TRPV Channels in Osteoarthritis: A Comprehensive Review
Abstract
1. Introduction to Osteoarthritis
2. Etiology of Osteoarthritis
3. Signal Pathways and Ion Channels Related to Osteoarthritis
4. The TRPV Channel Family Overview
5. The Expression and Role of TRPV Channels in Osteoarthritis
5.1. TRPV1
5.2. TRPV2
5.3. TRPV3
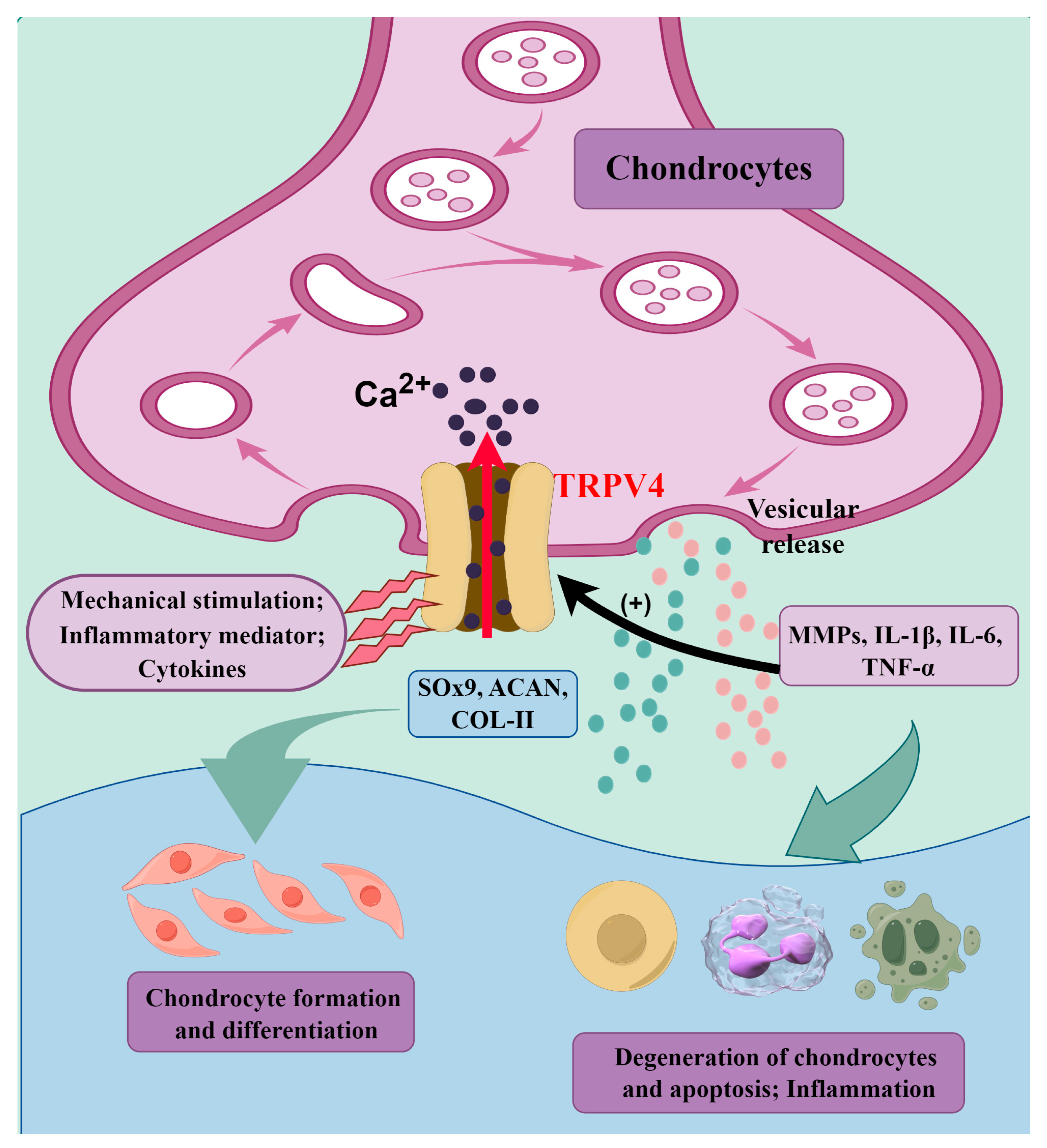
5.4. TRPV4
5.5. TRPV5
5.6. TRPV6
6. Potential Treatment Strategies of TRPV Channels in Osteoarthritis
7. Future Research Directions for TRPV Channels in Osteoarthritis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef] [PubMed]
- Allen, K.D.; Thoma, L.M.; Golightly, Y.M. Epidemiology of osteoarthritis. Osteoarthr. Cartil. 2022, 30, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Shin, J.; Cho, Y.; Kim, H.S.; Gu, Y.R.; Kim, H.; You, K.T.; Chang, M.J.; Chang, C.B.; Kang, S.B.; et al. Stress-activated miR-204 governs senescent phenotypes of chondrocytes to promote osteoarthritis development. Sci. Transl. Med. 2019, 11, eaar6659. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Wu, X.; Tao, C.; Gong, W.; Chen, M.; Qu, M.; Zhong, Y.; He, T.; Chen, S.; Xiao, G. Osteoarthritis: Pathogenic signaling pathways and therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 56. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Ma, F.; Yi, D.; Yu, H.; Tong, L.; Chen, D. Molecular signaling in temporomandibular joint osteoarthritis. J. Orthop. Transl. 2022, 32, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Leung, Y.Y.; Thumboo, J. An overview of OA research in two urban APLAR populations. Int. J. Rheum. Dis. 2011, 14, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Alhambra, D.; Judge, A.; Javaid, M.K.; Cooper, C.; Diez-Perez, A.; Arden, N.K. Incidence and risk factors for clinically diagnosed knee, hip and hand osteoarthritis: Influences of age, gender and osteoarthritis affecting other joints. Ann. Rheum. Dis. 2014, 73, 1659–1664. [Google Scholar] [CrossRef]
- Quicke, J.G.; Conaghan, P.G.; Corp, N.; Peat, G. Osteoarthritis year in review 2021: Epidemiology & therapy. Osteoarthr. Cartil. 2022, 30, 196–206. [Google Scholar]
- Wallace, I.J.; Worthington, S.; Felson, D.T.; Jurmain, R.D.; Wren, K.T.; Maijanen, H.; Woods, R.J.; Lieberman, D.E. Knee osteoarthritis has doubled in prevalence since the mid-20th century. Proc. Natl. Acad. Sci. USA 2017, 114, 9332–9336. [Google Scholar] [CrossRef]
- Turkiewicz, A.; Petersson, I.F.; Björk, J.; Hawker, G.; Dahlberg, L.E.; Lohmander, L.S.; Englund, M. Current and future impact of osteoarthritis on health care: A population-based study with projections to year 2032. Osteoarthr. Cartil. 2014, 22, 1826–1832. [Google Scholar] [CrossRef]
- Liu, Q.; Niu, J.; Li, H.; Ke, Y.; Li, R.; Zhang, Y.; Lin, J. Knee Symptomatic Osteoarthritis, Walking Disability, NSAIDs Use and All-cause Mortality: Population-based Wuchuan Osteoarthritis Study. Sci. Rep. 2017, 7, 3309. [Google Scholar] [CrossRef] [PubMed]
- Lo, J.; Chan, L.; Flynn, S. A Systematic Review of the Incidence, Prevalence, Costs, and Activity and Work Limitations of Amputation, Osteoarthritis, Rheumatoid Arthritis, Back Pain, Multiple Sclerosis, Spinal Cord Injury, Stroke, and Traumatic Brain Injury in the United States: A 2019 Update. Arch. Phys. Med. Rehabil. 2021, 102, 115–131. [Google Scholar] [PubMed]
- Long, H.; Zeng, X.; Liu, Q.; Wang, H.; Vos, T.; Hou, Y.; Lin, C.; Qiu, Y.; Wang, K.; Xing, D.; et al. Burden of osteoarthritis in China, 1990-2017: Findings from the Global Burden of Disease Study 2017. Lancet Rheumatol. 2020, 2, e164–e172. [Google Scholar] [CrossRef] [PubMed]
- Silverwood, V.; Blagojevic-Bucknall, M.; Jinks, C.; Jordan, J.L.; Protheroe, J.; Jordan, K.P. Current evidence on risk factors for knee osteoarthritis in older adults: A systematic review and meta-analysis. Osteoarthr. Cartil. 2015, 23, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.-T.; Chen, J.; Meng, Z.-L.; Ge, W.-Y.; Bian, Y.-Y.; Cheng, S.-W.; Xing, C.-K.; Yao, J.-L.; Fu, J.; Peng, L. Research progress on osteoarthritis treatment mechanisms. Biomed. Pharmacother. 2017, 93, 1246–1252. [Google Scholar] [CrossRef] [PubMed]
- Little, C.B.; Hunter, D.J. Post-traumatic osteoarthritis: From mouse models to clinical trials. Nat. Rev. Rheumatol. 2013, 9, 485–497. [Google Scholar] [CrossRef]
- Charlesworth, J.; Fitzpatrick, J.; Perera, N.K.P.; Orchard, J. Osteoarthritis—A systematic review of long-term safety implications for osteoarthritis of the knee. BMC Musculoskelet. Disord. 2019, 20, 151. [Google Scholar] [CrossRef] [PubMed]
- Warner, S.C.; Valdes, A.M. The Genetics of Osteoarthritis: A Review. J. Funct. Morphol. Kinesiol. 2016, 1, 140–153. [Google Scholar] [CrossRef]
- Roos, E.M.; Lohmander, L.S. The Knee injury and Osteoarthritis Outcome Score (KOOS): From joint injury to osteoarthritis. Health Qual. Life Outcomes 2003, 1, 64. [Google Scholar] [CrossRef]
- Lieberthal, J.; Sambamurthy, N.; Scanzello, C.R. Inflammation in joint injury and post-traumatic osteoarthritis. Osteoarthr. Cartil. 2015, 23, 1825–1834. [Google Scholar] [CrossRef]
- O’brien, M.S.; Mcdougall, J.J. Age and frailty as risk factors for the development of osteoarthritis. Mech. Ageing Dev. 2019, 180, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Loeser, R.F.; Collins, J.A.; Diekman, B.O. Ageing and the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, B.R.; Katz, J.N.; Solomon, D.H.; Yelin, E.H.; Hunter, D.J.; Messier, S.P.; Suter, L.G.; Losina, E. Number of Persons with Symptomatic Knee Osteoarthritis in the US: Impact of Race and Ethnicity, Age, Sex, and Obesity. Arthritis Care Res. 2016, 68, 1743–1750. [Google Scholar] [CrossRef] [PubMed]
- Greene, M.A.; Loeser, R.F. Aging-related inflammation in osteoarthritis. Osteoarthr. Cartil. 2015, 23, 1966–1971. [Google Scholar] [CrossRef] [PubMed]
- Blanco, F.J.; Rego, I.; Ruiz-Romero, C. The role of mitochondria in osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 161–169. [Google Scholar] [CrossRef]
- Hui, W.; Young, D.A.; Rowan, A.D.; Xu, X.; Cawston, T.E.; Proctor, C.J. Oxidative changes and signalling pathways are pivotal in initiating age-related changes in articular cartilage. Ann. Rheum. Dis. 2016, 75, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Felson, D.T. Osteoarthritis as a disease of mechanics. Osteoarthr. Cartil. 2013, 21, 10–15. [Google Scholar] [CrossRef]
- Laitner, M.H.; Erickson, L.C.; Ortman, E. Understanding the Impact of Sex and Gender in Osteoarthritis: Assessing Research Gaps and Unmet Needs. J. Women’s Health 2021, 30, 634–641. [Google Scholar] [CrossRef]
- Astephen Wilson, J.L.; Kobsar, D. Osteoarthritis year in review 2020: Mechanics. Osteoarthr. Cartil. 2021, 29, 161–169. [Google Scholar] [CrossRef]
- Andersson, J.K.; Hagert, E.; Brittberg, M. Cartilage Injuries and Posttraumatic Osteoarthritis in the Wrist: A Review. Cartilage 2021, 13 (Suppl. 1), 156s–168s. [Google Scholar] [CrossRef]
- Peshkova, M.; Lychagin, A.; Lipina, M.; Di Matteo, B.; Anzillotti, G.; Ronzoni, F.; Kosheleva, N.; Shpichka, A.; Royuk, V.; Fomin, V.; et al. Gender-Related Aspects in Osteoarthritis Development and Progression: A Review. Int. J. Mol. Sci. 2022, 23, 2767. [Google Scholar] [CrossRef] [PubMed]
- Tschon, M.; Contartese, D.; Pagani, S.; Borsari, V.; Fini, M. Gender and Sex Are Key Determinants in Osteoarthritis Not Only Confounding Variables. A Systematic Review of Clinical Data. J. Clin. Med. 2021, 10, 3178. [Google Scholar] [CrossRef]
- Raud, B.; Gay, C.; Guiguet-Auclair, C.; Bonnin, A.; Gerbaud, L.; Pereira, B.; Duclos, M.; Boirie, Y.; Coudeyre, E. Level of obesity is directly associated with the clinical and functional consequences of knee osteoarthritis. Sci. Rep. 2020, 10, 3601. [Google Scholar] [CrossRef]
- Reyes, C.; Leyland, K.M.; Peat, G.; Cooper, C.; Arden, N.K.; Prieto-Alhambra, D. Association Between Overweight and Obesity and Risk of Clinically Diagnosed Knee, Hip, and Hand Osteoarthritis: A Population-Based Cohort Study. Arthritis Rheumatol. 2016, 68, 1869–1875. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Pu, Y.; Li, Z.H.; Liu, W.; Deng, Y.; Liang, R.; Zhang, X.M.; Zuo, H.D. Adiponectin, May Be a Potential Protective Factor for Obesity-Related Osteoarthritis. Diabetes Metab. Syndr. Obes. Targets Ther. 2022, 15, 1305–1319. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Zhang, L.; Yan, Z.; Li, Z.; Fu, M.; Xue, C.; Wang, J. A low proportion n-6/n-3 PUFA diet supplemented with Antarctic krill (Euphausia superba) oil protects against osteoarthritis by attenuating inflammation in ovariectomized mice. Food Funct. 2021, 12, 6766–6779. [Google Scholar] [CrossRef]
- Sekar, S.; Wu, X.; Friis, T.; Crawford, R.; Prasadam, I.; Xiao, Y. Saturated fatty acids promote chondrocyte matrix remodeling through reprogramming of autophagy pathways. Nutrition 2018, 54, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Antony, B.; Singh, A. Imaging Biochem. Markers Osteoarthr. 2021, 11, 1205. [Google Scholar]
- Aubourg, G.; Rice, S.J.; Bruce-Wootton, P.; Loughlin, J. Genetics of osteoarthritis. Osteoarthr. Cartil. 2022, 30, 636–649. [Google Scholar] [CrossRef]
- Boer, C.G.; Hatzikotoulas, K.; Southam, L.; Stefánsdóttir, L.; Zhang, Y.; Coutinho de Almeida, R.; Wu, T.T.; Zheng, J.; Hartley, A.; Teder-Laving, M.; et al. Deciphering osteoarthritis genetics across 826,690 individuals from 9 populations. Cell 2021, 184, 4784–4818.e17. [Google Scholar] [CrossRef]
- Young, D.A.; Barter, M.J.; Soul, J. Osteoarthritis year in review: Genetics, genomics, epigenetics. Osteoarthr. Cartil. 2022, 30, 216–225. [Google Scholar] [CrossRef]
- Ratneswaran, A.; Kapoor, M. Osteoarthritis year in review: Genetics, genomics, epigenetics. Osteoarthr. Cartil. 2021, 29, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Yu, H.; Huang, X.; Shen, J.; Xiao, G.; Chen, L.; Wang, H.; Xing, L.; Chen, D. Current understanding of osteoarthritis pathogenesis and relevant new approaches. Bone Res. 2022, 10, 60. [Google Scholar] [CrossRef] [PubMed]
- Nalesso, G.; Thomas, B.L.; Sherwood, J.C.; Yu, J.; Addimanda, O.; Eldridge, S.E.; Thorup, A.S.; Dale, L.; Schett, G.; Zwerina, J.; et al. WNT16 antagonises excessive canonical WNT activation and protects cartilage in osteoarthritis. Ann. Rheum. Dis. 2017, 76, 218–226. [Google Scholar] [CrossRef]
- Tong, W.; Zeng, Y.; Chow, D.H.K.; Yeung, W.; Xu, J.; Deng, Y.; Chen, S.; Zhao, H.; Zhang, X.; Ho, K.K.; et al. Wnt16 attenuates osteoarthritis progression through a PCP/JNK-mTORC1-PTHrP cascade. Ann. Rheum. Dis. 2019, 78, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Chen, M.; Zuscik, M.; Wu, Q.; Wang, Y.J.; Rosier, R.N.; O’Keefe, R.J.; Chen, D. Inhibition of beta-catenin signaling in articular chondrocytes results in articular cartilage destruction. Arthritis Rheum. 2008, 58, 2053–2064. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, W.; Sun, M.; Deng, Z.; Zeng, C.; Li, H.; Yang, T.; Li, L.; Luo, W.; Lei, G. The Expression of Osteopontin and Wnt5a in Articular Cartilage of Patients with Knee Osteoarthritis and Its Correlation with Disease Severity. BioMed Res. Int. 2016, 2016, 9561058. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, T.; Hamilton, J.L.; Chen, D. Wnt/β-catenin Signaling in Osteoarthritis and in Other Forms of Arthritis. Curr. Rheumatol. Rep. 2017, 19, 53. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, J.; Mirando, A.J.; Wang, C.; Zuscik, M.J.; O’Keefe, R.J.; Hilton, M.J. A dual role for NOTCH signaling in joint cartilage maintenance and osteoarthritis. Sci. Signal. 2015, 8, ra71. [Google Scholar] [CrossRef]
- Monteagudo, S.; Lories, R.J. A Notch in the joint that exacerbates osteoarthritis. Nat. Rev. Rheumatol. 2018, 14, 563–564. [Google Scholar] [CrossRef]
- Zanotti, S.; Yu, J.; Bridgewater, D.; Wolf, J.M.; Canalis, E. Mice harboring a Hajdu Cheney Syndrome mutation are sensitized to osteoarthritis. Bone 2018, 114, 198–205. [Google Scholar] [CrossRef]
- Ge, Y.; Zhou, S.; Li, Y.; Wang, Z.; Chen, S.; Xia, T.; Shen, J.; Teng, H.; Jiang, Q. Estrogen prevents articular cartilage destruction in a mouse model of AMPK deficiency via ERK-mTOR pathway. Ann. Transl. Med. 2019, 7, 336. [Google Scholar] [CrossRef]
- Mével, E.; Shutter, J.A.; Ding, X.; Mattingly, B.T.; Williams, J.N.; Li, Y.; Huls, A.; Kambrath, A.V.; Trippel, S.B.; Wagner, D.; et al. Systemic inhibition or global deletion of CaMKK2 protects against post-traumatic osteoarthritis. Osteoarthr. Cartil. 2022, 30, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Tang, C.L.; Huang, S.Q.; Zhao, D.D.; Luo, A.; Wu, M.J.; An, H.Y.; Tan, C.F.; Yang, Z.X.; Zhu, Z.W.; et al. Effect of Electroacupuncture on Synovial M 1/M 2 Macrophage Polarization in Rats with Acute Gouty Arthritis. Acupunct. Res. 2018, 43, 767–772. [Google Scholar]
- Caron, M.M.; Emans, P.J.; Surtel, D.A.; Cremers, A.; Voncken, J.W.; Welting, T.J.; van Rhijn, L.W. Activation of NF-κB/p65 facilitates early chondrogenic differentiation during endochondral ossification. PLoS ONE 2012, 7, e33467. [Google Scholar] [CrossRef] [PubMed]
- Nakatomi, C.; Nakatomi, M.; Matsubara, T.; Komori, T.; Doi-Inoue, T.; Ishimaru, N.; Weih, F.; Iwamoto, T.; Matsuda, M.; Kokabu, S.; et al. Constitutive activation of the alternative NF-κB pathway disturbs endochondral ossification. Bone 2019, 121, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Ulivi, V.; Giannoni, P.; Gentili, C.; Cancedda, R.; Descalzi, F. p38/NF-kB-dependent expression of COX-2 during differentiation and inflammatory response of chondrocytes. J. Cell. Biochem. 2008, 104, 1393–1406. [Google Scholar] [CrossRef]
- Chen, S.; Qin, L.; Wu, X.; Fu, X.; Lin, S.; Chen, D.; Xiao, G.; Shao, Z.; Cao, H. Moderate Fluid Shear Stress Regulates Heme Oxygenase-1 Expression to Promote Autophagy and ECM Homeostasis in the Nucleus Pulposus Cells. Front. Cell Dev. Biol. 2020, 8, 127. [Google Scholar] [CrossRef]
- Rigoglou, S.; Papavassiliou, A.G. The NF-κB signalling pathway in osteoarthritis. Int. J. Biochem. Cell Biol. 2013, 45, 2580–2584. [Google Scholar] [CrossRef]
- Li, Z.; Dai, A.; Yang, M.; Chen, S.; Deng, Z.; Li, L. p38MAPK Signaling Pathway in Osteoarthritis: Pathological and Therapeutic Aspects. J. Inflamm. Res. 2022, 15, 723–734. [Google Scholar] [CrossRef]
- Zhang, Y.; Pizzute, T.; Pei, M. A review of crosstalk between MAPK and Wnt signals and its impact on cartilage regeneration. Cell Tissue Res. 2014, 358, 633–649. [Google Scholar] [CrossRef]
- Gao, S.-C.; Yin, H.-B.; Liu, H.-X.; Sui, Y.-H. Research progress on MAPK signal pathway in the pathogenesis of osteoarthritis. Zhongguo Gu Shang 2014, 27, 441–444. [Google Scholar]
- Deng, Y.; Lu, J.; Li, W.; Wu, A.; Zhang, X.; Tong, W.; Ho, K.K.; Qin, L.; Song, H.; Mak, K.K. Reciprocal inhibition of YAP/TAZ and NF-κB regulates osteoarthritic cartilage degradation. Nat. Commun. 2018, 9, 4564. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Huang, X.; Karperien, M.; Post, J.N. The Regulatory Role of Signaling Crosstalk in Hypertrophy of MSCs and Human Articular Chondrocytes. Int. J. Mol. Sci. 2015, 16, 19225–19247. [Google Scholar] [CrossRef] [PubMed]
- Gamer, L.W.; Pregizer, S.; Gamer, J.; Feigenson, M.; Ionescu, A.; Li, Q.; Han, L.; Rosen, V. The Role of Bmp2 in the Maturation and Maintenance of the Murine Knee Joint. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2018, 33, 1708–1717. [Google Scholar] [CrossRef] [PubMed]
- Pal, B.; Endisha, H.; Zhang, Y.; Kapoor, M. mTOR: A potential therapeutic target in osteoarthritis? Drugs RD 2015, 15, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, H.; Zeng, C.; Yan, B.; Ouyang, J.; Liu, X.; Sun, Q.; Zhao, C.; Fang, H.; Pan, J.; et al. mTORC1 activation downregulates FGFR3 and PTH/PTHrP receptor in articular chondrocytes to initiate osteoarthritis. Osteoarthr. Cartil. 2017, 25, 952–963. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Kuang, L.; Chen, C.; Yang, J.; Zeng, W.N.; Li, T.; Chen, H.; Huang, S.; Fu, Z.; Li, J.; et al. miR-100-5p-abundant exosomes derived from infrapatellar fat pad MSCs protect articular cartilage and ameliorate gait abnormalities via inhibition of mTOR in osteoarthritis. Biomaterials 2019, 206, 87–100. [Google Scholar] [CrossRef]
- Kovács, B.; Vajda, E.; Nagy, E.E. Regulatory Effects and Interactions of the Wnt and OPG-RANKL-RANK Signaling at the Bone-Cartilage Interface in Osteoarthritis. Int. J. Mol. Sci. 2019, 20, 4653. [Google Scholar] [CrossRef]
- Muratovic, D.; Atkins, G.J.; Findlay, D.M. Is RANKL a potential molecular target in osteoarthritis? Osteoarthr. Cartil. 2023, S1063–S4584. [Google Scholar] [CrossRef]
- Pei, F.; Liu, J.; Zhang, L.; Pan, X.; Huang, W.; Cen, X.; Huang, S.; Jin, Y.; Zhao, Z. The functions of mechanosensitive ion channels in tooth and bone tissues. Cell. Signal. 2021, 78, 109877. [Google Scholar] [CrossRef] [PubMed]
- Cox, C.D.; Bavi, N.; Martinac, B. Biophysical Principles of Ion-Channel-Mediated Mechanosensory Transduction. Cell Rep. 2019, 29, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Benítez-Angeles, M.; Morales-Lázaro, S.L.; Juárez-González, E.; Rosenbaum, T. TRPV1: Structure, Endogenous Agonists, and Mechanisms. Int. J. Mol. Sci. 2020, 21, 3421. [Google Scholar] [CrossRef]
- Kim, J.H. The Emerging Role of TRPV1 in Airway Inflammation. Allergy Asthma Immunol. Res. 2018, 10, 187–188. [Google Scholar] [CrossRef] [PubMed]
- Sasase, T.; Fatchiyah, F.; Ohta, T. Transient receptor potential vanilloid (TRPV) channels: Basal properties and physiological potential. Gen. Physiol. Biophys. 2022, 41, 165–190. [Google Scholar] [CrossRef]
- Nummenmaa, E.; Hämäläinen, M.; Moilanen, L.J.; Paukkeri, E.L.; Nieminen, R.M.; Moilanen, T.; Vuolteenaho, K.; Moilanen, E. Transient receptor potential ankyrin 1 (TRPA1) is functionally expressed in primary human osteoarthritic chondrocytes. Arthritis Res. Ther. 2016, 18, 185. [Google Scholar] [CrossRef] [PubMed]
- Hinata, M.; Imai, S.; Sanaki, T.; Tsuchida, J.; Yoshioka, T.; Higashino, K.; Yamamoto, M.; Imai, M.; Soga, M.; Horita, N.; et al. Sensitization of transient receptor potential vanilloid 4 and increasing its endogenous ligand 5,6-epoxyeicosatrienoic acid in rats with monoiodoacetate-induced osteoarthritis. Pain 2018, 159, 939–947. [Google Scholar] [CrossRef] [PubMed]
- Xing, R.; Wang, P.; Zhao, L.; Xu, B.; Zhang, N.; Li, X. Mechanism of TRPA1 and TRPV4 Participating in Mechanical Hyperalgesia of Rat Experimental Knee Osteoarthritis. Arch. Rheumatol. 2017, 32, 96–104. [Google Scholar] [CrossRef]
- Seebohm, G.; Schreiber, J.A. Beyond Hot and Spicy: TRPV Channels and their Pharmacological Modulation. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2021, 55, 108–130. [Google Scholar]
- Tomohiro, D.; Mizuta, K.; Fujita, T.; Nishikubo, Y.; Kumamoto, E. Inhibition by capsaicin and its related vanilloids of compound action potentials in frog sciatic nerves. Life Sci. 2013, 92, 368–378. [Google Scholar] [CrossRef]
- Malfait, A.-M.; Miller, R.J. Emerging Targets for the Management of Osteoarthritis Pain. Curr. Osteoporos. Rep. 2016, 14, 260–268. [Google Scholar] [CrossRef]
- Venkatachalam, K.; Montell, C. TRP channels. Annu. Rev. Biochem. 2007, 76, 387–417. [Google Scholar] [CrossRef]
- Phelps, C.B.; Huang, R.J.; Lishko, P.V.; Wang, R.R.; Gaudet, R. Structural analyses of the ankyrin repeat domain of TRPV6 and related TRPV ion channels. Biochemistry 2008, 47, 2476–2484. [Google Scholar] [CrossRef] [PubMed]
- Huynh, K.W.; Cohen, M.R.; Chakrapani, S.; Holdaway, H.A.; Stewart, P.L.; Moiseenkova-Bell, V.Y. Structural insight into the assembly of TRPV channels. Structure 2014, 22, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Satheesh, N.J.; Uehara, Y.; Fedotova, J.; Pohanka, M.; Büsselberg, D.; Kruzliak, P. TRPV currents and their role in the nociception and neuroplasticity. Neuropeptides 2016, 57, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hellwig, N.; Albrecht, N.; Harteneck, C.; Schultz, G.; Schaefer, M. Homo- and heteromeric assembly of TRPV channel subunits. J. Cell Sci. 2005, 118 Pt 5, 917–928. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Sun, C.; Li, H.; Tang, C.; Kan, H.; Yang, Z.; Mao, A.; Ma, X. TRPV4 (Transient Receptor Potential Vanilloid 4) Mediates Endothelium-Dependent Contractions in the Aortas of Hypertensive Mice. Hypertension 2018, 71, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Na, T.; Peng, J.B. TRPV5: A Ca(2+) channel for the fine-tuning of Ca(2+) reabsorption. Handb. Exp. Pharmacol. 2014, 222, 321–357. [Google Scholar]
- Szallasi, A.; Blumberg, P.M. Vanilloid (Capsaicin) receptors and mechanisms. Pharmacol. Rev. 1999, 51, 159–212. [Google Scholar]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 1997, 389, 816–824. [Google Scholar] [CrossRef]
- Bujak, J.K.; Kosmala, D.; Szopa, I.M.; Majchrzak, K.; Bednarczyk, P. Inflammation, Cancer and Immunity-Implication of TRPV1 Channel. Front. Oncol. 2019, 9, 1087. [Google Scholar] [CrossRef] [PubMed]
- Perálvarez-Marín, A.; Doñate-Macian, P.; Gaudet, R. What do we know about the transient receptor potential vanilloid 2 (TRPV2) ion channel? FEBS J. 2013, 280, 5471–5487. [Google Scholar] [CrossRef] [PubMed]
- Peier, A.M.; Reeve, A.J.; Andersson, D.A.; Moqrich, A.; Earley, T.J.; Hergarden, A.C.; Story, G.M.; Colley, S.; Hogenesch, J.B.; McIntyre, P.; et al. A heat-sensitive TRP channel expressed in keratinocytes. Science 2002, 296, 2046–2049. [Google Scholar] [CrossRef]
- Patapoutian, A.; Peier, A.M.; Story, G.M.; Viswanath, V. ThermoTRP channels and beyond: Mechanisms of temperature sensation. Nat. Rev. Neurosci. 2003, 4, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Morales-Lázaro, S.L.; Serrano-Flores, B.; Llorente, I.; Hernández-García, E.; González-Ramírez, R.; Banerjee, S.; Miller, D.; Gududuru, V.; Fells, J.; Norman, D.; et al. Structural determinants of the transient receptor potential 1 (TRPV1) channel activation by phospholipid analogs. J. Biol. Chem. 2014, 289, 24079–24090. [Google Scholar] [CrossRef] [PubMed]
- Plant, T.D. TRPs in mechanosensing and volume regulation. Handb. Exp. Pharmacol. 2014, 223, 743–766. [Google Scholar] [PubMed]
- Caterina, M.J.; Leffler, A.; Malmberg, A.B.; Martin, W.J.; Trafton, J.; Petersen-Zeitz, K.R.; Koltzenburg, M.; Basbaum, A.I.; Julius, D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 2000, 288, 306–313. [Google Scholar] [CrossRef]
- Cao, E.; Cordero-Morales, J.F.; Liu, B.; Qin, F.; Julius, D. TRPV1 channels are intrinsically heat sensitive and negatively regulated by phosphoinositide lipids. Neuron 2013, 77, 667–679. [Google Scholar] [CrossRef]
- Brito, R.; Sheth, S.; Mukherjea, D.; Rybak, L.P.; Ramkumar, V. TRPV1: A Potential Drug Target for Treating Various Diseases. Cells 2014, 3, 517–545. [Google Scholar] [CrossRef]
- Cavanaugh, D.J.; Chesler, A.T.; Jackson, A.C.; Sigal, Y.M.; Yamanaka, H.; Grant, R.; O’Donnell, D.; Nicoll, R.A.; Shah, N.M.; Julius, D.; et al. Trpv1 reporter mice reveal highly restricted brain distribution and functional expression in arteriolar smooth muscle cells. J. Neurosci. Off. J. Soc. Neurosci. 2011, 31, 5067–5077. [Google Scholar] [CrossRef]
- Kark, T.; Bagi, Z.; Lizanecz, E.; Pásztor, E.T.; Erdei, N.; Czikora, A.; Papp, Z.; Edes, I.; Pórszász, R.; Tóth, A. Tissue-specific regulation of microvascular diameter: Opposite functional roles of neuronal and smooth muscle located vanilloid receptor-1. Mol. Pharmacol. 2008, 73, 1405–1412. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.Y.; Gu, Q.; Lin, Y.S.; Lee, L.Y. Sensitivity of vagal afferent endings to chemical irritants in the rat lung. Respir. Physiol. 2001, 127, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Ristoiu, V.; Shibasaki, K.; Uchida, K.; Zhou, Y.; Ton, B.T.; Flonta, M.L.; Tominaga, M. Hypoxia-induced sensitization of transient receptor potential vanilloid 1 involves activation of hypoxia-inducible factor-1 alpha and PKC. Pain 2011, 152, 936–945. [Google Scholar] [CrossRef] [PubMed]
- Denda, M.; Fuziwara, S.; Inoue, K.; Denda, S.; Akamatsu, H.; Tomitaka, A.; Matsunaga, K. Immunoreactivity of VR1 on epidermal keratinocyte of human skin. Biochem. Biophys. Res. Commun. 2001, 285, 1250–1252. [Google Scholar] [CrossRef] [PubMed]
- Kanzaki, M.; Zhang, Y.Q.; Mashima, H.; Li, L.; Shibata, H.; Kojima, I. Translocation of a calcium-permeable cation channel induced by insulin-like growth factor-I. Nat. Cell Biol. 1999, 1, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.R.; Huynh, K.W.; Cawley, D.; Moiseenkova-Bell, V.Y. Understanding the cellular function of TRPV2 channel through generation of specific monoclonal antibodies. PLoS ONE 2013, 8, e85392. [Google Scholar] [CrossRef]
- Cohen, M.R.; Johnson, W.M.; Pilat, J.M.; Kiselar, J.; DeFrancesco-Lisowitz, A.; Zigmond, R.E.; Moiseenkova-Bell, V.Y. Nerve Growth Factor Regulates Transient Receptor Potential Vanilloid 2 via Extracellular Signal-Regulated Kinase Signaling To Enhance Neurite Outgrowth in Developing Neurons. Mol. Cell. Biol. 2015, 35, 4238–4252. [Google Scholar] [CrossRef]
- Liberati, S.; Morelli, M.B.; Amantini, C.; Santoni, M.; Nabissi, M.; Cardinali, C.; Santoni, G. Advances in transient receptor potential vanilloid-2 channel expression and function in tumor growth and progression. Curr. Protein Pept. Sci. 2014, 15, 732–737. [Google Scholar] [CrossRef]
- Kärki, T.; Tojkander, S. TRPV Protein Family-From Mechanosensing to Cancer Invasion. Biomolecules 2021, 11, 1019. [Google Scholar] [CrossRef]
- Shibasaki, K. Physiological significance of TRPV2 as a mechanosensor, thermosensor and lipid sensor. J. Physiol. Sci. JPS 2016, 66, 359–365. [Google Scholar] [CrossRef]
- Mercado, J.; Gordon-Shaag, A.; Zagotta, W.N.; Gordon, S.E. Ca2+-dependent desensitization of TRPV2 channels is mediated by hydrolysis of phosphatidylinositol 4,5-bisphosphate. J. Neurosci. Off. J. Soc. Neurosci. 2010, 30, 13338–13347. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Sobhan, U.; Tsumura, M.; Kuroda, H.; Soya, M.; Masamura, A.; Nishiyama, A.; Katakura, A.; Ichinohe, T.; Tazaki, M.; et al. Hypotonic-induced stretching of plasma membrane activates transient receptor potential vanilloid channels and sodium-calcium exchangers in mouse odontoblasts. J. Endod. 2013, 39, 779–787. [Google Scholar] [CrossRef]
- Aguettaz, E.; Bois, P.; Cognard, C.; Sebille, S. Stretch-activated TRPV2 channels: Role in mediating cardiopathies. Prog. Biophys. Mol. Biol. 2017, 130 Pt B, 273–280. [Google Scholar] [CrossRef]
- Naticchioni, M.; Karani, R.; Smith, M.A.; Onusko, E.; Robbins, N.; Jiang, M.; Radzyukevich, T.; Fulford, L.; Gao, X.; Apel, R.; et al. Transient Receptor Potential Vanilloid 2 Regulates Myocardial Response to Exercise. PLoS ONE 2015, 10, e0136901. [Google Scholar] [CrossRef] [PubMed]
- Koch, S.E.; Gao, X.; Haar, L.; Jiang, M.; Lasko, V.M.; Robbins, N.; Cai, W.; Brokamp, C.; Varma, P.; Tranter, M.; et al. Probenecid: Novel use as a non-injurious positive inotrope acting via cardiac TRPV2 stimulation. J. Mol. Cell. Cardiol. 2012, 53, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Iwata, Y.; Ito, S.; Wakabayashi, S.; Kitakaze, M. TRPV2 channel as a possible drug target for the treatment of heart failure. Lab. Investig. A J. Tech. Methods Pathol. 2020, 100, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Iwata, Y.; Katanosaka, Y.; Arai, Y.; Shigekawa, M.; Wakabayashi, S. Dominant-negative inhibition of Ca2+ influx via TRPV2 ameliorates muscular dystrophy in animal models. Hum. Mol. Genet. 2009, 18, 824–834. [Google Scholar] [CrossRef]
- Nagasawa, M.; Nakagawa, Y.; Tanaka, S.; Kojima, I. Chemotactic peptide fMetLeuPhe induces translocation of the TRPV2 channel in macrophages. J. Cell. Physiol. 2007, 210, 692–702. [Google Scholar] [CrossRef]
- Link, T.M.; Park, U.; Vonakis, B.M.; Raben, D.M.; Soloski, M.J.; Caterina, M.J. TRPV2 has a pivotal role in macrophage particle binding and phagocytosis. Nat. Immunol. 2010, 11, 232–239. [Google Scholar] [CrossRef]
- Santoni, G.; Amantini, C.; Maggi, F.; Marinelli, O.; Santoni, M.; Nabissi, M.; Morelli, M.B. The TRPV2 cation channels: From urothelial cancer invasiveness to glioblastoma multiforme interactome signature. Lab. Investig. A J. Tech. Methods Pathol. 2020, 100, 186–198. [Google Scholar] [CrossRef]
- Xu, H.; Ramsey, I.S.; Kotecha, S.A.; Moran, M.M.; Chong, J.A.; Lawson, D.; Ge, P.; Lilly, J.; Silos-Santiago, I.; Xie, Y.; et al. TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature 2002, 418, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Delling, M.; Jun, J.C.; Clapham, D.E. Oregano, thyme and clove-derived flavors and skin sensitizers activate specific TRP channels. Nat. Neurosci. 2006, 9, 628–635. [Google Scholar] [CrossRef]
- Yan, K.; Sun, X.; Wang, G.; Liu, Y.; Wang, K. Pharmacological Activation of Thermo-Transient Receptor Potential Vanilloid 3 Channels Inhibits Hair Growth by Inducing Cell Death of Hair Follicle Outer Root Sheath. J. Pharmacol. Exp. Ther. 2019, 370, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.D.; Gunthorpe, M.J.; Kelsell, R.E.; Hayes, P.D.; Reilly, P.; Facer, P.; Wright, J.E.; Jerman, J.C.; Walhin, J.P.; Ooi, L.; et al. TRPV3 is a temperature-sensitive vanilloid receptor-like protein. Nature 2002, 418, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Aijima, R.; Wang, B.; Takao, T.; Mihara, H.; Kashio, M.; Ohsaki, Y.; Zhang, J.Q.; Mizuno, A.; Suzuki, M.; Yamashita, Y.; et al. The thermosensitive TRPV3 channel contributes to rapid wound healing in oral epithelia. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2015, 29, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, T.; Petrus, M.J.; Dubin, A.E.; Patapoutian, A. TRPV3 regulates nitric oxide synthase-independent nitric oxide synthesis in the skin. Nat. Commun. 2011, 2, 369. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Jin, J.; Hu, L.; Shen, D.; Dong, X.P.; Samie, M.A.; Knoff, J.; Eisinger, B.; Liu, M.L.; Huang, S.M.; et al. TRP channel regulates EGFR signaling in hair morphogenesis and skin barrier formation. Cell 2010, 141, 331–343. [Google Scholar] [CrossRef]
- Asakawa, M.; Yoshioka, T.; Matsutani, T.; Hikita, I.; Suzuki, M.; Oshima, I.; Tsukahara, K.; Arimura, A.; Horikawa, T.; Hirasawa, T.; et al. Association of a mutation in TRPV3 with defective hair growth in rodents. J. Investig. Dermatol. 2006, 126, 2664–2672. [Google Scholar] [CrossRef]
- Zhong, W.; Hu, L.; Cao, X.; Zhao, J.; Zhang, X.; Lee, M.; Wang, H.; Zhang, J.; Chen, Q.; Feng, C.; et al. Genotype-Phenotype Correlation of TRPV3-Related Olmsted Syndrome. J. Investig. Dermatol. 2021, 141, 545–554. [Google Scholar] [CrossRef]
- Moqrich, A.; Hwang, S.W.; Earley, T.J.; Petrus, M.J.; Murray, A.N.; Spencer, K.S.; Andahazy, M.; Story, G.M.; Patapoutian, A. Impaired thermosensation in mice lacking TRPV3, a heat and camphor sensor in the skin. Science 2005, 307, 1468–1472. [Google Scholar] [CrossRef]
- Liu, N.; Wu, J.; Chen, Y.; Zhao, J. Channels that Cooperate with TRPV4 in the Brain. J. Mol. Neurosci. 2020, 70, 1812–1820. [Google Scholar] [CrossRef] [PubMed]
- Kassmann, M.; Harteneck, C.; Zhu, Z.; Nürnberg, B.; Tepel, M.; Gollasch, M. Transient receptor potential vanilloid 1 (TRPV1), TRPV4, and the kidney. Acta Physiol. 2013, 207, 546–564. [Google Scholar] [CrossRef]
- Filosa, J.A.; Yao, X.; Rath, G. TRPV4 and the regulation of vascular tone. J. Cardiovasc. Pharmacol. 2013, 61, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Liedtke, W.; Choe, Y.; Martí-Renom, M.A.; Bell, A.M.; Denis, C.S.; Sali, A.; Hudspeth, A.J.; Friedman, J.M.; Heller, S. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell 2000, 103, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, T.; Benítez-Angeles, M.; Sánchez-Hernández, R.; Morales-Lázaro, S.L.; Hiriart, M.; Morales-Buenrostro, L.E.; Torres-Quiroz, F. TRPV4: A Physio and Pathophysiologically Significant Ion Channel. Int. J. Mol. Sci. 2020, 21, 3837. [Google Scholar] [CrossRef] [PubMed]
- White, J.P.; Cibelli, M.; Urban, L.; Nilius, B.; McGeown, J.G.; Nagy, I. TRPV4: Molecular Conductor of a Diverse Orchestra. Physiol. Rev. 2016, 96, 911–973. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Guo, M.; Lv, X.; Wang, Z.; Yang, J.; Li, Y.; Yu, F.; Wen, X.; Feng, L.; Zhou, T. Role of Transient Receptor Potential Vanilloid 4 in Vascular Function. Front. Mol. Biosci. 2021, 8, 677661. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Mizusawa, M.; Sharmin, M.M.; Hayashi, S.; Yonekura, S. TRPV4 Increases the Expression of Tight Junction Protein-Encoding Genes via XBP1 in Mammary Epithelial Cells. Animals 2020, 10, 1174. [Google Scholar] [CrossRef]
- Liedtke, W.; Friedman, J.M. Abnormal osmotic regulation in trpv4-/- mice. Proc. Natl. Acad. Sci. USA 2003, 100, 13698–13703. [Google Scholar] [CrossRef]
- Corrigan, M.A.; Johnson, G.P.; Stavenschi, E.; Riffault, M.; Labour, M.N.; Hoey, D.A. TRPV4-mediates oscillatory fluid shear mechanotransduction in mesenchymal stem cells in part via the primary cilium. Sci. Rep. 2018, 8, 3824. [Google Scholar] [CrossRef]
- Denda, M.; Sokabe, T.; Fukumi-Tominaga, T.; Tominaga, M. Effects of skin surface temperature on epidermal permeability barrier homeostasis. J. Investig. Dermatol. 2007, 127, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Lu, P.; Luo, J.; Du, L.; Feng, J.; Cai, T.; Yuan, Y.; Cheng, H.; Hu, H. Transient stimulation of TRPV4-expressing keratinocytes promotes hair follicle regeneration in mice. Br. J. Pharmacol. 2020, 177, 4181–4192. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, Z.L.; Yeo, M.; Zhang, Q.J.; López-Romero, A.E.; Ding, H.P.; Zhang, X.; Zeng, Q.; Morales-Lázaro, S.L.; Moore, C.; et al. Epithelia-Sensory Neuron Cross Talk Underlies Cholestatic Itch Induced by Lysophosphatidylcholine. Gastroenterology 2021, 161, 301–317.e16. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Iida, T.; Mizuno, A.; Suzuki, M.; Caterina, M.J. Altered thermal selection behavior in mice lacking transient receptor potential vanilloid 4. J. Neurosci. Off. J. Soc. Neurosci. 2005, 25, 1304–1310. [Google Scholar] [CrossRef] [PubMed]
- Sonkusare, S.K.; Bonev, A.D.; Ledoux, J.; Liedtke, W.; Kotlikoff, M.I.; Heppner, T.J.; Hill-Eubanks, D.C.; Nelson, M.T. Elementary Ca2+ signals through endothelial TRPV4 channels regulate vascular function. Science 2012, 336, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Masuyama, R.; Vriens, J.; Voets, T.; Karashima, Y.; Owsianik, G.; Vennekens, R.; Lieben, L.; Torrekens, S.; Moermans, K.; Vanden Bosch, A.; et al. TRPV4-mediated calcium influx regulates terminal differentiation of osteoclasts. Cell Metab. 2008, 8, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Nilius, B.; Voets, T. The puzzle of TRPV4 channelopathies. EMBO Rep. 2013, 14, 152–163. [Google Scholar] [CrossRef]
- Peng, J.B.; Chen, X.Z.; Berger, U.V.; Vassilev, P.M.; Brown, E.M.; Hediger, M.A. A rat kidney-specific calcium transporter in the distal nephron. J. Biol. Chem. 2000, 275, 28186–28194. [Google Scholar] [CrossRef]
- Hoenderop, J.G.; Van Der Kemp, A.W.; Hartog, A.; van de Graaf, S.F.; van Os, C.H.; Willems, P.H.; Bindels, R.J. Molecular identification of the apical Ca2+ channel in 1, 25-dihydroxyvitamin D3-responsive epithelia. J. Biol. Chem. 1999, 274, 8375–8378. [Google Scholar] [CrossRef]
- Bernucci, L.; Henríquez, M.; Díaz, P.; Riquelme, G. Diverse calcium channel types are present in the human placental syncytiotrophoblast basal membrane. Placenta 2006, 27, 1082–1095. [Google Scholar] [CrossRef]
- Van Der Eerden, B.C.; Hoenderop, J.G.; De Vries, T.J.; Schoenmaker, T.; Buurman, C.J.; Uitterlinden, A.G.; Pols, H.A.; Bindels, R.J.; van Leeuwen, J.P. The epithelial Ca2+ channel TRPV5 is essential for proper osteoclastic bone resorption. Proc. Natl. Acad. Sci. USA 2005, 102, 17507–17512. [Google Scholar] [CrossRef] [PubMed]
- Hoenderop, J.G.; Van Leeuwen, J.P.; Van Der Eerden, B.C.; Kersten, F.F.; van der Kemp, A.W.; Mérillat, A.M.; Waarsing, J.H.; Rossier, B.C.; Vallon, V.; Hummler, E.; et al. Renal Ca2+ wasting, hyperabsorption, and reduced bone thickness in mice lacking TRPV5. J. Clin. Investig. 2003, 112, 1906–1914. [Google Scholar] [CrossRef] [PubMed]
- Renkema, K.Y.; Nijenhuis, T.; Van Der Eerden, B.C.; van der Kemp, A.W.; Weinans, H.; van Leeuwen, J.P.; Bindels, R.J.; Hoenderop, J.G. Hypervitaminosis D mediates compensatory Ca2+ hyperabsorption in TRPV5 knockout mice. J. Am. Soc. Nephrol. 2005, 16, 3188–3195. [Google Scholar] [CrossRef] [PubMed]
- Nijenhuis, T.; Van Der Eerden, B.C.; Hoenderop, J.G.; Weinans, H.; van Leeuwen, J.P.; Bindels, R.J. Bone resorption inhibitor alendronate normalizes the reduced bone thickness of TRPV5(-/-) mice. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2008, 23, 1815–1824. [Google Scholar] [CrossRef] [PubMed]
- Yelshanskaya, M.V.; Nadezhdin, K.D.; Kurnikova, M.G.; Sobolevsky, A.I. Structure and function of the calcium-selective TRP channel TRPV6. J. Physiol. 2021, 599, 2673–2697. [Google Scholar] [CrossRef] [PubMed]
- Bianco, S.D.; Peng, J.B.; Takanaga, H.; Suzuki, Y.; Crescenzi, A.; Kos, C.H.; Zhuang, L.; Freeman, M.R.; Gouveia, C.H.; Wu, J.; et al. Marked disturbance of calcium homeostasis in mice with targeted disruption of the Trpv6 calcium channel gene. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2007, 22, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Yao, D.; Huang, L.; Ke, J.; Zhang, M.; Xiao, Q.; Zhu, X. Bone metabolism regulation: Implications for the treatment of bone diseases. Biomed. Pharmacother. 2020, 129, 110494. [Google Scholar] [CrossRef]
- Srivastava, R.K.; Sapra, L.; Mishra, P.K. Osteometabolism: Metabolic Alterations in Bone Pathologies. Cells 2022, 11, 3943. [Google Scholar] [CrossRef]
- Gavva, N.R.; Treanor, J.J.; Garami, A.; Fang, L.; Surapaneni, S.; Akrami, A.; Alvarez, F.; Bak, A.; Darling, M.; Gore, A.; et al. Pharmacological blockade of the vanilloid receptor TRPV1 elicits marked hyperthermia in humans. Pain 2008, 136, 202–210. [Google Scholar] [CrossRef]
- Shibata, M.; Tang, C. Implications of Transient Receptor Potential Cation Channels in Migraine Pathophysiology. Neurosci. Bull. 2021, 37, 103–116. [Google Scholar] [CrossRef]
- Fischer, S.P.M.; Brusco, I.; Brum, E.S.; Fialho, M.F.P.; Camponogara, C.; Scussel, R.; Machado-de-Ávila, R.A.; Trevisan, G.; Oliveira, S.M. Involvement of TRPV1 and the efficacy of α-spinasterol on experimental fibromyalgia symptoms in mice. Neurochem. Int. 2020, 134, 104673. [Google Scholar] [CrossRef]
- Nahama, A.; Ramachandran, R.; Cisternas, A.F.; Ji, H. The role of afferent pulmonary innervation in ARDS associated with COVID-19 and potential use of resiniferatoxin to improve prognosis: A review. Med. Drug Discov. 2020, 5, 100033. [Google Scholar] [CrossRef] [PubMed]
- Caterina, M.J. Transient receptor potential ion channels as participants in thermosensation and thermoregulation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R64–R76. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Hu, H. Chapter Twelve—Thermally Activated TRPV3 Channels. In Current Topics in Membranes; Islas, L.D., Qin, F., Eds.; Academic Press: Cambridge, MA, USA, 2014; pp. 325–364. [Google Scholar]
- Yamada, T.; Ueda, T.; Ugawa, S.; Ishida, Y.; Imayasu, M.; Koyama, S.; Shimada, S. Functional expression of transient receptor potential vanilloid 3 (TRPV3) in corneal epithelial cells: Involvement in thermosensation and wound healing. Exp. Eye Res. 2010, 90, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Randhawa, P.K.; Jaggi, A.S. TRPV4 channels: Physiological and pathological role in cardiovascular system. Basic Res. Cardiol. 2015, 110, 54. [Google Scholar] [CrossRef] [PubMed]
- Itoh, Y.; Hatano, N.; Hayashi, H.; Onozaki, K.; Miyazawa, K.; Muraki, K. An environmental sensor, TRPV4 is a novel regulator of intracellular Ca2+ in human synoviocytes. Am. J. Physiol. Cell Physiol. 2009, 297, C1082–C1090. [Google Scholar] [CrossRef]
- Kang, S.S.; Shin, S.H.; Auh, C.K.; Chun, J. Human skeletal dysplasia caused by a constitutive activated transient receptor potential vanilloid 4 (TRPV4) cation channel mutation. Exp. Mol. Med. 2012, 44, 707–722. [Google Scholar] [CrossRef]
- Zhong, G.; Long, H.; Chen, F.; Yu, Y. Oxoglaucine mediates Ca2+ influx and activates autophagy to alleviate osteoarthritis through the TRPV5/calmodulin/CAMK-II pathway. Br. J. Pharmacol. 2021, 178, 2931–2947. [Google Scholar] [CrossRef]
- Lieben, L.; Benn, B.S.; Ajibade, D.; Stockmans, I.; Moermans, K.; Hediger, M.A.; Peng, J.B.; Christakos, S.; Bouillon, R.; Carmeliet, G. Trpv6 mediates intestinal calcium absorption during calcium restriction and contributes to bone homeostasis. Bone 2010, 47, 301–308. [Google Scholar] [CrossRef]
- Ho, K.W.; Ward, N.J.; Calkins, D.J. TRPV1: A stress response protein in the central nervous system. Am. J. Neurodegener. Dis. 2012, 1, 1–14. [Google Scholar]
- Cho, W.G.; Valtschanoff, J.G. Vanilloid receptor TRPV1-positive sensory afferents in the mouse ankle and knee joints. Brain Res 2008, 1219, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Yang, X.-Y.; Qian, F.-P.; Tang, M.; Ma, C.; Chiang, L.-Y. A novel analgesic approach to optogenetically and specifically inhibit pain transmission using TRPV1 promoter. Brain Res. 2015, 1609, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Korepanova, A.V.; Vos, M.H.; Moreland, R.B.; Chiu, M.L.; Faltynek, C.R. Quantification of TRPV1 protein levels in rat tissues to understand its physiological roles. J. Mol. Neurosci. 2013, 50, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Kochukov, M.Y.; Mcnearney, T.A.; Fu, Y.; Westlund, K.N. Thermosensitive TRP ion channels mediate cytosolic calcium response in human synoviocytes. Am. J. Physiol. Cell Physiol. 2006, 291, C424–C432. [Google Scholar] [CrossRef] [PubMed]
- Valdes, A.M.; De Wilde, G.; Doherty, S.A.; Lories, R.J.; Vaughn, F.L.; Laslett, L.L.; Maciewicz, R.A.; Soni, A.; Hart, D.J.; Zhang, W.; et al. The Ile585Val TRPV1 variant is involved in risk of painful knee osteoarthritis. Ann. Rheum. Dis. 2011, 70, 1556–1561. [Google Scholar] [CrossRef] [PubMed]
- El Karim, I.; Mccrudden, M.T.; Linden, G.J.; Abdullah, H.; Curtis, T.M.; McGahon, M.; About, I.; Irwin, C.; Lundy, F.T. TNF-α-induced p38MAPK activation regulates TRPA1 and TRPV4 activity in odontoblast-like cells. Am. J. Pathol. 2015, 185, 2994–3002. [Google Scholar] [CrossRef] [PubMed]
- Spahn, V.; Stein, C.; Zöllner, C. Modulation of transient receptor vanilloid 1 activity by transient receptor potential ankyrin 1. Mol. Pharmacol. 2014, 85, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Xiao, T.; Sun, M.; Kang, J.; Zhao, C. Transient Receptor Potential Vanilloid1 (TRPV1) Channel Opens Sesame of T Cell Responses and T Cell-Mediated Inflammatory Diseases. Front. Immunol. 2022, 13, 870952. [Google Scholar] [CrossRef]
- Majhi, R.K.; Sahoo, S.S.; Yadav, M.; Pratheek, B.M.; Chattopadhyay, S.; Goswami, C. Functional expression of TRPV channels in T cells and their implications in immune regulation. FEBS J. 2015, 282, 2661–2681. [Google Scholar] [CrossRef]
- Lv, Z.; Xu, X.; Sun, Z.; Yang, Y.X.; Guo, H.; Li, J.; Sun, K.; Wu, R.; Xu, J.; Jiang, Q.; et al. TRPV1 alleviates osteoarthritis by inhibiting M1 macrophage polarization via Ca(2+)/CaMKII/Nrf2 signaling pathway. Cell Death Dis. 2021, 12, 504. [Google Scholar] [CrossRef]
- Engler, A.; Aeschlimann, A.; Simmen, B.R.; Michel, B.A.; Gay, R.E.; Gay, S.; Sprott, H. Expression of transient receptor potential vanilloid 1 (TRPV1) in synovial fibroblasts from patients with osteoarthritis and rheumatoid arthritis. Biochem. Biophys. Res. Commun. 2007, 359, 884–888. [Google Scholar] [CrossRef] [PubMed]
- Dewaker, V.; Sharma, A.R.; Debnath, U.; Park, S.T.; Kim, H.S. Insights from molecular dynamics simulations of TRPV1 channel modulators in pain. Drug Discov. Today 2023, 28, 103798. [Google Scholar] [CrossRef] [PubMed]
- Gouin, O.; L’herondelle, K.; Lebonvallet, N.; Le Gall-Ianotto, C.; Sakka, M.; Buhé, V.; Plée-Gautier, E.; Carré, J.-L.; Lefeuvre, L.; Misery, L.; et al. TRPV1 and TRPA1 in cutaneous neurogenic and chronic inflammation: Pro-inflammatory response induced by their activation and their sensitization. Protein Cell 2017, 8, 644–661. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Matsuda, Y.; Sato, K.; de Jong, P.R.; Bertin, S.; Tabeta, K.; Yamazaki, K. Neuronal TRPV1 activation regulates alveolar bone resorption by suppressing osteoclastogenesis via CGRP. Sci. Rep. 2016, 6, 29294. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, H.; Katanosaka, Y.; Chijimatsu, R.; Mori, D.; Xuan, F.; Yano, F.; Omata, Y.; Maenohara, Y.; Murahashi, Y.; Kawaguchi, K.; et al. Involvement of Transient Receptor Potential Vanilloid Channel 2 in the Induction of Lubricin and Suppression of Ectopic Endochondral Ossification in Mouse Articular Cartilage. Arthritis Rheumatol. 2021, 73, 1441–1450. [Google Scholar] [CrossRef] [PubMed]
- Laragione, T.; Harris, C.; Gulko, P.S. TRPV2 suppresses Rac1 and RhoA activation and invasion in rheumatoid arthritis fibroblast-like synoviocytes. Int. Immunopharmacol. 2019, 70, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Somogyi, C.S.; Matta, C.; Foldvari, Z.; Juhász, T.; Katona, É.; Takács Á, R.; Hajdú, T.; Dobrosi, N.; Gergely, P.; Zákány, R. Polymodal Transient Receptor Potential Vanilloid (TRPV) Ion Channels in Chondrogenic Cells. Int. J. Mol. Sci. 2015, 16, 18412–18438. [Google Scholar] [CrossRef]
- Halonen, L.; Pemmari, A.; Nummenmaa, E.; Hämäläinen, M.; Moilanen, T.; Vuolteenaho, K.; Moilanen, E. Human Osteoarthritic Chondrocytes Express Nineteen Different TRP-Genes-TRPA1 and TRPM8 as Potential Drug Targets. Int. J. Mol. Sci. 2023, 24, 10057. [Google Scholar] [CrossRef]
- Yin, S.; Zhang, L.; Ding, L.; Huang, Z.; Xu, B.; Li, X.; Wang, P.; Mao, J. Transient receptor potential ankyrin 1 (trpa1) mediates il-1β-induced apoptosis in rat chondrocytes via calcium overload and mitochondrial dysfunction. J. Inflamm. 2018, 15, 27. [Google Scholar] [CrossRef]
- Willard, V.P.; Leddy, H.A.; Palmer, D.; Wu, C.L.; Liedtke, W.; Guilak, F. Transient receptor potential vanilloid 4 as a regulator of induced pluripotent stem cell chondrogenesis. Stem Cells 2021, 39, 1447–1456. [Google Scholar] [CrossRef]
- Muramatsu, S.; Wakabayashi, M.; Ohno, T.; Amano, K.; Ooishi, R.; Sugahara, T.; Shiojiri, S.; Tashiro, K.; Suzuki, Y.; Nishimura, R.; et al. Functional gene screening system identified TRPV4 as a regulator of chondrogenic differentiation. J. Biol. Chem. 2007, 282, 32158–32167. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.L.; Votta, B.J.; Kumar, S.; Liedtke, W.; Guilak, F. Chondroprotective role of the osmotically sensitive ion channel transient receptor potential vanilloid 4: Age-and sex-dependent progression of osteoarthritis in Trpv4-deficient mice. Arthritis Rheum. 2010, 62, 2973–2983. [Google Scholar] [CrossRef] [PubMed]
- Phan, M.N.; Leddy, H.A.; Votta, B.J.; Kumar, S.; Levy, D.S.; Lipshutz, D.B.; Lee, S.H.; Liedtke, W.; Guilak, F. Functional characterization of TRPV4 as an osmotically sensitive ion channel in porcine articular chondrocytes. Arthritis Rheum. 2009, 60, 3028–3037. [Google Scholar] [CrossRef] [PubMed]
- Masuyama, R.; Mizuno, A.; Komori, H.; Kajiya, H.; Uekawa, A.; Kitaura, H.; Okabe, K.; Ohyama, K.; Komori, T. Calcium/calmodulin-signaling supports TRPV4 activation in osteoclasts and regulates bone mass. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2012, 27, 1708–1721. [Google Scholar] [CrossRef] [PubMed]
- Pettenuzzo, S.; Arduino, A.; Belluzzi, E.; Pozzuoli, A.; Fontanella, C.G.; Ruggieri, P.; Salomoni, V.; Majorana, C.; Berardo, A. Biomechanics of Chondrocytes and Chondrons in Healthy Conditions and Osteoarthritis: A Review of the Mechanical Characterisations at the Microscale. Biomedicines 2023, 11, 1942. [Google Scholar] [CrossRef]
- Gao, W.; Hasan, H.; Anderson, D.E.; Lee, W. The Role of Mechanically-Activated Ion Channels Piezo1, Piezo2, and TRPV4 in Chondrocyte Mechanotransduction and Mechano-Therapeutics for Osteoarthritis. Front. Cell Dev. Biol. 2022, 10, 885224. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Meng, H.; Inamdar, S.; Das, B.; Gupta, H.; Wang, W.; Thompson, C.L.; Knight, M.M. Activation of TRPV4 by mechanical, osmotic or pharmaceutical stimulation is anti-inflammatory blocking IL-1β mediated articular cartilage matrix destruction. Osteoarthr. Cartil. 2021, 29, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Khatib, N.S.; Monsen, J.; Ahmed, S.; Huang, Y.; Hoey, D.A.; Nowlan, N.C. Mechanoregulatory role of TRPV4 in prenatal skeletal development. Sci. Adv. 2023, 9, eade2155. [Google Scholar] [CrossRef]
- Mizoguchi, F.; Mizuno, A.; Hayata, T.; Nakashima, K.; Heller, S.; Ushida, T.; Sokabe, M.; Miyasaka, N.; Suzuki, M.; Ezura, Y.; et al. Transient receptor potential vanilloid 4 deficiency suppresses unloading-induced bone loss. J. Cell. Physiol. 2008, 216, 47–53. [Google Scholar] [CrossRef]
- O’conor, C.J.; Leddy, H.A.; Benefield, H.C.; Liedtke, W.B.; Guilak, F. TRPV4-mediated mechanotransduction regulates the metabolic response of chondrocytes to dynamic loading. Proc. Natl. Acad. Sci. USA 2014, 111, 1316–1321. [Google Scholar] [CrossRef]
- Zhang, M.; Meng, N.; Wang, X.; Chen, W.; Zhang, Q. TRPV4 and PIEZO Channels Mediate the Mechanosensing of Chondrocytes to the Biomechanical Microenvironment. Membranes 2022, 12, 237. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Lin, C.; Lu, Y.; Guan, H.; Qi, W.; Zhang, H.; Shao, Y.; Zeng, C.; Zhang, R.; Zhang, H.; et al. FABP4 secreted by M1-polarized macrophages promotes synovitis and angiogenesis to exacerbate rheumatoid arthritis. Bone Res. 2022, 10, 45. [Google Scholar] [CrossRef]
- Sun, H.; Sun, Z.; Xu, X.; Lv, Z.; Li, J.; Wu, R.; Fei, Y.; Tan, G.; Liu, Z.; Liu, Y.; et al. Blocking TRPV4 Ameliorates Osteoarthritis by Inhibiting M1 Macrophage Polarization via the ROS/NLRP3 Signaling Pathway. Antioxidants 2022, 11, 2315. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wang, W.; Miao, J.; Bai, L. Expression and significance of transient receptor potential cation channel V5 in articular cartilage cells under exercise loads. Biomed. Rep. 2014, 2, 813–817. [Google Scholar] [CrossRef][Green Version]
- Chen, R.; Zhou, X.; Yin, S.; Lu, Z.; Nie, J.; Zhou, W.; Liu, X. Study on the protective mechanism of autophagy on cartilage by magnesium sulfate. Chin. J. Reparative Reconstr. Surg. 2018, 32, 1340–1345. [Google Scholar]
- Hdud, I.M.; El-Shafei, A.A.; Loughna, P.; Barrett-Jolley, R.; Mobasheri, A. Expression of Transient Receptor Potential Vanilloid (TRPV) channels in different passages of articular chondrocytes. Int. J. Mol. Sci. 2012, 13, 4433–4445. [Google Scholar] [CrossRef]
- Wei, Y.; Zheng, D.; Guo, X.; Zhao, M.; Gao, L.; Bai, L. Transient Receptor Potential Channel, Vanilloid 5, Induces Chondrocyte Apoptosis in a Rat Osteoarthritis Model through the Mediation of Ca2+ Influx. Cell. Physiol. Biochem. 2018, 46, 687–698. [Google Scholar] [CrossRef]
- Wei, Y.; Jin, Z.; Zhang, H.; Piao, S.; Lu, J.; Bai, L. The Transient Receptor Potential Channel, Vanilloid 5, Induces Chondrocyte Apoptosis via Ca2+ CaMKII-Dependent MAPK and Akt/ mTOR Pathways in a Rat Osteoarthritis Model. Cell. Physiol. Biochem. 2018, 51, 2309–2323. [Google Scholar] [CrossRef]
- Song, T.; Ma, J.; Guo, L.; Yang, P.; Zhou, X.; Ye, T. Regulation of chondrocyte functions by transient receptor potential cation channel V6 in osteoarthritis. J. Cell. Physiol. 2017, 232, 3170–3181. [Google Scholar] [CrossRef]
- Haywood, A.R.; Hathway, G.J.; Chapman, V. Differential contributions of peripheral and central mechanisms to pain in a rodent model of osteoarthritis. Sci. Rep. 2018, 8, 7122. [Google Scholar] [CrossRef]
- Rosenbaum, T.; Islas, L.D. Molecular Physiology of TRPV Channels: Controversies and Future Challenges. Annu. Rev. Physiol. 2023, 85, 293–316. [Google Scholar] [CrossRef] [PubMed]
- Yelshanskaya, M.V.; Sobolevsky, A.I. Ligand-Binding Sites in Vanilloid-Subtype TRP Channels. Front. Pharmacol. 2022, 13, 900623. [Google Scholar] [CrossRef] [PubMed]
- Logashina, Y.A.; Palikova, Y.A.; Palikov, V.A.; Kazakov, V.A.; Smolskaya, S.V.; Dyachenko, I.A.; Tarasova, N.V.; Andreev, Y.A. Anti-Inflammatory and Analgesic Effects of TRPV1 Polypeptide Modulator APHC3 in Models of Osteo- and Rheumatoid Arthritis. Mar. Drugs 2021, 19, 39. [Google Scholar] [CrossRef] [PubMed]
- Mlost, J.; Kostrzewa, M.; Malek, N.; Starowicz, K. Molecular Understanding of the Activation of CB1 and Blockade of TRPV1 Receptors: Implications for Novel Treatment Strategies in Osteoarthritis. Int. J. Mol. Sci. 2018, 19, 342. [Google Scholar] [CrossRef] [PubMed]
- Atobe, M.; Nagami, T.; Muramatsu, S.; Ohno, T.; Kitagawa, M.; Suzuki, H.; Ishiguro, M.; Watanabe, A.; Kawanishi, M. Discovery of Novel Transient Receptor Potential Vanilloid 4 (TRPV4) Agonists as Regulators of Chondrogenic Differentiation: Identification of Quinazolin-4(3 H)-ones and in Vivo Studies on a Surgically Induced Rat Model of Osteoarthritis. J. Med. Chem. 2019, 62, 1468–1483. [Google Scholar] [CrossRef]
- Xu, B.; Xing, R.; Huang, Z.; Yin, S.; Li, X.; Zhang, L.; Ding, L.; Wang, P. Excessive mechanical stress induces chondrocyte apoptosis through TRPV4 in an anterior cruciate ligament-transected rat osteoarthritis model. Life Sci. 2019, 228, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Brown, W.; Leff, R.L.; Griffin, A.; Hossack, S.; Aubray, R.; Walker, P.; Chiche, D.A. Safety, Pharmacokinetics, and Pharmacodynamics Study in Healthy Subjects of Oral NEO6860, a Modality Selective Transient Receptor Potential Vanilloid Subtype 1 Antagonist. J. Pain 2017, 18, 726–738. [Google Scholar] [CrossRef]
- Stevens, R.M.; Ervin, J.; Nezzer, J.; Nieves, Y.; Guedes, K.; Burges, R.; Hanson, P.D.; Campbell, J.N. Randomized, Double-Blind, Placebo-Controlled Trial of Intraarticular Trans-Capsaicin for Pain Associated With Osteoarthritis of the Knee. Arthritis Rheumatol. 2019, 71, 1524–1533. [Google Scholar] [CrossRef]
- Stevens, R.; Hanson, P.; Tiseo, P.; Guedes, K.; Campbell, J.; Connolly, J.; Ruggiero, S.; Corliss, M.; Smith, V.; Conaghan, P.G. Op0187 determining optimal cooling and administration methods for cntx-4975 intra-articular injection in subjects with moderate to severe osteoarthritis knee pain. Ann. Rheum. Dis. 2020, 79 (Suppl. 1), 116. [Google Scholar] [CrossRef]
- Othman, A.A.; Nothaft, W.; Awni, W.M.; Dutta, S. Effects of the TRPV1 antagonist ABT-102 on body temperature in healthy volunteers: Pharmacokinetic/ pharmacodynamic analysis of three phase 1 trials. Br. J. Clin. Pharmacol. 2013, 75, 1029–1040. [Google Scholar] [CrossRef]
- Othman, A.A.; Nothaft, W.; Awni, W.M.; Dutta, S. Pharmacokinetics of the TRPV1 antagonist ABT-102 in healthy human volunteers: Population analysis of data from 3 phase 1 trials. J. Clin. Pharmacol. 2012, 52, 1028–1041. [Google Scholar] [CrossRef] [PubMed]
- Manitpisitkul, P.; Flores, C.M.; Moyer, J.A.; Romano, G.; Shalayda, K.; Tatikola, K.; Hutchison, J.S.; Mayorga, A.J. A multiple-dose double-blind randomized study to evaluate the safety, pharmacokinetics, pharmacodynamics and analgesic efficacy of the TRPV1 antagonist JNJ-39439335 (mavatrep). Scand. J. Pain 2018, 18, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Mayorga, A.J.; Flores, C.M.; Trudeau, J.J.; Moyer, J.A.; Shalayda, K.; Dale, M.; Frustaci, M.E.; Katz, N.; Manitpisitkul, P.; Treister, R.; et al. A randomized study to evaluate the analgesic efficacy of a single dose of the TRPV1 antagonist mavatrep in patients with osteoarthritis. Scand. J. Pain 2017, 17, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Iftinca, M.; Defaye, M.; Altier, C. TRPV1-Targeted Drugs in Development for Human Pain Conditions. Drugs 2021, 81, 7–27. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.C. Resiniferatoxin: The Evolution of the “Molecular Scalpel” for Chronic Pain Relief. Pharmaceuticals 2016, 9, 47. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Ma, Y.; Ye, X.; Zhang, N.; Pan, L.; Wang, B. TRP (transient receptor potential) ion channel family: Structures, biological functions and therapeutic interventions for diseases. Signal Transduct. Target. Ther. 2023, 8, 261. [Google Scholar] [CrossRef] [PubMed]
- Miller, F.; Björnsson, M.; Svensson, O.; Karlsten, R. Experiences with an adaptive design for a dose-finding study in patients with osteoarthritis. Contemp. Clin. Trials 2014, 37, 189–199. [Google Scholar] [CrossRef]

| Channels | Distribution | Functions | Pathological Roles | Related Diseases | References |
|---|---|---|---|---|---|
| TRPV1 | Brain, DRG, Sensory nerve, Liver, Kidney, Brain, Urinary bladder, Pancreas, Testis | Mechanosensitivity; Thermosensitivity; Neurodepolarization | Inflammatory pain; Neuropathic pain; Aberrant thermosensitivity | Osteoarthritis; Migraine; ARDS; Fibromyalgia | [96,97,98,99,159,160,161,162] |
| TRPV2 | Brain, DRG, Sensory nerve, Spinal cord, Liver, Lung, Spleen, Muscle, Intestine, Urinary bladder, Immune cells | Thermosensitivity; Thermosensitivity; Cell cycle regulation | Cell proliferation abnormalities; Tumor growth; Mechanical injury sensitivity | Heart failure | [75,91,105,109,110,120,163] |
| TRPV3 | Keratinocytes, Brain, DRG, Sensory nerve, Spinal cord, Skin, Tongue, Nose, Palate, Colon, Testicles | Thermosensitivity; Maintain normal skin barrier | Skin injuries; Pain symptoms; Sensory abnormalities | Dermatitis; Psoriatic lesions; Olmsted syndrome | [75,93,123,127,128,129,164,165] |
| TRPV4 | Musculoskeletal tissue, Brain, Skin, Sensory neurons, DRG, Kidney, Liver, Lung, Spleen, Heart, Vascular endothelia | Mechanosensitivity; Thermosensitivity; Regulates the musculoskeletal system | Inflammation; Pain perception; Cellular deformation; Cell proliferation abnormalities | Osteoarthritis; Skeletal dysplasia; Neuromuscular disorders | [131,132,133,134,140,147,166,167,168] |
| TRPV5 | Kidney, Placenta, Pancreas, Prostate | Maintenance of Ca2+ homeostasis; Renal regulation | Calcium metabolism abnormalities; Renal diseases | Nephrolithiasis; Osteoporosis; | [75,88,148,149,152,153,154,169,170] |
| TRPV6 | Intestine, Kidney, Pancreas, Breast, Placenta, Testes, Prostate | Maintenance of Ca2+ homeostasis; Intestinal calcium regulation | Calcium metabolism abnormalities; Intestinal inflammation | Osteoporosis; Hyperparathyroidism; Cancer | [75,88,109,155,156,169,170] |
| Channels | Distribution | Species | Results/Conclusions | References |
|---|---|---|---|---|
| TRPV1 | Synovium; Cartilage; Nerve fibers associated with joints | Mouse | TRPV1 positive fibers regulate knee and ankle pain sensation in mice | [172] |
| Synovium | Synoviocytes; Patients with inflammatory arthropathies | TRP1 channels are functionally expressed in human synoviocytes | [175] | |
| Synovium | Rats and patients with OA | TRPV1 expression and M1 macrophage infiltration were simultaneously increased in both human and rat OA synovia | [181] | |
| Synovial fibroblasts | Patients with symptomatic OA and RA | TRPV1 is expressed in SF from symptomatic OA and RA patients | [182] | |
| TRPV2 | Articular cartilage Ectopic ossification lesions | Mouse; Human | Regulation of articular cartilage by TRPV2 through Prg4 induction and suppression of ectopic ossification | [186] |
| Fibroblast-Like Synoviocyte (FLS) | Patients with RA | Stimulation of TRPV2 in FLS is capable of suppressing the activation of RhoA and Rac1 | [187] | |
| TRPV3 | Cartilage | Chicken and mouse embryos | The mRNA level of TRPV3 was increased | [188] |
| TRPV4 | Cartilage | Murine induced pluripotent stem cells (iPSCs) | TRPV4 serves both as a marker and a regulator of iPSC chondrogenesis | [191] |
| Cartilage | TRPV4(−/−) mice | Deletion of TRPV4 leads to osteoarthritic joint degeneration | [193] | |
| Cartilage | Murine chondrogenic cell line (ATDC5) | TRPV4 regulates the SOX9 pathway and contributes to the process of chondrogenesis | [192] | |
| Cartilage | Porcines | TRPV4 is expressed in articular chondrocytes and mediates the hypoosmotic response | [194] | |
| Cartilage | Embryonic mouse | TRPV4 promotes joint cartilage growth and morphogenesis | [199] | |
| Synovium | Patients with RA | TRPV4 is a functional regulator of Ca2+ in human synoviocytes | [167] | |
| Synovium | Tumor-derived SW982 synoviocytes; Patients with inflammatory arthropathies | TRPV4 may play a critical role in adaptive or pathological changes in articular surfaces during arthritic inflammation | [175] | |
| Bone | Trpv4(R616Q/V620I) transgenic mice | Activation of TRPV4 can promote sufficient of osteoclast function | [195] | |
| Bone | Wild-type mice | TRPV4 protein is expressed in both osteoblasts and osteoclasts | [200] | |
| TRPV5 | Cartilage | Normal and OA SD rats | TRPV5 is expressed in all cartilage tissues | [205] |
| Cartilage | Rabbit | Reducing TRPV5 activity could be beneficial for cartilage health and regeneration | [206] | |
| Cartilage | Rats with osteoarthritis | Upregulated TRPV5 expression was observed in chondrocytes from rats with osteoarthritis | [209] | |
| TRPV6 | Cartilage | OA rat and OA patients; TRPV6 knockout mice | TRPV6 as a cartilage protective factor was involved in the pathogenesis of OA | [210] |
| Channels | Drug | Clinical Progress | Function | Adverse Events | Organization/NCT Number | References |
|---|---|---|---|---|---|---|
| TRPV1 | CNTX-4975 | Phase II/III | Reduce pain | / | NCT02558439; NCT03661996 | [219,220,225] |
| Resiniferatoxin (RTX) | Phase I/II/III | Reduce pain and improve mobility | Destroys nerve endings produces reversible analgesia | NCT02566564 2018-000818-37(EU); NCT04044742 | [225,226,227] | |
| AZD1386 | Phase II | / | No significant pain decrease | NCT00878501 | [225,227,228] | |
| JNJ-39439335 | Phase I/II | Analgesic | Thermal hypoesthesia; Paresthesia; feeling cold; minor thermal burns | NCT01006340 NCT00933582 NCT01343303 | [223,224,225,227] | |
| ABT-102 | Phase I | Analgesic | Dysesthesias; altered taste sensation | NCT00854659 | [221,225,227] | |
| NEO6860 | Phase I | Analgesic | Feeling of hotness; headache; paresthesia; nausea; dizziness | NCT02337543 | [218,225,227] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.; Yang, F.; Chen, R.; Yang, C.; Xiao, H.; Geng, B.; Xia, Y. TRPV Channels in Osteoarthritis: A Comprehensive Review. Biomolecules 2024, 14, 292. https://doi.org/10.3390/biom14030292
Chen C, Yang F, Chen R, Yang C, Xiao H, Geng B, Xia Y. TRPV Channels in Osteoarthritis: A Comprehensive Review. Biomolecules. 2024; 14(3):292. https://doi.org/10.3390/biom14030292
Chicago/Turabian StyleChen, Changshun, Fei Yang, Rongjin Chen, Chenhui Yang, Hefang Xiao, Bin Geng, and Yayi Xia. 2024. "TRPV Channels in Osteoarthritis: A Comprehensive Review" Biomolecules 14, no. 3: 292. https://doi.org/10.3390/biom14030292
APA StyleChen, C., Yang, F., Chen, R., Yang, C., Xiao, H., Geng, B., & Xia, Y. (2024). TRPV Channels in Osteoarthritis: A Comprehensive Review. Biomolecules, 14(3), 292. https://doi.org/10.3390/biom14030292








