Erythropoietin: A Personal Alice in Wonderland Trip in the Shadow of the Giants
Abstract
1. Introduction
2. The Discovery of the Active Principle
3. The Long Process to Obtain “Pure EPO”
4. The European Effort to Purify EPO Supported by the Volkswagen Foundation
5. Cloning and Production of Recombinant Human Erythropoietin
6. The First-in-Man Clinical Trial to Treat Anemia of Chronic Kidney Failure
7. EPO Becomes a Worldwide Pharmaceutical Business
8. The Year of the Successful Clinical Trial with EPO the Nobel Prize for Biopharmaceutics Was Awarded for Research on Nerve Growth Factor
9. Where There Is “Smoke” There Is “Fire”: The Case of Endless Lawsuits
10. Additional Clinical and Non-Clinical Uses of Recombinant Erythropoietin
11. The Clinical Use of EPO May Not Be So Benign after All: Development of Neutralizing Antibody and Acceleration of Disease Progression in Patients Treated with EPO
11.1. The Case of EPO Antibodies
11.2. Acceleration of Disease Progression in Cancer Patients Treated with EPO
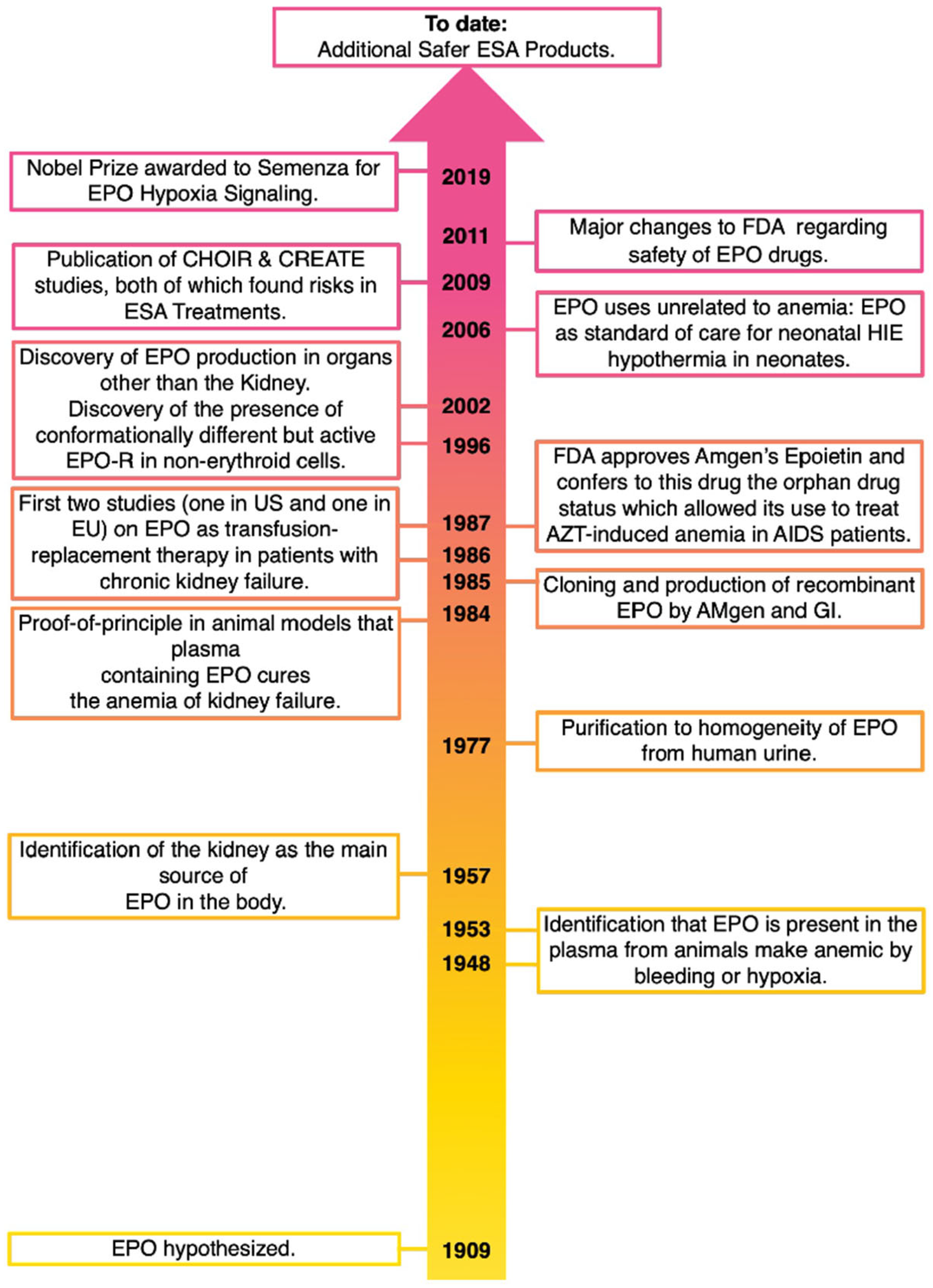
12. Overall Summary (See Also Figure 1)
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thompson, P.; Strandenes, G. The History of Fluid Resuscitation for Bleeding. In Damage Control Resuscitation; Springer International Publishing: Cham, Switzerland, 2020; pp. 3–29. [Google Scholar]
- Blundell, J. Experiments on the Transfusion of Blood by the Syringe. J. R. Soc. Med. 1818, MCT-9, 56–92. [Google Scholar] [CrossRef]
- Landsteine, K.L. Über Agglutinationserscheinungen Normalen Menschlichen Blutes. (Translation: On Agglutination Phenomena of Normal Human Blood). Wien. Klin. Wochenschr. 1901, 14, 1132–1134. [Google Scholar]
- Carnot, P. Sur l’activité Hémopoiétique Du Sérum Au Cours de La Régénération Du Sang. CR Acad. Sci. 1906, 143, 384–386. [Google Scholar]
- Bonsdorff, E.V.A.; Jalavisto, E. A Humoral Mechanism in Anoxic Erythrocytosis. Acta Physiol. Scand. 1948, 16, 150–170. [Google Scholar] [CrossRef]
- Erslev, A. Humoral Regulation of Red Cell Production. Blood 1953, 8, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, L.O.; Goldwasser, E.; Fried, W.; Plzak, L. Role of the Kidney in Erythropoiesis. Nature 1957, 179, 633–634. [Google Scholar] [CrossRef] [PubMed]
- American Society of Hematology. The Story of Erythropoietin. 2008. Available online: https://www.hematology.org/about/history/50-years/erythropoietin (accessed on 17 January 2024).
- Caro, J.; Erslev, A.J. Erythropoietin Assays and Their Use in the Study of Anemias. Contrib. Nephrol. 1988, 66, 54–62. [Google Scholar] [CrossRef]
- Schischlik, F.; Kralovics, R. Mutations in Myeloproliferative Neoplasms–Their Significance and Clinical Use. Expert Rev. Hematol. 2017, 10, 961–973. [Google Scholar] [CrossRef]
- Miyake, T.; Kung, C.K.; Goldwasser, E. Purification of Human Erythropoietin. J. Biol. Chem. 1977, 252, 5558–5564. [Google Scholar] [CrossRef]
- Gregory, C.J.; Tepperman, A.D.; McCulloch, E.A.; Till, J.E. Erythropoietic Progenitors Capable of Colony Formation in Culture: Response of Normal and Genetically Anemic W-W-V Mice to Manipulations of the Erythron. J. Cell. Physiol. 1974, 84, 1–12. [Google Scholar] [CrossRef]
- Iscove, N.N.; Sieber, F.; Winterhalter, K.H. Erythroid Colony Formation in Cultures of Mouse and Human Bone Marrow: Analysis of the Requirement for Erythropoietin by Gel Filtration and Affinity Chromatography on Agarose-concanavalin A. J. Cell. Physiol. 1974, 83, 309–320. [Google Scholar] [CrossRef]
- Rubinstein, P.; Dobrila, L.; Rosenfield, R.E.; Adamson, J.W.; Migliaccio, G.; Migliaccio, A.R.; Taylor, P.E.; Stevens, C.E. Processing and Cryopreservation of Placental/Umbilical Cord Blood for Unrelated Bone Marrow Reconstitution. Proc. Natl. Acad. Sci. USA 1995, 92, 10119–10122. [Google Scholar] [CrossRef]
- Goldwasser, E.; Sherwood, J.B. Radioimmunoassay of Erythropoietin. Br. J. Haematol. 1981, 48, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.F.; Sherwood, J.; Goldwasser, E. Radioimmunoassay of Erythropoietin. Blood Cells 1979, 5, 405–419. [Google Scholar]
- Egrie, J.C.; Cotes, P.M.; Lane, J.; Das, R.E.G.; Tam, R.C. Development of Radioimmunoassays for Human Erythropoietin Using Recombinant Erythropoietin as Tracer and Immunogen. J. Immunol. Methods 1987, 99, 235–241. [Google Scholar] [CrossRef]
- Goldwasser, E.; White, W.F.; Taylor, K.B. Further Purification of Sheep Plasma Erythropoietin. Biochim. Biophys. Acta 1962, 64, 487–496. [Google Scholar] [CrossRef]
- Goldwasser, E.; Kung, C.K.-H. Purification of Erythropoietin. Proc. Natl. Acad. Sci. USA 1971, 68, 697–698. [Google Scholar] [CrossRef] [PubMed]
- Gutnisky, A.; Malgor, L.A.; Nohr, M.L.; Van Dyke, D. Collection of Erythropoietin from Urine of Patients with Anemia Secondary to Hookworm. Ann. N. Y. Acad. Sci. 1968, 149, 564–568. [Google Scholar] [CrossRef]
- Dukes, P.P. Preparation of Ep Containing Protein Concentrates Suitable for Shipment to the Collection Center at the Hematopoiesis Research Laboratory-Childrens Hospital of Los Angeles. Exp. Hematol. 1980, 8 (Suppl. 8), 347–348. [Google Scholar] [PubMed]
- Lai, P.H.; Everett, R.; Wang, F.F.; Arakawa, T.; Goldwasser, E. Structural Characterization of Human Erythropoietin. J. Biol. Chem. 1986, 261, 3116–3121. [Google Scholar] [CrossRef] [PubMed]
- Papayannopoulou, T.H.; Brice, M.; Stamatoyannopoulos, G. Stimulation of Fetal Hemoglobin Synthesis in Bone Marrow Cultures from Adult Individuals. Proc. Natl. Acad. Sci. USA 1976, 73, 2033–2037. [Google Scholar] [CrossRef]
- Migliaccio, G.; Migliaccio, A.R.; Petti, S.; Mavilio, F.; Russo, G.; Lazzaro, D.; Testa, U.; Marinucci, M.; Peschle, C. Human Embryonic Hemopoiesis. Kinetics of Progenitors and Precursors Underlying the Yolk Sac-Liver Transition. J. Clin. Investig. 1986, 78, 51–60. [Google Scholar] [CrossRef]
- Peschle, C.; Migliaccio, A.R.; Migliaccio, G.; Ciccariello, R.; Lettieri, F.; Quattrin, S.; Russo, G.; Mastroberardino, G. Identification and Characterization of Three Classes of Erythroid Progenitors in Human Fetal Liver. Blood 1981, 58, 565–572. [Google Scholar] [CrossRef]
- Cheetham, J.C.; Smith, D.M.; Aoki, K.H.; Stevenson, J.L.; Hoeffel, T.J.; Syed, R.S.; Egrie, J.; Harvey, T.S. NMR Structure of Human Erythropoietin and a Comparison with Its Receptor Bound Conformation. Nat. Struct. Biol. 1998, 5, 861–866. [Google Scholar] [CrossRef]
- Peschle, C.; Rappaport, I.A.; Magli, M.C.; Marone, G.; Lettieri, F.; Cillo, C.; Gordon, A.S. Role of the Hypophysis in Erythropoietin Production during Hypoxia. Blood 1978, 51, 1117–1124. [Google Scholar] [CrossRef]
- Skloot, R. The Immortal Life of Henrietta Lacks; Crown editor: New York, NY, USA, 2010. [Google Scholar]
- Iscove, N.N. The Role of Erythropoietin in Regulation of Population Size and Cell Cycling of Early and Late Erythroid Precursors in Mouse Bone Marrow. Cell Tissue Kinet. 1977, 10, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.B.; Migliaccio, A.R.; Migliaccio, G.; Lettieri, F.; Di Rosa, M.; Peschle, C.; Mastroberardino, G. In Vitro Interactions of PGE and CAMP with Murine and Human Erythroid Precursors. Blood 1980, 56, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.K.; Suggs, S.; Lin, C.H.; Browne, J.K.; Smalling, R.; Egrie, J.C.; Chen, K.K.; Fox, G.M.; Martin, F.; Stabinsky, Z. Cloning and Expression of the Human Erythropoietin Gene. Proc. Natl. Acad. Sci. USA 1985, 82, 7580–7584. [Google Scholar] [CrossRef]
- Egrie, J. The Cloning and Production of Recombinant Human Erythropoietin. Pharmacother. J. Hum. Pharmacol. Drug Ther. 1990, 10, 3S–8S. [Google Scholar] [CrossRef]
- Binder, G.M.; Bashe, P. Science Lessons: What the Business of Biotech Taught Me about Management; Harvard Business Press: Brighton, MA, USA, 2008. [Google Scholar]
- Eschbach, J.W.; Funk, D.; Adamson, J.; Kuhn, I.; Scribner, B.H.; Finch, C.A. Erythropoiesis in Patients with Renal Failure Undergoing Chronic Dialysis. N. Engl. J. Med. 1967, 276, 653–658. [Google Scholar] [CrossRef]
- Adamson, J.W.; Eschbach, J.W. Erythropoietin for End-Stage Renal Disease. N. Engl. J. Med. 1998, 339, 625–627. [Google Scholar] [CrossRef]
- Eschbach, J.W.; Mladenovic, J.; Garcia, J.F.; Wahl, P.W.; Adamson, J.W. The Anemia of Chronic Renal Failure in Sheep. Response to Erythropoietin-Rich Plasma in Vivo. J. Clin. Investig. 1984, 74, 434–441. [Google Scholar] [CrossRef]
- Eschbach, J.W.; Egrie, J.C.; Downing, M.R.; Browne, J.K.; Adamson, J.W. Correction of the Anemia of End-Stage Renal Disease with Recombinant Human Erythropoietin. N. Engl. J. Med. 1987, 316, 73–78. [Google Scholar] [CrossRef]
- Finch, C.A. Erythropoiesis, Erythropoietin, and Iron. Blood 1982, 60, 1241–1246. [Google Scholar] [CrossRef]
- Barker, J.E.; Pierce, J.E.; Nienhuis, A.W. Hemoglobin Switching in Sheep: A Comparison of the Erythropoietin-Induced Switch to HbC and the Fetal to Adult Hemoglobin Switch. Blood 1980, 56, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Andrew Pollack George Rathmann, Amgen Chief, Dies at 84. The New York Times, 23 April 2012; Section B. 17.
- Winearls, C.; Pippard, M.; Downing, M.; Oliver, D.; Reid, C.; Mary Cotes, P. Effect of Human Erythropoietin Derived from Recombinant DNA on the Anaemia of Patients Maintained by Chronic Haemodialysis. Lancet 1986, 328, 1175–1178. [Google Scholar] [CrossRef]
- Eschbach, J.W.; Kelly, M.R.; Haley, N.R.; Abels, R.I.; Adamson, J.W. Treatment of the Anemia of Progressive Renal Failure with Recombinant Human Erythropoietin. N. Engl. J. Med. 1989, 321, 158–163. [Google Scholar] [CrossRef]
- Lynskey, M.J. The Locus of Corporate Entrepreneurship: Kirin Brewery’s Diversification into Biopharmaceuticals. Bus. Hist. Rev. 2006, 80, 689–723. [Google Scholar] [CrossRef]
- Urabe, A. Obituary of Professor Fumimaro Takaku: A Tribute to a Real Leader in Medicine in Japan (1931–2022). Int. J. Hematol. 2022, 115, 759. [Google Scholar] [CrossRef]
- Fumimaro, T. History Pictures Memorial. Available online: https://Medicalnote.Jp/Features/Memorial/Dr_takaku_fumimaro/Images/History_pc.Png (accessed on 17 January 2024).
- Takaku, F.; Dukes, P.; Goldwasser, E. In Vitro Studies of Cell Types Responsive to Erythropoietin. Endocrinology 1964, 74, 968–972. [Google Scholar] [CrossRef]
- Hirashima, K.; Takaku, F. Experimental Studies on Erythropoietin. II. The Relationship between Juxtaglomerular Cells and Erythropoietin. Blood 1962, 20, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, J.; Kumon, K.; Takanashi, S.; Kawashima, Y.; Eguchi, S.; Takaku, F.; Yamamura, H. Subcutaneous Administration of Recombinant Human Erythropoietin before Cardiac Surgery: A Double-blind, Multicenter Trial in Japan. Transfusion 1994, 34, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Baum, S.J.; Santos, G.W.; Takaku, F. Recent Advances and Future Directions in Bone Marrow Transplantation. In Proceedings of the A Symposium Held in Conjunction with the 16th Annual Meeting of the International Society for Experimental Hematology, Tokyo, Japan, 23–28 August 1987; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Akizawa, T.; Koshikawa, S.; Takaku, F.; Urabe, A.; Akiyama, N.; Mimura, N.; Otsubo, O.; Nihei, H.; Suzuki, Y.; Kawaguchi, Y. Clinical Effect of Recombinant Human Erythropoietin on Anemia Associated with Chronic Renal Failure. A Multi-Institutional Study in Japan. Int. J. Artif. Organs 1988, 11, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Fu-Kuen, L.; Chi-Hwei, L.; Por-Hsiung, L.; Browne, J.K.; Egrie, J.C.; Smalling, R.; Fox, G.M.; Chen, K.K.; Miguel, C.; Suggs, S. Monkey Erythropoietin Gene: Cloning, Expression and Comparison with the Human Erythropoietin Gene. Gene 1986, 44, 201–209. [Google Scholar] [CrossRef]
- Migliaccio, G.; Sanchez, M.; Masiello, F.; Tirelli, V.; Varricchio, L.; Whitsett, C.; Migliaccio, A.R. Humanized Culture Medium for Clinical Expansion of Human Erythroblasts. Cell Transplant. 2010, 19, 453–469. [Google Scholar] [CrossRef] [PubMed]
- Pearce, J.; Eschbach, J.W. 74, Dies; Developed Anemia Drug. The New York Times. 15 September 2007. Available online: https://www.nytimes.com/2007/09/15/health/15eschbach-backf-obt-33-28.html (accessed on 17 January 2024).
- Blagg, C.R. In Memory of Joseph Wetherill Eschbach. Kidney Int. 2008, 73, 665–666. [Google Scholar] [CrossRef] [PubMed]
- Wojchowski, D. Eugene Goldwasser (1922–2010). Nature 2011, 470, 40. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wardrop, K.J.; Kramer, J.W.; Abkowitz, J.L.; Clemons, G.; Adamson, J.W. Quantitative Studies of Erythropoiesis in the Clinically Normal, Phlebotomized, and Feline Leukemia Virus-Infected Cat. Am. J. Vet. Res. 1986, 47, 2274–2277. [Google Scholar] [PubMed]
- Broudy, V.C.; Tait, J.F.; Powell, J.S. Recombinant Human Erythropoietin: Purification and Analysis of Carbohydrate Linkage. Arch Biochem. Biophys. 1988, 265, 329–336. [Google Scholar] [CrossRef]
- Broudy, V.C.; Lin, N.; Egrie, J.; de Haën, C.; Weiss, T.; Papayannopoulou, T.; Adamson, J.W. Identification of the Receptor for Erythropoietin on Human and Murine Erythroleukemia Cells and Modulation by Phorbol Ester and Dimethyl Sulfoxide. Proc. Natl. Acad. Sci. USA 1988, 85, 6513–6517. [Google Scholar] [CrossRef]
- Umemura, T.; Papayannopoulou, T.; Stamatoyannopoulos, G. The Mechanism of Expansion of Late Erythroid Progenitors during Erythroid Regeneration: Target Cells and Effects of Erythropoietin and Interleukin-3. Blood 1989, 73, 1993–1998. [Google Scholar] [CrossRef] [PubMed]
- Umemura, T.; Al-Khatti, A.; Donahue, R.E.; Papayannopoulou, T.; Stamatoyannopoulos, G. Effects of Interleukin-3 and Erythropoietin on in Vivo Erythropoiesis and F-Cell Formation in Primates. Blood 1989, 74, 1571–1576. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, K.; Shoemaker, C.; Rudersdorf, R.; Neill, S.D.; Kaufman, R.J.; Mufson, A.; Seehra, J.; Jones, S.S.; Hewick, R.; Fritsch, E.F.; et al. Isolation and Characterization of Genomic and CDNA Clones of Human Erythropoietin. Nature 1985, 313, 806–810. [Google Scholar] [CrossRef]
- Powell, J.S.; Berkner, K.L.; Lebo, R.V.; Adamson, J.W. Human Erythropoietin Gene: High Level Expression in Stably Transfected Mammalian Cells and Chromosome Localization. Proc. Natl. Acad. Sci. USA 1986, 83, 6465–6469. [Google Scholar] [CrossRef] [PubMed]
- Rocco, M.L.; Soligo, M.; Manni, L.; Aloe, L. Nerve Growth Factor: Early Studies and Recent Clinical Trials. Curr. Neuropharmacol. 2018, 16, 1455–1465. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Levi-Montalcini, R.; Hamburger, V. A nerve growth-stimulating factor isolated from sarcom as 37 and 180. Proc. Natl. Acad. Sci. USA 1954, 40, 1014–1018. [Google Scholar] [CrossRef]
- Hefti, F. Pharmacology of Nerve Growth Factor and Discovery of Tanezumab, an Anti-Nerve Growth Factor Antibody and Pain Therapeutic. Pharmacol. Res. 2020, 154, 104240. [Google Scholar] [CrossRef]
- Levi-Montalcini, R. The Nerve Growth Factor: Thirty-Five Years Later. EMBO J. 1987, 6, 1145–1154. [Google Scholar] [CrossRef]
- Goldwasser, E. Erythropoietin and Its Mode of Action. Blood Cells 1984, 10, 147–162. [Google Scholar]
- Wang, G.L.; Jiang, B.H.; Rue, E.A.; Semenza, G.L. Hypoxia-Inducible Factor 1 Is a Basic-Helix-Loop-Helix-PAS Heterodimer Regulated by Cellular O2 Tension. Proc. Natl. Acad. Sci. USA 1995, 92, 5510–5514. [Google Scholar] [CrossRef]
- Kular, D.; Macdougall, I.C. HIF Stabilizers in the Management of Renal Anemia: From Bench to Bedside to Pediatrics. Pediatr. Nephrol. 2019, 34, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Sanghani, N.S.; Haase, V.H. Hypoxia-Inducible Factor Activators in Renal Anemia: Current Clinical Experience. Adv. Chronic Kidney Dis. 2019, 26, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Lappin, K.M.; Mills, K.I.; Lappin, T.R. Erythropoietin in Bone Homeostasis—Implications for Efficacious Anemia Therapy. Stem. Cells Transl. Med. 2021, 10, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Koury, S.T.; Bondurant, M.C.; Koury, M.J. Localization of Erythropoietin Synthesizing Cells in Murine Kidneys by in Situ Hybridization. Blood 1988, 71, 524–527. [Google Scholar] [CrossRef] [PubMed]
- Gershon, D. Court Battle Ends at the Start. Nature 1989, 342, 846. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gershon, D. Amgen Scores a Knockout. Nature 1991, 350, 99. [Google Scholar] [CrossRef] [PubMed]
- Robin Herman One Patented Gene’s Astounding Success Story. The Washington Post. 16 June 1992. Available online: https://www.washingtonpost.com/archive/lifestyle/wellness/1992/06/16/one-patented-genes-astounding-success-story/8e16d63f-ded2-4736-9e2f-bbdd7e9bb7ac/ (accessed on 17 January 2024).
- Amgen, Inc., Plaintiff/Cross-Appellant, v. Chugai Pharmaceutical Co., Ltd., and Genetics Institute, Inc., Defendants-Appellants, 927 F.2d 1200 (Fed. Cir. 1991). Available online: https://Law.Justia.Com/Cases/Federal/Appellate-Courts/F2/927/1200/109675 (accessed on 17 January 2024).
- Robertson, D. First Round to Amgen in EPO Battle. Nat. Biotechnol. 2000, 18, 483. [Google Scholar] [CrossRef]
- Chahine, K. Amgen Preserves Erythropoietin Monopoly for Now. Nat. Biotechnol. 2001, 19, 188. [Google Scholar] [CrossRef] [PubMed]
- Pollack, A. Amgen Wins Initial Battle in Legal War over Patent. The New York Times. 27 April 2000. Available online: https://www.nytimes.com/2000/04/27/business/amgen-wins-initial-battle-in-legal-war-over-patent.html (accessed on 17 January 2024).
- Amgen, Inc. v. F. Hoffman-La Roche Ltd. Available online: https://Case-Law.Vlex.Com/Vid/Amgen-Inc-v-f-892716156 (accessed on 17 January 2024).
- Lee-Huang, S. Cloning and Expression of Human Erythropoietin CDNA in Escherichia Coli. Proc. Natl. Acad. Sci. USA 1984, 81, 2708–2712. [Google Scholar] [CrossRef] [PubMed]
- Toledo, J.R.; Sánchez, O.; Seguí, R.M.; García, G.; Montañez, M.; Zamora, P.A.; Rodríguez, M.P.; Cremata, J.A. High Expression Level of Recombinant Human Erythropoietin in the Milk of Non-Transgenic Goats. J. Biotechnol. 2006, 123, 225–235. [Google Scholar] [CrossRef]
- Dubé, S.; Fisher, J.W.; Powell, J.S. Glycosylation at Specific Sites of Erythropoietin Is Essential for Biosynthesis, Secretion, and Biological Function. J. Biol. Chem. 1988, 263, 17516–17521. [Google Scholar] [CrossRef] [PubMed]
- Delorme, E.; Lorenzini, T.; Giffin, J.; Martin, F.; Jacobsen, F.; Boone, T.; Elliott, S. Role of Glycosylation on the Secretion and Biological Activity of Erythropoietin. Biochemistry 1992, 31, 9871–9876. [Google Scholar] [CrossRef] [PubMed]
- Elliott, S.; Egrie, J.; Browne, J.; Lorenzini, T.; Busse, L.; Rogers, N.; Ponting, I. Control of RHuEPO Biological Activity: The Role of Carbohydrate. Exp. Hematol. 2004, 32, 1146–1155. [Google Scholar] [CrossRef] [PubMed]
- Cazzola, M.; Mercuriali, F.; Brugnara, C. Use of Recombinant Human Erythropoietin Outside the Setting of Uremia. Blood 1997, 89, 4248–4267. [Google Scholar] [CrossRef] [PubMed]
- Robertson, H.T.; Haley, N.R.; Guthrie, M.; Cardenas, D.; Eschbach, J.W.; Adamson, J.W. Recombinant Erythropoietin Improves Exercise Capacity in Anemic Hemodialysis Patients. Am. J. Kidney Dis. 1990, 15, 325–332. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, A.D.; Lodish, H.F.; Wong, G.G. Expression Cloning of the Murine Erythropoietin Receptor. Cell 1989, 57, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.S.; D’Andrea, A.D.; Haines, L.L.; Wong, G.G. Human Erythropoietin Receptor: Cloning, Expression, and Biologic Characterization. Blood 1990, 76, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, C.T.; Bae, K.S.; Chin, K.; Wada, Y.; Schechter, A.N.; Hankins, W.D. Cloning of the Human Erythropoietin Receptor Gene. Blood 1991, 78, 2548–2556. [Google Scholar] [CrossRef] [PubMed]
- Broudy, V.C.; Lin, N.; Brice, M.; Nakamoto, B.; Papayannopoulou, T. Erythropoietin Receptor Characteristics on Primary Human Erythroid Cells. Blood 1991, 77, 2583–2590. [Google Scholar] [CrossRef]
- Migliaccio, A.R.; Migliaccio, G.; D’Andrea, A.; Baiocchi, M.; Crotta, S.; Nicolis, S.; Ottolenghi, S.; Adamson, J.W. Response to Erythropoietin in Erythroid Subclones of the Factor-Dependent Cell Line 32D Is Determined by Translocation of the Erythropoietin Receptor to the Cell Surface. Proc. Natl. Acad. Sci. USA 1991, 88, 11086–11090. [Google Scholar] [CrossRef]
- Wu, H.; Klingmüller, U.; Acurio, A.; Hsiao, J.G.; Lodish, H.F. Functional Interaction of Erythropoietin and Stem Cell Factor Receptors Is Essential for Erythroid Colony Formation. Proc. Natl. Acad. Sci. USA 1997, 94, 1806–1810. [Google Scholar] [CrossRef] [PubMed]
- Federici, G.; Varricchio, L.; Martelli, F.; Falchi, M.; Picconi, O.; Francescangeli, F.; Contavalli, P.; Girelli, G.; Tafuri, A.; Petricoin, E.F.; et al. Phosphoproteomic Landscaping Identifies Non-Canonical CKIT Signaling in Polycythemia Vera Erythroid Progenitors. Front. Oncol. 2019, 9, 1245. [Google Scholar] [CrossRef]
- Stellacci, E.; Di Noia, A.; Di Baldassarre, A.; Migliaccio, G.; Battistini, A.; Migliaccio, A.R. Interaction between the Glucocorticoid and Erythropoietin Receptors in Human Erythroid Cells. Exp. Hematol. 2009, 37, 559–572. [Google Scholar] [CrossRef]
- Kurata, H.; Mancini, G.C.; Alespeiti, G.; Migliaccio, A.R.; Migliaccio, G. Stem Cell Factor Induces Proliferation and Differentiation of Fetal Progenitor Cells in the Mouse. Br. J. Haematol. 1998, 101, 676–687. [Google Scholar] [CrossRef] [PubMed]
- Varricchio, L.; Geer, E.B.; Martelli, F.; Mazzarini, M.; Funnell, A.; Bieker, J.J.; Papayannopoulou, T.; Migliaccio, A.R. Patients with Hypercortisolemic Cushing Disease Possess a Distinct Class of Hematopoietic Progenitor Cells Leading to Erythrocytosis. Haematologica 2022, 108, 1053–1067. [Google Scholar] [CrossRef] [PubMed]
- Lifshitz, L.; Prutchi-Sagiv, S.; Avneon, M.; Gassmann, M.; Mittelman, M.; Neumann, D. Non-Erythroid Activities of Erythropoietin: Functional Effects on Murine Dendritic Cells. Mol. Immunol. 2009, 46, 713–721. [Google Scholar] [CrossRef][Green Version]
- Deshet-Unger, N.; Kolomansky, A.; Ben-Califa, N.; Hiram-Bab, S.; Gilboa, D.; Liron, T.; Ibrahim, M.; Awida, Z.; Gorodov, A.; Oster, H.S.; et al. Erythropoietin Receptor in B Cells Plays a Role in Bone Remodeling in Mice. Theranostics 2020, 10, 8744–8756. [Google Scholar] [CrossRef]
- Suresh, S.; Lee, J.; Noguchi, C.T. Effects of Erythropoietin in White Adipose Tissue and Bone Microenvironment. Front. Cell Dev. Biol. 2020, 8, 584696. [Google Scholar] [CrossRef]
- Brines, M.; Grasso, G.; Fiordaliso, F.; Sfacteria, A.; Ghezzi, P.; Fratelli, M.; Latini, R.; Xie, Q.; Smart, J.; Su-Rick, C.; et al. Erythropoietin Mediates Tissue Protection through an Erythropoietin and Common β-Subunit Heteroreceptor. Proc. Natl. Acad. Sci. USA 2004, 101, 14907–14912. [Google Scholar] [CrossRef]
- Jubinsky, P.T.; Krijanovski, O.I.; Nathan, D.G.; Tavernier, J.; Sieff, C.A. The Beta Chain of the Interleukin-3 Receptor Functionally Associates with the Erythropoietin Receptor. Blood 1997, 90, 1867–1873. [Google Scholar] [CrossRef]
- Collino, M.; Thiemermann, C.; Cerami, A.; Brines, M. Flipping the Molecular Switch for Innate Protection and Repair of Tissues: Long-Lasting Effects of a Non-Erythropoietic Small Peptide Engineered from Erythropoietin. Pharmacol. Ther. 2015, 151, 32–40. [Google Scholar] [CrossRef]
- Yu, X.; Shacka, J.J.; Eells, J.B.; Suarez-Quian, C.; Przygodzki, R.M.; Beleslin-Cokic, B.; Lin, C.-S.; Nikodem, V.M.; Hempstead, B.; Flanders, K.C.; et al. Erythropoietin Receptor Signalling Is Required for Normal Brain Development. Development 2002, 129, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Arcasoy, M.O. The Non-haematopoietic Biological Effects of Erythropoietin. Br. J. Haematol. 2008, 141, 14–31. [Google Scholar] [CrossRef]
- Peng, B.; Kong, G.; Yang, C.; Ming, Y. Erythropoietin and Its Derivatives: From Tissue Protection to Immune Regulation. Cell Death Dis. 2020, 11, 79. [Google Scholar] [CrossRef] [PubMed]
- Oorschot, D.E.; Sizemore, R.J.; Amer, A.R. Treatment of Neonatal Hypoxic-Ischemic Encephalopathy with Erythropoietin Alone, and Erythropoietin Combined with Hypothermia: History, Current Status, and Future Research. Int. J. Mol. Sci. 2020, 21, 1487. [Google Scholar] [CrossRef] [PubMed]
- Najfeld, V. Clonal Origin of Leukemia–Revisited. A Tribute to Philip J Fialkow, MD. Leukemia 1998, 12, 106–107. [Google Scholar] [CrossRef][Green Version]
- Columns Staff, Medical School Dean, Wife and Guides Killed in Nepal. University of Washington Magazine. 1 December 1996, p. 108. Available online: https://magazine.washington.edu/medical-school-dean-wife-and-guides-killed-in-nepal/ (accessed on 17 January 2024).
- Ottolenghi, S.; Comi, P.; Giglioni, B.; Williamson, R.; Vullo, G.; Conconi, F. Direct Demonstration of β-Globin MRNA in Homozygous Ferrara Β0-Thalassaemia Patients. Nature 1977, 266, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Conconi, F.; Grazzi, G.; Casoni, I.; Guglielmini, C.; Borsetto, C.; Ballarin, E.; Mazzoni, G.; Patracchini, M.; Manfredini, F. The Conconi Test: Methodology After 12 Years of Application. Int. J. Sports Med. 1996, 17, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Bonarrigo, M. Costruire Campioni Dal Cuore Pazzo. Corriere della Sera (Suppl. Corriere Innovazione), 26 March 2021; p. 20. [Google Scholar]
- Claudio, G. Archivio Storico-La Gazzetta dello Sport. 20 November 2003. Available online: https://archiviostorico.gazzetta.it/2003/novembre/20/Conconi_troppo_tardi_ga_0_0311208163.shtml (accessed on 17 January 2024).
- Imai, N.; Kawamura, A.; Higuchi, M.; Oh-Eda, M.; Orita, T.; Kawaguchi, T.; Ochi, N. Physicochemical and Biological Comparison of Recombinant Human Erythropoietin with Human Urinary Erythropoietin1. J. Biochem. 1990, 107, 352–359. [Google Scholar] [CrossRef]
- Caldini, A.; Moneti, G.; Fanelli, A.; Bruschettini, A.; Mercurio, S.; Pieraccini, G.; Cini, E.; Ognibene, A.; Luceri, F.; Messeri, G. Epoetin Alpha, Epoetin Beta and Darbepoetin Alfa: Two-dimensional Gel Electrophoresis Isoforms Characterization and Mass Spectrometry Analysis. Proteomics 2003, 3, 937–941. [Google Scholar] [CrossRef]
- Jelkmann, W.; Lundby, C. Blood Doping and Its Detection. Blood 2011, 118, 2395–2404. [Google Scholar] [CrossRef] [PubMed]
- Epstein, D. Eero Mäntyranta–Finland’s Champion. 1937–2013: Obituary. Sci. Sport. 31 December 2013. Available online: https://sportsscientists.com/2013/12/eero-mantyranta-finlands-champion-1937-2013-obituary/ (accessed on 17 January 2024).
- de la Chapelle, A.; Sistonen, P.; Lehväslaiho, H.; Ikkala, E.; Juvonen, E. Familial Erythrocytosis Genetically Linked to Erythropoietin Receptor Gene. Lancet 1993, 341, 82–84. [Google Scholar] [CrossRef] [PubMed]
- Longmore, G.D. Erythropoietin Receptor Mutations and Olympic Glory. Nat. Genet. 1993, 4, 108–110. [Google Scholar] [CrossRef] [PubMed]
- Perrotta, S.; Cucciolla, V.; Ferraro, M.; Ronzoni, L.; Tramontano, A.; Rossi, F.; Scudieri, A.C.; Borriello, A.; Roberti, D.; Nobili, B.; et al. EPO Receptor Gain-of-Function Causes Hereditary Polycythemia, Alters CD34+ Cell Differentiation and Increases Circulating Endothelial Precursors. PLoS ONE 2010, 5, e12015. [Google Scholar] [CrossRef] [PubMed]
- Sergeyeva, A.; Gordeuk, V.R.; Tokarev, Y.N.; Sokol, L.; Prchal, J.F.; Prchal, J.T. Congenital Polycythemia in Chuvashia. Blood 1997, 89, 2148–2154. [Google Scholar] [CrossRef] [PubMed]
- Ang, S.O.; Chen, H.; Hirota, K.; Gordeuk, V.R.; Jelinek, J.; Guan, Y.; Liu, E.; Sergueeva, A.I.; Miasnikova, G.Y.; Mole, D.; et al. Disruption of Oxygen Homeostasis Underlies Congenital Chuvash Polycythemia. Nat. Genet. 2002, 32, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.; Percy, M.J.; Amos, C.I.; Guan, Y.; Shete, S.; Stockton, D.W.; McMullin, M.F.; Polyakova, L.A.; Ang, S.O.; Pastore, Y.D.; et al. The Worldwide Distribution of the VHL 598C>T Mutation Indicates a Single Founding Event. Blood 2004, 103, 1937–1940. [Google Scholar] [CrossRef] [PubMed]
- Perrotta, S.; Nobili, B.; Ferraro, M.; Migliaccio, C.; Borriello, A.; Cucciolla, V.; Martinelli, V.; Rossi, F.; Punzo, F.; Cirillo, P.; et al. Von Hippel-Lindau–Dependent Polycythemia Is Endemic on the Island of Ischia: Identification of a Novel Cluster. Blood 2006, 107, 514–519. [Google Scholar] [CrossRef]
- Gordeuk, V.; Prchal, J. Vascular Complications in Chuvash Polycythemia. Semin. Thromb. Hemost. 2006, 32, 289–294. [Google Scholar] [CrossRef]
- Bergström, J. New Aspects of Erythropoietin Treatment. J. Intern. Med. 1993, 233, 445–462. [Google Scholar] [CrossRef]
- ITALIA-44 ANNI IN CINA. Available online: https://www.Cronologia.It/2005/Disastr3.Htm (accessed on 17 January 2024).
- Concessione Italiana Di Tientsin. Available online: https://it.wikipedia.org/wiki/Concessione_italiana_di_Tientsin (accessed on 17 January 2024).
- Camporesi, S.; Hämäläinen, M. The Construction of Categories in Sport: Unfair Advantages, Equality of Opportunity and Strict Attainability. Eur. J. Sport Sci. 2021, 21, 1492–1499. [Google Scholar] [CrossRef]
- Binley, K.; Askham, Z.; Iqball, S.; Spearman, H.; Martin, L.; de Alwis, M.; Thrasher, A.J.; Ali, R.R.; Maxwell, P.H.; Kingsman, S.; et al. Long-Term Reversal of Chronic Anemia Using a Hypoxia-Regulated Erythropoietin Gene Therapy. Blood 2002, 100, 2406–2413. [Google Scholar] [CrossRef] [PubMed]
- Brzeziańska, E.; Domańska, D.; Jegier, A. Gene doping in sport–perspectives and risks. Biol. Sport 2014, 31, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Chen, F. Exogenous Insulin Antibody Syndrome (EIAS): A Clinical Syndrome Associated with Insulin Antibodies Induced by Exogenous Insulin in Diabetic Patients. Endocr. Connect. 2018, 7, R47–R55. [Google Scholar] [CrossRef] [PubMed]
- Brooks-Worrell, B.M.; Nielson, D.; Palmer, J.P. Insulin Autoantibodies and Insulin Antibodies Have Similar Binding Characteristics. Proc. Assoc. Am. Physicians 1999, 111, 92–96. [Google Scholar] [CrossRef]
- Verhelst, D.; Rossert, J.; Casadevall, N.; Krüger, A.; Eckardt, K.-U.; Macdougall, I.C. Treatment of Erythropoietin-Induced Pure Red Cell Aplasia: A Retrospective Study. Lancet 2004, 363, 1768–1771. [Google Scholar] [CrossRef]
- Casadevall, N. Pure Red Cell Aplasia and Anti-Erythropoietin Antibodies in Patients Treated with Epoetin. Nephrol. Dial. Transplant. 2003, 18, 37viii–41viii. [Google Scholar] [CrossRef] [PubMed]
- Ratanji, K.D.; Derrick, J.P.; Dearman, R.J.; Kimber, I. Immunogenicity of Therapeutic Proteins: Influence of Aggregation. J. Immunotoxicol. 2014, 11, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Buckner, F.S.; Eschbach, J.W.; Haley, N.R.; Davidson, R.C.; Adamson, J.W. Hypertension Following Erythropoietin Therapy in Anemic Hemodialysis Patients. Am. J. Hypertens. 1990, 3, 947–955. [Google Scholar] [CrossRef]
- Adamson, J.W.; Eschbach, J.W. The Use of Recombinant Human Erythropoietin [RHuEpo] in Man. Prog. Clin. Biol. Res. 1990, 352, 505–517. [Google Scholar]
- D’Andrea, A.D.; Rup, B.J.; Fisher, M.J.; Jones, S. Anti-Erythropoietin Receptor (EPO-R) Monoclonal Antibodies Inhibit Erythropoietin Binding and Neutralize Bioactivity. Blood 1993, 82, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Elliott, S.; Busse, L.; Bass, M.B.; Lu, H.; Sarosi, I.; Sinclair, A.M.; Spahr, C.; Um, M.; Van, G.; Begley, C.G. Anti-Epo Receptor Antibodies Do Not Predict Epo Receptor Expression. Blood 2006, 107, 1892–1895. [Google Scholar] [CrossRef] [PubMed]
- Lamon, S.; Zacharewicz, E.; Stephens, A.N.; Russell, A.P. EPO-Receptor Is Present in Mouse C2C12 and Human Primary Skeletal Muscle Cells but EPO Does Not Influence Myogenesis. Physiol. Rep. 2014, 2, e00256. [Google Scholar] [CrossRef] [PubMed]
- Chamorro, M.E.; Maltaneri, R.; Schiappacasse, A.; Nesse, A.; Vittori, D. Role of Protein Tyrosine Phosphatase 1B (PTP1B) in the Increased Sensitivity of Endothelial Cells to a Promigratory Effect of Erythropoietin in an Inflammatory Environment. Biol. Chem. 2020, 401, 1167–1180. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.K.; Matchett, K.B.; Coulter, J.A.; Yuen, H.-F.; McCrudden, C.M.; Zhang, S.-D.; Irwin, G.W.; Davidson, M.A.; Rülicke, T.; Schober, S.; et al. Erythropoietin Drives Breast Cancer Progression by Activation of Its Receptor EPOR. Oncotarget 2017, 8, 38251–38263. [Google Scholar] [CrossRef]
- Dunlop, E.A.; Percy, M.J.; Boland, M.P.; Maxwell, A.P.; Lappin, T.R. Induction of Signalling in Non-Erythroid Cells by Pharmacological Levels of Erythropoietin. Neurodegener. Dis. 2006, 3, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Brown, W.M.; Maxwell, P.; Graham, A.N.J.; Yakkundi, A.; Dunlop, E.A.; Shi, Z.; Johnston, P.G.; Lappin, T.R.J. Erythropoietin Receptor Expression in Non-Small Cell Lung Carcinoma: A Question of Antibody Specificity. Stem Cells 2007, 25, 718–722. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Hodges, V.M.; Dunlop, E.A.; Percy, M.J.; Maxwell, A.P.; El-Tanani, M.; Lappin, T.R.J. Erythropoietin-Induced Activation of the JAK2/STAT5, PI3K/Akt, and Ras/ERK Pathways Promotes Malignant Cell Behavior in a Modified Breast Cancer Cell Line. Mol. Cancer Res. 2010, 8, 615–626. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lopez, T.V.; Lappin, T.R.J.; Maxwell, P.; Shi, Z.; Lopez-Marure, R.; Aguilar, C.; Rocha-Zavaleta, L. Autocrine/Paracrine Erythropoietin Signalling Promotes JAK/STAT-dependent Proliferation of Human Cervical Cancer Cells. Int. J. Cancer 2011, 129, 2566–2576. [Google Scholar] [CrossRef]
- Berdel, W.E.; Oberberg, D.; Reufi, B.; Thiel, E. Studies on the Role of Recombinant Human Erythropoietin in the Growth Regulation of Human Nonhematopoietic Tumor Cells in Vitro. Ann. Hematol. 1991, 63, 5–8. [Google Scholar] [CrossRef]
- Sytkowski, A.J. Does Erythropoietin Have a Dark Side? Epo Signaling and Cancer Cells. Sci. STKE 2007, 2007, pe38. [Google Scholar] [CrossRef] [PubMed]
- Debeljak, N.; Solár, P.; Sytkowski, A.J. Erythropoietin and Cancer: The Unintended Consequences of Anemia Correction. Front. Immunol. 2014, 5, 563. [Google Scholar] [CrossRef] [PubMed]
- Bohlius, J.; Schmidlin, K.; Brillant, C.; Schwarzer, G.; Trelle, S.; Seidenfeld, J.; Zwahlen, M.; Clarke, M.; Weingart, O.; Kluge, S.; et al. Recombinant Human Erythropoiesis-Stimulating Agents and Mortality in Patients with Cancer: A Meta-Analysis of Randomised Trials. Lancet 2009, 373, 1532–1542. [Google Scholar] [CrossRef] [PubMed]
- Drüeke, T.B.; Locatelli, F.; Clyne, N.; Eckardt, K.-U.; Macdougall, I.C.; Tsakiris, D.; Burger, H.-U.; Scherhag, A. Normalization of Hemoglobin Level in Patients with Chronic Kidney Disease and Anemia. N. Engl. J. Med. 2006, 355, 2071–2084. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Szczech, L.; Tang, K.L.; Barnhart, H.; Sapp, S.; Wolfson, M.; Reddan, D. Correction of Anemia with Epoetin Alfa in Chronic Kidney Disease. N. Engl. J. Med. 2006, 355, 2085–2098. [Google Scholar] [CrossRef] [PubMed]
- Pollack, A. Studies Show Anemia Drugs May Harm Cancer Patients. The New York Times. 27 February 2007. Available online: https://www.nytimes.com/2007/02/27/health/27drug.html (accessed on 17 January 2024).
- Maxwell, P.; Melendez-Rodríguez, F.; Matchett, K.B.; Aragones, J.; Ben-Califa, N.; Jaekel, H.; Hengst, L.; Lindner, H.; Bernardini, A.; Brockmeier, U.; et al. Novel Antibodies Directed against the Human Erythropoietin Receptor: Creating a Basis for Clinical Implementation. Br. J. Haematol. 2015, 168, 429–442. [Google Scholar] [CrossRef] [PubMed]
- Hammond, W.P.; Price, T.H.; Souza, L.M.; Dale, D.C. Treatment of Cyclic Neutropenia with Granulocyte Colony-Stimulating Factor. N. Engl. J. Med. 1989, 320, 1306–1311. [Google Scholar] [CrossRef] [PubMed]
- Migliaccio, A.R.; Migliaccio, G.; Dale, D.C.; Hammond, W.P. Hematopoietic Progenitors in Cyclic Neutropenia: Effect of Granulocyte Colony-Stimulating Factor in Vivo. Blood 1990, 75, 1951–1959. [Google Scholar] [CrossRef] [PubMed]
- Morris, E.S.; MacDonald, K.P.A.; Hill, G.R. Stem Cell Mobilization with G-CSF Analogs: A Rational Approach to Separate GVHD and GVL? Blood 2006, 107, 3430–3435. [Google Scholar] [CrossRef][Green Version]
- Welte, K. G-CSF: Filgrastim, Lenograstim and Biosimilars. Expert Opin. Biol. Ther. 2014, 14, 983–993. [Google Scholar] [CrossRef]
- Trivedi, M.; Martinez, S.; Corringham, S.; Medley, K.; Ball, E.D. Review and Revision of Clinical Practice of Using G-CSF after Autologous and Allogeneic Hematopoietic Stem Cell Transplantation at UCSD. J. Oncol. Pharm. Pract. 2011, 17, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Al-Khatti, A.; Veith, R.W.; Papayannopoulou, T.; Fritsch, E.F.; Goldwasser, E.; Stamatoyannopoulos, G. Stimulation of Fetal Hemoglobin Synthesis by Erythropoietin in Baboons. N. Engl. J. Med. 1987, 317, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Lavelle, D.; Molokie, R.; Ducksworth, J.; DeSimone, J. Effects of Hydroxurea, Stem Cell Factor, and Erythropoietin in Combination on Fetal Hemoglobin in the Baboon. Exp. Hematol. 2001, 29, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, F.; Del Vecchio, L.; Elliott, S. The Anaemia Treatment Journey of CKD Patients: From Epoetins to Hypoxia-Inducible Factor-Prolyl Hydroxylase Inhibitors. Clin. Kidney J. 2023, 16, 1563–1579. [Google Scholar] [CrossRef]
- Lappin, T.R.; Rich, I.; Goldwasser, E. The Effect of Erythropoietin and Other Factors on DNA Synthesis by Mouse Spleen Cells. Exp. Hematol. 1983, 11, 661–666. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Migliaccio, A.R. Erythropoietin: A Personal Alice in Wonderland Trip in the Shadow of the Giants. Biomolecules 2024, 14, 408. https://doi.org/10.3390/biom14040408
Migliaccio AR. Erythropoietin: A Personal Alice in Wonderland Trip in the Shadow of the Giants. Biomolecules. 2024; 14(4):408. https://doi.org/10.3390/biom14040408
Chicago/Turabian StyleMigliaccio, Anna Rita. 2024. "Erythropoietin: A Personal Alice in Wonderland Trip in the Shadow of the Giants" Biomolecules 14, no. 4: 408. https://doi.org/10.3390/biom14040408
APA StyleMigliaccio, A. R. (2024). Erythropoietin: A Personal Alice in Wonderland Trip in the Shadow of the Giants. Biomolecules, 14(4), 408. https://doi.org/10.3390/biom14040408





