The Chicken Egg: An Advanced Material for Tissue Engineering
Abstract
1. Introduction
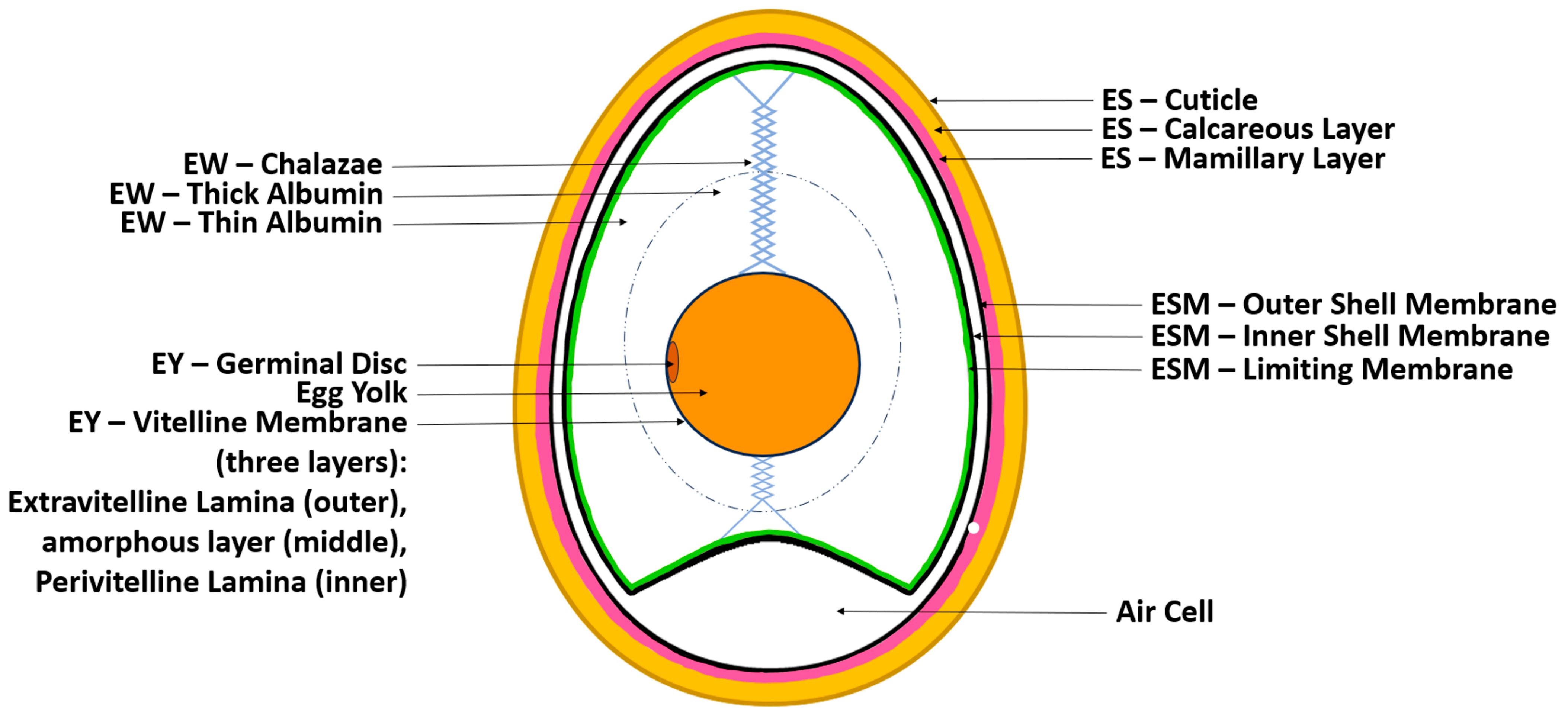
1.1. The Chicken Egg’s Structure and Physicochemical Properties
1.2. The Chicken Egg’s Composition
1.3. The Chicken Egg’s Biological Activity
2. Advanced Biomedical Applications of Egg Materials
2.1. Egg White (EW)
| Crosslinker/Gelation Methods | Crosslinks | Feature(s) | Application(s) | Reference(s) | |
|---|---|---|---|---|---|
| Electrospun egg white/polyvinyl alcohol fiber | 1-(3-dimethylaminopropyl)-3-ethyl carbodiimide (EDC) | Amide bonds between amines and carboxylic acid groups | Good spinnability (with 40–60% EW), mechanical strength, resistance to degradation, and good biocompatibility. | Wound healing | [48] |
| Ovalbumin-flavonoids self-assembled hydrogels | Flavonoids |
| Promoted the gelation of ovalbumin in low concentration; generated a hydrogel more elastic than viscous. | N/A | [68] |
| Ovalbumin hydrogel | 1,4-butanediol diglycidyl ether (BDE) | Amide bonds | Uniform pore size | N/A | [62] |
| EW-alginate hydrogel | Calcium chloride | Ionic bonds | Easily reversible from hydrogel to solution |
| [10,57,72] |
| EW-hydroxypropyl chitosan hydrogel | Chitosan | Intramolecular hydrogen bonding | Biocompatibility, Anti-inflammation Antibacterial property Self-healing |
| [49] |
| EW-gelatin hydrogel | Glutaraldehyde | Disulfide bonds | Tunable viscosity |
| [79] |
| EW hydrogel | Alkalization and ionic crosslinking | Hydrogen bonding and hydrophobic interactions, Ionic bonding. | Tunable mechanical properties (elongation ratio, tensile strength and Young’s Modulus) |
| [23,45,50] |
2.2. Egg Yolk (EY)
2.3. Eggshell (ES) and Eggshell Membrane (ESM)
3. Conclusions and Future Remarks
Funding
Conflicts of Interest
References
- Perez-Puyana, V.; Jiménez-Rosado, M.; Romero, A.; Guerrero, A. Polymer-Based Scaffolds for Soft-Tissue Engineering. Polymers 2020, 12, 1566. [Google Scholar] [CrossRef] [PubMed]
- Kalirajan, C.; Dukle, A.; Nathanael, A.J.; Oh, T.-H.; Manivasagam, G. A Critical Review on Polymeric Biomaterials for Biomedical Applications. Polymers 2021, 13, 3015. [Google Scholar] [CrossRef] [PubMed]
- Lynch, C.R.; Kondiah, P.P.D.; Choonara, Y.E. Advanced Strategies for Tissue Engineering in Regenerative Medicine: A Biofabrication and Biopolymer Perspective. Molecules 2021, 26, 2518. [Google Scholar] [CrossRef] [PubMed]
- Langer, R.; Vacanti, J.P. Tissue Engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Dawson, D.R.; El-Ghannam, A.; Van Sickels, J.E.; Naung, N.Y. Tissue Engineering: What is New? Dent. Clin. N. Am. 2019, 63, 433–445. [Google Scholar] [CrossRef] [PubMed]
- Aisenbrey, E.A.; Murphy, W.L. Synthetic alternatives to Matrigel. Nat. Rev. Mater. 2020, 5, 539–551. [Google Scholar] [CrossRef] [PubMed]
- Abdulghani, S.; Mitchell, G. Biomaterials for In Situ Tissue Regeneration: A Review. Biomolecules 2019, 9, 750. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, O.; Cooper-White, J.; Janmey, P.A.; Mooney, D.J.; Shenoy, V.B. Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature 2020, 584, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.; Lee, H.; Luo, L.; Kyriakides, T.R. Extracellular matrix-derived biomaterials in engineering cell function. Biotechnol. Adv. 2020, 42, 107421. [Google Scholar] [CrossRef]
- Pham, H.M.; Zhang, Y.; Munguia-Lopez, J.G.; Tran, S.D. Egg White Alginate as a Novel Scaffold Biomaterial for 3D Salivary Cell Culturing. Biomimetics 2021, 7, 5. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Rahmati, M.; Pennisi, C.P.; Mobasheri, A.; Mozafari, M. Bioengineered Scaffolds for Stem Cell Applications in Tissue Engineering and Regenerative Medicine. Adv. Exp. Med. Biol. 2018, 1107, 73–89. [Google Scholar] [CrossRef] [PubMed]
- Magazzù, A.; Marcuello, C. Investigation of Soft Matter Nanomechanics by Atomic Force Microscopy and Optical Tweezers: A Comprehensive Review. Nanomaterials 2023, 13, 963. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, M.P.; Chavali, M.S. Recent advances in biomaterials for 3D scaffolds: A review. Bioact. Mater. 2019, 4, 271–292. [Google Scholar] [CrossRef] [PubMed]
- Rahmati, M.; Pennisi, C.P.; Budd, E.; Mobasheri, A.; Mozafari, M. Biomaterials for Regenerative Medicine: Historical Perspectives and Current Trends. In Cell Biology and Translational Medicine; Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2018; Volume 1119, pp. 1–19. [Google Scholar] [CrossRef]
- Dong, X.; Zhang, Y.-Q. An insight on egg white: From most common functional food to biomaterial application. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 1045–1058. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.A.; Camci-Unal, G. Unconventional Tissue Engineering Materials in Disguise. Trends Biotechnol. 2020, 38, 178–190. [Google Scholar] [CrossRef] [PubMed]
- Jalili-Firoozinezhad, S.; Filippi, M.; Mohabatpour, F.; Letourneur, D.; Scherberich, A. Chicken egg white: Hatching of a new old biomaterial. Mater. Today 2020, 40, 193–214. [Google Scholar] [CrossRef]
- Hughes, C.S.; Postovit, L.M.; Lajoie, G.A. Matrigel: A complex protein mixture required for optimal growth of cell culture. Proteomics 2010, 10, 1886–1890. [Google Scholar] [CrossRef] [PubMed]
- Aswathy, S.H.; Narendrakumar, U.; Manjubala, I. Commercial hydrogels for biomedical applications. Heliyon 2020, 6, e03719. [Google Scholar] [CrossRef]
- Mahdavi, S.; Amirsadeghi, A.; Jafari, A.; Niknezhad, S.V.; Bencherif, S.A. Avian Egg: A Multifaceted Biomaterial for Tissue Engineering. Ind. Eng. Chem. Res. 2021, 60, 17348–17364. [Google Scholar] [CrossRef]
- Liu, T.; Zhao, Y.; Wu, N.; Chen, S.; Xu, M.; Du, H.; Yao, Y.; Tu, Y. Egg white protein-based delivery system for bioactive substances: A review. Crit. Rev. Food Sci. Nutr. 2022, 64, 617–637. [Google Scholar] [CrossRef] [PubMed]
- Chang, Q.; Darabi, M.A.; Liu, Y.; He, Y.; Zhong, W.; Mequanin, K.; Li, B.; Lu, F.; Xing, M.M.Q. Hydrogels from natural egg white with extraordinary stretchability, direct-writing 3D printability and self-healing for fabrication of electronic sensors and actuators. J. Mater. Chem. A 2019, 7, 24626–24640. [Google Scholar] [CrossRef]
- Croguennec, T.; Nau, F.; Brulé, G. Influence of pH and Salts on Egg White Gelation. J. Food Sci. 2002, 67, 608–614. [Google Scholar] [CrossRef]
- Rodríguez-Navarro, A.B.; Domínguez-Gasca, N.; Muñoz, A.; Ortega-Huertas, M. Change in the chicken eggshell cuticle with hen age and egg freshness. Poult. Sci. 2013, 92, 3026–3035. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.T.; Yang, J.M.; Lai, C.W.; Cheng, Y.H.; Lin, C.C.; Yeh, C.W. Characterization and adsorption properties of eggshells and eggshell membrane. Bioresour. Technol. 2006, 97, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Bellairs, R.; Osmond, M. Chapter 1—The Hen’s Egg and its Formation. In Atlas of Chick Development, 3rd ed.; Bellairs, R., Osmond, M., Eds.; Academic Press: Boston, MA, USA, 2014; pp. 1–6. [Google Scholar] [CrossRef]
- Mensah, R.A.; Jo, S.B.; Kim, H.; Park, S.M.; Patel, K.D.; Cho, K.J.; Cook, M.T.; Kirton, S.B.; Hutter, V.; Sidney, L.E.; et al. The eggshell membrane: A potential biomaterial for corneal wound healing. J. Biomater. Appl. 2021, 36, 912–929. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhou, K.; Li, D.; Guyonnet, V.; Hincke, M.T.; Mine, Y. Avian Eggshell Membrane as a Novel Biomaterial: A Review. Foods 2021, 10, 2178. [Google Scholar] [CrossRef] [PubMed]
- Sharp, P.F.; Powell, C.K. Increase in the pH of the White and Yolk of Hens’ Eggs. Ind. Eng. Chem. 1931, 23, 196–199. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Fan, Q.; Teng, C.; Xie, W.; Shi, Y.; Su, Y.; Yang, Y. Combination effects of NaOH and NaCl on the rheology and gel characteristics of hen egg white proteins. Food Chem. 2018, 250, 1–6. [Google Scholar] [CrossRef]
- Razi, S.M.; Fahim, H.; Amirabadi, S.; Rashidinejad, A. An overview of the functional properties of egg white proteins and their application in the food industry. Food Hydrocoll. 2023, 135, 108183. [Google Scholar] [CrossRef]
- Nys, Y.; Guyot, N. 6—Egg formation and chemistry. In Improving the Safety and Quality of Eggs and Egg Products; Nys, Y., Bain, M., Van Immerseel, F., Eds.; Woodhead Publishing: Cambridge, UK, 2011; pp. 83–132. [Google Scholar] [CrossRef]
- Lesnierowski, G.; Stangierski, J. What’s new in chicken egg research and technology for human health promotion?—A review. Trends Food Sci. Technol. 2018, 71, 46–51. [Google Scholar] [CrossRef]
- Chambers, J.R.; Zaheer, K.; Akhtar, H.; Abdel-Aal, E.-S.M. Chapter 1—Chicken Eggs. In Egg Innovations and Strategies for Improvements; Hester, P.Y., Ed.; Academic Press: San Diego, CA, USA, 2017; pp. 1–9. [Google Scholar] [CrossRef]
- Sugino, H.; Nitoda, T.; Juneja, L. General chemical composition of hen eggs. In Hen Eggs; CRC Press: Boca Raton, FL, USA, 1997; pp. 13–24. [Google Scholar]
- Samiullah, S.; Roberts, J.R. The eggshell cuticle of the laying hen. World’s Poult. Sci. J. 2014, 70, 693–708. [Google Scholar] [CrossRef]
- Abeyrathne, E.D.; Lee, H.Y.; Ahn, D.U. Egg white proteins and their potential use in food processing or as nutraceutical and pharmaceutical agents—A review. Poult. Sci. 2013, 92, 3292–3299. [Google Scholar] [CrossRef] [PubMed]
- Anton, M. Egg yolk: Structures, functionalities and processes. J. Sci. Food Agric. 2013, 93, 2871–2880. [Google Scholar] [CrossRef] [PubMed]
- Dadhich, P.; Das, B.; Pal, P.; Srivas, P.K.; Dutta, J.; Ray, S.; Dhara, S. A Simple Approach for an Eggshell-Based 3D-Printed Osteoinductive Multiphasic Calcium Phosphate Scaffold. ACS Appl. Mater. Interfaces 2016, 8, 11910–11924. [Google Scholar] [CrossRef]
- Xiao, N.; Huang, X.; He, W.; Yao, Y.; Wu, N.; Xu, M.; Du, H.; Zhao, Y.; Tu, Y. A review on recent advances of egg byproducts: Preparation, functional properties, biological activities and food applications. Food Res. Int. 2021, 147, 110563. [Google Scholar] [CrossRef] [PubMed]
- Xiao, N.; Zhao, Y.; Yao, Y.; Wu, N.; Xu, M.; Du, H.; Tu, Y. Biological Activities of Egg Yolk Lipids: A Review. J. Agric. Food Chem. 2020, 68, 1948–1957. [Google Scholar] [CrossRef] [PubMed]
- Zambrowicz, A.; Dąbrowska, A.; Bobak, Ł.; Szołtysik, M. Egg yolk proteins and peptides with biological activity. Adv. Hyg. Exp. Med. 2014, 68, 1524–1529. [Google Scholar] [CrossRef]
- Park, J.-M.; Kim, M.; Park, H.-S.; Jang, A.; Min, J.; Kim, Y.-H. Immobilization of lysozyme-CLEA onto electrospun chitosan nanofiber for effective antibacterial applications. Int. J. Biol. Macromol. 2013, 54, 37–43. [Google Scholar] [CrossRef]
- Guo, L.; Niu, X.; Chen, X.; Lu, F.; Gao, J.; Chang, Q. 3D direct writing egg white hydrogel promotes diabetic chronic wound healing via self-relied bioactive property. Biomaterials 2022, 282, 121406. [Google Scholar] [CrossRef] [PubMed]
- Vilcacundo, R.; Méndez, P.; Reyes, W.; Romero, H.; Pinto, A.; Carrillo, W. Antibacterial Activity of Hen Egg White Lysozyme Denatured by Thermal and Chemical Treatments. Sci. Pharm. 2018, 86, 48. [Google Scholar] [CrossRef]
- Patty, D.J.; Nugraheni, A.D.; Ana, I.D.; Yusuf, Y. In vitro bioactivity of 3D microstructure hydroxyapatite/collagen based-egg white as an antibacterial agent. J. Biomed. Mater. Res. Part B Appl. Biomater. 2022, 110, 1412–1424. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Zou, Q.; Zhu, K.; Yuan, D.; Ma, M.; Ye, C. Electrospun egg white/polyvinyl alcohol fiber dressing to accelerate wound healing. J. Polym. Res. 2021, 28, 67. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, Y.; Chu, Y.; Chang, Q. Facile synthesis of hydroxypropyl chitosan-egg white hydrogel dressing with antibacterial and antioxidative activities for accelerating the healing of burn wounds. J. Mater. Chem. B 2023, 11, 4330–4345. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ma, Y.; Niu, X.; Su, T.; Huang, X.; Lu, F.; Chang, Q. Direct three-dimensional printed egg white hydrogel wound dressing promotes wound healing with hitching adipose stem cells. Front. Bioeng. Biotechnol. 2022, 10, 930551. [Google Scholar] [CrossRef] [PubMed]
- Sirousazar, M.; Jahani-Javanmardi, A.; Kheiri, F.; Hassan, Z.M. In vitro and in vivo assays on egg white/polyvinyl alcohol/clay nanocomposite hydrogel wound dressings. J. Biomater. Sci. Polym. Ed. 2016, 27, 1569–1583. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.-H.; Kim, Y.K.; Kim, K.-H.; Kwon, T.-Y.; Vaezmomeni, S.Z.; Samiei, M.; Aghazadeh, M.; Davaran, S.; Mahkam, M.; Asadi, G.; et al. Synthesis, characterization, biocompatibility of hydroxyapatite-natural polymers nanocomposites for dentistry applications. Artif. Cells Nanomed. Biotechnol. 2016, 44, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Sangeetha, R.; Madheswari, D.; Priya, G. Fabrication of poly (methyl methacrylate)/Ce/Cu substituted apatite/Egg white (Ovalbumin) biocomposite owning adjustable properties: Towards bone tissue rejuvenation. J. Photochem. Photobiol. B Biol. 2018, 187, 162–169. [Google Scholar] [CrossRef]
- Singh, S.; Bhushan, S.; Khan, H.; Chaudhari, L.R.; Ali, A.; Das, A.; Barui, A.; Negi, Y.S.; Joshi, M.G.; Dutt, D. Surgical cotton microfibers loaded with proteins and apatite: A potential platform for bone tissue engineering. Int. J. Biol. Macromol. 2023, 236, 123812. [Google Scholar] [CrossRef]
- Renkler, N.Z.; Ergene, E.; Gokyer, S.; Tuzlakoglu Ozturk, M.; Yilgor Huri, P.; Tuzlakoglu, K. Facile modification of polycaprolactone nanofibers with egg white protein. J. Mater. Sci. Mater. Med. 2021, 32, 34. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Yin, J.; Jiang, W.; Liang, B.; Pehkonen, S.O.; Choong, C. Enhancing antibacterial activity of surface-grafted chitosan with immobilized lysozyme on bioinspired stainless steel substrates. Colloids Surf. B Biointerfaces 2013, 106, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Pham, H.M.; Munguia-Lopez, J.G.; Kinsella, J.M.; Tran, S.D. The Optimization of a Novel Hydrogel—Egg White-Alginate for 2.5D Tissue Engineering of Salivary Spheroid-Like Structure. Molecules 2020, 25, 5751. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Yang, D.; Cui, D.; Wang, Z.; Guo, L. Egg white-mediated green synthesis of silver nanoparticles with excellent biocompatibility and enhanced radiation effects on cancer cells. Int. J. Nanomed. 2012, 7, 2101–2107. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhu, H.; Liu, X.; Yang, F.; Yang, X. Egg white coagulum: A precisely tailorable membrane for biomimetic multilevel structured nanomaterials. Sci. Rep. 2013, 3, 1464. [Google Scholar] [CrossRef] [PubMed]
- Jalili-Firoozinezhad, S.; Rajabi-Zeleti, S.; Mohammadi, P.; Gaudiello, E.; Bonakdar, S.; Solati-Hashjin, M.; Marsano, A.; Aghdami, N.; Scherberich, A.; Baharvand, H.; et al. Facile Fabrication of Egg White Macroporous Sponges for Tissue Regeneration. Adv. Healthc. Mater. 2015, 4, 2281–2290. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.-Z.; Dong, X.; Zhang, Y.-Q. A mechanically robust egg white hydrogel scaffold with excellent biocompatibility by three-step green processing. Sci. China Technol. Sci. 2022, 65, 1599–1612. [Google Scholar] [CrossRef]
- Luo, B.; Choong, C. Porous ovalbumin scaffolds with tunable properties: A resource-efficient biodegradable material for tissue engineering applications. J. Biomater. Appl. 2015, 29, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Zhang, Y.-Q. A Novel Mechanically Robust and Biodegradable Egg White Hydrogel Membrane by Combined Unidirectional Nanopore Dehydration and Annealing. Int. J. Mol. Sci. 2023, 24, 12661. [Google Scholar] [CrossRef]
- Li, J.-X.; Zhang, Y.-Q. Mechanically Robust Poly(vinyl alcohol)-Egg White Composite Hydrogel with Enhancing Biocompatibility by Unidirectional Nanopore Dehydration. ACS Omega 2023, 8, 33763–33773. [Google Scholar] [CrossRef]
- Carpena, N.T.; Abueva, C.D.G.; Padalhin, A.R.; Lee, B.-T. Evaluation of egg white ovomucin-based porous scaffold as an implantable biomaterial for tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 2107–2117. [Google Scholar] [CrossRef] [PubMed]
- Offengenden, M.; Wu, J. Egg white ovomucin gels: Structured fluids with weak polyelectrolyte properties. RSC Adv. 2013, 3, 910–917. [Google Scholar] [CrossRef]
- Shojaee, M.; Navaee, F.; Jalili-Firoozinezhad, S.; Faturechi, R.; Majidi, M.; Bonakdar, S. Fabrication and characterization of ovalbumin films for wound dressing applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 48, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Yang, L.; Dai, T.; Zhu, Z.; Chen, H.; Wu, J.; Gong, E.S. Flavonoids enhance gel strength of ovalbumin: Properties, structures, and interactions. Food Chem. 2022, 387, 132892. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chen, Z.; Li, J.; Xu, M.; Shao, Y.; Tu, Y. Formation mechanism of ovalbumin gel induced by alkali. Food Hydrocoll. 2016, 61, 390–398. [Google Scholar] [CrossRef]
- Li, X.; Zhang, P.; Li, Q.; Wang, H.; Yang, C. Direct-ink-write printing of hydrogels using dilute inks. iScience 2021, 24, 102319. [Google Scholar] [CrossRef] [PubMed]
- Mousseau, Y.; Mollard, S.; Qiu, H.; Richard, L.; Cazal, R.; Nizou, A.; Vedrenne, N.; Rémi, S.; Baaj, Y.; Fourcade, L.; et al. In vitro 3D angiogenesis assay in egg white matrix: Comparison to Matrigel, compatibility to various species, and suitability for drug testing. Lab. Investig. 2014, 94, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Shen, Z.; Zhang, H.; Liu, S.; Feng, R.; Feng, J.; Ramalingam, M. Designed and fabrication of triple-layered vascular scaffold with microchannels. J. Biomater. Sci. Polym. Ed. 2021, 32, 714–734. [Google Scholar] [CrossRef]
- Patty, D.J.; Nugraheni, A.D.; Ana, I.D.; Yusuf, Y. Dual functional carbonate-hydroxyapatite nanocomposite from Pinctada maxima and egg-white for bone tissue engineering. J. Biomater. Sci. Polym. Ed. 2022, 33, 1043–1062. [Google Scholar] [CrossRef]
- Duan, B.; Yang, M.; Chao, Q.; Wang, L.; Zhang, L.; Gou, M.; Li, Y.; Liu, C.; Lu, K. Preparation and Properties of Egg White Dual Cross-Linked Hydrogel with Potential Application for Bone Tissue Engineering. Polymers 2022, 14, 5116. [Google Scholar] [CrossRef]
- Liu, S.; Kilian, D.; Ahlfeld, T.; Hu, Q.; Gelinsky, M. Egg white improves the biological properties of an alginate-methylcellulose bioink for 3D bioprinting of volumetric bone constructs. Biofabrication 2023, 15, 025013. [Google Scholar] [CrossRef] [PubMed]
- Augst, A.D.; Kong, H.J.; Mooney, D.J. Alginate hydrogels as biomaterials. Macromol. Biosci. 2006, 6, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Drury, J.L.; Dennis, R.G.; Mooney, D.J. The tensile properties of alginate hydrogels. Biomaterials 2004, 25, 3187–3199. [Google Scholar] [CrossRef] [PubMed]
- Pele, K.G.; Amaveda, H.; Mora, M.; Marcuello, C.; Lostao, A.; Alamán-Díez, P.; Pérez-Huertas, S.; Ángeles Pérez, M.; García-Aznar, J.M.; García-Gareta, E. Hydrocolloids of Egg White and Gelatin as a Platform for Hydrogel-Based Tissue Engineering. Gels 2023, 9, 505. [Google Scholar] [CrossRef] [PubMed]
- Charbonneau, A.M.; Kinsella, J.M.; Tran, S.D. 3D Cultures of Salivary Gland Cells in Native or Gelled Egg Yolk Plasma, Combined with Egg White and 3D-Printing of Gelled Egg Yolk Plasma. Materials 2019, 12, 3480. [Google Scholar] [CrossRef] [PubMed]
- Charbonneau, A.M.; Tran, S.D. 3D Cell Culture of Human Salivary Glands Using Nature-Inspired Functional Biomaterials: The Egg Yolk Plasma and Egg White. Materials 2020, 13, 4807. [Google Scholar] [CrossRef] [PubMed]
- Rodil, A.; Laca, A.; Paredes, B.; Rendueles, M.; Meana, Á.; Díaz, M. Gels prepared from egg yolk and its fractions for tissue engineering. Biotechnol. Prog. 2016, 32, 1577–1583. [Google Scholar] [CrossRef] [PubMed]
- Yenilmez, E.; Başaran, E.; Arslan, R.; Berkman, M.S.; Güven, U.M.; Bayçu, C.; Yazan, Y. Chitosan gel formulations containing egg yolk oil and epidermal growth factor for dermal burn treatment. Pharmazie 2015, 70, 67–73. [Google Scholar]
- Park, S.; Hong, S.; Kim, J.; Son, S.Y.; Lee, H.; Kim, S.J. Eco friendly nanofluidic platforms using biodegradable nanoporous materials. Sci. Rep. 2021, 11, 3804. [Google Scholar] [CrossRef]
- Pereira, E.P.V.; van Tilburg, M.F.; Florean, E.; Guedes, M.I.F. Egg yolk antibodies (IgY) and their applications in human and veterinary health: A review. Int. Immunopharmacol. 2019, 73, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Duan, S.; Liu, X.; Wang, H.; Ding, S.; Chen, Y.; Xie, J.; Tian, J.; Yu, N.; Ge, P.; et al. Chicken Egg Yolk Antibodies (IgYs) block the binding of multiple SARS-CoV-2 spike protein variants to human ACE2. Int. Immunopharmacol. 2021, 90, 107172. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Zhang, C.; Lyu, J.; Yi, P.; Shen, X.; Tang, B.; Zhao, H.; Ren, B.; Kuang, Y.; Zhou, L.; et al. Egg yolk immunoglobulin (IgY) targeting SARS-CoV-2 S1 as potential virus entry blocker. J. Appl. Microbiol. 2022, 132, 2421–2430. [Google Scholar] [CrossRef] [PubMed]
- Chuysinuan, P.; Nooeaid, P.; Thanyacharoen, T.; Techasakul, S.; Pavasant, P.; Kanjanamekanant, K. Injectable eggshell-derived hydroxyapatite-incorporated fibroin-alginate composite hydrogel for bone tissue engineering. Int. J. Biol. Macromol. 2021, 193, 799–808. [Google Scholar] [CrossRef]
- Huang, K.; Hou, J.; Gu, Z.; Wu, J. Egg-White-/Eggshell-Based Biomimetic Hybrid Hydrogels for Bone Regeneration. ACS Biomater. Sci. Eng. 2019, 5, 5384–5391. [Google Scholar] [CrossRef] [PubMed]
- Torres-Mansilla, A.; Hincke, M.; Voltes, A.; López-Ruiz, E.; Baldión, P.A.; Marchal, J.A.; Álvarez-Lloret, P.; Gómez-Morales, J. Eggshell Membrane as a Biomaterial for Bone Regeneration. Polymers 2023, 15, 1342. [Google Scholar] [CrossRef] [PubMed]
- Torres-Mansilla, A.; Alvarez-Lloret, P.; Voltes-Martinez, A.; Lopez-Ruiz, E.; Baldion, P.A.; Marchal, J.A.; Gomez-Morales, J. Apatite-coated outer layer eggshell membrane: A novel osteoinductive biohybrid composite for guided bone/tissue regeneration. Biomater. Adv. 2023, 154, 213605. [Google Scholar] [CrossRef] [PubMed]
- Mensah, R.A.; Salim, K.; Peszko, K.; Diop, S.; Wong, T.H.; Chau, D.Y. The chicken eggshell membrane: A versatile, sustainable, biological material for translational biomedical applications. Biomed. Mater. 2023, 18, 042001. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Liu, T.; Zhang, X.; Jian, Z.; Xia, H.; Yang, J.; Hu, X.; Xing, M.; Luo, G.; Wu, J. Fabrication of KR-12 peptide-containing hyaluronic acid immobilized fibrous eggshell membrane effectively kills multi-drug-resistant bacteria, promotes angiogenesis and accelerates re-epithelialization. Int. J. Nanomed. 2019, 14, 3345–3360. [Google Scholar] [CrossRef]
- Ahmed, T.A.E.; Suso, H.P.; Maqbool, A.; Hincke, M.T. Processed eggshell membrane powder: Bioinspiration for an innovative wound healing product. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 95, 192–203. [Google Scholar] [CrossRef]
- Ohto-Fujita, E.; Shimizu, M.; Sano, S.; Kurimoto, M.; Yamazawa, K.; Atomi, T.; Sakurai, T.; Murakami, Y.; Takami, T.; Murakami, T.; et al. Solubilized eggshell membrane supplies a type III collagen-rich elastic dermal papilla. Cell Tissue Res. 2019, 376, 123–135. [Google Scholar] [CrossRef]
- You, R.; Zhang, J.; Gu, S.; Zhou, Y.; Li, X.; Ye, D.; Xu, W. Regenerated egg white/silk fibroin composite films for biomedical applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 79, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Guérin-Dubiard, C.; Pasco, M.; Mollé, D.; Désert, C.; Croguennec, T.; Nau, F. Proteomic analysis of hen egg white. J. Agric. Food Chem. 2006, 54, 3901–3910. [Google Scholar] [CrossRef] [PubMed]
- Omana, D.A.; Liang, Y.; Kav, N.N.; Wu, J. Proteomic analysis of egg white proteins during storage. Proteomics 2011, 11, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Feng, Y.; Cui, J.; Hou, Q.; Li, T.; Jia, M.; Lv, Z.; Jiang, Q.; Wang, Y.; Zhang, M.; et al. The ionome and proteome landscape of aging in laying hens and relation to egg white quality. Sci. China Life Sci. 2023, 66, 2020–2040. [Google Scholar] [CrossRef]
- Alavi, F.; Emam-Djomeh, Z.; Salami, M.; Mohammadian, M. Effect of microbial transglutaminase on the mechanical properties and microstructure of acid-induced gels and emulsion gels produced from thermal denatured egg white proteins. Int. J. Biol. Macromol. 2020, 153, 523–532. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Pham, H.M.; Tran, S.D. The Chicken Egg: An Advanced Material for Tissue Engineering. Biomolecules 2024, 14, 439. https://doi.org/10.3390/biom14040439
Zhang Y, Pham HM, Tran SD. The Chicken Egg: An Advanced Material for Tissue Engineering. Biomolecules. 2024; 14(4):439. https://doi.org/10.3390/biom14040439
Chicago/Turabian StyleZhang, Yuli, Hieu M. Pham, and Simon D. Tran. 2024. "The Chicken Egg: An Advanced Material for Tissue Engineering" Biomolecules 14, no. 4: 439. https://doi.org/10.3390/biom14040439
APA StyleZhang, Y., Pham, H. M., & Tran, S. D. (2024). The Chicken Egg: An Advanced Material for Tissue Engineering. Biomolecules, 14(4), 439. https://doi.org/10.3390/biom14040439








