Electroceuticals and Magnetoceuticals in Gastroenterology
Abstract
:1. Introduction
2. Vagal Nerve Modulation
2.1. Mechanisms of Action of Vagal Nerve Modulation
2.2. Devices, Parameters, and Treatment Regimens in Use
2.3. Impact on Major GI Diseases
2.4. Review of Key Clinical Studies
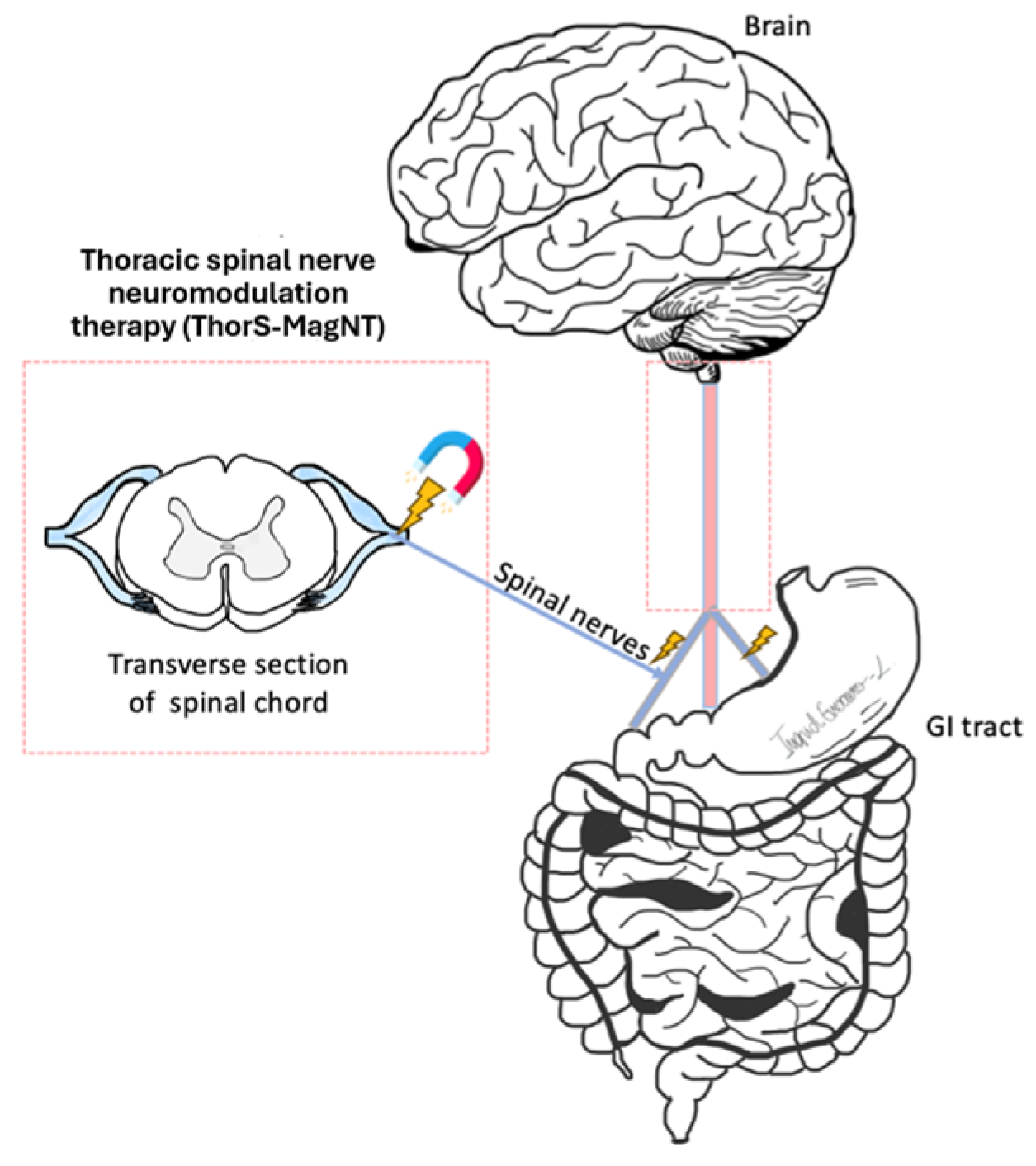
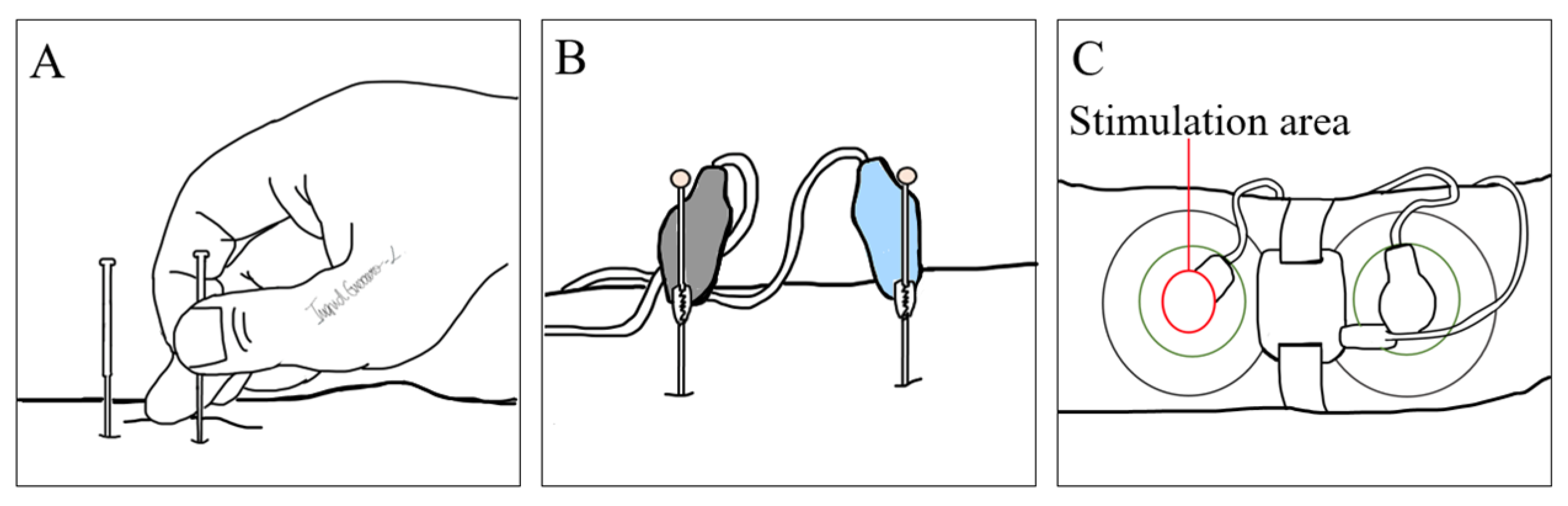
3. Magnetic Neuromodulation
3.1. Mechanisms of Action of Magnetic Stimulation
3.2. Devices, Parameters, and Treatment Regimens in Use
3.3. Impact on Major GI Diseases
3.4. Review of Key Clinical Studies
4. Acupuncture, Electroacupuncture, and Transcutaneous Electrical Acustimulation (TEA)
4.1. Mechanism of Action of Acupuncture, Electroacupuncture, and TEA
4.2. Devices, Parameters, and Treatment Regimen in Use
4.3. Impact on Major GI Diseases
4.4. Review of Key Clinical Studies
5. Disadvantages and Drawbacks of Electroceuticals and Magnetoceuticals
5.1. Stimulation Parameters
5.2. Safety Concerns
5.3. Drawbacks
6. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Song, G.; Trujillo, S.; Fu, Y.; Shibi, F.; Chen, J.; Fass, R. Transcutaneous electrical stimulation for gastrointestinal motility disorders. Neurogastroenterol. Motil. 2023, 35, e14618. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Soffer, E. Electroceuticals for Neurogastroenterology and Motility Disorders. Curr. Gastroenterol. Rep. 2023, 25, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Flatscher, J.; Loriè, E.P.; Mittermayr, R.; Meznik, P.; Slezak, P.; Redl, H.; Slezak, C. Pulsed Electromagnetic Fields (PEMF)—Physiological Response and Its Potential in Trauma Treatment. Int. J. Mol. Sci. 2023, 24, 11239. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Shen, H.; Chowdhury, R.; Abdi, T.; Selaru, F.; Chen, J.D.Z. Potential of Electrical Neuromodulation for Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2020, 26, 1119–1130. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Fiocchi, C.; Achkar, J.-P. Acupuncture in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2018, 25, 1129–1139. [Google Scholar] [CrossRef] [PubMed]
- Lefaucheur, J.-P.; Aleman, A.; Baeken, C.; Benninger, D.H.; Brunelin, J.; Di Lazzaro, V.; Filipović, S.R.; Grefkes, C.; Hasan, A.; Hummel, F.C.; et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): An update (2014–2018). Clin. Neurophysiol. 2020, 131, 474–528. [Google Scholar] [CrossRef]
- Rao, S.S.; Yan, Y.; Xiang, X.; Sharma, A.; Ayyala, D.; Hamdy, S. Effects of Translumbosacral Neuromodulation Therapy on Gut and Brain Interactions and Anorectal Neuropathy in Fecal Incontinence: A Randomized Study. Neuromodulation Technol. Neural Interface 2021, 24, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Yap, J.Y.Y.; Keatch, C.; Lambert, E.; Woods, W.; Stoddart, P.R.; Kameneva, T. Critical Review of Transcutaneous Vagus Nerve Stimulation: Challenges for Translation to Clinical Practice. Front. Neurosci. 2020, 14, 284. [Google Scholar] [CrossRef]
- Frøkjær, J.B.; Bergmann, S.; Brock, C.; Madzak, A.; Farmer, A.D.; Ellrich, J.; Drewes, A.M. Modulation of vagal tone enhances gastroduodenal motility and reduces somatic pain sensitivity. Neurogastroenterol. Motil. 2016, 28, 592–598. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, B.; Shi, X.; Ning, B.; Shi, J.; Zeng, X.; Liu, F.; Chen, J.D.; Xie, W.-F. Ameliorating Effects and Autonomic Mechanisms of Transcutaneous Electrical Acustimulation in Patients with Gastroesophageal Reflux Disease. Neuromodulation: Technol. Neural Interface 2019, 23, 1207–1214. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, T.; Dong, Y.; Chen, Y.; Chen, J.D.Z. Auricular vagal nerve stimulation enhances gastrointestinal motility and improves interstitial cells of Cajal in rats treated with loperamide. Neurogastroenterol. Motil. 2021, 33, e14163. [Google Scholar] [CrossRef] [PubMed]
- Bonaz, B. Anti-inflammatory effects of vagal nerve stimulation with a special attention to intestinal barrier dysfunction. Neurogastroenterol. Motil. 2022, 34, e14456. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Xu, F.; Lu, D.; Rong, P.; Cheng, J.; Li, M.; Gong, Y.; Sun, C.; Wei, W.; Lin, L.; et al. Transcutaneous auricular vagal nerve stimulation improves functional dyspepsia by enhancing vagal efferent activity. Am. J. Physiol. Liver Physiol. 2021, 320, G700–G711. [Google Scholar] [CrossRef] [PubMed]
- Farmer, A.D.; Albusoda, A.; Amarasinghe, G.; Ruffle, J.K.; Fitzke, H.E.; Idrees, R.; Fried, R.; Brock, C.; Aziz, Q. Transcutaneous vagus nerve stimulation prevents the development of, and reverses, established oesophageal pain hypersensitivity. Aliment. Pharmacol. Ther. 2020, 52, 988–996. [Google Scholar] [CrossRef] [PubMed]
- Sclocco, R.; Garcia, R.G.; Kettner, N.W.; Fisher, H.P.; Isenburg, K.; Makarovsky, M.; Stowell, J.A.; Goldstein, J.; Barbieri, R.; Napadow, V. Stimulus frequency modulates brainstem response to respiratory-gated transcutaneous auricular vagus nerve stimulation. Brain Stimul. 2020, 13, 970–978. [Google Scholar] [CrossRef] [PubMed]
- Gottfried-Blackmore, A.; Adler, E.P.; Fernandez-Becker, N.; Clarke, J.; Habtezion, A.; Nguyen, L. Open-label pilot study: Non-invasive vagal nerve stimulation improves symptoms and gastric emptying in patients with idiopathic gastroparesis. Neurogastroenterol. Motil. 2020, 32, e13769. [Google Scholar] [CrossRef] [PubMed]
- Fornaro, R.; Actis, G.C.; Caviglia, G.P.; Pitoni, D.; Ribaldone, D.G. Inflammatory Bowel Disease: Role of Vagus Nerve Stimulation. J. Clin. Med. 2022, 11, 5690. [Google Scholar] [CrossRef] [PubMed]
- Mogilevski, T.; Rosella, S.; Aziz, Q.; Gibson, P.R. Transcutaneous vagal nerve stimulation protects against stress-induced intestinal barrier dysfunction in healthy adults. Neurogastroenterol. Motil. 2022, 34, e14382. [Google Scholar] [CrossRef] [PubMed]
- Sinniger, V.; Pellissier, S.; Fauvelle, F.; Trocmé, C.; Hoffmann, D.; Vercueil, L.; Cracowski, J.; David, O.; Bonaz, B. A 12-month pilot study outcomes of vagus nerve stimulation in Crohn’s disease. Neurogastroenterol. Motil. 2020, 32, e13911. [Google Scholar] [CrossRef]
- Paulon, E.; Nastou, D.; Jaboli, F.; Marin, J.; Liebler, E.; Epstein, O. Proof of concept: Short-term non-invasive cervical vagus nerve stimulation in patients with drug-refractory gastroparesis. Front. Gastroenterol. 2017, 8, 325–330. [Google Scholar] [CrossRef]
- Bonaz, B.; Sinniger, V.; Pellissier, S. Therapeutic Potential of Vagus Nerve Stimulation for Inflammatory Bowel Diseases. Front. Neurosci. 2021, 15, 650971. [Google Scholar] [CrossRef] [PubMed]
- Karrento, K.; Zhang, L.; Conley, W.; Qazi, Z.; Venkatesan, T.; Simpson, P.; Li, B.U. Percutaneous electrical nerve field stimulation improves comorbidities in children with cyclic vomiting syndrome. Front. Pain Res. 2023, 4, 1203541. [Google Scholar] [CrossRef]
- Shi, X.; Hu, Y.; Zhang, B.; Li, W.; Chen, J.D.; Liu, F. Ameliorating effects and mechanisms of transcutaneous auricular vagal nerve stimulation on abdominal pain and constipation. J. Clin. Investig. 2021, 6, e150052. [Google Scholar] [CrossRef] [PubMed]
- A Bassett, C. Fundamental and practical aspects of therapeutic uses of pulsed electromagnetic fields (PEMFs). Crit. Rev. Biomed. Eng. 1989, 17, 451–529. [Google Scholar] [PubMed]
- Tepper, O.M.; Callaghan, M.J.; Chang, E.I.; Galiano, R.D.; Bhatt, K.A.; Baharestani, S.; Gan, J.; Simon, B.; Hopper, R.A.; Levine, J.P.; et al. Electromagnetic fields increase in vitro and in vivo angiogenesis through endothelial release of FGF-2. FASEB J. 2004, 18, 1231–1233. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, M.J.M.; Chang, E.I.M.; Seiser, N.M.; Aarabi, S.B.; Ghali, S.M.; Kinnucan, E.R.B.; Simon, B.J.; Gurtner, G.C.M. Pulsed Electromagnetic Fields Accelerate Normal and Diabetic Wound Healing by Increasing Endogenous FGF-2 Release. Plast. Reconstr. Surg. 2008, 121, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Piacentini, R.; Ripoli, C.; Mezzogori, D.; Azzena, G.B.; Grassi, C. Extremely low-frequency electromagnetic fields promote in vitro neurogenesis via upregulation of Cav1-channel activity. J. Cell. Physiol. 2007, 215, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Cuccurazzu, B.; Leone, L.; Podda, M.V.; Piacentini, R.; Riccardi, E.; Ripoli, C.; Azzena, G.B.; Grassi, C. Exposure to extremely low-frequency (50Hz) electromagnetic fields enhances adult hippocampal neurogenesis in C57BL/6 mice. Exp. Neurol. 2010, 226, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Chen, C.; Deng, P.; Zhu, G.; Lin, M.; Zhang, L.; Xu, S.; He, M.; Lu, Y.; Duan, W.; et al. Extremely Low-Frequency Electromagnetic Fields Promote In Vitro Neuronal Differentiation and Neurite Outgrowth of Embryonic Neural Stem Cells via Up-Regulating TRPC1. PLoS ONE 2016, 11, e0150923. [Google Scholar] [CrossRef]
- Cheng, Y.; Dai, Y.; Zhu, X.; Xu, H.; Cai, P.; Xia, R.; Mao, L.; Zhao, B.-Q.; Fan, W. Extremely low-frequency electromagnetic fields enhance the proliferation and differentiation of neural progenitor cells cultured from ischemic brains. NeuroReport 2015, 26, 896–902. [Google Scholar] [CrossRef]
- Gramowski-Voß, A.; Schwertle, H.-J.; Pielka, A.-M.; Schultz, L.; Steder, A.; Jügelt, K.; Axmann, J.; Pries, W. Enhancement of Cortical Network Activity in vitro and Promotion of GABAergic Neurogenesis by Stimulation with an Electromagnetic Field with a 150 MHz Carrier Wave Pulsed with an Alternating 10 and 16 Hz Modulation. Front. Neurol. 2015, 6, 00158. [Google Scholar] [CrossRef] [PubMed]
- Daskalakis, Z.J.; Möller, B.; Christensen, B.K.; Fitzgerald, P.B.; Gunraj, C.; Chen, R. The effects of repetitive transcranial magnetic stimulation on cortical inhibition in healthy human subjects. Exp. Brain Res. 2006, 174, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Payne, S.C.; Furness, J.B.; Stebbing, M.J. Bioelectric neuromodulation for gastrointestinal disorders: Effectiveness and mechanisms. Nat. Rev. Gastroenterol. Hepatol. 2018, 16, 89–105. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M.; Saslow, S.B.; Bharucha, A.E. GASTROINTESTINAL SENSATION. Gastroenterol. Clin. North Am. 1996, 25, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Cervero, F.; Laird, J.M. Understanding the signaling and transmission of visceral nociceptive events. J. Neurobiol. 2004, 61, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Furness, J.B.; Callaghan, B.P.; Rivera, L.R.; Cho, H.-J. The Enteric Nervous System and Gastrointestinal Innervation: Integrated Local and Central Control. In Microbial Endocrinology: The Microbiota-Gut-Brain Axis in Health and Disease; Springer: Berlin, Germany, 2014; Volume 817, pp. 39–71. [Google Scholar] [CrossRef]
- Zhang, T.; Perkins, M.H.; Chang, H.; Han, W.; de Araujo, I.E. An inter-organ neural circuit for appetite suppression. Cell 2022, 185, 2478–2494. [Google Scholar] [CrossRef] [PubMed]
- Willemze, R.A.; Welting, O.; van Hamersveld, H.P.; Meijer, S.L.; A Folgering, J.H.; Darwinkel, H.; Witherington, J.; Sridhar, A.; Vervoordeldonk, M.J.; Seppen, J.; et al. Neuronal control of experimental colitis occurs via sympathetic intestinal innervation. Neurogastroenterol. Motil. 2017, 30, e13163. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M.; Coulie, B.; Tack, J.F. Visceral hypersensitivity: Facts, speculations, and challenges. Gut 2001, 48, 125–131. [Google Scholar] [CrossRef]
- Rao, S.S.; Xiang, X.; Sharma, A.; Patcharatrakul, T.; Yan, Y.; Parr, R.; Ayyala, D.; Hamdy, S. Translumbosacral Neuromodulation Therapy for Fecal Incontinence: A Randomized Frequency Response Trial. Am. J. Gastroenterol. 2020, 116, 162–170. [Google Scholar] [CrossRef]
- Karunaratne, T.; Yan, Y.; Eubanks, A.; Inman, B.; Rao, S.; Sharma, A. Thoracic Spinal Nerve Neuromodulation Therapy for Diabetic Gastroparesis: A Proof-of-Concept Study. Clin. Gastroenterol. Hepatol. 2023, 21, 2958–2959. [Google Scholar] [CrossRef]
- Polak, T.; Markulin, F.; Ehlis, A.-C.; Langer, J.B.M.; Ringel, T.M.; Fallgatter, A.J. Far field potentials from brain stem after transcutaneous Vagus nerve stimulation: Optimization of stimulation and recording parameters. J. Neural Transm. 2009, 116, 1237–1242. [Google Scholar] [CrossRef] [PubMed]
- Hatton, K.W.; McLarney, J.T.; Pittman, T.; Fahy, B.G. Vagal Nerve Stimulation: Overview and Implications for Anesthesiologists. Obstet. Anesthesia Dig. 2006, 103, 1241–1249. [Google Scholar] [CrossRef] [PubMed]
- Rossini, P.M.; Burke, D.; Chen, R.; Cohen, L.G.; Daskalakis, Z.; Di Iorio, R.; Di Lazzaro, V.; Ferreri, F.; Fitzgerald, P.B.; George, M.S.; et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin. Neurophysiol. 2015, 126, 1071–1107. [Google Scholar] [CrossRef] [PubMed]
- Spagnolo, P.A.; Wang, H.; Srivanitchapoom, P.; Schwandt, M.; Heilig, M.; Hallett, M. Lack of Target Engagement Following Low-Frequency Deep Transcranial Magnetic Stimulation of the Anterior Insula. Neuromodulation Technol. Neural Interface 2018, 22, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Opitz, A.; Legon, W.; Rowlands, A.; Bickel, W.K.; Paulus, W.; Tyler, W.J. Physiological observations validate finite element models for estimating subject-specific electric field distributions induced by transcranial magnetic stimulation of the human motor cortex. NeuroImage 2013, 81, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Rao, S.S.C. Epidemiologic Trends and Diagnostic Evaluation of Fecal Incontinence. Gastroenterol. Hepatol. 2020, 16, 302–309. [Google Scholar] [PubMed]
- Xiang, X.; Sharma, A.; Patcharatrakul, T.; Yan, Y.; Karunaratne, T.; Parr, R.; Ayyala, D.N.; Hall, P.; Rao, S.S.C. Randomized controlled trial of home biofeedback therapy versus office biofeedback therapy for fecal incontinence. Neurogastroenterol. Motil. 2021, 33, e14168. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Yan, Y.; Rao, S. Fecal incontinence. In Clinical and Basic Neurogastroenterology and Motility; Academic Press: Cambridge, MA, USA, 2020; pp. 493–504. [Google Scholar]
- Xiang, X.; Patcharatrakul, T.; Sharma, A.; Parr, R.; Hamdy, S.; Rao, S.S. Cortico-anorectal, Spino-anorectal, and Cortico-spinal Nerve Conduction and Locus of Neuronal Injury in Patients with Fecal Incontinence. Clin. Gastroenterol. Hepatol. 2018, 17, 1130–1137. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Coles, M.; Parkman, H.P. Gastroparesis in the 2020s: New Treatments, New Paradigms. Curr. Gastroenterol. Rep. 2020, 22, 1–10. [Google Scholar] [CrossRef]
- Wise, J.L.; Ingrosso, M.R.; Ianiro, G.; Black, C.J.; Ford, A.C.; Lacy, B.E. Response and Adverse Event Rates with Placebo in Gastroparesis: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2023, 21, 1447–1461. [Google Scholar] [CrossRef]
- Pasricha, P.J.; Yates, K.P.; Nguyen, L.; Clarke, J.; Abell, T.L.; Farrugia, G.; Hasler, W.L.; Koch, K.L.; Snape, W.J.; McCallum, R.W.; et al. Outcomes and Factors Associated with Reduced Symptoms in Patients With Gastroparesis. Gastroenterology 2015, 149, 1762–1774. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.M.; Sharma, A.M.; Herekar, A.M.; Jimenez, E.; Hudgi, A.M.; Gu, Q.G.; Rao, S.S. Translumbosacral Anorectal Magnetic Stimulation Test for Fecal Incontinence. Dis. Colon Rectum 2021, 65, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Chen, J.D. Gastrointestinal motility disorders and acupuncture. Auton. Neurosci. 2010, 157, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.D.Z.; Ni, M.; Yin, J. Electroacupuncture treatments for gut motility disorders. Neurogastroenterol. Motil. 2018, 30, e13393. [Google Scholar] [CrossRef]
- Bonaz, B.; Sinniger, V.; Pellissier, S. Anti-inflammatory properties of the vagus nerve: Potential therapeutic implications of vagus nerve stimulation. J. Physiol. 2016, 594, 5781–5790. [Google Scholar] [CrossRef]
- Borovikova, L.V.; Ivanova, S.; Zhang, M.; Yang, H.; Botchkina, G.I.; Watkins, L.R.; Wang, H.; Abumrad, N.; Eaton, J.W.; Tracey, K.J. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 2000, 405, 458–462. [Google Scholar] [CrossRef]
- Zitnik, R.J. Treatment of chronic inflammatory diseases with implantable medical devices*. Clevel. Clin. J. Med. 2011, 78, S30–S34. [Google Scholar] [CrossRef]
- Xing, J.; Larive, B.; Mekhail, N.; Soffer, E. Transcutaneous electrical acustimulation can reduce visceral perception in patients with the irritable bowel syndrome: A pilot study. Altern. Ther. Health Med. 2004, 10, 38–42. [Google Scholar]
- Deng, D.; Guo, K.; Tan, J.; Huang, G.; Li, S.; Jiang, Q.; Xie, J.; Xie, H.; Zhang, Z.; Chen, Y.; et al. Acupuncture for diarrhea-predominant irritable bowel syndrome: A meta-analysis. Zhongguo Zhen Jiu = Chin. Acupunct. Moxibustion 2017, 37, 907–912. [Google Scholar]
- Qin, Y.; Yi, W.; Lin, S.; Yang, C.; Zhuang, Z. Clinical effect of abdominal acupuncture for diarrhea irritable bowel syndrome. Zhongguo Zhen Jiu= Chin. Acupunct. Moxibustion 2017, 37, 1265–1268. [Google Scholar] [CrossRef]
- Hu, S.; Du, M.-H.; Luo, H.-M.; Wang, H.; Lv, Y.; Ma, L.; Lin, Z.-L.; Shi, X.; Gaischek, I.; Wang, L.; et al. Electroacupuncture at Zusanli (ST36) Prevents Intestinal Barrier and Remote Organ Dysfunction following Gut Ischemia through Activating the Cholinergic Anti-Inflammatory-Dependent Mechanism. Evidence-Based Complement. Altern. Med. 2013, 2013, 1–10. [Google Scholar] [CrossRef]
- Hu, S.; Zhao, Z.-K.; Liu, R.; Wang, H.-B.; Gu, C.-Y.; Luo, H.-M.; Wang, H.; Du, M.-H.; Lv, Y.; Shi, X. Electroacupuncture activates enteric glial cells and protects the gut barrier in hemorrhaged rats. World J. Gastroenterol. 2015, 21, 1468–1478. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.J.; Lu, Y.S.; Zhou, X.; Chen, H.Y.; Xue, S.Y.; Kuai, L. Protective Effects of Acupuncture Intervention Against Damage of Intesti-nal Mucosal Barrier Induced by 5-Fluorouracil in Rats. Zhen Ci Yan Jiu. 2016, 41, 95–99. [Google Scholar] [PubMed]
- Wang, X.-M.; Lu, Y.; Wu, L.-Y.; Yu, S.-G.; Zhao, B.-X.; Hu, H.-Y.; Wu, H.-G.; Bao, C.-H.; Liu, H.-R.; Wang, J.-H.; et al. Moxibustion inhibits interleukin-12 and tumor necrosis factor alpha and modulates intestinal flora in rat with ulcerative colitis. World J. Gastroenterol. 2012, 18, 6819–6828. [Google Scholar] [CrossRef]
- Hou, T.S.; Han, X.X.; Yang, Y.; Zhao, J.L.; Ren, Y.D.; Yu, S.G.; Wu, Q.F. Effect of electroacupuncture intervention on enteric microecol-ogy in ulcerative colitis rats. Zhen Ci Yan Jiu 2014, 39, 27–34. [Google Scholar] [PubMed]
- Xu, J.; Zheng, X.; Cheng, K.-K.; Chang, X.; Shen, G.; Liu, M.; Wang, Y.; Shen, J.; Zhang, Y.; He, Q.; et al. NMR-based metabolomics Reveals Alterations of Electro-acupuncture Stimulations on Chronic Atrophic Gastritis Rats. Sci. Rep. 2017, 7, srep45580. [Google Scholar] [CrossRef]
- Xu, Z.; Li, R.; Zhu, C.; Li, M. Effect of Acupuncture Treatment for Weight Loss on Gut Flora in Patients with Simple Obesity. Acupunct. Med. 2013, 31, 116–117. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, H.; Xing, J.; Chen, J. Electroacupuncture Restores Impaired Gastric Accommodation in Vagotomized Dogs. Dig. Dis. Sci. 2004, 49, 1418–1424. [Google Scholar] [CrossRef]
- Zhang, B.; Hu, Y.; Shi, X.; Li, W.; Zeng, X.; Liu, F.; Chen, J.D.; Xie, W.-F. Integrative Effects and Vagal Mechanisms of Transcutaneous Electrical Acustimulation on Gastroesophageal Motility in Patients with Gastroesophageal Reflux Disease. Am. J. Gastroenterol. 2021, 116, 1495–1505. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wei, R.; Liu, Z.; Xu, J.; Xu, C.; Chen, J.D. Ameliorating Effects of Transcutaneous Electrical Acustimulation Combined with Deep Breathing Training on Refractory Gastroesophageal Reflux Disease Mediated via the Autonomic Pathway. Neuromodulation Technol. Neural Interface 2019, 22, 751–757. [Google Scholar] [CrossRef]
- Meng, L.N.; Chen, S.; Chen, J.D.; Jin, H.F.; Lu, B. Effects of transcutaneous electrical acustimulation on refractory gastroesophage-al reflux disease. Evid.-Based Complement. Altern. Med. 2016, 2, 8246171. [Google Scholar] [CrossRef]
- Liu, Z.; Lu, D.; Guo, J.; Liu, Y.; Shi, Z.; Xu, F.; Lin, L.; Chen, J.D. Elevation of Lower Esophageal Sphincter Pressure with Acute Transcutaneous Electrical Acustimulation Synchronized with Inspiration. Neuromodulation Technol. Neural Interface 2019, 22, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Song, G.-Q.; Yin, J.; Koothan, T.; Jin, H.; Guo, J.; Liu, J.; Lyu, B.; Foreman, R.D.; Shi, Z.; et al. Electroacupuncture improves impaired gastric motility and slow waves induced by rectal distension in dogs. Am. J. Physiol. Liver Physiol. 2008, 295, G614–G620. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Chen, J.D.Z.; Jin, H.; Liu, J.; Foreman, R.D.; Kuang, J.; Chandalia, M.; Tuvdendorj, D.; Tumurbaatar, B.; Abate, N.; et al. Electroacupuncture improves rectal distension-induced delay in solid gastric emptying in dogs. Am. J. Physiol. Integr. Comp. Physiol. 2011, 301, R465–R472. [Google Scholar] [CrossRef] [PubMed]
- Iwa, M.; Nakade, Y.; Pappas, T.N.; Takahashi, T. Electroacupuncture Elicits Dual Effects: Stimulation of Delayed Gastric Emptying and Inhibition of Accelerated Colonic Transit Induced by Restraint Stress in Rats. Dig. Dis. Sci. 2006, 51, 1493–1500. [Google Scholar] [CrossRef] [PubMed]
- Tabosa, A.; Yamamura, Y.; Forno, E.R.; Mello, L.E.A.M. A comparative study of the effects of electroacupuncture and moxibustion in the gastrointestinal motility of the rat. Dig. Dis. Sci. 2004, 49, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-P.; Kao, C.-H.; Chen, W.-K.; Lo, W.-Y.; Hsieh, C.-L. A Single-Blinded, Randomized Pilot Study Evaluating Effects of Electroacupuncture in Diabetic Patients with Symptoms Suggestive of Gastroparesis. J. Altern. Complement. Med. 2008, 14, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Hou, X.; Zha, H.; Gao, Z.; Zhang, Y.; Chen, J.D.Z. Electroacupuncture Accelerates Solid Gastric Emptying and Improves Dyspeptic Symptoms in Patients with Functional Dyspepsia. Dig. Dis. Sci. 2006, 51, 2154–2159. [Google Scholar] [CrossRef] [PubMed]
- Ji, T.; Li, X.; Lin, L.; Jiang, L.; Wang, M.; Zhou, X.; Zhang, R.; Chen, J.D. An Alternative to Current Therapies of Functional Dyspepsia: Self-Administrated Transcutaneous Electroacupuncture Improves Dyspeptic Symptoms. Evidence-Based Complement. Altern. Med. 2014, 2014, 1–7. [Google Scholar] [CrossRef]
- Cheong, K.B.; Zhang, J.-P.; Huang, Y. The effectiveness of acupuncture in postoperative gastroparesis syndrome—A systematic review and meta-analysis. Complement. Ther. Med. 2014, 22, 767–786. [Google Scholar] [CrossRef]
- Sun, Y.; Song, G.; Yin, J.; Song, J.; Chen, J.D.Z. Effects and mechanisms of electroacupuncture on glucagon-induced small intestinal hypomotility in dogs. Neurogastroenterol. Motil. 2010, 22, 1217-e318. [Google Scholar] [CrossRef] [PubMed]
- Chae, M.; Jung, J.; Seo, M.; Lee, K.; Nam, T.; Yang, I.; Yoon, Y.; Yoon, J. Ultrasonographic observation of intestinal mobility of dogs after acupunctural stimulation on acupoints ST-36 and BL-27. J. Veter- Sci. 2001, 2, 221–226. [Google Scholar] [CrossRef]
- Yuxue, Z.; Changxiang, C.; Qingguang, Q.; Hui, B.; Junhong, G.; Xiaochun, Y.; Bing, Z. Effect of manual acupuncture on bowel mo-tility in normal kunming mouse. J Tradit Chin Med. 2015, 35, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Wang, K.; Gong, M.; Li, Q.; Yu, Z.; Xu, B. Electro-acupuncture at ST37 and ST25 induce different effects on colonic motility via the enteric nervous system by affecting excitatory and inhibitory neurons. Neurogastroenterol. Motil. 2018, 30, e13318. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H.; Ryu, Y.; Hahm, D.H.; Sohn, B.Y.; Shim, I.; Kwon, O.S.; Chang, S.; Gwak, Y.S.; Kim, M.S.; Kim, J.H.; et al. Acupuncture points can be identified as cutaneous neurogenic inflammatory spots. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Huang, Y.-X.; Tian, M.; Gao, W.; Chang, Q. Downregulation of electroacupuncture at ST36 on TNF-α in rats with ulcerative colitis. World J. Gastroenterol. 2003, 9, 1028–1033. [Google Scholar] [CrossRef]
- Goes, A.C.A.D.M.; Pinto, F.M.M.; Fernandes, G.C.; Barbosa, J.S.; Correia, E.S.; Ribeiro, R.A.; Guimaraes, S.B.; Júnior, R.C.P.L.; Brito, G.A.D.C.; Rodrigues, L.V. Electroacupuncture ameliorates experimental colitis induced by TNBS through activation of interleukin-10 and inhibition of iNOS in mice. Acta Cir. Bras. 2014, 29, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Guo, J.; Liu, J.; Lyu, B.; Foreman, R.D.; Yin, J.; Shi, Z.; Chen, J.D.Z. Anti-inflammatory effects and mechanisms of vagal nerve stimulation combined with electroacupuncture in a rodent model of TNBS-induced colitis. Am. J. Physiol. Liver Physiol. 2017, 313, G192–G202. [Google Scholar] [CrossRef] [PubMed]
- Bao, C.-H.; Wu, L.-Y.; Wu, H.-G.; Shi, Y.; Liu, H.-R.; Zhang, R.; Yu, L.-Q.; Wang, J.-H. Moxibustion Inhibits Apoptosis and Tumor Necrosis Factor-Alpha/Tumor Necrosis Factor Receptor 1 in the Colonic Epithelium of Crohn’s Disease Model Rats. Dig. Dis. Sci. 2012, 57, 2286–2295. [Google Scholar] [CrossRef]
- Fass, R.; Zerbib, F.; Gyawali, C.P. AGA Clinical Practice Update on Functional Heartburn: Expert Review. Gastroenterology 2020, 158, 2286–2293. [Google Scholar] [CrossRef]
- De Winter, B.Y.; Deiteren, A.; De Man, J.G. Novel nervous system mechanisms in visceral pain. Neurogastroenterol. Motil. 2016, 28, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Miwa, H. The Role of Esophageal Hypersensitivity in Functional Heartburn. J. Clin. Gastroenterol. 2017, 51, 571–578. [Google Scholar] [CrossRef]
- Gil, D.W.; Wang, J.; Gu, C.; Donello, J.E.; Cabrera, S.; Al-Chaer, E.D. Role of sympathetic nervous system in rat model of chronic visceral pain. Neurogastroenterol. Motil. 2015, 28, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cheng, J.; Zhang, Y.; Chen, J.D.; Selaru, F.M. Electroacupuncture at ST36 Relieves Visceral Hypersensitivity via the NGF/TrkA/TRPV1 Peripheral Afferent Pathway in a Rodent Model of Post-Inflammation Rectal Hypersensitivity. J. Inflamm. Res. 2021, ume 14, 325–339. [Google Scholar] [CrossRef]
- Xu, G.; Winston, J.H.; Chen, J.D.Z. Electroacupuncture attenuates visceral hyperalgesia and inhibits the enhanced excitability of colon specific sensory neurons in a rat model of irritable bowel syndrome. Neurogastroenterol. Motil. 2009, 21, 1302-e125. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Guo, J.; Liu, J.; Lyu, B.; Foreman, R.D.; Shi, Z.; Yin, J.; Chen, J.D.Z. Autonomically mediated anti-inflammatory effects of electrical stimulation at acupoints in a rodent model of colonic inflammation. Neurogastroenterol. Motil. 2019, 31, e13615. [Google Scholar] [CrossRef] [PubMed]
- Murakami, H.; Li, S.; Foreman, R.; Yin, J.; Hirai, T.; Chen, J.D.Z. Ameliorating Effects of Electroacupuncture on Dysmotility, Inflammation, and Pain Mediated via the Autonomic Mechanism in a Rat Model of Postoperative Ileus. J. Neurogastroenterol. Motil. 2019, 25, 286–299. [Google Scholar] [CrossRef]
- Shang, H.-X. Moxibustion combined with acupuncture increases tight junction protein expression in Crohn’s disease patients. World J. Gastroenterol. 2015, 21, 4986–4996. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.-L.; Lin, J.-G.; Li, T.-C.; Chang, Q.-Y. Changes of Pulse Rate and Skin Temperature Evoked by Electroacupuncture Stimulation with Different Frequency on both Zusanli Acupoints in Humans. Am. J. Chin. Med. 1999, 27, 11–18. [Google Scholar] [CrossRef]
- Chou, J.W.; Chang, Y.H.; Chang, C.S.; Chen, G.H. The effect of different frequency electrical acu-stimulation on gastric myoelectri-cal activity in healthy subjects. Hepatogastroenterology 2003, 50, 582–586. [Google Scholar] [PubMed]
- Song, J.; Yin, J.; Sallam, H.; Chen, J. Needleless Transcutaneous Electroacupuncture (TEA) is as Effective as Electroacupuncture (EA) in Improving Rectal Distention-Induced Intestinal Dysmotility in Dogs. Gastroenterology 2011, 140. [Google Scholar] [CrossRef]
- Nishida, A.; Inoue, R.; Inatomi, O.; Bamba, S.; Naito, Y.; Andoh, A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin. J. Gastroenterol. 2017, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Wu, G.D.; Albenberg, L.; Tomov, V.T. Gut microbiota and IBD: Causation or correlation? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 573–584. [Google Scholar] [CrossRef]
- Li, D.; Zhu, L.; Liu, D. Acupuncture for refractory gastro-oesophageal reflux disease: A systematic review and meta-analysis protocol. BMJ Open 2019, 9, e030713. [Google Scholar] [CrossRef]
- Kwon, C.-Y.; Ko, S.-J.; Lee, B.; Cha, J.M.; Yoon, J.Y.; Park, J.-W. Acupuncture as an Add-On Treatment for Functional Dyspepsia: A Systematic Review and Meta-Analysis. Front. Med. 2021, 8, 682783. [Google Scholar] [CrossRef]
- Liu, S.; Peng, S.; Hou, X.; Ke, M.; Chen, J.D.Z. Transcutaneous electroacupuncture improves dyspeptic symptoms and increases high frequency heart rate variability in patients with functional dyspepsia. Neurogastroenterol. Motil. 2008, 20, 1204–1211. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Hu, P.; Zhang, B.; Xu, F.; Yin, J.; Yang, X.; Lin, L.; Chen, J.D.Z. Transcutaneous electrical acustimulation synchronized with inspiration improves gastric accommodation impaired by cold stress in healthy subjects. Neurogastroenterol. Motil. 2018, 31, e13491. [Google Scholar] [CrossRef] [PubMed]
- Jie, L.; Shiping, L.; Yue, X.; Fuli, Z. Efficacy and safety of electroacupuncture for secondary constipation: A systematic review and meta-analysis. Int. J. Color. Dis. 2023, 38, 1–13. [Google Scholar] [CrossRef]
- Tong, Y.M.; Yu, Y.M.; Yin, S.B.; Lin, S.M.; Chen, Y.M.; Su, X.M. Efficacy and safety of acupoint application in the treatment of ulcerative colitis: A systematic review and meta-analysis. Medicine 2023, 102, e34489. [Google Scholar] [CrossRef]
- Joos, S.; Brinkhaus, B.; Maluche, C.; Maupai, N.; Kohnen, R.; Kraehmer, N.; Hahn, E.G.; Schuppan, D. Acupuncture and Moxibustion in the Treatment of Active Crohn’s Disease: A Randomized Controlled Study. Digestion 2004, 69, 131–139. [Google Scholar] [CrossRef]
- Bao, C.-H.; Zhao, J.-M.; Liu, H.-R.; Lu, Y.; Zhu, Y.-F.; Shi, Y.; Weng, Z.-J.; Feng, H.; Guan, X.; Li, J.; et al. Randomized controlled trial: Moxibustion and acupuncture for the treatment of Crohn’s disease. World J. Gastroenterol. 2014, 20, 11000–11011. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, G.; Sclocco, R.; Sharma, A.; Guerrero-López, I.; Kuo, B. Electroceuticals and Magnetoceuticals in Gastroenterology. Biomolecules 2024, 14, 760. https://doi.org/10.3390/biom14070760
Song G, Sclocco R, Sharma A, Guerrero-López I, Kuo B. Electroceuticals and Magnetoceuticals in Gastroenterology. Biomolecules. 2024; 14(7):760. https://doi.org/10.3390/biom14070760
Chicago/Turabian StyleSong, Gengqing, Roberta Sclocco, Amol Sharma, Ingrid Guerrero-López, and Braden Kuo. 2024. "Electroceuticals and Magnetoceuticals in Gastroenterology" Biomolecules 14, no. 7: 760. https://doi.org/10.3390/biom14070760
APA StyleSong, G., Sclocco, R., Sharma, A., Guerrero-López, I., & Kuo, B. (2024). Electroceuticals and Magnetoceuticals in Gastroenterology. Biomolecules, 14(7), 760. https://doi.org/10.3390/biom14070760





