Strategies for Treating Traumatic Neuromas with Tissue-Engineered Materials
Abstract
1. Introduction
2. Peripheral Nerve Injury, Regeneration, and Traumatic Neuroma Formation
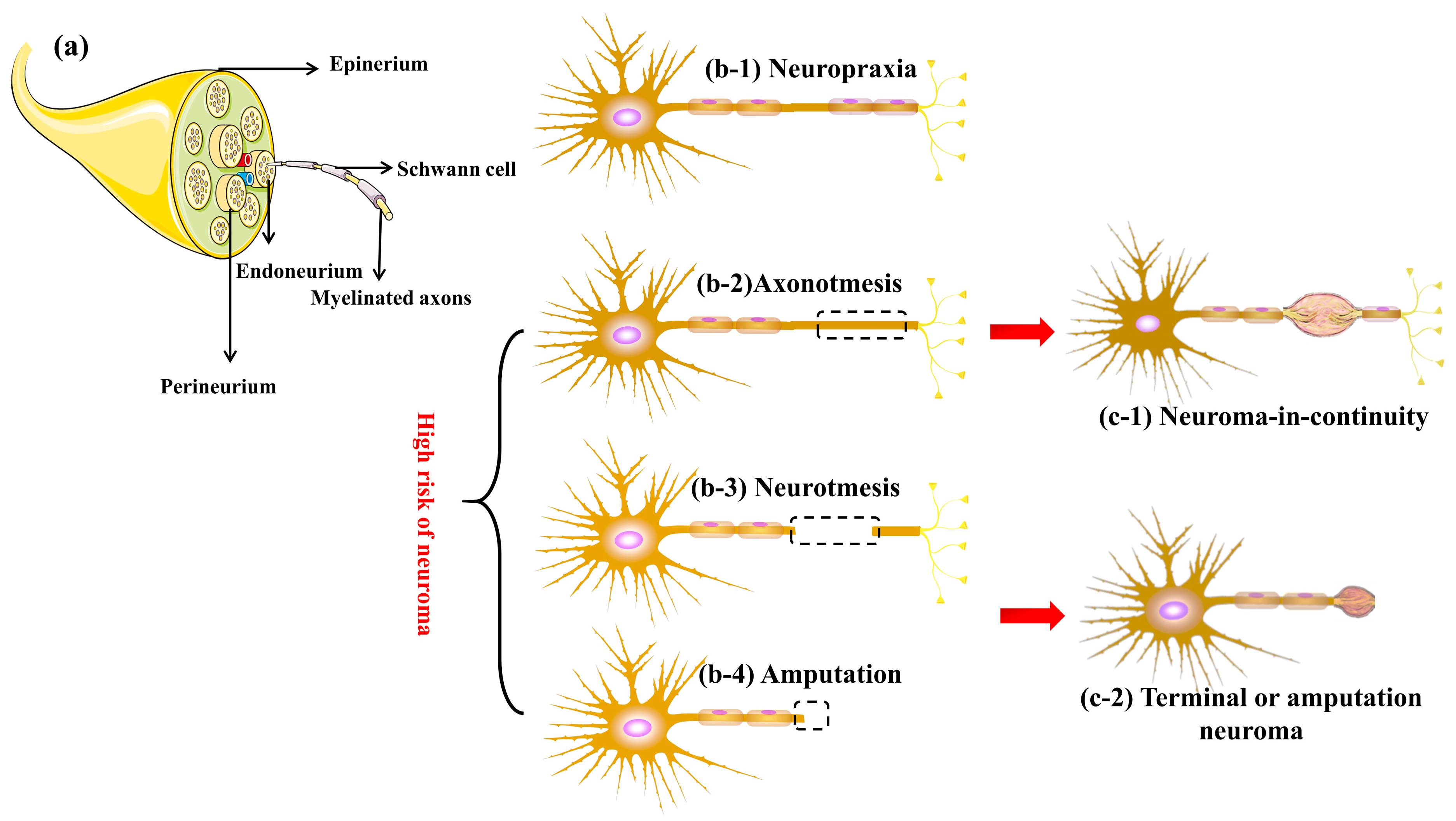
2.1. Peripheral Nerve Injuries and Neuroma
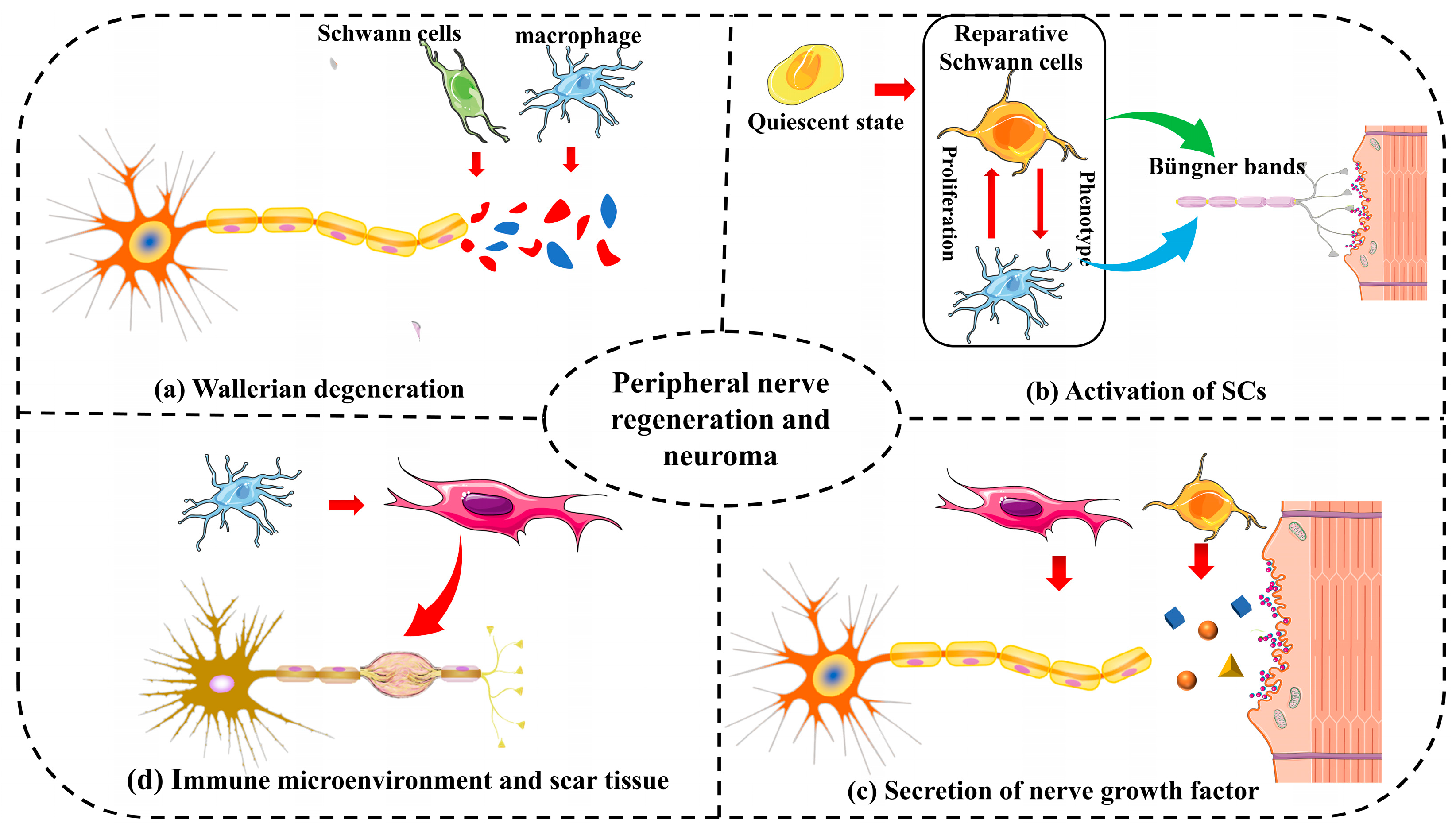
2.2. Peripheral Nerve Regeneration and Neuroma
2.3. Mechanisms of Neuroma Formation
| Factors | Study and Methods | Source | Observations and Conclusions |
|---|---|---|---|
| NGF | The expression of NGF was quantified by immunohistochemistry [36]. | Damaged nerve axons | The expression of NGF was significantly elevated in patients with traumatic neuroma compared to normal individuals |
| The NGF receptors p75 and trkA and the BDNF receptor GFRalpha-1 were semiquantitatively analyzed by immunohistochemistry [37]. | Glial cells | The immunoreactivity of trkA receptor was significantly increased in the traumatic neuroma group | |
| A mouse model of severe limb injury was used to study the role of sensory nerve fibers in fibrous scar tissue travel [38]. | Myofibroblasts | NGF expressed by myofibroblasts is an important factor in the formation of neuroma after severe limb trauma | |
| BDNF | Expression of BDNF and its receptor trkB after sciatic nerve transection in wild-type and heterozygous trkB-deficient mice [31]. | Injured peripheral nerves | Trkb-deficient mice did not develop traumatic neuromas after long-stage peripheral nerve injury |
| After sciatic nerve transection, the rats were given BDNF-containing and antagonistic BDNF-containing connective tissue chambers, respectively, and the incidence of traumatic neuroma was observed [39]. | Endogenous secretion | BDNF plays a key role in the development of neuropathic pain after peripheral nerve injury, and its local inactivation reduces the incidence and severity of neuroma formation | |
| The formation of a neuroma 2 weeks after complete transection of the inferior alveolar nerve was confirmed by histological analysis [40]. | Damaged nerve axons | Local administration of DNF antibody inhibited the proliferation of connective tissue at the injured site, promoted the integrity of nerve fibers, and reduced the formation of traumatic neuroma | |
| Neuroinflammatory peptides | IL-6 antiserum or CGRP receptor antagonist was administered at the sciatic nerve ligation site [41]. | Calcitonin gene-related peptide (CGRP) is expressed by the axons of neuromas | CGRP is involved in the formation of neuroma by upregulating the secretion of IL-6 in macrophages |
| Neuroma model animals were treated daily with histidine and loratadine [42]. | Histamine; mast cell | Endogenous histamine reduces neuropathic pain caused by traumatic neuromas | |
| Immunostaining was used to compare the contents of substance P and CGRP in axons of normal and neuroma sides [33]. | Substance P; mast cell | The neuroma showed a large number of disorganized axonal contours and positive immunostaining for CGRP or Substance P | |
| Inflammatory factor | To compare the expression of anti-inflammatory factors and proinflammatory factors in neuroma [43]. | TNF-α, IL-1β, IL-6, and IL-10 | IL-6 and IL-1β may play a role in the formation of traumatic neuroma, while IL-10 may inhibit neuroma formation |
| The expression of inflammatory factor genes in the injured sciatic nerve was detected [44]. | IL-1β, IL-10, IFN-γ, and TNF-α; macrophage | The high expression of proinflammatory and anti-inflammatory cytokines may be associated with the formation of fibrosis caused by irreversible nerve injury and, therefore, may be associated with the formation of traumatic neuroma | |
| Quantitative analysis of the expression of inflammatory factors in peripheral nerve stump [30]. | TNF-α, IL-6, and IL-1β | Expression of proinflammatory cytokines TNF-α, IL-6, and IL-1β was significantly increased in the dorsal root ganglia of traumatic neuromas | |
| α-SMA | The expression of α-SMA in neuroma was observed by immunofluorescence staining [35]. | Myofibroblasts | The expression of α-SMA was positively correlated with the pain index of patients |
| A rat model of amputated neuroma was used to quantitatively analyze the expression of α-SMA [45]. | Myofibroblasts | Levels of α-SMA and the pain marker c-fos were significantly higher in the amputation group | |
| Quantitative analysis of α-SMA expression in the terminal of neuroma was performed [46]. | Myofibroblasts | Capping transected rat sciatic nerves while concurrently administering myelin-associated glycoprotein was linked to reduced levels of α-SMA and autotomy behavior |
3. Traditional Treatment Options and Challenges
3.1. Nonsurgical Treatments
3.2. Surgical Treatments
4. Tissue-Engineered Materials for Neuromas
4.1. Composition and Types of Tissue-Engineered Materials
4.1.1. Composition of Tissue-Engineered Materials
4.1.2. Type of Tissue-Engineered Materials
4.2. Biomaterial-Based Scaffolds
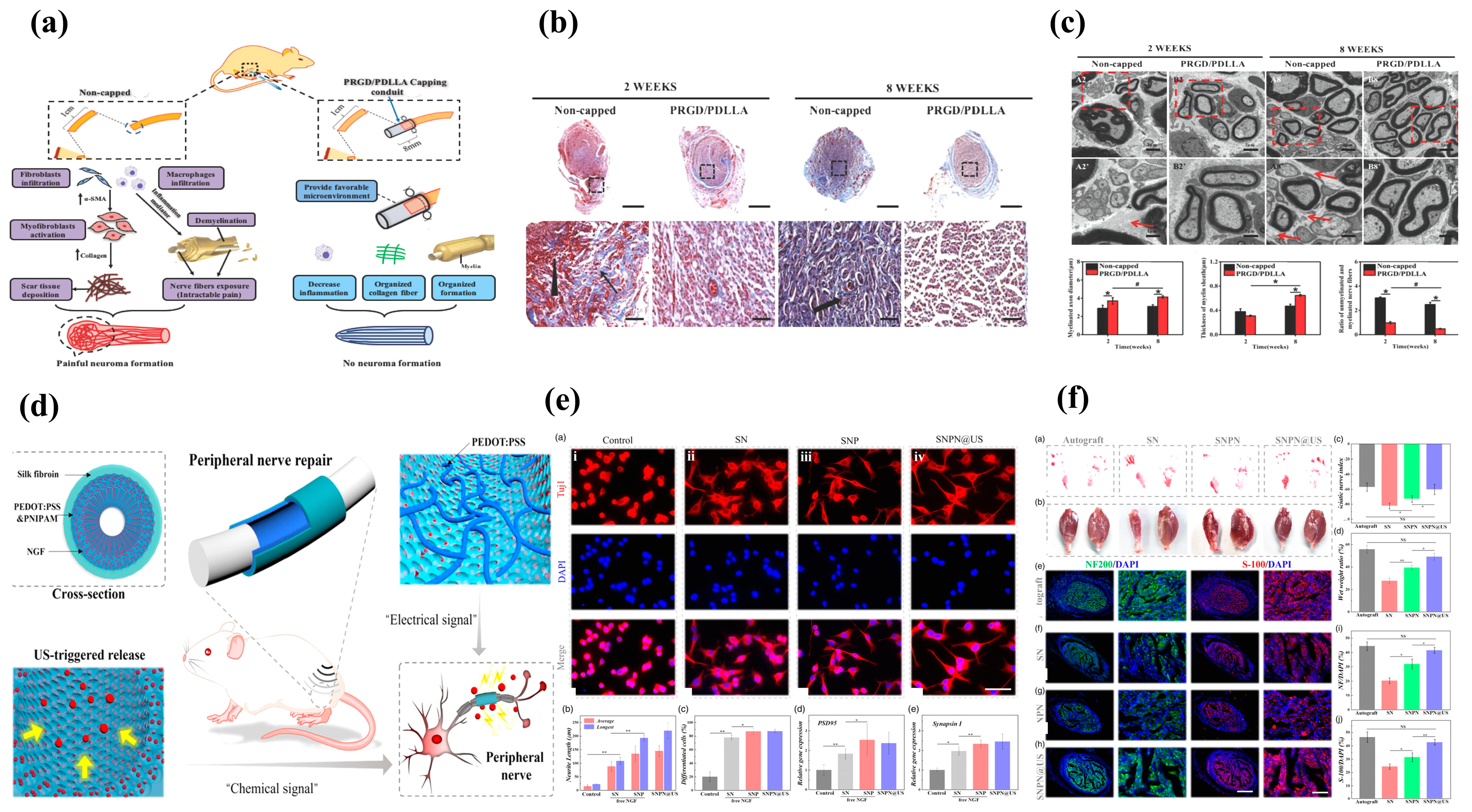
4.3. Growth Factor Incorporation
| Growth Factors | Receptors | Signaling Pathways | Bio-Function in Promoting Peripheral Nerve Regeneration |
|---|---|---|---|
| Nerve growth factor (NGF) [83] | TrkA, p75NTR |
|
|
| Brain-derived neurotrophic factor (BDNF) [84] | TrkB, p75NTR |
|
|
| Glial-derived neurotrophic factor (GDNF) [85] | GFRα 1~4 |
|
|
| Neurotrophin-3 (NT-3) [86] | TrkC, p75NTR |
|
|
| Insulin-like growth factor (IGF-1) [87] | IGF 1R, and 2R |
|
|
| Basic fibroblast growth factor (bFGF) [88] | FGFR I-IIIc |
|
|
| Vascular endothelial growth factor (VEGF) [89] | VEGF R1~R3 NP1, NP2 |
|
|
4.4. Cell-Based Approaches
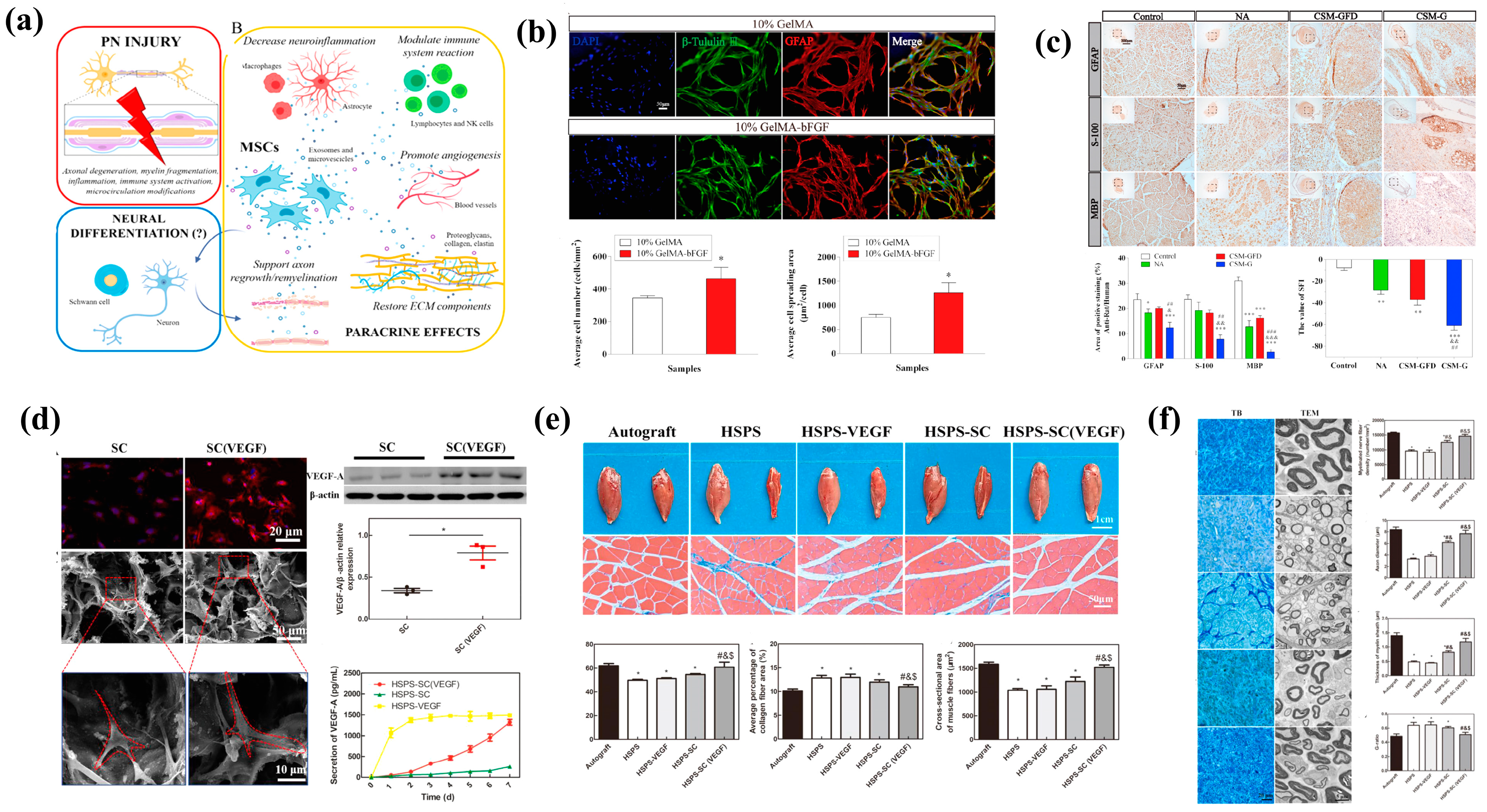
4.5. Electrical Stimulation
5. Summary and Challenge
Author Contributions
Funding
Conflicts of Interest
References
- List, E.B.; Krijgh, D.D.; Martin, E.; Coert, J.H. Prevalence of residual limb pain and symptomatic neuromas after lower extremity amputation: A systematic review and meta-analysis. Pain 2021, 162, 1906–1913. [Google Scholar] [CrossRef]
- Hwang, C.D.; Hoftiezer, Y.A.J.; Raasveld, F.V.; Gomez-Eslava, B.; van der Heijden, E.P.A.; Jayakar, S.; Black, B.J.; Johnston, B.R.; Wainger, B.J.; Renthal, W.; et al. Biology and pathophysiology of symptomatic neuromas. Pain 2022, 60, 550–564. [Google Scholar] [CrossRef] [PubMed]
- Tal, M.; Minert, A.; Devor, M. Resurgent neuropathic discharge: An obstacle to the therapeutic use of neuroma resection? Pain 2023, 164, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Janes, L.E.; Fracol, M.E.; Dumanian, G.A.; Ko, J.H. Targeted Muscle Reinnervation for the Treatment of Neuroma. Hand Clin. 2021, 37, 345–359. [Google Scholar] [CrossRef] [PubMed]
- Starr, B.W.; Chung, K.C. Traditional Neuroma Management. Hand Clin. 2021, 37, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Shamoun, F.; Shamoun, V.; Akhavan, A.; Tuffaha, S.H. Target Receptors of Regenerating Nerves: Neuroma Formation and Current Treatment Options. Front. Mol. Neurosci. 2022, 15, 859221. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Cheng, Y.; Cai, J.; Zhao, X.; Ouyang, Y.; Yuan, W.E.; Fan, C. Advances in electrical and magnetic stimulation on nerve regeneration. Regen. Med. 2019, 14, 969–979. [Google Scholar] [CrossRef] [PubMed]
- Scott, B.B.; Winograd, J.M.; Redmond, R.W. Surgical Approaches for Prevention of Neuroma at Time of Peripheral Nerve Injury. Front. Surg. 2022, 9, 819608. [Google Scholar] [CrossRef] [PubMed]
- Dumanian, G.A.; Potter, B.K.; Mioton, L.M.; Ko, J.H.; Cheesborough, J.E.; Souza, J.M.; Ertl, W.J.; Tintle, S.M.; Nanos, G.P.; Valerio, I.L.; et al. Targeted Muscle Reinnervation Treats Neuroma and Phantom Pain in Major Limb Amputees: A Randomized Clinical Trial. Ann. Surg. 2019, 270, 238–246. [Google Scholar] [CrossRef]
- Yang, Q.; Su, S.; Liu, S.; Yang, S.; Xu, J.; Zhong, Y.; Yang, Y.; Tian, L.; Tan, Z.; Wang, J.; et al. Exosomes-loaded electroconductive nerve dressing for nerve regeneration and pain relief against diabetic peripheral nerve injury. Bioact. Mater. 2023, 26, 194–215. [Google Scholar] [CrossRef]
- Sisti, A.; Uygur, S.; Lopez-Schultz, S.D.; Konofaos, P. Nerve Capping Techniques for Neuroma Management: A Comprehensive Literature Review. Ann. Plast. Surg. 2024, 92, 106–119. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Deng, P.J.; He, F.L.; Yan, L.W.; Tu, Z.H.; Liu, X.L.; Quan, D.P.; Bai, Y.; Zheng, C.B.; Zhu, Q.T. A decellularized nerve matrix scaffold inhibits neuroma formation in the stumps of transected peripheral nerve after peripheral nerve injury. Neural Regen. Res. 2023, 18, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Wan, T.; Zhang, F.-S.; Qin, M.-Y.; Jiang, H.-R.; Zhang, M.; Qu, Y.; Wang, Y.-L.; Zhang, P.-X. Growth factors: Bioactive macromolecular drugs for peripheral nerve injury treatment—Molecular mechanisms and delivery platforms. Biomed. Pharmacother. 2024, 170, 116024. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Li, B.; Yan, Q.; Dai, H.; Wang, X.; Huang, J.; Li, S. Promotion of peripheral nerve regeneration and prevention of neuroma formation by PRGD/PDLLA/β-TCP conduit: Report of two cases. Regen. Biomater. 2015, 2, 119–124. [Google Scholar] [CrossRef]
- Yang, X.; Huang, L.; Yi, X.; Huang, S.; Duan, B.; Yu, A. Multifunctional chitin-based hollow nerve conduit for peripheral nerve regeneration and neuroma inhibition. Carbohydr. Polym. 2022, 289, 119443. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Lang, Y.; Chang, M.-W.; Zhao, M.; Li, C.; Liu, S.; Wang, B. Leveraging Oriented Lateral Walls of Nerve Guidance Conduit with Core–Shell MWCNTs Fibers for Peripheral Nerve Regeneration. Adv. Healthc. Mater. 2024, 2303867. [Google Scholar] [CrossRef] [PubMed]
- Kocher, M.; Yilmaz, S.; Visoiu, M. Sciatic nerve neuropraxia following embolization therapy in a patient receiving quadratus lumborum nerve block. J. Clin. Anesth. 2022, 78, 110601. [Google Scholar] [CrossRef]
- Kim, H.W.; Shim, S.W.; Zhao, A.M.; Roh, D.; Han, H.M.; Middleton, S.J.; Kim, W.; Chung, S.; Johnson, E.; Prentice, J.; et al. Long-term tactile hypersensitivity after nerve crush injury in mice is characterized by the persistence of intact sensory axons. Pain 2023, 164, 2327–2342. [Google Scholar] [CrossRef] [PubMed]
- Fordington, S.; Manford, M. A review of seizures and epilepsy following traumatic brain injury. J. Neurol. 2020, 267, 3105–3111. [Google Scholar] [CrossRef]
- Chhabra, A.; Ahlawat, S.; Belzberg, A.; Andreseik, G. Peripheral nerve injury grading simplified on MR neurography: As referenced to Seddon and Sunderland classifications. Indian J. Radiol. Imaging 2014, 24, 217–224. [Google Scholar] [CrossRef]
- Barberá, J.; Garcia, G.; Lopez-Orta, A.; Gil-Salu, J.L. The role of the neuroma in autotomy following sciatic nerve section in rats. Pain 1988, 33, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Wan, T.; Wang, Y.L.; Zhang, F.S.; Zhang, X.M.; Zhang, Y.C.; Jiang, H.R.; Zhang, M.; Zhang, P.X. The Porous Structure of Peripheral Nerve Guidance Conduits: Features, Fabrication, and Implications for Peripheral Nerve Regeneration. Int. J. Mol. Sci. 2023, 24, 14132. [Google Scholar] [CrossRef] [PubMed]
- Han, G.H.; Peng, J.; Liu, P.; Ding, X.; Wei, S.; Lu, S.; Wang, Y. Therapeutic strategies for peripheral nerve injury: Decellularized nerve conduits and Schwann cell transplantation. Neural Regen. Res. 2019, 14, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
- Min, Q.; Parkinson, D.B.; Dun, X.P. Migrating Schwann cells direct axon regeneration within the peripheral nerve bridge. Glia 2021, 69, 235–254. [Google Scholar] [CrossRef]
- Jha, M.K.; Passero, J.V.; Rawat, A.; Ament, X.H.; Yang, F.; Vidensky, S.; Collins, S.L.; Horton, M.R.; Hoke, A.; Rutter, G.A.; et al. Macrophage monocarboxylate transporter 1 promotes peripheral nerve regeneration after injury in mice. J. Clin. Investig. 2021, 131, e141964. [Google Scholar] [CrossRef] [PubMed]
- Lindenlaub, T.; Sommer, C. Partial sciatic nerve transection as a model of neuropathic pain: A qualitative and quantitative neuropathological study. Pain 2000, 89, 97–106. [Google Scholar] [CrossRef]
- Mathieu, L.; Diner, C.; Aries, P.; Thomas, M.; Truffaut, S.; de L’escalopier, N. Preemptive targeted muscle reinnervation: The single incision approach should be avoided in trans-tibial traumatic amputation. Military Med. Res. 2022, 9, 60. [Google Scholar] [CrossRef]
- Foltán, R.; Klíma, K.; Spacková, J.; Sedy, J. Mechanism of traumatic neuroma development. Med. Hypotheses 2008, 71, 572–576. [Google Scholar] [CrossRef] [PubMed]
- Ji, F.; Zhang, Y.Y.; Cui, P.; Li, Y.; Li, C.X.; Du, D.P.; Xu, H. Preventive Effect of Local Lidocaine Administration on the Formation of Traumatic Neuroma. J. Clin. Med. 2023, 12, 2476. [Google Scholar] [CrossRef]
- Pu, S.; Wu, Y.; Tong, F.; Du, W.-J.; Liu, S.; Yang, H.; Zhang, C.; Zhou, B.; Chen, Z.; Zhou, X.; et al. Mechanosensitive Ion Channel TMEM63A Gangs Up with Local Macrophages to Modulate Chronic Post-amputation Pain. Neurosci. Bull. 2023, 39, 177–193. [Google Scholar] [CrossRef]
- Kotulska, K.; Larysz-Brysz, M.; Marcol, W.; Grajkowska, W.; Józwiak, S.; Lewin-Kowalik, J. The role of trkB receptor in the formation of post-traumatic neuroma. Folia Neuropathol. 2006, 44, 221–227. [Google Scholar]
- Jimenez-Andrade, J.M.; Ghilardi, J.R.; Castañeda-Corral, G.; Kuskowski, M.A.; Mantyh, P.W. Preventive or late administration of anti-NGF therapy attenuates tumor-induced nerve sprouting, neuroma formation, and cancer pain. Pain 2011, 152, 2564–2574. [Google Scholar] [CrossRef] [PubMed]
- Zochodne, D.W.; Theriault, M.; Sharkey, K.A.; Cheng, C.; Sutherland, G. Peptides and neuromas: Calcitonin gene-related peptide, substance P, and mast cells in a mechanosensitive human sural neuroma. Muscle Nerve 1997, 20, 875–880. [Google Scholar] [CrossRef]
- Xie, W.R.; Strong, J.A.; Zhang, J.M. Localized sympathectomy reduces peripheral nerve regeneration and pain behaviors in 2 rat neuropathic pain models. Pain 2020, 161, 1925–1936. [Google Scholar] [CrossRef]
- Yan, H.D.; Gao, W.Y.; Pan, Z.J.; Zhang, F.; Fan, C.Y. The Expression of α-SMA in the Painful Traumatic Neuroma: Potential Role in the Pathobiology of Neuropathic Pain. J. Neurotrauma 2012, 29, 2791–2797. [Google Scholar] [CrossRef]
- Atherton, D.D.; Taherzadeh, O.; Facer, P.; Elliot, D.; Anand, P. The potential role of nerve growth factor (NGF) in painful neuromas and the mechanism of pain relief by their relocation to muscle. J. Hand Surg.-Br. Eur. Vol. 2006, 31B, 652–656. [Google Scholar] [CrossRef]
- Harpf, C.; Dabernig, J.; Humpel, C. Receptors for NGF and GDNF are highly expressed in human peripheral nerve neuroma. Muscle Nerve 2002, 25, 612–615. [Google Scholar] [CrossRef]
- Lee, S.Y.; Hwang, C.; Marini, S.; Tower, R.J.; Qin, Q.Z.; Negri, S.; Pagani, C.A.; Sun, Y.X.; Stepien, D.M.; Sorkin, M.; et al. NGF-TrkA signaling dictates neural ingrowth and aberrant osteochondral differentiation after soft tissue trauma. Nat. Commun. 2021, 12, 4939. [Google Scholar] [CrossRef] [PubMed]
- Marcol, W.; Kotulska, K.; Larysz-Brysz, M.; Kowalik, J.L. BDNF contributes to animal model neuropathic pain after peripheral nerve transection. Neurosurg. Rev. 2007, 30, 235–243. [Google Scholar] [CrossRef]
- Guevara, Y.M.V.; Yoshikawa, H.; Saito, I.; Maeda, T.; Seo, K. Effect of local application of an antibody against brain-derived neurotrophic factor on neuroma formation after transection of the inferior alveolar nerve in the rat. Neuroreport 2014, 25, 1069–1074. [Google Scholar] [CrossRef]
- Ma, W.Y.; Quirion, R. Increased calcitonin gene-related peptide in neuroma and invading macrophages is involved in the up-regulation of interleukin-6 and thermal hyperalgesia in a rat model of mononeuropathy. J. Neurochem. 2006, 98, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Lou, G.D.; Yue, J.X.; Tang, Y.Y.; Hou, W.W.; Shou, W.T.; Ohtsu, H.; Zhang, S.H.; Chen, Z. Effects of histamine on spontaneous neuropathic pain induced by peripheral axotomy. Neurosci. Bull. 2013, 29, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Khan, J.; Noboru, N.; Young, A.; Thomas, D. Pro and anti-inflammatory cytokine levels (TNF-α, IL-1β, IL-6 and IL-10) in rat model of neuroma. Pathophysiology 2017, 24, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Ruohonen, S.; Khademi, M.; Jagodic, M.; Taskinen, H.S.; Olsson, T.; Röyttä, M. Cytokine responses during chronic denervation. J. Neuroinflamm. 2005, 2, 26. [Google Scholar] [CrossRef] [PubMed]
- Weng, W.D.; Zhao, B.; Lin, D.S.; Gao, W.Y.; Li, Z.J.; Yan, H.D. Significance of alpha smooth muscle actin expression in traumatic painful neuromas: A pilot study in rats. Sci. Rep. 2016, 6, 23828. [Google Scholar] [CrossRef] [PubMed]
- Pi, W.; Li, C.; Zhang, M.; Zhang, W.; Zhang, P.X. Myelin-associated glycoprotein combined with chitin conduit inhibits painful neuroma formation after sciatic nerve transection. Neural Regen. Res. 2022, 17, 1343–1347. [Google Scholar] [CrossRef] [PubMed]
- Morrison, D.R.; Moore, L.S.; Walsh, E.M. Perioperative Pain Management Following Otologic Surgery. Otolaryngol. Clin. N. Am. 2020, 53, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Lans, J.; Westenberg, R.F.; Gottlieb, R.E.; Valerio, I.L.; Chen, N.C.; Eberlin, K.R. Long-Term Opioid Use Following Surgery for Symptomatic Neuroma. J. Reconstr. Microsurg. 2022, 38, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Kent, M.L.; Hsia, H.L.J.; Van de Ven, T.J.; Buchheit, T.E. Perioperative Pain Management Strategies for Amputation: A Topical Review. Pain Med. 2017, 18, 504–519. [Google Scholar] [CrossRef][Green Version]
- Sperry, B.P.; Cheney, C.W.; Conger, A.; Shipman, H.; McCormick, Z.L. Cooled Radiofrequency Ablation of a Large Sciatic Neuroma at the Infrapiriformis Foramen for Recalcitrant Phantom Limb Pain in a Below-Knee Amputee. Pain Med. 2020, 22, 223–226. [Google Scholar] [CrossRef]
- Gougoulias, N.; Lampridis, V.; Sakellariou, A. Morton’s interdigital neuroma: Instructional review. EFORT Open Rev. 2019, 4, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Ernberg, L.A.; Adler, R.S.; Lane, J. Ultrasound in the detection and treatment of a painful stump neuroma. Skeletal Radiol. 2003, 32, 306–309. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.B.; Jacobs, A.; Williams, K.L.; Bour, R.K.; Gyftopoulos, S. Ultrasound-Guided Injection Treatments Versus Surgical Neurectomy for Morton Neuroma: A Cost-Effectiveness Analysis. Am. J. Roentgenol. 2022, 218, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Hertzano, R.; Eisenman, D.J. Large, Symptomatic Tension Pneumocele: 23 Years After Translabyrinthine Resection of an Acoustic Neuroma. Otolaryngol.-Head Neck Surg. 2011, 144, 477–478. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Dip, T.M.; Padhye, R.; Houshyar, S. Review on electrically conductive smart nerve guide conduit for peripheral nerve regeneration. J. Biomed. Mater. Res. Part A 2023, 111, 1916–1950. [Google Scholar] [CrossRef] [PubMed]
- Kerasnoudis, A.; Yoon, M.S. Relapsing Neuromas: A Therapeutic Challenge? Clin. J. Pain 2013, 29, 280. [Google Scholar] [CrossRef] [PubMed]
- Wolvetang, N.H.A.; Lans, J.; Verhiel, S.H.W.L.; Notermans, B.J.W.; Chen, N.C.; Eberlin, K.R. Surgery for Symptomatic Neuroma: Anatomic Distribution and Predictors of Secondary Surgery. Plast. Reconstr. Surg. 2019, 143, 1762–1771. [Google Scholar] [CrossRef]
- Roth, E.; Linehan, A.; Weihrauch, D.; Stucky, C.; Hogan, Q.; Hoben, G. Targeted muscle reinnervation prevents and reverses rat pain behaviors after nerve transection. Pain 2023, 164, 316–324. [Google Scholar] [CrossRef] [PubMed]
- González-Prieto, J.; Cristóbal, L.; Arenillas, M.; Giannetti, R.; Muñoz Frías, J.D.; Alonso Rivas, E.; Sanz Barbero, E.; Gutiérrez-Pecharromán, A.; Díaz Montero, F.; Maldonado, A.A. Regenerative Peripheral Nerve Interfaces (RPNIs) in Animal Models and Their Applications: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 1141. [Google Scholar] [CrossRef]
- Liu, K.; Yan, L.S.; Li, R.T.; Song, Z.M.; Ding, J.X.; Liu, B.; Chen, X.S. 3D Printed Personalized Nerve Guide Conduits for Precision Repair of Peripheral Nerve Defects. Adv. Sci. 2022, 9, 2103875. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, J.H.; Wang, Y.; Shang, L.R.; Chai, R.J.; Zhao, Y.J. Natural Polymer-Derived Bioscaffolds for Peripheral Nerve Regeneration. Adv. Funct. Mater. 2022, 32, 2203829. [Google Scholar] [CrossRef]
- Deng, P.; Chen, F.; Zhang, H.; Chen, Y.; Zhou, J. Multifunctional Double-Layer Composite Hydrogel Conduit Based on Chitosan for Peripheral Nerve Repairing. Adv. Healthc. Mater. 2022, 11, 2200115. [Google Scholar] [CrossRef] [PubMed]
- Maksoud, F.J.; Velázquez de la Paz, M.F.; Hann, A.J.; Thanarak, J.; Reilly, G.C.; Claeyssens, F.; Green, N.H.; Zhang, Y.S. Porous biomaterials for tissue engineering: A review. J. Mater. Chem. B 2022, 10, 8111–8165. [Google Scholar] [CrossRef]
- Valentino, C.; Vigani, B.; Zucca, G.; Ruggeri, M.; Marrubini, G.; Boselli, C.; Icaro Cornaglia, A.; Sandri, G.; Rossi, S. Design of Novel Mechanically Resistant and Biodegradable Multichannel Platforms for the Treatment of Peripheral Nerve Injuries. Biomacromolecules 2023, 24, 1731–1743. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Wang, G.; Qian, T.; Cai, X.; Zhang, P.; Li, M.; Shen, Y.; Xue, C.; Wang, H. The balanced microenvironment regulated by the degradants of appropriate PLGA scaffolds and chitosan conduit promotes peripheral nerve regeneration. Mater. Today Bio 2021, 12, 100158. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Yuan, W.; Xu, J.; Tong, W.; Mi, J.; Ho, P.-C.; Chow, D.H.K.; Li, Y.; Yao, H.; Li, X.; et al. Magnesium-Encapsulated Injectable Hydrogel and 3D-Engineered Polycaprolactone Conduit Facilitate Peripheral Nerve Regeneration. Adv. Sci. 2022, 9, 2202102. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Gong, J.; Zhang, J.; Zhu, Z.; Qian, Y.; Lu, K.; Zhou, S.; Gu, T.; Wang, H.; He, Y.; et al. Three Potential Elements of Developing Nerve Guidance Conduit for Peripheral Nerve Regeneration. Adv. Funct. Mater. 2023, 33, 2302251. [Google Scholar] [CrossRef]
- Fang, Y.; Wang, C.; Liu, Z.; Ko, J.; Chen, L.; Zhang, T.; Xiong, Z.; Zhang, L.; Sun, W. 3D Printed Conductive Multiscale Nerve Guidance Conduit with Hierarchical Fibers for Peripheral Nerve Regeneration. Adv. Sci. 2023, 10, 2205744. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Xiao, C.; Liu, B. Engineered hydrogels for peripheral nerve repair. Mater. Today Bio 2023, 20, 100668. [Google Scholar] [CrossRef]
- Xu, W.; Wu, Y.; Lu, H.; Zhang, X.; Zhu, Y.; Liu, S.; Zhang, Z.; Ye, J.; Yang, W. Injectable hydrogel encapsulated with VEGF-mimetic peptide-loaded nanoliposomes promotes peripheral nerve repair in vivo. Acta Biomater. 2023, 160, 225–238. [Google Scholar] [CrossRef]
- Cai, C.; Zhu, H.; Chen, Y.; Chen, C.; Li, H.; Yang, Z.; Liu, H. Conductive nerve guide conduits based on wet-adhesive hydrogel to accelerate peripheral nerve repair. Appl. Mater. Today 2022, 27, 101491. [Google Scholar] [CrossRef]
- Li, X.; He, N.; Li, X.; Wang, X.; Zhan, L.; Yuan, W.-E.; Song, J.; Ouyang, Y. Graphdiyne-loaded polycaprolactone nanofiber scaffold for peripheral nerve regeneration. J. Colloid Interface Sci. 2023, 646, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Thow, S.Y.; Abdullah, S.; Ng, M.H.; Haflah, N.H.M. Advancement of Electrospun Nerve Conduit for Peripheral Nerve Regeneration: A Systematic Review (2016–2021). Int. J. Nanomed. 2022, 17, 6723–6758. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.; Yang, Y.; Chen, Y.; Xie, J.; Liu, S.; Tan, Z.; Tian, L.; Yu, Z.; Shi, Z.; Xie, P.; et al. Ropivacaine microsphere-loaded electroconductive nerve dressings for long-acting analgesia and functional recovery following diabetic peripheral nerve injury. Mater. Today Bio 2023, 21, 100712. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Chang, B.; Dong, H.; Liu, X. Functional microspheres for tissue regeneration. Bioact. Mater. 2023, 25, 485–499. [Google Scholar] [CrossRef]
- Yi, J.; Jiang, N.; Li, B.; Yan, Q.; Qiu, T.; Swaminatha Iyer, K.; Yin, Y.; Dai, H.; Yetisen, A.K.; Li, S. Painful Terminal Neuroma Prevention by Capping PRGD/PDLLA Conduit in Rat Sciatic Nerves. Adv. Sci. 2018, 5, 1700876. [Google Scholar] [CrossRef] [PubMed]
- Fadia, N.B.; Bliley, J.M.; DiBernardo, G.A.; Crammond, D.J.; Schilling, B.K.; Sivak, W.N.; Spiess, A.M.; Washington, K.M.; Waldner, M.; Liao, H.T.; et al. Long-gap peripheral nerve repair through sustained release of a neurotrophic factor in nonhuman primates. Sci. Transl. Med. 2020, 12, eaav7753. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Ai, J.; Xiao, A.; Wu, P.; Wu, M.; Liu, X.; Huselstein, C.; Cai, L.; Feng, X.; Chen, Y. Nerve Defect Treatment with a Capping Hydroxyethyl Cellulose/Soy Protein Isolate Sponge Conduit for Painful Neuroma Prevention. ACS Omega 2023, 8, 30850–30858. [Google Scholar] [CrossRef]
- Millán, D.; Jiménez, R.A.; Nieto, L.E.; Poveda, I.Y.; Torres, M.A.; Silva, A.S.; Ospina, L.F.; Mano, J.F.; Fontanilla, M.R. Adjustable conduits for guided peripheral nerve regeneration prepared from bi-zonal unidirectional and multidirectional laminar scaffold of type I collagen. Mater. Sci. Eng. C 2021, 121, 111838. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, H.; Wen, B.; Lu, L.; Zhao, Y.; Chai, R. Ultrasound-Responsive Composited Conductive Silk Conduits for Peripheral Nerve Regeneration. Small Struct. 2023, 4, 2300045. [Google Scholar] [CrossRef]
- Xu, H.X.; Yu, Y.; Zhang, L.X.; Zheng, F.R.; Yin, Y.X.; Gao, Y.X.; Li, K.B.; Xu, J.Y.; Wen, J.; Chen, H.; et al. Sustainable release of nerve growth factor for peripheral nerve regeneration using nerve conduits laden with Bioconjugated hyaluronic acid-chitosan hydrogel. Compos. Part B-Eng. 2022, 230, 109509. [Google Scholar] [CrossRef]
- Zhu, L.; Jia, S.; Liu, T.; Yan, L.; Huang, D.; Wang, Z.; Chen, S.; Zhang, Z.; Zeng, W.; Zhang, Y.; et al. Aligned PCL Fiber Conduits Immobilized with Nerve Growth Factor Gradients Enhance and Direct Sciatic Nerve Regeneration. Adv. Funct. Mater. 2020, 30, 2002610. [Google Scholar] [CrossRef]
- Jiao, J.; Wang, F.; Huang, J.-J.; Huang, J.-J.; Li, Z.-A.; Kong, Y.; Zhang, Z.-J. Microfluidic hollow fiber with improved stiffness repairs peripheral nerve injury through non-invasive electromagnetic induction and controlled release of NGF. Chem. Eng. J. 2021, 426, 131826. [Google Scholar] [CrossRef]
- Huang, L.Y.; Jin, J.H.; Chen, K.; You, S.K.; Zhang, H.Y.; Sideris, A.; Norcini, M.; Recio-Pinto, E.; Wang, J.; Gan, W.B.; et al. BDNF produced by cerebral microglia promotes cortical plasticity and pain hypersensitivity after peripheral nerve injury. PLoS Biol. 2021, 19, e3001337. [Google Scholar] [CrossRef] [PubMed]
- Cintron-Colon, A.F.; Almeida-Alves, G.; VanGyseghem, J.M.; Spitsbergen, J.M. GDNF to the rescue: GDNF delivery effects on motor neurons and nerves, and muscle re-innervation after peripheral nerve injuries. Neural Regen. Res. 2022, 17, 748–753. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Song, L.; Li, Y.; Guo, J.; Huang, S.; Du, S.; Li, W.; Cao, R.; Cui, S. Neurotrophin-3 promotes peripheral nerve regeneration by maintaining a repair state of Schwann cells after chronic denervation via the TrkC/ERK/c-Jun pathway. J. Transl. Med. 2023, 21, 733. [Google Scholar] [CrossRef] [PubMed]
- Hanwright, P.J.; Qiu, C.H.; Rath, J.; Zhou, Y.; von Guionneau, N.; Sarhane, K.A.; Harris, T.G.W.; Howard, G.P.; Malapati, H.; Lan, M.J.; et al. Sustained IGF-1 delivery ameliorates effects of chronic denervation and improves functional recovery after peripheral nerve injury and repair. Biomaterials 2022, 280, 121244. [Google Scholar] [CrossRef] [PubMed]
- Idrisova, K.F.; Zeinalova, A.K.; Masgutova, G.A.; Bogov, A.A.; Allegrucci, C.; Syromiatnikova, V.Y.; Salafutdinov, I.I.; Garanina, E.E.; Andreeva, D.I.; Kadyrov, A.A.; et al. Application of neurotrophic and proangiogenic factors as therapy after peripheral nervous system injury. Neural Regen. Res. 2022, 17, 1240–1247. [Google Scholar] [CrossRef]
- Gao, Y.S.; Dai, C.L.; Zhang, M.; Zhang, J.Y.; Yin, L.; Li, W.H.; Zhang, K.Y.; Yang, Y.M.; Zhao, Y.H. Biomimetic Silk Fibroin Hydrogel for Enhanced Peripheral Nerve Regeneration: Synergistic Effects of Graphene Oxide and Fibroblast Exosome. Adv. Funct. Mater. 2023, 2314610. [Google Scholar] [CrossRef]
- Lei, W.-L.; Peng, C.-W.; Chiu, S.-C.; Lu, H.-E.; Wu, C.-W.; Cheng, T.-Y.; Huang, W.-C. All Biodisintegratable Hydrogel Biohybrid Neural Interfaces with Synergistic Performances of Microelectrode Array Technologies, Tissue Scaffolding, and Cell Therapy. Adv. Funct. Mater. 2024, 34, 2307365. [Google Scholar] [CrossRef]
- Muangsanit, P.; Roberton, V.; Costa, E.; Phillips, J.B. Engineered aligned endothelial cell structures in tethered collagen hydrogels promote peripheral nerve regeneration. Acta Biomater. 2021, 126, 224–237. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Zhang, F.; Zheng, Y.; Liu, Z.; Wang, D.; Wei, Z.; Deng, C. Synergistic effect of nanofat and mouse nerve-growth factor for promotion of sensory recovery in anterolateral thigh free flaps. Stem Cells Transl. Med. 2021, 10, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Tong, Z.; Luo, L.H.; Zhao, Y.N.; Chen, F.X.; Li, Y.P.; Huselstein, C.; Ye, Q.F.; Ye, Q.S.; Chen, Y. Comprehensive strategy of conduit guidance combined with VEGF producing Schwann cells accelerates peripheral nerve repair. Bioact. Mater. 2021, 6, 3515–3527. [Google Scholar] [CrossRef] [PubMed]
- Palombella, S.; Guiotto, M.; Higgins, G.C.; Applegate, L.L.; Raffoul, W.; Cherubino, M.; Hart, A.; Riehle, M.O.; di Summa, P.G. Human platelet lysate as a potential clinical-translatable supplement to support the neurotrophic properties of human adipose-derived stem cells. Stem Cell Res. Ther. 2020, 11, 432. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Chang, W.; Zhou, X.Q.; Chen, Y.F.; Dai, F.T.; Anwar, A.; Yu, X.J. Nanofibrous Nerve Conduits with Nerve Growth Factors and Bone Marrow Stromal Cells Pre-Cultured in Bioreactors for Peripheral Nerve Regeneration. ACS Appl. Mater. Interfaces 2020, 12, 16168–16177. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; He, Y.; Jin, L.; Zhang, Y.; Guastaldi, F.P.; Albashari, A.A.; Hu, F.; Wang, X.; Wang, L.; Xiao, J.; et al. Application of bioactive hydrogels combined with dental pulp stem cells for the repair of large gap peripheral nerve injuries. Bioact. Mater. 2021, 6, 638–654. [Google Scholar] [CrossRef] [PubMed]
- Lavorato, A.; Raimondo, S.; Boido, M.; Muratori, L.; Durante, G.; Cofano, F.; Vincitorio, F.; Petrone, S.; Titolo, P.; Tartara, F.; et al. Mesenchymal Stem Cell Treatment Perspectives in Peripheral Nerve Regeneration: Systematic Review. Int. J. Mol. Sci. 2021, 22, 572. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Jeon, J.; Kim, B.; Lee, M.S.; Park, S.; Lim, J.; Yi, J.; Lee, H.; Yang, H.S.; Lee, J.Y. Electrically Conductive Hydrogel Nerve Guidance Conduits for Peripheral Nerve Regeneration. Adv. Funct. Mater. 2020, 30, 2003759. [Google Scholar] [CrossRef]
- Chu, X.L.; Song, X.Z.; Li, Q.; Li, Y.R.; He, F.; Gu, X.S.; Ming, D. Basic mechanisms of peripheral nerve injury and treatment via electrical stimulation. Neural Regen. Res. 2022, 17, 2185–2193. [Google Scholar] [CrossRef]
- Song, S.; McConnell, K.W.; Amores, D.; Levinson, A.; Vogel, H.; Quarta, M.; Rando, T.A.; George, P.M. Electrical stimulation of human neural stem cells via conductive polymer nerve guides enhances peripheral nerve recovery. Biomaterials 2021, 275, 120982. [Google Scholar] [CrossRef]
- He, L.; Xiao, Q.; Zhao, Y.; Li, J.; Reddy, S.; Shi, X.; Su, X.; Chiu, K.; Ramakrishna, S. Engineering an Injectable Electroactive Nanohybrid Hydrogel for Boosting Peripheral Nerve Growth and Myelination in Combination with Electrical Stimulation. ACS Appl. Mater. Interfaces 2020, 12, 53150–53163. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yin, X.; Wang, H.; Qiu, W.; Li, L.; Li, F.; Shan, Y.; Zhao, Z.; Li, Z.; Guo, J.; et al. Engineering a wirelessly self-powered and electroconductive scaffold to promote peripheral nerve regeneration. Nano Energy 2023, 107, 108145. [Google Scholar] [CrossRef]
- Wang, Q.Q.; Wang, H.; Ma, Y.; Cao, X.D.; Gao, H.C. Effects of electroactive materials on nerve cell behaviors and applications in peripheral nerve repair. Biomater. Sci. 2022, 10, 6061–6076. [Google Scholar] [CrossRef] [PubMed]
| Therapeutic Regimen | Methods | Objectives | Advantages | Disadvantages |
|---|---|---|---|---|
| Nonsurgical treatment |
| Relieve the pain symptoms of patients |
|
|
| Surgical treatment |
|
|
|
|
| Tissue-engineered materials |
|
|
| Development of a nerve repair material that aligns with the microenvironment of peripheral nerve regeneration, which requires further exploration |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, T.; Li, Q.-C.; Qin, M.-Y.; Wang, Y.-L.; Zhang, F.-S.; Zhang, X.-M.; Zhang, Y.-C.; Zhang, P.-X. Strategies for Treating Traumatic Neuromas with Tissue-Engineered Materials. Biomolecules 2024, 14, 484. https://doi.org/10.3390/biom14040484
Wan T, Li Q-C, Qin M-Y, Wang Y-L, Zhang F-S, Zhang X-M, Zhang Y-C, Zhang P-X. Strategies for Treating Traumatic Neuromas with Tissue-Engineered Materials. Biomolecules. 2024; 14(4):484. https://doi.org/10.3390/biom14040484
Chicago/Turabian StyleWan, Teng, Qi-Cheng Li, Ming-Yu Qin, Yi-Lin Wang, Feng-Shi Zhang, Xiao-Meng Zhang, Yi-Chong Zhang, and Pei-Xun Zhang. 2024. "Strategies for Treating Traumatic Neuromas with Tissue-Engineered Materials" Biomolecules 14, no. 4: 484. https://doi.org/10.3390/biom14040484
APA StyleWan, T., Li, Q.-C., Qin, M.-Y., Wang, Y.-L., Zhang, F.-S., Zhang, X.-M., Zhang, Y.-C., & Zhang, P.-X. (2024). Strategies for Treating Traumatic Neuromas with Tissue-Engineered Materials. Biomolecules, 14(4), 484. https://doi.org/10.3390/biom14040484






