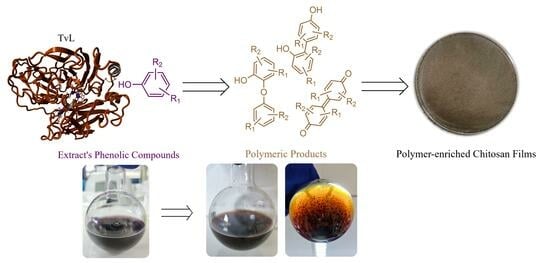
Laccase-Mediated Oxidation of Phenolic Compounds from Wine Lees Extract towards the Synthesis of Polymers with Potential Applications in Food Packaging
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Wine Lees Extraction
2.3. TvL Activity and Stability
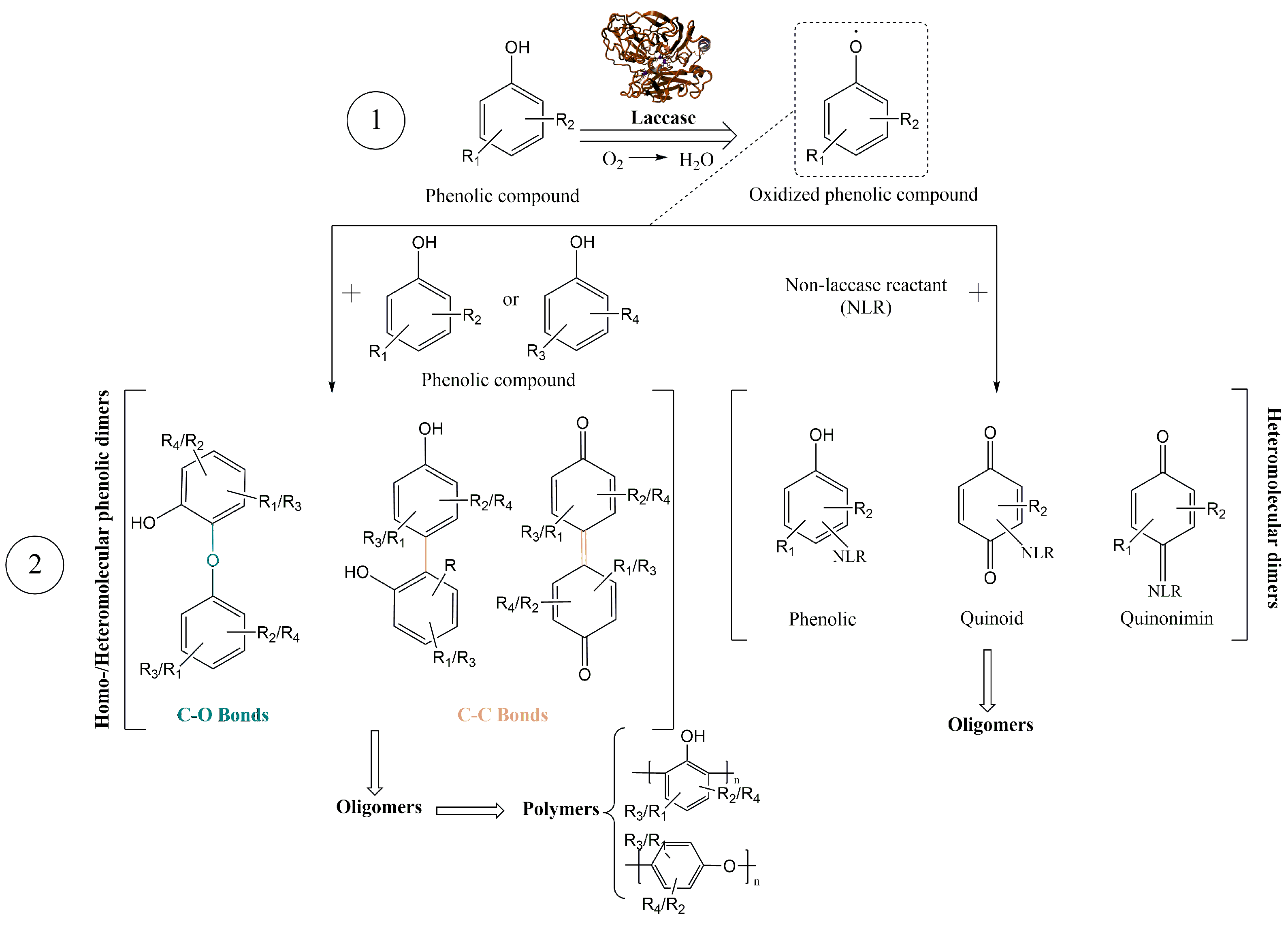
2.4. Enzymatic Synthesis of Phenolic Polymers
2.5. Dephenolization of the Extract during Polymer Synthesis
2.6. Analytical and Spectroscopic Characterization
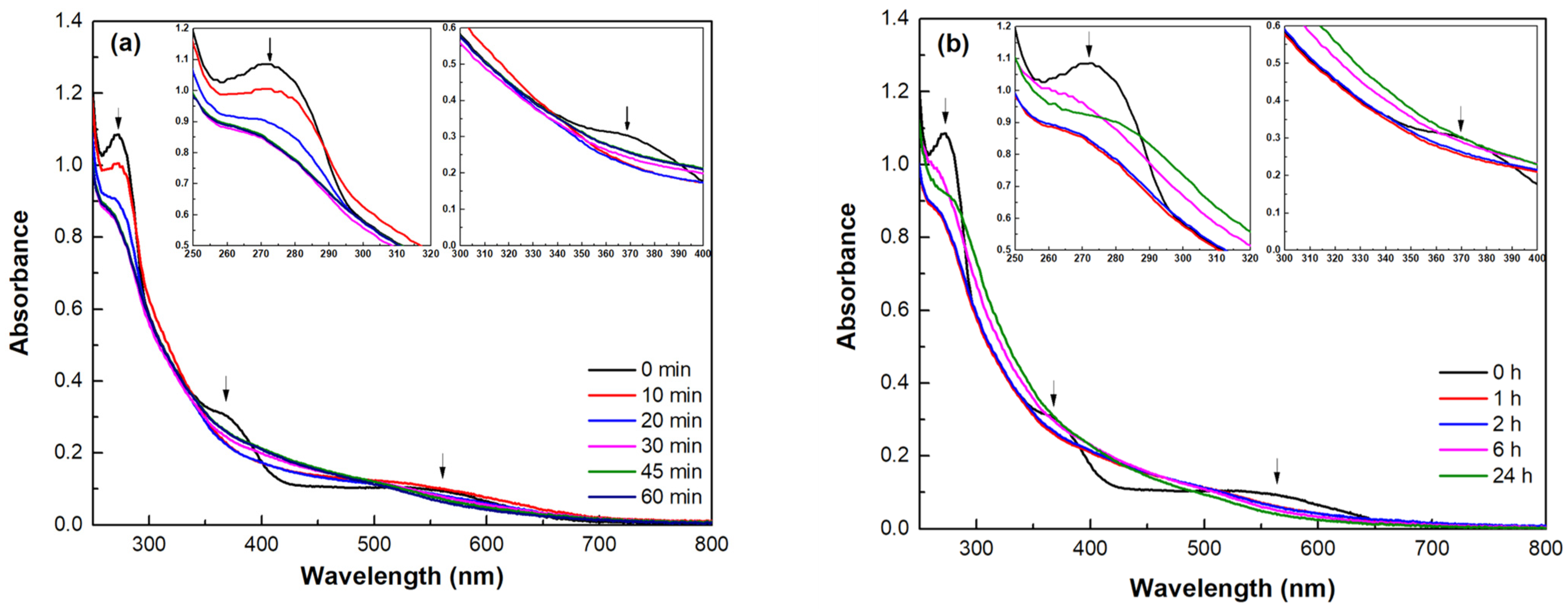
2.6.1. Ultraviolet–Visible Spectroscopy
2.6.2. HPLC Analysis
2.6.3. NMR Analysis
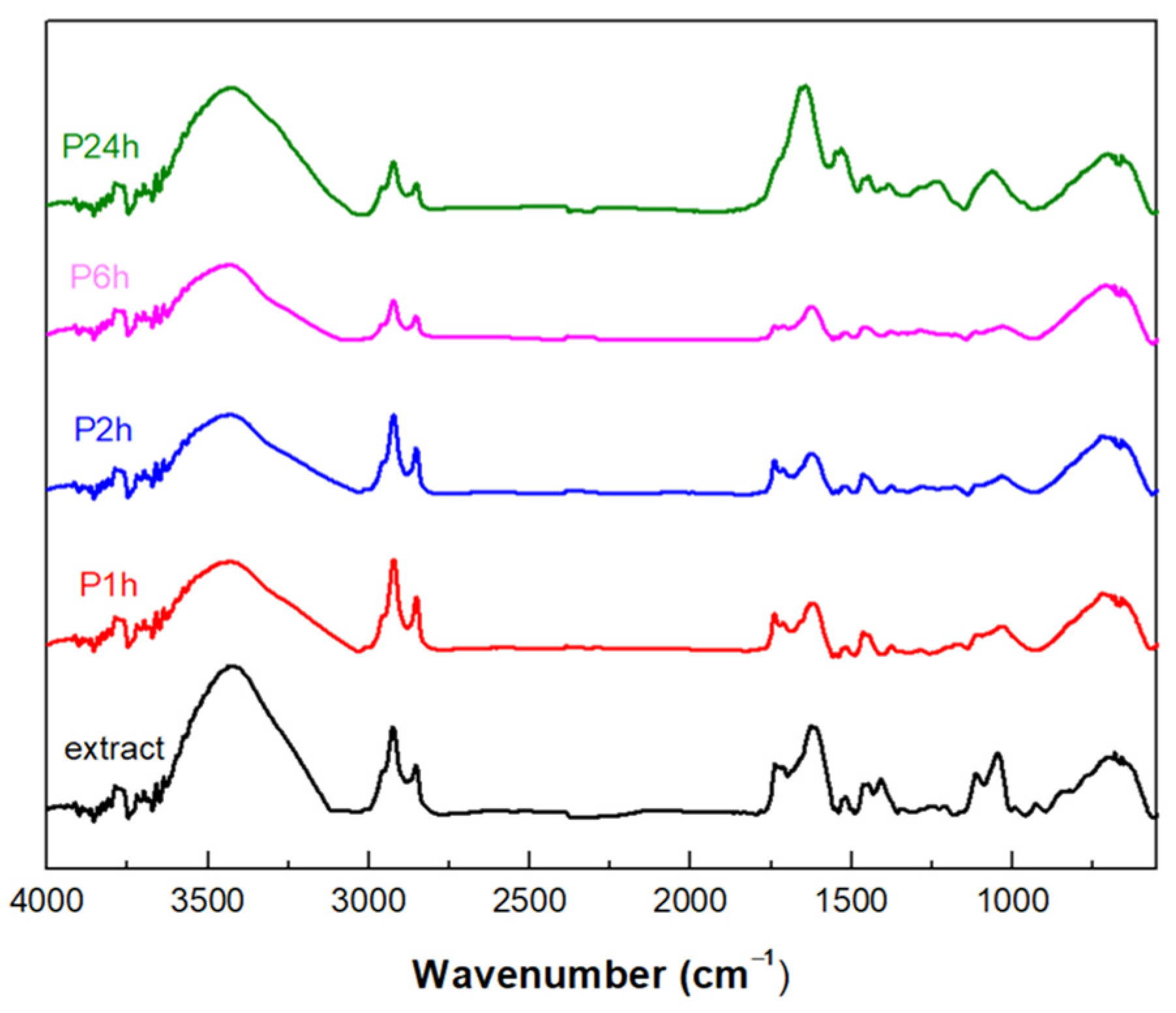
2.6.4. Fourier-Transform Infrared Spectroscopy
2.6.5. Gel Permeation Chromatography Analysis
2.6.6. Differential Scanning Calorimetry Measurements
2.6.7. Thermogravimetric Analysis
2.7. Formation of Polymers-Enriched CS Films
2.8. Antioxidant Activity
2.9. Moisture Content, Water Swelling, and Water Solubility Assays
3. Results and Discussion
3.1. Evaluation of the Polymerization Process
3.2. Thermal Analysis of the Formed Polymers
3.3. Antioxidant Activity of the Formed Polymers
3.4. Properties of the Polymer-Enriched CS Films
3.4.1. Antioxidant Activity of the CS-Polymer Films
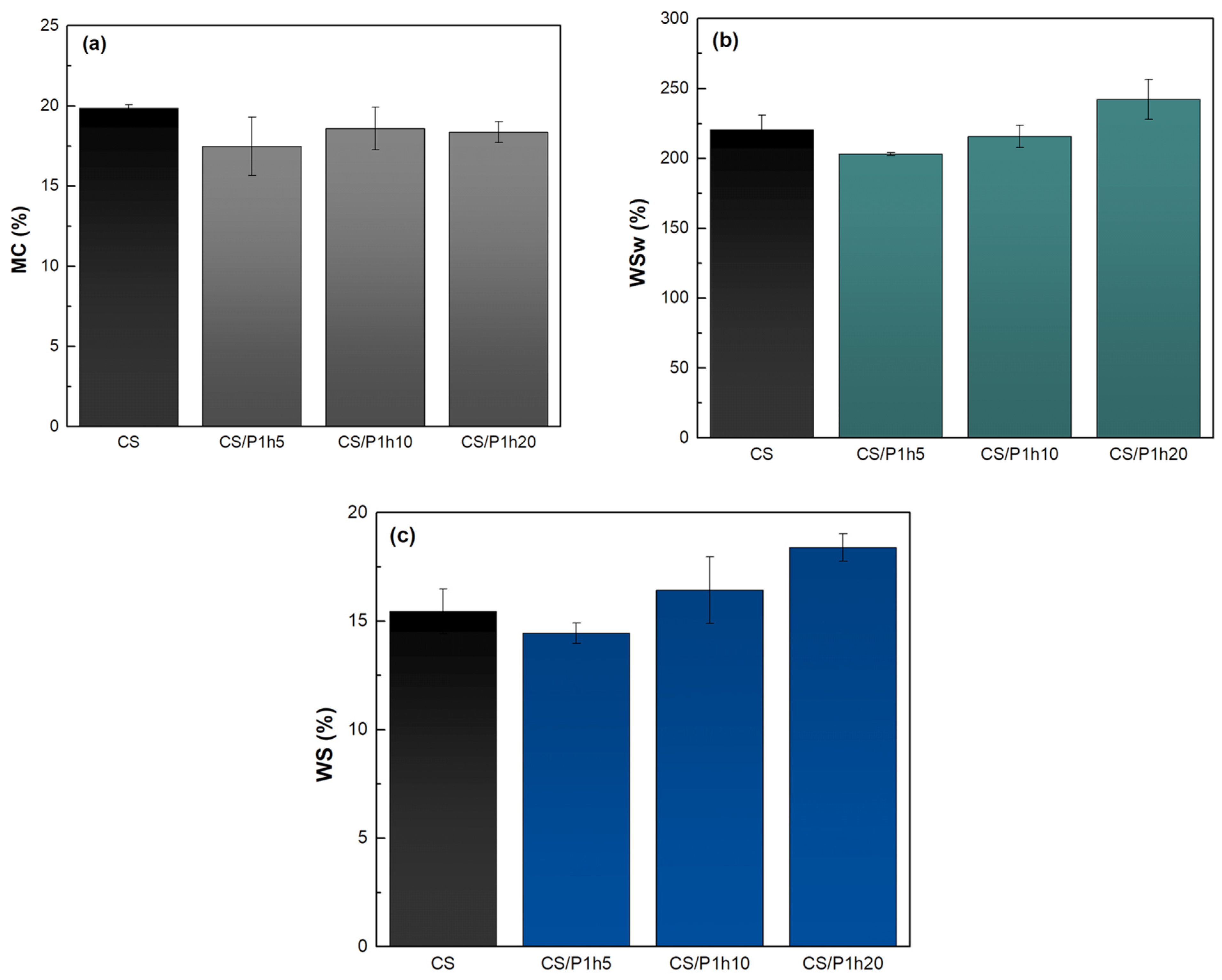
3.4.2. Moisture Content, Water Swelling, and Water Solubility of the Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Patila, M.; Athanasiou, P.E.; Kortessis, L.; Potsi, G.; Kouloumpis, A.; Gournis, D.; Stamatis, H. Immobilization of Laccase on Hybrid Super-Structured Nanomaterials for the Decolorization of Phenolic Dyes. Processes 2022, 10, 233. [Google Scholar] [CrossRef]
- Viswanath, B.; Rajesh, B.; Janardhan, A.; Kumar, A.P.; Narasimha, G. Fungal Laccases and Their Applications in Bioremediation. Enzyme Res. 2014, 2014, 163242. [Google Scholar] [CrossRef]
- Wang, H.X.; Ng, T.B. Purification of a Laccase from Fruiting Bodies of the Mushroom Pleurotus Eryngii. Appl. Microbiol. Biotechnol. 2006, 69, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Panwar, V.; Dey, B.; Sheikh, J.N.; Dutta, T. Thermostable Bacterial Laccase for Sustainable Dyeing Using Plant Phenols. RSC Adv. 2022, 12, 18168–18180. [Google Scholar] [CrossRef]
- Bakratsas, G.; Antoniadis, K.; Athanasiou, P.E.; Katapodis, P.; Stamatis, H. Laccase and Biomass Production via Submerged Cultivation of Pleurotus ostreatus Using Wine Lees. Biomass 2023, 4, 1–22. [Google Scholar] [CrossRef]
- Spyrou, S.; Chatzikonstantinou, A.; Giannakopoulou, A.; Fotiadou, R.; Priska, S.; Simos, Y.; Tsakni, A.; Peschos, D.; Houhoula, D.; Voutsas, E.; et al. Fungal Laccase-Mediated Enhancement of the Bioactivity of Green Algae Extracts. Catal. Res. 2023, 3, 004. [Google Scholar] [CrossRef]
- Su, J.; Fu, J.; Wang, Q.; Silva, C.; Cavaco-Paulo, A. Laccase: A Green Catalyst for the Biosynthesis of Poly-Phenols. Crit. Rev. Biotechnol. 2018, 38, 294–307. [Google Scholar] [CrossRef] [PubMed]
- Desentis-Mendoza, R.M.; Hernández-Sánchez, H.; Moreno, A.; del Rojas, C.E.; Chel-Guerrero, L.; Tamariz, J.; Jaramillo-Flores, M.E. Enzymatic Polymerization of Phenolic Compounds Using Laccase and Tyrosinase from Ustilago maydis. Biomacromolecules 2006, 7, 1845–1854. [Google Scholar] [CrossRef]
- Tarasov, A.; Stozhko, N.; Bukharinova, M.; Khamzina, E. Biosensors Based on Phenol Oxidases (Laccase, Tyrosinase, and Their Mixture) for Estimating the Total Phenolic Index in Food-Related Samples. Life 2023, 13, 291. [Google Scholar] [CrossRef]
- Catherine, H.; Penninckx, M.; Frédéric, D. Product Formation from Phenolic Compounds Removal by Laccases: A Review. Environ. Technol. Innov. 2016, 5, 250–266. [Google Scholar] [CrossRef]
- Mogharabi, M.; Faramarzi, M.A. Laccase and Laccase-Mediated Systems in the Synthesis of Organic Compounds. Adv. Synth. Catal. 2014, 356, 897–927. [Google Scholar] [CrossRef]
- Mikolasch, A.; Schauer, F. Fungal Laccases as Tools for the Synthesis of New Hybrid Molecules and Biomaterials. Appl. Microbiol. Biotechnol. 2009, 82, 605–624. [Google Scholar] [CrossRef] [PubMed]
- Adelakun, O.E.; Kudanga, T.; Parker, A.; Green, I.R.; le Roes-Hill, M.; Burton, S.G. Laccase-Catalyzed Dimerization of Ferulic Acid Amplifies Antioxidant Activity. J. Mol. Catal. B Enzym. 2012, 74, 29–35. [Google Scholar] [CrossRef]
- Nagarajan, S.; Nagarajan, R.; Kumar, J.; Salemme, A.; Togna, A.R.; Saso, L.; Bruno, F. Antioxidant Activity of Synthetic Polymers of Phenolic Compounds. Polymers 2020, 12, 1646. [Google Scholar] [CrossRef]
- Latos-Brozio, M.; Masek, A.; Piotrowska, M. Effect of Enzymatic Polymerization on the Thermal Stability of Flavonoids. J. Therm. Anal. Calorim. 2023, 148, 5357–5374. [Google Scholar] [CrossRef]
- Sun, X.; Bai, R.; Zhang, Y.; Wang, Q.; Fan, X.; Yuan, J.; Cui, L.; Wang, P. Laccase-Catalyzed Oxidative Polymerization of Phenolic Compounds. Appl. Biochem. Biotechnol. 2013, 171, 1673–1680. [Google Scholar] [CrossRef] [PubMed]
- Mita, N.; Tawaki, S.; Hiroshi, U.; Kobayashi, S. Laccase-Catalyzed Oxidative Polymerization of Phenols. Macromol. Biosci. 2003, 3, 253–257. [Google Scholar] [CrossRef]
- Su, J.; Wang, C.; Noro, J.; Cavaco-Paulo, A.; Silva, C.; Fu, J. Polymers from Bamboo Extracts Produced by Laccase. Polymers 2018, 10, 1141. [Google Scholar] [CrossRef]
- Li, N.; Freitas, D.S.; Santos, J.; Venâncio, A.; Noro, J.; Su, J.; Wang, H.; Silva, C.; Cavaco-Paulo, A. Laccase-Catalyzed Synthesis of Added-Value Polymers from Cork and Grape Extracts. J. Agric. Food Chem. 2023, 71, 18877–18889. [Google Scholar] [CrossRef]
- Chatzikonstantinou, A.V.; Giannakopoulou, A.; Spyrou, S.; Simos, Y.V.; Kontogianni, V.G.; Peschos, D.; Katapodis, P.; Polydera, A.C.; Stamatis, H. Production of Hydroxytyrosol Rich Extract from Olea Europaea Leaf with Enhanced Biological Activity Using Immobilized Enzyme Reactors. Environ. Sci. Pollut. Res. 2022, 29, 29624–29637. [Google Scholar] [CrossRef]
- Zuorro, A. Optimization of Polyphenol Recovery from Espresso Coffee Residues Using Factorial Design and Response Surface Methodology. Sep. Purif. Technol. 2015, 152, 64–69. [Google Scholar] [CrossRef]
- Tagkouli, D.; Tsiaka, T.; Kritsi, E.; Soković, M.; Sinanoglou, V.J.; Lantzouraki, D.Z.; Zoumpoulakis, P. Towards the Optimization of Microwave-Assisted Extraction and the Assessment of Chemical Profile, Antioxidant and Antimicrobial Activity of Wine Lees Extracts. Molecules 2022, 27, 2189. [Google Scholar] [CrossRef]
- Matos, M.S.; Romero-Díez, R.; Álvarez, A.; Bronze, M.R.; Rodríguez-Rojo, S.; Mato, R.B.; Cocero, M.J.; Matias, A.A. Polyphenol-Rich Extracts Obtained from Winemaking waste Streams as Natural Ingredients with Cosmeceutical Potential. Antioxidants 2019, 8, 355. [Google Scholar] [CrossRef] [PubMed]
- Panzella, L.; Moccia, F.; Nasti, R.; Marzorati, S.; Verotta, L.; Napolitano, A. Bioactive Phenolic Compounds From Agri-Food Wastes: An Update on Green and Sustainable Extraction Methodologies. Front. Nutr. 2020, 7, 60. [Google Scholar] [CrossRef]
- Botta, L.; Brunori, F.; Tulimieri, A.; Piccinino, D.; Meschini, R.; Saladino, R. Laccase-Mediated Enhancement of the Antioxidant Activity of Propolis and Poplar Bud Exudates. ACS Omega 2017, 2, 2515–2523. [Google Scholar] [CrossRef]
- Flórez, M.; Guerra-Rodríguez, E.; Cazón, P.; Vázquez, M. Chitosan for Food Packaging: Recent Advances in Active and Intelligent Films. Food Hydrocoll. 2022, 124, 107328. [Google Scholar] [CrossRef]
- Ferreira, A.S.; Nunes, C.; Castro, A.; Ferreira, P.; Coimbra, M.A. Influence of Grape Pomace Extract Incorporation on Chitosan Films Properties. Carbohydr. Polym. 2014, 113, 490–499. [Google Scholar] [CrossRef]
- Kahya, N.; Kestir, S.M.; Öztürk, S.; Yolaç, A.; Torlak, E.; Kalaycıoğlu, Z.; Akın-Evingür, G.; Erim, F.B. Antioxidant and Antimicrobial Chitosan Films Enriched with Aqueous Sage and Rosemary Extracts as Food Coating Materials: Characterization of the Films and Detection of Rosmarinic Acid Release. Int. J. Biol. Macromol. 2022, 217, 470–480. [Google Scholar] [CrossRef]
- Kaya, M.; Khadem, S.; Cakmak, Y.S.; Mujtaba, M.; Ilk, S.; Akyuz, L.; Salaberria, A.M.; Labidi, J.; Abdulqadir, A.H.; Deligöz, E. Antioxidative and Antimicrobial Edible Chitosan Films Blended with Stem, Leaf and Seed Extracts of Pistacia Terebinthus for Active Food Packaging. RSC Adv. 2018, 8, 3941–3950. [Google Scholar] [CrossRef]
- Kaynarca, G.B.; Kamer, D.D.A.; Gumus, T.; Sagdıc, O. Characterization of Poly(Vinyl Alcohol)/Gelatin Films Made with Winery Solid by-Product (Vinasse) Extract. Food Packag. Shelf Life 2023, 35, 101013. [Google Scholar] [CrossRef]
- Etxabide, A.; Yang, Y.; Maté, J.I.; de la Caba, K.; Kilmartin, P.A. Developing Active and Intelligent Films through the Incorporation of Grape Skin and Seed Tannin Extracts into Gelatin. Food Packag. Shelf Life 2022, 33, 100896. [Google Scholar] [CrossRef]
- Panzella, L.; Napolitano, A. Natural Phenol Polymers: Recent Advances in Food and Health Applications. Antioxidants 2017, 6, 30. [Google Scholar] [CrossRef] [PubMed]
- Meini, M.R.; Cabezudo, I.; Boschetti, C.E.; Romanini, D. Recovery of Phenolic Antioxidants from Syrah Grape Pomace through the Optimization of an Enzymatic Extraction Process. Food Chem. 2019, 283, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Delgado De La Torre, M.P.; Priego-Capote, F.; Luque De Castro, M.D. Characterization and Comparison of Wine Lees by Liquid Chromatography-Mass Spectrometry in High-Resolution Mode. J. Agric. Food Chem. 2015, 63, 1116–1125. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Wu, D.; Zhang, Q.A.; Sun, D.W. Ultrasound-Assisted Extraction of Phenolics from Wine Lees: Modeling, Optimization and Stability of Extracts during Storage. Ultrason. Sonochem. 2014, 21, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Guardado, A.L.P.; Druon-Bocquet, S.; Belleville, M.-P.; Sanchez-Marcano, J. A Novel Process for the Covalent Immobilization of Laccases on Silica Gel and Its Application for the Elimination of Pharmaceutical Micropollutants. Environ. Sci. Pollut. Res. 2021, 28, 25579–25593. [Google Scholar] [CrossRef] [PubMed]
- Siripatrawan, U.; Vitchayakitti, W. Improving Functional Properties of Chitosan Films as Active Food Packaging by Incorporating with Propolis. Food Hydrocoll. 2016, 61, 695–702. [Google Scholar] [CrossRef]
- Liu, T.; Wang, J.; Chi, F.; Tan, Z.; Liu, L. Development and Characterization of Novel Active Chitosan Films Containing Fennel and Peppermint Essential Oils. Coatings 2020, 10, 936. [Google Scholar] [CrossRef]
- Piontek, K.; Antorini, M.; Choinowski, T. Crystal Structure of a Laccase from the Fungus trametes Versicolor at 1.90-Å Resolution Containing a Full Complement of Coppers. J. Biol. Chem. 2002, 277, 37663–37669. [Google Scholar] [CrossRef]
- Kudanga, T.; Nemadziva, B.; Le Roes-Hill, M. Laccase Catalysis for the Synthesis of Bioactive Compounds. Appl. Microbiol. Biotechnol. 2017, 101, 13–33. [Google Scholar] [CrossRef]
- Meneghetti, M.; Talon, A.; Cattaruzza, E.; Celotti, E.; Bellantuono, E.; Rodríguez-Castellón, E.; Meneghetti, S.; Moretti, E. Sustainable Organic Dyes from Winemaking Lees for Photoelectrochemical Dye-Sensitized Solar Cells. Appl. Sci. 2020, 10, 2149. [Google Scholar] [CrossRef]
- Philippidis, A.; Poulakis, E.; Kontzedaki, R.; Orfanakis, E.; Symianaki, A.; Zoumi, A.; Velegrakis, M. Application of Ultraviolet-Visible Absorption Spectroscopy with Machine Learning Techniques for the Classification of Cretan Wines. Foods 2020, 10, 9. [Google Scholar] [CrossRef]
- Dujmić, F.; Kovačević Ganić, K.; Ćurić, D.; Karlović, S.; Bosiljkov, T.; Ježek, D.; Vidrih, R.; Hribar, J.; Zlatić, E.; Prusina, T.; et al. Non-Thermal Ultrasonic Extraction of Polyphenolic Compounds from Red Wine Lees. Foods 2020, 9, 472. [Google Scholar] [CrossRef]
- Romero-Díez, R.; Rodríguez-Rojo, S.; Cocero, M.J.; Duarte, C.M.M.; Matias, A.A.; Bronze, M.R. Phenolic Characterization of Aging Wine Lees: Correlation with Antioxidant Activities. Food Chem. 2018, 259, 188–195. [Google Scholar] [CrossRef]
- Latos-Brozio, M.; Masek, A.; Piotrowska, M. Polymeric Forms of Plant Flavonoids Obtained by Enzymatic Reactions. Molecules 2022, 27, 3702. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Noro, J.; Fu, J.; Wang, Q.; Silva, C.; Cavaco-Paulo, A. Exploring PEGylated and Immobilized Laccases for Catechol Polymerization. AMB Express 2018, 8, 134. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.; Yu, Y.; Wang, Q.; Shen, J.; Yuan, J.; Fan, X. Laccase-Catalyzed Polymerization of Hydroquinone Incorporated with Chitosan Oligosaccharide for Enzymatic Coloration of Cotton. Appl. Biochem. Biotechnol. 2020, 191, 605–622. [Google Scholar] [CrossRef] [PubMed]
- Andreo-Martínez, P.; Ortiz-Martínez, V.M.; García-Martínez, N.; Hernández-Fernández, F.J.; Pérez de los Ríos, A.; Quesada-Medina, J. A Simple Fractionation Method and GPC Analysis of Organosolv Extracts Obtained from Lignocellulosic Materials. Biomass Convers. Biorefinery 2021, 11, 1807–1821. [Google Scholar] [CrossRef]
- Wang, Q.; Sarkar, A.; Kurokawa, H.; Sekiguchi, K. Investigation of Condensation Reaction during Phenol Liquefaction of Waste Woody Materials. Int. J. Sustain. Dev. Plan. 2014, 9, 658–668. [Google Scholar] [CrossRef]
- Araújo, R.G.; Rodríguez-Jasso, R.M.; Ruiz, H.A.R.; Govea-Salas, M.; Rosas-Flores, W.; Aguilar-González, M.A.; Pintado, M.E.; Lopez-Badillo, C.; Luevanos, C.; Aguilar, C.N. Hydrothermal–Microwave Processing for Starch Extraction from Mexican Avocado Seeds: Operational Conditions and Characterization. Processes 2020, 8, 759. [Google Scholar] [CrossRef]
- Li, P.; Coleman, D.W.; Spaulding, K.M.; Mcclennen, W.H.; Stafford, P.R.; Fife, D.J. Fractionation and Characterization of Phenolic Resins by High-Performance Liquid Chromatography and Gel-Permeation Chromatography Combined with Ultraviolet, Refractive Index, Mass Spectrometry and Light-Scattering Detection. J. Chromatogr. A 2001, 914, 147–159. [Google Scholar] [CrossRef]
- He, F.; Liang, N.-N.; Mu, L.; Pan, Q.-H.; Wang, J.; Reeves, M.J.; Duan, C.-Q. Anthocyanins and Their Variation in Red Wines I. Monomeric Anthocyanins and Their Color Expression. Molecules 2012, 17, 1571–1601. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, D.; Li, W.; Mou, Y.; Geng, Y.; Chen, F.; Hu, X.; Ji, J.; Ma, L. Quinone Reactivity: Investigation of Their Contribution to Nonenzymatic Browning. Food Front. 2023, 4, 945–954. [Google Scholar] [CrossRef]
- Mustafa, R.; Muniglia, L.; Rovel, B.; Girardin, M. Phenolic Colorants Obtained by Enzymatic Synthesis Using a Fungal Laccase in a Hydro-Organic Biphasic System. Food Res. Int. 2005, 38, 995–1000. [Google Scholar] [CrossRef]
- Zerva, A.; Manos, N.; Vouyiouka, S.; Christakopoulos, P.; Topakas, E. Bioconversion of Biomass-Derived Phenols Catalyzed by Myceliophthora thermophila Laccase. Molecules 2016, 21, 550. [Google Scholar] [CrossRef] [PubMed]
- David, G.; Vannini, M.; Sisti, L.; Marchese, P.; Celli, A.; Gontard, N.; Angellier-Coussy, H. Eco-Conversion of Two Winery Lignocellulosic Wastes into Fillers for Biocomposites: Vine Shoots and Wine Pomaces. Polymers 2020, 12, 1530. [Google Scholar] [CrossRef]
- Hiller, B.T.; Azzi, J.L.; Rennert, M. Improvement of the Thermo-Oxidative Stability of Biobased Poly(Butylene Succinate) (PBS) Using Biogenic Wine By-Products as Sustainable Functional Fillers. Polymers 2023, 15, 2533. [Google Scholar] [CrossRef]
- Gkountela, C.; Rigopoulou, M.; Barampouti, E.M.; Vouyiouka, S. Enzymatic Prepolymerization Combined with Bulk Post-Polymerization towards the Production of Bio-Based Polyesters: The Case of Poly(Butylene Succinate). Eur. Polym. J. 2021, 143, 110197. [Google Scholar] [CrossRef]
- Gkountela, C.I.; Markoulakis, D.; Mathioudaki, M.; Pitterou, I.; Detsi, A.; Vouyiouka, S.N. Scalable Enzymatic Polymerization and Low-Temperature Post-Polymerization of Poly(Butylene Succinate): Process Parameters and Application. Eur. Polym. J. 2023, 198, 112423. [Google Scholar] [CrossRef]
- Chrissafis, K.; Paraskevopoulos, K.M.; Bikiaris, D.N. Effect of Molecular Weight on Thermal Degradation Mechanism of the Biodegradable Polyester Poly(Ethylene Succinate). Thermochim. Acta 2006, 440, 166–175. [Google Scholar] [CrossRef]
- Unger, M.; Vogel, C.; Siesler, H.W. Molecular Weight Dependence of the Thermal Degradation of Poly(ε-Caprolactone): A Thermogravimetric Differential Thermal Fourier Transform Infrared Spectroscopy Study. Appl. Spectrosc. 2010, 64, 805–809. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Liu, C.; Geng, S. Laccase Catalyzed Oxidative Polymerization of Phloridzin: Polymer Characterization, Antioxidant Capacity and α-Glucosidase Inhibitory Activity. Nat. Prod. Commun. 2021, 16, 1934578X2110523. [Google Scholar] [CrossRef]
- Platzer, M.; Kiese, S.; Tybussek, T.; Herfellner, T.; Schneider, F.; Schweiggert-Weisz, U.; Eisner, P. Radical Scavenging Mechanisms of Phenolic Compounds: A Quantitative Structure-Property Relationship (QSPR) Study. Front. Nutr. 2022, 9, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Platzer, M.; Kiese, S.; Herfellner, T.; Schweiggert-Weisz, U.; Miesbauer, O.; Eisner, P. Common Trends and Differences in Antioxidant Activity Analysis of Phenolic Substances Using Single Electron Transfer Based Assays. Molecules 2021, 26, 1244. [Google Scholar] [CrossRef]
- Xiao, Z.; He, L.; Hou, X.; Wei, J.; Ma, X.; Gao, Z.; Yuan, Y.; Xiao, J.; Li, P.; Yue, T. Relationships between Structure and Antioxidant Capacity and Activity of Glycosylated Flavonols. Foods 2021, 10, 849. [Google Scholar] [CrossRef]
- Cerretani, L.; Bendini, A. Rapid Assays to Evaluate the Antioxidant Capacity of Phenols in Virgin Olive Oil. In Olives and Olive Oil in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2010; pp. 625–635. ISBN 9780123744203. [Google Scholar]
- Rachtanapun, P.; Klunklin, W.; Jantrawut, P.; Jantanasakulwong, K.; Phimolsiripol, Y.; Seesuriyachan, P.; Leksawasdi, N.; Chaiyaso, T.; Ruksiriwanich, W.; Phongthai, S.; et al. Characterization of Chitosan Film Incorporated with Curcumin Extract. Polymers 2021, 13, 963. [Google Scholar] [CrossRef]
- Rambabu, K.; Bharath, G.; Banat, F.; Show, P.L.; Cocoletzi, H.H. Mango Leaf Extract Incorporated Chitosan Antioxidant Film for Active Food Packaging. Int. J. Biol. Macromol. 2019, 126, 1234–1243. [Google Scholar] [CrossRef]
- Halász, K.; Csóka, L. Black Chokeberry (Aronia melanocarpa) Pomace Extract Immobilized in Chitosan for Colorimetric PH Indicator Film Application. Food Packag. Shelf Life 2018, 16, 185–193. [Google Scholar] [CrossRef]
- Terzioğlu, P.; Güney, F.; Parın, F.N.; Şen, İ.; Tuna, S. Biowaste Orange Peel Incorporated Chitosan/Polyvinyl Alcohol Composite Films for Food Packaging Applications. Food Packag. Shelf Life 2021, 30, 100742. [Google Scholar] [CrossRef]
- De Carli, C.; Aylanc, V.; Mouffok, K.M.; Santamaria-Echart, A.; Barreiro, F.; Tomás, A.; Pereira, C.; Rodrigues, P.; Vilas-Boas, M.; Falcão, S.I. Production of Chitosan-Based Biodegradable Active Films Using Bio-Waste Enriched with Polyphenol Propolis Extract Envisaging Food Packaging Applications. Int. J. Biol. Macromol. 2022, 213, 486–497. [Google Scholar] [CrossRef]
- Wrońska, N.; Katir, N.; Nowak-Lange, M.; El Kadib, A.; Lisowska, K. Biodegradable Chitosan-Based Films as an Alternative to Plastic Packaging. Foods 2023, 12, 3519. [Google Scholar] [CrossRef] [PubMed]


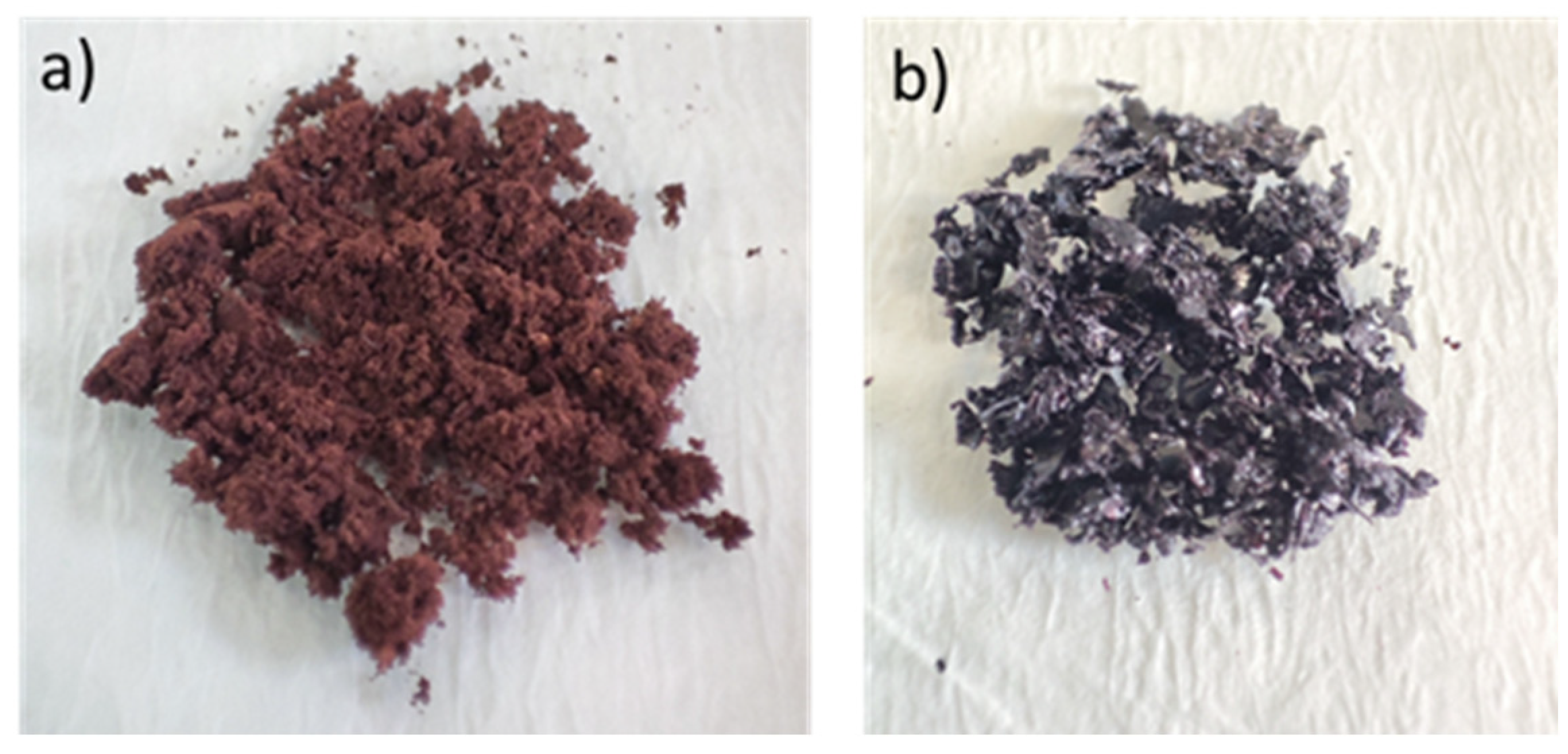



| Polymeric Products | Abbreviation | Amount of Polymeric Products (mg) | Chitosan Solution (mL) |
|---|---|---|---|
| - | CS | - | 10 |
| P1h | CS/P1h5 | 5 | 10 |
| CS/P1h10 | 10 | 10 | |
| CS/P1h20 | 20 | 10 | |
| P2h | CS/P2h5 | 5 | 10 |
| CS/P2h10 | 10 | 10 | |
| CS/P2h20 | 20 | 10 | |
| P6h | CS/P6h5 | 5 | 10 |
| CS/P6h10 | 10 | 10 | |
| CS/P6h20 | 20 | 10 | |
| P24h | CS/P24h5 | 5 | 10 |
| CS/P24h10 | 10 | 10 | |
| CS/P24h20 | 20 | 10 |
| Sample Name | Dephenolization (%) | Amount of Polymeric Products (mg) | Polymeric Products Recovery * (%) |
|---|---|---|---|
| P1h | 82.4 ± 0.1 | 27.5 ± 0.5 | 22.0 ± 0.4 |
| P2h | 82.2 ± 0.6 | 27.9 ± 0.6 | 22.4 ± 0.5 |
| P6h | 81.1 ± 0.3 | 30.2 ± 1.0 | 24.2 ± 0.8 |
| P24h | 80.4 ± 0.7 | 32.1 ± 1.3 | 25.7 ± 1.1 |
| Compound | Reduction (%) |
|---|---|
| Gallic acid | 98.1 ± 1.2 |
| Syringic acid | 50.0 ± 4.9 |
| p-Coumaric acid | 97.3 ± 2.1 |
| Caffeic acid | 81.3 ± 2.8 |
| Vanillic acid | 98.8 ± 0.9 |
| Hydroxytyrosol | 96.3 ± 3.1 |
| Homovanillyl alcohol | 42.3 ± 2.3 |
| Tyrosol | 76.0 ± 3.7 |
| Catechin | 97.6 ± 1.8 |
| Vanillyl alcohol | 98.3 ± 0.9 |
| Catechol | 97.9 ± 1.3 |
| Polymeric Products | ABTS (μg TE mg−1 Polymers) | DPPH (μg TE mg−1 Polymers) |
|---|---|---|
| P1h | 133.75 ± 1.53 | 164.77 ± 3.14 |
| P2h | 99.39 ± 3.67 | 122.28 ± 2.45 |
| P6h | 95.94 ± 3.06 | 120.80 ± 1.05 |
| P24h | 96.59 ± 4.32 | 124.51 ± 2.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Athanasiou, P.E.; Gkountela, C.I.; Patila, M.; Fotiadou, R.; Chatzikonstantinou, A.V.; Vouyiouka, S.N.; Stamatis, H. Laccase-Mediated Oxidation of Phenolic Compounds from Wine Lees Extract towards the Synthesis of Polymers with Potential Applications in Food Packaging. Biomolecules 2024, 14, 323. https://doi.org/10.3390/biom14030323
Athanasiou PE, Gkountela CI, Patila M, Fotiadou R, Chatzikonstantinou AV, Vouyiouka SN, Stamatis H. Laccase-Mediated Oxidation of Phenolic Compounds from Wine Lees Extract towards the Synthesis of Polymers with Potential Applications in Food Packaging. Biomolecules. 2024; 14(3):323. https://doi.org/10.3390/biom14030323
Chicago/Turabian StyleAthanasiou, Panagiotis E., Christina I. Gkountela, Michaela Patila, Renia Fotiadou, Alexandra V. Chatzikonstantinou, Stamatina N. Vouyiouka, and Haralambos Stamatis. 2024. "Laccase-Mediated Oxidation of Phenolic Compounds from Wine Lees Extract towards the Synthesis of Polymers with Potential Applications in Food Packaging" Biomolecules 14, no. 3: 323. https://doi.org/10.3390/biom14030323
APA StyleAthanasiou, P. E., Gkountela, C. I., Patila, M., Fotiadou, R., Chatzikonstantinou, A. V., Vouyiouka, S. N., & Stamatis, H. (2024). Laccase-Mediated Oxidation of Phenolic Compounds from Wine Lees Extract towards the Synthesis of Polymers with Potential Applications in Food Packaging. Biomolecules, 14(3), 323. https://doi.org/10.3390/biom14030323








_Stamatis.png)