Paradoxes: Cholesterol and Hypoxia in Preeclampsia
Abstract
1. Introduction
2. Changes in Dietary Lipids in the United States of America (US) over the Last Century
3. Environmental Lipids and Membrane Modifiers Affect Preeclampsia Risk
4. Placental Hypoxia and Hypoxia-Inducible Factors (HIF)
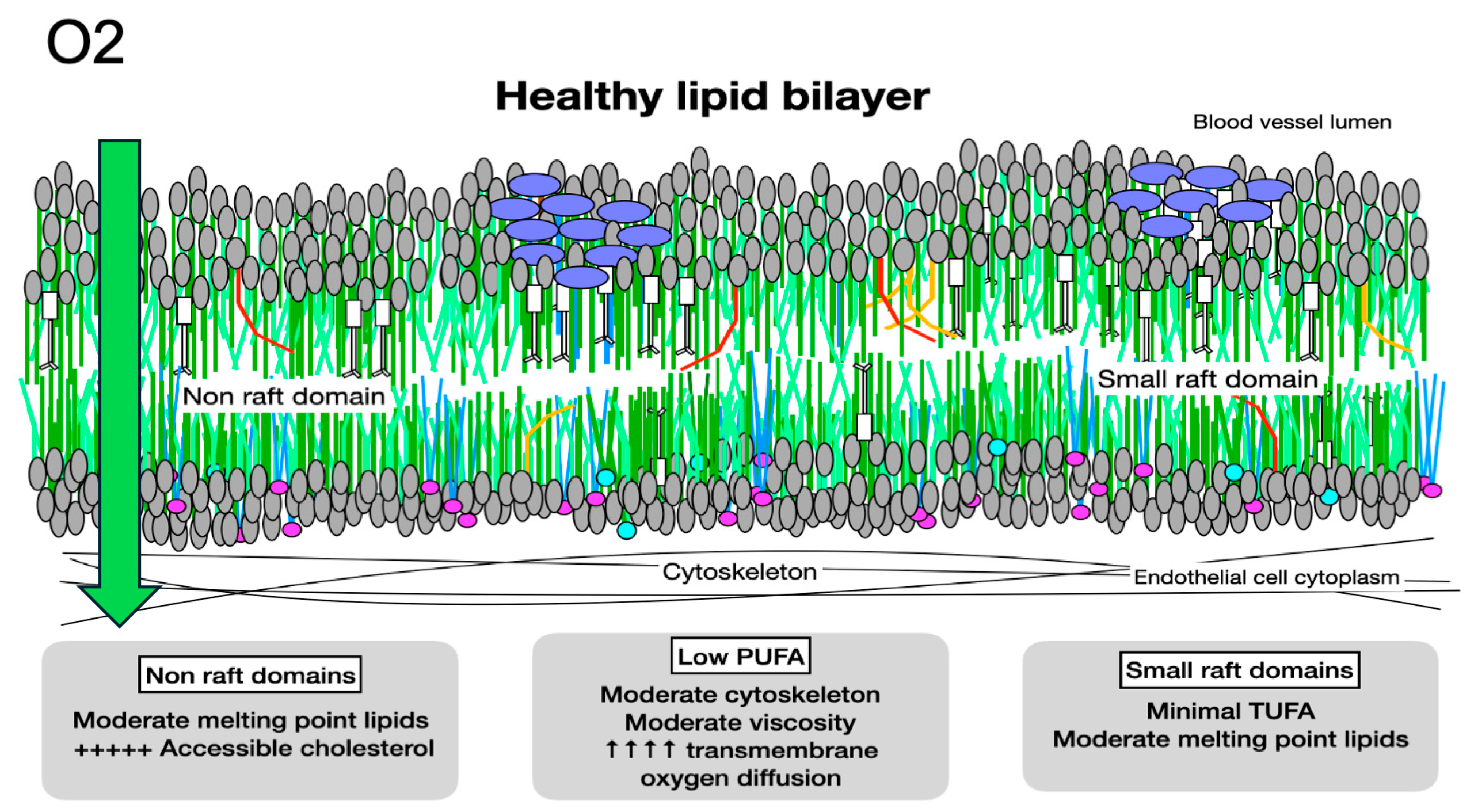
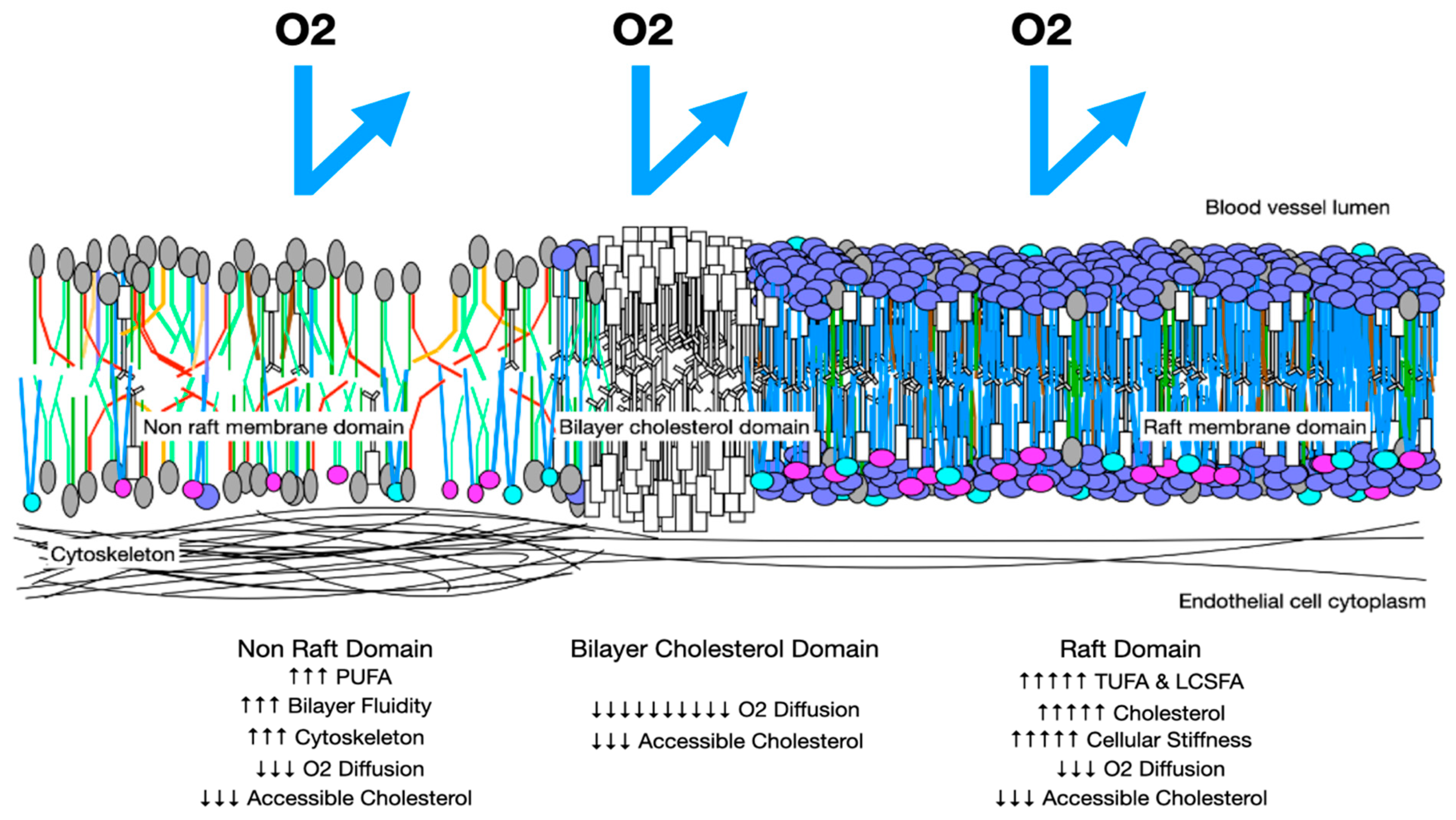
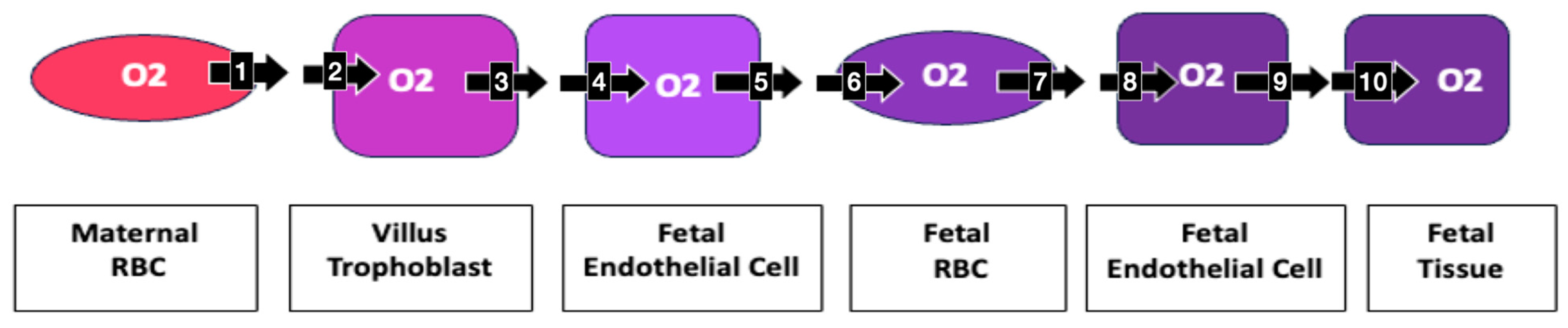
5. Impedance to Transmembrane Oxygen Diffusion
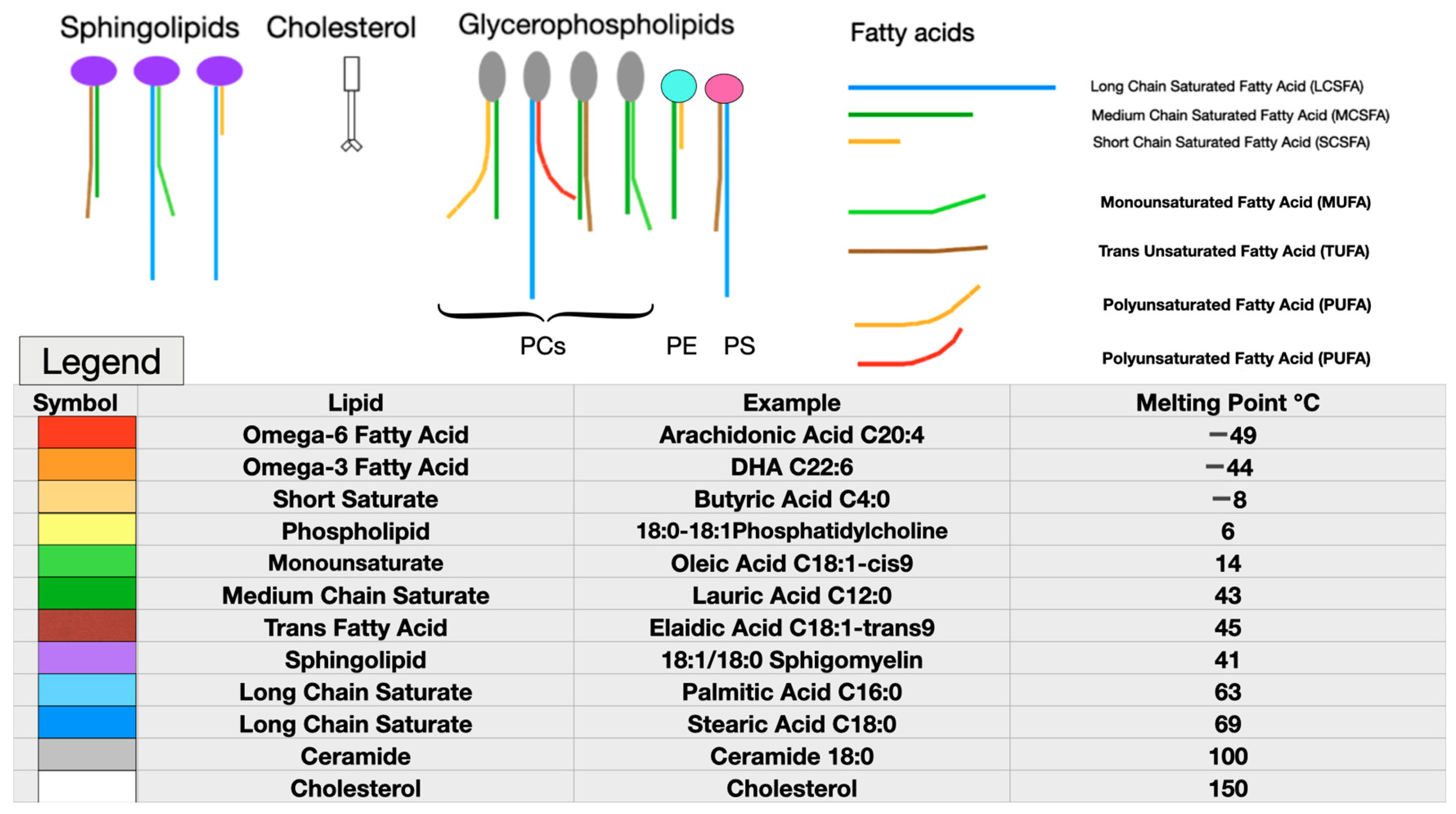
6. Effects of Molecular Components on Membrane Biophysics and Phase Separation
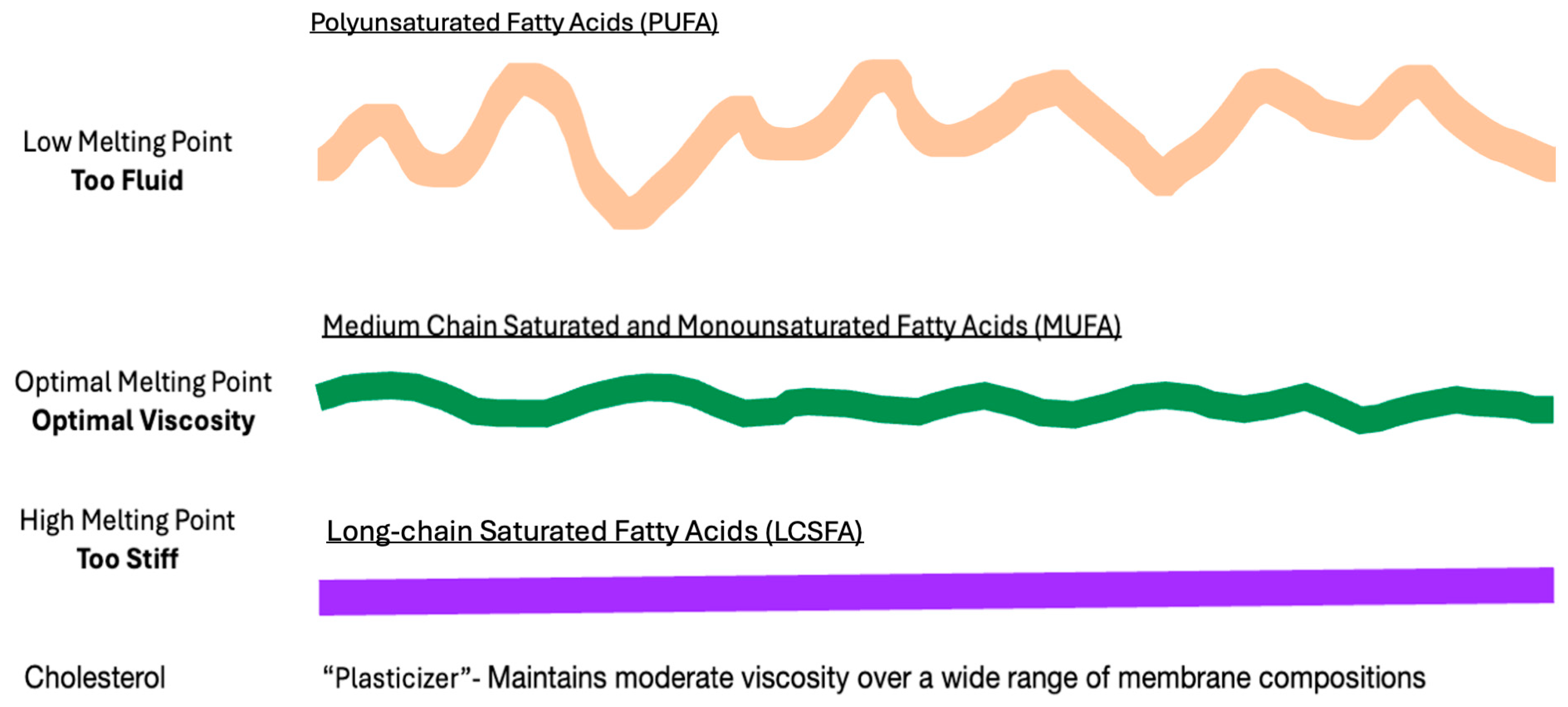
7. Dietary Challenges to Membrane Homeoviscosity
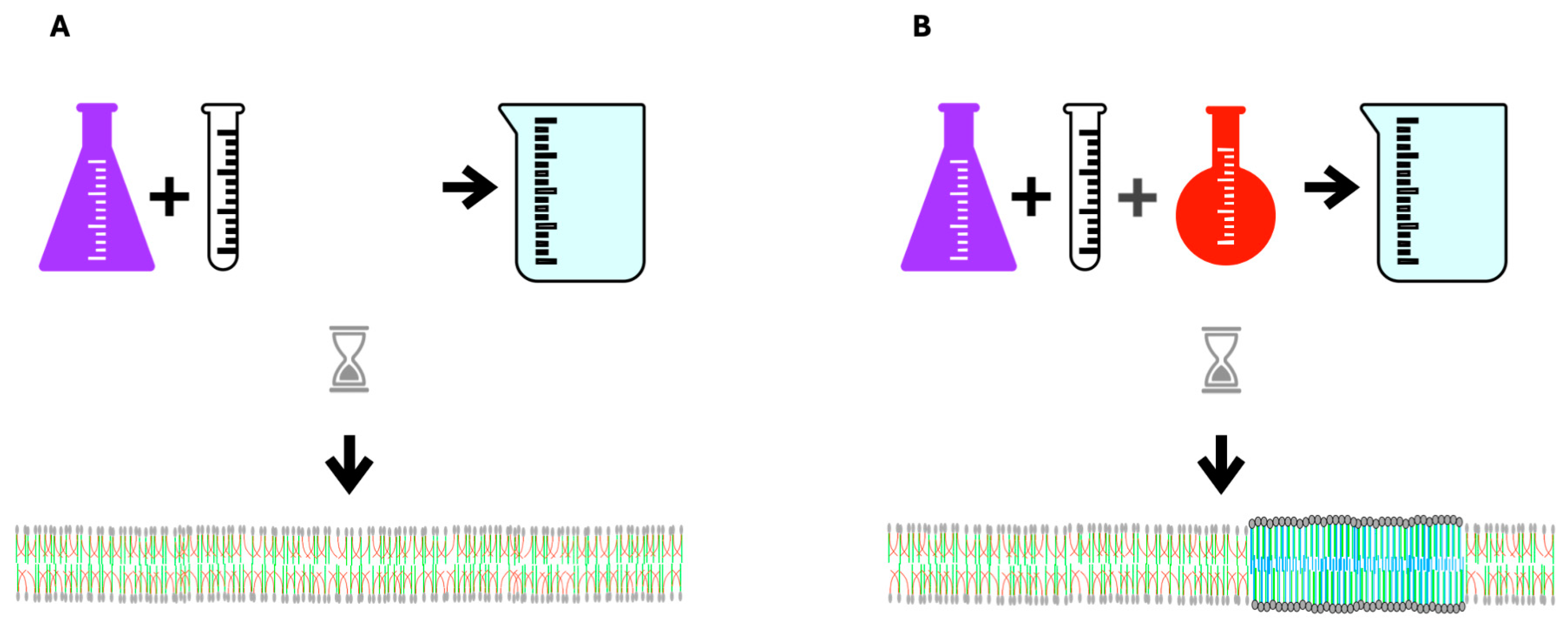
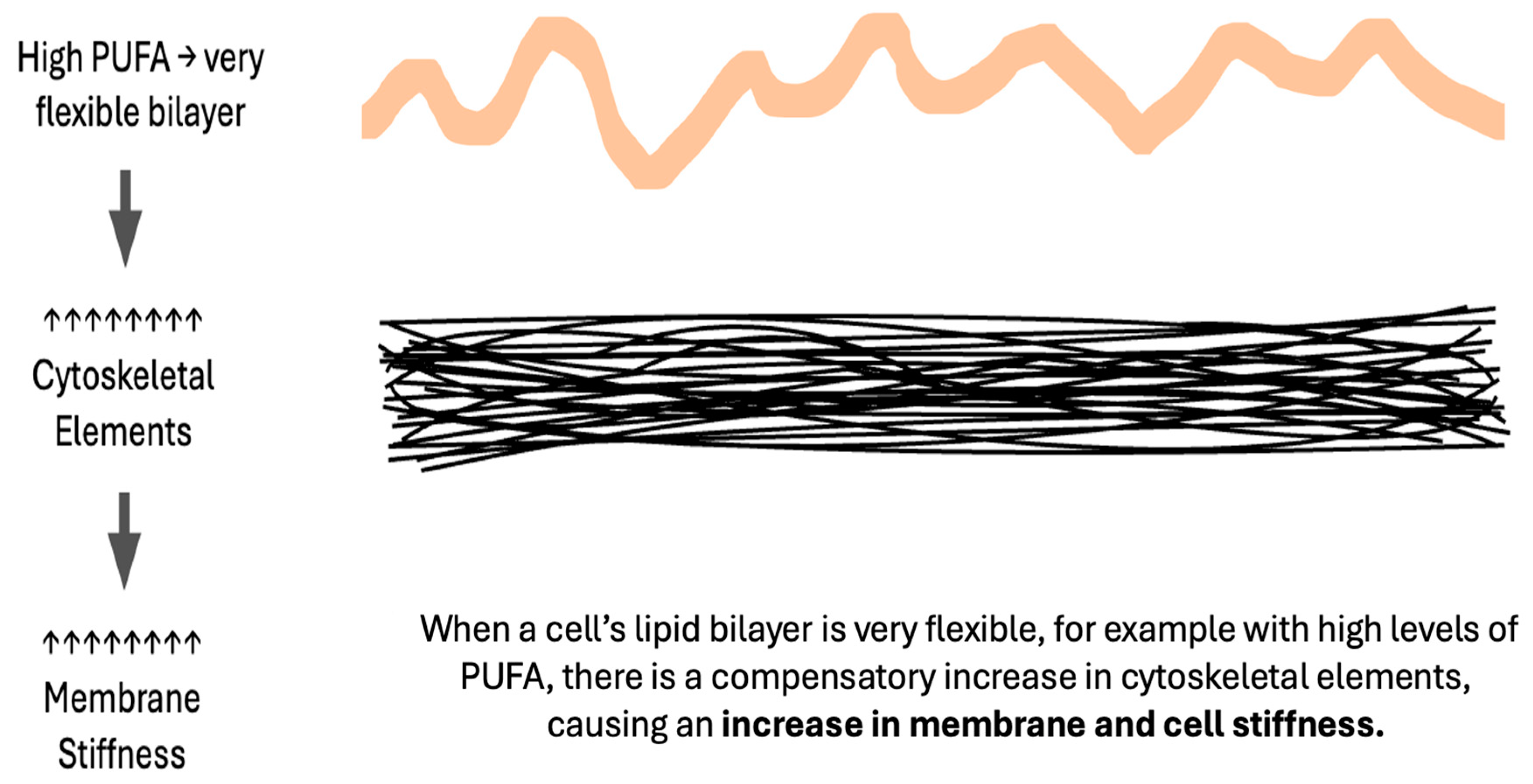
8. Excessively Fluid Lipid Bilayers Increase Membrane Stiffness
9. Melting Point Disparity, Membrane Rafts, and Cholesterol Content
10. Dyslipidemia: Increased Total Cholesterol, Reduced Cholesterol-Dependent Signaling
10.1. Accessible Membrane Cholesterol
10.2. Hedgehog Signaling in Preeclampsia
10.3. Wnt/βcatenin Signaling
10.4. Endothelial Nitric Oxide Synthase Signaling
10.5. Dyslipidemia and Accessible Cholesterol
11. Dietary Lipids and Oxidative Stress
12. Lipids in Preeclampsia Screening and Epidemiology
13. Diets and Preeclampsia
13.1. Diets Affect Preeclampsia Incidence
13.2. Increased Dietary PUFAs Increase Preeclampsia Incidence
13.3. Dietary Intervention Studies
13.4. Choline Deficiency and Preeclampsia
13.5. Preeclampsia with PUFA Deficiency
13.6. Dietary Cholesterol
14. Discussion
- Reduce oxidative stress: With very-low-melting-point PUFA as the driving force for oxidative stress, replacing dietary PUFAs with medium-chain saturated fatty acids, such as lauric acid (C12:0) in coconut oil, monounsaturated fatty acids, such as oleic acid (C18:1) in olive oil, or short-chain saturated fatty acids, such as butyric (C2-5:0), which are found in milk products or as products of gut bacteria, should markedly reduce oxidative stress, as these lipids are less vulnerable to oxidative damage. Avoiding dietary oxysterols, which are found in dehydrated milk, eggs, and proteins, also reduces oxidative stress [204]. These measures should lower blood pressure, restore normal angiogenic signaling, and reduce protein misfolding.
- Reduce lipid raft formation and hypoxia: Lowering dietary PUFAs reduces the disparity in membrane lipid melting points and the need for endogenous LCSFA synthesis, thus lessening lipid raft formation and hypoxia. A reduction in membrane PUFAs also reduces the formation of cholesterol crystals and cholesterol/cholesterol bilayer domains, which markedly impair the movement of oxygen across placental membranes. LCSFAs, such as palmitoleic (C:16) and stearic acid (C18:0) found in beef, pork, lamb, and chicken, may also stimulate excessive lipid raft formation, and are elevated in some with preeclampsia [205,206], so intake should be low or moderate. Supplementation with moderate-melting-point fatty acids to inhibit raft formation and with choline in those who are deficient should be beneficial [103].
- Increase accessible membrane cholesterol: Increasing dietary cholesterol allows for esterification and the removal of damaged fatty acids, as well as reductions in oxidative stress. Reducing dietary TUFAs by avoiding foods cooked at high temperature in oil and meats produced with feeds using spent deep fryer oils should reduce cholesterol sequestration in rafts and cholesterol esters. Supplementing with dietary cholesterol provides accessible cholesterol to restore normal Wnt, Hh, and eNOS signaling, aids in angiogenesis, improves vasodilation, and restores normal membrane rheology and permeability.
- Reduce the omega 6:3 ratio to less than 4:1: Monitoring the omega 6:3 ratio allows for the identification of cases in which an elevated 6:3 ratio (>4:1) is clinically significant and whether supplementation has been effective. This measure should reduce inflammation and thrombosis.
15. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Rana, S.; Lemoine, E.; Granger, J.P.; Karumanchi, S.A. Preeclampsia: Pathophysiology, Challenges, and Perspectives. Circ. Res. 2019, 124, 1094–1112, Erratum in Circ. Res. 2020, 126, e8. [Google Scholar] [CrossRef] [PubMed]
- Inversetti, A.; Pivato, C.A.; Cristodoro, M.; Latini, A.C.; Condorelli, G.; Di Simone, N.; Stefanini, G. Update on long-term cardiovascular risk after pre-eclampsia: A systematic review and meta-analysis. Eur. Heart J. Qual. Care Clin. Outcomes 2024, 10, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.A.; King, I.B.; Sorensen, T.K.; Zingheim, R.W.; Troyer, B.L.; Zebelman, A.M.; Luthy, D.A. Risk of preeclampsia in relation to elaidic acid (trans fatty acid) in maternal erythrocytes. Gynecol. Obstet. Investig. 1998, 46, 84–87. [Google Scholar] [CrossRef]
- Clausen, T.; Slott, M.; Solvoll, K.; Drevon, C.A.; Vollset, S.E.; Henriksen, T. High intake of energy, sucrose, and polyunsaturated fatty acids is associated with increased risk of preeclampsia. Am. J. Obstet. Gynecol. 2001, 185, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.A.; Zingheim, R.W.; King, I.B.; Zebelman, A.M. Omega-3 fatty acids in maternal erythrocytes and risk of preeclampsia. Epidemiology 1995, 6, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Wojcik-Baszko, D.; Charkiewicz, K.; Laudanski, P. Role of dyslipidemia in preeclampsia-A review of lipidomic analysis of blood, placenta, syncytiotrophoblast microvesicles and umbilical cord artery from women with preeclampsia. Prostaglandins Other Lipid Mediat. 2018, 139, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Poornima, I.G.; Indaram, M.; Ross, J.D.; Agarwala, A.; Wild, R.A. Hyperlipidemia and risk for preclampsia. J. Clin. Lipidol. 2022, 16, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Odenkirk, M.T.; Stratton, K.G.; Gritsenko, M.A.; Bramer, L.M.; Webb-Robertson, B.-J.M.; Bloodsworth, K.J.; Weitz, K.K.; Lipton, A.K.; Monroe, M.E.; Ash, J.R.; et al. Unveiling molecular signatures of preeclampsia and gestational diabetes mellitus with multi-omics and innovative cheminformatics visualization tools. Mol. Omics 2020, 16, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Melland-Smith, M.; Ermini, L.; Chauvin, S.; Craig-Barnes, H.; Tagliafetto, A.; Todros, T.; Post, M.; Caniggia, I. Disruption of sphingolipid metabolism augments ceramide-induced autophagy in preclampsia. Autophagy 2015, 11, 653–669. [Google Scholar] [CrossRef]
- Brown, S.H.; Eather, S.R.; Freeman, D.J.; Meyer, B.J.; Mitchell, T.W. A Lipidomic Analysis of Placenta in Preeclampsia: Evidence for Lipid Storage. PLoS ONE 2016, 11, e0163972. [Google Scholar] [CrossRef]
- Del Gaudio, I.; Sasset, L.; Lorenzo, A.D.; Wadsack, C. Sphingolipid signature of human feto- placental vasculature in preeclampsia. Int. J. Mol. Sci. 2020, 21, 1019. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, Y.; Lv, Y.; Ding, H. Dissecting the Roles of Lipids in Preeclampsia. Metabolites 2022, 12, 590. [Google Scholar] [CrossRef] [PubMed]
- Al, M.D.; van Houwelingen, A.C.; Badart-Smook, A.; Hasaart, T.H.; Roumen, F.J.; Hornstra, G. The essential fatty acid status of mother and child in pregnancy-induced hypertension: A prospective longitudinal study. Am. J. Obstet. Gynecol. 1995, 172, 1605–1614. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.; Young, S.; Esplin, M.S.; Peaden, B.; Tolley, H.D.; Porter, T.F.; Varner, M.W.; D’Alton, M.E.; Jackson, B.J.; Graves, S.W. Detection and confirmation of serum lipid biomarkers for preeclampsia using direct infusion mass spectrometry. J. Lipid Res. 2016, 57, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Korkes, H.A.; Sass, N.; Moron, A.F.; Câmara, N.O.S.; Bonetti, T.; Cerdeira, A.S.; Da Silva, I.D.C.G.; De Oliveira, L. Lipidomic assessment of plasma and placenta of women with early-onset preeclampsia. PLoS ONE 2014, 9, e110747. [Google Scholar] [CrossRef] [PubMed]
- Baig, S.; Lim, J.Y.; Fernandis, A.Z.; Wenk, M.R.; Kale, A.; Su, L.L.; Biswas, A.; Vasoo, S.; Shui, G.; Choolani, M. Lipidomic analysis of human placental syncytiotrophoblast microvesicles in adverse pregnancy outcomes. Placenta 2013, 34, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Thangaratinam, S.; Rogozińska, E.; Jolly, K.; Glinkowski, S.; Duda, W.; Borowiack, E.; Roseboom, T.; Tomlinson, J.; Walczak, J.; Kunz, R.; et al. Interventions to reduce or prevent obesity in pregnant women: A systematic review. Health Technol. Assess. 2012, 16, iii–iv, 1–191. [Google Scholar] [CrossRef] [PubMed]
- Alamolhoda, S.H.; Simbar, M.; Mirmiran, P.; Mirabi, P. Effect of low trans-fatty acid intakes on preeclampsia: A randomized controlled trial. J. Res. Med. Sci. 2020, 25, 112. [Google Scholar] [CrossRef]
- Hedegaard, S.; Nohr, E.A.; Olsen, S.F.; Halldorsson, T.I.; Renault, K.M. Adherence to different forms of plant-based diets and pregnancy outcomes in the Danish National Birth Cohort: A prospective observational study. Acta Obstet. Gynecol. Scand. 2024, 103, 1046–1053. [Google Scholar] [CrossRef] [PubMed]
- Irwinda, R.; Hiksas, R.; Siregar, A.A.; Saroyo, Y.B.; Wibowo, N. Long-chain polyunsaturated fatty acid (LC-PUFA) status in severe preeclampsia and preterm birth: A cross sectional study. Sci. Rep. 2021, 11, 14701. [Google Scholar] [CrossRef]
- Kinshella, M.W.; Omar, S.; Scherbinsky, K.; Vidler, M.; Magee, L.A.; von Dadelszen, P.; Moore, S.E.; Elango, R. The Precise Conceptual Framework Working Group. Effects of Maternal Nutritional Supplements and Dietary Interventions on Placental Complications: An Umbrella Review, Meta-Analysis and Evidence Map. Nutrients 2021, 13, 472. [Google Scholar] [CrossRef] [PubMed]
- Li, S.N.; Liu, Y.H.; Luo, Z.Y.; Cui, Y.F.; Cao, Y.; Fu, W.J.; Dou, W.F.; Duan, D.D.; Zhao, X.L.; Chen, Y.M.; et al. The association between dietary fatty acid intake and the risk of developing preeclampsia: A matched case-control study. Sci. Rep. 2021, 11, 4048. [Google Scholar] [CrossRef] [PubMed]
- Bakrania, B.A.; George, E.M.; Granger, J.P. Animal models of preeclampsia: Investigating pathophysiology and therapeutic targets. Am. J. Obstet. Gynecol. 2022, 226, S973–S987. [Google Scholar] [CrossRef] [PubMed]
- Colson, A.; Depoix, C.L.; Lambert, I.; Leducq, C.; Bedin, M.; De Beukelaer, M.; Gatto, L.; Loriot, A.; Peers de Nieuwburgh, M.; Bouhna, K.; et al. Specific HIF-2α (Hypoxia-Inducible Factor-2) Inhibitor PT2385 Mitigates Placental Dysfunction In Vitro and in a Rat Model of Preeclampsia (RUPP). Hypertension 2023, 80, 1011–1023. [Google Scholar] [CrossRef] [PubMed]
- Rajakumar, A.; Whitelock, K.A.; Weissfeld, L.A.; Daftary, A.R.; Markovic, N.; Conrad, K.P. Selective overexpression of the hypoxia-inducible transcription factor, HIF-2alpha, in placentas from women with preeclampsia. Biol. Reprod. 2001, 64, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Huppertz, B. Placental physioxia is based on spatial and temporal variations of placental oxygenation throughout pregnancy. J. Reprod. Immunol. 2023, 158, 103985. [Google Scholar] [CrossRef] [PubMed]
- Blasbalg, T.L.; Hibbeln, J.R.; Ramsden, C.E.; Majchrzak, S.F.; Rawlings, R.R. Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century. Am. J. Clin. Nutr. 2011, 93, 950–962. [Google Scholar] [CrossRef]
- Gerrior, S.; Bente, L.; Hiza, H. Nutrient Content of the U.S. Food Supply, 1909–2000; Home Economics Research Report No. 56; U.S. Department of Agriculture, Center for Nutrition Policy and Promotion: Washington, DC, USA, 2004.
- USDA/ERS. U.S. Department of Agriculture, Food Availability (per Capita) Data System; Updated 21 July 2021; USDA/ERS: Washington, DC, USA, 2021.
- Craig-Schmidt, M.C. World-wide consumption of trans fatty acids. Atheroscler. Suppl. 2006, 7, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Wanders, A.J.; Zock, P.L.; Brouwer, I.A. Trans fat intake and its dietary sources in general populations worldwide: A systematic review. Nutrients 2017, 9, 840. [Google Scholar] [CrossRef]
- Federal Register, Department of Health and Human Services, Food and Drug Administration. Revocation of Uses of Partially Hydrogenated Oils in Foods; Rules and Regulations, Action: Direct final rule; FDA: Rockville, MD, USA, 2023; Volume 88, pp. 53827–53836.
- World Health Organization Replace Trans Fat; an Action Pacakage to Eliminate Industrially Produced Trans-Fatty Acids Executive Summary WHO/NMH/NHD/18.4. Available online: https://www.who.int/docs/default-source/documents/replace-transfats/replace-action-package.pdf (accessed on 27 May 2024).
- Bhardwaj, S.; Passi, S.J.; Misra, A.; Pant, K.K.; Anwar, K.; Pandey, R.M.; Kardam, V. Effect of heating/reheating of fats/oils, as used by Asian Indians, on trans fatty acid formation. Food Chem. 2016, 212, 663–670. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Cordain, L.; Miller, J.B.; Eaton, S.B.; Mann, N.; Holt, S.H.; Speth, J.D. Plant–animal subsistence ratios and macronutrient energy estimations in worldwide hunter-gatherer diets. Am. J. Clin. Nutr. 2000, 71, 682–692. [Google Scholar] [CrossRef] [PubMed]
- Cordain, L.; Eaton, S.B.; Miller, J.B.; Mann, N.; Hill, K. The paradoxical nature of hunter-gatherer diets: Meat-based, yet non-atherogenic. Eur. J. Clin. Nutr. 2002, 56, S42–S52. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; McClure, S.T.; Appel, L.J. Dietary cholesterol intake and sources among U.S adults: Results from national health and nutrition examination surveys (NHANES), 2001–2014. Nutrients 2018, 10, 771. [Google Scholar] [CrossRef] [PubMed]
- Gogna, P.; Villeneuve, P.J.; Borghese, M.M.; King, W.D. An exposure-response meta-analysis of ambient PM2.5 during pregnancy and preeclampsia. Environ. Res. 2022, 210, 112934. [Google Scholar] [CrossRef]
- Pedersen, M.; Stayner, L.; Slama, R.; Sørensen, M.; Figueras, F.; Nieuwenhuijsen, M.J.; Raaschou-Nielsen, O.; Dadvand, P. Ambient air pollution and pregnancy-induced hypertensive disorders: A systematic review and meta-analysis. Hypertension 2014, 64, 494–500. [Google Scholar] [CrossRef] [PubMed]
- van den Hooven, E.H.; de Kluizenaar, Y.; Pierik, F.H.; Hofman, A.; van Ratingen, S.W.; Zandveld, P.Y.; Mackenbach, J.P.; Steegers, E.A.; Miedema, H.M.; Jaddoe, V.W. Air pollution, blood pressure, and the risk of hypertensive complications during pregnancy: The generation R study. Hypertension 2011, 57, 406–412. [Google Scholar] [CrossRef] [PubMed]
- National Toxicology Program. NTP monograph on the systematic review of traffic-related air pollution and hypertensive disorders of pregnancy. NTP Monogr. 2019, NTP-MGRAPH-7. [Google Scholar] [CrossRef] [PubMed]
- Pereira, G.; Haggar, F.; Shand, A.W.; Bower, C.; Cook, A.; Nassar, N. Association between pre-eclampsia and locally derived traffic-related air pollution: A retrospective cohort study. J. Epidemiol. Community Health 2013, 67, 147–152. [Google Scholar] [CrossRef]
- Agrawal, S.; Yamamoto, S. Effect of indoor air pollution from biomass and solid fuel combustion on symptoms of preeclampsia/eclampsia in Indian women. Indoor Air 2015, 25, 341–352. [Google Scholar] [CrossRef]
- Robbins, T.; Kuhrt, K.; Vousden, N.; Seed, P.; Shennan, A. Household air pollution and incidence of eclampsia in eight low- and middle-income countries. Int. J. Gynaecol. Obstet. 2023, 160, 449–450. [Google Scholar] [CrossRef] [PubMed]
- Nobles, C.J.; Williams, A.; Ouidir, M.; Sherman, S.; Mendola, P. Differential Effect of Ambient Air Pollution Exposure on Risk of Gestational Hypertension and Preeclampsia. Hypertension 2019, 74, 384–390. [Google Scholar] [CrossRef]
- Assibey-Mensah, V.; Glantz, J.C.; Hopke, P.K.; Jusko, T.A.; Thevenet-Morrison, K.; Chalupa, D.; Rich, D.Q. Wintertime Wood Smoke, Traffic Particle Pollution, and Preeclampsia. Hypertension 2020, 75, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Dadvand, P.; Basagaña, X.; Figueras, F.; Amoly, E.; Tobias, A.; de Nazelle, A.; Querol, X.; Sunyer, J.; Nieuwenhuijsen, M.J. Saharan dust episodes and pregnancy. J. Environ. Monit. 2011, 13, 3222–3228. [Google Scholar] [CrossRef] [PubMed]
- Bogan, M.; Al, B.; Kul, S.; Zengin, S.; Oktay, M.; Sabak, M.; Gümüşboğa, H.; Bayram, H. The effects of desert dust storms, air pollution, and temperature on morbidity due to spontaneous abortions and toxemia of pregnancy: 5-year analysis. Int. J. Biometeorol. 2021, 65, 1733–1739. [Google Scholar] [CrossRef]
- Michikawa, T.; Morokuma, S.; Yamazaki, S.; Takami, A.; Sugata, S.; Yoshino, A.; Takeda, Y.; Nakahara, K.; Saito, S.; Hoshi, J.; et al. Exposure to chemical components of fine particulate matter and ozone, and placenta-mediated pregnancy complications in Tokyo: A register-based study. J. Expo. Sci. Environ. Epidemiol. 2022, 32, 135–145. [Google Scholar] [CrossRef]
- Wrońska-Nofer, T.; Rosin, J.; Bartosz, G. Interaction of ethanol and xylene in their effects on erythrocytes and other haematological parameters in the rat. J. Appl. Toxicol. 1991, 11, 289–292. [Google Scholar] [CrossRef]
- Moghe, A.; Ghare, S.; Lamoreau, B.; Mohammad, M.; Barve, S.; McClain, C.; Joshi-Barve, S. Molecular mechanisms of acrolein toxicity: Relevance to human disease. Toxicol. Sci. 2015, 143, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Shan, Q.; Li, H.; Chen, N.; Qu, F.; Guo, J. Understanding the Multiple Effects of PCBs on Lipid Metabolism. Diabetes Metab. Syndr. Obes. 2020, 13, 3691–3702. [Google Scholar] [CrossRef]
- Wikström, S.; Lindh, C.H.; Shu, H.; Bornehag, C.-G. Early pregnancy serum levels of perfluoroalkyl substances and risk of preeclampsia in Swedish women. Sci. Rep. 2019, 9, 9179. [Google Scholar] [CrossRef]
- Huang, R.; Chen, Q.; Zhang, L.; Luo, K.; Chen, L.; Zhao, S.; Feng, L.; Zhang, J. Prenatal exposure to perfluoroalkyl and polyfluoroalkyl substances and the risk of hypertensive disorders of pregnancy. Environ. Health 2019, 18, 5. [Google Scholar] [CrossRef] [PubMed]
- Bommarito, P.A.; Ferguson, K.K.; Meeker, J.D.; McElrath, T.F.; Cantonwine, D.E. Maternal Levels of Perfluoroalkyl Substances (PFAS) during Early Pregnancy in Relation to Preeclampsia Subtypes and Biomarkers of Preeclampsia Risk. Environ. Health Perspect. 2021, 129, 107004. [Google Scholar] [CrossRef] [PubMed]
- Eslami, B.; Malekafzali, H.; Rastkari, N.; Rashidi, B.H.; Djazayeri, A.; Naddafi, K. Association of serum concentrations of persistent organic pollutants (POPs) and risk of pre-eclampsia: A case-control study. J. Environ. Health Sci. Eng. 2016, 14, 17. [Google Scholar] [CrossRef] [PubMed]
- Soleymanlou, N.; Jurisica, I.; Nevo, O.; Ietta, F.; Zhang, X.; Zamudio, S.; Post, M.; Caniggia, I. Molecular evidence of placental hypoxia in preeclampsia. J. Clin. Endocrinol. Metab. 2005, 90, 4299–4308. [Google Scholar] [CrossRef] [PubMed]
- Higgins, D.F.; Kimura, K.; Iwano, M.; Haase, V.H. Hypoxia-inducible factor signaling in the development of tissue fibrosis. Cell Cycle 2008, 7, 1128–1132. [Google Scholar] [CrossRef] [PubMed]
- Tirpe, A.A.; Gulei, D.; Ciortea, S.M.; Crivii, C.; Berindan-Neagoe, I. Hypoxia: Overview on Hypoxia-Mediated Mechanisms with a Focus on the Role of HIF Genes. Int. J. Mol. Sci. 2019, 20, 6140. [Google Scholar] [CrossRef] [PubMed]
- Korkes, H.A.; De Oliveira, L.; Sass, N.; Salahuddin, S.; Karumanchi, S.A.; Rajakumar, A. Relationship between hypoxia and downstream pathogenic pathways in preeclampsia. Hypertens. Pregnancy 2017, 36, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Chang, C.; Hao, M.; Chen, M.; Woodley, D.T.; Schönthal, A.H.; Li, W. Heat shock protein-90alpha (Hsp90α) stabilizes hypoxia-inducible factor-1α (HIF-1α) in support of spermatogenesis and tumorigenesis. Cancer Gene Ther. 2021, 28, 1058–1070. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.S.; Qiao, X.; Reslan, O.M.; Xia, Y.; Raffetto, J.D.; Paleolog, E.; Davies, A.H.; Khalil, R.A. Prolonged mechanical stretch is associated with upregulation of hypoxia-inducible factors and reduced contraction in rat inferior vena cava. J. Vasc. Surg. 2011, 53, 764–773. [Google Scholar] [CrossRef]
- Tafani, M.; Sansone, L.; Limana, F.; Arcangeli, T.; De Santis, E.; Polese, M.; Fini, M.; Russo, M.A. The Interplay of Reactive Oxygen Species, Hypoxia, Inflammation, and Sirtuins in Cancer Initiation and Progression. Oxid. Med. Cell Longev. 2016, 2016, 3907147. [Google Scholar] [CrossRef]
- Lum, J.J.; Bui, T.; Gruber, M.; Gordan, J.D.; DeBerardinis, R.J.; Covello, K.L.; Simon, M.C.; Thompson, C.B. The transcription factor HIF-1alpha plays a critical role in the growth factor-dependent regulation of both aerobic and anaerobic glycolysis. Genes Dev. 2007, 21, 1037–1049. [Google Scholar] [CrossRef]
- Zera, K.; Zastre, J. Stabilization of the hypoxia-inducible transcription Factor-1 alpha (HIF-1α) in thiamine deficiency is mediated by pyruvate accumulation. Toxicol. Appl. Pharmacol. 2018, 355, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Watts, E.R.; Walmsley, S.R. Inflammation and Hypoxia: HIF and PHD Isoform Selectivity. Trends Mol. Med. 2019, 25, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Déry, M.A.; Michaud, M.D.; Richard, D.E. Hypoxia-inducible factor 1: Regulation by hypoxic and non-hypoxic activators. Int. J. Biochem. Cell Biol. 2005, 37, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Schönenberger, M.J.; Kovacs, W.J. Hypoxia signaling pathways: Modulators of oxygen-related organelles. Front. Cell Dev. Biol. 2015, 3, 42. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, S.J.; Lin, Q.D. Study on the expressions of PHD and HIF in placentas from normal pregnant women and patients with preeclampsia. Int. J. Biol. Sci. 2014, 10, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Fuenzalida, B.; Yañez, M.J.; Mueller, M.; Mistry, H.D.; Leiva, A.; Albrecht, C. Evidence for hypoxia-induced dysregulated cholesterol homeostasis in preeclampsia: Insights into the mechanisms from human placental cells and tissues. FASEB J. 2024, 38, e23431. [Google Scholar] [CrossRef] [PubMed]
- Coates, H.W.; Capell-Hattam, I.M.; Olzomer, E.M.; Du, X.; Farrell, R.; Yang, H.; Byrne, F.L.; Brown, A.J. Hypoxia truncates and constitutively activates the key cholesterol synthesis enzyme squalene monooxygenase. Elife 2023, 12, e82843. [Google Scholar] [CrossRef]
- Diet induced experimental toxemia and cation transport. Nutr. Rev. 1965, 23, 309–311. [CrossRef] [PubMed]
- Wang, G.L.; Semenza, G.L. General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia. Proc. Natl. Acad. Sci. USA 1993, 90, 4304–4308. [Google Scholar] [CrossRef]
- Caniggia, I.; Winter, J.; Lye, S.J.; Post, M. Oxygen and placental development during the first trimester: Implications for the pathophysiology of pre-eclampsia. Placenta 2000, 21 (Suppl. A), S25–S30. [Google Scholar] [CrossRef]
- Carter, A.M. Placental Gas Exchange and the Oxygen Supply to the Fetus. Compr. Physiol. 2015, 5, 1381–1403. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, E.S.; Brownbill, P.; Jones, N.W.; Abrahams, V.M.; Baker, P.N.; Sibley, C.P.; Crocker, I.P. Utero-placental haemodynamics in the pathogenesis of pre-eclampsia. Placenta 2009, 30, 634–641. [Google Scholar] [CrossRef]
- Mayhew, T.M.; Manwani, R.; Ohadike, C.; Wijesekara, J.; Baker, P.N. The placenta in pre-eclampsia and intrauterine growth restriction: Studies on exchange surface areas, diffusion distances and villous membrane diffusive conductances. Placenta 2007, 28, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Subczynski, W.K.; Widomska, J.; Stein, N.; Swartz, H.M. Factors determining barrier properties to oxygen transport across model and cell plasma membranes based on EPR spin-label oximetry. Appl. Magn. Reson. 2021, 52, 1237–1260. [Google Scholar] [CrossRef] [PubMed]
- Plesnar, E.; Szczelina, R.; Subczynski, W.K.; Pasenkiewicz-Gierula, M. Is the cholesterol bilayer domain a barrier to oxygen transport into the eye lens? Biochim. Biophys. Acta Biomembr. 2018, 1860, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Albert, A.D.; Boesze-Battaglia, K. The role of cholesterol in rod outer segment membranes. Prog. Lipid Res. 2005, 44, 99–124. [Google Scholar] [CrossRef]
- Zuniga-Hertz, J.P.; Patel, H.H. The Evolution of Cholesterol-Rich Membrane in Oxygen Adaption: The Respiratory System as a Model. Front. Physiol. 2019, 10, 1340. [Google Scholar] [CrossRef]
- Quehenberger, O.; Armando, A.M.; Brown, A.H.; Milne, S.B.; Myers, D.S.; Merrill, A.H.; Bandyopadhyay, S.; Jones, K.N.; Kelly, S.; Shaner, R.L.; et al. Lipidomics reveals a remarkable diversity of lipids in human plasma. J. Lipid Res. 2010, 51, 3299–3305. [Google Scholar] [CrossRef]
- Niu, S.L.; Mitchell, D.C.; Litman, B.J. Trans fatty acid derived phospholipids show increased membrane cholesterol and reduced receptor activation as compared to their cis analogs. Biochemistry 2005, 44, 4458–4465. [Google Scholar] [CrossRef][Green Version]
- Baumer, Y.; McCurdy, S.; Weatherby, T.M.; Mehta, N.N.; Halbherr, S.; Halbherr, P.; Yamazaki, N.; Boisvert, W.A. Hyperlipidemia-induced cholesterol crystal production by endothelial cells promotes atherogenesis. Nat. Commun. 2017, 8, 1129. [Google Scholar] [CrossRef] [PubMed]
- Ekroos, K.; Ejsing, C.S.; Bahr, U.; Karas, M.; Simons, K.; Shevchenko, A. Charting molecular composition of phosphatidylcholines by fatty acid scanning and ion trap MS3 fragmentation. J. Lipid Res. 2003, 44, 2181–2192. [Google Scholar] [CrossRef] [PubMed]
- Hart, N.R. A theoretical model of dietary lipid variance as the origin of primary ciliary dysfunction in preeclampsia. Front. Mol. Biosci. 2023, 10, 1173030. [Google Scholar] [CrossRef]
- Sinensky, M. Homeoviscous adaptation--a homeostatic process that regulates the viscosity of membrane lipids in Escherichia coli. Proc. Natl. Acad. Sci. USA 1974, 71, 522–525. [Google Scholar] [CrossRef] [PubMed]
- Levental, K.R.; Malmberg, E.; Symons, J.L.; Fan, Y.Y.; Chapkin, R.S.; Ernst, R.; Levental, I. Lipidomic and biophysical homeostasis of mammalian membranes counteracts dietary lipid perturbations to maintain cellular fitness. Nat. Commun. 2020, 11, 1339. [Google Scholar] [CrossRef] [PubMed]
- Schauf, B.; Lang, U.; Stute, P.; Schneider, S.; Dietz, K.; Aydeniz, B.; Wallwiener, D. Reduced red blood cell deformability, an indicator for high fetal or maternal risk, is found in preeclampsia and IUGR. Hypertens. Pregnancy 2002, 21, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Csiszar, B.; Galos, G.; Funke, S.; Kevey, D.K.; Meggyes, M.; Szereday, L.; Kenyeres, P.; Toth, K.; Sandor, B. Peripartum Investigation of Red Blood Cell Properties in Women Diagnosed with Early-Onset Preeclampsia. Cells 2021, 10, 2714. [Google Scholar] [CrossRef] [PubMed]
- Heilmann, L. Blood rheology and pregnancy. Baillieres Clin. Haematol. 1987, 1, 777–799. [Google Scholar] [CrossRef]
- Tsuda, K.; Nishio, I. Membrane fluidity and hypertension. Am. J. Hypertens. 2003, 16, 259–261. [Google Scholar] [CrossRef]
- Lühr, J.J.; Alex, N.; Amon, L.; Kräter, M.; Kubánková, M.; Sezgin, E.; Lehmann, C.H.; Heger, L.; Heidkamp, G.F.; Smith, A.S.; et al. Maturation of monocyte derived DCs leads to increased cellular stiffness, higher membrane fluidity, and changed lipid composition. Front. Immunol. 2020, 11, 590121. [Google Scholar] [CrossRef]
- Efremov, Y.M.; Velay-Lizancos, M.; Weaver, C.J.; Athamneh, A.I.; Zavattieri, P.D.; Suter, D.M.; Raman, A. Anisotropy vs isotropy in living cell indentation with AFM. Sci. Rep. 2019, 9, 5757. [Google Scholar] [CrossRef] [PubMed]
- Shentu, T.P.; Singh, D.K.; Oh, M.J.; Sun, S.; Sadaat, L.; Makino, A.; Mazzone, T.; Subbaiah, P.V.; Cho, M.; Levitan, I. The role of oxysterols in control of endothelial stiffness. J. Lipid Res. 2012, 53, 1348–1358. [Google Scholar] [CrossRef] [PubMed]
- Byfield, F.J.; Aranda-Espinoza, H.; Romanenko, V.G.; Rothblat, G.H.; Levitan, I. Cholesterol depletion increases membrane stiffness of aortic endothelial cells. Biophys. J. 2004, 87, 3336–3343. [Google Scholar] [CrossRef] [PubMed]
- Schauf, B.; Becker, S.; Abele, H.; Klever, T.; Wallwiener, D.; Aydeniz, B. Effect of magnesium on red blood cell deformability in pregnancy. Hypertens. Pregnancy 2005, 24, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Prescott, A.R.; Comerford, J.G.; Magrath, R.; Lamb, N.J.; Warn, R.M. Effects of elevated intracellular magnesium on cytoskeletal integrity. J. Cell Sci. 1988, 89 Pt 3, 321–329. [Google Scholar] [CrossRef]
- Simons, K.; Toomre, D. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell. Biol. 2000, 1, 31–39. [Google Scholar] [CrossRef]
- Lin, X.; Lorent, J.H.; Skinkle, A.D.; Levental, K.R.; Waxham, M.N.; Gorfe, A.A.; Levental, I. Domain stability in biomimetic membranes driven by lipid polyunsaturation. J. Phys. Chem. B 2016, 120, 11930–11941. [Google Scholar] [CrossRef]
- Turk, H.F.; Chapkin, R.S. Membrane lipid raft organization is uniquely modified by n-3 polyunsaturated fatty acids. Prostaglandins Leukot. Essent. Fat. Acids 2013, 88, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Sviridov, D.; Mukhamedova, N.; Miller, Y.I. Lipid rafts as a therapeutic target. J. Lipid Res. 2020, 61, 687–695. [Google Scholar] [CrossRef]
- Riquelme, G.; Vallejos, C.; de Gregorio, N.; Morales, B.; Godoy, V.; Berrios, M.; Bastías, N.; Rodríguez, C. Lipid rafts and cytoskeletal proteins in placental microvilli membranes from preeclamptic and IUGR pregnancies. J. Membr. Biol. 2011, 241, 127–140. [Google Scholar] [CrossRef]
- Lee, S.M.; Moon, J.Y.; Lim, B.Y.; Kim, S.M.; Park, C.W.; Kim, B.J.; Jun, J.K.; Norwitz, E.R.; Choi, M.H.; Park, J.S. Increased biosynthesis and accumulation of cholesterol in maternal plasma, but not amniotic fluid in pre-eclampsia. Sci. Rep. 2019, 9, 1550. [Google Scholar] [CrossRef] [PubMed]
- Olsen, B.N.; Bielska, A.A.; Lee, T.; Daily, M.D.; Covey, D.F.; Schlesinger, P.H.; Baker, N.A.; Ory, D.S. The structural basis of cholesterol accessibility in membranes. Biophys. J. 2013, 105, 1838–1847. [Google Scholar] [CrossRef] [PubMed]
- Woollett, L.A. Review: Transport of maternal cholesterol to the fetal circulation. Placenta 2011, 32 (Suppl. S2), S218–S221. [Google Scholar] [CrossRef] [PubMed]
- Horne, H.; Holme, A.M.; Roland, M.C.P.; Holm, M.B.; Haugen, G.; Henriksen, T.; Michelsen, T.M. Maternal-fetal cholesterol transfer in human term pregnancies. Placenta 2019, 87, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhu, L.; Wang, M.; Sun, Q. Associations between Per- and Polyfluoroalkyl Substances Exposures and Blood Lipid Levels among Adults-A Meta-Analysis. Environ. Health Perspect. 2023, 131, 56001. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.H.; Soh, S.X.H.; Wang, M.X.; Ong, J.; Seah, A.; Wong, Y.; Fang, Z.; Sim, S.; Lim, J.T. Perfluoroalkyl substances and lipid concentrations in the blood: A systematic review of epidemiological studies. Sci. Total Environ. 2022, 850, 158036. [Google Scholar] [CrossRef] [PubMed]
- Tazuma, S.; Ochi, H.; Teramen, K.; Yamashita, Y.; Horikawa, K.; Miura, H.; Hirano, N.; Sasaki, M.; Aihara, N.; Hatsushika, S.; et al. Degree of fatty acyl chain unsaturation in biliary lecithin dictates cholesterol nucleation and crystal growth. Biochim. Biophys. Acta 1994, 1215, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, G.N.; Subramanian, S.; Chait, A.; Haigh, W.G.; Yeh, M.M.; Farrell, G.C.; Lee, S.P.; Savard, C. Cholesterol crystallization within hepatocyte lipid droplets and its role in murine NASH. J. Lipid Res. 2017, 58, 1067–1079. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Huang, S.; Song, T.; Yin, Y.; Tan, C. Placental Angiogenesis in Mammals: A Review of the Regulatory Effects of Signaling Pathways and Functional Nutrients. Adv. Nutr. 2021, 12, 2415–2434. [Google Scholar] [CrossRef]
- Wang, C.Y.; Tsai, P.Y.; Chen, T.Y.; Tsai, H.L.; Kuo, P.L.; Su, M.T. Elevated miR-200a and miR-141 inhibit endocrine gland-derived vascular endothelial growth factor expression and ciliogenesis in preeclampsia. J. Physiol. 2019, 597, 3069–3083. [Google Scholar] [CrossRef]
- Lawson, N.D.; Vogel, A.M.; Weinstein, B.M. Sonic hedgehog and vascular endothelial growth factor act upstream of the Notch pathway during arterial endothelial differentiation. Dev. Cell. 2002, 3, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Takai, H.; Kondoh, E.; Mogami, H.; Kawasaki, K.; Chigusa, Y.; Sato, M.; Kawamura, Y.; Murakami, R.; Matsumura, N.; Konishi, I.; et al. Placental Sonic Hedgehog Pathway Regulates Fetal Growth via the IGF Axis in Preeclampsia. J. Clin. Endocrinol. Metab. 2019, 104, 4239–4252. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, A.; Rohatgi, R.; Siebold, C. Cholesterol access in cellular membranes controls Hedgehog signaling. Nat. Chem. Biol. 2020, 16, 1303–1313. [Google Scholar] [CrossRef] [PubMed]
- Kinnebrew, M.; Iverson, E.J.; Patel, B.B.; Pusapati, G.V.; Kong, J.H.; Johnson, K.A.; Luchetti, G.; Eckert, K.M.; McDonald, J.G.; Covey, D.F.; et al. Cholesterol accessibility at the ciliary membrane controls hedgehog signaling. Elife 2019, 8, e50051. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, X.; Zhang, L.; Shi, Y.; Wang, J.; Yan, H. Wnt/β-catenin signaling pathway in trophoblasts and abnormal activation in preeclampsia (Review). Mol. Med. Rep. 2017, 16, 1007–1013. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Z.; Zeng, X.; Wang, J.; Zhang, L.; Song, W.; Shi, Y. Wnt/β-catenin signaling pathway in severe preeclampsia. J. Mol. Histol. 2018, 49, 317–327, Erratum in J. Mol. Histol. 2022, 53, 145–147. [Google Scholar] [CrossRef] [PubMed]
- DiFederico, E.; Genbacev, O.; Fisher, S.J. Preeclampsia is associated with widespread apoptosis of placental cytotrophoblasts within the uterine wall. Am. J. Pathol. 1999, 155, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Sheng, R.; Kim, H.; Lee, H.; Xin, Y.; Chen, Y.; Tian, W.; Cui, Y.; Choi, J.C.; Doh, J.; Han, J.K.; et al. Cholesterol selectively activates canonical Wnt signalling over non-canonical Wnt signalling. Nat. Commun. 2014, 5, 4393. [Google Scholar] [CrossRef]
- Osol, G.; Ko, N.L.; Mandalà, M. Altered Endothelial Nitric Oxide Signaling as a Paradigm for Maternal Vascular Maladaptation in Preeclampsia. Curr. Hypertens. Rep. 2017, 19, 82. [Google Scholar] [CrossRef]
- Guerby, P.; Tasta, O.; Swiader, A.; Pont, F.; Bujold, E.; Parant, O.; Vayssiere, C.; Salvayre, R.; Negre-Salvayre, A. Role of oxidative stress in the dysfunction of the placental endothelial nitric oxide synthase in preeclampsia. Redox Biol. 2021, 40, 101861. [Google Scholar] [CrossRef]
- Goligorsky, M.S.; Li, H.; Brodsky, S.; Chen, J. Relationships between caveolae and eNOS: Everything in proximity and the proximity of everything. Am. J. Physiol. Ren. Physiol. 2002, 283, F1–F10. [Google Scholar] [CrossRef] [PubMed]
- Rodal, S.K.; Skretting, G.; Garred, O.; Vilhardt, F.; van Deurs, B.; Sandvig, K. Extraction of cholesterol with methyl-beta-cyclodextrin perturbs formation of clathrin-coated endocytic vesicles. Mol. Biol. Cell. 1999, 10, 961–974. [Google Scholar] [CrossRef] [PubMed]
- Plenz, G.A.; Hofnagel, O.; Robenek, H. Differential modulation of caveolin-1 expression in cells of the vasculature by statins. Circulation 2004, 109, e7–e8. [Google Scholar] [CrossRef] [PubMed]
- Salvary, T.; Gambert-Nicot, S.; Brindisi, M.C.; Meneveau, N.; Schiele, F.; Séronde, M.F.; Lorgis, L.; Zeller, M.; Cottin, Y.; Kantelip, J.P.; et al. Pravastatin reverses the membrane cholesterol reorganization induced by myocardial infarction within lipid rafts in CD14(+)/CD16(-) circulating monocytes. Biochim. Biophys. Acta 2012, 1821, 1287–1294. [Google Scholar] [CrossRef]
- Blair, A.; Shaul, P.W.; Yuhanna, I.S.; Conrad, P.A.; Smart, E.J. Oxidized low density lipoprotein displaces endothelial nitric-oxide synthase (eNOS) from plasmalemmal caveolae and impairs eNOS activation. J. Biol. Chem. 1999, 274, 32512–32519. [Google Scholar] [CrossRef] [PubMed]
- Levitan, I.; Shentu, T.P. Impact of oxLDL on Cholesterol-Rich Membrane Rafts. J. Lipids 2011, 2011, 730209. [Google Scholar] [CrossRef] [PubMed]
- Gliozzi, M.; Scicchitano, M.; Bosco, F.; Musolino, V.; Carresi, C.; Scarano, F.; Maiuolo, J.; Nucera, S.; Maretta, A.; Paone, S.; et al. Modulation of Nitric Oxide Synthases by Oxidized LDLs: Role in Vascular Inflammation and Atherosclerosis Development. Int. J. Mol. Sci. 2019, 20, 3294. [Google Scholar] [CrossRef]
- Palei, A.C.; Granger, J.P.; Spradley, F.T. Placental Ischemia Says “NO” to Proper NOS-Mediated Control of Vascular Tone and Blood Pressure in Preeclampsia. Int. J. Mol. Sci. 2021, 22, 11261. [Google Scholar] [CrossRef] [PubMed]
- Sizar, O.; Khare, S.; Jamil, R.T.; Talati, R. Statin Medications. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar] [PubMed]
- Goldberg, I.J.; Holleran, S.; Ramakrishnan, R.; Adams, M.; Palmer, R.H.; Dell, R.B.; Goodman, D.S. Lack of effect of lovastatin therapy on the parameters of whole-body cholesterol metabolism. J. Clin. Investig. 1990, 86, 801–808. [Google Scholar] [CrossRef]
- Schonewille, M.; de Boer, J.F.; Mele, L.; Wolters, H.; Bloks, V.W.; Wolters, J.C.; Kuivenhoven, J.A.; Tietge, U.J.; Brufau, G.; Groen, A.K. Statins increase hepatic cholesterol synthesis and stimulate fecal cholesterol elimination in mice. J. Lipid Res. 2016, 57, 1455–1464. [Google Scholar] [CrossRef]
- Afonso, M.S.; Machado, R.M.; Lavrador, M.S.; Quintao, E.C.R.; Moore, K.J.; Lottenberg, A.M. Molecular Pathways Underlying Cholesterol Homeostasis. Nutrients 2018, 10, 760. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Mott, M.M.; Yiannakou, I.; Bradlee, M.L.; Singer, M.R.; Moore, L.L. Eggs and a Fiber-Rich Diet Are Beneficially Associated with Lipid Levels in Framingham Offspring Study Adults. Curr. Dev. Nutr. 2024, 8, 102062. [Google Scholar] [CrossRef] [PubMed]
- Dias, I.H.K.; Milic, I.; Lip, G.Y.H.; Devitt, A.; Polidori, M.C.; Griffiths, H.R. Simvastatin reduces circulating oxysterol levels in men with hypercholesterolaemia. Redox Biol. 2018, 16, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Enssle, J.; Pietzner, A.; Schmöcker, C.; Weiland, L.; Ritter, O.; Jaensch, M.; Elbelt, U.; Pagonas, N.; Weylandt, K.H. Essential Polyunsaturated Fatty Acids in Blood from Patients with and without Catheter-Proven Coronary Artery Disease. Int. J. Mol. Sci. 2022, 23, 766. [Google Scholar] [CrossRef] [PubMed]
- Stark, J.M. Pre-eclampsia and cytokine induced oxidative stress. Br. J. Obstet. Gynaecol. 1993, 100, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Goulopoulou, S.; Davidge, S.T. Molecular mechanisms of maternal vascular dysfunction in preeclampsia. Trends Mol. Med. 2015, 21, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Grzeszczak, K.; Łanocha-Arendarczyk, N.; Malinowski, W.; Ziętek, P.; Kosik-Bogacka, D. Oxidative Stress in Pregnancy. Biomolecules 2023, 13, 1768. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef] [PubMed]
- McCord, J.M. The evolution of free radicals and oxidative stress. Am. J. Med. 2000, 108, 652–659. [Google Scholar] [CrossRef]
- Bogdanov, M.; Mileykovskaya, E.; Dowhan, W. Lipids in the assembly of membrane proteins and organization of protein supercomplexes: Implications for lipid-linked disorders. Subcell. Biochem. 2008, 49, 197–239. [Google Scholar] [CrossRef]
- Koshy, C.; Ziegler, C. Structural insights into functional lipid-protein interactions in secondary transporters. Biochim. Biophys. Acta 2015, 1850, 476–487. [Google Scholar] [CrossRef] [PubMed]
- Mahler, C.A.; Snoke, D.B.; Cole, R.M.; Angelotti, A.; Sparagna, G.C.; Baskin, K.K.; Ni, A.; Belury, M.A. Consuming a Linoleate-Rich Diet Increases Concentrations of Tetralinoleoyl Cardiolipin in Mouse Liver and Alters Hepatic Mitochondrial Respiration. J. Nutr. 2024, 154, 856–865. [Google Scholar] [CrossRef] [PubMed]
- Funai, K.; Summers, S.A.; Rutter, J. Reign in the membrane: How common lipids govern mitochondrial function. Curr. Opin. Cell Biol. 2020, 63, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Paradies, G.; Ruggiero, F.M.; Petrosillo, G.; Quagliariello, E. Peroxidative damage to cardiac mitochondria: Cytochrome oxidase and cardiolipin alterations. FEBS Lett. 1998, 424, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Bellanti, F.; Villani, R.; Tamborra, R.; Blonda, M.; Iannelli, G.; di Bello, G.; Facciorusso, A.; Poli, G.; Iuliano, L.; Avolio, C.; et al. Synergistic interaction of fatty acids and oxysterols impairs mitochondrial function and limits liver adaptation during nafld progression. Redox Biol. 2018, 15, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Pamplona, R.; Portero-Otin, M.; Sanz, A.; Requena, J.; Barja, G. Modification of the longevity-related degree of fatty acid unsaturation modulates oxidative damage to proteins and mitochondrial DNA in liver and brain. Exp. Gerontol. 2004, 39, 725–733. [Google Scholar] [CrossRef]
- Halliwell, B. Reactive oxygen species in living systems: Source, biochemistry, and role in human disease. Am. J. Med. 1991, 91, 14S–22S. [Google Scholar] [CrossRef]
- Saito, Y.; Noguchi, N.; Niki, E. Cholesterol is more readily oxidized than phospholipid linoleates in cell membranes to produce cholesterol hydroperoxides. Free Radic. Biol. Med. 2024, 211, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Girao, H.; Mota, C.; Pereira, P. Cholesterol may act as an antioxidant in lens membranes. Curr. Eye Res. 1999, 18, 448–454. [Google Scholar] [CrossRef]
- Smith, L.L. Another cholesterol hypothesis: Cholesterol as antioxidant. Free Radic. Biol. Med. 1991, 11, 47–61. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.J.; Jauniaux, E. Oxidative stress. Best Pract. Res. Clin. Obstet. Gynaecol. 2011, 25, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef]
- Schäfer, G.; Cramer, T.; Suske, G.; Kemmner, W.; Wiedenmann, B.; Höcker, M. Oxidative stress regulates vascular endothelial growth factor-A gene transcription through Sp1- and Sp3-dependent activation of two proximal GC-rich promoter elements. J. Biol. Chem. 2003, 278, 8190–8198. [Google Scholar] [CrossRef] [PubMed]
- Rumbold, A.; Duley, L.; Crowther, C.A.; Haslam, R.R. Antioxidants for preventing pre-eclampsia. Cochrane Database Syst Rev. 2008, 2008, CD004227. [Google Scholar] [CrossRef] [PubMed]
- Salles, A.M.; Galvao, T.F.; Silva, M.T.; Motta, L.C.; Pereira, M.G. Antioxidants for preventing preeclampsia: A systematic review. ScientificWorldJournal 2012, 2012, 243476. [Google Scholar] [CrossRef] [PubMed]
- Tenório, M.B.; Ferreira, R.C.; Moura, F.A.; Bueno, N.B.; Goulart, M.O.F.; Oliveira, A.C.M. Oral antioxidant therapy for prevention and treatment of preeclampsia: Meta-analysis of randomized controlled trials. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 865–876. [Google Scholar] [CrossRef] [PubMed]
- Di Fabrizio, C.; Giorgione, V.; Khalil, A.; Murdoch, C.E. Antioxidants in Pregnancy: Do We Really Need More Trials? Antioxidants 2022, 11, 812. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.M.; Beddaoui, M.; Kramer, M.S.; Platt, R.W.; Basso, O.; Kahn, S.R. Maternal Antioxidant Levels in Pregnancy and Risk of Preeclampsia and Small for Gestational Age Birth: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0135192. [Google Scholar] [CrossRef]
- Giannubilo, S.R.; Marzioni, D.; Tossetta, G.; Montironi, R.; Meccariello, M.L.; Ciavattini, A. The “Bad Father”: Paternal Role in Biology of Pregnancy and in Birth Outcome. Biology 2024, 13, 165. [Google Scholar] [CrossRef]
- Qiu, C.; Sanchez, S.E.; Larrabure, G.; David, R.; Bralley, J.A.; Williams, M.A. Erythrocyte omega-3 and omega-6 polyunsaturated fatty acids and preeclampsia risk in Peruvian women. Arch. Gynecol. Obstet. 2006, 274, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zu, L.; Cai, W.; Cheng, Q.; Hua, T.; Peng, L.; Li, G.; Zhang, X. Metabolomics revealed decreased level of omega-3 PUFA-derived protective eicosanoids in pregnant women with pre-eclampsia. Clin. Exp. Pharmacol. Physiol. 2019, 46, 705–710. [Google Scholar] [CrossRef] [PubMed]
- Mahomed, K.; Williams, M.A.; King, I.B.; Mudzamiri, S.; Woelk, G.B. Erythrocyte omega-3, omega-6 and trans fatty acids in relation to risk of preeclampsia among women delivering at Harare Maternity Hospital, Zimbabwe. Physiol. Res. 2007, 56, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Wada, Y.; Yoshida-Yamamoto, S.; Wada, Y.; Nakayama, M.; Mitsuda, N.; Kitajima, H. Trans fatty acid accumulation in the human placenta. J. Mass. Spectrom. 2017, 52, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Chavarro, J.E.; Halldorsson, T.I.; Leth, T.; Bysted, A.; Olsen, S.F. A prospective study of trans fat intake and risk of preeclampsia in Denmark. Eur. J. Clin. Nutr. 2011, 65, 944–951. [Google Scholar] [CrossRef] [PubMed]
- Arvizu, M.; Minguez-Alarcon, L.; Wang, S.; Mitsunami, M.; Stuart, J.J.; Rich-Edwards, J.W.; Rosner, B.; Chavarro, J.E. Pre-pregnancy fat intake in relation to hypertensive disorders of pregnancy. Am. J. Clin. Nutr. 2022, 116, 750–758. [Google Scholar] [CrossRef] [PubMed]
- Bartho, L.A.; Keenan, E.; Walker, S.P.; MacDonald, T.M.; Nijagal, B.; Tong, S.; Kaitu’u-Lino, T.J. Plasma lipids are dysregulated preceding diagnosis of preeclampsia or delivery of a growth restricted infant. EBioMedicine 2023, 94, 104704. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- He, B.; Liu, Y.; Maurya, M.R.; Benny, P.; Lassiter, C.; Li, H.; Subramaniam, S.; Garmire, L.X. The maternal blood lipidome is indicative of the pathogenesis of severe preeclampsia. J. Lipid Res. 2021, 62, 100118. [Google Scholar] [CrossRef]
- Lantzanaki, M.; Vavilis, T.; Harizopoulou, V.C.; Bili, H.; Goulis, D.G.; Vavilis, D. Ceramides during Pregnancy and Obstetrical Adverse Outcomes. Metabolites 2023, 13, 1136. [Google Scholar] [CrossRef]
- Dobierzewska, A.; Soman, S.; Illanes, S.E.; Morris, A.J. Plasma cross-gestational sphingolipidomic analyses reveal potential first trimester biomarkers of preeclampsia. PLoS ONE 2017, 12, e0175118. [Google Scholar] [CrossRef]
- Patanapirunhakit, P.; Karlsson, H.; Mulder, M.; Ljunggren, S.; Graham, D.; Freeman, D. Sphingolipids in HDL—Potential markers for adaptation to pregnancy? Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2021, 1866, 158955. [Google Scholar] [CrossRef]
- Huang, Q.; Hao, S.; You, J.; Yao, X.; Li, Z.; Schilling, J.; Thyparambil, S.; Liao, W.L.; Zhou, X.; Mo, L.; et al. Early-pregnancy prediction of risk for pre-eclampsia using maternal blood leptin/ceramide ratio: Discovery and confirmation. BMJ Open 2021, 11, e050963. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Kang, Y.; Lee, E.M.; Jung, Y.M.; Hong, S.; Park, S.J.; Park, C.W.; Norwitz, E.R.; Lee, D.Y.; Park, J.S. Metabolomic biomarkers in midtrimester maternal plasma can accurately predict the development of preeclampsia. Sci. Rep. 2020, 10, 16142. [Google Scholar] [CrossRef]
- Kummerow, F.A. Modification of cell membrane composition by dietary lipids and its implications for atherosclerosis. Ann. N. Y. Acad. Sci. 1983, 414, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.; Zhu, L.; Zhang, J.; Fu, Y.; Qi, X.; Zhao, M. Association of dietary inflammatory index with risk of gestational diabetes mellitus and preeclampsia: A systematic review and meta-analysis. Br. J. Nutr. 2024, 131, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Mu, T.S.; Duran-Stanton, A.; Stone, E.A.; Zarzabal, L.A.; Loewendorf, A. Rates of Preeclampsia and Post-preeclamptic Cardiovascular Disease Among US Military Servicewomen: A Retrospective Case-cohort Study. Mil. Med. 2024, 189, 1210–1215. [Google Scholar] [CrossRef]
- Yang, J.; Song, Y.; Gaskins, A.J.; Li, L.J.; Huang, Z.; Eriksson, J.G.; Hu, F.B.; Chong, Y.S.; Zhang, C. Mediterranean diet and female reproductive health over lifespan: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2023, 229, 617–631. [Google Scholar] [CrossRef] [PubMed]
- Scholl, T.O.; Leskiw, M.; Chen, X.; Sims, M.; Stein, T.P. Oxidative stress, diet, and the etiology of preeclampsia. Am. J. Clin. Nutr. 2005, 81, 1390–1396. [Google Scholar] [CrossRef]
- Godhamgaonkar, A.A.; Wadhwani, N.S.; Randhir, K.N.; Selukar, S.S.; Dalvi, S.; Dangat, K.; Wagh, G.N.; Lalwani, S.; Chandhiok, N.; Kulkarni, B.; et al. Erythrocyte fatty acids and desaturase indices in early pregnancy are associated with risk of preeclampsia. Prostaglandins Leukot. Essent. Fat. Acids 2023, 196, 102583. [Google Scholar] [CrossRef] [PubMed]
- Nordqvist, M.; Jacobsson, B.; Brantsæter, A.L.; Myhre, R.; Nilsson, S.; Sengpiel, V. Timing of probiotic milk consumption during pregnancy and effects on the incidence of preeclampsia and preterm delivery: A prospective observational cohort study in Norway. BMJ Open 2018, 8, e018021. [Google Scholar] [CrossRef]
- Cortez-Ribeiro, A.C.; Meireles, M.; Ferro-Lebres, V.; Almeida-de-Souza, J. Olive oil consumption confers protective effects on maternal-fetal outcomes: A systematic review of the evidence. Nutr. Res. 2023, 110, 87–95. [Google Scholar] [CrossRef]
- Frederick, I.O.; Williams, M.A.; Dashow, E.; Kestin, M.; Zhang, C.; Leisenring, W.M. Dietary fiber, potassium, magnesium and calcium in relation to the risk of preeclampsia. J. Reprod. Med. 2005, 50, 332–344. [Google Scholar] [PubMed]
- Luqman, A.; Hassan, A.; Ullah, M.; Naseem, S.; Ullah, M.; Zhang, L.; Din, A.U.; Ullah, K.; Ahmad, W.; Wang, G. Role of the intestinal microbiome and its therapeutic intervention in cardiovascular disorder. Front. Immunol. 2024, 15, 1321395. [Google Scholar] [CrossRef]
- Zong, Y.; Wang, X.; Wang, J. Research progress on the correlation between gut microbiota and preeclampsia: Microbiome changes, mechanisms and treatments. Front. Cell Infect. Microbiol. 2023, 13, 1256940. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Chen, Y.; Zhou, Q.; Wang, C.; Chen, L.; Di, W.; Zhang, Y. Short-chain fatty acids accompanying changes in the gut microbiome contribute to the development of hypertension in patients with preeclampsia. Clin. Sci. 2020, 134, 289–302. [Google Scholar] [CrossRef]
- Middleton, P.; Gomersall, J.C.; Gould, J.F.; Shepherd, E.; Olsen, S.F.; Makrides, M. Omega-3 fatty acid addition during pregnancy. Cochrane Database Syst. Rev. 2018, 11, CD003402. [Google Scholar] [CrossRef]
- Al Wattar, B.H.; Dodds, J.; Placzek, A.; Beresford, L.; Spyreli, E.; Moore, A.; Gonzalez Carreras, F.J.; Austin, F.; Murugesu, N.; Roseboom, T.J.; et al. Mediterranean-style diet in pregnant women with metabolic risk factors (ESTEEM): A pragmatic multicentre randomised trial. PLoS Med. 2019, 16, e1002857. [Google Scholar] [CrossRef] [PubMed]
- Assaf-Balut, C.; García de la Torre, N.; Duran, A.; Fuentes, M.; Bordiú, E.; Del Valle, L.; Familiar, C.; Valerio, J.; Jiménez, I.; Herraiz, M.A.; et al. A Mediterranean Diet with an Enhanced Consumption of Extra Virgin Olive Oil and Pistachios Improves Pregnancy Outcomes in Women Without Gestational Diabetes Mellitus: A Sub-Analysis of the St. Carlos Gestational Diabetes Mellitus Prevention Study. Ann. Nutr. Metab. 2019, 74, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Sherriff, J.L.; O’Sullivan, T.A.; Properzi, C.; Oddo, J.L.; Adams, L.A. Choline, Its Potential Role in Nonalcoholic Fatty Liver Disease, and the Case for Human and Bacterial Genes. Adv. Nutr. 2016, 7, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Sanders, L.M.; Zeisel, S.H. Choline: Dietary Requirements and Role in Brain Development. Nutr. Today 2007, 42, 181–186. [Google Scholar] [CrossRef]
- Wallace, T.C.; Fulgoni, V.L., 3rd. Assessment of Total Choline Intakes in the United States. J. Am. Coll. Nutr. 2016, 35, 108–112. [Google Scholar] [CrossRef]
- Caudill, M.A. Pre- and postnatal health: Evidence of increased choline needs. J. Am. Diet. Assoc. 2010, 110, 1198–1206. [Google Scholar] [CrossRef]
- Chai, C.; Chen, L.; Deng, M.G.; Liang, Y.; Liu, F.; Nie, J.Q. Dietary choline intake and non-alcoholic fatty liver disease (NAFLD) in U.S. adults: National Health and Nutrition Examination Survey (NHANES) 2017–2018. Eur. J. Clin. Nutr. 2023, 77, 1160–1166. [Google Scholar] [CrossRef]
- Costa, R.S.; Rossi, M.A.; Oliveira, J.S. Pathogenesis of the renal injury in choline deficiency: The role of catecholamines and acetylcholine. Br. J. Exp. Pathol. 1979, 60, 613–619. [Google Scholar] [PubMed]
- Golzarand, M.; Bahadoran, Z.; Mirmiran, P.; Azizi, F. Dietary choline and betaine intake and risk of hypertension development: A 7. 4-year follow-up. Food Funct. 2021, 12, 4072–4078. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zang, X.; Lv, J.; Zhang, Y.; Lv, Z.; Yu, M. Changes in Lipidomics, Metabolomics, and the Gut Microbiota in CDAA-Induced NAFLD Mice after Polyene Phosphatidylcholine Treatment. Int. J. Mol. Sci. 2023, 24, 1502. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, N.S.; Nazli, R.; Zafar, H.; Fatima, S. Effects of lipid based Multiple Micronutrients Supplement on the birth outcome of underweight pre-eclamptic women: A randomized clinical trial. Pak. J. Med. Sci. 2022, 38, 219–226. [Google Scholar] [CrossRef]
- Fernandez, M.L.; Murillo, A.G. Is There a Correlation between Dietary and Blood Cholesterol? Evidence from Epidemiological Data and Clinical Interventions. Nutrients 2022, 14, 2168. [Google Scholar] [CrossRef] [PubMed]
- Soliman, G.A. Dietary Cholesterol and the Lack of Evidence in Cardiovascular Disease. Nutrients 2018, 10, 780. [Google Scholar] [CrossRef]
- Addis, P.B. Occurrence of lipid oxidation products in foods. Food Chem. Toxicol. 1986, 24, 1021–1030. [Google Scholar] [CrossRef]
- Herlambang, H.; Puspasari, A.; Maharani, C.; Enis, R.N.; Tarawifa, S.; Fitri, A.D.; Harahap, H.; Harahap, A.H.; Kusdiyah, E.; Syamsunarno, M.R.A.A. Comprehensive fatty acid fractionation profilling in preeclampsia: A case control study with multivariable analysis. BMC Pregnancy Childbirth 2022, 22, 8. [Google Scholar] [CrossRef]
- Tan, B.; Ma, Y.; Zhang, L.; Li, N.; Zhang, J. The application of metabolomics analysis in the research of gestational diabetes mellitus and preeclampsia. J. Obstet. Gynaecol. Res. 2020, 46, 1310–1318. [Google Scholar] [CrossRef] [PubMed]
- Esquivel, M.K. Nutritional Status and Nutrients Related to Pre-Eclampsia Risk. Am. J. Lifestyle Med. 2022, 17, 41–45. [Google Scholar] [CrossRef] [PubMed]
| Condition | Relative Risk |
|---|---|
| Previous pregnancy with preeclampsia | 8.4 |
| Chronic hypertension | 5.1 |
| Pregestational Diabetes | 3.7 |
| Multiple gestation | 2.9 |
| Pre-pregnancy BMI > 30 | 2.8 |
| Anti-phospholipid Antibodies | 2.8 |
| Reduced maternal serum omega-3 fatty acids | 7.6 |
| Increased maternal RBC TUFA | 3 to 7.4 |
| Increased dietary PUFA | 2.6 to 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hart, N.R. Paradoxes: Cholesterol and Hypoxia in Preeclampsia. Biomolecules 2024, 14, 691. https://doi.org/10.3390/biom14060691
Hart NR. Paradoxes: Cholesterol and Hypoxia in Preeclampsia. Biomolecules. 2024; 14(6):691. https://doi.org/10.3390/biom14060691
Chicago/Turabian StyleHart, Nancy R. 2024. "Paradoxes: Cholesterol and Hypoxia in Preeclampsia" Biomolecules 14, no. 6: 691. https://doi.org/10.3390/biom14060691
APA StyleHart, N. R. (2024). Paradoxes: Cholesterol and Hypoxia in Preeclampsia. Biomolecules, 14(6), 691. https://doi.org/10.3390/biom14060691






