Effect of Health Status and Heat-Induced Inactivation on the Proteomic Profile of Plasma Rich in Growth Factors Obtained from Donors with Chronic Inflammatory Skin Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of PRGF Supernatants
2.2. Immunosafe Treatment
2.3. Proteomic Analysis
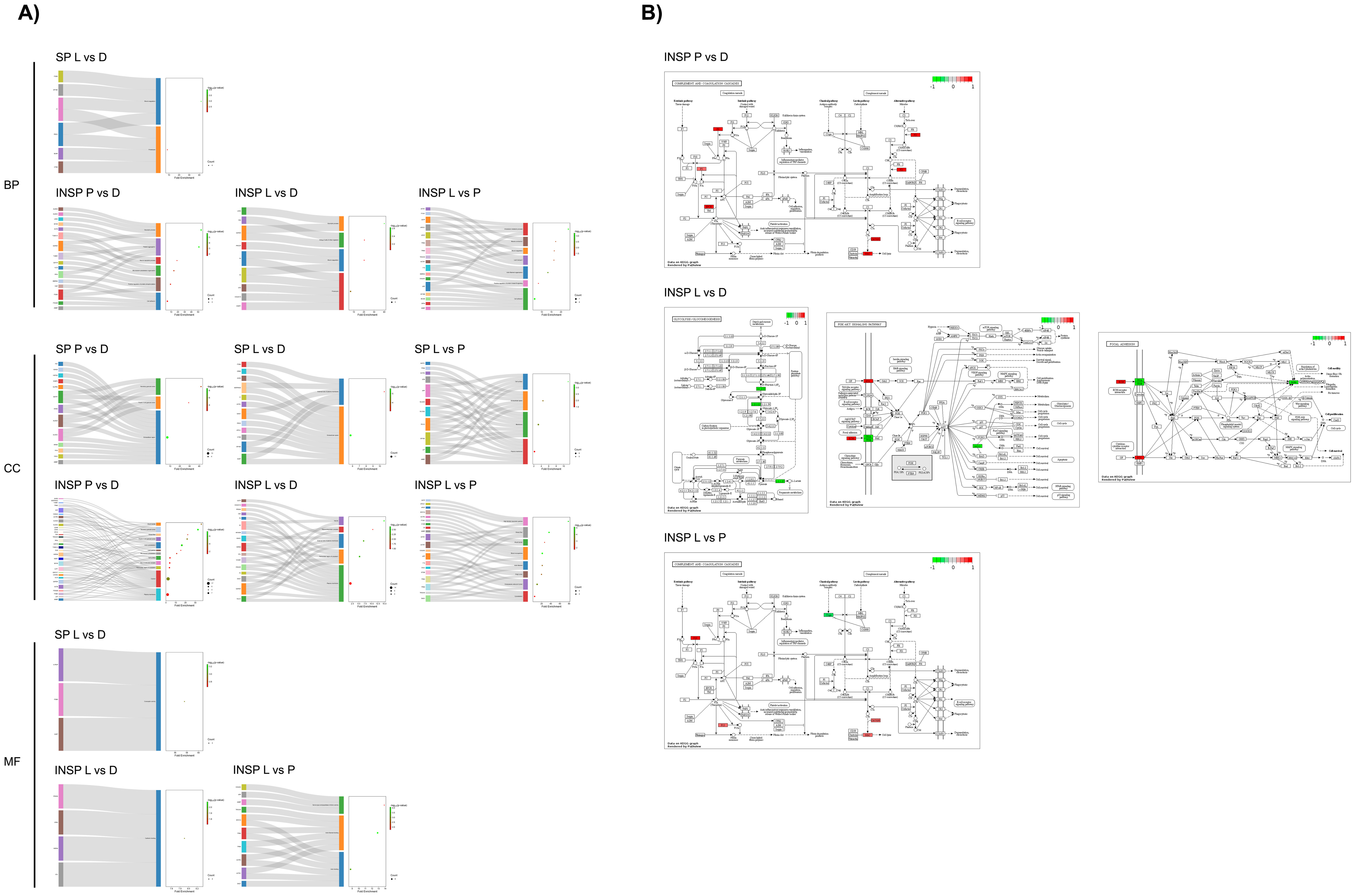
2.4. Functional Analyses
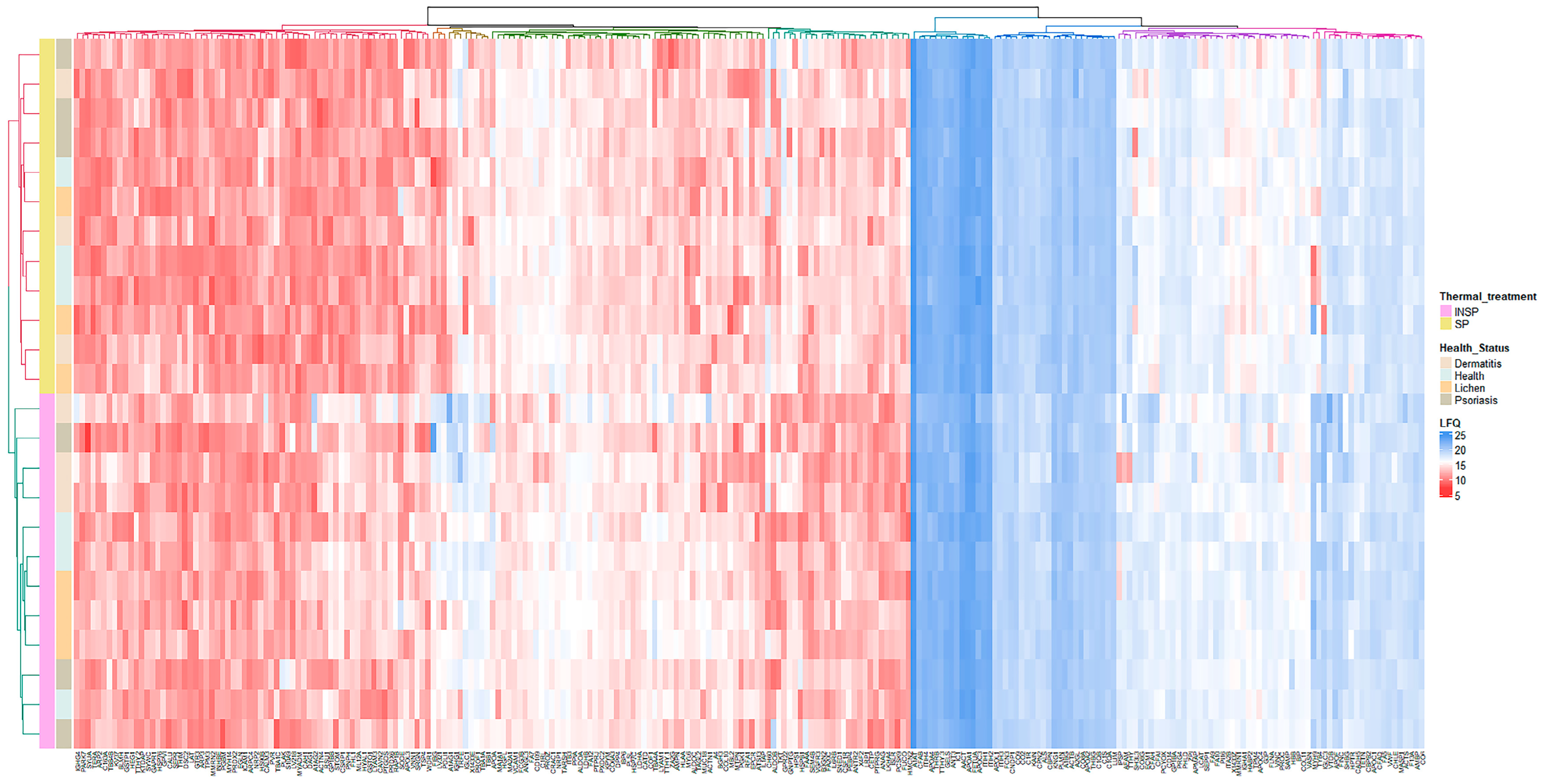
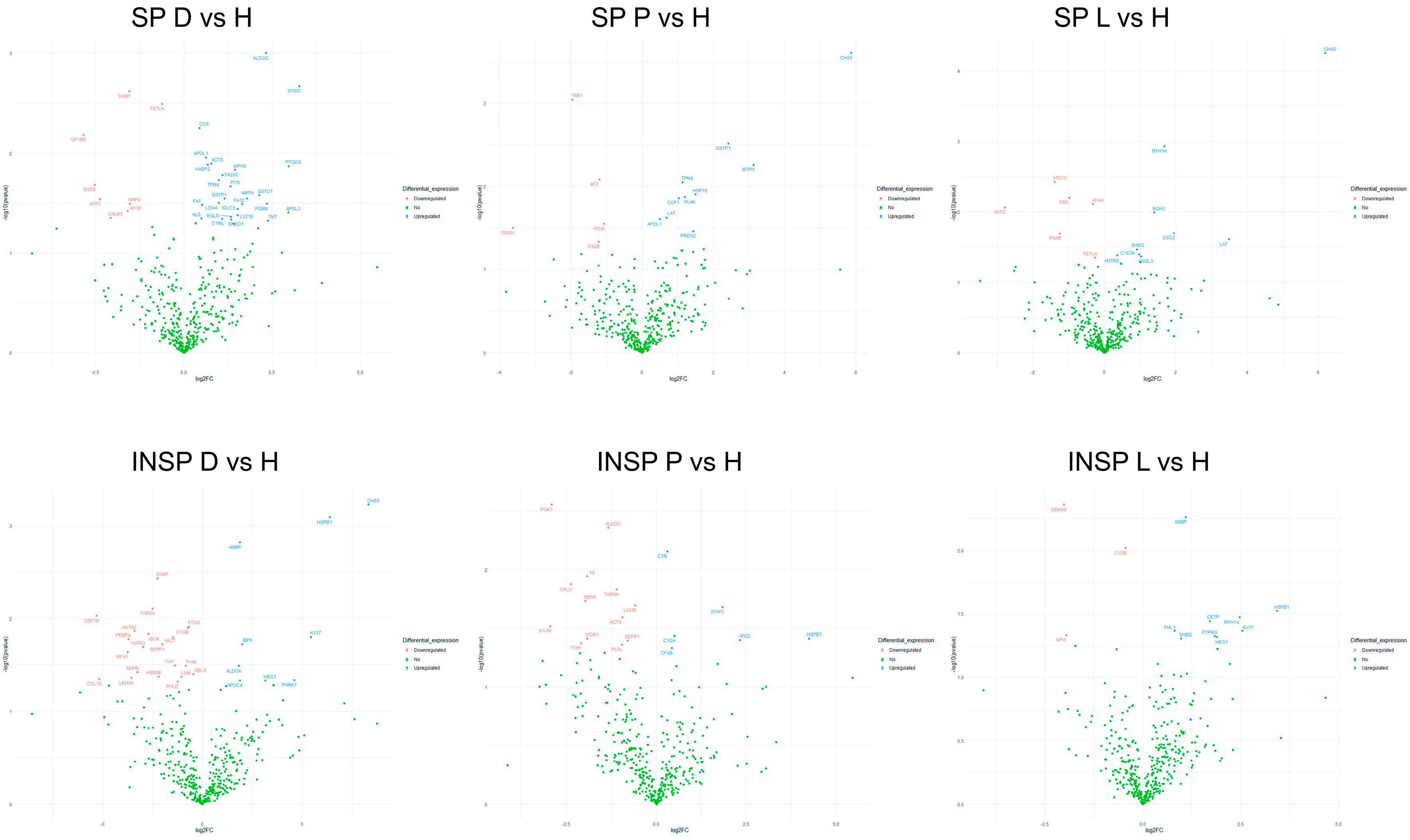
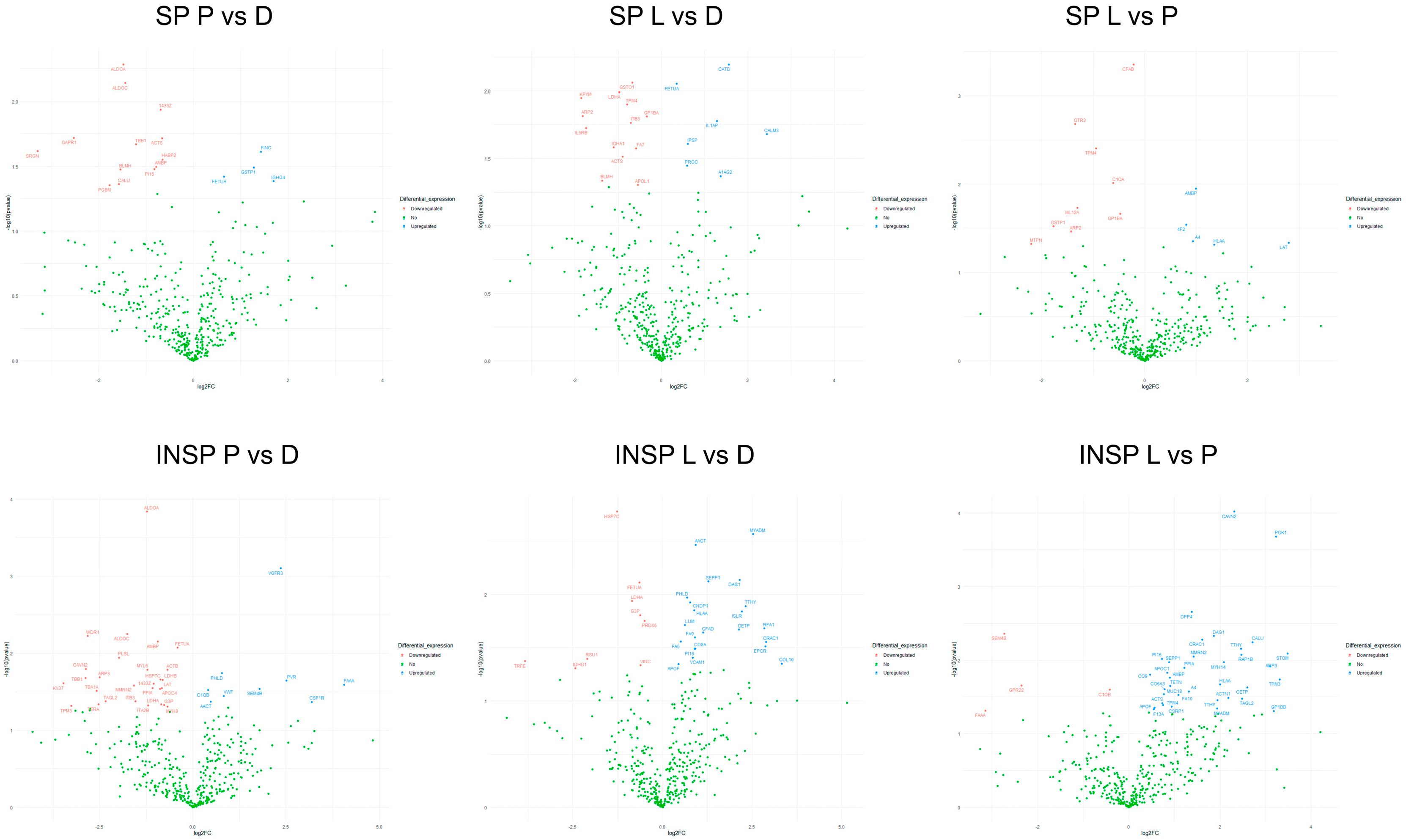
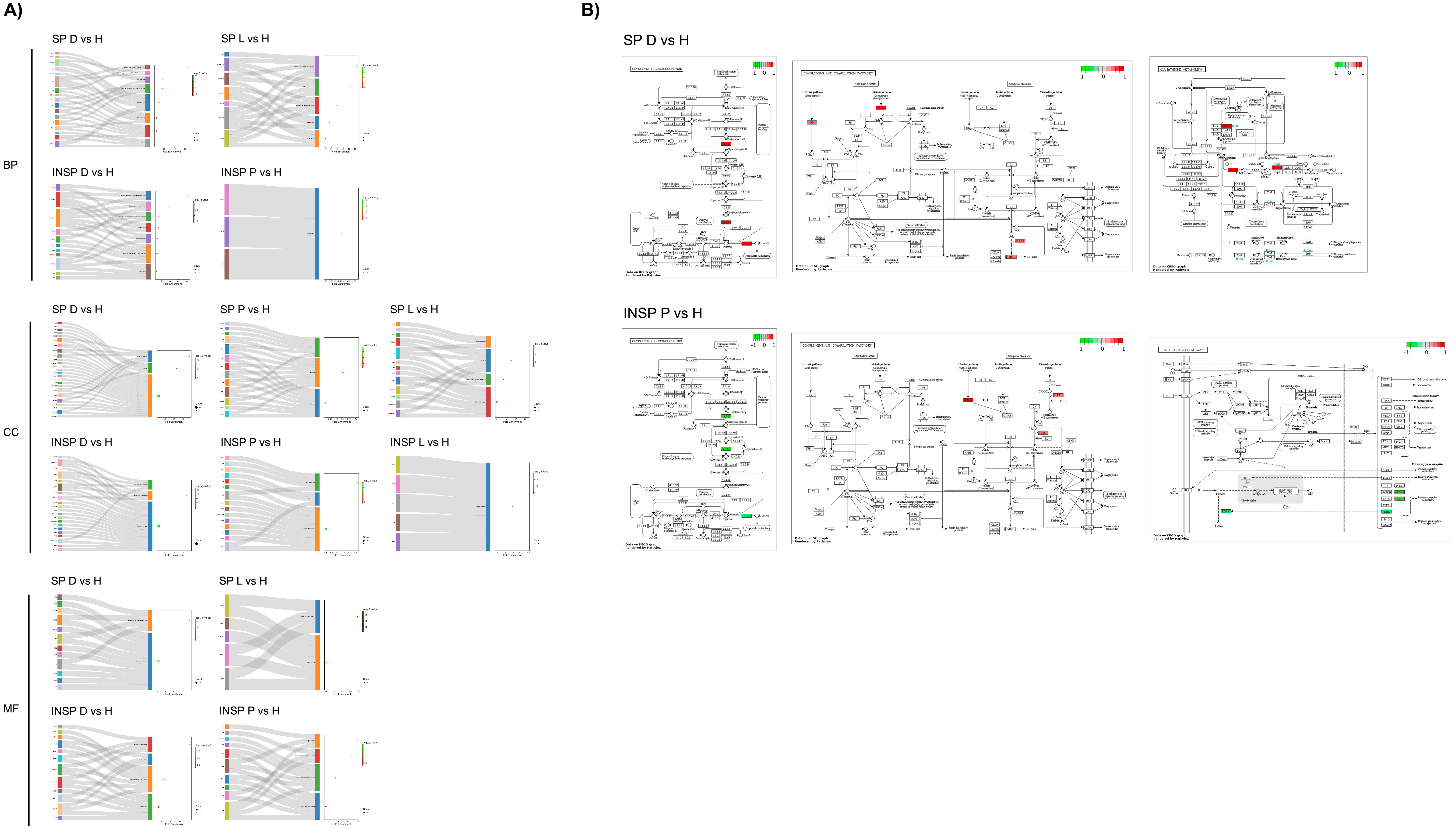
3. Results
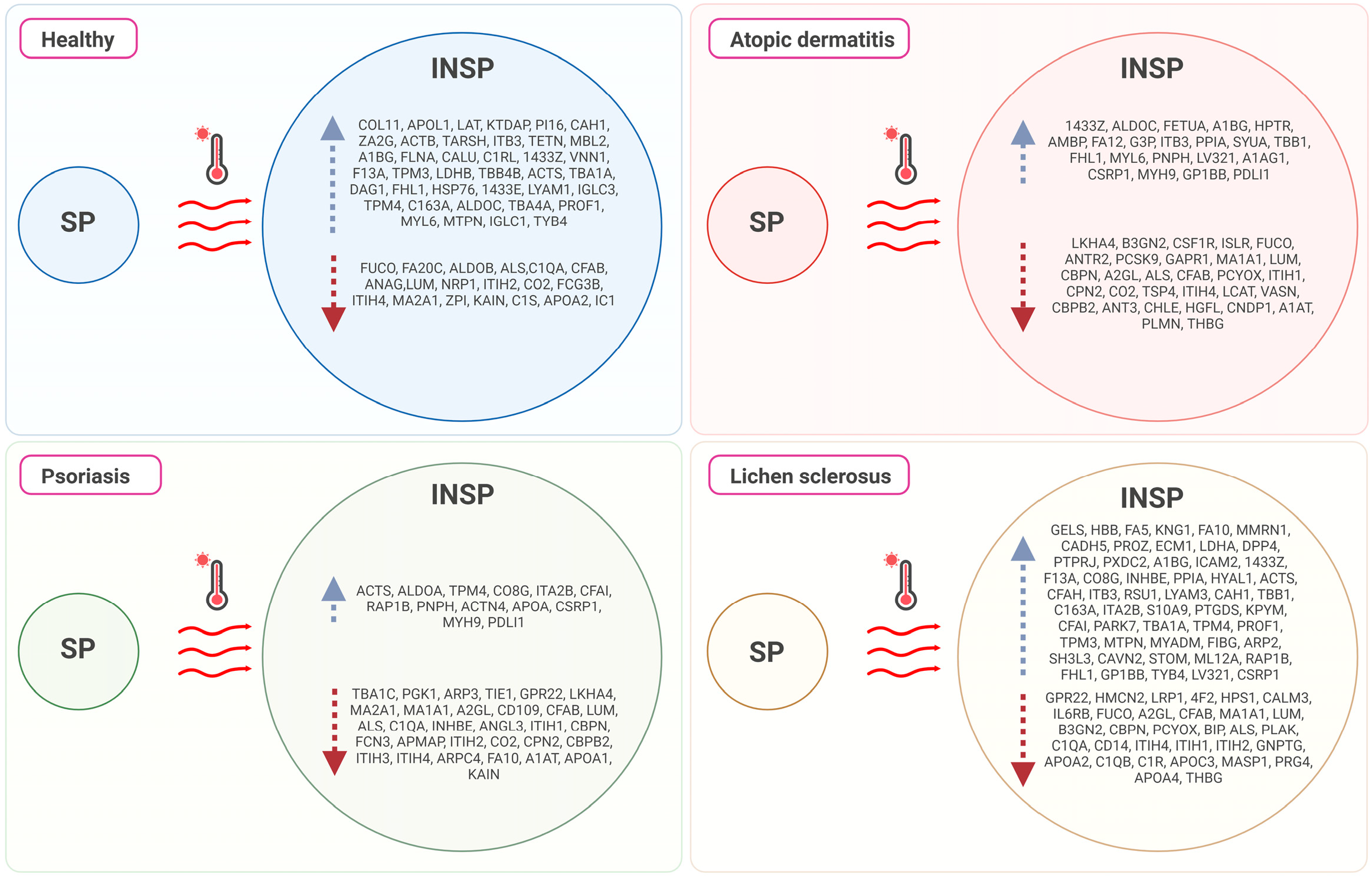
3.1. Effect of Skin Condition
3.2. Effect of the Immunosafe Treatment
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ujiie, H.; Rosmarin, D.; Schön, M.P.; Ständer, S.; Boch, K.; Metz, M.; Maurer, M.; Thaci, D.; Schmidt, E.; Cole, C.; et al. Unmet Medical Needs in Chronic, Non-communicable Inflammatory Skin Diseases. Front. Med. 2022, 9, 875492. [Google Scholar] [CrossRef]
- Song, A.; Lee, S.E.; Kim, J.H. Immunopathology and Immunotherapy of Inflammatory Skin Diseases. Immune Netw. 2022, 22, e7. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, H.; Taylor, M.; Cook, C.; Martínez-Berdeja, A.; North, J.P.; Harirchian, P.; Hailer, A.A.; Zhao, Z.; Ghadially, R.; et al. Classification of human chronic inflammatory skin disease based on single-cell immune profiling. Sci. Immunol. 2022, 7, eabl9165. [Google Scholar] [CrossRef]
- Peterson, D.M.; Damsky, W.E.; Vesely, M.D. Treatment of lichen sclerosus and hypertrophic scars with dupilumab. JAAD Case Rep. 2022, 23, 76–78. [Google Scholar] [CrossRef]
- Ferreira, S.; Guttman-Yassky, E.; Torres, T. Selective JAK1 Inhibitors for the Treatment of Atopic Dermatitis: Focus on Upadacitinib and Abrocitinib. Am. J. Clin. Dermatol. 2020, 21, 783–798. [Google Scholar] [CrossRef]
- Menter, A.; Strober, B.E.; Kaplan, D.H.; Kivelevitch, D.; Prater, E.F.; Stoff, B.; Armstrong, A.W.; Connor, C.; Cordoro, K.M.; Davis, D.M.; et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J. Am. Acad. Dermatol. 2019, 80, 1029–1072. [Google Scholar] [CrossRef]
- Simpson, E.L.; Bieber, T.; Guttman-Yassky, E.; Beck, L.A.; Blauvelt, A.; Cork, M.J.; Silverberg, J.I.; Deleuran, M.; Kataoka, Y.; Lacour, J.-P.; et al. Two Phase 3 Trials of Dupilumab versus Placebo in Atopic Dermatitis. N. Engl. J. Med. 2016, 375, 2335–2348. [Google Scholar] [CrossRef]
- Kauhl, W.; Pototschnig, H.; Paasch, U. Can Platelet-Rich Plasma Reduce the Burden of Inflammatory Skin Diseases such as Psoriasis and Atopic Dermatitis? Cureus 2021, 13, e18472. [Google Scholar] [CrossRef]
- Emer, J. Platelet-Rich Plasma (PRP): Current Applications in Dermatology. Ski. Ther. Lett. 2019, 24, 1–6. [Google Scholar]
- Alves, R.; Grimalt, R. A Review of Platelet-Rich Plasma: History, Biology, Mechanism of Action, and Classification. Ski. Appendage Disord. 2018, 4, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Huber, S.C.; de Lima Montalvão, S.A.; Sachetto, Z.; Santos Duarte Lana, J.F.; Annichino-Bizzacchi, J.M. Characterization of autologous platelet rich plasma (PRP) and its biological effects in patients with Behçet’s Disease. Regen. Ther. 2021, 18, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Vafaei-Nodeh, S.; Kabiri-Abyaneh, S. Long-term control of atopic dermatitis with platelet-rich plasma. JAAD Case Rep. 2021, 7, 54–56. [Google Scholar] [CrossRef] [PubMed]
- Yosef, A.; Elkady, N.; Khattab, F. Possible clinical efficacy and tolerability of platelet-rich plasma with atopic dermatitis. J. Cosmet. Dermatol. 2021, 20, 3264–3269. [Google Scholar] [CrossRef]
- Lin, C.S.; Cheng, M.S.; Wu, C.J.; Lin, K.T. A Randomized Standard-of-Care Controlled Trial of Xenogeneic Platelet-Rich Plasma Lotion to Reduce Acute Radiation Dermatitis in Breast Cancer Patients. Int. J. Radiat. Oncol.-Biol.-Phys. 2022, 17, S115. [Google Scholar] [CrossRef]
- Chakravdhanula, U.; Anbarasu, K.; Verma, V.K.; Beevi, S.S. Clinical efficacy of platelet rich plasma in combination with methotrexate in chronic plaque psoriatic patients. Dermatol. Ther. 2016, 29, 446–450. [Google Scholar] [CrossRef]
- Anwar, M.; Siddiqui, S.; Shahzad, A.; Rasheed, T.; Anwar, M. Efficacy of intralesional injection of platlet rich plasma in combination with methoterexate in chronic plaque psoriasis. J. Pak. Assoc. Dermatol. 2022, 32, 233–238. [Google Scholar]
- Mona, A.; Mohammed, E.S.; Nader, A.I.; Sahar, F.M. Interleukin 17 expression in psoriatic skin lesions before and after treatment with platelet rich plasma (PrP). Med. J. Cairo Univ. 2018, 86, 1447–1456. [Google Scholar] [CrossRef]
- Rahman, A.E.; Salem, R.M.; Ahmed, A.H. Evaluation of Platelet Rich Plasma in the Management of Psoriasis. Benha J. Appl. Sci. 2018, 5, 21–25. [Google Scholar] [CrossRef]
- Villalpando, B.K.; Wyles, S.P.; Schaefer, L.A.; Bodiford, K.J.; Bruce, A.J. Platelet-rich plasma for the treatment of lichen sclerosus. Plast. Aesthetic Res. 2021, 8, 63. [Google Scholar] [CrossRef]
- Goldstein, A.T.; King, M.; Runels, C.; Gloth, M.; Pfau, R. Intradermal injection of autologous platelet-rich plasma for the treatment of vulvar lichen sclerosus. J. Am. Acad. Dermatol. 2017, 76, 158–160. [Google Scholar] [CrossRef]
- Paganelli, A.; Contu, L.; Condorelli, A.; Ficarelli, E.; Motolese, A.; Paganelli, R.; Motolese, A. Platelet-Rich Plasma (PRP) and Adipose-Derived Stem Cell (ADSC) Therapy in the Treatment of Genital Lichen Sclerosus: A Comprehensive Review. Int. J. Mol. Sci. 2023, 24, 16107. [Google Scholar] [CrossRef]
- Anitua, E.; Alkhraisat, M.H.; Orive, G. Perspectives and challenges in regenerative medicine using plasma rich in growth factors. J. Control. Release Off. J. Control. Release Soc. 2012, 157, 29–38. [Google Scholar] [CrossRef]
- Anitua, E.; Pino, A.; Aspe, L.; Martínez, M.; García, A.; Goñi, F.; Troya, M. Anti-inflammatory effect of different PRGF formulations on cutaneous surface. J. Tissue Viability 2021, 30, 183–189. [Google Scholar] [CrossRef]
- Daha, N.A.; Banda, N.K.; Roos, A.; Beurskens, F.J.; Bakker, J.M.; Daha, M.R.; Trouw, L.A. Complement activation by (auto-) antibodies. Mol. Immunol. 2011, 48, 1656–1665. [Google Scholar] [CrossRef]
- Niemann, M.; Ort, M.; Lauterbach, L.; Streitz, M.; Wilhelm, A.; Grütz, G.; Fleckenstein, F.N.; Graef, F.; Blankenstein, A.; Reinke, S.; et al. Individual immune cell and cytokine profiles determine platelet-rich plasma composition. Arthritis Res. Ther. 2023, 25, 6. [Google Scholar] [CrossRef]
- Patriarca, F.; Skert, C.; Sperotto, A.; Zaja, F.; Falleti, E.; Mestroni, R.; Kikic, F.; Calistri, E.; Filì, C.; Geromin, A.; et al. The development of autoantibodies after allogeneic stem cell transplantation is related with chronic graft-vs-host disease and immune recovery. Exp. Hematol. 2006, 34, 389–396. [Google Scholar] [CrossRef]
- Yi, P.; Jiang, J.; Wang, Z.; Wang, X.; Zhao, M.; Wu, H.; Ding, Y. Comparison of mean platelet volume (MPV) and red blood cell distribution width (RDW) between psoriasis patients and controls: A systematic review and meta-analysis. PLoS ONE 2022, 17, e0264504. [Google Scholar] [CrossRef]
- Nakahara, T.; Kido-Nakahara, M.; Tsuji, G.; Furue, M. Basics and recent advances in the pathophysiology of atopic dermatitis. J. Dermatol. 2021, 48, 130–139. [Google Scholar] [CrossRef]
- Cianciulli, A.; Calvello, R.; Porro, C.; Lofrumento, D.D.; Panaro, M.A. Inflammatory Skin Diseases: Focus on the Role of Suppressors of Cytokine Signaling (SOCS) Proteins. Cells 2024, 13, 505. [Google Scholar] [CrossRef]
- Parisi, R.; Iskandar, I.Y.K.; Kontopantelis, E.; Augustin, M.; Griffiths, C.E.M.; Ashcroft, D.M. National, regional, and worldwide epidemiology of psoriasis: Systematic analysis and modelling study. BMJ (Clin. Res. Ed.) 2020, 369, m1590. [Google Scholar] [CrossRef]
- Orsmond, A.; Bereza-Malcolm, L.; Lynch, T.; March, L.; Xue, M. Skin Barrier Dysregulation in Psoriasis. Int. J. Mol. Sci. 2021, 22, 10841. [Google Scholar] [CrossRef]
- Farrell, A.M.; Dean, D.; Millard, P.R.; Charnock, F.M.; Wojnarowska, F. Cytokine alterations in lichen sclerosus: An immunohistochemical study. Br. J. Dermatol. 2006, 155, 931–940. [Google Scholar] [CrossRef]
- Johnson, B.Z.; Stevenson, A.W.; Prele, C.M.; Fear, M.W.; Wood, F.M. The Role of IL-6 in Skin Fibrosis and Cutaneous Wound Healing. Biomedicines 2020, 8, 101. [Google Scholar] [CrossRef]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef] [PubMed]
- Matarazzo, L.; Hernandez Santana, Y.E.; Walsh, P.T.; Fallon, P.G. The IL-1 cytokine family as custodians of barrier immunity. Cytokine 2022, 154, 155890. [Google Scholar] [CrossRef]
- Bergman, R.; Ramon, M.; Wildbaum, G.; Avitan-Hersh, E.; Mayer, E.; Shemer, A.; Karin, N. Psoriasis patients generate increased serum levels of autoantibodies to tumor necrosis factor-alpha and interferon-alpha. J. Dermatol. Sci. 2009, 56, 163–167. [Google Scholar] [CrossRef]
- Bernadetta; Hutagalung, M.R.; Hariani, L. Clinical manifestations of allergic reactions and immunoglobulin E levels on allogeneic freeze-dried platelet-rich plasma (PRP) application. Bali Med. J. 2023, 12, 1167–1173. [Google Scholar] [CrossRef]
- Anitua, E.; de la Fuente, M.; Muruzábal, F.; Merayo-Lloves, J. Short- and Long-Term Stability of Plasma Rich in Growth Factors Eye Drops. Cornea 2021, 40, 107–112. [Google Scholar] [CrossRef]
- Anitua, E.; Muruzabal, F.; De la Fuente, M.; Merayo-Lloves, J.; Orive, G. Effects of heat-treatment on plasma rich in growth factors-derived autologous eye drop. Exp. Eye Res. 2014, 119, 27–34. [Google Scholar] [CrossRef]
- Wiśniewski, J.R.; Zougman, A.; Nagaraj, N.; Mann, M. Universal sample preparation method for proteome analysis. Nat. Methods 2009, 6, 359–362. [Google Scholar] [CrossRef]
- Cox, J.; Mann, M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 2008, 26, 1367–1372. [Google Scholar] [CrossRef] [PubMed]
- Tyanova, S.; Temu, T.; Sinitcyn, P.; Carlson, A.; Hein, M.Y.; Geiger, T.; Mann, M.; Cox, J. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat. Methods 2016, 13, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Everitt, B.S.; Landau, S.; Leese, M. Cluster Analysis; Wiley: Hoboken, NJ, USA, 2001. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing, Version 4.2.3; R Foundation for Statistical Computing: Vienna, Austria, 2023.
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Bioinformatics enrichment tools: Paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009, 37, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Chen, M.; Huang, X.; Zhang, G.; Zeng, L.; Zhang, G.; Wu, S.; Wang, Y. SRplot: A free online platform for data visualization and graphing. PLoS ONE 2023, 18, e0294236. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Pant, G.; Bhavnasi, Y.K.; Blanchard, S.G., Jr.; Brouwer, C. Pathview Web: User friendly pathway visualization and data integration. Nucleic Acids Res. 2017, 45, W501–W508. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.; Rodrigues, P.M.; Pintado, M.; Tavaria, F.K. A systematic review of natural products for skin applications: Targeting inflammation, wound healing, and photo-aging. Phytomed. Int. J. Phytother. Phytopharm. 2023, 115, 154824. [Google Scholar] [CrossRef]
- Prasannanjaneyulu, V.; Nene, S.; Jain, H.; Nooreen, R.; Otavi, S.; Chitlangya, P.; Srivastava, S. Old drugs, new tricks: Emerging role of drug repurposing in the management of atopic dermatitis. Cytokine Growth Factor Rev. 2022, 65, 12–26. [Google Scholar] [CrossRef]
- Assfalg, M.; Bortoletti, E.; D’Onofrio, M.; Pigozzi, R.; Molinari, H.; Boner, A.L.; Peroni, D.; Piacentini, G. An exploratory 1H-nuclear magnetic resonance metabolomics study reveals altered urine spectral profiles in infants with atopic dermatitis. Br. J. Dermatol. 2012, 166, 1123–1125. [Google Scholar] [CrossRef]
- Ottas, A.; Fishman, D.; Okas, T.L.; Püssa, T.; Toomik, P.; Märtson, A.; Kingo, K.; Soomets, U. Blood serum metabolome of atopic dermatitis: Altered energy cycle and the markers of systemic inflammation. PLoS ONE 2017, 12, e0188580. [Google Scholar] [CrossRef]
- Cibrian, D.; de la Fuente, H.; Sánchez-Madrid, F. Metabolic Pathways That Control Skin Homeostasis and Inflammation. Trends Mol. Med. 2020, 26, 975–986. [Google Scholar] [CrossRef]
- Zhang, L.; Wen, X.; Hou, Y.; Yang, Y.; Song, W.; Zeng, Y.; Sun, J. Integrated metabolomics and lipidomics study of patients with atopic dermatitis in response to dupilumab. Front. Immunol. 2022, 13, 1002536. [Google Scholar] [CrossRef]
- Farhana, A.; Lappin, S.L. Biochemistry, Lactate Dehydrogenase. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Napolitano, M.; Megna, M.; Monfrecola, G. Insulin resistance and skin diseases. Sci. World J. 2015, 2015, 479354. [Google Scholar] [CrossRef]
- Savaş Erdoğan, S.; Falay Gür, T.; Özkur, E.; Doğan, B. Insulin Resistance and Metabolic Syndrome in Patients with Seborrheic Dermatitis: A Case-Control Study. Metab. Syndr. Relat. Disord. 2022, 20, 50–56. [Google Scholar] [CrossRef]
- Cho, H.-R.; Uhm, Y.-K.; Kim, H.-J.; Ban, J.-Y.; Chung, J.-H.; Yim, S.-V.; Choi, B.-K.; Lee, M.-H. Glutathione S-transferase M1 (GSTM1) polymorphism is associated with atopic dermatitis susceptibility in a Korean population. Int. J. Immunogenet. 2011, 38, 145–150. [Google Scholar] [CrossRef]
- Hu, Y.; Zhu, Y.; Lian, N.; Chen, M.; Bartke, A.; Yuan, R. Metabolic Syndrome and Skin Diseases. Front. Endocrinol. 2019, 10, 788. [Google Scholar] [CrossRef]
- Giang, J.; Seelen, M.A.J.; van Doorn, M.B.A.; Rissmann, R.; Prens, E.P.; Damman, J. Complement Activation in Inflammatory Skin Diseases. Front. Immunol. 2018, 9, 639. [Google Scholar] [CrossRef]
- Kapp, A.; Schopf, E. Involvement of complement in atopic dermatitis. Acta Derm. Venereol. Suppl. 1985, 114, 152–154. [Google Scholar]
- Panelius, J.; Meri, S. Complement system in dermatological diseases—Fire under the skin. Front. Med. 2015, 2, 3. [Google Scholar]
- Park, J.S.; Park, J.Y. Dermatomyositis sine dermatitis, a rare phenotype of idiopathic inflammatory myopathy. J. Yeungnam Med. Sci. 2017, 34, 137–139. [Google Scholar] [CrossRef]
- Dunkelberger, J.R.; Song, W.C. Complement and its role in innate and adaptive immune responses. Cell Res. 2010, 20, 34–50. [Google Scholar] [CrossRef]
- Janeway, C. Immunobiology: The Immune System in Health and Disease; Garland Pub.: New York, NY, USA, 2001. [Google Scholar]
- Schartz, N.D.; Tenner, A.J. The good, the bad, and the opportunities of the complement system in neurodegenerative disease. J. Neuroinflamm. 2020, 17, 354. [Google Scholar] [CrossRef]
- Xie, C.B.; Jane-Wit, D.; Pober, J.S. Complement Membrane Attack Complex: New Roles, Mechanisms of Action, and Therapeutic Targets. Am. J. Pathol. 2020, 190, 1138–1150. [Google Scholar] [CrossRef]
- Dahl, M.V.; Falk, R.J.; Carpenter, R.; Michael, A.F. Membrane attack complex of complement in dermatitis herpetiformis. Arch. Dermatol. 1985, 121, 70–72. [Google Scholar] [CrossRef]
- Cugno, M.; Borghi, A.; Garcovich, S.; Marzano, A.V. Coagulation and Skin Autoimmunity. Front. Immunol. 2019, 10, 1407. [Google Scholar] [CrossRef]
- Atkins, P.C.; Miragliotta, G.; Talbot, S.F.; Zweiman, B.; Kaplan, A.P. Activation of plasma Hageman factor and kallikrein in ongoing allergic reactions in the skin. J. Immunol. 1987, 139, 2744–2748. [Google Scholar] [CrossRef]
- Wollenberg, A.; Thomsen, S.F.; Lacour, J.P.; Jaumont, X.; Lazarewicz, S. Targeting immunoglobulin E in atopic dermatitis: A review of the existing evidence. World Allergy Organ. J. 2021, 14, 100519. [Google Scholar] [CrossRef]
- Nebert, D.W.; Vasiliou, V. Analysis of the glutathione S-transferase (GST) gene family. Hum. Genom. 2004, 1, 460–464. [Google Scholar] [CrossRef]
- Campione, E.; Mazzilli, S.; Lanna, C.; Cosio, T.; Palumbo, V.; Cesaroni, G.; Lozzi, F.; Diluvio, L.; Bianchi, L. The Effectiveness of a New Topical Formulation Containing GSH-C4 and Hyaluronic Acid in Seborrheic Dermatitis: Preliminary Results of an Exploratory Pilot Study. Clin. Cosmet. Investig. Dermatol. 2019, 12, 881–885. [Google Scholar] [CrossRef]
- Chung, J.; Oh, S.Y.; Shin, Y.K. Association of glutathione-S-transferase polymorphisms with atopic dermatitis risk in preschool age children. Clin. Chem. Lab. Med. 2009, 47, 1475–1481. [Google Scholar] [CrossRef]
- Hsu, C.H.; Chua, K.Y.; Huang, S.K.; Chiang, I.P.; Hsieh, K.H. Glutathione-S-transferase induces murine dermatitis that resembles human atopic dermatitis. Clin. Exp. Allergy 1996, 26, 1329–1337. [Google Scholar] [CrossRef]
- Lutz, W.; Tarkowski, M.; Nowakowska, E. Genetic polymorphism of glutathione s-transferase as a factor predisposing to allergic dermatitis. Med. Pr. 2001, 52, 45–51. [Google Scholar] [PubMed]
- Cho, Y.W.; Song, K.Y.; Park, S.C.; Ro, B.I. Immunohistochemical Study of Pi Class of Glutathione S-Transferase in Psoriasis and Eczematous Dermatitis. Ann. Dermatol. 1994, 6, 136–139. [Google Scholar] [CrossRef]
- Davoine, C.; Bouckaert, C.; Fillet, M.; Pochet, L. Factor XII/XIIa inhibitors: Their discovery, development, and potential indications. Eur. J. Med. Chem. 2020, 208, 112753. [Google Scholar] [CrossRef] [PubMed]
- Didiasova, M.; Wujak, L.; Schaefer, L.; Wygrecka, M. Factor XII in coagulation, inflammation and beyond. Cell Signal. 2018, 51, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Matus, C.E.; Bhoola, K.D.; Figueroa, C.D. Kinin B1 Receptor Signaling in Skin Homeostasis and Wound Healing. Yale J. Biol. Med. 2020, 93, 175–185. [Google Scholar]
- Pail, P.B.; Neculqueo, G.W.; Maccari, G.P.; Chagastelles, P.C.; Freitas, R.D.; Dagnino, A.P.A.; Campos, M.M. The role of kinin B1 and B2 receptors in the mouse model of oxazolone-induced atopic dermatitis. Int. Immunopharmacol. 2019, 72, 62–73. [Google Scholar] [CrossRef]
- Cichon, S.; Martin, L.; Hennies, H.C.; Müller, F.; Van Driessche, K.; Karpushova, A.; Stevens, W.; Colombo, R.; Renne, T.; Drouet, C.; et al. Increased activity of coagulation factor XII (Hageman factor) causes hereditary angioedema type III. Am. J. Hum. Genet. 2006, 79, 1098–1104. [Google Scholar] [CrossRef]
- Deroux, A.; Boccon-Gibod, I.; Fain, O.; Pralong, P.; Ollivier, Y.; Pagnier, A.; Djenouhat, K.; Du-Thanh, A.; Gompel, A.; Faisant, C.; et al. Hereditary angioedema with normal C1 inhibitor and factor XII mutation: A series of 57 patients from the French National Center of Reference for Angioedema. Clin. Exp. Immunol. 2016, 185, 332–337. [Google Scholar] [CrossRef]
- Renné, T.; Stavrou, E.X. Roles of Factor XII in Innate Immunity. Front. Immunol. 2019, 10, 2011. [Google Scholar] [CrossRef]
- Carbone, C.; Piro, G.; Merz, V.; Simionato, F.; Santoro, R.; Zecchetto, C.; Tortora, G.; Melisi, D. Angiopoietin-Like Proteins in Angiogenesis, Inflammation and Cancer. Int. J. Mol. Sci. 2018, 19, 431. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Olesen, C.M.; Pavel, A.B.; Clausen, M.-L.; Wu, J.; Estrada, Y.; Zhang, N.; Agner, T.; Guttman-Yassky, E. Tape-Strip Proteomic Profiling of Atopic Dermatitis on Dupilumab Identifies Minimally Invasive Biomarkers. Front. Immunol. 2020, 11, 1768. [Google Scholar] [CrossRef]
- Mesko, B.; Poliska, S.; Szegedi, A.; Szekanecz, Z.; Palatka, K.; Papp, M.; Nagy, L. Peripheral blood gene expression patterns discriminate among chronic inflammatory diseases and healthy controls and identify novel targets. BMC Med. Genom. 2010, 3, 15. [Google Scholar] [CrossRef] [PubMed]
- Mezu-Ndubuisi, O.J.; Maheshwari, A. The role of integrins in inflammation and angiogenesis. Pediatr. Res. 2021, 89, 1619–1626. [Google Scholar] [CrossRef]
- Yamaguchi, H.L.; Yamaguchi, Y.; Peeva, E. Role of Innate Immunity in Allergic Contact Dermatitis: An Update. Int. J. Mol. Sci. 2023, 24, 12975. [Google Scholar] [CrossRef]
- Hunyadi, J.; Simon, M.; Jr Kenderessy, A.S.; Dobozy, A. Expression of monocyte/macrophage markers (CD13, CD14, CD68) on human keratinocytes in healthy and diseased skin. J. Dermatol. 1993, 20, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Mina-Osorio, P. The moonlighting enzyme CD13: Old and new functions to target. Trends Mol. Med. 2008, 14, 361–371. [Google Scholar] [CrossRef]
- Bhagwat, S.V.; Lahdenranta, J.; Giordano, R.; Arap, W.; Pasqualini, R.; Shapiro, L.H. CD13/APN is activated by angiogenic signals and is essential for capillary tube formation. Blood 2001, 97, 652–659. [Google Scholar] [CrossRef]
- Rangel, R.; Sun, Y.; Guzman-Rojas, L.; Ozawa, M.G.; Sun, J.; Giordano, R.J.; Van Pelt, C.S.; Tinkey, P.T.; Behringer, R.R.; Sidman, R.L.; et al. Impaired angiogenesis in aminopeptidase N-null mice. Proc. Natl. Acad. Sci. USA 2007, 104, 4588–4593. [Google Scholar] [CrossRef]
- Chan, I. The role of extracellular matrix protein 1 in human skin. Clin. Exp. Dermatol. 2004, 29, 52–56. [Google Scholar] [CrossRef]
- Sercu, S.; Zhang, M.; Oyama, N.; Hansen, U.; Ghalbzouri, A.E.; Jun, G.; Geentjens, K.; Zhang, L.; Merregaert, J.H. Interaction of extracellular matrix protein 1 with extracellular matrix components: ECM1 is a basement membrane protein of the skin. J. Investig. Dermatol. 2008, 128, 1397–1408. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.A.; Tan, X.; Macri, C.J.; Goldstein, A.T.; Fu, S.W. Lichen Sclerosus: An autoimmunopathogenic and genomic enigma with emerging genetic and immune targets. Int. J. Biol. Sci. 2019, 15, 1429–1439. [Google Scholar] [CrossRef] [PubMed]
- Muszbek, L.; Bereczky, Z.; Bagoly, Z.; Komáromi, I.; Katona, É. Factor XIII: A coagulation factor with multiple plasmatic and cellular functions. Physiol. Rev. 2011, 91, 931–972. [Google Scholar] [CrossRef] [PubMed]
- Lucientes-Continente, L.; Marquez-Tirado, B.; Goicoechea de Jorge, E. The Factor H protein family: The switchers of the complement alternative pathway. Immunol. Rev. 2023, 313, 25–45. [Google Scholar] [CrossRef] [PubMed]
- Peoples, N.; Strang, C. Complement Activation in the Central Nervous System: A Biophysical Model for Immune Dysregulation in the Disease State. Front. Mol. Neurosci. 2021, 14, 620090. [Google Scholar] [CrossRef]
- Krishnan, V.; Xu, Y.; Macon, K.; Volanakis, J.E.; Narayana, S.V. The structure of C2b, a fragment of complement component C2 produced during C3 convertase formation. Acta Crystallogr. D Biol. Crystallogr. 2009, 65 Pt 3, 266–274. [Google Scholar] [CrossRef]
- Barnum, S.R.; Schein, T.N. The Complement FactsBook; Elsevier Science: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Rus, H.; Cudrici, C.; Niculescu, F. The role of the complement system in innate immunity. Immunol. Res. 2005, 33, 103–112. [Google Scholar] [CrossRef]
- Morley, B.J.; Walport, M. The Complement FactsBook; Elsevier Science: Amsterdam, The Netherlands, 2000. [Google Scholar]
- Le, G.T.; Abbenante, G.; Fairlie, D.P. Profiling the enzymatic properties and inhibition of human complement factor B. J. Biol. Chem. 2007, 282, 34809–34816. [Google Scholar] [CrossRef]
- Hourcade, D.E.; Mitchell, L.M. Access to the complement factor B scissile bond is facilitated by association of factor B with C3b protein. J. Biol. Chem. 2011, 286, 35725–35732. [Google Scholar] [CrossRef]
- Heesterbeek, D.A.; Bardoel, B.W.; Parsons, E.S.; Bennett, I.; Ruyken, M.; Doorduijn, D.J.; Gorham, R.D.; Berends, E.T.; Pyne, A.L.; Hoogenboom, B.W.; et al. Bacterial killing by complement requires membrane attack complex formation via surface-bound C5 convertases. EMBO J. 2019, 38, e99852. [Google Scholar] [CrossRef]
- Barthel, D.; Schindler, S.; Zipfel, P.F. Plasminogen is a complement inhibitor. J. Biol. Chem. 2012, 287, 18831–18842. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.F.; Ward, P.A. Role of C5a in inflammatory responses. Annu. Rev. Immunol. 2005, 23, 821–852. [Google Scholar] [CrossRef] [PubMed]
- Ruest, M.K.; Dennis, J.J. The Exploration of Complement-Resistance Mechanisms of Pathogenic Gram-Negative Bacteria to Support the Development of Novel Therapeutics. Pathogens 2022, 11, 931. [Google Scholar] [CrossRef] [PubMed]
- Morser, J.; Gabazza, E.C.; Myles, T.; Leung, L.L. What has been learnt from the thrombin-activatable fibrinolysis inhibitor-deficient mouse? J. Thromb. Haemost. JTH 2010, 8, 868–876. [Google Scholar] [CrossRef] [PubMed]
- Morser, J.; Shao, Z.; Nishimura, T.; Zhou, Q.; Zhao, L.; Higgins, J.; Leung, L.L.K. Carboxypeptidase B2 and N play different roles in regulation of activated complements C3a and C5a in mice. J. Thromb. Haemost. JTH 2018, 16, 991–1002. [Google Scholar] [CrossRef] [PubMed]
- Kapp, A.; Wokalek, H.; Schopf, E. Involvement of complement in psoriasis and atopic dermatitis—Measurement of C3a and C5a, C3, C4 and C1 inactivator. Arch. Dermatol. Res. 1985, 277, 359–361. [Google Scholar] [CrossRef]
- Tagami, H. The role of complement-derived mediators in inflammatory skin diseases. Arch. Dermatol. Res. 1992, 284 (Suppl. S1), S2–S9. [Google Scholar] [CrossRef]



| Study Group | Mean Age (Years) | Sex (Number of Patients) | |
|---|---|---|---|
| Male | Female | ||
| Healthy | 51 (range: 33 to 69) | 2 | 1 |
| Atopic Dermatitis | 28 (range: 15 to 52) | 1 | 2 |
| Psoriasis | 50 (range: 36 to 65) | 0 | 3 |
| Lichen sclerosus | 41 (range: 20 to 61) | 0 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anitua, E.; Tierno, R.; Azkargorta, M.; Elortza, F.; Alkhraisat, M.H. Effect of Health Status and Heat-Induced Inactivation on the Proteomic Profile of Plasma Rich in Growth Factors Obtained from Donors with Chronic Inflammatory Skin Conditions. Biomolecules 2024, 14, 763. https://doi.org/10.3390/biom14070763
Anitua E, Tierno R, Azkargorta M, Elortza F, Alkhraisat MH. Effect of Health Status and Heat-Induced Inactivation on the Proteomic Profile of Plasma Rich in Growth Factors Obtained from Donors with Chronic Inflammatory Skin Conditions. Biomolecules. 2024; 14(7):763. https://doi.org/10.3390/biom14070763
Chicago/Turabian StyleAnitua, Eduardo, Roberto Tierno, Mikel Azkargorta, Félix Elortza, and Mohammad H. Alkhraisat. 2024. "Effect of Health Status and Heat-Induced Inactivation on the Proteomic Profile of Plasma Rich in Growth Factors Obtained from Donors with Chronic Inflammatory Skin Conditions" Biomolecules 14, no. 7: 763. https://doi.org/10.3390/biom14070763






