Agro-Industrial By-Products of Plant Origin: Therapeutic Uses as well as Antimicrobial and Antioxidant Activity
Abstract
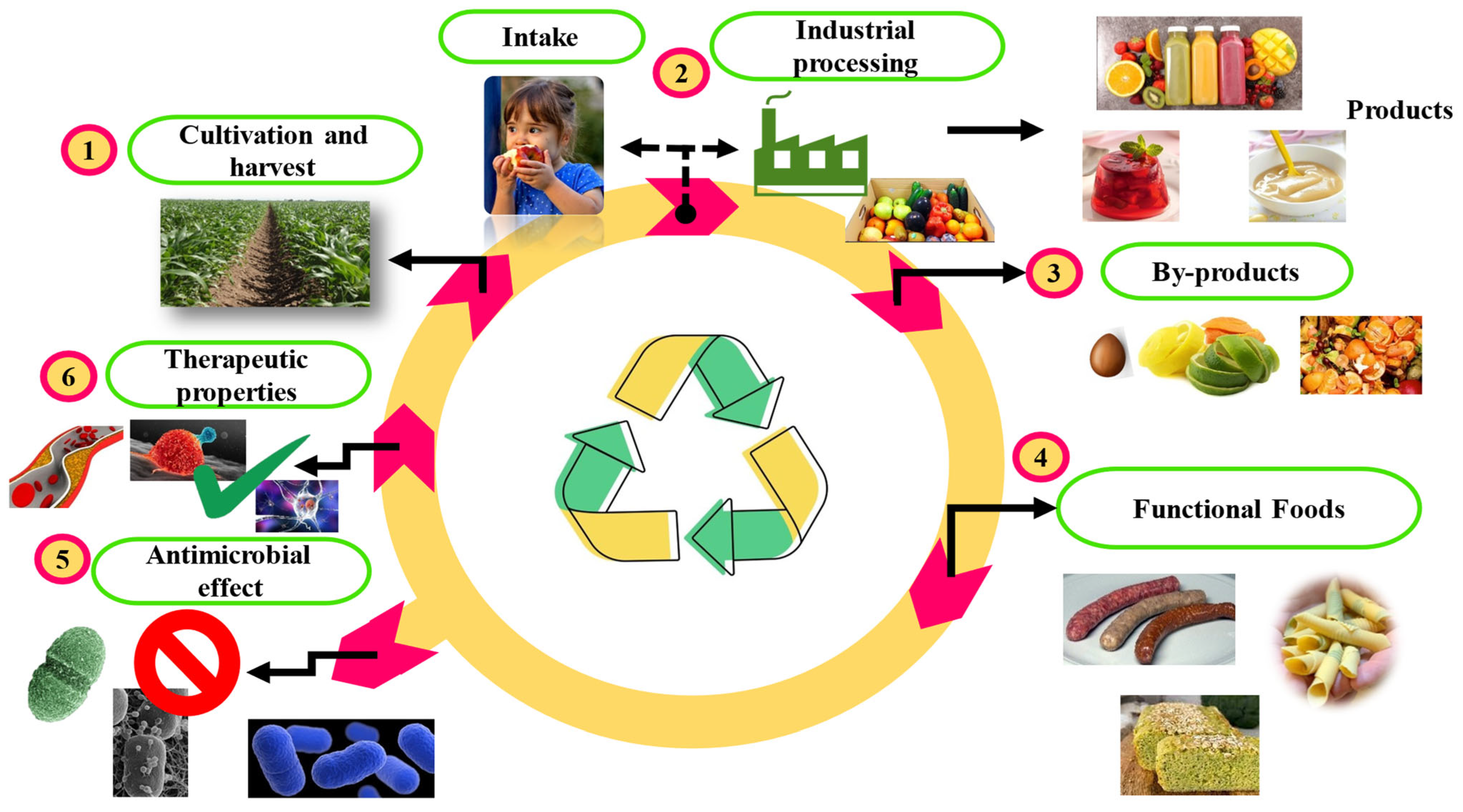
:1. Introduction
2. Bioactive Compounds in Agro-Industrial By-Products
3. Antimicrobial Action of Agro-Industrial By-Products of Plant Origin
4. Antioxidant Activity of Agro-Industrial By-Products of Plant Origin
5. Therapeutic Uses of Bioactive Compounds Present in Agro-Industrial By-Products of Plant Origin
5.1. Dyslipidemia
5.2. Diabetes
5.3. Cancer
5.4. Neuroprotective Effect
6. Trends in Food and Sustainability
7. Future Prospects and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rațu, R.N.; Veleșcu, I.D.; Stoica, F.; Usturoi, A.; Arsenoaia, V.N.; Crivei, I.C.; Postolache, A.N.; Lipșa, F.D.; Filipov, F.; Florea, A.M.; et al. Application of agri-food by-products in the food industry. Agriculture 2023, 13, 1559. [Google Scholar] [CrossRef]
- Ravindran, R.; Hassan, S.; Williams, G.; Jaiswal, A. A Review on bioconversion of agro-industrial wastes to industrially important enzymes. Bioengineering 2018, 5, 93. [Google Scholar] [CrossRef] [PubMed]
- Villamil-Galindo, E.; Van de Velde, F.; Piagentini, A.M. Strawberry agro-industrial by-products as a source of bioactive compounds: Effect of cultivar on the phenolic profile and the antioxidant capacity. Bioresour. Bioprocess. 2021, 8, 61. [Google Scholar] [CrossRef] [PubMed]
- Sadh, P.K.; Duhan, S.; Duhan, J.S. Agro-industrial wastes and their utilization using solid state fermentation: A review. Bioresour. Bioprocess. 2018, 5, 1. [Google Scholar] [CrossRef]
- Freitas, L.C.; Barbosa, J.R.; da Costa, A.L.C.; Bezerra, F.W.F.; Pinto, R.H.H.; de Carvalho Junior, R.N. From waste to sustainable industry: How can agro-industrial wastes help in the development of new products? Resour. Conserv. Recycl. 2021, 169, 105466. [Google Scholar] [CrossRef]
- Coman, V.; Teleky, B.-E.; Mitrea, L.; Martău, G.A.; Szabo, K.; Călinoiu, L.-F.; Vodnar, D.C. Bioactive potential of fruit and vegetable wastes. Adv. Food Nutr. Res. 2020, 91, 157–225. [Google Scholar] [PubMed]
- Lopes, F.C.; Ligabue-Braun, R. Agro-industrial residues: Eco-friendly and inexpensive substrates for microbial pigments production. Front. Sustain. Food Syst. 2021, 5, 589414. [Google Scholar] [CrossRef]
- Lemes, A.C.; Egea, M.B.; de Oliveira Filho, J.G.; Gautério, G.V.; Ribeiro, B.D.; Coelho, M.A.Z. Biological approaches for extraction of bioactive compounds from agro-industrial by-products: A review. Front. Bioeng. Biotechnol. 2022, 9, 802543. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, R.M.; Pimentel, T.C.; de Rezende, T.A.M.; Silva, J.D.S.; Falcão, H.G.; Ida, E.I.; Egea, M.B. Gluten-free bread: Effect of soy and corn co-products on the quality parameters. Eur. Food Res. Technol. 2019, 245, 1365–1376. [Google Scholar] [CrossRef]
- Guil-Guerrero, J.L.; Ramos, L.; Moreno, C.; Zúñiga-Paredes, J.C.; Carlosama-Yepez, M.; Ruales, P. Antimicrobial activity of plant-food by-products: A review focusing on the tropics. Livest. Sci. 2016, 189, 32–49. [Google Scholar] [CrossRef]
- Socas-Rodríguez, B.; Álvarez-Rivera, G.; Valdés, A.; Ibáñez, E.; Cifuentes, A. Food by-products and food wastes: Are they safe enough for their valorization? Trends Food Sci. Technol. 2021, 114, 133–147. [Google Scholar] [CrossRef]
- Borrajo, P.; Pateiro, M.; Barba, F.J.; Mora, L.; Franco, D.; Toldrá, F.; Lorenzo, J.M. Antioxidant and antimicrobial activity of peptides extracted from meat by-products: A review. Food Anal. Methods 2019, 12, 2401–2415. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Neveu, V.; Vos, F.; Scalbert, A. Identification of the 100 richest dietary sources of polyphenols: An application of the phenol-Explorer database. Eur. J. Clin. Nutr. 2010, 64, S112–S120. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-W.; Cheng, M.-C.; Chen, B.-Y.; Wang, C.-Y. Effects of high pressure extraction on the extraction yield, phenolic compounds, antioxidant and anti-tyrosinase activity of Djulis hull. J. Food Sci. Technol. 2019, 56, 4016–4024. [Google Scholar] [CrossRef]
- Tanase, C.; Coșarcă, S.; Muntean, D.L. A Critical review of phenolic compounds extracted from the bark of woody vascular plants and their potential biological activity. Molecules 2019, 24, 1182. [Google Scholar] [CrossRef] [PubMed]
- Dzah, C.S.; Duan, Y.; Zhang, H.; Serwah Boateng, N.A.; Ma, H. Latest developments in polyphenol recovery and purification from plant by-products: A review. Trends Food Sci. Technol. 2020, 99, 375–388. [Google Scholar] [CrossRef]
- Llerena, W.; Samaniego, I.; Vallejo, C.; Arreaga, A.; Zhunio, B.; Coronel, Z.; Quiroz, J.; Angós, I.; Carrillo, W. Profile of bioactive components of cocoa (Theobroma cacao L.) by-products from Ecuador and evaluation of their antioxidant activity. Foods 2023, 12, 2583. [Google Scholar] [CrossRef] [PubMed]
- Abbasi-Parizad, P.; De Nisi, P.; Scaglia, B.; Scarafoni, A.; Pilu, S.; Adani, F. Recovery of phenolic compounds from agro-industrial by-products: Evaluating antiradical activities and immunomodulatory properties. Food Bioprod. Process. 2021, 127, 338–348. [Google Scholar] [CrossRef]
- Fernández-Prior, Á.; Bermúdez-Oria, A.; Fernández-Bolaños, J.; Espejo-Calvo, J.A.; López-Maestro, F.; Rodríguez-Gutiérrez, G. Evolution of hydroxytyrosol, hydroxytyrosol 4-β-d-glucoside, 3,4-dihydroxyphenylglycol and tyrosol in olive oil solid waste or “Alperujo”. Molecules 2022, 27, 8380. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Prior, Á.; Bermúdez-Oria, A.; Millán-Linares, M.d.C.; Fernández-Bolaños, J.; Espejo-Calvo, J.A.; Rodríguez-Gutiérrez, G. Anti-inflammatory and antioxidant activity of hydroxytyrosol and 3,4-dihydroxyphenyglycol purified from table olive effluents. Foods 2021, 10, 227. [Google Scholar] [CrossRef] [PubMed]
- Toledo-Merma, P.R.; Cornejo-Figueroa, M.H.; Crisosto-Fuster, A.d.R.; Strieder, M.M.; Chañi-Paucar, L.O.; Náthia-Neves, G.; Rodríguez-Papuico, H.; Rostagno, M.A.; Meireles, M.A.A.; Alcázar-Alay, S.C. Phenolic compounds recovery from pomegranate (Punica granatum L.) by-products of pressurized liquid extraction. Foods 2022, 11, 1070. [Google Scholar] [CrossRef] [PubMed]
- Vella, F.M.; Cautela, D.; Laratta, B. Characterization of polyphenolic compounds in Cantaloupe melon by-products. Foods 2019, 8, 196. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.M.; Santos, L. Incorporation of phenolic extracts from different by-products in yoghurts to create fortified and sustainable foods. Food Biosci. 2023, 51, 102293. [Google Scholar] [CrossRef]
- Rodriguez-Lopez, A.D.; Melgar, B.; Conidi, C.; Barros, L.; Ferreira, I.C.F.R.; Cassano, A.; Garcia-Castello, E.M. Food industry by-products valorization and new ingredients. In Sustainability of the Food System; Elsevier: Amsterdam, The Netherlands, 2020; pp. 71–99. [Google Scholar]
- Rao, A.; Rao, L. Carotenoids and human health. Pharmacol. Res. 2007, 55, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Gea-Botella, S.; Agulló, L.; Martí, N.; Martínez-Madrid, M.C.; Lizama, V.; Martín-Bermudo, F.; Berná, G.; Saura, D.; Valero, M. Carotenoids from persimmon juice processing. Food Res. Int. 2021, 141, 109882. [Google Scholar] [CrossRef] [PubMed]
- Lara-Abia, S.; Welti-Chanes, J.; Cano, M.P. Effect of ultrasound-assisted extraction of carotenoids from papaya (Carica papaya L. cv. Sweet Mary) Using Vegetable Oils. Molecules 2022, 27, 638. [Google Scholar] [CrossRef] [PubMed]
- Szabo, K.; Emőke Teleky, B.; Ranga, F.; Simon, E.; Lelia Pop, O.; Babalau-Fuss, V.; Kapsalis, N.; Cristian Vodnar, D. Bioaccessibility of microencapsulated carotenoids, recovered from tomato processing industrial by-products, using in vitro digestion model. LWT 2021, 152, 112285. [Google Scholar] [CrossRef]
- da Silva Lima, R.; Nunes, I.L.; Block, J.M. Ultrasound-assisted extraction for the recovery of carotenoids from Guava’s pulp and waste powders. Plant Foods Hum. Nutr. 2020, 75, 63–69. [Google Scholar] [CrossRef] [PubMed]
- da Silva, A.C.; Jorge, N. Bioactive compounds of the lipid fractions of agro-industrial waste. Food Res. Int. 2014, 66, 493–500. [Google Scholar] [CrossRef]
- Mallek-Ayadi, S.; Bahloul, N.; Kechaou, N. Characterization, phenolic compounds and functional properties of Cucumis melo L. peels. Food Chem. 2017, 221, 1691–1697. [Google Scholar] [CrossRef] [PubMed]
- Barros, R.G.C.; Andrade, J.K.S.; Denadai, M.; Nunes, M.L.; Narain, N. Evaluation of bioactive compounds potential and antioxidant activity in some Brazilian exotic fruit residues. Food Res. Int. 2017, 102, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Garzón, G.A.; Narváez-Cuenca, C.-E.; Vincken, J.-P.; Gruppen, H. Polyphenolic composition and antioxidant activity of açai (Euterpe oleracea Mart.) from Colombia. Food Chem. 2017, 217, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Xi, W.; Zhang, G.; Jiang, D.; Zhou, Z. Phenolic compositions and antioxidant activities of grapefruit (Citrus paradisi Macfadyen) varieties cultivated in China. Int. J. Food Sci. Nutr. 2015, 66, 858–866. [Google Scholar] [CrossRef]
- Tremocoldi, M.A.; Rosalen, P.L.; Franchin, M.; Massarioli, A.P.; Denny, C.; Daiuto, É.R.; Paschoal, J.A.R.; Melo, P.S.; de Alencar, S.M. Exploration of avocado by-products as natural sources of bioactive compounds. PLoS ONE 2018, 13, e0192577. [Google Scholar] [CrossRef] [PubMed]
- da Silva, L.M.R.; de Figueiredo, E.A.T.; Ricardo, N.M.P.S.; Vieira, I.G.P.; de Figueiredo, R.W.; Brasil, I.M.; Gomes, C.L. Quantification of bioactive compounds in pulps and by-products of tropical fruits from Brazil. Food Chem. 2014, 143, 398–404. [Google Scholar] [CrossRef]
- Morais, D.R.; Rotta, E.M.; Sargi, S.C.; Schmidt, E.M.; Bonafe, E.G.; Eberlin, M.N.; Sawaya, A.C.H.F.; Visentainer, J.V. Antioxidant activity, phenolics and UPLC–ESI(–)–MS of extracts from different tropical fruits parts and processed peels. Food Res. Int. 2015, 77, 392–399. [Google Scholar] [CrossRef]
- Tlais, A.Z.A.; Fiorino, G.M.; Polo, A.; Filannino, P.; Di Cagno, R. High-value compounds in fruit, vegetable and cereal byproducts: An overview of potential sustainable reuse and exploitation. Molecules 2020, 25, 2987. [Google Scholar] [CrossRef] [PubMed]
- Donadio, G.; Mensitieri, F.; Santoro, V.; Parisi, V.; Bellone, M.L.; De Tommasi, N.; Izzo, V.; Dal Piaz, F. Interactions with microbial proteins driving the antibacterial activity of flavonoids. Pharmaceutics 2021, 13, 660. [Google Scholar] [CrossRef]
- López-García, G.; Dublan-García, O.; Arizmendi-Cotero, D.; Gómez Oliván, L.M. Antioxidant and antimicrobial peptides derived from food proteins. Molecules 2022, 27, 1343. [Google Scholar] [CrossRef]
- Hajam, Y.A.; Lone, R.; Kumar, R. Role of plant phenolics against Reactive Oxygen Species (ROS) induced oxidative stress and biochemical alterations. In Plant Phenolics in Abiotic Stress Management; Springer: Singapore, 2023; pp. 125–147. [Google Scholar]
- Giordano, M.; Pinela, J.; Dias, M.I.; Calhelha, R.C.; Stojković, D.; Soković, M.; Tavares, D.; Cánepa, A.L.; Ferreira, I.C.F.R.; Caleja, C.; et al. Ultrasound-assisted extraction of flavonoids from kiwi peel: Process optimization and bioactivity assessment. Appl. Sci. 2021, 11, 6416. [Google Scholar] [CrossRef]
- Archundia Velarde, E.D.; Pinzón Martínez, D.L.; Salem, A.Z.M.; Mendoza García, P.G.; Mariezcurrena Berasain, M.D. Antioxidant and antimicrobial capacity of three agroindustrial residues as animal feeds. Agrofor. Syst. 2020, 94, 1393–1402. [Google Scholar] [CrossRef]
- Alexandre, E.M.C.; Silva, S.; Santos, S.A.O.; Silvestre, A.J.D.; Duarte, M.F.; Saraiva, J.A.; Pintado, M. Antimicrobial activity of pomegranate peel extracts performed by high pressure and enzymatic assisted extraction. Food Res. Int. 2019, 115, 167–176. [Google Scholar] [CrossRef]
- Lou, Z.; Wang, H.; Zhu, S.; Ma, C.; Wang, Z. Antibacterial activity and mechanism of action of chlorogenic acid. J. Food Sci. 2011, 76, M398–M403. [Google Scholar] [CrossRef]
- Silva-Beltrán, N.P.; Ruiz-Cruz, S.; Cira-Chávez, L.A.; Estrada-Alvarado, M.I.; Ornelas-Paz, J.d.J.; López-Mata, M.A.; Del-Toro-Sánchez, C.L.; Ayala-Zavala, J.F.; Márquez-Ríos, E. Total Phenolic, flavonoid, tomatine, and tomatidine contents and antioxidant and antimicrobial activities of extracts of tomato plant. Int. J. Anal. Chem. 2015, 2015, 284071. [Google Scholar] [CrossRef]
- Vodnar, D.C.; Călinoiu, L.F.; Dulf, F.V.; Ştefănescu, B.E.; Crişan, G.; Socaciu, C. Identification of the bioactive compounds and antioxidant, antimutagenic and antimicrobial activities of thermally processed agro-industrial waste. Food Chem. 2017, 231, 131–140. [Google Scholar] [CrossRef]
- Mejri, F.; Baati, T.; Martins, A.; Selmi, S.; Luisa Serralheiro, M.; Falé, P.L.; Rauter, A.; Casabianca, H.; Hosni, K. Phytochemical analysis and in vitro and in vivo evaluation of biological activities of artichoke (Cynara scolymus L.) floral stems: Towards the valorization of food by-products. Food Chem. 2020, 333, 127506. [Google Scholar] [CrossRef]
- Albuquerque, B.R.; Pereira, C.; Calhelha, R.C.; José Alves, M.; Abreu, R.M.V.; Barros, L.; Oliveira, M.B.P.P.; Ferreira, I.C.F.R. Jabuticaba residues (Myrciaria jaboticaba (Vell.) Berg) are rich sources of valuable compounds with bioactive properties. Food Chem. 2020, 309, 125735. [Google Scholar] [CrossRef]
- Gullón, B.; Gullón, P.; Eibes, G.; Cara, C.; De Torres, A.; López-Linares, J.C.; Ruiz, E.; Castro, E. Valorisation of olive agro-industrial by-products as a source of bioactive compounds. Sci. Total Environ. 2018, 645, 533–542. [Google Scholar] [CrossRef]
- Nazeam, J.A.; AL-Shareef, W.A.; Helmy, M.W.; El-Haddad, A.E. Bioassay-guided isolation of potential bioactive constituents from pomegranate agrifood by-product. Food Chem. 2020, 326, 126993. [Google Scholar] [CrossRef]
- Sorrenti, V.; Burò, I.; Consoli, V.; Vanella, L. Recent Advances in health benefits of bioactive compounds from food wastes and by-products: Biochemical aspects. Int. J. Mol. Sci. 2023, 24, 2019. [Google Scholar] [CrossRef]
- Aguiar, S.; Enríquez Estrella, M.; Uvidia Cabadiana, H. Residuos agroindustriales: Su impacto, manejo y aprovechamiento. Axioma 2022, 1, 5–11. [Google Scholar] [CrossRef]
- Ueda, J.M.; Pedrosa, M.C.; Heleno, S.A.; Carocho, M.; Ferreira, I.C.F.R.; Barros, L. Food additives from fruit and vegetable by-products and bio-residues: A comprehensive review focused on sustainability. Sustainability 2022, 14, 5212. [Google Scholar] [CrossRef]
- Soares Gettens, C.; Heberle, T.; Carbonera, N.; Avila Gandra, E.; Machado Pereira, A.; Arocha Gularte, M. Antimicrobial potential and chemical and bioactive compounds in agroindustrial by-products from peach. In Ciência e Tecnologia de Alimentos: O Avanço da Ciência no Brasil—Volume 3; Editora Científica Digital Brasil: Sao Paulo, Brazil, 2023; pp. 71–83. [Google Scholar]
- Sandoval-Cárdenas, D.I.; Feregrino-Pérez, A.A.; Favela-Camacho, S.E.; Arredondo-Ochoa, T.; Escamilla-García, M.; Regalado, C.; Amaro-Reyes, A. Potential antioxidant activity of multi enzymatically hydrolyzed corncob. Biologia 2022, 77, 803–813. [Google Scholar] [CrossRef]
- Suleria, H.A.R.; Barrow, C.J.; Dunshea, F.R. Screening and characterization of phenolic compounds and their antioxidant capacity in different fruit peels. Foods 2020, 9, 1206. [Google Scholar] [CrossRef]
- Gualberto, N.C.; de Oliveira, C.S.; Nogueira, J.P.; de Jesus, M.S.; Araujo, H.C.S.; Rajan, M.; Neta, M.T.S.L.; Narain, N. Bioactive compounds and antioxidant activities in the agro-industrial residues of acerola (Malpighia emarginata L.), guava (Psidium guajava L.), genipap (Genipa americana L.) and umbu (Spondias tuberosa L.) fruits assisted by ultrasonic or shaker extracti. Food Res. Int. 2021, 147, 110538. [Google Scholar] [CrossRef]
- Rodrigues, C.; Silva, V.; Loyola, A.; Silva, M.; Augusti, R.; Melo, J.; Carlos, L.; Fante, C. Characterization and identification of bioactive compounds in agro-food waste flours. Quim. Nova 2021, 45, 403–409. [Google Scholar] [CrossRef]
- Melo, P.S.; Selani, M.M.; Gonçalves, R.H.; Paulino, J.D.O.; Massarioli, A.P.; de Alencar, S.M. Açaí seeds: An unexplored agro-industrial residue as a potential source of lipids, fibers, and antioxidant phenolic compounds. Ind. Crops Prod. 2021, 161, 113204. [Google Scholar] [CrossRef]
- Da Costa, R.S.; Dos Santos, O.V.; Lannes, S.C.D.S.; Casazza, A.A.; Aliakbarian, B.; Perego, P.; Ribeiro-Costa, R.M.; Converti, A.; Silva Júnior, J.O.C. Bioactive compounds and value-added applications of cupuassu (Theobroma grandiflorum Schum.) agroindustrial by-product. Food Sci. Technol. 2020, 40, 401–407. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Sicari, V.; Pellicanò, T.; Xiao, J.; Poiana, M.; Tundis, R. Comparative analysis of chemical composition, antioxidant and anti-proliferative activities of Italian Vitis vinifera by-products for a sustainable agro-industry. Food Chem. Toxicol. 2019, 127, 127–134. [Google Scholar] [CrossRef]
- Zuñiga-Martínez, B.S.; Domínguez-Avila, J.A.; Wall-Medrano, A.; Ayala-Zavala, J.F.; Hernández-Paredes, J.; Salazar-López, N.J.; Villegas-Ochoa, M.A.; González-Aguilar, G.A. Avocado paste from industrial byproducts as an unconventional source of bioactive compounds: Characterization, in vitro digestion and in silico interactions of its main phenolics with cholesterol. J. Food Meas. Charact. 2021, 15, 5460–5476. [Google Scholar] [CrossRef]
- Antonic, B.; Dordevic, D.; Jancikova, S.; Holeckova, D.; Tremlova, B.; Kulawik, P. Effect of grape seed flour on the antioxidant profile, textural and sensory properties of waffles. Processes 2021, 9, 131. [Google Scholar] [CrossRef]
- Mandha, J.; Shumoy, H.; Matemu, A.O.; Raes, K. Valorization of mango by-products to enhance the nutritional content of maize complementary porridges. Foods 2021, 10, 1635. [Google Scholar] [CrossRef]
- Joymak, W.; Ngamukote, S.; Chantarasinlapin, P.; Adisakwattana, S. Unripe papaya by-product: From food wastes to functional ingredients in pancakes. Foods 2021, 10, 615. [Google Scholar] [CrossRef]
- Lasunon, P.; Phonkerd, N.; Tettawong, P.; Sengkhamparn, N. Total phenolic compound and its antioxidant activity of by-product from pineapple. Food Res. 2022, 6, 107–112. [Google Scholar] [CrossRef]
- Tumbas Šaponjac, V.; Gironés-Vilaplana, A.; Djilas, S.; Mena, P.; Ćetković, G.; Moreno, D.A.; Čanadanović-Brunet, J.; Vulić, J.; Stajčić, S.; Vinčić, M. Chemical composition and potential bioactivity of strawberry pomace. RSC Adv. 2015, 5, 5397–5405. [Google Scholar] [CrossRef]
- Suárez, B.; Álvarez, Á.L.; García, Y.D.; del Barrio, G.; Lobo, A.P.; Parra, F. Phenolic profiles, antioxidant activity and in vitro antiviral properties of apple pomace. Food Chem. 2010, 120, 339–342. [Google Scholar] [CrossRef]
- Duangjai, A.; Suphrom, N.; Wungrath, J.; Ontawong, A.; Nuengchamnong, N.; Yosboonruang, A. Comparison of antioxidant, antimicrobial activities and chemical profiles of three coffee (Coffea arabica L.) pulp aqueous extracts. Integr. Med. Res. 2016, 5, 324–331. [Google Scholar] [CrossRef]
- Barbosa, P.d.P.M.; Ruviaro, A.R.; Macedo, G.A. Comparison of different Brazilian citrus by-products as source of natural antioxidants. Food Sci. Biotechnol. 2018, 27, 1301–1309. [Google Scholar] [CrossRef]
- Oszmiański, J.; Wojdylo, A. Aronia melanocarpa phenolics and their antioxidant activity. Eur. Food Res. Technol. 2005, 221, 809–813. [Google Scholar] [CrossRef]
- Ghasemi, F.; Navab, F.; Rouhani, M.H.; Amini, P.; Shokri-Mashhadi, N. The effect of lutein and Zeaxanthine on dyslipidemia: A meta-analysis study. Prostaglandins Other Lipid Mediat. 2023, 164, 106691. [Google Scholar] [CrossRef]
- Ahmad, R.S.; Butt, M.S.; Sultan, M.T.; Mushtaq, Z.; Ahmad, S.; Dewanjee, S.; De Feo, V.; Zia-Ul-Haq, M. Preventive role of green tea catechins from obesity and related disorders especially hypercholesterolemia and hyperglycemia. J. Transl. Med. 2015, 13, 79. [Google Scholar] [CrossRef]
- Chao, J.; Cheng, H.-Y.; Chang, M.-L.; Huang, S.-S.; Liao, J.-W.; Cheng, Y.-C.; Peng, W.-H.; Pao, L.-H. Gallic acid ameliorated impaired lipid homeostasis in a mouse model of high-fat diet—And streptozotocin-induced NAFLD and diabetes through improvement of β-oxidation and ketogenesis. Front. Pharmacol. 2021, 11, 606759. [Google Scholar] [CrossRef]
- Salamat, S.; Sharif, S.S.; Nazary-Vanani, A.; Kord-Varkaneh, H.; Clark, C.C.T.; Mohammadshahi, M. The effect of green coffee extract supplementation on serum oxidized LDL cholesterol and total antioxidant capacity in patients with dyslipidemia: A randomized, double-blind, placebo-controlled trial. Eur. J. Integr. Med. 2019, 28, 109–113. [Google Scholar] [CrossRef]
- Luo, Z.; Li, M.; Yang, J.; Li, J.; Zhang, Y.; Liu, F.; El-Omar, E.; Han, L.; Bian, J.; Gong, L.; et al. Ferulic acid attenuates high-fat diet-induced hypercholesterolemia by activating classic bile acid synthesis pathway. Front. Nutr. 2022, 9, 976638. [Google Scholar] [CrossRef]
- Wang, K.; Liang, C.; Cao, W.; Luo, G.; Zhong, S.; Zeng, Z.; Dai, L.; Song, J. Dietary sinapic acid attenuated high-fat diet-induced lipid metabolism and oxidative stress in male Syrian hamsters. J. Food Biochem. 2022, 46, e14203. [Google Scholar] [CrossRef]
- Demir, Y.; Durmaz, L.; Taslimi, P.; Gulçin, İ. Antidiabetic properties of dietary phenolic compounds: Inhibition effects on α-amylase, aldose reductase, and α-glycosidase. Biotechnol. Appl. Biochem. 2019, 66, 781–786. [Google Scholar] [CrossRef]
- Martin, M.A.; Goya, L.; Ramos, S. Protective effects of tea, red wine and cocoa in diabetes. Evidences from human studies. Food Chem. Toxicol. 2017, 109, 302–314. [Google Scholar] [CrossRef]
- Rahimifard, M.; Baeeri, M.; Bahadar, H.; Moini-Nodeh, S.; Khalid, M.; Haghi-Aminjan, H.; Mohammadian, H.; Abdollahi, M. Therapeutic effects of gallic acid in regulating senescence and diabetes; an in vitro study. Molecules 2020, 25, 5875. [Google Scholar] [CrossRef]
- Oršolić, N.; Sirovina, D.; Odeh, D.; Gajski, G.; Balta, V.; Šver, L.; Jazvinšćak Jembrek, M. Efficacy of caffeic acid on diabetes and its complications in the mouse. Molecules 2021, 26, 3262. [Google Scholar] [CrossRef]
- Chowdhury, S.; Ghosh, S.; Das, A.K.; Sil, P.C. Ferulic acid protects hyperglycemia-induced kidney damage by regulating oxidative insult, inflammation and autophagy. Front. Pharmacol. 2019, 10, 27. [Google Scholar] [CrossRef]
- Mani, A.; Kushwaha, K.; Khurana, N.; Gupta, J. p-Coumaric acid attenuates high-fat diet-induced oxidative stress and nephropathy in diabetic rats. J. Anim. Physiol. Anim. Nutr. 2022, 106, 872–880. [Google Scholar] [CrossRef]
- Altındağ, F.; Rağbetli, M.Ç.; Özdek, U.; Koyun, N.; Ismael Alhalboosi, J.K.; Elasan, S. Combined treatment of sinapic acid and ellagic acid attenuates hyperglycemia in streptozotocin-induced diabetic rats. Food Chem. Toxicol. 2021, 156, 112443. [Google Scholar] [CrossRef]
- Rašković, A.; Ćućuz, V.; Torović, L.; Tomas, A.; Gojković-Bukarica, L.; Ćebović, T.; Milijašević, B.; Stilinović, N.; Cvejić Hogervorst, J. Resveratrol supplementation improves metabolic control in rats with induced hyperlipidemia and type 2 diabetes. Saudi Pharm. J. 2019, 27, 1036–1043. [Google Scholar] [CrossRef]
- Neuhouser, M.L. Review: Dietary flavonoids and cancer risk: Evidence from human population studies. Nutr. Cancer 2004, 50, 1–7. [Google Scholar] [CrossRef]
- Bondonno, N.P.; Dalgaard, F.; Kyrø, C.; Murray, K.; Bondonno, C.P.; Lewis, J.R.; Croft, K.D.; Gislason, G.; Scalbert, A.; Cassidy, A.; et al. Flavonoid intake is associated with lower mortality in the Danish diet cancer and health cohort. Nat. Commun. 2019, 10, 3651. [Google Scholar] [CrossRef]
- Moccia, S.; Russo, M.; Durante, M.; Lenucci, M.S.; Mita, G.; Russo, G.L. A carotenoid-enriched extract from pumpkin delays cell proliferation in a human chronic lymphocytic leukemia cell line through the modulation of autophagic flux. Curr. Res. Biotechnol. 2020, 2, 74–82. [Google Scholar] [CrossRef]
- Alazzouni, A.S.; Dkhil, M.A.; Gadelmawla, M.H.A.; Gabri, M.S.; Farag, A.H.; Hassan, B.N. Ferulic acid as anticarcinogenic agent against 1,2-dimethylhydrazine induced colon cancer in rats. J. King Saud Univ. Sci. 2021, 33, 101354. [Google Scholar] [CrossRef]
- Sawata, Y.; Matsukawa, T.; Doi, S.; Tsunoda, T.; Arikawa, N.; Matsunaga, N.; Ohnuki, K.; Shirasawa, S.; Kotake, Y. A novel compound, ferulic acid-bound resveratrol, induces the tumor suppressor gene p15 and inhibits the three-dimensional proliferation of colorectal cancer cells. Mol. Cell. Biochem. 2019, 462, 25–31. [Google Scholar] [CrossRef]
- Jang, M.G.; Ko, H.C.; Kim, S.-J. Effects of p-coumaric acid on microRNA expression profiles in SNU-16 human gastric cancer cells. Genes Genom. 2020, 42, 817–825. [Google Scholar] [CrossRef]
- Mohd Sairazi, N.S.; Sirajudeen, K.N.S. Natural products and their bioactive compounds: Neuroprotective potentials against neurodegenerative diseases. Evid. Based Complement. Altern. Med. 2020, 2020, 6565396. [Google Scholar] [CrossRef]
- Lamport, D.J.; Pal, D.; Macready, A.L.; Barbosa-Boucas, S.; Fletcher, J.M.; Williams, C.M.; Spencer, J.P.E.; Butler, L.T. The effects of flavanone-rich citrus juice on cognitive function and cerebral blood flow: An acute, randomised, placebo-controlled cross-over trial in healthy, young adults. Br. J. Nutr. 2016, 116, 2160–2168. [Google Scholar] [CrossRef]
- Baek, S.Y.; Kim, M.R. Neuroprotective Effect of carotenoid-rich enteromorpha prolifera extract via TrkB/Akt pathway against oxidative stress in hippocampal neuronal cells. Mar. Drugs 2020, 18, 372. [Google Scholar] [CrossRef]
- Nakayama, H.; Nakahara, M.; Matsugi, E.; Soda, M.; Hattori, T.; Hara, K.; Usami, A.; Kusumoto, C.; Higashiyama, S.; Kitaichi, K. Protective Effect of ferulic acid against hydrogen peroxide induced apoptosis in PC12 cells. Molecules 2020, 26, 90. [Google Scholar] [CrossRef]
- Dou, Z.; Rong, X.; Zhao, E.; Zhang, L.; Lv, Y. Neuroprotection of resveratrol against focal cerebral ischemia/reperfusion injury in mice through a mechanism targeting gut-brain axis. Cell. Mol. Neurobiol. 2019, 39, 883–898. [Google Scholar] [CrossRef]
- Colonnello, A.; Aguilera-Portillo, G.; Rubio-López, L.C.; Robles-Bañuelos, B.; Rangel-López, E.; Cortez-Núñez, S.; Evaristo-Priego, Y.; Silva-Palacios, A.; Galván-Arzate, S.; García-Contreras, R.; et al. Comparing the neuroprotective effects of caffeic acid in rat cortical slices and caenorhabditis elegans: Involvement of Nrf2 and SKN-1 signaling pathways. Neurotox. Res. 2020, 37, 326–337. [Google Scholar] [CrossRef]
- Verma, V.; Singh, D.; Kh, R. Sinapic acid alleviates oxidative stress and neuro-inflammatory changes in sporadic model of Alzheimer’s disease in rats. Brain Sci. 2020, 10, 923. [Google Scholar] [CrossRef]
- Paraíso, A.F.; Sousa, J.N.; Andrade, J.M.O.; Mangabeira, E.S.; Lelis, D.d.F.; de Paula, A.M.B.; Martins, A.M.E.B.L.; Lima, W.J.N.; Guimarães, A.L.S.; Melo, G.A.; et al. Oral gallic acid improves metabolic profile by modulating SIRT1 expression in obese mice brown adipose tissue: A molecular and bioinformatic approach. Life Sci. 2019, 237, 116914. [Google Scholar] [CrossRef]
- Ong, K.W.; Hsu, A.; Tan, B.K.H. Anti-diabetic and anti-lipidemic effects of chlorogenic acid are mediated by ampk activation. Biochem. Pharmacol. 2013, 85, 1341–1351. [Google Scholar] [CrossRef]
- Chang, W.-C.; Hsieh, C.-H.; Hsiao, M.-W.; Lin, W.-C.; Hung, Y.-C.; Ye, J.-C. Caffeic acid induces apoptosis in human cervical cancer cells through the mitochondrial pathway. Taiwan. J. Obstet. Gynecol. 2010, 49, 419–424. [Google Scholar] [CrossRef]
- Gao, J.; Yu, H.; Guo, W.; Kong, Y.; Gu, L.; Li, Q.; Yang, S.; Zhang, Y.; Wang, Y. The anticancer effects of ferulic acid is associated with induction of cell cycle arrest and autophagy in cervical cancer cells. Cancer Cell Int. 2018, 18, 102. [Google Scholar] [CrossRef]
- Sun, J.; Li, Y.; Ding, Y.; Wang, J.; Geng, J.; Yang, H.; Ren, J.; Tang, J.; Gao, J. Neuroprotective effects of gallic acid against hypoxia/reoxygenation-induced mitochondrial dysfunctions in vitro and cerebral ischemia/reperfusion injury in vivo. Brain Res. 2014, 1589, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Mukherjee, S.; Paliwal, P.; Singh, S.S.; Birla, H.; Singh, S.P.; Krishnamurthy, S.; Patnaik, R. Neuroprotective effect of chlorogenic acid in global cerebral ischemia-reperfusion rat model. Naunyn. Schmiedebergs. Arch. Pharmacol. 2019, 392, 1293–1309. [Google Scholar] [CrossRef] [PubMed]
- Kerman, M.; Kanter, M.; Coşkun, K.K.; Erboga, M.; Gurel, A. Neuroprotective effects of Caffeic acid phenethyl ester on experimental traumatic brain injury in rats. J. Mol. Histol. 2012, 43, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Haque, E.; Javed, H.; Azimullah, S.; Abul Khair, S.B.; Ojha, S. Neuroprotective potential of ferulic acid in the rotenone model of Parkinson’s disease. Drug Des. Devel. Ther. 2015, 9, 5499. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.-C.; Ou, T.-T.; Wu, C.-H.; Wang, C.-J. Prevention of diet-induced hyperlipidemia and obesity by caffeic acid in C57BL/6 mice through regulation of hepatic lipogenesis gene expression. J. Agric. Food Chem. 2013, 61, 11082–11088. [Google Scholar] [CrossRef] [PubMed]
- Sri Balasubashini, M.; Rukkumani, R.; Menon, V.P. Protective effects of ferulic acid on hyperlipidemic diabetic rats. Acta Diabetol. 2003, 40, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Dovale-Rosabal, G.; Espinosa, A.; Rodríguez, A.; Barriga, A.; Palomino-Calderón, A.; Romero, N.; Troncoso, R.H.; Aubourg, S.P. Effect of structured phenolic lipids with EPA/DHA and gallic acid against metabolic-associated fatty liver disease (MAFLD) in Mice. Molecules 2022, 27, 7702. [Google Scholar] [CrossRef] [PubMed]
- Ong, K.W.; Hsu, A.; Tan, B.K.H. Chlorogenic acid stimulates glucose transport in skeletal muscle via AMPK activation: A contributor to the beneficial effects of coffee on diabetes. PLoS ONE 2012, 7, e32718. [Google Scholar] [CrossRef] [PubMed]
- Oboh, G.; Agunloye, O.M.; Adefegha, S.A.; Akinyemi, A.J.; Ademiluyi, A.O. Caffeic and chlorogenic acids inhibit key enzymes linked to type 2 diabetes (in vitro): A comparative study. J. Basic Clin. Physiol. Pharmacol. 2015, 26, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Choi, R.; Kim, B.H.; Naowaboot, J.; Lee, M.Y.; Hyun, M.R.; Cho, E.J.; Lee, E.S.; Lee, E.Y.; Yang, Y.C.; Chung, C.H. Effects of ferulic acid on diabetic nephropathy in a rat model of type 2 diabetes. Exp. Mol. Med. 2011, 43, 676. [Google Scholar] [CrossRef] [PubMed]
- You, B.R.; Moon, H.J.; Han, Y.H.; Park, W.H. Gallic acid inhibits the growth of HeLa cervical cancer cells via apoptosis and/or necrosis. Food Chem. Toxicol. 2010, 48, 1334–1340. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Wang, L.-L.; Xue, N.-N.; Li, C.; Guo, H.-H.; Ren, T.-K.; Zhan, Y.; Li, W.-B.; Zhang, J.; Chen, X.-G.; et al. Chlorogenic acid effectively treats cancers through induction of cancer cell differentiation. Theranostics 2019, 9, 6745–6763. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Cui, W.; Wang, L.; Xiong, Y.; Liu, L.; Sun, X.; Hao, L. Lutein Prevents high fat diet-induced atherosclerosis in apoe-deficient mice by inhibiting NADPH oxidase and increasing PPAR expression. Lipids 2015, 50, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Anila, L.; Vijayalakshmi, N. Flavonoids from Emblica officinalis and Mangifera indica—Effectiveness for dyslipidemia. J. Ethnopharmacol. 2002, 79, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Alkhalidy, H.; Moore, W.; Wang, Y.; Luo, J.; McMillan, R.; Zhen, W.; Zhou, K.; Liu, D. The flavonoid kaempferol ameliorates streptozotocin-induced diabetes by suppressing hepatic glucose production. Molecules 2018, 23, 2338. [Google Scholar] [CrossRef] [PubMed]
- Kotake-Nara, E.; Miyashita, K.; Nagao, A.; Kushiro, M.; Zhang, H.; Sugawara, T. Carotenoids affect proliferation of human prostate cancer cells. J. Nutr. 2001, 131, 3303–3306. [Google Scholar] [CrossRef] [PubMed]
- Tavsan, Z.; Kayali, H.A. Flavonoids showed anticancer effects on the ovarian cancer cells: Involvement of reactive oxygen species, apoptosis, cell cycle and invasion. Biomed. Pharmacother. 2019, 116, 109004. [Google Scholar] [CrossRef] [PubMed]
- Bei, W.; Zang, L.; Guo, J.; Peng, W.; Xu, A.; Good, D.A.; Hu, Y.; Wu, W.; Hu, D.; Zhu, X.; et al. Neuroprotective effects of a standardized flavonoid extract from Diospyros kaki leaves. J. Ethnopharmacol. 2009, 126, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Perles, R.; Moreno, D.A.; García-Viguera, C. Waking up from four decades’ long dream of valorizing agro-food byproducts: Toward practical applications of the gained knowledge. J. Agric. Food Chem. 2018, 66, 3069–3073. [Google Scholar] [CrossRef] [PubMed]
- Ng, H.S.; Kee, P.E.; Yim, H.S.; Chen, P.-T.; Wei, Y.-H.; Chi-Wei Lan, J. Recent advances on the sustainable approaches for conversion and reutilization of food wastes to valuable bioproducts. Bioresour. Technol. 2020, 302, 122889. [Google Scholar] [CrossRef] [PubMed]
- Reguengo, L.M.; Salgaço, M.K.; Sivieri, K.; Maróstica Júnior, M.R. Agro-industrial by-products: Valuable sources of bioactive compounds. Food Res. Int. 2022, 152, 110871. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Kristo, E.; LaPointe, G. Adding apple pomace as a functional ingredient in stirred-type yogurt and yogurt drinks. Food Hydrocoll. 2020, 100, 105453. [Google Scholar] [CrossRef]
- de Toledo, N.; de Camargo, A.; Ramos, P.; Button, D.; Granato, D.; Canniatti-Brazaca, S. Potentials and pitfalls on the use of passion fruit by-products in drinkable yogurt: Physicochemical, technological, microbiological, and sensory aspects. Beverages 2018, 4, 47. [Google Scholar] [CrossRef]
- Skwarek, P.; Karwowska, M. Fatty Acids profile and antioxidant properties of raw fermented sausages with the addition of tomato pomace. Biomolecules 2022, 12, 1695. [Google Scholar] [CrossRef] [PubMed]
- Tolve, R.; Simonato, B.; Rainero, G.; Bianchi, F.; Rizzi, C.; Cervini, M.; Giuberti, G. Wheat bread fortification by grape pomace powder: Nutritional, technological, antioxidant, and sensory properties. Foods 2021, 10, 75. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, B.E.; Contini, L.; Garcia, V.A.D.S.; Cilli, L.P.D.L.; Chagas, E.G.L.; Andreo, M.A.; Vanin, F.M.; Carvalho, R.A.; Sinnecker, P.; Venturini, A.C.; et al. Valorization of grape by-products as functional and nutritional ingredients for healthy pasta development. J. Food Process. Preserv. 2022, 46, e17245. [Google Scholar] [CrossRef]
- Panza, O.; Conte, A.; Del Nobile, M.A. Recycling of fig peels to enhance the quality of handmade pasta. LWT 2022, 168, 113872. [Google Scholar] [CrossRef]
- Drabińska, N.; Nogueira, M.; Szmatowicz, B. Valorisation of broccoli by-products: Technological, sensory and flavour properties of durum pasta fortified with broccoli leaf powder. Molecules 2022, 27, 4672. [Google Scholar] [CrossRef] [PubMed]
- Rainero, G.; Bianchi, F.; Rizzi, C.; Cervini, M.; Giuberti, G.; Simonato, B. Breadstick fortification with red grape pomace: Effect on nutritional, technological and sensory properties. J. Sci. Food Agric. 2022, 102, 2545–2552. [Google Scholar] [CrossRef]
- Garzón, G.A.; Medina, J.L.; Montana, T.L.; Sánchez, M.; Novoa, C.F.; Gutiérrez, L. Utilization of Vaccinium meridionale S. pomace as an eco-friendly and functional colorant in Greek-style yogurt. J. Food Sci. 2021, 86, 3896–3908. [Google Scholar] [CrossRef] [PubMed]
- Sah, B.N.P.; Vasiljevic, T.; McKechnie, S.; Donkor, O.N. Physicochemical, textural and rheological properties of probiotic yogurt fortified with fibre-rich pineapple peel powder during refrigerated storage. LWT Food Sci. Technol. 2016, 65, 978–986. [Google Scholar] [CrossRef]
- Vénica, C.I.; Spotti, M.J.; Pavón, Y.L.; Molli, J.S.; Perotti, M.C. Influence of carrot fibre powder addition on rheological, microstructure and sensory characteristics of stirred-type yogurt. Int. J. Food Sci. Technol. 2020, 55, 1916–1923. [Google Scholar] [CrossRef]
- Mileriene, J.; Serniene, L.; Kasparaviciene, B.; Lauciene, L.; Kasetiene, N.; Zakariene, G.; Kersiene, M.; Leskauskaite, D.; Viskelis, J.; Kourkoutas, Y.; et al. Exploring the potential of sustainable acid whey cheese supplemented with apple pomace and GABA-Producing indigenous Lactococcus lactis Strain. Microorganisms 2023, 11, 436. [Google Scholar] [CrossRef] [PubMed]
- Milinčić, D.D.; Kostić, A.Ž.; Gašić, U.M.; Lević, S.; Stanojević, S.P.; Barać, M.B.; Tešić, Ž.L.; Nedović, V.; Pešić, M.B. Skimmed goat’s milk powder enriched with grape pomace seed extract: Phenolics and protein characterization and antioxidant properties. Biomolecules 2021, 11, 965. [Google Scholar] [CrossRef] [PubMed]
- Salazar, D.; Arancibia, M.; Raza, K.; López-Caballero, M.E.; Montero, M.P. Influence of underutilized unripe banana (Cavendish) flour in the formulation of healthier chorizo. Foods 2021, 10, 1486. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Ospina, J.; Martuscelli, M.; Grande-Tovar, C.D.; Lucas-González, R.; Molina-Hernandez, J.B.; Viuda-Martos, M.; Fernández-López, J.; Pérez-Álvarez, J.Á.; Chaves-López, C. Cacao pod husk flour as an ingredient for reformulating frankfurters: Effects on quality properties. Foods 2021, 10, 1243. [Google Scholar] [CrossRef] [PubMed]
- Bedrníček, J.; Kadlec, J.; Laknerová, I.; Mráz, J.; Samková, E.; Petrášková, E.; Hasoňová, L.; Vácha, F.; Kron, V.; Smetana, P. Onion peel powder as an antioxidant-rich material for sausages prepared from mechanically separated fish meat. Antioxidants 2020, 9, 974. [Google Scholar] [CrossRef] [PubMed]
| Chemical Class | Bioactive Compounds | Concentration (µg/g d.w.) | By-Product | Ref. |
|---|---|---|---|---|
| Anthocyanin | Anthocyanin | 147.4 ± 2.9 | Cashew apple (peel and leftover pulp) | [36] |
| 9.0 ± 0.3 | Guava (peel, leftover pulp, and seed) | |||
| 22.9 ± 2.3 | Mango (peel and leftover pulp) | |||
| 37.0 ± 3.9 | Passion fruit (seed) | |||
| 101.0 ± 2.7 | Pineapple (peel and leftover pulp) | |||
| Carotenoids | β-carotene | 1791.4 ± 179.2 | Cashew apple (peel and leftover pulp) | [36] |
| 266.7 ± 26.7 | Guava (peel, leftover pulp, and seed) | |||
| 582.6 ± 58.3 | Mango (peel and leftover pulp) | |||
| 579.3 ± 58.0 | Passion fruit (seed) | |||
| 1561.0 ± 156.3 | Pineapple (peel and leftovers pulp) | |||
| Flavanols | Catechin | 3640 ± 30 | ‘Hass’ avocado (seed) | [35] |
| 8130 ± 38 | ‘Fuerte’ avocado (seed) | |||
| 132.27 ± 7.10 | Bacaba (seed, peel, and pulp) | [32] | ||
| 53.6 ± 1.2 | Grape (seed oil) | [30] | ||
| Epicatechin | 10,270 ± 80 | ‘Hass’ avocado (seed) | [35] | |
| 11,060 ± 30 | ‘Fuerte’ avocado (seed) | |||
| 73.4 ± 15.1 | Achachairú (seed, peel, and pulp) | [32] | ||
| 1.67 ± 0.29 | Aracá-boi (seed, peel, and pulp) | |||
| 122.65 ± 4.89 | Bacaba (seed, peel, and pulp) | |||
| 1.29 ± 0.01 | Avocado (peel) | [37] | ||
| 0.02 ± 0.0 | Banana (peel) | |||
| Rutin | 6.26 ± 0.04 | Bacaba (seed, peel, and pulp) | [32] | |
| 34.0 ± 7.0 | Colombian acaí (pulp) | [33] | ||
| Flavanone glycosides | Naringenin | 115.8 ± 1.1 | Melon (peel) | [31] |
| 1.09± 0.31 | Achachairú (seed, peel, and pulp) | [32] | ||
| 0.28 ± 0.0 | Bacaba (seed, peel, and pulp) | |||
| 5380.3 ± 182.3 | Pummelo ‘Jiwei’ (flavedo) | [34] | ||
| 58.8 ± 27.9 | Pummelo ‘Aolangbulangke’ (seed) | |||
| Flavones | Apigenin-7-glycoside | 293.4 ± 1.7 | Melon (peel) | [31] |
| Flavone | 135.1 ± 3.2 | Melons (peel) | [31] | |
| Luteolin | 67.3 ± 1.8 | Melons (peel) | [31] | |
| 9.0 ± 3.0 | Colombian acaí (pulp) | [33] | ||
| Luteolin-7-glycoside | 165.1 ± 1.5 | Melon (peel) | [31] | |
| Hydroxycinnamic acids | Caffeic acid | 0.60 ± 0.0 | Bacaba (seed, peel, and pulp) | [32] |
| 19.0 ± 0.80 | Colombian acaí (pulp) | [33] | ||
| 0.43 ± 0.7 | Pummelo ‘Jiwei’ (flavedo) | [34] | ||
| 3.65 ± 0.29 | Pummelo ‘Aolangbulangke’ (seed) | |||
| 7.9 ± 0.1 | Grape (seed oil) | [30] | ||
| 6.9 ± 0.0 | Passion fruit (seed oil) | |||
| Chlorogenic acid | 82.5 ± 10.1 | Melon (peel) | [31] | |
| 0.79 ± 0.60 | Bacaba (seed, peel, and pulp) | [32] | ||
| 33.57 ± 2.69 | Pummelo ‘Jiwei’ (flavedo) | [34] | ||
| 7.57 ± 0.55 | Pummelo ‘Aolangbulangke’ (seed) | |||
| p-coumaric acid | 0.95 ± 0.10 | Achachairú (seed, peel, and pulp) | [32] | |
| 0.47 ± 0.02 | Aracá-boi (seed, peel, and pulp) | |||
| 0.56 ± 0.02 | Bacaba (seed, peel, and pulp) | |||
| 48.4 ± 0.4 | Guava (seed oils) | [30] | ||
| 7.3 ± 0.4 | Passion fruit (seed oil) | |||
| 8.0 ± 0.1 | Soursop (seed oil) | |||
| Ferulic acid | 0.76 ± 0.01 | Bacaba (seed, peel, and pulp) | [32] | |
| 1.12 ± 0.11 | Pummelo ‘Jiwei’ (flavedo) | [34] | ||
| 0.67 ± 0.04 | Pummelo ‘Aolangbulangke’ (seed) | |||
| 3-hydroxybenzoic acid | 334.5 ± 3.7 | Melon (peel) | [31] | |
| Isovanillic acid | 237.0 ± 0.4 | Melon (peel) | [31] | |
| Protocatechuic acid | 34.6 ± 8.1 | Melon (peel) | [31] | |
| 2.78 ± 0.31 | Bacaba (seed, peel, and pulp) | [32] | ||
| 17.0 ± 4.0 | Colombian acaí (pulp) | [33] | ||
| Syringic acid | 48.0 ± 11.0 | Colombian acaí (pulp) | [33] | |
| Lignans | Pinoresinol | 19.2 ± 0.7 | Melon (peel) | [31] |
| Phenolic acids | Tyrosol | 113.5 ± 0.3 | Melon (peel) | [31] |
| Phenylethanoids | Hydroxytyrosol | 91.1 ± 2.6 | Melon (peel) | [31] |
| Tannins | Gallic acid | 8.130 ± 1 | Melon (peel) | [31] |
| 8.26 ± 0.17 | Aracá-boi (seed, peel, and pulp) | [32] | ||
| 130.9 ± 9.6 | Pummelo flavedo | [34] | ||
| 20.66 ± 2.58 | Pummelo seed | |||
| Tocopherols | α-tocopherol | 11.8 ± 0.0 | Grape (seed oil) | [30] |
| 45.8 ± 0.2 | Guava (seed oil) | |||
| 20.5 ± 0.3 | Melon (seed oil) | |||
| 7.3 ± 0.00 | Pumpkin (seed oil) | |||
| 22.1 ± 0.0 | Soursop (seed oil) | |||
| ϒ-tocopherol | 60.1 ± 0.1 | Grape (seed oil) | [30] | |
| 93.1 ± 0.2 | Guava (seed oil) | |||
| 249.6 ± 0.0 | Melon (seed oil) | |||
| 107.2 ± 0.2 | Passion fruit (seed oil) | |||
| 294.5 ± 0.3 | Pumpkin (seed oil) | |||
| 7.1 ± 0.0 | Soursop (seed oil) | |||
| 328.7 ± 0.3 | Tomato (seed oil) |
| By-Product | Bioactive Compound | Antimicrobial Activity against | Outcomes (mg/mL) | Ref. |
|---|---|---|---|---|
| Apple peels | Phenolic compounds | Enterobacter faecium | MIC = 15.6 MBC = 31.2 | [47] |
| Escherichia coli | MIC = 15.6 MBC = 31.2 | |||
| Listeria monocytogenes | MIC = 62.5 MBC = 125.0 | |||
| Pseudomonas aeruginosa | MIC = 15.6 MBC = 31.2 | |||
| Salmonellatyphimurium | MIC = 31.2 MBC = 62.5 | |||
| Staphylococcus aureus | MIC = 3.9 MBC = 7.8 | |||
| Artichoke (Cynara scolymus L.) floral stems | Luteolin, apigenin derivaties,1-O-, 3-O, 4-O, and 5-O-caffeoylquimic acids, and procyanidin dimer | Candida albicans | MIC = 1.0 MBC = 1.0–2.0 | [48] |
| Enterococcus faecium | MIC = 1.0–1.5 MBC = 1.5–2.0 | |||
| Escherichia coli | MIC = 1.0–1.5 MBC = 1.0–1.5 | |||
| S. typhimurium | MIC = 1.0–1.5 MBC = 1.5–2.0 | |||
| S. aureus | MIC = 1.0–1.5 MBC = 1.5–2.0 | |||
| Jaboticaba (Myrciaria jaboticaba Vell. Berg) peels | Bis-HHDP-glucose, galloyl-bis-HHDP-glucose, pentagalloyl glucose, trisgalloyl-HHPD-glucose, and bis-HHDP-glucose | Enterococcus faecalis | MIC = 10.0 MBC = > 20.0 | [49] |
| E. coli | MIC = 20.0 MBC = > 20.0 | |||
| Klebsiella pneumoniae | MIC = 20.0 MBC = > 20.0 | |||
| Listeria monocytogenes | MIC = 10.0 MBC = > 20.0 | |||
| Pseudomonas aeruginosa | MIC = 20.0 MBC = > 20.0 | |||
| MRSA | MIC = 10.0 MBC = > 20.0 | |||
| Kiwi (Actinidia deliciosa cv. ‘Hayward’) | Epicatechin, B-type (epi)catechin, and quercetin | Bacillus cereus | MIC = 2.0 MBC = 4.0 | [42] |
| peels | Enterobacter cloacae | MIC = 2.0 MBC = 4.0 | ||
| E. coli | MIC = 1.0 MBC = 2.0 | |||
| L. monocytogenes | MIC = 2.0 MBC = 4.0 | |||
| S. typhimurium | MIC = 2.0 MBC = 4.0 | |||
| S. aureus | MIC = 1.0 MBC = 2.0 | |||
| Olive (Olea europaea) leaves and branches | Luteolin and tyrosol | E. coli | MIC = 40.0 MBC = 45.0 | [50] |
| Listeria innocua | MIC = 20.0 MBC = 25.0 | |||
| Salmonella sp. | MIC = 35.0 MBC = 40.0 | |||
| S. aureus | MIC = 20.0 MBC = 25.0 | |||
| Pomegranate (Punica granatum L.) | Phloretin, quercetin, indolamine, coutaric acid, isohydroxymatairesinol, and punicatannin C | Staphylococcus epidermidis | MIC = 0.1 MBC = 0.3 | [51] |
| E. coli | MIC = 0.3 MBC = 0.7 | |||
| Pseudomonas aeruginosa | MIC = 0.1 MBC = 1.5 | |||
| E. faecalis | MIC = 0.2 MBC = 0.7 |
| By-Products | Chemical Class | Method | Antioxidant Activity | Ref. |
|---|---|---|---|---|
| Avocado paste | Hydroxycinnamic acids | FRAP | 3.52 ± 0.33 mg TE/g d.w. | [63] |
| DPPH | 1.57 ± 0.14 mg TE/g d.w. | |||
| ABTS | 5.89 ± 0.34 mg TE/g d.w. | |||
| Grape seed flour | Phenolic acids, flavonoids, and procyanidins | FRAP | 225.23 ± 1.89 µmol TE/g | [64] |
| DPPH | 65.66 ± 5.03% | |||
| Mango seed | Hydroxycinnamic acids | ABTS | 10,568 ± 73.05 mg TE/100 g d.w. | [65] |
| DPPH | 10,659 ± 419.69 mg TE/100 g d.w. | |||
| Unripe papaya powder | Carotenoids and hydroxycinnamic acids | FRAP | 411.58 ± 38.0 mmol FeSO4/100 g | [66] |
| DPPH | 37.87 ± 3.69 mg ascorbic acid/100 g | |||
| Pineapple peel | Flavonoids and hydroxycinnamic acids | DPPH | 93.12 ± 0.43% | [67] |
| ABTS | 3.19 ± 0.02 mg TE/g d.w. extract | |||
| β-carotene blanching | 5.74 ± 0.10% | |||
| Pineapple core | DPPH | 93.22 ± 3.11% | ||
| ABTS | 3.07 ± 0.01 mg TE/g dry extract | |||
| β-carotene blanching | 5.62 ± 0.02% | |||
| Pineapple pomace | DPPH | 27.03 ± 1.18% | ||
| ABTS | 3.04 ± 0.01 mg TE/g d.w. | |||
| β-carotene blanching | 5.51 ± 0.03% | |||
| Raspberry pomace | Anthocyanins, ellagitannins | FRAP | 772.73 ± 10.50 µmol/L | [68] |
| DPPH | 361.27 ± 5.65 µmol TE/100 g | |||
| Apple pomace | Flavonoids and hydroxycinnamic acids | DPPH | 9.75 ± 1.15 g ascorbic acid/kg d.w. | [69] |
| FRAP | 10.87 ± 0.26 g ascorbic acid/kg d.w. | |||
| Coffee pulp extracts | Hydroxycinnamic acids and anthocyanins | ABTS | 27.0 ± 1.2 IC50 μg/mL | [70] |
| DPPH | 140.0 ± 9.2 IC50 μg/mL | |||
| Citrus juice by-products | Hydroxycinnamic acids and flavones | DPPH | 11,035 ± 549 µmol TE/g d.w. | [71] |
| ORAC | 91.570 ± 12.153 µmol TE/g d.w. | |||
| Chokeberry pomace | Hydroxycinnamic acids and anthocyanin | DPPH | 301.89 ± NR μM TE/100 g d.w. | [72] |
| ABTS | 779.58 ± NR μM TE/100 g d.w. |
| Therapeutic Uses | Compound | Model/ Intervention | Main Results | Ref. |
|---|---|---|---|---|
| Dyslipidemia | Gallic acid | Swiss male mice fed high-fat diet, 100 mg/kg/body weight, 60 days | Improved glucose tolerance and metabolic parameters. Bioinformatic analyses showed that SIRT1 is the main target in the thermogenesis process, which was confirmed by higher mRNA expression of SIRT1 in brown adipose tissue | [100] |
| Chlorogenic acid | Lepr db/db mice, ip 250 mg/kg, 2 weeks | Inhibited hepatic glucose 6-phosphatase expression and activity, attenuated hepatic steatosis, improved lipid profile, skeletal muscle glucose uptake, fasting glucose, glucose tolerance, insulin sensitivity, and dyslipidemia | [101] | |
| Caffeic acid | C57BL/6 mice fed high-fat diet, 0.02 and 0.08% w/w, 6 weeks | Reduced plasma and liver triglyceride and cholesterol concentrations, increased phosphorylation of AMPK, and decreased acetyl carboxylase, a downstream target of AMPK, which are related to hepatic β-oxidation of fatty acids | [108] | |
| Ferulic acid | Diabetic female Wistar rats, 40 mg/kg, 45 days | Significantly reduced elevated plasma lipid and blood glucose | [109] | |
| Carotenoids | Male C57BL/6J apoE knockout mice, 25, 50 and 100 mg/kg, 24 weeks | Decreased serum total cholesterol, triglyceride, and LDL concentrations | [116] | |
| Flavonoids | Male Sprague–Dawley rats, 10 mg/kg BW/day, orally, 90 days | Reduced lipid levels in serum and tissues. Inhibited hepatic activity of 3-hydroxy-3-methylglutaryl coenzyme A reductase | [117] | |
| Diabetes | Gallic acid | Male C57BL/6J mice fed high-fat diet, 70 mg/kg, orally, one month | Decreased visceral fat, fasting blood glucose, and fasting insulin | [110] |
| Chlorogenic acid | Male db/db mice, ip 250 mg/kg, 15, 30, 60, and 90 min | An acute decrease in fasting blood glucose 10 min after the compound was administered; the effect persisted for a further 30 min | [111] | |
| Caffeic acid | In vitro, 3.68 and 4.98 μg/mL | Inhibited α-amylase and α-glucosidase activities | [112] | |
| Ferulic acid | Male OLETF rats fed high-fat diet, 10 mg/kg/day, oral gavage, 45 weeks | Decreased blood glucose and markers of oxidative stress | [113] | |
| Flavonoids | Diabetic C57BL/6 male mice, 50 mg/kg/day, oral, 7 weeks | Decreased hyperglycemia and hepatic glucose production and increased glucose oxidation in muscle | [118] | |
| Cancer | Gallic acid | HeLa cells and HUVEC, 10–400 μM, 24 h | Inhibited growth of HeLa cells by apoptosis and necrosis | [114] |
| Chlorogenic acid | Various cell lines, 25 or 50 μM, 6 days. Male NOD/SCID mice with xenographed tumors, ip 25–200 mg/kg/day, 30 days. Male Wistar rats with gliomas, ip 75 mg/kg | Inhibited tumor growth and prevented the development of new tumors | [115] | |
| Caffeic acid | HeLa cells, 0.5, 1, 2.5, 5, or 10 mM, 24 h | Significantly reduced cell proliferation, decreased levels of uncleaved caspase-3 and Bcl-2, and induced cleaved caspase-3 and p53 | [102] | |
| Ferulic acid | HeLa and Caski cells, 2.0 mM, 48 h | Inhibited cell invasion by reducing MMP-9 mRNA expression | [103] | |
| Carotenoids | Prostate cancer cells (PC-3, DU 145, and LNCaP), 20 μmol/L, 72 h | Reduced cell viability by inducing apoptosis | [119] | |
| Flavonoids | Various cell lines, 0–100 μM, 24 h | Suppression of cell growth, induction of apoptosis, cell cycle arrest, and inhibition of cell invasion | [120] | |
| Neuroprotective | Chlorogenic acid | Inbred male Charles foster albino rats, 10 mg/kg, intranasal | Significantly reduced the area of cerebral infarction as well as the expression of TNF-α, iNOS, and caspase-3 | [105] |
| Caffeic acid | Male Sprague–Dawley rats, 10 μmol/kg, 15 min | Effective for lipid peroxidation, antioxidant enzyme activity, and neuronal protection | [106] | |
| Carotenoids | HT-22 cells, 100 μg/mL, 24 h | Neuroprotective effects on oxidative stress-induced neuronal cells. Improved cell viability and attenuated the formation of intracellular reactive oxygen species (ROS) and apoptotic bodies in hippocampal neuronal cells | [95] | |
| Flavonoids | Rats with middle cerebral artery occlusion and four-vessel occlusion, 40 and 80 mg/kg, 7 days | Reduction in ischemic injury and protection of hippocampal and cortical neurons | [121] | |
| Ferulic acid | Male Wistar rats, ip 50 mg/kg, 4 weeks | Re-establishment of antioxidant enzymes, prevented glutathione depletion, inhibited lipid peroxidation, and reduced inflammatory mediators, like cyclooxygenase-2 and iNOS, and pro-inflammatory cytokines | [107] |
| Food Group | Product | Fruit | By-Product Used | Addition (%) | Effects | Ref. |
|---|---|---|---|---|---|---|
| Dairy products | Yogurt | Apple | Pomace | 3.0 | Increased total phenolics, dietary fiber, firmness, viscosity, and cohesion | [125] |
| Passion fruit | Husk and seed | 2.0 | Increased fiber (soluble and insoluble), mineral content (K, Mg, and Mn), and viscosity; changes in color parameters | [126] | ||
| Blueberry | Pomace | 0.7 | Significant increase in anthocyanins, total phenolic content, antioxidant activity, conjugated linoleic acid, and sensory acceptance | [133] | ||
| Pineapple | Peel | 1.0 | Significantly reduced the fermentation time of milk co-fermented with probiotic organisms and increased fiber content | [134] | ||
| Carrot | Fiber | 1.0 and 2.0 | Increased fiber content | [135] | ||
| Cheese | Apple | Pomace | 3.0 | Texture and flavor enhancer increases daily fiber intake | [136] | |
| Powdered milk | Grape | Seed extract | 0.1, 0.5, and 1.0 | Significantly improved antioxidant activity (FRAP, DPPH, and ABTS) | [137] | |
| Meat products | Chorizo | Plantain | Peel | 6.9 and 7.2 | Reducing pig fat incorporation results in excellent sensory characteristics due to technological parameters and sensory acceptance | [138] |
| Sausage | Cacao | Pod shell | 1.5 and 3.0 | Starch substitution showed emulsion stability, increased fiber content | [139] | |
| Fish meat sausages | Onion | Peel | 1.0 and 2.0 | Improved sensory properties, extended shelf life | [140] | |
| Sausage | Tomato | Pomace | 0.5, 1.0, and 1.5 | Reduced nitrite levels, antimicrobial properties | [127] | |
| Cereals | Wheat bread | Grape | Pomace | 5.0 and 10.0 | Increased fiber and phenolic compounds | [128] |
| Spaghetti | Grape | Pomace | 50.0 | High fiber, antioxidant activity, and nutritional benefit with good consumer acceptability | [129] | |
| Breadstick | Red grape | Pomace | 5.0 and 10.0 | High fiber and antioxidant content inhibited microbial growth | [132] | |
| Dough | Fig | Shell | 10, 13, and 16 | Increased total phenolics, flavonoids, and antioxidant activity; pH control and preservation of sensory quality during storage | [130] | |
| Broccoli | Leaves | 5.0 | Extended service life, improved mineral content and its appearance without compromising cooking, texture, or sensory characteristics | [131] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Enciso-Martínez, Y.; Zuñiga-Martínez, B.S.; Ayala-Zavala, J.F.; Domínguez-Avila, J.A.; González-Aguilar, G.A.; Viuda-Martos, M. Agro-Industrial By-Products of Plant Origin: Therapeutic Uses as well as Antimicrobial and Antioxidant Activity. Biomolecules 2024, 14, 762. https://doi.org/10.3390/biom14070762
Enciso-Martínez Y, Zuñiga-Martínez BS, Ayala-Zavala JF, Domínguez-Avila JA, González-Aguilar GA, Viuda-Martos M. Agro-Industrial By-Products of Plant Origin: Therapeutic Uses as well as Antimicrobial and Antioxidant Activity. Biomolecules. 2024; 14(7):762. https://doi.org/10.3390/biom14070762
Chicago/Turabian StyleEnciso-Martínez, Yessica, B. Shain Zuñiga-Martínez, Jesús Fernando Ayala-Zavala, J. Abraham Domínguez-Avila, Gustavo A. González-Aguilar, and Manuel Viuda-Martos. 2024. "Agro-Industrial By-Products of Plant Origin: Therapeutic Uses as well as Antimicrobial and Antioxidant Activity" Biomolecules 14, no. 7: 762. https://doi.org/10.3390/biom14070762
APA StyleEnciso-Martínez, Y., Zuñiga-Martínez, B. S., Ayala-Zavala, J. F., Domínguez-Avila, J. A., González-Aguilar, G. A., & Viuda-Martos, M. (2024). Agro-Industrial By-Products of Plant Origin: Therapeutic Uses as well as Antimicrobial and Antioxidant Activity. Biomolecules, 14(7), 762. https://doi.org/10.3390/biom14070762











