Med. 2023, 19, 415. [Google Scholar] [CrossRef]
- Del Fabbro, E.; Dev, R.; Hui, D.; Palmer, L.; Bruera, E. Effects of melatonin on appetite and other symptoms in patients with advanced cancer and cachexia: A double-blind placebo-controlled trial. J. Clin. Oncol. 2013, 31, 1271–1276. [Google Scholar] [CrossRef] [Green Version]
- Seely, D.; Wu, P.; Fritz, H.; Kennedy, D.A.; Tsui, T.; Seely, A.J.E.; Mills, E. Melatonin as adjuvant cancer care with and without chemotherapy: A systematic review and meta-analysis of randomized trials. Integr. Cancer Ther. 2012, 11, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Lund Rasmussen, C.; Klee Olsen, M.; Thit Johnsen, A.; Petersen, M.A.; Lindholm, H.; Andersen, L.; Villadsen, B.; Groenvold, M.; Pedersen, L. Effects of melatonin on physical fatigue and other symptoms in patients with advanced cancer receiving palliative care: A double-blind placebo-controlled crossover trial. Cancer 2015, 121, 3727–3736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skene, D.J.; Papagiannidou, E.; Hashemi, E.; Snelling, J.; Lewis, D.F.; Fernandez, M.; Ioannides, C. Contribution of CYP1A2 in the hepatic metabolism of melatonin: Studies with isolated microsomal preparations and liver slices. J. Pineal Res. 2001, 31, 333–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, X.; Chen, C.; Krausz, K.W.; Idle, J.R.; Gonzalez, F.J. A metabolomic perspective of melatonin metabolism in the mouse. Endocrinology 2008, 149, 1869–1879. [Google Scholar] [CrossRef] [Green Version]
- Ferry, G.; Ubeaud, C.; Lambert, P.-H.; Bertin, S.; Cogé, F.; Chomarat, P.; Delagrange, P.; Serkiz, B.; Bouchet, J.-P.; Truscott, R.J.W.; et al. Molecular evidence that melatonin is enzymatically oxidized in a different manner than tryptophan: Investigations with both indoleamine 2,3-dioxygenase and myeloperoxidase. Biochem. J. 2005, 388, 205–215. [Google Scholar] [CrossRef] [Green Version]
- Pond, S.M.; Tozer, T.N. First-pass elimination. Basic concepts and clinical consequences. Clin. Pharmacokinet. 1984, 9, 1–25. [Google Scholar] [CrossRef]
- Rowland, M. Influence of route of administration on drug availability. J. Pharm. Sci. 1972, 61, 70–74. [Google Scholar] [CrossRef]
- Domínguez-Rodríguez, A.; Abreu-González, P.; Báez-Ferrer, N.; Reiter, R.J.; Avanzas, P.; Hernández-Vaquero, D. Melatonin and Cardioprotection in Humans: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Cardiovasc. Med. 2021, 8, 635083. [Google Scholar] [CrossRef]
- Ekeloef, S.; Halladin, N.; Fonnes, S.; Jensen, S.E.; Zaremba, T.; Rosenberg, J.; Jonsson, G.; Aarøe, J.; Gasbjerg, L.S.; Rosenkilde, M.M.; et al. Effect of Intracoronary and Intravenous Melatonin on Myocardial Salvage Index in Patients with ST-Elevation Myocardial Infarction: A Randomized Placebo Controlled Trial. J. Cardiovasc. Transl. Res. 2017, 10, 470–479. [Google Scholar] [CrossRef]
- Dominguez-Rodriguez, A.; Abreu-Gonzalez, P.; de La Torre-Hernandez, J.M.; Consuegra-Sanchez, L.; Piccolo, R.; Gonzalez-Gonzalez, J.; Garcia-Camarero, T.; Del Mar Garcia-Saiz, M.; Aldea-Perona, A.; Reiter, R.J. Usefulness of Early Treatment with Melatonin to Reduce Infarct Size in Patients With ST-Segment Elevation Myocardial Infarction Receiving Percutaneous Coronary Intervention (From the Melatonin Adjunct in the Acute Myocardial Infarction Treated with Angioplasty Trial). Am. J. Cardiol. 2017, 120, 522–526. [Google Scholar] [CrossRef]
- Espezel, H.; Jan, J.E.; O’Donnell, M.E.; Milner, R. The Use of Melatonin to Treat Sleep-Wake-Rhythm Disorders in Children who are Visually Impaired. J. Vis. Impair. Blind. 1996, 90, 43–50. [Google Scholar] [CrossRef]
- Jan, J.E.; Espezel, H.; Appleton, R.E. The treatment of sleep disorders with melatonin. Dev. Med. Child Neurol. 1994, 36, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Jan, J.E.; O’Donnell, M.E. Use of melatonin in the treatment of paediatric sleep disorders. J. Pineal Res. 1996, 21, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Goldman, R.D.; Bongiorno, P.B.; Olcese, J.M.; Witt-Enderby, P.A.; Shatkin, J.P. Myths and Evidence Regarding Melatonin Supplementation for Occasional Sleeplessness in the Pediatric Population. Pediatr. Ann. 2021, 50, e391–e395. [Google Scholar] [CrossRef] [PubMed]
- Williams Buckley, A.; Hirtz, D.; Oskoui, M.; Armstrong, M.J.; Batra, A.; Bridgemohan, C.; Coury, D.; Dawson, G.; Donley, D.; Findling, R.L.; et al. Practice guideline: Treatment for insomnia and disrupted sleep behavior in children and adolescents with autism spectrum disorder: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology 2020, 94, 392–404. [Google Scholar] [CrossRef] [Green Version]
- Lelak, K.; Vohra, V.; Neuman, M.I.; Toce, M.S.; Sethuraman, U. Pediatric Melatonin Ingestions—United States, 2012–2021. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 725–729. [Google Scholar] [CrossRef]
- Poeggeler, B.; Reiter, R.J.; Tan, D.X.; Chen, L.D.; Manchester, L.C. Melatonin, hydroxyl radical-mediated oxidative damage, and aging: A hypothesis. J. Pineal Res. 1993, 14, 151–168. [Google Scholar] [CrossRef]
- Forman, H.J.; Davies, K.J.A.; Ursini, F. How do nutritional antioxidants really work: Nucleophilic tone and para-hormesis versus free radical scavenging in vivo. Free Radic. Biol. Med. 2014, 66, 24–35. [Google Scholar] [CrossRef] [Green Version]
- Forman, H.J.; Augusto, O.; Brigelius-Flohe, R.; Dennery, P.A.; Kalyanaraman, B.; Ischiropoulos, H.; Mann, G.E.; Radi, R.; Roberts, L.J.; Vina, J.; et al. Even free radicals should follow some rules: A guide to free radical research terminology and methodology. Free Radic. Biol. Med. 2015, 78, 233–235. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef]
- Nimse, S.B.; Pal, D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef] [Green Version]
- Treml, J.; Šmejkal, K. Flavonoids as Potent Scavengers of Hydroxyl Radicals. Compr. Rev. Food Sci. Food Saf. 2016, 15, 720–738. [Google Scholar] [CrossRef] [PubMed]
- Kostov, R.V.; Knatko, E.V.; McLaughlin, L.A.; Henderson, C.J.; Zheng, S.; Huang, J.T.-J.; Honda, T.; Dinkova-Kostova, A.T. Pharmacokinetics and pharmacodynamics of orally administered acetylenic tricyclic bis(cyanoenone), a highly potent Nrf2 activator with a reversible covalent mode of action. Biochem. Biophys. Res. Commun. 2015, 465, 402–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinkova-Kostova, A.T.; Talalay, P.; Sharkey, J.; Zhang, Y.; Holtzclaw, W.D.; Wang, X.J.; David, E.; Schiavoni, K.H.; Finlayson, S.; Mierke, D.F.; et al. An exceptionally potent inducer of cytoprotective enzymes: Elucidation of the structural features that determine inducer potency and reactivity with Keap1. J. Biol. Chem. 2010, 285, 33747–33755. [Google Scholar] [CrossRef] [Green Version]
- Couch, R.D.; Browning, R.G.; Honda, T.; Gribble, G.W.; Wright, D.L.; Sporn, M.B.; Anderson, A.C. Studies on the reactivity of CDDO, a promising new chemopreventive and chemotherapeutic agent: Implications for a molecular mechanism of action. Bioorg. Med. Chem. Lett. 2005, 15, 2215–2219. [Google Scholar] [CrossRef]
- Balogun, E.; Hoque, M.; Gong, P.; Killeen, E.; Green, C.J.; Foresti, R.; Alam, J.; Motterlini, R. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem. J. 2003, 371, 887–895. [Google Scholar] [CrossRef] [Green Version]
- Shahcheraghi, S.H.; Salemi, F.; Peirovi, N.; Ayatollahi, J.; Alam, W.; Khan, H.; Saso, L. Nrf2 Regulation by Curcumin: Molecular Aspects for Therapeutic Prospects. Molecules 2021, 27, 167. [Google Scholar] [CrossRef]
- Auf Dem Keller, U.; Kümin, A.; Braun, S.; Werner, S. Reactive oxygen species and their detoxification in healing skin wounds. J. Investig. Dermatol. Symp. Proc. 2006, 11, 106–111. [Google Scholar] [CrossRef] [Green Version]
- Amoroso, R.; Maccallini, C.; Bellezza, I. Activators of Nrf2 to Counteract Neurodegenerative Diseases. Antioxidants 2023, 12, 778. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, C.; Meng, C.-J.; Zhu, G.-Q.; Sun, X.-B.; Huo, L.; Zhang, J.; Liu, H.-X.; He, W.-C.; Shen, X.-M.; et al. Melatonin activates the Nrf2-ARE pathway when it protects against early brain injury in a subarachnoid hemorrhage model. J. Pineal Res. 2012, 53, 129–137. [Google Scholar] [CrossRef]
- Kryl’skii, E.D.; Popova, T.N.; Safonova, O.A.; Stolyarova, A.O.; Razuvaev, G.A.; de Carvalho, M.A.P. Transcriptional Regulation of Antioxidant Enzymes Activity and Modulation of Oxidative Stress by Melatonin in Rats Under Cerebral Ischemia/Reperfusion Conditions. Neuroscience 2019, 406, 653–666. [Google Scholar] [CrossRef]
- Kang, J.-W.; Lee, S.-M. Melatonin inhibits type 1 interferon signaling of toll-like receptor 4 via heme oxygenase-1 induction in hepatic ischemia/reperfusion. J. Pineal Res. 2012, 53, 67–76. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, Y.; Hou, M.; Liu, H.; Yang, H.; Chen, X.; Liu, T.; He, F.; Zhu, X. Melatonin Prevents Cartilage Degradation in Early-Stage Osteoarthritis Through Activation of miR-146a/NRF2/HO-1 Axis. J. Bone Miner. Res. 2022, 37, 1056–1072. [Google Scholar] [CrossRef] [PubMed]
- Becker-André, M.; Wiesenberg, I.; Schaeren-Wiemers, N.; André, E.; Missbach, M.; Saurat, J.H.; Carlberg, C. Pineal gland hormone melatonin binds and activates an orphan of the nuclear receptor superfamily. J. Biol. Chem. 1994, 269, 28531–28534. [Google Scholar] [CrossRef]
- Wiesenberg, I.; Missbach, M.; Kahlen, J.P.; Schräder, M.; Carlberg, C. Transcriptional activation of the nuclear receptor RZR alpha by the pineal gland hormone melatonin and identification of CGP 52608 as a synthetic ligand. Nucleic Acids Res. 1995, 23, 327–333. [Google Scholar] [CrossRef] [Green Version]
- Becker-André, M.; Wiesenberg, I.; Schaeren-Wiemers, N.; André, E.; Missbach, M.; Saurat, J.H.; Carlberg, C. Pineal gland hormone melatonin binds and activates an orphan of the nuclear receptor superfamily. J. Biol. Chem. 1997, 272, 16707. [Google Scholar] [CrossRef] [Green Version]
- Pierrefiche, G.; Topall, G.; Courboin, G.; Henriet, I.; Laborit, H. Antioxidant activity of melatonin in mice. Res. Commun. Chem. Pathol. Pharmacol. 1993, 80, 211–223. [Google Scholar] [PubMed]
- Reiter, R.J.; Rosales-Corral, S.; Tan, D.X.; Jou, M.J.; Galano, A.; Xu, B. Melatonin as a mitochondria-targeted antioxidant: One of evolution’s best ideas. Cell. Mol. Life Sci. 2017, 74, 3863–3881. [Google Scholar] [CrossRef] [PubMed]
- Que, Z.; Ma, T.; Shang, Y.; Ge, Q.; Zhang, Q.; Xu, P.; Zhang, J.; Francoise, U.; Liu, X.; Sun, X. Microorganisms: Producers of Melatonin in Fermented Foods and Beverages. J. Agric. Food Chem. 2020, 68, 4799–4811. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R.; Balzer, I.; Fuhrberg, B.; Behrmann, G. Melatonin in Unicellular Organisms and Plants1. In Melatonin: A Universal Photoperiodic Signal with Diverse Actions; Pang, S.F., Reiter, R.J., Tang, P.L., Eds.; S. Karger AG: Basel, Switzerland, 1996; pp. 1–6. ISBN 978-3-8055-6344-4. [Google Scholar]
- Hardeland, R.; Poeggeler, B. Non-vertebrate melatonin. J. Pineal Res. 2003, 34, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Byeon, Y.; Back, K. Melatonin production in Escherichia coli by dual expression of serotonin N-acetyltransferase and caffeic acid O-methyltransferase. Appl. Microbiol. Biotechnol. 2016, 100, 6683–6691. [Google Scholar] [CrossRef]
- Manchester, L.C.; Poeggeler, B.; Alvares, F.L.; Ogden, G.B.; Reiter, R.J. Melatonin immunoreactivity in the photosynthetic prokaryote Rhodospirillum rubrum: Implications for an ancient antioxidant system. Cell. Mol. Biol. Res. 1995, 41, 391–395. [Google Scholar] [PubMed]
- Jiao, J.; Ma, Y.; Chen, S.; Liu, C.; Song, Y.; Qin, Y.; Yuan, C.; Liu, Y. Melatonin-Producing Endophytic Bacteria from Grapevine Roots Promote the Abiotic Stress-Induced Production of Endogenous Melatonin in Their Hosts. Front. Plant Sci. 2016, 7, 1387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, D.-X.; Hardeland, R.; Manchester, L.C.; Rosales-Corral, S.; Coto-Montes, A.; Boga, J.A.; Reiter, R.J. Emergence of naturally occurring melatonin isomers and their proposed nomenclature. J. Pineal Res. 2012, 53, 113–121. [Google Scholar] [CrossRef]
- Tilden, A.R.; Becker, M.A.; Amma, L.L.; Arciniega, J.; McGaw, A.K. Melatonin production in an aerobic photosynthetic bacterium: An evolutionarily early association with darkness. J. Pineal Res. 1997, 22, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Martin, W.F.; Garg, S.; Zimorski, V. Endosymbiotic theories for eukaryote origin. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20140330. [Google Scholar] [CrossRef] [Green Version]
- Dipasquale, V.; Palermo, L.; Barbalace, A.; Tumminello, G.; Romano, C. Randomised controlled trial of melatonin for paediatric functional abdominal pain disorders. J. Paediatr. Child Health 2023, 59, 458–463. [Google Scholar] [CrossRef]
- Park, Y.S.; Kim, S.H.; Park, J.W.; Kho, Y.; Seok, P.R.; Shin, J.-H.; Choi, Y.J.; Jun, J.-H.; Jung, H.C.; Kim, E.K. Melatonin in the colon modulates intestinal microbiota in response to stress and sleep deprivation. Intest. Res. 2020, 18, 325–336. [Google Scholar] [CrossRef]
- Atroshi, F.; Rizzo, A.; Westermarck, T.; Ali-vehmas, T. Effects of tamoxifen, melatonin, coenzyme Q10, and L-carnitine supplementation on bacterial growth in the presence of mycotoxins. Pharmacol. Res. 1998, 38, 289–295. [Google Scholar] [CrossRef]
- Kennaway, D.J. Can we believe results obtained from plasma melatonin ELISA kits? Chronobiol. Int. 2021, 38, 616–619. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, Y.; Laborda, P.; Yang, Y.; Liu, F. Molecular Cloning and Characterization of a Serotonin N-Acetyltransferase Gene, xoSNAT3, from Xanthomonas oryzae pv. oryzae. Int. J. Environ. Res. Public Health 2023, 20, 1865. [Google Scholar] [CrossRef] [PubMed]
- Coon, S.L.; Klein, D.C. Evolution of arylalkylamine N-acetyltransferase: Emergence and divergence. Mol. Cell. Endocrinol. 2006, 252, 2–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferry, G.; Loynel, A.; Kucharczyk, N.; Bertin, S.; Rodriguez, M.; Delagrange, P.; Galizzi, J.P.; Jacoby, E.; Volland, J.P.; Lesieur, D.; et al. Substrate specificity and inhibition studies of human serotonin N-acetyltransferase. J. Biol. Chem. 2000, 275, 8794–8805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferry, G.; Ubeaud, C.; Dauly, C.; Mozo, J.; Guillard, S.; Berger, S.; Jimenez, S.; Scoul, C.; Leclerc, G.; Yous, S.; et al. Purification of the recombinant human serotonin N-acetyltransferase (EC 2.3.1.87): Further characterization of and comparison with AANAT from other species. Protein Expr. Purif. 2004, 38, 84–98. [Google Scholar] [CrossRef]
- Venegas, C.; García, J.A.; Escames, G.; Ortiz, F.; López, A.; Doerrier, C.; García-Corzo, L.; López, L.C.; Reiter, R.J.; Acuña-Castroviejo, D. Extrapineal melatonin: Analysis of its subcellular distribution and daily fluctuations. J. Pineal Res. 2012, 52, 217–227. [Google Scholar] [CrossRef]
- Xing, D.; Meng, Y.; Yuan, X.; Jin, S.; Song, X.; Zare, R.N.; Zhang, X. Capture of Hydroxyl Radicals by Hydronium Cations in Water Microdroplets. Angew. Chem. Int. Ed. Engl. 2022, 61, e202207587. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Gong, C.; Wang, J.; Xing, D.; Zhao, L.; Li, D.; Zhang, X. Unravelling Melatonin’s Varied Antioxidizing Protection of Membrane Lipids Determined by its Spatial Distribution. J. Phys. Chem. Lett. 2021, 12, 7387–7393. [Google Scholar] [CrossRef]
- Lee, H.Y.; Back, K. 2-Hydroxymelatonin Promotes Seed Germination by Increasing Reactive Oxygen Species Production and Gibberellin Synthesis in Arabidopsis thaliana. Antioxidants 2022, 11, 737. [Google Scholar] [CrossRef]
- Semak, I.; Korik, E.; Antonova, M.; Wortsman, J.; Slominski, A. Metabolism of melatonin by cytochrome P450s in rat liver mitochondria and microsomes. J. Pineal Res. 2008, 45, 515–523. [Google Scholar] [CrossRef] [Green Version]
- Pérez-González, A.; Galano, A.; Alvarez-Idaboy, J.R.; Tan, D.X.; Reiter, R.J. Radical-trapping and preventive antioxidant effects of 2-hydroxymelatonin and 4-hydroxymelatonin: Contributions to the melatonin protection against oxidative stress. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 2206–2217. [Google Scholar] [CrossRef]
- Szabò, I.; Zoratti, M. Membrane Transport|Potassium Channels in the Inner Membrane of Mitochondria in Various Organisms: From Unicellular Eukaryotes to Higher Plants and Mammals. In Encyclopedia of Biological Chemistry III; Elsevier: Amsterdam, The Netherlands, 2013; pp. 986–989. ISBN 9780128220405. [Google Scholar]
- Arendt, J.; Aulinas, A. Endotext: Physiology of the Pineal Gland and Melatonin; Endotext: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Legros, C.; Chesneau, D.; Boutin, J.A.; Barc, C.; Malpaux, B. Melatonin from cerebrospinal fluid but not from blood reaches sheep cerebral tissues under physiological conditions. J. Neuroendocrinol. 2014, 26, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Dickson, E.J.; Jung, S.-R.; Koh, D.-S.; Hille, B. High membrane permeability for melatonin. J. Gen. Physiol. 2016, 147, 63–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suofu, Y.; Li, W.; Jean-Alphonse, F.G.; Jia, J.; Khattar, N.K.; Li, J.; Baranov, S.V.; Leronni, D.; Mihalik, A.C.; He, Y.; et al. Dual role of mitochondria in producing melatonin and driving GPCR signaling to block cytochrome c release. Proc. Natl. Acad. Sci. USA 2017, 114, E7997–E8006. [Google Scholar] [CrossRef] [Green Version]
- Duncan, M.J.; Takahashi, J.S.; Dubocovich, M.L. 2-125Iiodomelatonin binding sites in hamster brain membranes: Pharmacological characteristics and regional distribution. Endocrinology 1988, 122, 1825–1833. [Google Scholar] [CrossRef]
- Dubocovich, M.L. Melatonin receptors: Are there multiple subtypes? Trends Pharmacol. Sci. 1995, 16, 50–56. [Google Scholar] [CrossRef]
- Molinari, E.J.; North, P.C.; Dubocovich, M.L. 2-125Iiodo-5-methoxycarbonylamino-N-acetyltryptamine: A selective radioligand for the characterization of melatonin ML2 binding sites. Eur. J. Pharmacol. 1996, 301, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Nosjean, O.; Ferro, M.; Coge, F.; Beauverger, P.; Henlin, J.M.; Lefoulon, F.; Fauchere, J.L.; Delagrange, P.; Canet, E.; Boutin, J.A. Identification of the melatonin-binding site MT3 as the quinone reductase 2. J. Biol. Chem. 2000, 275, 31311–31317. [Google Scholar] [CrossRef] [Green Version]
- Boutin, J.A.; Ferry, G. Is There Sufficient Evidence that the Melatonin Binding Site MT3 Is Quinone Reductase 2? J. Pharmacol. Exp. Ther. 2019, 368, 59–65. [Google Scholar] [CrossRef] [Green Version]
- Tan, D.-X.; Manchester, L.C.; Terron, M.P.; Flores, L.J.; Tamura, H.; Reiter, R.J. Melatonin as a naturally occurring co-substrate of quinone reductase-2, the putative MT3 melatonin membrane receptor: Hypothesis and significance. J. Pineal Res. 2007, 43, 317–320. [Google Scholar] [CrossRef]
- Boutin, J.A.; Marcheteau, E.; Hennig, P.; Moulharat, N.; Berger, S.; Delagrange, P.; Bouchet, J.-P.; Ferry, G. MT3/QR2 melatonin binding site does not use melatonin as a substrate or a co-substrate. J. Pineal Res. 2008, 45, 524–531. [Google Scholar] [CrossRef]
- Sinha, B.; Wu, Q.; Li, W.; Tu, Y.; Sirianni, A.C.; Chen, Y.; Jiang, J.; Zhang, X.; Chen, W.; Zhou, S.; et al. Protection of melatonin in experimental models of newborn hypoxic-ischemic brain injury through MT1 receptor. J. Pineal Res. 2018, 64, e12443. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Schutz, L.F.; Morrell, B.C.; Perego, M.C.; Spicer, L.J. Effect of melatonin on bovine theca cells in vitro. Reprod. Fertil. Dev. 2018, 30, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, L.; Li, Y.; Gao, J. Melatonin increases human cervical cancer HeLa cells apoptosis induced by cisplatin via inhibition of JNK/Parkin/mitophagy axis. In Vitro Cell. Dev. Biol. Anim. 2018, 54, 1–10. [Google Scholar] [CrossRef]
- Liu, Z.; Gan, L.; Zhang, T.; Ren, Q.; Sun, C. Melatonin alleviates adipose inflammation through elevating α-ketoglutarate and diverting adipose-derived exosomes to macrophages in mice. J. Pineal Res. 2018, 64, e12455. [Google Scholar] [CrossRef]
- Zhou, H.; Cheang, T.; Su, F.; Zheng, Y.; Chen, S.; Feng, J.; Pei, Z.; Chen, L. Melatonin inhibits rotenone-induced SH-SY5Y cell death via the downregulation of Dynamin-Related Protein 1 expression. Eur. J. Pharmacol. 2018, 819, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Casado, M.E.; Rusanova, I.; Aranda, P.; Fernández-Ortiz, M.; Sayed, R.K.A.; Fernández-Gil, B.I.; Hidalgo-Gutiérrez, A.; Escames, G.; López, L.C.; Acuña-Castroviejo, D. In Vivo Determination of Mitochondrial Respiration in 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine-Treated Zebrafish Reveals the Efficacy of Melatonin in Restoring Mitochondrial Normalcy. Zebrafish 2018, 15, 15–26. [Google Scholar] [CrossRef]
- Shen, Y.-Q.; Guerra-Librero, A.; Fernandez-Gil, B.I.; Florido, J.; García-López, S.; Martinez-Ruiz, L.; Mendivil-Perez, M.; Soto-Mercado, V.; Acuña-Castroviejo, D.; Ortega-Arellano, H.; et al. Combination of melatonin and rapamycin for head and neck cancer therapy: Suppression of AKT/mTOR pathway activation, and activation of mitophagy and apoptosis via mitochondrial function regulation. J. Pineal Res. 2018, 64, e12461. [Google Scholar] [CrossRef]
- Proietti, S.; Catizone, A.; Masiello, M.G.; Dinicola, S.; Fabrizi, G.; Minini, M.; Ricci, G.; Verna, R.; Reiter, R.J.; Cucina, A.; et al. Increase in motility and invasiveness of MCF7 cancer cells induced by nicotine is abolished by melatonin through inhibition of ERK phosphorylation. J. Pineal Res. 2018, 64, e12467. [Google Scholar] [CrossRef]
- Wang, M.-L.; Wei, C.-H.; Wang, W.-D.; Wang, J.-S.; Zhang, J.; Wang, J.-J. Melatonin attenuates lung ischaemia-reperfusion injury via inhibition of oxidative stress and inflammation. Interact. Cardiovasc. Thorac. Surg. 2018, 26, 761–767. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Lu, X.; Hu, Y.; Yang, B.; Tsui, C.-K.; Yu, S.; Lu, L.; Liang, X. Melatonin attenuated retinal neovascularization and neuroglial dysfunction by inhibition of HIF-1α-VEGF pathway in oxygen-induced retinopathy mice. J. Pineal Res. 2018, 64, e12473. [Google Scholar] [CrossRef]
- Tao, L.; Zhu, Y. Melatonin regulates CRE-dependent gene transcription underlying osteoblast proliferation by activating Src and PKA in parallel. Am. J. Transl. Res. 2018, 10, 86–100. [Google Scholar] [PubMed]
- Li, T.; Ni, L.; Zhao, Z.; Liu, X.; Lai, Z.; Di, X.; Xie, Z.; Song, X.; Wang, X.; Zhang, R.; et al. Melatonin attenuates smoking-induced hyperglycemia via preserving insulin secretion and hepatic glycogen synthesis in rats. J. Pineal Res. 2018, 64, e12475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Ji, H.; Wang, J.; Sun, Y.; Qian, Z.; Jiang, X.; Snutch, T.P.; Sun, Y.; Tao, J. Melatonin-mediated inhibition of Cav3.2 T-type Ca2+ channels induces sensory neuronal hypoexcitability through the novel protein kinase C-eta isoform. J. Pineal Res. 2018, 64, e12476. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Zhou, Q.; Niu, J.; Wang, Y.; Yan, Q.; Wu, C.; Qian, J.; Yang, H.; Zou, J. Melatonin Protects Intervertebral Disc from Degeneration by Improving Cell Survival and Function via Activation of the ERK1/2 Signaling Pathway. Oxid. Med. Cell. Longev. 2019, 2019, 5120275. [Google Scholar] [CrossRef]
- Li, H.-R.; Wang, C.; Sun, P.; Liu, D.-D.; Du, G.-Q.; Tian, J.-W. Melatonin attenuates doxorubicin-induced cardiotoxicity through preservation of YAP expression. J. Cell. Mol. Med. 2020, 24, 3634–3646. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, M.-J.; Lin, C.-W.; Su, S.-C.; Reiter, R.J.; Chen, A.W.-G.; Chen, M.-K.; Yang, S.-F. Effects of miR-34b/miR-892a Upregulation and Inhibition of ABCB1/ABCB4 on Melatonin-Induced Apoptosis in VCR-Resistant Oral Cancer Cells. Mol. Ther. Nucleic Acids 2020, 19, 877–889. [Google Scholar] [CrossRef]
- Lee, J.; Yoo, Y.-M.; Lee, Y.H.; Kim, C.H. Melatonin Induces Apoptotic Cell Death in 3T3-L1 Preadipocytes. Mol. Biol. 2020, 54, 233–243. [Google Scholar] [CrossRef]
- Lee, S.; Byun, J.-K.; Park, M.; Woo Kim, S.; Lee, S.; Kim, J.-G.; Lee, I.-K.; Choi, Y.-K.; Park, K.-G. Melatonin inhibits vascular smooth muscle cell proliferation and apoptosis through upregulation of Sestrin2. Exp. Ther. Med. 2020, 19, 3454–3460. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.-N.; Kong, L.-H.; Ding, P.; Liu, Y.; Fan, Z.-G.; Gao, E.-H.; Yang, J.; Yang, L.-F. Melatonin ameliorates pressure overload-induced cardiac hypertrophy by attenuating Atg5-dependent autophagy and activating the Akt/mTOR pathway. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165848. [Google Scholar] [CrossRef]
- Wu, J.; Yang, Y.; Gao, Y.; Wang, Z.; Ma, J. Melatonin Attenuates Anoxia/Reoxygenation Injury by Inhibiting Excessive Mitophagy through the MT2/SIRT3/FoxO3a Signaling Pathway in H9c2 Cells. Drug Des. Devel. Ther. 2020, 14, 2047–2060. [Google Scholar] [CrossRef]
- He, M.; Zhou, C.; Lu, Y.; Mao, L.; Xi, Y.; Mei, X.; Wang, X.; Zhang, L.; Yu, Z.; Zhou, Z. Melatonin Antagonizes Nickel-Induced Aerobic Glycolysis by Blocking ROS-Mediated HIF-1α/miR210/ISCU Axis Activation. Oxid. Med. Cell. Longev. 2020, 2020, 5406284. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Zhao, C.; Xiang, H.; Jia, G.; Zhong, R. Melatonin improves cryopreservation of ram sperm by inhibiting mitochondrial permeability transition pore opening. Reprod. Domest. Anim. 2020, 55, 1240–1249. [Google Scholar] [CrossRef] [PubMed]
- Bu, S.; Wang, Q.; Sun, J.; Li, X.; Gu, T.; Lai, D. Melatonin suppresses chronic restraint stress-mediated metastasis of epithelial ovarian cancer via NE/AKT/β-catenin/SLUG axis. Cell Death Dis. 2020, 11, 644. [Google Scholar] [CrossRef]
- Özşimşek, A.; Nazıroğlu, M. The involvement of TRPV4 on the hypoxia-induced oxidative neurotoxicity and apoptosis in a neuronal cell line: Protective role of melatonin. Neurotoxicology 2021, 87, 136–148. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Sang, X.; Wang, M.; Liu, Y.; Liu, J.; Wang, X.; Liu, P.; Cheng, H. Melatonin potentiates the cytotoxic effect of Neratinib in HER2+ breast cancer through promoting endocytosis and lysosomal degradation of HER2. Oncogene 2021, 40, 6273–6283. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Tan, Z.; Li, H.; Lin, M.; Jiang, Y.; Liang, L.; Ma, Q.; Gou, J.; Ning, L.; Li, X.; et al. Melatonin reduces proliferation and promotes apoptosis of bladder cancer cells by suppressing O-GlcNAcylation of cyclin-dependent-like kinase 5. J. Pineal Res. 2021, 71, e12765. [Google Scholar] [CrossRef]
- Qin, D.-Z.; Cai, H.; He, C.; Yang, D.-H.; Sun, J.; He, W.-L.; Li, B.-L.; Hua, J.-L.; Peng, S. Melatonin relieves heat-induced spermatocyte apoptosis in mouse testes by inhibition of ATF6 and PERK signaling pathways. Zool. Res. 2021, 42, 514–524. [Google Scholar] [CrossRef]
- Zhang, Y.; Cong, P.; Tong, C.; Jin, H.; Liu, Y.; Hou, M. Melatonin pretreatment alleviates blast-induced oxidative stress in the hypothalamic-pituitary-gonadal axis by activating the Nrf2/HO-1 signaling pathway. Life Sci. 2021, 280, 119722. [Google Scholar] [CrossRef]
- Wang, L.; He, C. Nrf2-mediated anti-inflammatory polarization of macrophages as therapeutic targets for osteoarthritis. Front. Immunol. 2022, 13, 967193. [Google Scholar] [CrossRef]
- Sadoughi, F.; Dana, P.M.; Asemi, Z.; Shafabakhash, R.; Mohammadi, S.; Heidar, Z.; Mirzamoradi, M.; Targhazeh, N.; Mirzaei, H. Molecular and cellular mechanisms of melatonin in breast cancer. Biochimie 2022, 202, 26–33. [Google Scholar] [CrossRef]
- Zhao, C.-N.; Wang, P.; Mao, Y.-M.; Dan, Y.-L.; Wu, Q.; Li, X.-M.; Wang, D.-G.; Davis, C.; Hu, W.; Pan, H.-F. Potential role of melatonin in autoimmune diseases. Cytokine Growth Factor Rev. 2019, 48, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Labani, N.; Cecon, E.; Jockers, R. Melatonin Target Proteins: Too Many or Not Enough? Front. Endocrinol. 2019, 10, 791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srinivasan, V.; Spence, D.W.; Trakht, I.; Pandi-Perumal, S.R.; Cardinali, D.P.; Maestroni, G.J. Immunomodulation by melatonin: Its significance for seasonally occurring diseases. Neuroimmunomodulation 2008, 15, 93–101. [Google Scholar] [CrossRef]
- Cecon, E.; Izabelle, C.; Le Poder, S.; Real, F.; Zhu, A.; Tu, L.; Ghigna, M.R.; Klonjkowski, B.; Bomsel, M.; Jockers, R.; et al. Therapeutic potential of melatonin and melatonergic drugs on K18-hACE2 mice infected with SARS-CoV-2. J. Pineal Res. 2022, 72, e12772. [Google Scholar] [CrossRef] [PubMed]
- Cecon, E.; Fernandois, D.; Renault, N.; Coelho, C.F.F.; Wenzel, J.; Bedart, C.; Izabelle, C.; Gallet, S.; Le Poder, S.; Klonjkowski, B.; et al. Melatonin drugs inhibit SARS-CoV-2 entry into the brain and virus-induced damage of cerebral small vessels. Cell. Mol. Life Sci. 2022, 79, 361. [Google Scholar] [CrossRef]
- Cecon, E.; Legros, C.; Boutin, J.A.; Jockers, R. Journal of pineal research guideline for authors: Defining and characterizing melatonin targets. J. Pineal Res. 2021, 70, e12712. [Google Scholar] [CrossRef]
- Hornedo-Ortega, R.; Da Costa, G.; Cerezo, A.B.; Troncoso, A.M.; Richard, T.; Garcia-Parrilla, M.C. In Vitro Effects of Serotonin, Melatonin, and Other Related Indole Compounds on Amyloid-β Kinetics and Neuroprotection. Mol. Nutr. Food Res. 2018, 62, 1700383. [Google Scholar] [CrossRef] [PubMed]
- Wölfler, A.; Abuja, P.M.; Schauenstein, K.; Liebmann, P.M. N-acetylserotonin is a better extra- and intracellular antioxidant than melatonin. FEBS Lett. 1999, 449, 206–210. [Google Scholar] [CrossRef] [Green Version]
- Lezoualc’h, F.; Sparapani, M.; Behl, C. N-acetyl-serotonin (normelatonin) and melatonin protect neurons against oxidative challenges and suppress the activity of the transcription factor NF-kappaB. J. Pineal Res. 1998, 24, 168–178. [Google Scholar] [CrossRef]
| Pathological Conditions | Title | Exp/Rev | Melatonin Concentration | |
|---|---|---|---|---|
| Aging | Melatonin as an Anti-Aging Therapy | [20] | Rev | |
| Aging | Protective Role of Melatonin and Its Metabolites in Skin Aging | [21] | Rev | |
| Alzheimer’s Disease | Mechanisms of Melatonin in Alleviating Alzheimer’s Disease. | [22] | Rev | |
| Anxiety | Melatonin as a Potential Approach to Anxiety Treatment. | [23] | Rev | |
| Atherosclerosis | Melatonin-based therapeutics for atherosclerotic lesions and beyond: | [24] | Rev | |
| Bacteria infection | Melatonin inhibits Gram-negative pathogens | [25] | Exp | 1 to 4 mM * |
| Cancer | Melatonin and cancer suppression: … | [26] | Rev | |
| Cancer | Melatonin Reverses the Warburg-Type Metabolism | [27] | Exp | 3.2 mM |
| Cancer | Oncostatic activities of melatonin: … | [28] | Rev | |
| Cardiovascular diseases | Evidence for the Benefits of Melatonin in Cardiovascular Disease | [29] | Rev | |
| Coronavirus infection | Melatonin and other indoles show antiviral activities | [30] | Exp | 3 mM ** |
| Diabetes | Coadministration of Melatonin and Insulin Improves Diabetes-Induced… | [31] | Exp | ~40 µM ** |
| Dislipidemia | The Mechanism of Oral Melatonin Ameliorates Intestinal and Adipose Lipid Dysmetabolis | [32] | Exp | 0.4 mg/mL *** |
| Ischemy (cellular) | Melatonin Attenuates Ischemic-like Cell Injury | [33] | Exp | 50 µM |
| Neurodegeneration | …New insights into the role of melatonin in neuronal recovery | [34] | Rev | |
| Neurodegeneration | Melatonin: Regulation of Biomolecular Condensates in Neurodegenerative Disorders | [35] | Rev | |
| Parkinson’s Disease | Melatonin and Parkinson Disease: Current Status… | [36] | Rev | |
| SARS infection | Melatonin use for SARS-CoV-2 infection: … | [37] | Rev | |
| SARS infection | Melatonin: highlighting its use as a potential treatment for SARS-CoV-2 infection | [38] | Rev |
| Abnormalities, Multiple | Colitis | Genetic Diseases, Inborn | Musculoskeletal Abnormalities | REM Sleep Parasomnias |
| Acute Graft versus Host Disease | Colitis, Ulcerative | Genetic Diseases, X-Linked | Musculoskeletal Diseases | Renal Insufficiency |
| Acute Kidney Injury | Collagen Diseases | Genital Neoplasms, Female | Musculoskeletal Pain | Renal Insufficiency, Chronic |
| Acute Lung Injury | Colonic Diseases | Glioma | Myalgia | Reperfusion Injury |
| Acute Respiratory Distress Syndrome | Colonic Diseases, Functional | Glucose Intolerance | Myocardial Infarction | Respiration Disorders |
| Adnexal Diseases | Communicable Diseases | Glucose Metabolism Disorders | Myocardial Ischemia | Respiratory Aspiration |
| Alcoholism | Confusion | Gonadal Disorders | Narcolepsy | Respiratory Distress Syndrome |
| Alcohol-Related Disorders | Congenital Abnormalities | Growth Disorders | Necrosis | Respiratory Distress Syndrome, Infant |
| Alzheimer’s Disease | Connective Tissue Diseases | Head and Neck Neoplasms | Necrotizing Enterocolitis | Respiratory Distress Syndrome, Newborn |
| Anaplastic Astrocytoma | Consciousness Disorders | Head Injuries, Closed | Neoplasm Metastasis | Respiratory Hypersensitivity |
| Anaplastic Ependymoma | Constriction, Pathologic | Headache | Neoplasms by Histologic Type | Respiratory Tract Diseases |
| Anaplastic Oligodendroglioma | Coronary Artery Disease | Headache Disorders | Neoplasms, Germ Cell and Embryonal | Respiratory Tract Infections |
| Aneurysm | Coronary Disease | Headache Disorders, Primary | Neoplasms, Glandular and Epithelial | Respiratory Tract Neoplasms |
| Angelman Syndrome | Coronaviridae Infections | Heart Diseases | Neoplasms, Nerve Tissue | Retinal Diseases |
| Anorexia | Coronavirus Infections | Heart Failure | Neoplastic Processes | Rheumatic Diseases |
| Anxiety Disorders | COVID-19 | Hemorrhage | Nervous System Neoplasms | RNA Virus Infections |
| Apnea | Cranial Nerve Diseases | Heredodegenerative Disorders, Nervous System | Neurobehavioral Manifestations | Schizophrenia |
| Arrhythmias, Cardiac | Craniocerebral Trauma | Hot Flashes | Neurocognitive Disorders | Schizophrenia Spectrum and Other Psychotic Disorders |
| Arterial Occlusive Diseases | Craniofacial Abnormalities | Hyperglycemia | Neurodegenerative Diseases | Sclerosis |
| Arteriosclerosis | Critical Illness | Hyperinsulinism | Neurodevelopmental Disorders | Seasonal Affective Disorder |
| Arthritis | Cysts | Hyperkinesis | Neuroectodermal Tumors | Seizures |
| Asphyxia | Death | Hyperlipidemias | Neuroectodermal Tumors, Primitive | Sensation Disorders |
| Asphyxia Neonatorum | Deglutition Disorders | Hyperlipoproteinemias | Neuroendocrine Tumors | Sepsis |
| Asthma | Delirium | Hyperphagia | Neuroepithelioma | Shock |
| Astrocytoma | Dementia | Hyperplasia | Neurologic Manifestations | Shock, Septic |
| Atrophy | Demyelinating Autoimmune Diseases, CNS | Hypersensitivity | Neuromuscular Diseases | Signs and Symptoms, Digestive |
| Attention Deficit and Disruptive Behavior Disorders | Demyelinating Diseases | Hypersensitivity, Immediate | Neurotoxicity Syndromes | Signs and Symptoms, Respiratory |
| Attention Deficit Disorder with Hyperactivity | Depression | Hypertension | Nevi and Melanomas | Skin Diseases |
| Autism Spectrum Disorder | Depression, Postpartum | Hyperthermia | Nevus | Skin Diseases, Eczematous |
| Autistic Disorder | Depressive Disorder | Hypothermia | Nevus, Pigmented | Skin Diseases, Genetic |
| Autoimmune Diseases | Depressive Disorder, Major | Hypoxia | Nidovirales Infections | Skin Manifestations |
| Autoimmune, Diseases of the Nervous System | Dermatitis | Hypoxia, Brain | Non-24-Hour Sleep-Wake Disorder | Sleep Apnea Syndromes |
| Autonomic Nervous System Diseases | Dermatitis, Atopic | Hypoxia-Ischemia, Brain | Nutrition Disorders | Sleep Apnea, Obstructive |
| Basal Ganglia Diseases | Developmental Disabilities | Immune System Diseases | Obesity | Sleep Deprivation |
| Behavioral Symptoms | Diabetes Complications | Infant, Newborn Diseases | Obstetric Labor Complications | Sleep Disorders, Circadian Rhythm |
| Bipolar and Related Disorders | Diabetes Mellitus | Infant, Premature, Diseases | Obstetric Labor, Premature | Sleep Disorders, Intrinsic |
| Bipolar Disorder | Diabetes Mellitus, Type 1 | Infarction | Occupational Diseases | Sleep Initiation and Maintenance Disorders |
| Blindness | Diabetes Mellitus, Type 2 | Infections | Oculo-cerebral Syndrome With Hypopigmentation | Sleep-Wake Disorders |
| Body Temperature Changes | Diabetic Angiopathies | Infertility | Oligodendroglioma | Sleepiness |
| Body Weight | Diabetic Retinopathy | Infertility, Female | Orthostatic Intolerance | Smith-Magenis Syndrome |
| Body Weight Changes | Digestive System Diseases | Inflammation | Osteoporosis | Spinal Cord Diseases |
| Bone Diseases | Digestive System Neoplasms | Inflammatory Bowel Diseases | Ovarian Cysts | Spinal Cord Injuries |
| Bone Diseases, Metabolic | Disease Attributes | Insulin Resistance | Ovarian Diseases | ST Elevation |
| Brain Concussion | Disease Progression | Intellectual Disability | Overnutrition | Myocardial Infarction |
| Brain Diseases | Disorders of Excessive Somnolence | Intestinal Diseases | Overweight | Stomatitis |
| Brain Diseases, Metabolic | Dyskinesias | Intracranial Hemorrhages | Pain | Stomatognathic Diseases |
| Brain Infarction | Dyslipidemias | Irritable Bowel Syndrome | Pain, Post-operative | Stomatognathic System |
| Brain Injuries | Dyspepsia | Ischemia | Paralysis | Abnormalities |
| Brain Injuries, Traumatic | Dyssomnias | Ischemic Stroke | Parasomnias | Stress Disorders, Traumatic |
| Brain Ischemia | Eczema | Jet Lag Syndrome | Parkinson’s Disease | Stress, Psychological |
| Brain Neoplasms | Emergence Delirium | Joint Diseases | Parkinsonian Disorders | Stroke |
| Breast Diseases | Emergencies | Kidney Diseases | Pathological Conditions, Anatomical | Substance-Related Disorders |
| Breast Neoplasms | Encephalomyelitis | Kidney Failure, Chronic | Pediatric Obesity | Syndrome |
| Bronchial Diseases | Endocrine System Diseases | Lens Diseases | Periodontal Diseases | Synovial Cyst |
| Burning Mouth Syndrome | Endometriosis | Lipid Metabolism Disorders | Periodontitis | Synucleinopathies |
| Burns | Enterocolitis | Liver Cirrhosis | Personality Disorders | Systemic Inflammatory Response Syndrome |
| Cachexia | Enterocolitis, Necrotizing | Liver Diseases | Photophobia | Tauopathies |
| Calcinosis | Ependymoma | Lower Urinary Tract Symptoms | Pneumonia | Thoracic Neoplasms |
| Calcium Metabolism Disorders | Epilepsy | Lung Diseases | Pneumonia, Viral | Tooth Diseases |
| Carcinoma | Esophageal Diseases | Lung Diseases, Obstructive | Poisoning | Toxemia |
| Carcinoma, Non-Small-Cell Lung | Esophageal Motility Disorders | Lung Injury | Polycystic Ovary Syndrome | Trauma, Nervous System |
| Cardiomyopathies | Esophageal Spasm, Diffuse | Lung Neoplasms | Post-operative Complications | Travel-Related Illness |
| Carotid Artery Diseases | Essential Hypertension | Mania | Precancerous Conditions | Tuberous Sclerosis |
| Carotid Stenosis | Eye Diseases | Maxillofacial Abnormalities | Prediabetic State | Tuberous Sclerosis Complex |
| Cataract | Familial Alzheimer Disease | Melanoma | Pregnancy Complications | Ulcer |
| Central Nervous System Diseases | Fatigue | Menopause | Premature Birth | Urogenital Neoplasms |
| Central Nervous System Infections | Fatigue Syndrome, Chronic | Menstruation Disturbances | Primary Dysautonomias | Urologic Diseases |
| Central Nervous System Neoplasms | Feeding and Eating Disorders | Mental Disorders | Problem Behavior | Urological Manifestations |
| Cerebral Infarction | Fetal Diseases | Metabolic Diseases | Prostatic Diseases | Uterine Cervical Diseases |
| Cerebral Palsy | Fetal Growth Retardation | Metabolic Syndrome | Pruritus | Uterine Cervical Neoplasms |
| Cerebrovascular Disorders | Fever | Migraine Disorders | Psychomotor Agitation | Uterine Diseases |
| Chemically-Induced Disorders | Fibrosis | Mood Disorders | Psychomotor Disorders | Uterine Neoplasms |
| Child Development Disorders, Pervasive | Flaviviridae Infections | Mouth Diseases | Psychotic Disorders | Vascular Diseases |
| Chromosome Disorders | Fractures, Bone | Movement Disorders | Puerperal Disorders | Vascular System Injuries |
| Chronic Graft Versus Host Disease | Frailty | Mucinoses | Quadriplegia | Virus Diseases |
| Chronic Pain | Ganglion Cysts | Mucositis | Quality of Life | Vision Disorders |
| Chronic Periodontitis | Gastroenteritis | Multiple Sclerosis | Radiation Injuries | Wasting Syndrome |
| Chronobiology Disorders | Gastroesophageal Reflux | Multiple Sclerosis, Relapsing-Remitting | Radiodermatitis | Weight Gain |
| Cognition Disorders | Gastrointestinal Diseases | Muscular Atrophy | Recurrence | Weight Loss |
| Cognitive Dysfunction | Gastrointestinal Neoplasms | Muscular Diseases | REM Sleep Behavior Disorder |
| Acanthosis Nigricans | Chromosome Deletion | Hepatitis | Marijuana Abuse | Prognathism |
| Acid-Base Imbalance | Chronic Disease | Hepatitis A | Melanosis | Prostatic Hyperplasia |
| Acidosis | Cluster Headache | Hepatitis C | MELAS Syndrome | Prostatic Neoplasms |
| Acquired Immunodeficiency Syndrome | Colic | Hepatitis C, Chronic | Memory Disorders | Psychophysiologic Disorders |
| ACTH-Secreting Pituitary Adenoma | Colonic Neoplasms | Hepatitis, Chronic | Mental Retardation, X-Linked | Rare Diseases |
| Acute Coronary Syndrome | Colorectal Neoplasms | Hepatitis, Viral, Human | Metabolism, Inborn Errors | Retinal Degeneration |
| Acute Mountain Sickness | Communication Disorders | Herpes Genitalis | Microvascular Angina | Retrognathia |
| Adamantinoma | Constipation | Herpes Simplex | Migraine with Aura | Rupture |
| Adrenal Gland Diseases | Contusions | Herpesviridae Infections | Migraine without Aura | Salivary Gland Diseases |
| Adrenal Insufficiency | Craniopharyngioma | Hip Fractures | Monosomy | Sarcopenia |
| Adrenocortical Hyperfunction | Crohn Disease | Hip Injuries | Mouth Neoplasms | Scoliosis |
| Aggression | Cushing Syndrome | HIV Infections | Mouth, Edentulous | Seizures, Febrile |
| Alcohol Drinking | Delayed Emergence from Anesthesia | Hodgkin Disease | Multiple Myeloma | Self-Injurious Behavior |
| Alternating Hemiplegia of Childhood | Dengue | Hodgkin Lymphoma | Multiple System Atrophy | Septo-Optic Dysplasia |
| Altitude Sickness | Dengue Fever | Huntington Disease | Myocardial Reperfusion Injury | Septo-optic Dysplasia Spectrum |
| Alveolar Bone Loss | Dentofacial Deformities | Hyperadrenalism | Myofascial Pain Syndromes | Severe Acute Respiratory Syndrome |
| Ameloblastoma | Depressive Disorder, Treatment-Resistant | Hyperandrogenism | Narcotic-Related Disorders | Sex Chromosome Disorders |
| Amino Acid Metabolism, Inborn Errors | Diabetes Insipidus | Hyperpigmentation | Neonatal Sepsis | Sexually Transmitted Diseases |
| Anaplastic Oligoastrocytoma | Diabetes Insipidus, Neurogenic | Hypertension, Portal | Neoplasms, Neuroepithelial | Sexually Transmitted Diseases, Viral |
| Aneuploidy | Diarrhea | Hypertension, Pregnancy-Induced | Neoplasms, Plasma Cell | Shy-Drager Syndrome |
| Anodontia | Diffuse Large B-Cell Lymphoma | Hypo-hidrotic Ectodermal Dysplasia | Neoplasms, Squamous Cell | Skin Abnormalities |
| Anorexia Nervosa | DNA Virus Infections | Hypokinesia | Nervous System Malformations | Skin Diseases, Infectious |
| Aortic Aneurysm | Drug-Resistant Epilepsy | Hypopituitarism | Neuromuscular Manifestations | Skin Diseases, Viral |
| Aortic Aneurysm, Abdominal | Drug-Related Side Effects and Adverse Reactions | Hypotension | Night Eating Syndrome | Somatoform Disorders |
| Aortic Diseases | Dysbiosis | Hypotension, Orthostatic | Nocturia | Speech Disorders |
| Aphasia | Dyskinesia, Drug-Induced | Hypothalamic Diseases | Nocturnal Enuresis | Spinal Curvatures |
| Arbovirus Infections | Dysmenorrhea | Hypothalamic Obesity | Obesity, Morbid | Spinal Diseases |
| Arthritis, Juvenile | Ear Diseases | Idiopathic Hypersomnia | Oligoastrocytoma | Squamous Cell Carcinoma of Head and Neck |
| Arthritis, Rheumatoid | Ectodermal Dysplasia | Immunoproliferative Disorders | Opioid-Related Disorders | Stillbirth |
| Atrial Fibrillation | Ectodermal Dysplasia 1, Anhidrotic | Inborn Amino Acid Metabolism Disorder | Optic Nerve Diseases | Stress Disorders, Traumatic, Acute |
| B-cell Lymphoma | Emaciation | Influenza, Human | Optic Nerve Hypoplasia | Substance Withdrawal Syndrome |
| Back Pain | Endotoxemia | Intestinal Neoplasms | Oral Cancer | Sunburn |
| Bacteremia | Enterovirus Infections | Intracranial Aneurysm | Oral Leukoplakia | Tachycardia |
| Barrett Esophagus | Enuresis | Intracranial Arterial Diseases | Oral Squamous Cell Carcinoma | Tachycardia, Sinus |
| Binge-Eating Disorder | Epilepsy, Generalized | Intracranial Hemorrhage, Traumatic | Osteoarthritis | Tardive Dyskinesia |
| Birth Weight | Epileptic Syndromes | Intraocular Melanoma | Osteoarthritis, Knee | Thoracic Injuries |
| Blood-Borne Infections | Erythema | Jaw Abnormalities | Osteoporosis, Postmenopausal | Tic Disorders |
| Bone Neoplasms | Eye Diseases, Hereditary | Jaw Diseases | Otorhinolaryngologic Diseases | Tics |
| Bone Resorption | Eye Neoplasms | Lacerations | Pancreatic Cancer | Tinnitus |
| Borderline Personality Disorder | Facies | Language Disorders | Pancreatic Neoplasms | Tobacco Use Disorder |
| Brain Damage, Chronic | Femoral Fractures | Leg Injuries | Pelvic Pain | Tooth Abnormalities |
| Brain Diseases, Metabolic, Inborn | Fetal Alcohol Spectrum Disorders | Lennox Gastaut Syndrome | Periodontal Atrophy | Tooth Loss |
| Bronchial Neoplasms | Fetal Death | Leukoencephalopathies | Phenylketonurias | Tooth, Impacted |
| Bronchitis | Fetal Membranes, Premature Rupture | Leukoplakia | Photosensitivity Disorders | Tourette Syndrome |
| Bulimia | Fibromyalgia | Leukoplakia, Oral | Picornaviridae Infections | Trauma and Stressor Related Disorders |
| Bulimia Nervosa | Flavivirus Infections | Lewy Body Disease | Pigmentation Disorders | Trigeminal Autonomic Cephalalgias |
| Burnout, Psychological | Flushing | Lightning Injuries | Pinealoma | Unconsciousness |
| Carcinogenesis | Fragile X Syndrome | Liver Failure | Pineocytoma | Urinary Incontinence |
| Carcinoma, Bronchogenic | Genital Neoplasms, Male | Low Back Pain | Pituitary ACTH Hypersecretion | Urination Disorders |
| Carcinoma, Squamous Cell | Headache Disorders, Secondary | Lymphatic Diseases | Pituitary Diseases | Uterine Hemorrhage |
| Cardiac Conduction System Disease | Hearing Disorders | Lymphoma | Post-Concussion Syndrome | Uveal Diseases |
| Caregiver Burden | Heat Stress Disorders | Lymphoma, B-Cell | Post-operative Cognitive Complications | Uveal Neoplasms |
| Central Diabetes Insipidus | Hematoma | Lymphoma, Large B-Cell, Diffuse | Postpartum Hemorrhage | Vector Borne Diseases |
| Cerebral Hemorrhage | Hematoma, Subdural | Lymphoma, Non-Hodgkin | Postural Orthostatic Tachycardia Syndrome | Viral Hemorrhagic Fever |
| Childhood Acute Lymphoblastic Leukemia | Hematoma, Subdural, Chronic | Lymphoproliferative Disorders | Prader-Willi Syndrome | Vision, Low |
| Chorea | Hemiplegia | Lymphosarcoma | Prehypertension | X-linked Hypo-hidrotic Ectodermal Dysplasia |
| Chorioamnionitis | Hemorrhagic Fevers, Viral | Macular Degeneration | Premenstrual Dysphoric Disorder | Xerostomia |
| Chromosome 17p Deletion | Hepatic Encephalopathy | Malocclusion, Angle Class III | Premenstrual Syndrome | |
| Chromosome Aberrations | Hepatic Insufficiency | Mandibular Diseases | Primary Orthostatic Hypotension |
| Conditions | Number Patients | Treatment | Duration (Days) (Years) | Observational | |
|---|---|---|---|---|---|
| Smith-Magenis syndrome | 8 | 1 | Mel measure | NCT02180451 | |
| Autism | 105 | 3 | Mel measure | NCT02878499 | |
| Cushing disease | 15 | 90 | Mel measure | NCT03343470 | |
| Breast Neoplasms | 27 | 270 | Mel measure | NCT04364347 | |
| Alzheimer’s Disease | 60 | * | Mel measure | NCT04522960 | |
| Sleep deprivation | 20 | 32 | Mel measure | NCT04868539 | |
| Frail Elderly | 300 | 3 | Mel measure | NCT05107947 | |
| Hypo-hidrotic Ectodermal Dysplasia | 80 | 10 | Mel measure | NCT05378932 | |
| Bipolar disorder | 80 | 2 | Mel measure | NCT05413486 | |
| Alzheimer’s Disease | 164 | 42 | Mel measure | NCT05543681 | |
| Sleep Disturbance | 60 | 3 | Mel measure | NCT05647148 | |
| Hypoxic-ischemic Encephalopathy | 70 | 0.5–5 mg | 14 | NCT02621944 | |
| Delirium | 190 | 4 mg | 28 | NCT03438526 | |
| Multiple Progressive Primary Sclerosis | 50 | 100 mg daily | 2 | NCT03540485 | |
| Alzheimer’s Disease | 230 | 5 mg daily | 231 | NCT03954899 | |
| Osteopenia | 40 | 5 mg daily | 1 | NCT04233112 | |
| Attention Deficit Hyperactivity Disorder | 80 | 3 mg daily | 0.5 | NCT04318067 | |
| Postoperative delirium | 790 | 4 mg daily | 10 | NCT04335968 | |
| Huntington’s Disease | 20 | 5 mg daily | 63 | NCT04421339 | |
| COVID-19 infection | 30 | 3 × 10 mg daily | 28 | NCT04474483 | |
| Diabetic Eye Problems | 36 | 3 mg daily | 14 | NCT04547439 | |
| Post-operative pain | 60 | 10 mg 3 days | 21 | NCT04791943 | |
| Osteoarthritis | 252 | 5 mg daily | 60 | NCT04795336 | |
| Traumatic Brain Injury | 110 | 3–5 mg daily | 30 | NCT04932096 | |
| Necrotizing Enterocolitis | 100 | 6 mg daily | 150 | NCT05033639 | |
| Emergence Agitation | 117 | 5 mg pre-op *** | 1 | NCT05223010 | |
| Ischemic Stroke | 80 | 3 mg daily | 14 | NCT05247125 | |
| Hypertension | 23 | 1 mg daily | 1 | NCT05257291 | |
| PD | 50 | 5 mg daily | 28 | NCT05307770 | |
| Epilepsy | 120 | 5 mg daily | 28 | NCT05439876 | |
| Post-menopause insomnia | 14 | 2 mg daily | 15 | NCT05440734 | |
| Chronic Fatigue Syndrome | 106 | 1 mg daily | 28 | NCT05454683 | |
| Uveal Melanoma | 100 | 20 mg daily | 5 | NCT05502900 | |
| Severe Preterm Fetal Growth Restriction | 336 | 3 × 10 mg daily ** | 126 | NCT05651347 | |
| Bacteria Type | Reference | Comments (See Text) |
|---|---|---|
| Cyanobacteria, | [95] | Comment A |
| Rhodospirillum rubrum | [95,96] | Comments A & B |
| Bacillus cereus CS-17 | [97] | Comment C |
| Ensifer sp. VA11, | [97] | Comment C |
| Pseudomonas sp. | [97] | Comment C |
| Variovorax sp. | [97] | Comment C |
| Agrobacterium tumefaciens | [97] | Comment C |
| Bacillus amyloliquefaciens | [97] | Comment C |
| Bacillus thuringiensis | [97] | Comment C |
| Sphingomonas sp. | [97] | Comment C |
| Bifidobacterium breve, | [98] | Comment D |
| Lactobacillus brevis, | [98] | Comment D |
| Lactobacillus casei, | [98] | Comment D |
| Bifidobacterium longum, | [98] | Comment D |
| Enterococcus faecalis TH10, | [98] | Comment D |
| Lactobacillus acidophilus, | [98] | Comment D |
| Lactobacillus bulgaricus, | [98] | Comment D |
| Lactobacillus fermentum, | [98] | Comment D |
| Lactobacillus helveticus | [98] | Comment D |
| Lactobacillus plantarum, | [98] | Comment D |
| Streptococcus thermophilus, | [98] | Comment D |
| Erythrobacter longus, | [99] | Comment E |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boutin, J.A.; Kennaway, D.J.; Jockers, R. Melatonin: Facts, Extrapolations and Clinical Trials. Biomolecules 2023, 13, 943. https://doi.org/10.3390/biom13060943
Boutin JA, Kennaway DJ, Jockers R. Melatonin: Facts, Extrapolations and Clinical Trials. Biomolecules. 2023; 13(6):943. https://doi.org/10.3390/biom13060943
Chicago/Turabian StyleBoutin, J. A., D. J. Kennaway, and R. Jockers. 2023. "Melatonin: Facts, Extrapolations and Clinical Trials" Biomolecules 13, no. 6: 943. https://doi.org/10.3390/biom13060943
APA StyleBoutin, J. A., Kennaway, D. J., & Jockers, R. (2023). Melatonin: Facts, Extrapolations and Clinical Trials. Biomolecules, 13(6), 943. https://doi.org/10.3390/biom13060943







