Plasticity of Adipose Tissues: Interconversion among White, Brown, and Beige Fat and Its Role in Energy Homeostasis
Abstract
1. Introduction
2. The Roles of Different Types of Adipose Tissue in Metabolism
3. Interconversion between Different Adipose Tissues
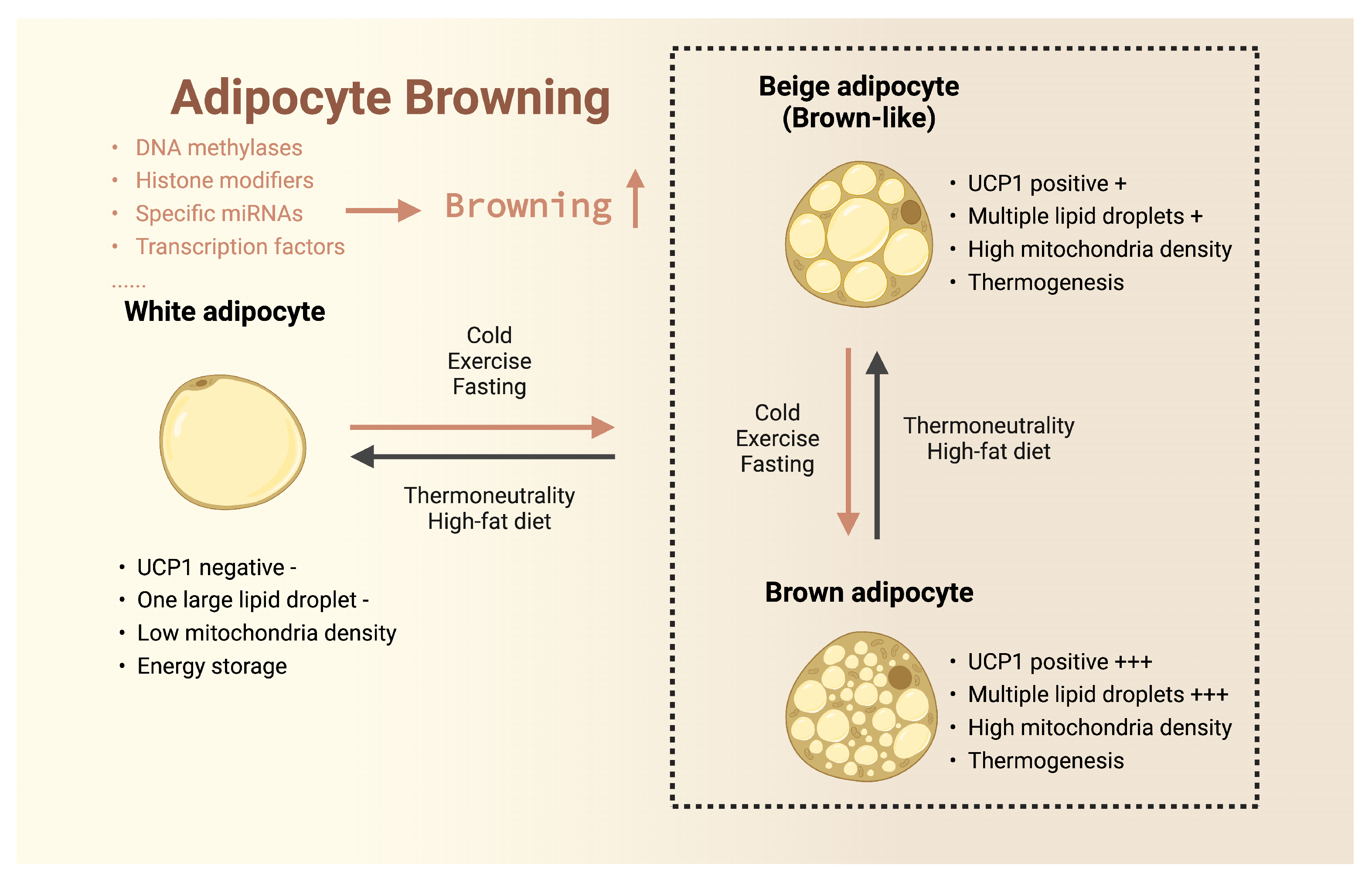
3.1. Browning of White Adipose Tissue
3.2. Browning of Beige Adipose Tissue
3.3. Activation of Brown Adipose Tissue
3.4. Bioactive Compounds
4. Conclusions
Funding
Conflicts of Interest
References
- Zhang, X.; Ha, S.; Lau, H.C.-H.; Yu, J. Excess Body Weight: Novel Insights into Its Roles in Obesity Comorbidities. Semin. Cancer Biol. 2023, 92, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Feinberg, A.P.; Levchenko, A. Epigenetics as a Mediator of Plasticity in Cancer. Science 2023, 379, eaaw3835. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, K.N.; Hodges, R.; Hanes, D.; Stack, E.; Cheishvili, D.; Szyf, M.; Henkel, J.; Twedt, M.W.; Giannopoulou, D.; Herdell, J.; et al. Correction for: Potential Reversal of Epigenetic Age Using a Diet and Lifestyle Intervention: A Pilot Randomized Clinical Trial. Aging 2022, 14, 5959. [Google Scholar] [CrossRef] [PubMed]
- Tanimura, K.; Suzuki, T.; Vargas, D.; Shibata, H.; Inagaki, T. Epigenetic Regulation of Beige Adipocyte Fate by Histone Methylation. Endocr. J. 2019, 66, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Nanduri, R. Epigenetic Regulators of White Adipocyte Browning. Epigenomes 2021, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- You, D.; Nilsson, E.; Tenen, D.E.; Lyubetskaya, A.; Lo, J.C.; Jiang, R.; Deng, J.; Dawes, B.A.; Vaag, A.; Ling, C.; et al. Dnmt3a Is an Epigenetic Mediator of Adipose Insulin Resistance. eLife 2017, 6, e30766. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, T.; Sakai, J.; Kajimura, S. Transcriptional and Epigenetic Control of Brown and Beige Adipose Cell Fate and Function. Nat. Rev. Mol. Cell. Biol. 2016, 17, 480–495. [Google Scholar] [CrossRef] [PubMed]
- Bradford, S.T.; Nair, S.S.; Statham, A.L.; Van Dijk, S.J.; Peters, T.J.; Anwar, F.; French, H.J.; Von Martels, J.Z.H.; Sutcliffe, B.; Maddugoda, M.P.; et al. Methylome and Transcriptome Maps of Human Visceral and Subcutaneous Adipocytes Reveal Key Epigenetic Differences at Developmental Genes. Sci. Rep. 2019, 9, 9511. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, W.; Li, H.; Xu, F.; Yinwang, E.; Xue, Y.; Chen, T.; Wang, S.; Wang, Z.; Sun, H.; et al. Osteocyte Mitochondria Inhibit Tumor Development via STING-Dependent Antitumor Immunity. Sci. Adv. 2024, 10, eadi4298. [Google Scholar] [CrossRef]
- Cox, S.L.; O’Siorain, J.R.; Fagan, L.E.; Curtis, A.M.; Carroll, R.G. Intertwining Roles of Circadian and Metabolic Regulation of the Innate Immune Response. Semin. Immunopathol. 2022, 44, 225–237. [Google Scholar] [CrossRef]
- Kawada-Horitani, E.; Kita, S.; Okita, T.; Nakamura, Y.; Nishida, H.; Honma, Y.; Fukuda, S.; Tsugawa-Shimizu, Y.; Kozawa, J.; Sakaue, T.; et al. Human Adipose-Derived Mesenchymal Stem Cells Prevent Type 1 Diabetes Induced by Immune Checkpoint Blockade. Diabetologia 2022, 65, 1185–1197. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, V.; Hogan, A.E.; Fallon, P.G.; Schwartz, C. Obesity-Mediated Immune Modulation: One Step Forward, (Th)2 Steps Back. Front. Immunol. 2022, 13, 932893. [Google Scholar] [CrossRef]
- Ma, Y.; Jun, H.; Wu, J. Immune Cell Cholinergic Signaling in Adipose Thermoregulation and Immunometabolism. Trends Immunol. 2022, 43, 718–727. [Google Scholar] [CrossRef] [PubMed]
- Latteri, S.; Sofia, M.; Puleo, S.; Di Vincenzo, A.; Cinti, S.; Castorina, S. Mechanisms Linking Bariatric Surgery to Adipose Tissue, Glucose Metabolism, Fatty Liver Disease and Gut Microbiota. Langenbecks Arch. Surg. 2023, 408, 101. [Google Scholar] [CrossRef]
- Sankararaman, S.; Noriega, K.; Velayuthan, S.; Sferra, T.; Martindale, R. Gut Microbiome and Its Impact on Obesity and Obesity-Related Disorders. Curr. Gastroenterol. Rep. 2023, 25, 31–44. [Google Scholar] [CrossRef]
- Sbierski-Kind, J.; Grenkowitz, S.; Schlickeiser, S.; Sandforth, A.; Friedrich, M.; Kunkel, D.; Glauben, R.; Brachs, S.; Mai, K.; Thürmer, A.; et al. Effects of Caloric Restriction on the Gut Microbiome Are Linked with Immune Senescence. Microbiome 2022, 10, 57. [Google Scholar] [CrossRef]
- Sung, H.; Siegel, R.L.; Torre, L.A.; Pearson-Stuttard, J.; Islami, F.; Fedewa, S.A.; Goding Sauer, A.; Shuval, K.; Gapstur, S.M.; Jacobs, E.J.; et al. Global Patterns in Excess Body Weight and the Associated Cancer Burden. CA Cancer J. Clin. 2019, 69, 88–112. [Google Scholar] [CrossRef] [PubMed]
- Bosello, O.; Vanzo, A. Obesity Paradox and Aging. Eat. Weight. Disord. 2021, 26, 27–35. [Google Scholar] [CrossRef]
- Lv, M.; Zhang, S.; Jiang, B.; Cao, S.; Dong, Y.; Cao, L.; Guo, S. Adipose-derived Stem Cells Regulate Metabolic Homeostasis and Delay Aging by Promoting Mitophagy. FASEB J. 2021, 35, e21709. [Google Scholar] [CrossRef]
- Feng, X.; Wang, L.; Zhou, R.; Zhou, R.; Chen, L.; Peng, H.; Huang, Y.; Guo, Q.; Luo, X.; Zhou, H. Senescent Immune Cells Accumulation Promotes Brown Adipose Tissue Dysfunction during Aging. Nat. Commun. 2023, 14, 3208. [Google Scholar] [CrossRef]
- Mau, T.; Yung, R. Adipose Tissue Inflammation in Aging. Exp. Gerontol. 2018, 105, 27–31. [Google Scholar] [CrossRef]
- Fabbrini, E.; Yoshino, J.; Yoshino, M.; Magkos, F.; Tiemann Luecking, C.; Samovski, D.; Fraterrigo, G.; Okunade, A.L.; Patterson, B.W.; Klein, S. Metabolically Normal Obese People Are Protected from Adverse Effects Following Weight Gain. J. Clin. Investig. 2015, 125, 787–795. [Google Scholar] [CrossRef]
- Stanford, K.I.; Middelbeek, R.J.W.; Goodyear, L.J. Exercise Effects on White Adipose Tissue: Beiging and Metabolic Adaptations. Diabetes 2015, 64, 2361–2368. [Google Scholar] [CrossRef]
- Scheel, A.K.; Espelage, L.; Chadt, A. Many Ways to Rome: Exercise, Cold Exposure and Diet—Do They All Affect BAT Activation and WAT Browning in the Same Manner? Int. J. Mol. Sci. 2022, 23, 4759. [Google Scholar] [CrossRef]
- Hall, K.D.; Kahan, S. Maintenance of Lost Weight and Long-Term Management of Obesity. Med. Clin. 2018, 102, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Rubino, D.M.; Greenway, F.L.; Khalid, U.; O’Neil, P.M.; Rosenstock, J.; Sørrig, R.; Wadden, T.A.; Wizert, A.; Garvey, W.T.; STEP 8 Investigators; et al. Effect of Weekly Subcutaneous Semaglutide vs Daily Liraglutide on Body Weight in Adults With Overweight or Obesity Without Diabetes: The STEP 8 Randomized Clinical Trial. JAMA 2022, 327, 138. [Google Scholar] [CrossRef] [PubMed]
- Wilding, J.P.H.; Batterham, R.L.; Calanna, S.; Davies, M.; Van Gaal, L.F.; Lingvay, I.; McGowan, B.M.; Rosenstock, J.; Tran, M.T.D.; Wadden, T.A.; et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N. Eng. J. Med. 2021, 384, 989–1002. [Google Scholar] [CrossRef] [PubMed]
- Maurer, S.; Harms, M.; Boucher, J. The Colorful Versatility of Adipocytes: White-to-brown Transdifferentiation and Its Therapeutic Potential in Humans. FEBS J. 2021, 288, 3628–3646. [Google Scholar] [CrossRef]
- Lee, K.Y.; Luong, Q.; Sharma, R.; Dreyfuss, J.M.; Ussar, S.; Kahn, C.R. Developmental and Functional Heterogeneity of White Adipocytes within a Single Fat Depot. EMBO J. 2019, 38, e99291. [Google Scholar] [CrossRef]
- Tang, H.; Wang, J.; Deng, P.; Li, Y.; Cao, Y.; Yi, B.; Zhu, L.; Zhu, S.; Lu, Y. Transcriptome-Wide Association Study-Derived Genes as Potential Visceral Adipose Tissue-Specific Targets for Type 2 Diabetes. Diabetologia 2023, 66, 2087–2100. [Google Scholar] [CrossRef]
- Ahmad, B.; Vohra, M.S.; Saleemi, M.A.; Serpell, C.J.; Fong, I.L.; Wong, E.H. Brown/Beige Adipose Tissues and the Emerging Role of Their Secretory Factors in Improving Metabolic Health: The Batokines. Biochimie 2021, 184, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Wang, J.; Dai, H.; Duan, Y.; An, Y.; Shi, L.; Lv, Y.; Li, H.; Wang, C.; Ma, Q.; et al. Brown and Beige Adipose Tissue: A Novel Therapeutic Strategy for Obesity and Type 2 Diabetes Mellitus. Adipocyte 2021, 10, 48–65. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Porras, M.A.; Stojkova, K.; Acosta, F.M.; Rathbone, C.R.; Brey, E.M. Engineering Human Beige Adipose Tissue. Front. Bioeng. Biotechnol. 2022, 10, 906395. [Google Scholar] [CrossRef] [PubMed]
- Castro, É.; Silva, T.E.O.; Festuccia, W.T. Critical Review of Beige Adipocyte Thermogenic Activation and Contribution to Whole-Body Energy Expenditure. Horm. Mol. Biol. Clin. Investig. 2017, 31, 20170042. [Google Scholar] [CrossRef] [PubMed]
- Yuko, O.-O.; Saito, M. Brown Fat as a Regulator of Systemic Metabolism beyond Thermogenesis. Diabetes Metab. J. 2021, 45, 840–852. [Google Scholar] [CrossRef] [PubMed]
- Angueira, A.R.; Sakers, A.P.; Holman, C.D.; Cheng, L.; Arbocco, M.N.; Shamsi, F.; Lynes, M.D.; Shrestha, R.; Okada, C.; Batmanov, K.; et al. Defining the Lineage of Thermogenic Perivascular Adipose Tissue. Nat. Metab. 2021, 3, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Sakers, A.; De Siqueira, M.K.; Seale, P.; Villanueva, C.J. Adipose-Tissue Plasticity in Health and Disease. Cell 2022, 185, 419–446. [Google Scholar] [CrossRef]
- Wu, J.; Jun, H.; McDermott, J.R. Formation and Activation of Thermogenic Fat. Trends Genet. 2015, 31, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.E. Thermoregulatory and Adaptive Behavior of Brown Adipose Tissue. Science 1964, 146, 1686–1689. [Google Scholar] [CrossRef]
- Cohen, P.; Kajimura, S. The Cellular and Functional Complexity of Thermogenic Fat. Nat. Rev. Mol. Cell. Biol. 2021, 22, 393–409. [Google Scholar] [CrossRef]
- Abe, Y.; Fujiwara, Y.; Takahashi, H.; Matsumura, Y.; Sawada, T.; Jiang, S.; Nakaki, R.; Uchida, A.; Nagao, N.; Naito, M.; et al. Histone Demethylase JMJD1A Coordinates Acute and Chronic Adaptation to Cold Stress via Thermogenic Phospho-Switch. Nat. Commun. 2018, 9, 1566. [Google Scholar] [CrossRef] [PubMed]
- Valdivia, L.F.G.; Castro, É.; Eichler, R.A.D.S.; Moreno, M.F.; De Sousa, É.; Jardim, G.F.R.; Peixoto, Á.S.; Moraes, M.N.; Castrucci, A.M.D.L.; Nedergaard, J.; et al. Cold Acclimation and Pioglitazone Combined Increase Thermogenic Capacity of Brown and White Adipose Tissues but This Does Not Translate into Higher Energy Expenditure in Mice. Am. J. Physiol.-Endocrinol. Metab. 2023, 324, E358–E373. [Google Scholar] [CrossRef]
- Xiao, F.; Jiang, H.; Li, Z.; Jiang, X.; Chen, S.; Niu, Y.; Yin, H.; Shu, Y.; Peng, B.; Lu, W.; et al. Reduced Hepatic Bradykinin Degradation Accounts for Cold-Induced BAT Thermogenesis and WAT Browning in Male Mice. Nat. Commun. 2023, 14, 2523. [Google Scholar] [CrossRef] [PubMed]
- Lahesmaa, M.; Oikonen, V.; Helin, S.; Luoto, P.; U Din, M.; Pfeifer, A.; Nuutila, P.; Virtanen, K.A. Regulation of Human Brown Adipose Tissue by Adenosine and A2A Receptors—Studies with [15O]H2O and [11C]TMSX PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Müller, S.; Perdikari, A.; Dapito, D.H.; Sun, W.; Wollscheid, B.; Balaz, M.; Wolfrum, C. ESRRG and PERM1 Govern Mitochondrial Conversion in Brite/Beige Adipocyte Formation. Front. Endocrinol. 2020, 11, 387. [Google Scholar] [CrossRef] [PubMed]
- Kazak, L.; Chouchani, E.T.; Jedrychowski, M.P.; Erickson, B.K.; Shinoda, K.; Cohen, P.; Vetrivelan, R.; Lu, G.Z.; Laznik-Bogoslavski, D.; Hasenfuss, S.C.; et al. A Creatine-Driven Substrate Cycle Enhances Energy Expenditure and Thermogenesis in Beige Fat. Cell 2015, 163, 643–655. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Rahbani, J.F.; Jedrychowski, M.P.; Riley, C.L.; Vidoni, S.; Bogoslavski, D.; Hu, B.; Dumesic, P.A.; Zeng, X.; Wang, A.B.; et al. Mitochondrial TNAP Controls Thermogenesis by Hydrolysis of Phosphocreatine. Nature 2021, 593, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Lee, J.H.; Yeung, J.L.-H.; Das, E.; Kim, R.Y.; Jiang, Y.; Moon, J.H.; Jeong, H.; Thakkar, N.; Son, J.E.; et al. Thermogenesis-Independent Metabolic Benefits Conferred by Isocaloric Intermittent Fasting in Ob/Ob Mice. Sci. Rep. 2019, 9, 2479. [Google Scholar] [CrossRef] [PubMed]
- Giordano, A.; Cinti, F.; Canese, R.; Carpinelli, G.; Colleluori, G.; Di Vincenzo, A.; Palombelli, G.; Severi, I.; Moretti, M.; Redaelli, C.; et al. The Adipose Organ Is a Unitary Structure in Mice and Humans. Biomedicines 2022, 10, 2275. [Google Scholar] [CrossRef]
- Gavaldà-Navarro, A.; Villarroya, J.; Cereijo, R.; Giralt, M.; Villarroya, F. The Endocrine Role of Brown Adipose Tissue: An Update on Actors and Actions. Rev. Endocr. Metab. Disord. 2022, 23, 31–41. [Google Scholar] [CrossRef]
- Lynch, L.; Hogan, A.E.; Duquette, D.; Lester, C.; Banks, A.; LeClair, K.; Cohen, D.E.; Ghosh, A.; Lu, B.; Corrigan, M.; et al. iNKT Cells Induce FGF21 for Thermogenesis and Are Required for Maximal Weight Loss in GLP1 Therapy. Cell Metab. 2016, 24, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, G.-X.; Ma, S.L.; Jung, D.Y.; Ha, H.; Altamimi, T.; Zhao, X.-Y.; Guo, L.; Zhang, P.; Hu, C.-R.; et al. Nrg4 Promotes Fuel Oxidation and a Healthy Adipokine Profile to Ameliorate Diet-Induced Metabolic Disorders. Mol. Metab. 2017, 6, 863–872. [Google Scholar] [CrossRef]
- Velickovic, K.; Leija, H.A.L.; Kosic, B.; Sacks, H.; Symonds, M.E.; Sottile, V. Leptin Deficiency Impairs Adipogenesis and Browning Response in Mouse Mesenchymal Progenitors. Eur. J. Cell Biol. 2023, 102, 151342. [Google Scholar] [CrossRef] [PubMed]
- Comeau, K.; Caillon, A.; Paradis, P.; Schiffrin, E.L. Determination of Interleukin-17A and Interferon-γ Production in γδ, CD4+, and CD8+ T Cells Isolated from Murine Lymphoid Organs, Perivascular Adipose Tissue, Kidney, and Lung. Bio Protoc. 2023, 13, e4679. [Google Scholar] [CrossRef] [PubMed]
- Ziqubu, K.; Dludla, P.V.; Moetlediwa, M.T.; Nyawo, T.A.; Pheiffer, C.; Jack, B.U.; Nkambule, B.; Mazibuko-Mbeje, S.E. Disease Progression Promotes Changes in Adipose Tissue Signatures in Type 2 Diabetic (Db/Db) Mice: The Potential Pathophysiological Role of Batokines. Life Sci. 2023, 313, 121273. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.; Becerril, S.; Hernández-Pardos, A.W.; Frühbeck, G. Adipose Tissue Depot Differences in Adipokines and Effects on Skeletal and Cardiac Muscle. Curr. Opin. Pharmacol. 2020, 52, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wu, W.; Ma, C.; Du, C.; Huang, Y.; Xu, H.; Li, C.; Cheng, X.; Hao, R.; Xu, Y. RNA-Binding Proteins in the Regulation of Adipogenesis and Adipose Function. Cells 2022, 11, 2357. [Google Scholar] [CrossRef] [PubMed]
- Baca, P.; Barajas-Olmos, F.; Mirzaeicheshmeh, E.; Zerrweck, C.; Guilbert, L.; Sánchez, E.C.; Flores-Huacuja, M.; Villafán, R.; Martínez-Hernández, A.; Carlos Sánchez, E.; et al. DNA Methylation and Gene Expression Analysis in Adipose Tissue to Identify New Loci Associated with T2D Development in Obesity. Nutr. Diabetes 2022, 12, 50. [Google Scholar] [CrossRef] [PubMed]
- MacCannell, A.D.; Roberts, L.D. Metabokines in the Regulation of Systemic Energy Metabolism. Curr. Opin. Pharmacol. 2022, 67, 102286. [Google Scholar] [CrossRef]
- Zou, T.; Chen, D.; Yang, Q.; Wang, B.; Zhu, M.-J.; Nathanielsz, P.W.; Du, M. Resveratrol Supplementation of High-Fat Diet-Fed Pregnant Mice Promotes Brown and Beige Adipocyte Development and Prevents Obesity in Male Offspring: Maternal Resveratrol Promotes Beige Adipogenesis in Offspring. J. Physiol. 2017, 595, 1547–1562. [Google Scholar] [CrossRef]
- Gong, H.; Sun, L.; Chen, B.; Han, Y.; Pang, J.; Wu, W.; Qi, R.; Zhang, T. Evaluation of Candidate Reference Genes for RT-qPCR Studies in Three Metabolism Related Tissues of Mice after Caloric Restriction. Sci. Rep. 2016, 6, 38513. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Huang, R.; Zhou, M.; Chen, J.; Zhang, J.; Zhou, T.; Ji, S.; Liu, X.; Tian, H.; Lam, S.M.; et al. Daytime-Restricted Feeding Enhances Running Endurance without Prior Exercise in Mice. Nat. Metab. 2023, 5, 1236–1251. [Google Scholar] [CrossRef] [PubMed]
- Blondin, D.P.; Labbé, S.M.; Noll, C.; Kunach, M.; Phoenix, S.; Guérin, B.; Turcotte, É.E.; Haman, F.; Richard, D.; Carpentier, A.C. Selective Impairment of Glucose but Not Fatty Acid or Oxidative Metabolism in Brown Adipose Tissue of Subjects with Type 2 Diabetes. Diabetes 2015, 64, 2388–2397. [Google Scholar] [CrossRef] [PubMed]
- Carey, A.L.; Kingwell, B.A. Brown Adipose Tissue in Humans: Therapeutic Potential to Combat Obesity. Pharmacol. Ther. 2013, 140, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Barrios, A.; Dirakvand, G.; Pervin, S. Human Brown Adipose Tissue and Metabolic Health: Potential for Therapeutic Avenues. Cells 2021, 10, 3030. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-H.; Wei, Y.-H. Therapeutic Perspectives of Thermogenic Adipocytes in Obesity and Related Complications. Int. J. Mol. Sci. 2021, 22, 7177. [Google Scholar] [CrossRef] [PubMed]
- Paulo, E.; Wang, B. Towards a Better Understanding of Beige Adipocyte Plasticity. Cells 2019, 8, 1552. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhang, M.; Duan, Y.; Shuai, L.; Jiang, H.; Li, J.; Nan, F.; Li, J. Pyrazolone Derivative C29 Protects against HFD-Induced Obesity in Mice via Activation of AMPK in Adipose Tissue. Acta Pharmacol. Sin. 2021, 42, 964–974. [Google Scholar] [CrossRef] [PubMed]
- Lundgren, P.; Sharma, P.V.; Dohnalová, L.; Coleman, K.; Uhr, G.T.; Kircher, S.; Litichevskiy, L.; Bahnsen, K.; Descamps, H.C.; Demetriadou, C.; et al. A Subpopulation of Lipogenic Brown Adipocytes Drives Thermogenic Memory. Nat. Metab. 2023, 5, 1691–1705. [Google Scholar] [CrossRef]
- Wu, D.; Bang, I.H.; Park, B.-H.; Bae, E.J. Loss of Sirt6 in Adipocytes Impairs the Ability of Adipose Tissue to Adapt to Intermittent Fasting. Exp. Mol. Med. 2021, 53, 1298–1306. [Google Scholar] [CrossRef]
- Li, G.; Xie, C.; Lu, S.; Nichols, R.G.; Tian, Y.; Li, L.; Patel, D.; Ma, Y.; Brocker, C.N.; Yan, T.; et al. Intermittent Fasting Promotes White Adipose Browning and Decreases Obesity by Shaping the Gut Microbiota. Cell Metab. 2017, 26, 672–685.e4. [Google Scholar] [CrossRef]
- Mu, W.-J.; Zhu, J.-Y.; Chen, M.; Guo, L. Exercise-Mediated Browning of White Adipose Tissue: Its Significance, Mechanism and Effectiveness. Int. J. Mol. Sci. 2021, 22, 11512. [Google Scholar] [CrossRef]
- Tam, B.T.; Siu, P.M. Autophagic Cellular Responses to Physical Exercise in Skeletal Muscle. Sport. Med. 2014, 44, 625–640. [Google Scholar] [CrossRef] [PubMed]
- Hepler, C.; Weidemann, B.J.; Waldeck, N.J.; Marcheva, B.; Cedernaes, J.; Thorne, A.K.; Kobayashi, Y.; Nozawa, R.; Newman, M.V.; Gao, P.; et al. Time-Restricted Feeding Mitigates Obesity through Adipocyte Thermogenesis. Science 2022, 378, 276–284. [Google Scholar] [CrossRef]
- Kaisanlahti, A.; Glumoff, T. Browning of White Fat: Agents and Implications for Beige Adipose Tissue to Type 2 Diabetes. J. Physiol. Biochem. 2019, 75, 1–10. [Google Scholar] [CrossRef]
- Ziqubu, K.; Dludla, P.V.; Mthembu, S.X.H.; Nkambule, B.B.; Mabhida, S.E.; Jack, B.U.; Nyambuya, T.M.; Mazibuko-Mbeje, S.E. An Insight into Brown/Beige Adipose Tissue Whitening, a Metabolic Complication of Obesity with the Multifactorial Origin. Front. Endocrinol. 2023, 14, 1114767. [Google Scholar] [CrossRef]
- Park, W.Y.; Choe, S.-K.; Park, J.; Um, J.-Y. Black Raspberry (Rubus Coreanus Miquel) Promotes Browning of Preadipocytes and Inguinal White Adipose Tissue in Cold-Induced Mice. Nutrients 2019, 11, 2164. [Google Scholar] [CrossRef] [PubMed]
- Aladag, T.; Mogulkoc, R.; Baltaci, K.A. Irisin and Energy Metabolism and the Role of Irisin on Metabolic Syndrome. Mini-Rev. Med. Chem. 2023, 23, 1942–1958. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.-Y.; Li, B.-Y.; Xiao, G.; Liu, Y.; Guo, L.; Tang, Q.-Q. Cdo1 Promotes PPARγ-Mediated Adipose Tissue Lipolysis in Male Mice. Nat. Metab. 2022, 4, 1352–1368. [Google Scholar] [CrossRef]
- Bean, C.; Audano, M.; Varanita, T.; Favaretto, F.; Medaglia, M.; Gerdol, M.; Pernas, L.; Stasi, F.; Giacomello, M.; Herkenne, S.; et al. The Mitochondrial Protein Opa1 Promotes Adipocyte Browning That Is Dependent on Urea Cycle Metabolites. Nat. Metab. 2021, 3, 1633–1647. [Google Scholar] [CrossRef]
- Rabiee, A.; Plucińska, K.; Isidor, M.S.; Brown, E.L.; Tozzi, M.; Sidoli, S.; Petersen, P.S.S.; Agueda-Oyarzabal, M.; Torsetnes, S.B.; Chehabi, G.N.; et al. White Adipose Remodeling during Browning in Mice Involves YBX1 to Drive Thermogenic Commitment. Mol. Metab. 2021, 44, 101137. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhang, R.; Yu, L.; Zahr, T.; Li, X.; Kim, T.-W.; Qiang, L. PPARγ Acetylation in Adipocytes Exacerbates BAT Whitening and Worsens Age-Associated Metabolic Dysfunction. Cells 2023, 12, 1424. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.A.; Ramachandran, D.; Roh, H.C.; DiSpirito, J.R.; Belchior, T.; Zushin, P.-J.H.; Palmer, C.; Hong, S.; Mina, A.I.; Liu, B.; et al. Obesity-Linked PPARγ S273 Phosphorylation Promotes Insulin Resistance through Growth Differentiation Factor 3. Cell Metab. 2020, 32, 665–675.e6. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, E.; Mitani, T.; Nakashima, M.; Yonemoto, E.; Fujii, H.; Ashida, H. Theobromine Enhances the Conversion of White Adipocytes into Beige Adipocytes in a PPARγ Activation-Dependent Manner. J. Nutr. Biochem. 2022, 100, 108898. [Google Scholar] [CrossRef]
- Recinella, L.; De Filippis, B.; Libero, M.L.; Ammazzalorso, A.; Chiavaroli, A.; Orlando, G.; Ferrante, C.; Giampietro, L.; Veschi, S.; Cama, A.; et al. Anti-Inflammatory, Antioxidant, and WAT/BAT-Conversion Stimulation Induced by Novel PPAR Ligands: Results from Ex Vivo and In Vitro Studies. Pharmaceuticals 2023, 16, 346. [Google Scholar] [CrossRef] [PubMed]
- Nanduri, R.; Furusawa, T.; Lobanov, A.; He, B.; Xie, C.; Dadkhah, K.; Kelly, M.C.; Gavrilova, O.; Gonzalez, F.J.; Bustin, M. Epigenetic Regulation of White Adipose Tissue Plasticity and Energy Metabolism by Nuc leosome Binding HMGN Proteins. Nat. Commun. 2022, 13, 7303. [Google Scholar] [CrossRef] [PubMed]
- Arpón, A.; Milagro, F.I.; Ramos-Lopez, O.; Mansego, M.L.; Santos, J.L.; Riezu-Boj, J.-I.; Martínez, J.A. Epigenome-Wide Association Study in Peripheral White Blood Cells Involving Insulin Resistance. Sci. Rep. 2019, 9, 2445. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wu, Z.; Huang, S.; Wang, X.; He, S.; Liu, L.; Hu, Y.; Chen, L.; Chen, P.; Liu, S.; et al. Adipocyte IRE1α Promotes PGC1α mRNA Decay and Restrains Adaptive Thermogenesis. Nat. Metab. 2022, 4, 1166–1184. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, F.S.; Cavalcanti de Araújo, P.H.; Mota, R.F.; Carvalho, A.J.R.; Santos de Queiroz, M.; Baldo de Almeida, B.; Ferreira, K.C. de O.S.; Metzner, R.J.M.; Ferrari, G.D.; et al. RANKL Induces Beige Adipocyte Differentiation in Preadipocytes. Am. J. Physiol. Endocrinol. Metab. 2020, 318, E866–E877. [Google Scholar] [CrossRef]
- Lin, Y.-W.; Park, S.W.; Lin, Y.-L.; Burton, F.H.; Wei, L.-N. Cellular Retinoic Acid Binding Protein 1 Protects Mice from High-Fat Diet-Induced Obesity by Decreasing Adipocyte Hypertrophy. Int. J. Obes. 2020, 44, 466–474. [Google Scholar] [CrossRef]
- Chen, H.; Sun, L.; Feng, L.; Mulholland, M.; Zhang, W.; Yin, Y. Peptidoglycan Inhibits Beigeing of Adipose Tissue. Acta Pharm. Sin. B 2022, 12, 990–993. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Yang, G.; Yoneshiro, T.; Abe, Y.; Ito, R.; Yang, C.; Nakazono, J.; Okamoto-Katsuyama, M.; Uchida, A.; Arai, M.; et al. MYPT1-PP1β Phosphatase Negatively Regulates Both Chromatin Landscape and Co-Activator Recruitment for Beige Adipogenesis. Nat. Commun. 2022, 13, 5715. [Google Scholar] [CrossRef]
- Li, Y.; Wang, D.; Ping, X.; Zhang, Y.; Zhang, T.; Wang, L.; Jin, L.; Zhao, W.; Guo, M.; Shen, F.; et al. Local Hyperthermia Therapy Induces Browning of White Fat and Treats Obesity. Cell 2022, 185, 949–966.e19. [Google Scholar] [CrossRef] [PubMed]
- Antonyshyn, J.; Mazzoli, V.; McFadden, M.; Gramolini, A.; Hofer, S.; Simmons, C.; Santerre, J.P. Immunomagnetic Isolation and Enrichment of Microvascular Endothelial Cells from Human Adipose Tissue. Bio Protoc. 2022, 12, e4422. [Google Scholar] [CrossRef] [PubMed]
- Schneider, D.B. Brown Adipose Tissue (BAT) Activation and Its Potential Utilization as a Treatment Option for Obesity and Diabetes. Student Publications. 2022, p. 1006. Available online: https://cupola.gettysburg.edu/student_scholarship/1006 (accessed on 1 March 2024).
- Carpentier, A.C.; Blondin, D.P.; Haman, F.; Richard, D. Brown Adipose Tissue—A Translational Perspective. Endocr. Rev. 2023, 44, 143–192. [Google Scholar] [CrossRef] [PubMed]
- Till, A.; Fries, C.; Fenske, W.K. Brain-to-BAT - and Back?: Crosstalk between the Central Nervous System and Thermogenic Adipose Tissue in Development and Therapy of Obesity. Brain Sci. 2022, 12, 1646. [Google Scholar] [CrossRef]
- Gu, X.; Wang, L.; Liu, S.; Shan, T. Adipose Tissue Adipokines and Lipokines: Functions and Regulatory Mechanism in Skeletal Muscle Development and Homeostasis. Metabolism 2023, 139, 155379. [Google Scholar] [CrossRef] [PubMed]
- Baskaran, P.; Krishnan, V.; Fettel, K.; Gao, P.; Zhu, Z.; Ren, J.; Thyagarajan, B. TRPV1 Activation Counters Diet-Induced Obesity through Sirtuin-1 Activation and PRDM-16 Deacetylation in Brown Adipose Tissue. Int. J. Obes. 2017, 41, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Gu, H.; Gan, L.; Xu, Y.; Feng, F.; Saeed, M.; Sun, C. Reducing Smad3/ATF4 Was Essential for Sirt1 Inhibiting ER Stress-Induced Apoptosis in Mice Brown Adipose Tissue. Oncotarget 2017, 8, 9267–9279. [Google Scholar] [CrossRef]
- Fougerat, A.; Schoiswohl, G.; Polizzi, A.; Régnier, M.; Wagner, C.; Smati, S.; Fougeray, T.; Lippi, Y.; Lasserre, F.; Raho, I.; et al. ATGL-Dependent White Adipose Tissue Lipolysis Controls Hepatocyte PPARα Activity. Cell Rep. 2022, 39, 110910. [Google Scholar] [CrossRef]
- Cairó, M.; Villarroya, J. The Role of Autophagy in Brown and Beige Adipose Tissue Plasticity. J. Physiol. Biochem. 2020, 76, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Kaikaew, K.; Grefhorst, A.; Visser, J.A. Sex Differences in Brown Adipose Tissue Function: Sex Hormones, Glucocorticoids, and Their Crosstalk. Front. Endocrinol. 2021, 12, 652444. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Guo, Y.; Si, W.; Zhong, Q.; Mei, Y.; Feng, Y.; Zhang, X. Detection of Brown Adipose Tissue in Rats with Acute Cold Stimulation Using Quantitative Susceptibility Mapping. Chin. Med. J. 2022, 136, 2137–2139. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, Y.; Yu, J.; Gan, Z.; Wei, W.; Wang, C.; Zhang, L.; Wang, T.; Zhong, X. Resveratrol Attenuates High-Fat Diet Induced Hepatic Lipid Homeostasis Disorder and Decreases m6A RNA Methylation. Front. Pharmacol. 2020, 11, 568006. [Google Scholar] [CrossRef]
- Zhou, L.; Xiao, X.; Zhang, Q.; Zheng, J.; Deng, M. Deciphering the Anti-Obesity Benefits of Resveratrol: The “Gut Microbiota-Adipose Tissue” Axis. Front. Endocrinol. 2019, 10, 413. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Zhai, M.; Yan, D.; Li, D.; Li, C.; Zhang, Y.; Xiao, L.; Xiong, D.; Deng, Q.; Sun, W. Dietary Menthol-Induced TRPM8 Activation Enhances WAT “Browning” and Ameliorates Diet-Induced Obesity. Oncotarget 2017, 8, 75114–75126. [Google Scholar] [CrossRef] [PubMed]
- Lone, J.; Choi, J.H.; Kim, S.W.; Yun, J.W. Curcumin Induces Brown Fat-like Phenotype in 3T3-L1 and Primary White Adipocytes. J. Nutr. Biochem. 2016, 27, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.-Q.; Xiao, G.; Li, B.-Y.; Guo, Y.-Y.; Guo, L.; Tang, Q.-Q. L-Theanine Activates the Browning of White Adipose Tissue Through the AMPK/α-Ketoglutarate/Prdm16 Axis and Ameliorates Diet-Induced Obesity in Mice. Diabetes 2021, 70, 1458–1472. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Liu, X.; Peng, C.; Tan, C.; Sun, H.; Liu, H.; Zhang, Y.; Wu, P.; Cui, C.; Liu, C.; et al. The Phytochemical Hyperforin Triggers Thermogenesis in Adipose Tissue via a Dlat-AMPK Signaling Axis to Curb Obesity. Cell Metab. 2021, 33, 565–580.e7. [Google Scholar] [CrossRef]
- Aouichat, S.; Raya, E.; Molina-Carballo, A.; Munoz-Hoyos, A.; Aloweidi, A.S.; Elmahallawy, E.K.; Agil, A. Dose-Dependent Effect of Melatonin on BAT Thermogenesis in Zücker Diabetic Fatty Rat: Future Clinical Implications for Obesity. Antioxidants 2022, 11, 1646. [Google Scholar] [CrossRef]
- Liu, X.; Huang, Y.; Liang, X.; Wu, Q.; Wang, N.; Zhou, L.; Liu, W.; Ma, Q.; Hu, B.; Gao, H.; et al. Atractylenolide III from Atractylodes Macrocephala Koidz Promotes the Activation of Brown and White Adipose Tissue through SIRT1/PGC-1α Signaling Pathway. Phytomedicine 2022, 104, 154289. [Google Scholar] [CrossRef] [PubMed]
| Biomolecule | Species | Function | Tissue | Methods | Citation |
|---|---|---|---|---|---|
| A2AR | human | + | WAT/BAT/muscle | CE | [44] |
| NRG4 | mice | + | WAT/BAT | HFD/HIEC | [52] |
| CDO1 | mice | + | iWAT/eWAT/BAT | HFD/CE | [79] |
| Opa1 | mice | + | WAT/BAT | HFD/CE | [80] |
| YBX1 | mice | + | scWAT | CE | [81] |
| TZD | mice | + | WAT/BAT | HFD/HIEC | [83] |
| TB | mice | + | WAT/beige AT | CE | [84] |
| HMGN | mice | + | iWAT/eWAT | HFD | [86] |
| IRE1 | mice | + | iWAT/eWAT/beige AT | HFD/CE/3-adrenergic | [88] |
| PGN | mice | - | iWAT/eWAT | HFD | [91] |
| MYPT1 | mice | + | scWAT/beige AT | HFD/CE | [92] |
| HSF1 | human/mice | + | iWAT/BAT | HFD/CE | [93] |
| Sirt1 | mice | + | WAT/BAT | HFD/CE/3-adrenergic | [99,100] |
| ATGL | mice | + | BAT | Fasting/CE/3-adrenergic | [101] |
| Resveratrol | mice | + | pWAT/abWAT/eWAT | HFD | [105,106] |
| Menthol | mice | + | sWAT/beige AT/WAT | HFD | [107] |
| Curcumin | mice | + | WAT | None | [108] |
| L-theanine | mice | + | iWAT | HFD | [109] |
| HPF | human/mice | + | iWAT/BAT | HFD/CE | [110] |
| Melatonin | rat | + | iBAT | CE | [111] |
| AE | mice | + | iWAT/BAT | CE | [112] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, Y.; Zhao, L.; Li, M.; Liu, Y.; Shi, Y.; Zhang, J. Plasticity of Adipose Tissues: Interconversion among White, Brown, and Beige Fat and Its Role in Energy Homeostasis. Biomolecules 2024, 14, 483. https://doi.org/10.3390/biom14040483
Peng Y, Zhao L, Li M, Liu Y, Shi Y, Zhang J. Plasticity of Adipose Tissues: Interconversion among White, Brown, and Beige Fat and Its Role in Energy Homeostasis. Biomolecules. 2024; 14(4):483. https://doi.org/10.3390/biom14040483
Chicago/Turabian StylePeng, Yanqiu, Lixia Zhao, Min Li, Yunfei Liu, Yuke Shi, and Jian Zhang. 2024. "Plasticity of Adipose Tissues: Interconversion among White, Brown, and Beige Fat and Its Role in Energy Homeostasis" Biomolecules 14, no. 4: 483. https://doi.org/10.3390/biom14040483
APA StylePeng, Y., Zhao, L., Li, M., Liu, Y., Shi, Y., & Zhang, J. (2024). Plasticity of Adipose Tissues: Interconversion among White, Brown, and Beige Fat and Its Role in Energy Homeostasis. Biomolecules, 14(4), 483. https://doi.org/10.3390/biom14040483






