On Interactions of Sulfamerazine with Cyclodextrins from Coupled Diffusometry and Toxicity Tests
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Taylor Dispersion Technique: Diffusion Measurements
2.3. Toxicity Tests: Kiwi Pollen Germination
2.4. Toxicity Tests: BY-2 Cellular Tests
2.5. Statistical Analysis
3. Results
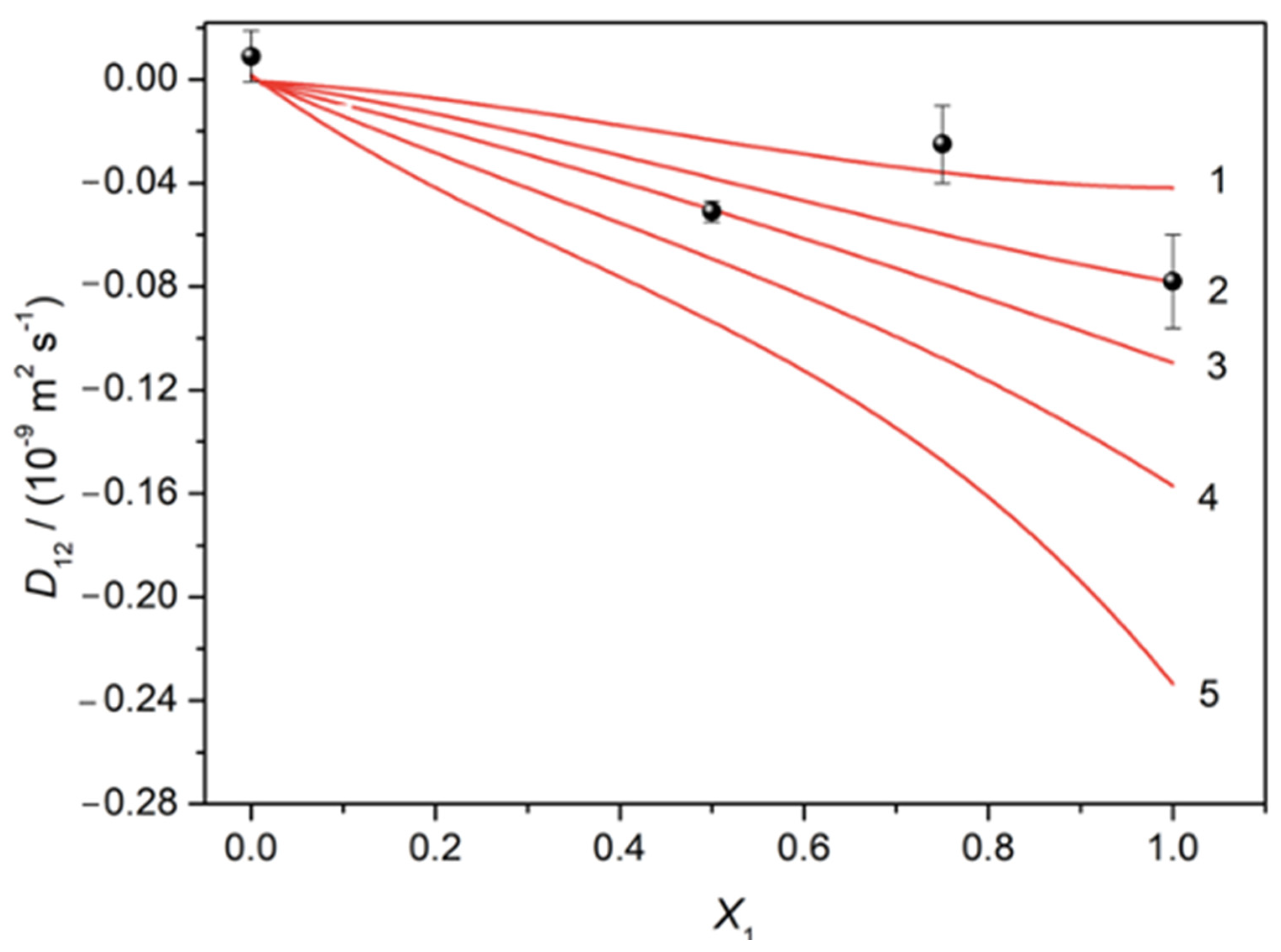
3.1. Diffusion Measurements
3.2. Toxicity Tests
3.2.1. Kiwi Pollen Germination
3.2.2. BY-2 Cellular Tests
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
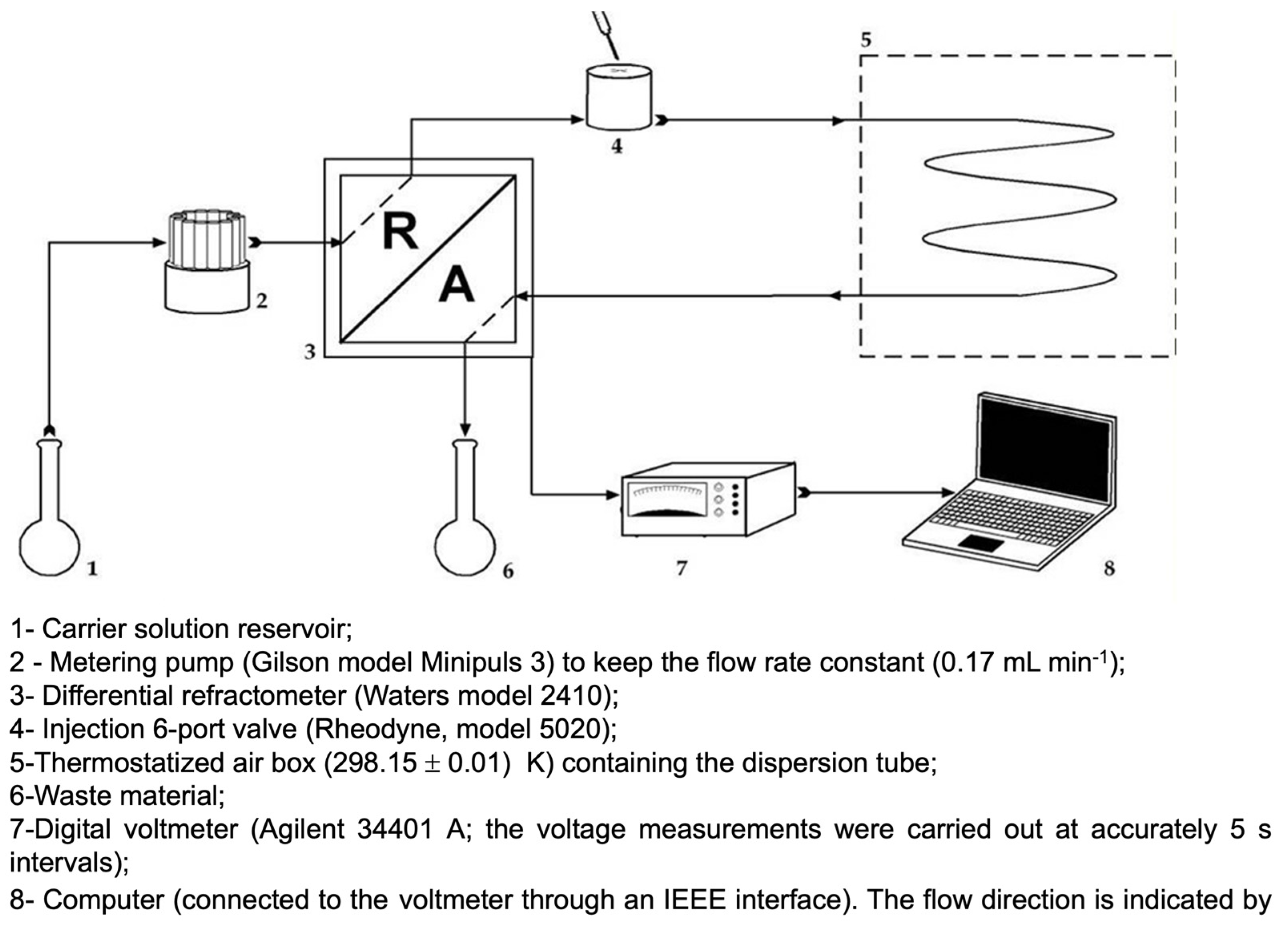
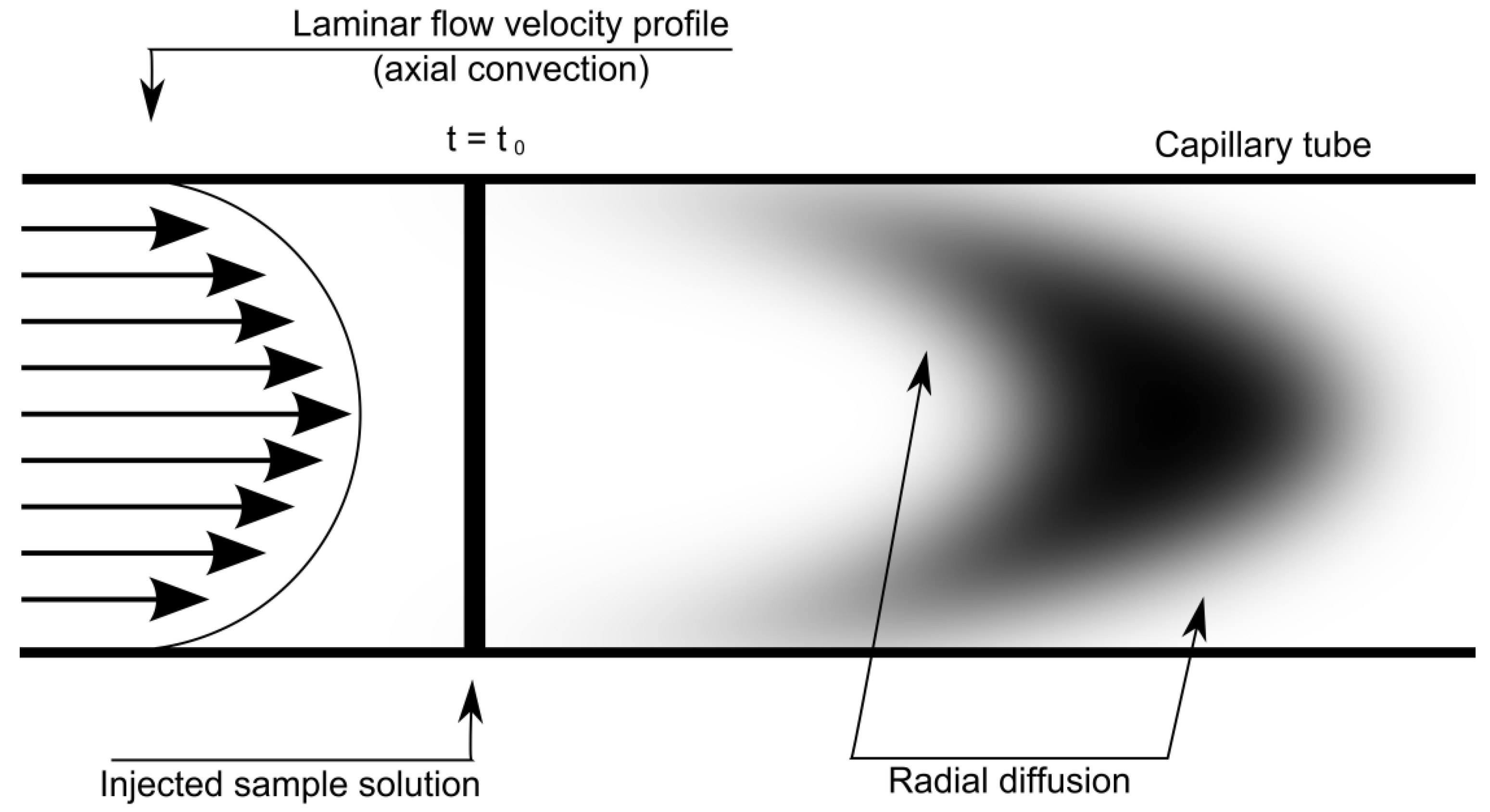
Appendix B
| Aqueous System | D ± SD/(10−9 m2/s) | DLit/(10−9 m2/s) |
|---|---|---|
| KCl 0.100 mol dm−3 | 1.843 ± 0.009 | 1.849 b |
| 1.846 c | ||
| Glycine 0.100 mol dm−3 | 1.048 ± 0.005 | 1.047 d |
| 1.061 e | ||
| 1.049 f |
References
- Vargason, A.M.; Anselmo, A.C.; Mitragotri, S. The evolution of commercial drug delivery technologies. Nat. Biomed. Eng. 2021, 5, 951–967. [Google Scholar] [CrossRef] [PubMed]
- Trucillo, P. Drug Carriers: Classification, Administration, Release Profiles, and Industrial Approach. Processes 2021, 9, 470. [Google Scholar] [CrossRef]
- Rajendiran, N.; Mohandoss, T.; Venkatesh, G. Investigation of inclusion complexes of sulfamerazine with α-and β-cyclodextrins: An experimental and theoretical study. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 124, 441–450. [Google Scholar] [CrossRef]
- Saokham, P.; Muankaew, C.; Jansook, P.; Loftsson, T. Solubility of Cyclodextrins and Drug/Cyclodextrin Complexes. Molecules 2018, 23, 1161. [Google Scholar] [CrossRef] [PubMed]
- Mu, K.; Jiang, K.; Wang, Y.; Zhao, Z.; Cang, S.; Bi, K.; Li, Q.; Liu, R. The biological fate of pharmaceutical excipient β-cyclodextrin: Pharmacokinetics, tissue distribution, excretion, and metabolism of β-cyclodextrin in rats. Molecules 2022, 27, 1138. [Google Scholar] [CrossRef]
- Gould, S.; Scott, R.C. 2-Hydroxypropyl-β-cyclodextrin (HP-β-CD): A toxicology review. Food Chem. Toxicol. 2005, 43, 1451–1459. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, L.; Yao, C. Optimization of Antibacterial Activity and Biosafety through Ultrashort Peptide/Cyclodextrin Inclusion Complexes. Int. J. Mol. Sci. 2023, 24, 14801. [Google Scholar] [CrossRef]
- Jiang, Y.; Jiang, X.; Law, K.; Chen, Y.; Gu, J.; Zhang, W.; Xin, H.; Sha, X.; Fang, X. Enhanced anti-tumor effect of 9-nitro-camptothecin complexed by hydroxypropyl-β-cyclodextrin and safety evaluation. Int. J. Pharm. 2011, 415, 252–258. [Google Scholar] [CrossRef]
- Gidwani, B.; Vyas, A. A comprehensive review on cyclodextrin-based carriers for delivery of chemotherapeutic cytotoxic anticancer drugs. BioMed Res. Int. 2015, 2015, 198268. [Google Scholar] [CrossRef]
- Ramos-Martínez, B.; Dávila-Pousa, C.; Merino-Bohórquez, V.; García-Palomo, M. Use of cyclodextrins as excipients in pharmaceutical products: Why not in extemporaneous preparations? Farm. Hosp. 2022, 46, 31–39. [Google Scholar]
- Kali, G.; Haddadzadegan, S.; Bernkop-Schnürch, A. Cyclodextrins and derivatives in drug delivery: New developments, relevant clinical trials, and advanced products. Carbohydr. Polym. 2024, 324, 121500. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Guo, S.; Wei, S.; Liu, J.; Tian, B. Pharmacokinetics and pharmacodynamics of cyclodextrin-based oral drug delivery formulations for disease therapy. Carbohydr. Polym. 2024, 329, 121763. [Google Scholar] [CrossRef] [PubMed]
- Rincón-López, J.; Almanza-Arjona, Y.C.; Riascos, A.P.; Rojas-Aguirre, Y. Technological evolution of cyclodextrins in the pharmaceutical field. J. Drug Deliv. Sci. Technol. 2021, 61, 102156. [Google Scholar] [CrossRef] [PubMed]
- Biwer, A.; Antranikian, G.; Heinzle, E. Enzymatic production of cyclodextrins. Appl. Microbiol. Biotechnol. 2002, 59, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Kurkov, S.V.; Loftsson, T. Cyclodextrins. Int. J. Pharm. 2013, 453, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Jansook, P.; Ogawa, N.; Loftsson, T. Cyclodextrins: Structure, physicochemical properties and pharmaceutical applications. Int. J. Pharm. 2018, 535, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Aiassa, V.; Garnero, C.; Zoppi, A.; Longhi, M.R. Cyclodextrins and Their Derivatives as Drug Stability Modifiers. Pharmaceuticals 2023, 16, 1074. [Google Scholar] [CrossRef]
- Utzeri, G.; Matias, P.M.C.; Murtinho, D.; Valente, A.J.M. Cyclodextrin-Based Nanosponges: Overview and Opportunities. Front. Chem. 2022, 10, 859406. [Google Scholar] [CrossRef]
- Sandilya, A.A.; Natarajan, U.; Priya, M.H. Molecular View into the Cyclodextrin Cavity: Structure and Hydration. ACS Omega 2020, 5, 25655–25667. [Google Scholar] [CrossRef]
- Aday, B.; Sola, P.; Çolak, F.; Kaya, M. Synthesis of novel sulfonamide analogs containing sulfamerazine/sulfaguanidine and their biological activities. J. Enzyme Inhib. Med. Chem. 2016, 31, 1005–1010. [Google Scholar] [CrossRef]
- Zoppi, A.; Quevedo, M.A.; Delrivo, A.; Longhi, M.R. Complexation of sulfonamides with β-cyclodextrin studied by experimental and theoretical methods. J. Pharm. Sci. 2010, 99, 3166–3176. [Google Scholar] [CrossRef]
- Mohamed Ameen, H.; Kunsági-Máté, S.; Bognár, B.; Szente, L.; Poór, M.; Lemli, B. Thermodynamic characterization of the interaction between the antimicrobial drug sulfamethazine and two selected cyclodextrins. Molecules 2019, 24, 4565. [Google Scholar] [CrossRef]
- Nagata, T.; Nemoto, Y.; Hasezawa, S. Tobacco BY-2 Cell Line as the “HeLa” Cell in the Cell Biology of Higher Plants; Academic Press: Cambridge, MA, USA, 1992; pp. 1–30. [Google Scholar]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Tyrrell, H.J.V.; Harris, K.R. Diffusion in Liquids: A Theoretical and Experimental Study; Butterworth: London, UK, 1984. [Google Scholar]
- Erdey-Gruz, T. Transport Phenomena in Aqueous Solutions, 2nd ed.; Adam Hilger: London, UK, 1974. [Google Scholar]
- Robinson, R.; Stokes, R. Electrolyte Solutions, 2nd ed.; Dover: London, UK, 2002. [Google Scholar]
- Loh, W. A técnica de dispersão de taylor para estudos de difusão em líquidos e suas aplicações. Quim. Nova 1997, 20, 541–545. [Google Scholar] [CrossRef]
- Callendar, R.; Leaist, D.G. Diffusion Coefficients for Binary, Ternary, and Polydisperse Solutions from Peak-Width Analysis of Taylor Dispersion Profiles. J. Solut. Chem. 2006, 35, 353–379. [Google Scholar] [CrossRef]
- Barthel, J.; Gores, H.J.; Lohr, C.M.; Seidl, J.J. Taylor dispersion measurements at low electrolyte concentrations. I. Tetraalkylammonium perchlorate aqueous solutions. J. Solut. Chem. 1996, 25, 921–935. [Google Scholar] [CrossRef]
- Ribeiro, A.C.F.; Natividade, J.J.S.; Esteso, M.A. Differential mutual diffusion coefficients of binary and ternary aqueous systems measured by the open ended conductometric capillary cell and by the Taylor technique. J. Mol. Liq. 2010, 156, 58–64. [Google Scholar] [CrossRef]
- Ribeiro, A.C.F.; Barros, M.C.F.; Verissimo, L.M.P.; Esteso, M.A.; Leaist, D.G. Coupled mutual diffusion in aqueous sodium (salicylate + sodium chloride) solutions at 25 °C. J. Chem. Thermodyn. 2019, 138, 282–287. [Google Scholar] [CrossRef]
- Vitagliano, V.; Sartorio, R. Diffusion in ternary systems. J. Phys. Chem. 1970, 74, 2949–2956. [Google Scholar] [CrossRef]
- Ribeiro, A.C.F.; Santos, C.I.A.V.; Lobo, V.M.M.; Esteso, M.A. Quaternary Diffusion Coefficients of β-Cyclodextrin + KCl + Caffeine + Water at 298.15 K Using a Taylor Dispersion Method. J. Chem. Eng. Data 2010, 55, 2610–2612. [Google Scholar] [CrossRef]
- Ribeiro, A.C.F.; Leaist, D.G.; Esteso, M.A.; Lobo, V.M.M.; Valente, A.J.M.; Santos, C.I.A.V.; Cabral, A.M.T.D.P.V.; Veiga, F.J.B. Binary Mutual Diffusion Coefficients of Aqueous Solutions of β-Cyclodextrin at Temperatures from 298.15 to 312.15 K. J. Chem. Eng. Data 2006, 51, 1368–1371. [Google Scholar] [CrossRef]
- Williams, J.H. The evolution of pollen germination timing in flowering plants: Austrobaileya scandens (Austrobaileyaceae). AoB Plants 2012, 2012, pls010. [Google Scholar] [CrossRef] [PubMed]
- Breygina, M.; Klimenko, E.; Schekaleva, O. Pollen Germination and Pollen Tube Growth in Gymnosperms. Plants 2021, 10, 1301. [Google Scholar] [CrossRef] [PubMed]
- Fragallah, S.; Lin, S.; Li, N.; Ligate, E.; Chen, Y. Effects of Sucrose, Boric Acid, pH, and Incubation Time on in Vitro Germination of Pollen and Tube Growth of Chinese fir (Cunnighamial lanceolata L.). Forests 2019, 10, 102. [Google Scholar] [CrossRef]
- Zheng, R.; Su, S.; Xiao, H.; Tian, H. Calcium: A Critical Factor in Pollen Germination and Tube Elongation. Int. J. Mol. Sci. 2019, 20, 420. [Google Scholar] [CrossRef] [PubMed]
- Prochowicz, D.; Kornowicz, A.; Lewiński, J. Interactions of Native Cyclodextrins with Metal Ions and Inorganic Nanoparticles: Fertile Landscape for Chemistry and Materials Science. Chem. Rev. 2017, 117, 13461–13501. [Google Scholar] [CrossRef]
- Chen, X.; Chu, Y.; Gu, L.; Zhou, M.; Ding, C.-F. The non-covalent complexes of α-or γ-cyclodextrin with divalent metal cations determined by mass spectrometry. Carbohydr. Res. 2020, 492, 107987. [Google Scholar] [CrossRef]
- Stachowicz, A.; Styrcz, A.; Korchowiec, J.; Modaressi, A.; Rogalski, M. DFT studies of cation binding by β-cyclodextrin. Theor. Chem. Acc. 2011, 130, 939–953. [Google Scholar] [CrossRef]
- Gorelova, V.; Ambach, L.; Rébeillé, F.; Stove, C.; Van Der Straeten, D. Folates in Plants: Research Advances and Progress in Crop Biofortification. Front. Chem. 2017, 5, 21. [Google Scholar] [CrossRef]
- Sabater-Jara, A.B.; Marín-Marín, M.J.; Almagro, L.; Pedreño, M.A. Cyclodextrins Increase Triterpene Production in Solanum lycopersicum Cell Cultures by Activating Biosynthetic Genes. Plants 2022, 11, 2782. [Google Scholar] [CrossRef]
- Yamamoto, K.; Tanikawa, T.; Tomita, J.; Ishida, Y.; Nakata, D.; Terao, K.; Inoue, Y. Characterization, Preparation, and Promotion of Plant Growth of 1,3-Diphenylurea/β-Cyclodextrin Derivatives Inclusion Complexes. ACS Omega 2023, 8, 34972–34981. [Google Scholar] [CrossRef] [PubMed]
- Lobo, V.M.M.; Ribeiro, A.C.F.; Verissimo, L.M.P. Diffusion coefficients in aqueous solutions of potassium chloride at high and low concentrations. J. Mol. Liq. 1998, 78, 139–149. [Google Scholar] [CrossRef]
- Harned, H.S.; Nuttall, R.L. The differential diffusion coefficient of potassium chloride in aqueous solutions. J. Am. Chem. Soc. 1949, 71, 1460–1463. [Google Scholar] [CrossRef]
- Ribeiro, A.C.F.; Lobo, V.M.M.; Leaist, D.G.; Natividade, J.J.S.; Veríssimo, L.P.; Barros, M.C.F.; Cabral, A.M. Binary diffusion coefficients for aqueous solutions of lactic acid. J. Solut. Chem. 2005, 34, 1009–1016. [Google Scholar] [CrossRef]
- Lyons, M.S.; Thomas, J.V. Diffusion Studies on Dilute Aqueous Glycine Solutions at 1 and 25 with the Gouy Interference Method. J. Am. Chem. Soc. 1950, 72, 4506–4511. [Google Scholar] [CrossRef]
- Changwei, Z.; Jiding, L.I.; Peisheng, M.A.; Shuqian, X.I.A. Measurement of Liquid Diffusion Coefficients of Aqueous Solutions of Glycine, L-Alanine, L-Valine and L-Isoleucine by Holographic Interferometry. Chin. J. Chem. Eng. 2005, 13, 285. [Google Scholar]


| Chemical Name | Source | CAS Number | Mass Fraction Purity 1 |
|---|---|---|---|
| Sulfamerazine sodium salt | Sigma-Aldrich | 127-58-2 | 0.99 |
| β-Cyclodextrin | Sigma-Aldrich (water mass fraction 0.131) 2 | 7585-39-9 | >0.97 |
| HP-β-Cyclodextrin | Sigma-Aldrich (water mass fraction 0.03) 3 | 128446-35-5 | >0.97 |
| Water | Millipore-Q water (18.2 mΩ·cm at 25.00 °C) | ||
| Potassium nitrate | Sigma-Aldrich | 7757-79-1 | 0.99 |
| Magnesium sulphate heptahydrate | Sigma-Aldrich | 10034-99-8 | 0.98 |
| Calcium nitrate tetrahydrate | Merck | 13477-34-4 | 0.99 |
| Boric acid | Merck | 10043-35-3 | 0.995 |
| Sucrose | Duchefa Biochemie B.V | 57-50-1 | 0.98 |
| Plant agar | Duchefa Biochemie B.V | 9002-18-0 |
| C/(mol dm−3) | D ± SD/(10−9 m2s−1) 1 |
|---|---|
| 0.000 | 0.944 ± 0.003 |
| 0.005 | 0.927 ± 0.005 |
| 0.008 | 0.917 ± 0.002 |
| 0.010 | 0.906 ± 0.003 |
| C1 a | C2 a | X1 b | D11 ± SD c | D12 ± SD c | D21 ± SD c | D22 ± SD c |
|---|---|---|---|---|---|---|
| NaSMR (component 1) + β-CD (component 2) | ||||||
| 0.000 | 0.010 | 0.000 | 0.878 ± 0.004 | 0.010 ± 0.010 | 0.022 ± 0.015 | 0.330 ± 0.004 |
| 0.005 | 0.005 | 0.500 | 0.888 ± 0.004 | 0.011 ± 0.002 | 0.024 ± 0.014 | 0.335 ± 0.006 |
| 0.0075 | 0.0025 | 0.750 | 0.892 ± 0.002 | −0.050 ± 0.003 | 0.030 ± 0.013 | 0.339 ± 0.001 |
| 0.010 | 0.000 | 1.000 | 0.946 ± 0.005 | −0.080 ± 0.002 | −0.008 ± 0.003 | 0.338 ± 0.012 |
| NaSMR (component 1) + HP-β-CD (component 2) | ||||||
| 0.000 | 0.010 | 0.000 | 0.864 ± 0.008 | 0.021 ± 0.028 | 0.008 ± 0.001 | 0.325 ± 0.009 |
| 0.005 | 0.005 | 0.500 | 0.909 ± 0.006 | 0.026 ± 0.030 | 0.010 ± 0.015 | 0.323 ± 0.004 |
| 0.0075 | 0.0025 | 0.750 | 0.920 ± 0.006 | 0.015 ± 0.030 | 0.016 ± 0.011 | 0.320 ± 0.003 |
| 0.010 | 0.000 | 1.000 | 0.930 ± 0.016 | 0.020 ± 0.010 | 0.002 ± 0.004 | 0.327 ± 0.015 |
| C1 a | C2 a | D12/D22 b | D21/D11 c |
|---|---|---|---|
| NaSMR (component 1) + β-CD (component 2) | |||
| 0.0000 | 0.0100 | 0.030 | 0.025 |
| 0.0050 | 0.0050 | 0.032 | 0.027 |
| 0.0100 | 0.0090 | −0.148 | 0.034 |
| 0.0100 | 0.0000 | −0.237 | −0.010 |
| NaSMR (component 1) + HP-β-CD (component 2) | |||
| 0.000 | 0.010 | 0.065 | 0.009 |
| 0.005 | 0.005 | 0.080 | 0.011 |
| 0.0075 | 0.0025 | 0.046 | 0.012 |
| 0.010 | 0.000 | 0.061 | 0.002 |
| Species | Di/(10−9 m2s−1) |
|---|---|
| NaSMR | 0.944 |
| β-CD | 0.326 [35] |
| NaSMR:β-CD | 0.322 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sofio, S.P.C.; Caeiro, A.; Ribeiro, A.C.F.; Cabral, A.M.T.D.P.V.; Valente, A.J.M.; Canhoto, J.; Esteso, M.A. On Interactions of Sulfamerazine with Cyclodextrins from Coupled Diffusometry and Toxicity Tests. Biomolecules 2024, 14, 462. https://doi.org/10.3390/biom14040462
Sofio SPC, Caeiro A, Ribeiro ACF, Cabral AMTDPV, Valente AJM, Canhoto J, Esteso MA. On Interactions of Sulfamerazine with Cyclodextrins from Coupled Diffusometry and Toxicity Tests. Biomolecules. 2024; 14(4):462. https://doi.org/10.3390/biom14040462
Chicago/Turabian StyleSofio, Sara P. C., André Caeiro, Ana C. F. Ribeiro, Ana M. T. D. P. V. Cabral, Artur J. M. Valente, Jorge Canhoto, and Miguel A. Esteso. 2024. "On Interactions of Sulfamerazine with Cyclodextrins from Coupled Diffusometry and Toxicity Tests" Biomolecules 14, no. 4: 462. https://doi.org/10.3390/biom14040462
APA StyleSofio, S. P. C., Caeiro, A., Ribeiro, A. C. F., Cabral, A. M. T. D. P. V., Valente, A. J. M., Canhoto, J., & Esteso, M. A. (2024). On Interactions of Sulfamerazine with Cyclodextrins from Coupled Diffusometry and Toxicity Tests. Biomolecules, 14(4), 462. https://doi.org/10.3390/biom14040462











