Redefining Immune Dynamics in Acute Pancreatitis: The Protective Role of Galectin-3 Deletion and Treg Cell Enhancement
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Induction of Acute Pancreatitis by Bile-Pancreatic Duct (BPD) Ligation
2.3. Histological Evaluation of Pancreatic, Lung, and Kidney Injury
2.4. Levels of Amylase
2.5. Trypsin Activity
2.6. Isolation of Leukocytes from Pancreata
2.7. Flow Cytometry
2.8. Statistical Analysis
3. Results
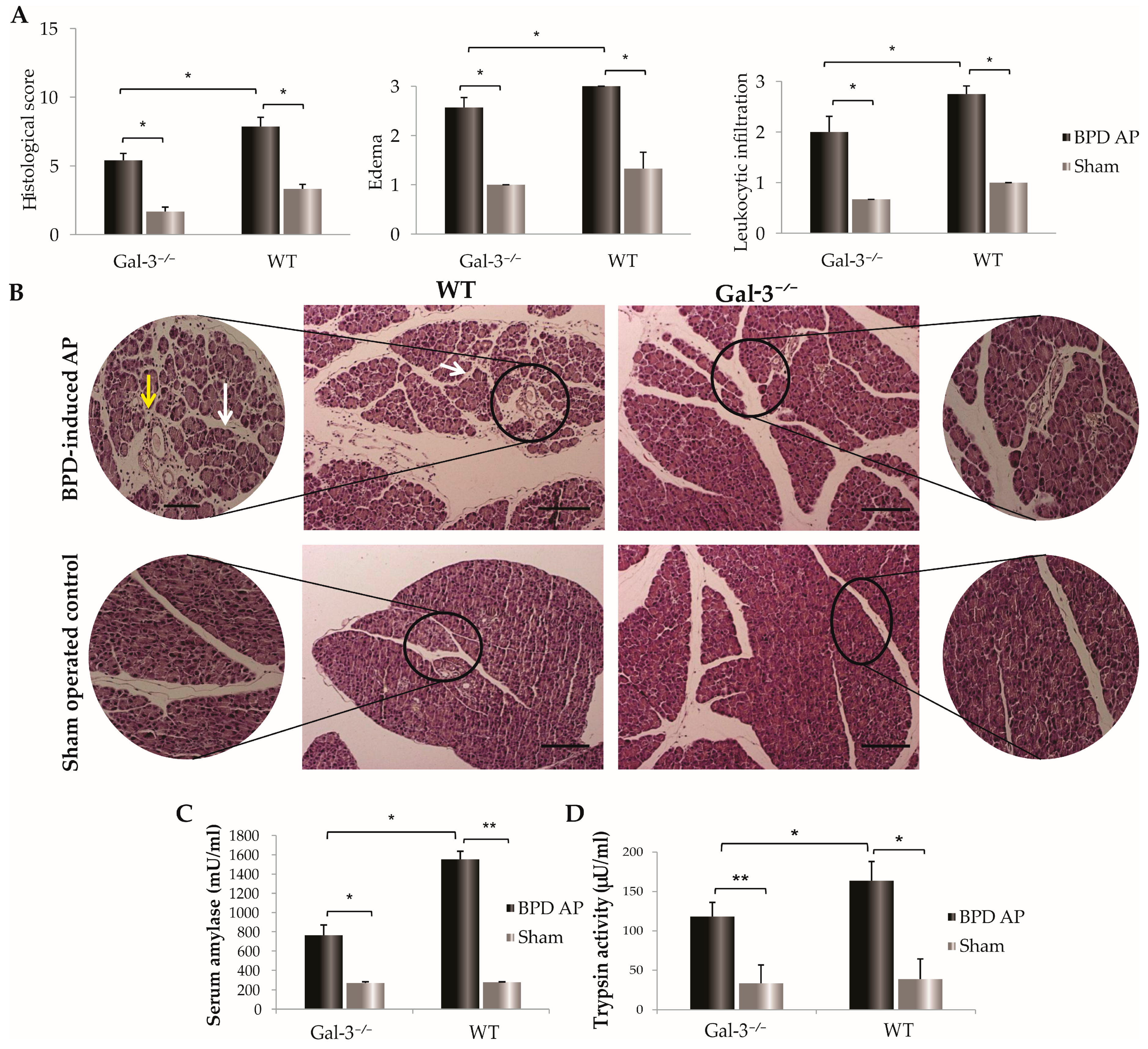
3.1. Reduced Pancreatic Damage in Galectin-3-Deficient Mice Post-BPD Ligation
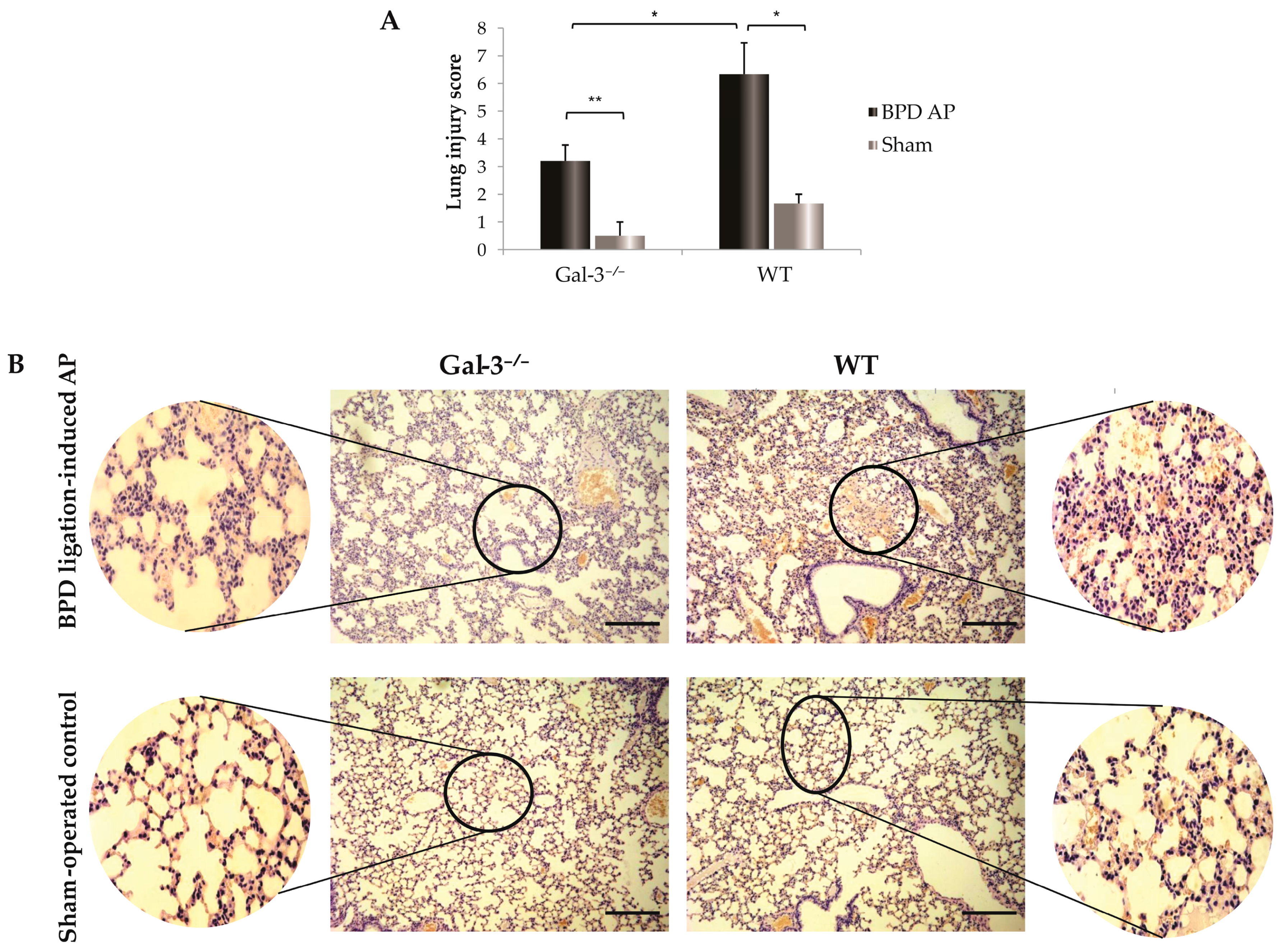
3.2. Galectin-3 Deficiency and Its Protective Effects against Lung Injury in Acute Pancreatitis
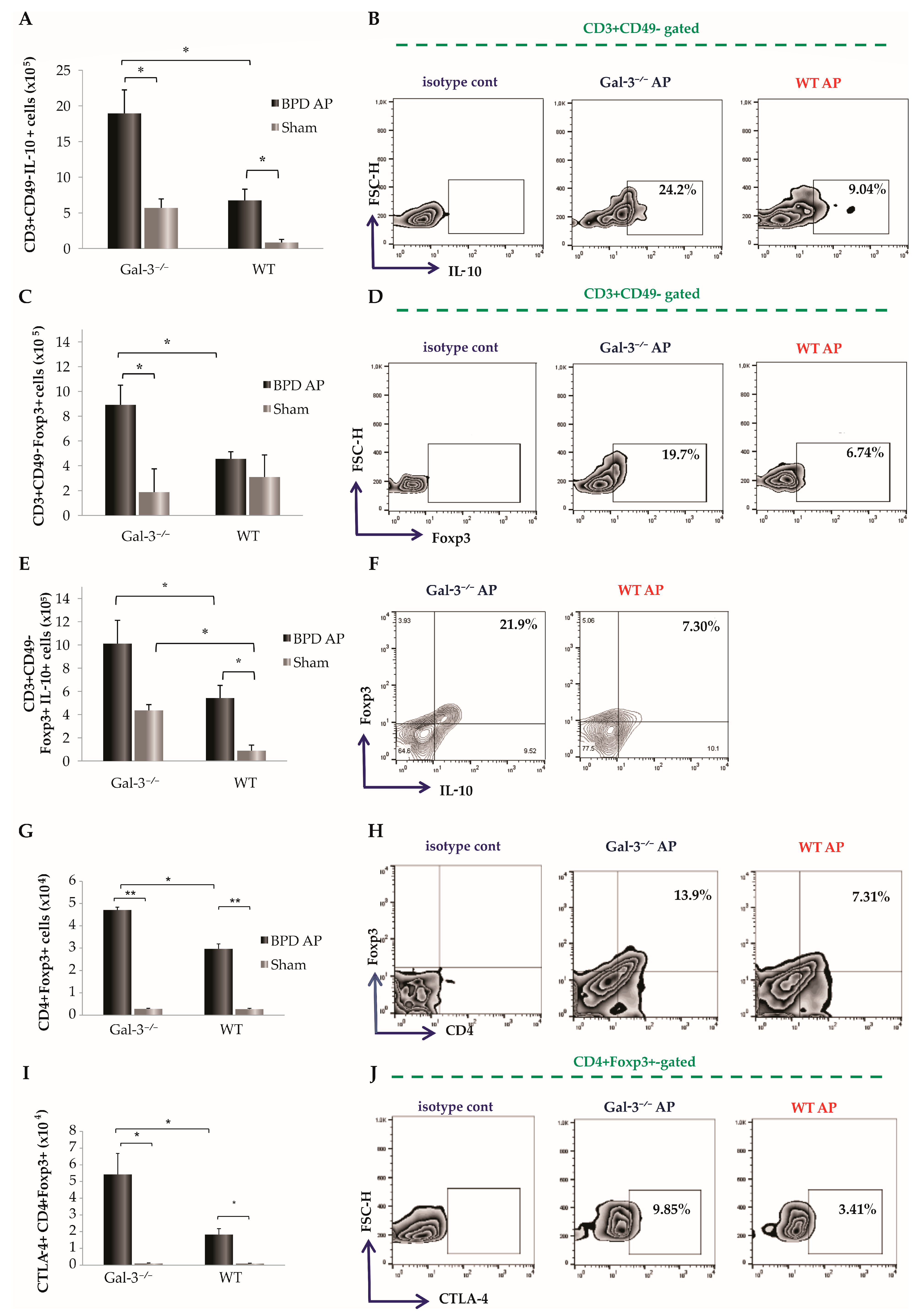
3.3. Impact of Galectin-3 Deletion on T Cell Dynamics in Acute Pancreatitis
3.4. Pronounced Infiltration of Regulatory T Cells in Pancreatic Tissue of Diseased Gal-3-Deficient Mice
3.5. Influence of Galectin-3 Deletion on Tolerogenic Dendritic Cell Infiltration in the Pancreas
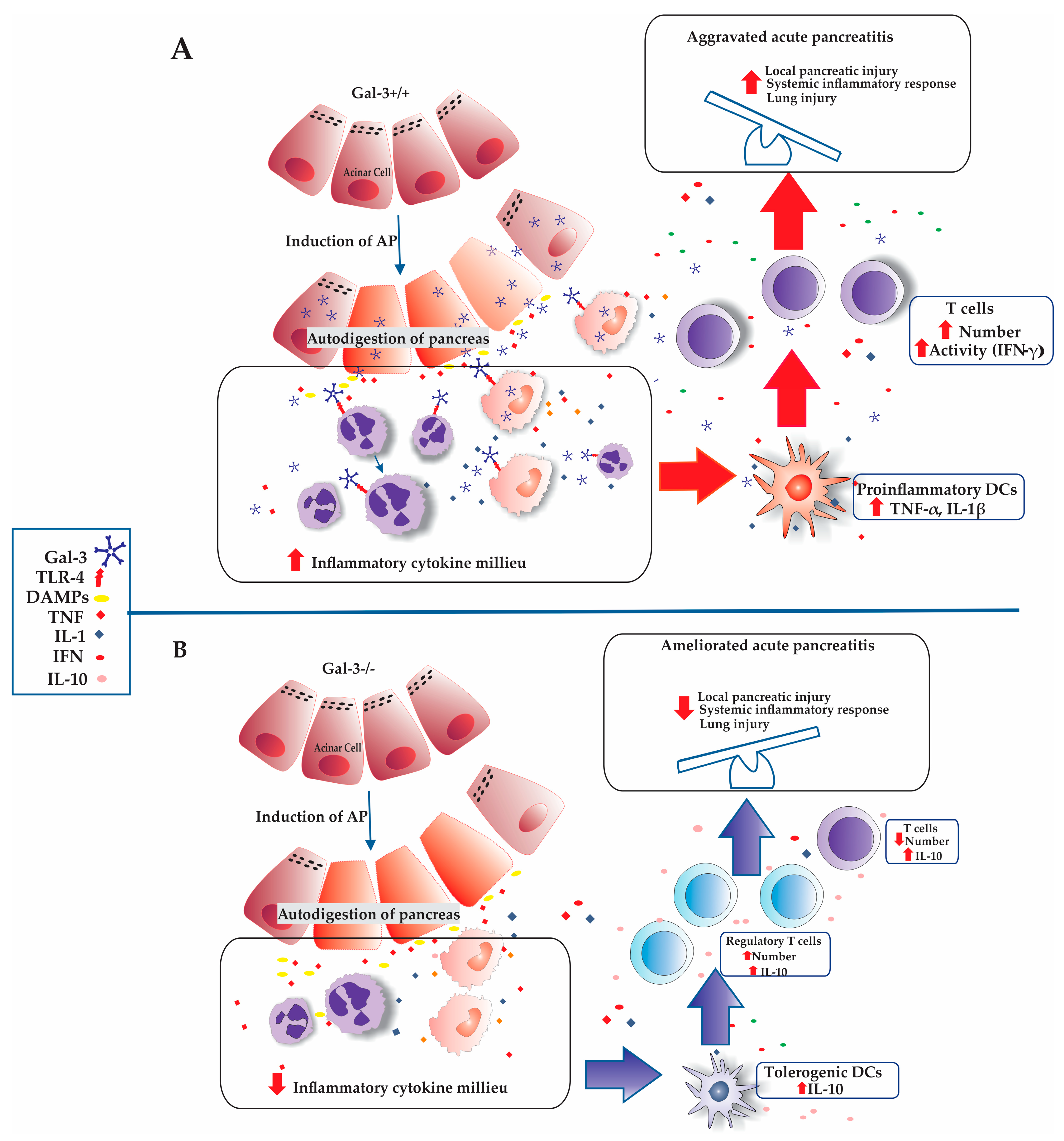
4. Discussion
4.1. The Role of Galectin-3 in Modulating Inflammation and Organ Injury in Acute Pancreatitis
4.2. Impact of T Cell Dynamics and Galectin-3 Deficiency on Acute Pancreatitis
4.3. Impact of Galectin-3 Deficiency on T Cell Activation and IFN-γ Production in Acute Pancreatitis
4.4. Regulatory Influence of Galectin-3 Deficiency on Tregs Dynamics in Acute Pancreatitis
4.5. Impact of Galectin-3 Deficiency on Tolerogenic DCs and Treg Infiltration in AP
4.6. Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, C.; Li, G.; Lu, T.; Liu, L.; Sui, Y.; Bai, R.; Li, L.; Sun, B. The Interaction of Microbiome and Pancreas in Acute Pancreatitis. Biomolecules 2024, 14, 59. [Google Scholar] [CrossRef] [PubMed]
- Goodchild, G.; Chouhan, M.; Johnson, G.J. Practical guide to the management of acute pancreatitis. Frontline Gastroenterol. 2019, 10, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Petrov, M.S.; Yadav, D. Global epidemiology and holistic prevention of pancreatitis. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 175–184. [Google Scholar] [CrossRef]
- Song, Y.; Lee, S.-H. Recent Treatment Strategies for Acute Pancreatitis. J. Clin. Med. 2024, 13, 978. [Google Scholar] [CrossRef] [PubMed]
- Boxhoorn, L.; Voermans, R.P.; Bouwense, S.A.; Bruno, M.J.; Verdonk, R.C.; Boermeester, M.A.; van Santvoort, H.C.; Besselink, M.G. Acute pancreatitis. Lancet 2020, 396, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Stojanovic, B.; Jovanovic, I.P.; Stojanovic, M.D.; Jovanovic, M.; Vekic, B.; Milosevic, B.; Cvetkovic, A.; Spasic, M.; Stojanovic, B.S. The Emerging Roles of the Adaptive Immune Response in Acute Pancreatitis. Cells 2023, 12, 1495. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Li, Z.; Yu, X. The Role of Pancreatic Infiltrating Innate Immune Cells in Acute Pancreatitis. Int. J. Med. Sci. 2021, 18, 534–545. [Google Scholar] [CrossRef] [PubMed]
- Okeke, E.B.; Uzonna, J.E. The Pivotal Role of Regulatory T Cells in the Regulation of Innate Immune Cells. Front. Immunol. 2019, 10, 680. [Google Scholar] [CrossRef] [PubMed]
- Fonteh, P.; Smith, M.; Brand, M. Adaptive Immune Cell Dysregulation and Role in Acute Pancreatitis Disease Progression and Treatment. Arch. Immunol. Ther. Exp. 2018, 66, 199–209. [Google Scholar] [CrossRef]
- Stojanovic, B.S.; Stojanovic, B.; Milovanovic, J.; Arsenijević, A.; Dimitrijevic Stojanovic, M.; Arsenijevic, N.; Milovanovic, M. The Pivotal Role of Galectin-3 in Viral Infection: A Multifaceted Player in Host–Pathogen Interactions. Int. J. Mol. Sci. 2023, 24, 9617. [Google Scholar] [CrossRef]
- Radosavljevic, G.; Volarevic, V.; Jovanovic, I.; Milovanovic, M.; Pejnovic, N.; Arsenijevic, N.; Hsu, D.K.; Lukic, M.L. The roles of Galectin-3 in autoimmunity and tumor progression. Immunol. Res. 2012, 52, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Aureli, A.; Del Cornò, M.; Marziani, B.; Gessani, S.; Conti, L. Highlights on the Role of Galectin-3 in Colorectal Cancer and the Preventive/Therapeutic Potential of Food-Derived Inhibitors. Cancers 2023, 15, 52. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, F.L.; Gatto, M.; Bassi, N.; Luisetto, R.; Ghirardello, A.; Punzi, L.; Doria, A. Galectin-3 in autoimmunity and autoimmune diseases. Exp. Biol. Med. 2015, 240, 1019–1028. [Google Scholar] [CrossRef] [PubMed]
- Gilson, R.C.; Gunasinghe, S.D.; Johannes, L.; Gaus, K. Galectin-3 modulation of T-cell activation: Mechanisms of membrane remodelling. Prog. Lipid Res. 2019, 76, 101010. [Google Scholar] [CrossRef] [PubMed]
- Stojanovic, B.; Jovanovic, I.; Stojanovic, B.S.; Stojanovic, M.D.; Gajovic, N.; Radosavljevic, G.; Pantic, J.; Arsenijevic, N.; Lukic, M.L. Deletion of Galectin-3 attenuates acute pancreatitis in mice by affecting activation of innate inflammatory cells. Eur. J. Immunol. 2019, 49, 940–946. [Google Scholar] [CrossRef] [PubMed]
- Stojanovic, M.D.; Stojanovic, B.; Arsenijevic, N.; Stojanovic, B. The Role of TLR-4 and Galectin-3 Interaction in Acute Pancreatitis. Exp. Appl. Biomed. Res. (EABR) 2020. [Google Scholar] [CrossRef]
- Dimitrijevic Stojanovic, M.; Stojanovic, B.; Radosavljevic, I.; Kovacevic, V.; Jovanovic, I.; Stojanovic, B.S.; Prodanovic, N.; Stankovic, V.; Jocic, M.; Jovanovic, M. Galectin-3’s Complex Interactions in Pancreatic Ductal Adenocarcinoma: From Cellular Signaling to Therapeutic Potential. Biomolecules 2023, 13, 1500. [Google Scholar] [CrossRef] [PubMed]
- Hsu, D.K.; Yang, R.Y.; Pan, Z.; Yu, L.; Salomon, D.R.; Fung-Leung, W.P.; Liu, F.T. Targeted disruption of the galectin-3 gene results in attenuated peritoneal inflammatory responses. Am. J. Pathol. 2000, 156, 1073–1083. [Google Scholar] [CrossRef] [PubMed]
- Samuel, I.; Yuan, Z.; Meyerholz, D.K.; Twait, E.; Williard, D.E.; Kempuraj, D. A novel model of severe gallstone pancreatitis: Murine pancreatic duct ligation results in systemic inflammation and substantial mortality. Pancreatology 2010, 10, 536–544. [Google Scholar] [CrossRef]
- Dembinski, A.; Warzecha, Z.; Ceranowicz, P.; Warzecha, A.M.; Pawlik, W.W.; Dembinski, M.; Rembiasz, K.; Sendur, P.; Kusnierz-Cabala, B.; Tomaszewska, R.; et al. Dual, time-dependent deleterious and protective effect of anandamide on the course of cerulein-induced acute pancreatitis. Role of sensory nerves. Eur. J. Pharmacol. 2008, 591, 284–292. [Google Scholar] [CrossRef]
- Cao, C.; Yin, C.; Shou, S.; Wang, J.; Yu, L.; Li, X.; Chai, Y. Ulinastatin Protects Against LPS-Induced Acute Lung Injury by Attenuating TLR4/NF-kappaB Pathway Activation and Reducing Inflammatory Mediators. Shock 2018, 50, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Markakis, C.; Tsaroucha, A.; Papalois, A.E.; Lambropoulou, M.; Spartalis, E.; Tsigalou, C.; Romanidis, K.; Simopoulos, C. The Role of Eugenol in the Prevention of Acute Pancreatitis-Induced Acute Kidney Injury: Experimental Study. HPB Surg. World J. Hepatic Pancreat. Biliary Surg. 2016, 2016, 3203147. [Google Scholar] [CrossRef] [PubMed]
- Mondragon, A.; Davidsson, D.; Kyriakoudi, S.; Bertling, A.; Gomes-Faria, R.; Cohen, P.; Rothery, S.; Chabosseau, P.; Rutter, G.A.; da Silva Xavier, G. Divergent effects of liraglutide, exendin-4, and sitagliptin on beta-cell mass and indicators of pancreatitis in a mouse model of hyperglycaemia. PLoS ONE 2014, 9, e104873. [Google Scholar] [CrossRef] [PubMed]
- LeBleu, V.S.; Teng, Y.; O’Connell, J.T.; Charytan, D.; Muller, G.A.; Muller, C.A.; Sugimoto, H.; Kalluri, R. Identification of human epididymis protein-4 as a fibroblast-derived mediator of fibrosis. Nat. Med. 2013, 19, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Nguyen, D.T.; Habtezion, A. Aryl hydrocarbon receptor regulates pancreatic IL-22 production and protects mice from acute pancreatitis. Gastroenterology 2012, 143, 1670–1680. [Google Scholar] [CrossRef] [PubMed]
- Barnes, M.J.; Griseri, T.; Johnson, A.M.; Young, W.; Powrie, F.; Izcue, A. CTLA-4 promotes Foxp3 induction and regulatory T cell accumulation in the intestinal lamina propria. Mucosal Immunol. 2013, 6, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Raker, V.K.; Domogalla, M.P.; Steinbrink, K. Tolerogenic Dendritic Cells for Regulatory T Cell Induction in Man. Front. Immunol. 2015, 6, 569. [Google Scholar] [CrossRef] [PubMed]
- Glaubitz, J.; Wilden, A.; van den Brandt, C.; Weiss, F.U.; Bröker, B.M.; Mayerle, J.; Lerch, M.M.; Sendler, M. Experimental pancreatitis is characterized by rapid T cell activation, Th2 differentiation that parallels disease severity, and improvement after CD4(+) T cell depletion. Pancreatology 2020, 20, 1637–1647. [Google Scholar] [CrossRef]
- Szatmary, P.; Grammatikopoulos, T.; Cai, W.; Huang, W.; Mukherjee, R.; Halloran, C.; Beyer, G.; Sutton, R. Acute Pancreatitis: Diagnosis and Treatment. Drugs 2022, 82, 1251–1276. [Google Scholar] [CrossRef]
- Samanta, J.; Singh, S.; Arora, S.; Muktesh, G.; Aggarwal, A.; Dhaka, N.; Kant Sinha, S.; Gupta, V.; Sharma, V.; Kochhar, R. Cytokine profile in prediction of acute lung injury in patients with acute pancreatitis. Pancreatology 2018, 18, 878–884. [Google Scholar] [CrossRef]
- Liu, D.; Wen, L.; Wang, Z.; Hai, Y.; Yang, D.; Zhang, Y.; Bai, M.; Song, B.; Wang, Y. The Mechanism of Lung and Intestinal Injury in Acute Pancreatitis: A Review. Front. Med. 2022, 9, 904078. [Google Scholar] [CrossRef]
- Rubenfeld, G.D.; Caldwell, E.; Peabody, E.; Weaver, J.; Martin, D.P.; Neff, M.; Stern, E.J.; Hudson, L.D. Incidence and outcomes of acute lung injury. N. Engl. J. Med. 2005, 353, 1685–1693. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, R.; Thompson, C.; Ganguly, K.; Singh, S.; Batra, S.K.; Kumar, S. Alcohol and Smoking Mediated Modulations in Adaptive Immunity in Pancreatitis. Cells 2020, 9, 1880. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Yang, Y.; Li, H.; Wang, H.; Gao, P. Circulating Lymphocyte Subsets Induce Secondary Infection in Acute Pancreatitis. Front. Cell Infect. Microbiol. 2020, 10, 128. [Google Scholar] [CrossRef] [PubMed]
- Mayerle, J.; Dummer, A.; Sendler, M.; Malla, S.R.; van den Brandt, C.; Teller, S.; Aghdassi, A.; Nitsche, C.; Lerch, M.M. Differential roles of inflammatory cells in pancreatitis. J. Gastroenterol. Hepatol. 2012, 27 (Suppl. S2), 47–51. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.P.; Chen, H.Q.; Liu, F.; Zhang, J. Advances in researches on the immune dysregulation and therapy of severe acute pancreatitis. J. Zhejiang Univ. Sci. B 2009, 10, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Gittens, B.R.; Bodkin, J.V.; Nourshargh, S.; Perretti, M.; Cooper, D. Galectin-3: A Positive Regulator of Leukocyte Recruitment in the Inflamed Microcirculation. J. Immunol. 2017, 198, 4458–4469. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Tao, X.; Xia, S.; Guo, F.; Pan, C.; Xiang, H.; Shang, D. T Lymphocytes: A Promising Immunotherapeutic Target for Pancreatitis and Pancreatic Cancer? Front. Oncol. 2020, 10, 382. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhang, Y.; Dong, L.; Yang, C.; Gou, S.; Yin, T.; Wu, H.; Wang, C. The Reduction of Peripheral Blood CD4+ T Cell Indicates Persistent Organ Failure in Acute Pancreatitis. PLoS ONE 2015, 10, e0125529. [Google Scholar] [CrossRef]
- Pietruczuk, M.; Dabrowska, M.I.; Wereszczynska-Siemiatkowska, U.; Dabrowski, A. Alteration of peripheral blood lymphocyte subsets in acute pancreatitis. World J. Gastroenterol. 2006, 12, 5344–5351. [Google Scholar] [CrossRef]
- Demols, A.; Le Moine, O.; Desalle, F.; Quertinmont, E.; Van Laethem, J.L.; Devière, J. CD4(+ )T cells play an important role in acute experimental pancreatitis in mice. Gastroenterology 2000, 118, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Habtezion, A. Inflammation in acute and chronic pancreatitis. Curr. Opin. Gastroenterol. 2015, 31, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.S.; Wu, Z.S.; Zhang, L.Y.; Ke, L.; Li, W.Q.; Li, N.; Li, J.S. Nicotine ameliorates experimental severe acute pancreatitis via enhancing immunoregulation of CD4+ CD25+ regulatory T cells. Pancreas 2015, 44, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Volarevic, V.; Markovic, B.S.; Bojic, S.; Stojanovic, M.; Nilsson, U.; Leffler, H.; Besra, G.S.; Arsenijevic, N.; Paunovic, V.; Trajkovic, V.; et al. Gal-3 regulates the capacity of dendritic cells to promote NKT-cell-induced liver injury. Eur. J. Immunol. 2015, 45, 531–543. [Google Scholar] [CrossRef] [PubMed]
- Fermino, M.L.; Dias, F.C.; Lopes, C.D.; Souza, M.A.; Cruz, Â.K.; Liu, F.T.; Chammas, R.; Roque-Barreira, M.C.; Rabinovich, G.A.; Bernardes, E.S. Galectin-3 negatively regulates the frequency and function of CD4(+) CD25(+) Foxp3(+) regulatory T cells and influences the course of Leishmania major infection. Eur. J. Immunol. 2013, 43, 1806–1817. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Sharma, V.; Habtezion, A. Immune cells and immune-based therapy in pancreatitis. Immunol. Res. 2014, 58, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, M.C.; Quintana, F.J. Tolerogenic dendritic cells. Semin. Immunopathol. 2017, 39, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and tumor progression: Signaling pathways and targeted intervention. Signal Transduct. Target. Ther. 2021, 6, 263. [Google Scholar] [CrossRef]
- Bedrosian, A.S.; Nguyen, A.H.; Hackman, M.; Connolly, M.K.; Malhotra, A.; Ibrahim, J.; Cieza-Rubio, N.E.; Henning, J.R.; Barilla, R.; Rehman, A.; et al. Dendritic cells promote pancreatic viability in mice with acute pancreatitis. Gastroenterology 2011, 141, 1915–1926.e14. [Google Scholar] [CrossRef]
- Iberg, C.A.; Hawiger, D. Natural and Induced Tolerogenic Dendritic Cells. J. Immunol. 2020, 204, 733–744. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milivojcevic Bevc, I.; Tasic-Uros, D.; Stojanovic, B.S.; Jovanovic, I.; Dimitrijevic Stojanovic, M.; Gajovic, N.; Jurisevic, M.; Radosavljevic, G.; Pantic, J.; Stojanovic, B. Redefining Immune Dynamics in Acute Pancreatitis: The Protective Role of Galectin-3 Deletion and Treg Cell Enhancement. Biomolecules 2024, 14, 642. https://doi.org/10.3390/biom14060642
Milivojcevic Bevc I, Tasic-Uros D, Stojanovic BS, Jovanovic I, Dimitrijevic Stojanovic M, Gajovic N, Jurisevic M, Radosavljevic G, Pantic J, Stojanovic B. Redefining Immune Dynamics in Acute Pancreatitis: The Protective Role of Galectin-3 Deletion and Treg Cell Enhancement. Biomolecules. 2024; 14(6):642. https://doi.org/10.3390/biom14060642
Chicago/Turabian StyleMilivojcevic Bevc, Ivana, Danijela Tasic-Uros, Bojana S. Stojanovic, Ivan Jovanovic, Milica Dimitrijevic Stojanovic, Nevena Gajovic, Milena Jurisevic, Gordana Radosavljevic, Jelena Pantic, and Bojan Stojanovic. 2024. "Redefining Immune Dynamics in Acute Pancreatitis: The Protective Role of Galectin-3 Deletion and Treg Cell Enhancement" Biomolecules 14, no. 6: 642. https://doi.org/10.3390/biom14060642





