The Rhododendron Chrysanthum Pall.s’ Acetylation Modification of Rubisco Enzymes Controls Carbon Cycling to Withstand UV−B Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Treatment
2.2. Measurement of Chlorophyll Fluorescence
2.3. Quantitative Proteomic Study of Acetylated Modification
2.3.1. Protein Extraction
2.3.2. Trypsin Digestion
2.3.3. Affinity Enrichment
2.3.4. LC-MS/MS Analysis and Database Search
2.3.5. Bioinformatics Analysis
2.4. Modeling Acetylated Proteins Homology
2.5. Statistics and Analysis of Data
3. Results
3.1. Quantitative Proteomic Analysis of Acetylated Modification in R. chrysanthum
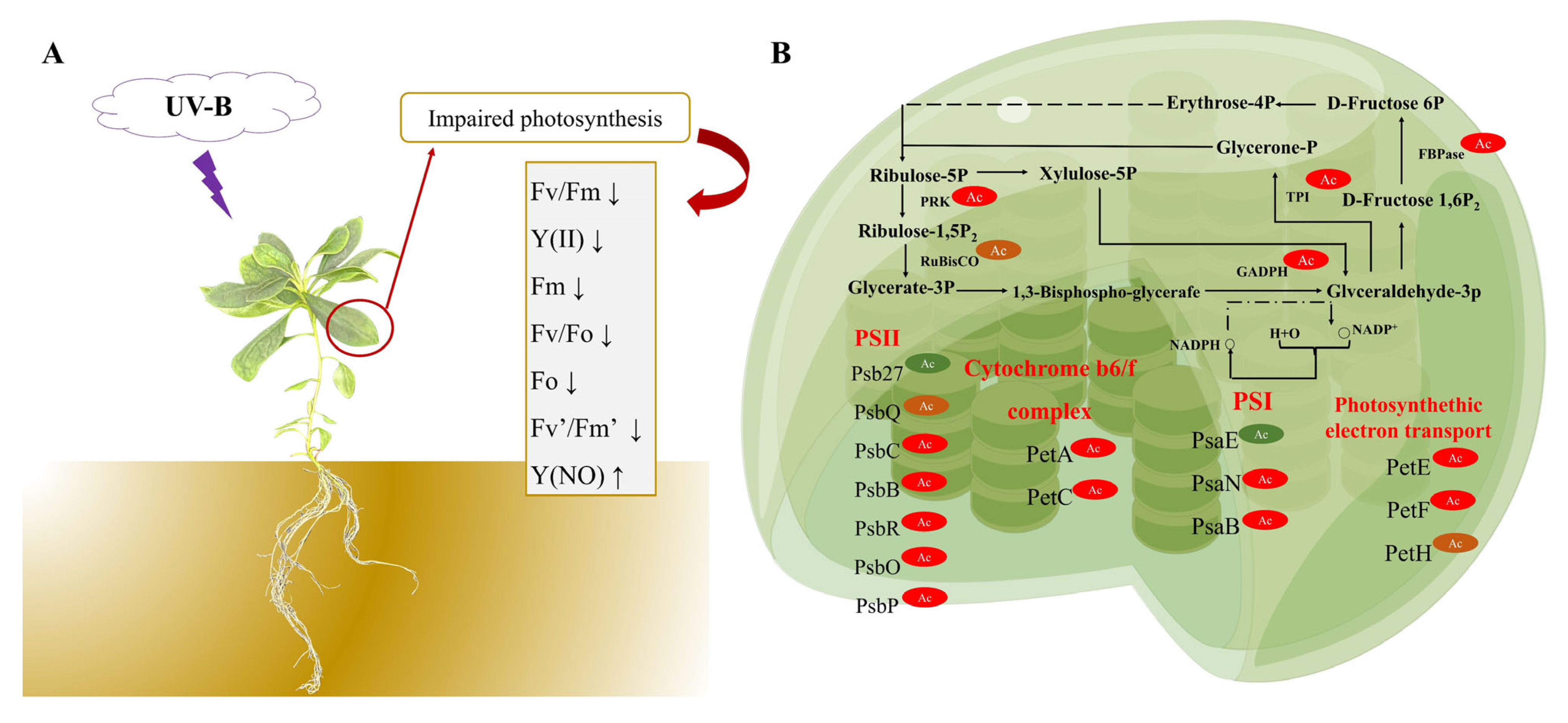
3.2. Plant Carbon Assimilation in R. chrysanthum Is Impacted by Photodamage under UV−B Stress
3.3. Analysis of Distribution and Motif of Acetylated Sites in Photosynthetic Carbon Cycle of R. chrysanthum
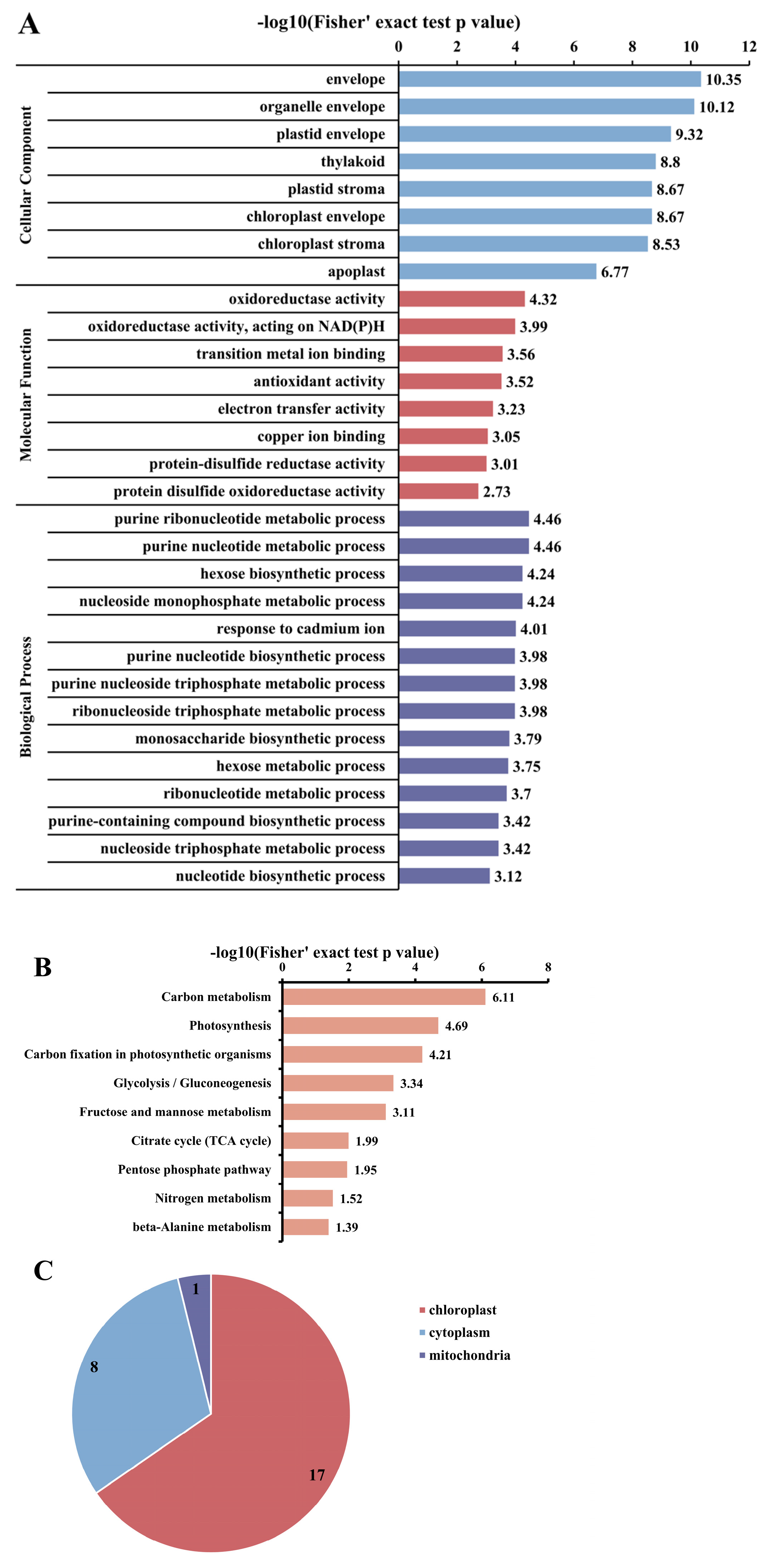
3.4. Analysis of Functional Enrichment of Acetylated Proteins and Sites in Photosynthetic Carbon Cycle of R. chrysanthum
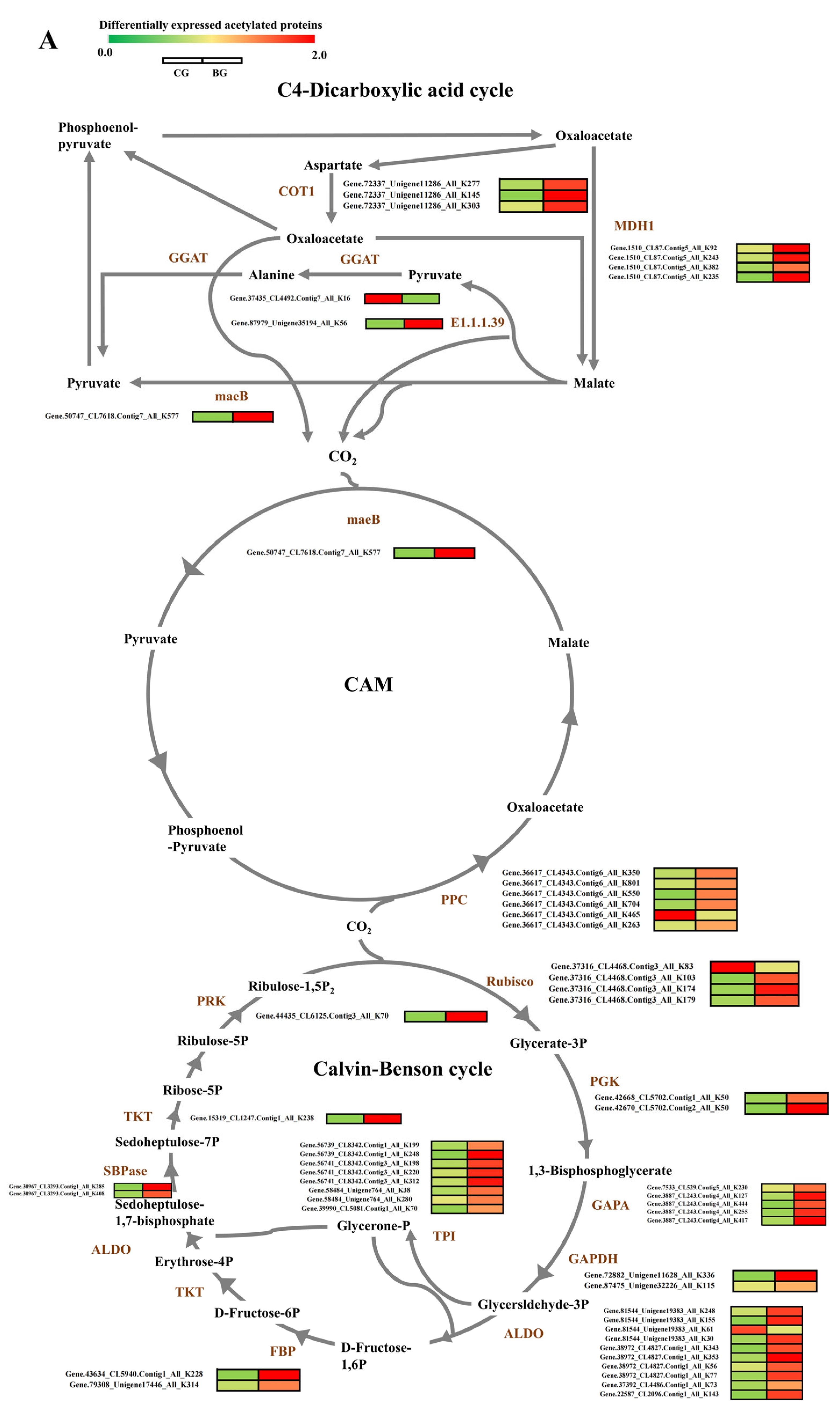
3.5. Analysis of DEPs and DAPs in Carbon Fixation in Photosynthetic Organisms of R. chrysanthum under UV−B Stress
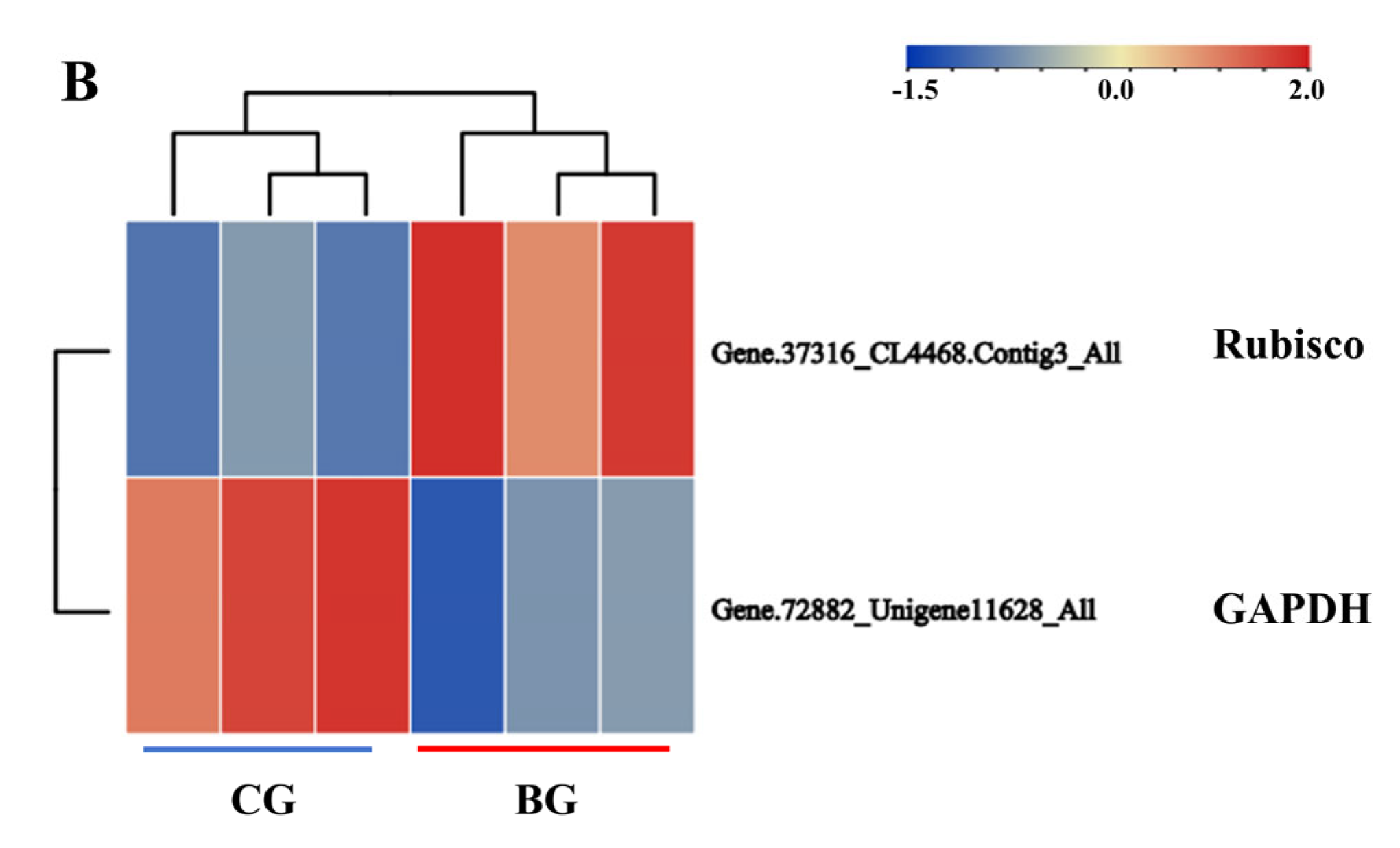
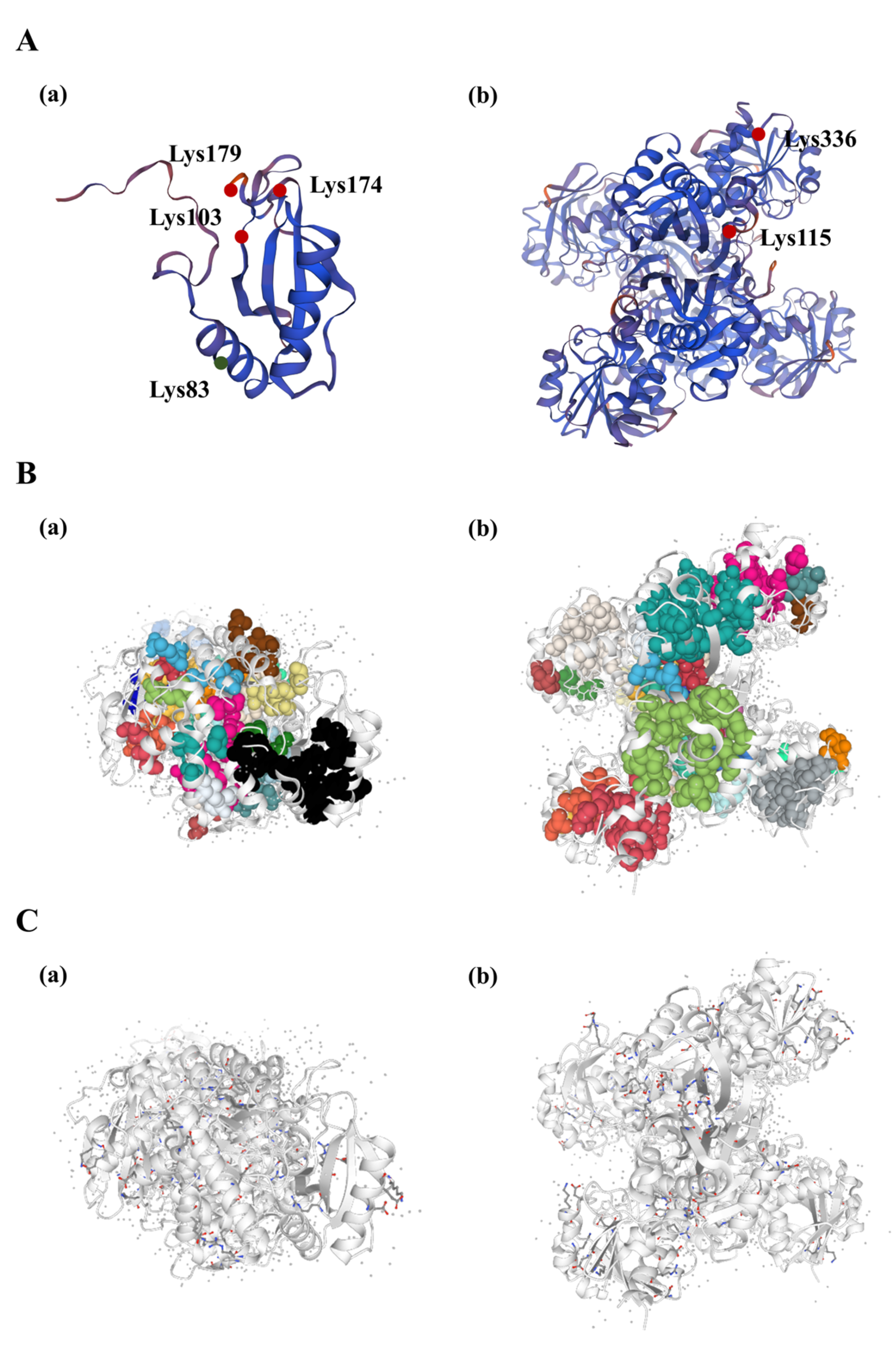
3.6. Protein Structure Analysis of Rubisco and GAPDH Enzymes Undergoing Acetylation Modification in R. chrysanthum under UV−B Stress
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, X.; Chen, S.; Wu, H.; Yang, Y.; Xu, H. Biochemical and proteomics analyses of antioxidant enzymes reveal the potential stress tolerance in Rhododendron chrysanthum Pall. Biol. Direct. 2017, 12, 10. [Google Scholar] [CrossRef] [PubMed]
- Lyu, J.; Wang, C.; Liang, D.Y.; Liu, L.; Pandey, L.K.; Xu, H.W.; Zhou, X.F. Sensitivity of wild and domesticated Rhododendron chrysanthum to different light regime (UVA, UVB, and PAR). Photosynthetica 2019, 57, 841–849. [Google Scholar] [CrossRef]
- Bassman, J.H. Ecosystem consequences of enhanced solar ultraviolet radiation: Secondary plant metabolites as mediators of multiple trophic interactions in terrestrial plant communities. Photochem. Photobiol. 2004, 79, 382–398. [Google Scholar] [CrossRef]
- Knox, P.P.; Lukashev, E.P.; Gorokhov, V.V.; Grishanova, N.P.; Paschenko, V.Z. Hybrid complexes of photosynthetic reaction centers and quantum dots in various matrices: Resistance to UV irradiation and heating. Photosynth. Res. 2019, 139, 295–305. [Google Scholar] [CrossRef]
- Widel, M.; Krzywon, A.; Gajda, K.; Skonieczna, M.; Rzeszowska-Wolny, J. Induction of bystander effects by UVA, UVB, and UVC radiation in human fibroblasts and the implication of reactive oxygen species. Free Radic. Biol. Med. 2014, 68, 278–287. [Google Scholar] [CrossRef]
- Hideg, E.; Jansen, M.A.; Strid, A. UV−B exposure, ROS, and stress: Inseparable companions or loosely linked associates? Trends Plant Sci. 2013, 18, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-E.; Su, Y.-Q.; Zhang, C.-M.; Ma, J.; Mao, H.-T.; Yang, Z.-H.; Yuan, M.; Zhang, Z.-W.; Yuan, S.; Zhang, H.-Y. Comparison of Photosynthetic Characteristics and Antioxidant Systems in Different Wheat Strains. J. Plant Growth Regul. 2018, 37, 347–359. [Google Scholar] [CrossRef]
- Barber, J. Photosystem two. Biochim. Biophys. Acta Bioenerg. 1998, 1365, 269–277. [Google Scholar] [CrossRef]
- Yoon, H.I.; Kim, D.; Son, J.E. Spatial and Temporal Bioactive Compound Contents and Chlorophyll Fluorescence of Kale (Brassica oleracea L.) Under UV−B Exposure Near Harvest Time in Controlled Environments. Photochem. Photobiol. 2020, 96, 845–852. [Google Scholar] [CrossRef]
- Piccini, C.; Cai, G.; Dias, M.C.; Romi, M.; Longo, R.; Cantini, C. UV−B Radiation Affects Photosynthesis-Related Processes of Two Italian Olea europaea (L.) Varieties Differently. Plants 2020, 9, 1712. [Google Scholar] [CrossRef]
- Liu, M.; Sun, Q.; Cao, K.; Xu, H.; Zhou, X. Acetylated Proteomics of UV−B Stress-Responsive in Photosystem II of Rhododendron chrysanthum. Cells 2023, 12, 478. [Google Scholar] [CrossRef] [PubMed]
- UniProt Consortium, T. UniProt: The universal protein knowledgebase. Nucleic Acids Res. 2018, 46, 2699. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, C.; Weinert, B.T.; Nishida, Y.; Verdin, E.; Mann, M. The growing landscape of lysine acetylation links metabolism and cell signalling. Nat. Rev. Mol. Cell Biol. 2014, 15, 536–550. [Google Scholar] [CrossRef] [PubMed]
- Narita, T.; Weinert, B.T.; Choudhary, C. Functions and mechanisms of non-histone protein acetylation. Nat. Rev. Mol. Cell Biol. 2019, 20, 156–174. [Google Scholar] [CrossRef] [PubMed]
- Colville, A.; Alhattab, R.; Hu, M.; Labbé, H.; Xing, T.; Miki, B. Role of HD2 genes in seed germination and early seedling growth in Arabidopsis. Plant Cell Rep. 2011, 30, 1969–1979. [Google Scholar] [CrossRef]
- Kim, W.; Latrasse, D.; Servet, C.; Zhou, D.X. Arabidopsis histone deacetylase HDA9 regulates flowering time through repression of AGL19. Biochem. Biophys. Res. Commun. 2013, 432, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Wang, Y.Y.; Liu, X.; Yang, S.; Lu, Q.; Cui, Y.; Wu, K. HD2C interacts with HDA6 and is involved in ABA and salt stress response in Arabidopsis. J. Exp. Bot. 2012, 63, 3297–3306. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Baek, D.; Cha, J.Y.; Liao, X.; Kang, S.H.; McClung, C.R.; Lee, S.Y.; Yun, D.J.; Kim, W.Y. HOS15 Interacts with the Histone Deacetylase HDA9 and the Evening Complex to Epigenetically Regulate the Floral Activator GIGANTEA. Plant Cell 2019, 31, 37–51. [Google Scholar] [CrossRef] [PubMed]
- van Zanten, M.; Zöll, C.; Wang, Z.; Philipp, C.; Carles, A.; Li, Y.; Kornet, N.G.; Liu, Y.; Soppe, W.J. HISTONE DEACETYLASE 9 represses seedling traits in Arabidopsis thaliana dry seeds. Plant J. 2014, 80, 475–488. [Google Scholar] [CrossRef]
- Yuan, L.; Chen, X.; Chen, H.; Wu, K.; Huang, S. Histone deacetylases HDA6 and HDA9 coordinately regulate valve cell elongation through affecting auxin signaling in Arabidopsis. Biochem. Biophys. Res. Commun. 2019, 508, 695–700. [Google Scholar] [CrossRef]
- Liao, X.; Li, Y.; Hu, Z.; Lin, Y.; Zheng, B.; Ding, J. Poplar acetylome profiling reveals lysine acetylation dynamics in seasonal bud dormancy release. Plant Cell Environ. 2021, 44, 1830–1845. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.R.; Yan, X.; Zhu, D.; Deng, X.; Wu, J.S.; Xia, J.; Yan, Y.M. Lysine acetylproteome profiling under water deficit reveals key acetylated proteins involved in wheat grain development and starch biosynthesis. J. Proteom. 2018, 185, 8–24. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Huang, Y.; Liang, Y.; Yuan, Y.; Liu, Y.; Han, T.; Li, S.; Gao, H.; Lv, B.; Huang, X.; et al. OsHYPK-mediated protein N-terminal acetylation coordinates plant development and abiotic stress responses in rice. Mol. Plant 2022, 15, 740–754. [Google Scholar] [CrossRef]
- Du, Q.; Qu, Z.; Wang, L.; Jiang, J.; Fu, X.; Fang, Y.; Li, X.; Xie, X. Histone deacetylase SbHDT701 in Sorghum bicolor reveals functions in response to stress factors by enhancing acetylation. Pestic. Biochem. Physiol. 2021, 178, 104908. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Oh, M.H.; Schwarz, E.M.; Larue, C.T.; Sivaguru, M.; Imai, B.S.; Yau, P.M.; Ort, D.R.; Huber, S.C. Lysine acetylation is a widespread protein modification for diverse proteins in Arabidopsis. Plant Physiol. 2011, 155, 1769–1778. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Gai, Z.; Wang, Y.; Fan, K.; Sun, L.; Wang, H.; Ding, Z. Comprehensive proteome analyses of lysine acetylation in tea leaves by sensing nitrogen nutrition. BMC Genom. 2018, 19, 840. [Google Scholar] [CrossRef]
- König, A.C.; Hartl, M.; Boersema, P.J.; Mann, M.; Finkemeier, I. The mitochondrial lysine acetylome of Arabidopsis. Mitochondrion 2014, 19, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Song, L.; Liang, W.; Mu, P.; Wang, S.; Lin, Q. Comprehensive profiling of lysine acetylproteome analysis reveals diverse functions of lysine acetylation in common wheat. Sci. Rep. 2016, 6, 21069. [Google Scholar] [CrossRef]
- Nallamilli, B.R.; Edelmann, M.J.; Zhong, X.; Tan, F.; Mujahid, H.; Zhang, J.; Nanduri, B.; Peng, Z. Global analysis of lysine acetylation suggests the involvement of protein acetylation in diverse biological processes in rice (Oryza sativa). PLoS ONE 2014, 9, e89283. [Google Scholar] [CrossRef]
- Liu, Z.; Song, J.; Miao, W.; Yang, B.; Zhang, Z.; Chen, W.; Tan, F.; Suo, H.; Dai, X.; Zou, X.; et al. Comprehensive Proteome and Lysine Acetylome Analysis Reveals the Widespread Involvement of Acetylation in Cold Resistance of Pepper (Capsicum annuum L.). Front. Plant Sci. 2021, 12, 730489. [Google Scholar] [CrossRef]
- Savitch, L.V.; Leonardos, E.D.; Krol, M.; Jansson, S.; Öquist, G. Two different strategies for light utilization in photosynthesis in relation to growth and cold acclimation. Plant Cell Environ. 2002, 25, 761–771. [Google Scholar] [CrossRef]
- Wu, J.-b.; Guan, D.-x.; Yuan, F.-h.; Zhang, X.-j. Research advances on the biological effects of elevated ultraviolet-B radiation on terrestrial plants. J. For. Res. 2009, 20, 383–390. [Google Scholar] [CrossRef]
- Lidon, F.J.C. Micronutrients’ accumulation in rice after supplemental UV−B irradiation. J. Plant Interact. 2012, 7, 19–28. [Google Scholar] [CrossRef]
- Zhang, Z.S.; Jin, L.Q.; Li, Y.T.; Tikkanen, M.; Li, Q.M.; Ai, X.Z.; Gao, H.Y. Ultraviolet−B Radiation (UV−B) Relieves Chilling-Light-Induced PSI Photoinhibition And Accelerates The Recovery Of CO2 Assimilation In Cucumber (Cucumis sativus L.) Leaves. Sci. Rep. 2016, 6, 34455. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Chen, H.; Tang, M.J.; Yang, P.F.; Shen, S.H. Responses of Jatropha curcas seedlings to cold stress: Photosynthesis-related proteins and chlorophyll fluorescence characteristics. Physiol. Plant. 2007, 131, 508–517. [Google Scholar] [CrossRef]
- Belkhodja, R.; Morales, F.; Abadia, A.; Gomez-Aparisi, J.; Abadia, J. Chlorophyll Fluorescence as a Possible Tool for Salinity Tolerance Screening in Barley (Hordeum vulgare L.). Plant Physiol. 1994, 104, 667–673. [Google Scholar] [CrossRef]
- Martínez-Abaigar, J.; Núñez-Olivera, E.; Beaucourt, N.; García-Álvaro, M.A.; Tomás, R.; Arróniz, M. Different physiological responses of two aquatic bryophytes to enhanced ultraviolet−B radiation. J. Bryol. 2003, 25, 17–30. [Google Scholar] [CrossRef]
- Ifuku, K.; Ido, K.; Sato, F. Molecular functions of PsbP and PsbQ proteins in the photosystem II supercomplex. J. Photochem. Photobiol. B Biol. 2011, 104, 158–164. [Google Scholar] [CrossRef] [PubMed]
- McFarlane, C.R.; Shah, N.R.; Kabasakal, B.V.; Echeverria, B.; Cotton, C.A.R.; Bubeck, D.; Murray, J.W. Structural basis of light-induced redox regulation in the Calvin-Benson cycle in cyanobacteria. Proc. Natl. Acad. Sci. USA 2019, 116, 20984–20990. [Google Scholar] [CrossRef]
- Howard, T.P.; Lloyd, J.C.; Raines, C.A. Inter-species variation in the oligomeric states of the higher plant Calvin cycle enzymes glyceraldehyde-3-phosphate dehydrogenase and phosphoribulokinase. J. Exp. Bot. 2011, 62, 3799–3805. [Google Scholar] [CrossRef]
- Martínez-Lüscher, J.; Morales, F.; Sánchez-Díaz, M.; Delrot, S.; Aguirreolea, J.; Gomès, E.; Pascual, I. Climate change conditions (elevated CO2 and temperature) and UV−B radiation affect grapevine (Vitis vinifera cv. Tempranillo) leaf carbon assimilation, altering fruit ripening rates. Plant Sci. 2015, 236, 168–176. [Google Scholar] [CrossRef] [PubMed]
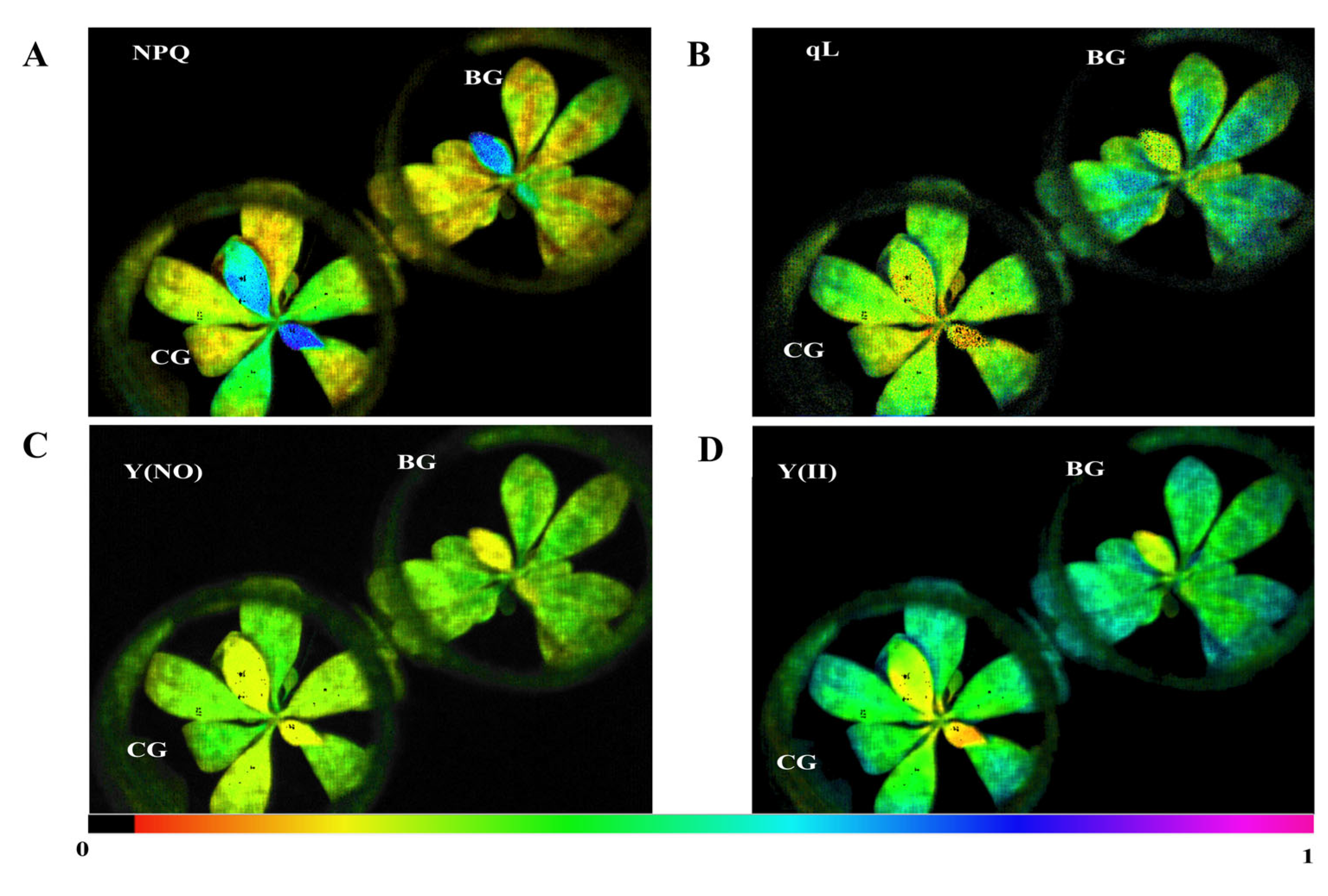


| Group | Fv′/Fm′ | Y(II) | Y(NO) | NPQ | qL | ETR |
|---|---|---|---|---|---|---|
| CG | 0.660 ± 0.015a | 0.489 ± 0.008a | 0.310 ± 0.019b | 0.193 ± 0.039a | 0.49 ± 0.038b | 12.8 ± 0.163a |
| BG | 0.599 ± 0.014b | 0.465 ± 0.016a | 0.366 ± 0.009a | 0.118 ± 0.006a | 0.59 ± 0.005a | 12.6 ± 0.205a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; Gong, F.; Yu, W.; Cao, K.; Xu, H.; Zhou, X. The Rhododendron Chrysanthum Pall.s’ Acetylation Modification of Rubisco Enzymes Controls Carbon Cycling to Withstand UV−B Stress. Biomolecules 2024, 14, 732. https://doi.org/10.3390/biom14060732
Liu M, Gong F, Yu W, Cao K, Xu H, Zhou X. The Rhododendron Chrysanthum Pall.s’ Acetylation Modification of Rubisco Enzymes Controls Carbon Cycling to Withstand UV−B Stress. Biomolecules. 2024; 14(6):732. https://doi.org/10.3390/biom14060732
Chicago/Turabian StyleLiu, Meiqi, Fushuai Gong, Wang Yu, Kun Cao, Hongwei Xu, and Xiaofu Zhou. 2024. "The Rhododendron Chrysanthum Pall.s’ Acetylation Modification of Rubisco Enzymes Controls Carbon Cycling to Withstand UV−B Stress" Biomolecules 14, no. 6: 732. https://doi.org/10.3390/biom14060732







