Skin Surface Sebum Analysis by ESI-MS
Abstract
:1. Introduction
1.1. Potential of Sebum Sampling
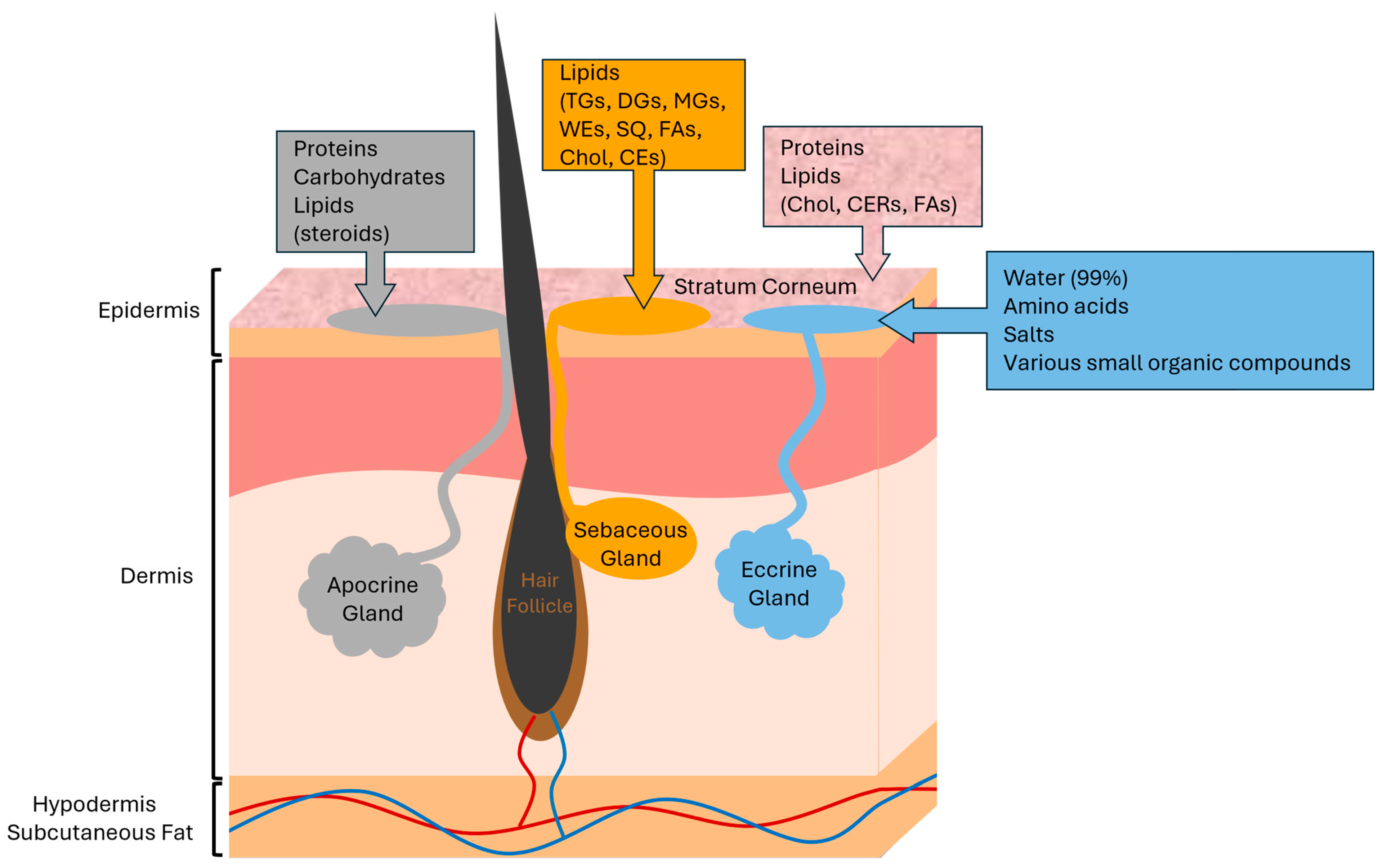
1.2. The Skin Surface
1.3. Sebaceous Glands
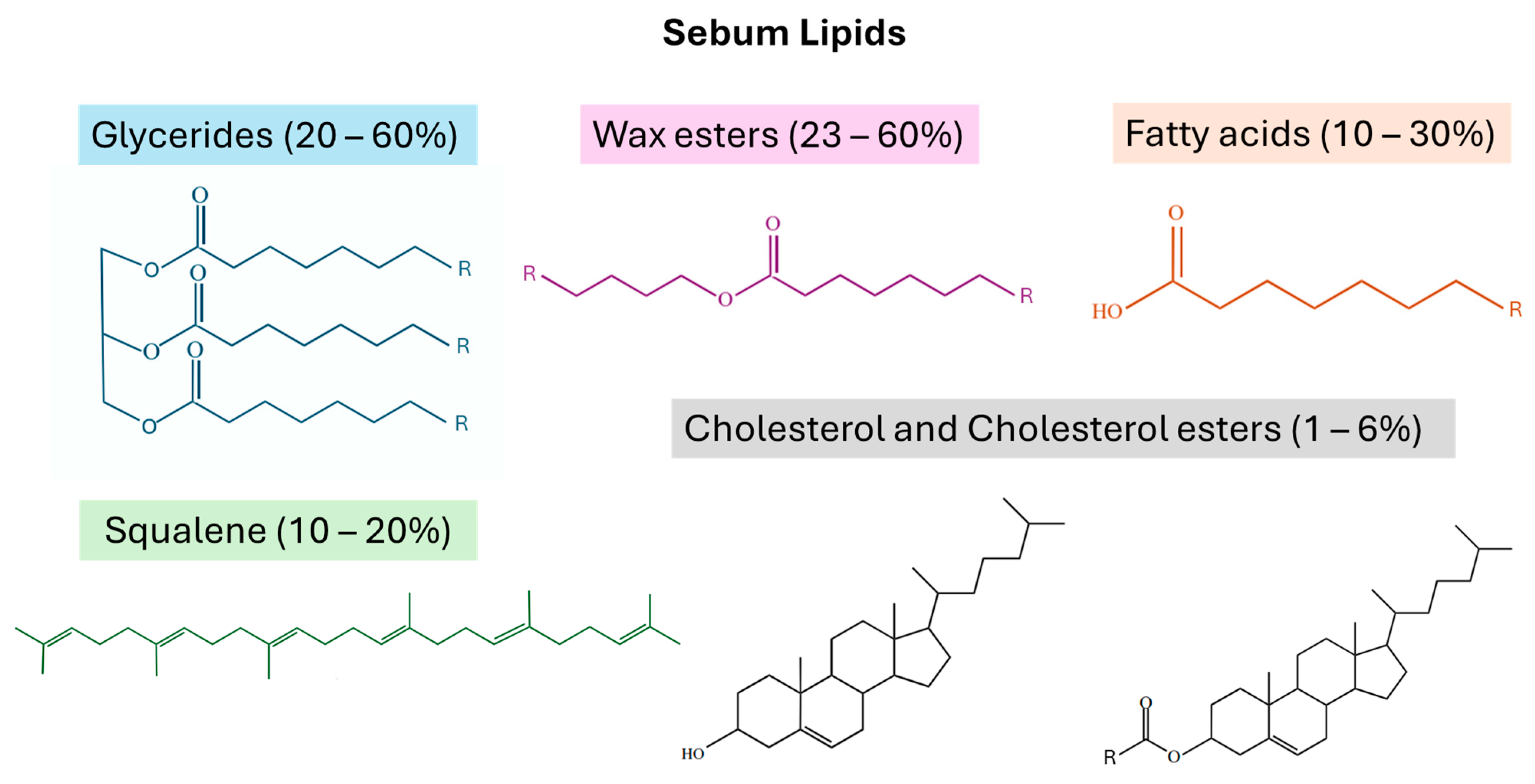
1.4. Sebum Lipids and Contaminants
1.5. ESI-MS Analysis of Sebum Lipids
2. Sebum Sampling from the Skin
2.1. Overview of Skin Surface Sampling Techniques
2.2. Participant Selection and Regimen Prior to Sampling
2.3. Anatomical Collection Regions
2.4. Collection Methods for Skin Surface Lipids
2.5. Sample Storage
3. Sample Preparation Prior to ESI-MS
3.1. Lipid Extraction Methods
3.2. Sample Preparation Prior to ESI-MS
3.3. Liquid Chromatography Separation
3.4. Shotgun Lipidomics
4. ESI-MS and ESI-MS/MS of Sebum Lipids
4.1. ESI-MS
4.2. ESI-MS/MS
5. Ambient ESI-MS Approaches to Sebum Analysis
5.1. Overview of Ambient ESI-MS
5.2. DESI-MS
5.3. SESI-MS
5.4. PSI-MS
6. Data Analysis of Sebum Lipids
6.1. Overview of Data Analysis Approaches
6.2. Types of Variability
6.3. Normalization Strategies
6.4. Machine Learning Methods
6.5. Lipid Identification
7. Applications
7.1. Clinical Biomarkers
7.2. Forensic Implications
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ACE | acetone |
| ANOVA | analysis of variance |
| AUC | area under the ROC curve |
| CE | cholesterol ester |
| CER | ceramide |
| Chol | cholesterol |
| DCM | dichloromethane |
| DESI | desorption electrospray ionization |
| DG | diglyceride |
| ESI | electrospray ionization |
| EtOH | ethanol |
| FA | fatty acid |
| GDBT | gradient boosting tree ensemble |
| GL | glycerolipid |
| GP | glycerophospholipid |
| HILIC | hydrophilic interaction liquid chromatography |
| HPLC | High-performance liquid chromatography |
| IM | ion mobility |
| IPA | isopropanol |
| LC | liquid chromatography |
| LOOCV | leave-one-out cross-validation |
| MeOH | methanol |
| MG | monoglyceride |
| NP | normal-phase |
| OPLS-DA | orthogonal partial least squares discriminant analysis |
| PCA | principal component analysis |
| PK | polyketide |
| PL | phospholipid |
| PLS-DA | partial least squares discriminant analysis |
| PR | prenol lipid |
| PSI | paper spray ionization |
| Q | single quadrupole |
| QqQ | triple quadrupole |
| RF | random forest |
| ROC | receiver operator characteristic |
| RP | reversed-phase |
| RR | rapid resolution |
| SESI | secondary electrospray ionization |
| SL | saccharolipid |
| SM | sphingomyelin |
| SP | sphingolipid |
| SPE | solid-phase extraction |
| SQ | squalene |
| ST | sterol lipid |
| SVM | support vector machine |
| TD | thermal desorption |
| TG | triglyceride |
| TOF | time-of-flight |
| TCM | trichloromethane (chloroform) |
| UPLC | ultra performance liquid chromatography |
| VIP | variable importance of projection |
| WE | wax ester |
| XGBoost | extreme gradient boosting |
| zvPSI | zero-volt paper spray ionization |
References
- Zouboulis, C.C.; Picardo, M.; Ju, Q.; Kurokawa, I.; Törőcsik, D.; Bíró, T.; Schneider, M.R. Beyond Acne: Current Aspects of Sebaceous Gland Biology and Function. Rev. Endocr. Metab. Disord. 2016, 17, 319–334. [Google Scholar] [CrossRef] [PubMed]
- Pappas, A. Epidermal surface lipids. Dermato-Endocrinology 2009, 1, 72–76. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhou, Z.; Zare, R.N. Personal Information from Latent Fingerprints Using Desorption Electrospray Ionization Mass Spectrometry and Machine Learning. Anal. Chem. 2017, 89, 1369–1372. [Google Scholar] [CrossRef]
- Sadowski, T.; Klose, C.; Gerl, M.J.; Wójcik-Maciejewicz, A.; Herzog, R.; Simons, K.; Reich, A.; Surma, M.A. Large-Scale Human Skin Lipidomics by Quantitative, High-Throughput Shotgun Mass Spectrometry. Sci. Rep. 2017, 7, 43761. [Google Scholar] [CrossRef]
- Agrawal, K.; Hassoun, L.A.; Foolad, N.; Borkowski, K.; Pedersen, T.L.; Sivamani, R.K.; Newman, J.W. Effects of Atopic Dermatitis and Gender on Sebum Lipid Mediator and Fatty Acid Profiles. Prostaglandins Leukot. Essent. Fatty Acids 2018, 134, 7–16. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, K.C.; Hinners, P.; Lee, Y.J. Potential of Triacylglycerol Profiles in Latent Fingerprints to Reveal Individual Diet, Exercise, or Health Information for Forensic Evidence. Anal. Methods 2020, 12, 792–798. [Google Scholar] [CrossRef]
- Bouslimani, A.; Melnik, A.V.; Xu, Z.; Amir, A.; Da Silva, R.R.; Wang, M.; Bandeira, N.; Alexandrov, T.; Knight, R.; Dorrestein, P.C. Lifestyle Chemistries from Phones for Individual Profiling. Proc. Natl. Acad. Sci. USA 2016, 113, E7645. [Google Scholar] [CrossRef]
- Hinners, P.; Thomas, M.; Lee, Y.J. Determining Fingerprint Age with Mass Spectrometry Imaging via Ozonolysis of Triacylglycerols. Anal. Chem. 2020, 92, 3125–3132. [Google Scholar] [CrossRef]
- Pleik, S.; Spengler, B.; Ram Bhandari, D.; Luhn, S.; Schäfer, T.; Urbach, D.; Kirsch, D. Ambient-Air Ozonolysis of Triglycerides in Aged Fingerprint Residues. Analyst 2018, 143, 1197–1209. [Google Scholar] [CrossRef]
- Sinclair, E.; Trivedi, D.K.; Sarkar, D.; Walton-Doyle, C.; Milne, J.; Kunath, T.; Rijs, A.M.; de Bie, R.M.A.; Goodacre, R.; Silverdale, M.; et al. Metabolomics of Sebum Reveals Lipid Dysregulation in Parkinson’s Disease. Nat. Commun. 2021, 12, 1592. [Google Scholar] [CrossRef]
- Sarkar, D.; Sinclair, E.; Lim, S.H.; Walton-Doyle, C.; Jafri, K.; Milne, J.; Vissers, J.P.C.; Richardson, K.; Trivedi, D.K.; Silverdale, M.; et al. Paper Spray Ionization Ion Mobility Mass Spectrometry of Sebum Classifies Biomarker Classes for the Diagnosis of Parkinson’s Disease. JACS Au 2022, 2, 2013–2022. [Google Scholar] [CrossRef]
- Li, H.; Ma, Y.; Feng, N.; Wang, W.; He, C. Exploration of Potential Biomarkers for Type 2 Diabetes by UPLC-QTOF-MS and WGCNA of Skin Surface Lipids. Clin. Cosmet. Investig. Dermatol. 2022, 15, 87–96. [Google Scholar] [CrossRef]
- Delafiori, J.; Siciliano, R.F.; Noin De Oliveira, A.; Nicolau, J.C.; Sales, G.M.; Dalçóquio, T.F.; Brandt Busanello, E.N.; Eguti, A.; Noin De Oliveira, D.; Bertolin, A.J.; et al. Skin Imprints to Provide Noninvasive Metabolic Profiling of COVID-19 Patients. MedRxiv 2021. [Google Scholar] [CrossRef]
- Spick, M.; Longman, K.; Frampas, C.; Lewis, H.; Costa, C.; Walters, D.D.; Stewart, A.; Wilde, M.; Greener, D.; Evetts, G.; et al. Changes to the Sebum Lipidome upon COVID-19 Infection Observed via Rapid Sampling from the Skin. EClinicalMedicine 2021, 33, 100786. [Google Scholar] [CrossRef] [PubMed]
- Spick, M.; Lewis, H.M.; Frampas, C.F.; Longman, K.; Costa, C.; Stewart, A.; Dunn-Walters, D.; Greener, D.; Evetts, G.; Wilde, M.J.; et al. An Integrated Analysis and Comparison of Serum, Saliva and Sebum for COVID-19 Metabolomics. Sci. Rep. 2022, 12, 11867. [Google Scholar] [CrossRef]
- Esteves, C.Z.; de Aguiar Dias, L.; de Oliveira Lima, E.; de Oliveira, D.N.; Odir Rodrigues Melo, C.F.; Delafiori, J.; Souza Gomez, C.C.; Ribeiro, J.D.; Ribeiro, A.F.; Levy, C.E.; et al. Skin Biomarkers for Cystic Fibrosis: A Potential Non-Invasive Approach for Patient Screening. Front. Pediatr. 2018, 5, 290. [Google Scholar] [CrossRef]
- Zhou, Z.; Alvarez, D.; Milla, C.; Zare, R.N.; Agar, N.Y.R.; Jarmusch, A.K.; Sharma, B. Proof of Concept for Identifying Cystic Fibrosis from Perspiration Samples. Proc. Natl. Acad. Sci. USA 2019, 116, 24408–24412. [Google Scholar] [CrossRef]
- Ishikawa, J.; Narita, H.; Kondo, N.; Hotta, M.; Takagi, Y.; Masukawa, Y.; Kitahara, T.; Takema, Y.; Koyano, S.; Yamazaki, S.; et al. Changes in the Ceramide Profile of Atopic Dermatitis Patients. J. Investig. Dermatol. 2010, 130, 2511–2514. [Google Scholar] [CrossRef]
- Yin, H.; Qiu, Z.; Zhu, R.; Wang, S.; Gu, C.; Yao, X.; Li, W. Dysregulated Lipidome of Sebum in Patients with Atopic Dermatitis. Allergy Eur. J. Allergy Clin. Immunol. 2023, 78, 1524–1537. [Google Scholar] [CrossRef]
- Berdyshev, E.; Goleva, E.; Bronova, I.; Dyjack, N.; Rios, C.; Jung, J.; Taylor, P.; Jeong, M.; Hall, C.F.; Richers, B.N.; et al. Lipid Abnormalities in Atopic Skin Are Driven by Type 2 Cytokines. JCI Insight 2018, 3, e98006. [Google Scholar] [CrossRef]
- Berdyshev, E.; Kim, J.; Kim, B.E.; Goleva, E.; Lyubchenko, T.; Bronova, I.; Bronoff, A.S.; Xiao, O.; Kim, J.; Kim, S.; et al. Stratum Corneum Lipid and Cytokine Biomarkers at Age 2 Months Predict the Future Onset of Atopic Dermatitis. J. Allergy Clin. Immunol. 2023, 151, 1307–1316. [Google Scholar] [CrossRef]
- Lima, E.D.O.; De Macedo, C.S.; Esteves, C.Z.; De Oliveira, D.N.; Pessolani, M.C.V.; Nery, J.A.D.C.; Sarno, E.N.; Catharino, R.R. Skin Imprinting in Silica Plates: A Potential Diagnostic Methodology for Leprosy Using High-Resolution Mass Spectrometry. Anal. Chem. 2015, 87, 3585–3592. [Google Scholar] [CrossRef]
- Shores, D.R.; Everett, A.D. Children as Biomarker Orphans: Progress in the Field of Pediatric Biomarkers. J. Pediatr. 2018, 193, 14–20.e31. [Google Scholar] [CrossRef]
- Isom, M.; Go, E.P.; Desaire, H. Enabling Lipidomic Biomarker Studies for Protected Populations by Combining Noninvasive Fingerprint Sampling with MS Analysis and Machine Learning. J. Proteome Res. 2024. [Google Scholar] [CrossRef]
- Knox, S.; O’Boyle, N.M. Skin Lipids in Health and Disease: A Review. Chem. Phys. Lipids 2021, 236, 105055. [Google Scholar] [CrossRef]
- Köfeler, H.C.; Ahrends, R.; Baker, E.S.; Ekroos, K.; Han, X.; Hoffmann, N.; Holcapek, M.; Wenk, M.R.; Liebisch, G. Recommendations for Good Practice in Ms-Based Lipidomics. J. Lipid Res. 2021, 62, 100138. [Google Scholar] [CrossRef]
- Smirnov, D.; Mazin, P.; Osetrova, M.; Stekolshchikova, E.; Khrameeva, E. The Hitchhiker’s Guide to Untargeted Lipidomics Analysis: Practical Guidelines. Metabolites 2021, 11, 713. [Google Scholar] [CrossRef]
- Girod, A.; Ramotowski, R.; Weyermann, C. Composition of Fingermark Residue: A Qualitative and Quantitative Review. Forensic Sci. Int. 2012, 223, 10–24. [Google Scholar] [CrossRef]
- Géhin, C.; Tokarska, J.; Fowler, S.J.; Barran, P.E.; Trivedi, D.K. No Skin off Your Back: The Sampling and Extraction of Sebum for Metabolomics. Metabolomics 2023, 19, 21. [Google Scholar] [CrossRef]
- Elpa, D.P.; Chiu, H.Y.; Wu, S.P.; Urban, P.L. Skin Metabolomics. Trends Endocrinol. Metab. 2021, 32, 66–75. [Google Scholar] [CrossRef]
- Li, S.; Ganguli-Indra, G.; Indra, A.K. Lipidomic Analysis of Epidermal Lipids: A Tool to Predict Progression of Inflammatory Skin Disease in Humans. Expert. Rev. Proteom. 2016, 13, 451–456. [Google Scholar] [CrossRef]
- Nicolaou, A.; Harwood, J.L. Skin Lipids in Health and Disease. Lipid Technol. 2016, 28, 36–39. [Google Scholar] [CrossRef]
- Kendall, A.C.; Koszyczarek, M.M.; Jones, E.A.; Hart, P.J.; Towers, M.; Griffiths, C.E.M.; Morris, M.; Nicolaou, A. Lipidomics for Translational Skin Research: A Primer for the Uninitiated. Exp. Dermatol. 2018, 27, 721–728. [Google Scholar] [CrossRef]
- Ludovici, M.; Kozul, N.; Materazzi, S.; Risoluti, R.; Picardo, M.; Camera, E. Influence of the Sebaceous Gland Density on the Stratum Corneum Lipidome. Sci. Rep. 2018, 8, 11500. [Google Scholar] [CrossRef]
- Semkova, K.; Gergovska, M.; Kazandjieva, J.; Tsankov, N. Hyperhidrosis, Bromhidrosis, and Chromhidrosis: Fold (Intertriginous) Dermatoses. Clin. Dermatol. 2015, 33, 483–491. [Google Scholar] [CrossRef]
- Baker, L.B. Physiology of Sweat Gland Function: The Roles of Sweating and Sweat Composition in Human Health. Temperature 2019, 6, 211–259. [Google Scholar] [CrossRef]
- Knowles, A.M. Aspects of Physicochemical Methods for the Detection of Latent Fingerprints. J. Phys. E Sci. Instrum. 1978, 11, 713. [Google Scholar] [CrossRef]
- Cho, Y.T.; Su, H.; Wu, C.Y.; Huang, T.L.; Jeng, J.; Huang, M.Z.; Wu, D.C.; Shiea, J. Molecular Mapping of Sebaceous Squalene by Ambient Mass Spectrometry. Anal. Chem. 2021, 93, 16608–16617. [Google Scholar] [CrossRef]
- Cho, H.J.; Chung, B.Y.; Lee, H.B.; Kim, H.O.; Park, C.W.; Lee, C.H. Quantitative Study of Stratum Corneum Ceramides Contents in Patients with Sensitive Skin. J. Dermatol. 2012, 39, 295–300. [Google Scholar] [CrossRef]
- Ishikawa, J.; Shimotoyodome, Y.; Ito, S.; Miyauchi, Y.; Fujimura, T.; Kitahara, T.; Hase, T. Variations in the Ceramide Profile in Different Seasons and Regions of the Body Contribute to Stratum Corneum Functions. Arch. Dermatol. Res. 2013, 305, 151–162. [Google Scholar] [CrossRef]
- Masukawa, Y.; Narita, H.; Sato, H.; Naoe, A.; Kondo, N.; Sugai, Y.; Oba, T.; Homma, R.; Ishikawa, J.; Takagi, Y.; et al. Comprehensive Quantification of Ceramide Species in Human Stratum Corneum. J. Lipid Res. 2009, 50, 1708–1719. [Google Scholar] [CrossRef] [PubMed]
- Ifa, D.R.; Manicke, N.E.; Dill, A.L.; Cooks, R.G. Latent Fingerprint Chemical Imaging by Mass Spectrometry. Science 2008, 321, 805. [Google Scholar] [CrossRef] [PubMed]
- Szynkowska, M.I.; Czerski, K.; Rogowski, J.; Paryjczak, T.; Parczewski, A. ToF-SIMS Application in the Visualization and Analysis of Fingerprints after Contact with Amphetamine Drugs. Forensic Sci. Int. 2009, 184, e24–e26. [Google Scholar] [CrossRef] [PubMed]
- Zouboulis, C.C.; Coenye, T.; He, L.; Kabashima, K.; Kobayashi, T.; Niemann, C.; Nomura, T.; Oláh, A.; Picardo, M.; Quist, S.R.; et al. Sebaceous Immunobiology—Skin Homeostasis, Pathophysiology, Coordination of Innate Immunity and Inflammatory Response and Disease Associations. Front. Immunol. 2022, 13, 1029818. [Google Scholar] [CrossRef] [PubMed]
- Misra, N.; Clavaud, C.; Guinot, F.; Bourokba, N.; Nouveau, S.; Mezzache, S.; Palazzi, P.; Appenzeller, B.M.R.; Tenenhaus, A.; Leung, M.H.Y.; et al. Multi-Omics Analysis to Decipher the Molecular Link between Chronic Exposure to Pollution and Human Skin Dysfunction. Sci. Rep. 2021, 11, 18302. [Google Scholar] [CrossRef] [PubMed]
- Crowther, J.M. Method for Quantification of Oils and Sebum Levels on Skin Using the Sebumeter®. Int. J. Cosmet. Sci. 2016, 38, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Wei, F.; Xie, Y.; Lv, X.; Dong, X.; Chen, H. Research Advances Based on Mass Spectrometry for Profiling of Triacylglycerols in Oils and Fats and Their Applications. Electrophoresis 2018, 39, 1558–1568. [Google Scholar] [CrossRef]
- Chiu, H.H.; Kuo, C.H. Gas Chromatography-Mass Spectrometry-Based Analytical Strategies for Fatty Acid Analysis in Biological Samples. J. Food Drug Anal. 2020, 28, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Kiss, A.; Hopfgartner, G. Laser-Based Methods for the Analysis of Low Molecular Weight Compounds in Biological Matrices. Methods 2016, 104, 142–153. [Google Scholar] [CrossRef]
- Wilm, M. Principles of Electrospray Ionization. Mol. Cell. Proteom. 2011, 10. [Google Scholar] [CrossRef]
- Cech, N.B.; Enke, C.G. Practical Implications of Some Recent Studies in Electrospray Ionization Fundamentals. Mass. Spectrom. Rev. 2001, 20, 362–387. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Duan, Q.; Han, X. Strategies to Improve/Eliminate the Limitations in Shotgun Lipidomics. Proteomics 2020, 20, e1900070. [Google Scholar] [CrossRef] [PubMed]
- Märtens, A.; Holle, J.; Mollenhauer, B.; Wegner, A.; Kirwan, J.; Hiller, K. Instrumental Drift in Untargeted Metabolomics: Optimizing Data Quality with Intrastudy QC Samples. Metabolites 2023, 13, 665. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Bao, X.; Jiang, J.; Li, J. Evaluation and Correction of Injection Order Effects in LC-MS/MS Based Targeted Metabolomics. J. Chromatogr. B Analyt Technol. Biomed. Life Sci. 2022, 1212, 123513. [Google Scholar] [CrossRef] [PubMed]
- Desaire, H.; Stepler, K.E.; Robinson, R.A.S. Exposing the Brain Proteomic Signatures of Alzheimer’s Disease in Diverse Racial Groups: Leveraging Multiple Data Sets and Machine Learning. J. Proteome Res. 2022, 21, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Lozano, P.; de la Mora, J.F. On-Line Detection of Human Skin Vapors. J. Am. Soc. Mass. Spectrom. 2009, 20, 1060–1063. [Google Scholar] [CrossRef] [PubMed]
- Martin, H.J.; Reynolds, J.C.; Riazanskaia, S.; Thomas, C.L.P. High Throughput Volatile Fatty Acid Skin Metabolite Profiling by Thermal Desorption Secondary Electrospray Ionisation Mass Spectrometry. Analyst 2014, 139, 4279–4286. [Google Scholar] [CrossRef] [PubMed]
- Afghani, J.; Huelpuesch, C.; Schmitt-Kopplin, P.; Traidl-Hoffmann, C.; Reiger, M.; Mueller, C. Enhanced Access to the Health-Related Skin Metabolome by Fast, Reproducible and Non-Invasive Wet Prep Sampling. Metabolites 2021, 11, 415. [Google Scholar] [CrossRef] [PubMed]
- Minami-Hori, M.; Honma, M.; Fujii, M.; Nomura, W.; Kanno, K.; Hayashi, T.; Nakamura, E.; Nagaya, K.; Miyauchi, Y.; Fujimura, T.; et al. Developmental Alterations of Physical Properties and Components of Neonatal-Infantile Stratum Corneum of Upper Thighs and Diaper-Covered Buttocks during the 1st Year of Life. J. Dermatol. Sci. 2014, 73, 67–73. [Google Scholar] [CrossRef]
- Kendall, A.C.; Pilkington, S.M.; Wray, J.R.; Newton, V.L.; Griffiths, C.E.M.; Bell, M.; Watson, R.E.B.; Nicolaou, A. Menopause Induces Changes to the Stratum Corneum Ceramide Profile, Which Are Prevented by Hormone Replacement Therapy. Sci. Rep. 2022, 12, 21715. [Google Scholar] [CrossRef]
- Zhou, M.; Gan, Y.; He, C.; Chen, Z.; Jia, Y. Lipidomics Reveals Skin Surface Lipid Abnormity in Acne in Young Men. Br. J. Dermatol. 2018, 179, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Hu, Y.; Yu, X.; He, C.; Tian, Y. Analysis on the Difference of Skin Surface Lipids during Blue Light Therapy for Acne by Lipidomics. Biomed. Opt. Express 2022, 13, 3434. [Google Scholar] [CrossRef]
- Striesow, J.; Lackmann, J.W.; Ni, Z.; Wenske, S.; Weltmann, K.D.; Fedorova, M.; von Woedtke, T.; Wende, K. Oxidative Modification of Skin Lipids by Cold Atmospheric Plasma (CAP): A Standardizable Approach Using RP-LC/MS2 and DI-ESI/MS2. Chem. Phys. Lipids 2020, 226, 104786. [Google Scholar] [CrossRef]
- Ma, Y.; Cui, L.; Tian, Y.; He, C. Lipidomics Analysis of Facial Lipid Biomarkers in Females with Self-Perceived Skin Sensitivity. Health Sci. Rep. 2022, 5, e632. [Google Scholar] [CrossRef]
- Kawana, M.; Miyamoto, M.; Ohno, Y.; Kihara, A. Comparative Profiling and Comprehensive Quantification of Stratum Corneum Ceramides in Humans and Mice by LC/MS/MS. J. Lipid Res. 2020, 61, 884–895. [Google Scholar] [CrossRef] [PubMed]
- Camera, E.; Ludovici, M.; Tortorella, S.; Sinagra, J.L.; Capitanio, B.; Goracci, L.; Picardo, M. Use of Lipidomics to Investigate Sebum Dysfunction in Juvenile Acne. J. Lipid Res. 2016, 57, 1051–1058. [Google Scholar] [CrossRef]
- Camera, E.; Ludovici, M.; Galante, M.; Sinagra, J.L.; Picardo, M. Comprehensive Analysis of the Major Lipid Classes in Sebum by Rapid Resolution High-Performance Liquid Chromatography and Electrospray Mass Spectrometry. J. Lipid Res. 2010, 51, 3377–3388. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.H.; Shon, J.C.; Lee, K.; Kim, S.; Park, C.S.; Choi, E.H.; Lee, C.H.; Lee, H.S.; Liu, K.H. A Lipidomic Platform Establishment for Structural Identification of Skin Ceramides with Non-Hydroxyacyl Chains. Anal. Bioanal. Chem. 2014, 406, 1917–1932. [Google Scholar] [CrossRef]
- T’Kindt, R.; Jorge, L.; Dumont, E.; Couturon, P.; David, F.; Sandra, P.; Sandra, K. Profiling and Characterizing Skin Ceramides Using Reversed-Phase Liquid Chromatography-Quadrupole Time-of-Flight Mass Spectrometry. Anal. Chem. 2012, 84, 403–411. [Google Scholar] [CrossRef]
- Masukawa, Y.; Narita, H.; Shimizu, E.; Kondo, N.; Sugai, Y.; Oba, T.; Homma, R.; Ishikawa, J.; Takagi, Y.; Kitahara, T.; et al. Characterization of Overall Ceramide Species in Human Stratum Corneum. J. Lipid Res. 2008, 49, 1466–1476. [Google Scholar] [CrossRef]
- Dapic, I.; Kobetic, R.; Brkljacic, L.; Kezic, S.; Jakasa, I. Quantification of Free Fatty Acids in Human Stratum Corneum Using Tandem Mass Spectrometry and Surrogate Analyte Approach. Biomed. Chromatogr. 2018, 32, e4056. [Google Scholar] [CrossRef]
- Vietzke, J.-R.; Stragner, M.; Hintze, U. Separation and Identification of Ceramides in the Human Stratum Corneum by High-Performance Liquid Chromatography Coupled with Electrospray Ionization Mass Spectrometry and Electrospray Multiple-Stage Mass Spectrometry Profiling. Chromatographia 1999, 50, 15–20. [Google Scholar] [CrossRef]
- Vietzke, J.P.; Brandt, O.; Abeck, D.; Rapp, C.; Strassner, M.; Schreiner, V.; Hintze, U. Comparative Investigation of Human Stratum Corneum Ceramides. Lipids 2001, 36, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Berdyshev, E.; Goleva, E.; Bissonnette, R.; Bronova, I.; Bronoff, A.S.; Richers, B.N.; Garcia, S.; Ramirez-Gama, M.; Taylor, P.; Praestgaard, A.; et al. Dupilumab Significantly Improves Skin Barrier Function in Patients with Moderate-to-Severe Atopic Dermatitis. Allergy Eur. J. Allergy Clin. Immunol. 2022, 77, 3388–3397. [Google Scholar] [CrossRef]
- Pappas, A.; Kendall, A.C.; Brownbridge, L.C.; Batchvarova, N.; Nicolaou, A. Seasonal Changes in Epidermal Ceramides Are Linked to Impaired Barrier Function in Acne Patients. Exp. Dermatol. 2018, 27, 833–836. [Google Scholar] [CrossRef]
- Zeng, J.; Mekic, M.; Xu, X.; Loisel, G.; Zhou, Z.; Gligorovski, S.; Li, X. A Novel Insight into the Ozone-Skin Lipid Oxidation Products Observed by Secondary Electrospray Ionization High-Resolution Mass Spectrometry. Environ. Sci. Technol. 2020, 54, 13478–13487. [Google Scholar] [CrossRef]
- Raith, K.; Zellmer, S.; Lasch, J.; Neubert, R.H.H. Profiling of Human Stratum Corneum Ceramides by Liquid Chromatography-Electrospray Mass Spectrometry. Anal. Chim. Acta 2000, 418, 167–173. [Google Scholar] [CrossRef]
- Katona, M.; Dénes, J.; Skoumal, R.; Tóth, M.; Takáts, Z. Intact Skin Analysis by Desorption Electrospray Ionization Mass Spectrometry. Analyst 2011, 136, 835–840. [Google Scholar] [CrossRef]
- Motoyama, A.; Kihara, K. Zero Volt Paper Spray Ionization Mass Spectrometry for Direct Analysis of Samples on Filter Paper Substrate. Rapid Commun. Mass Spectrom. 2015, 29, 1905–1916. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A Rapid Method of Total Lipid Extraction and Purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Liebisch, G.; Binder, M.; Schifferer, R.; Langmann, T.; Schulz, B.; Schmitz, G. High Throughput Quantification of Cholesterol and Cholesteryl Ester by Electrospray Ionization Tandem Mass Spectrometry (ESI-MS/MS). Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2006, 1761, 121–128. [Google Scholar] [CrossRef]
- Takáts, Z.; Wiseman, J.M.; Gologan, B.; Graham Cooks, R. Mass Spectrometry Sampling Under Ambient Conditions with Desorption Electrospray Ionization. Science 2004, 306, 471–473. [Google Scholar] [CrossRef]
- Motoyama, A.; Kihara, K. Mass Spectrometry in Cosmetic Science: Advanced Ionization Techniques for Detecting Trace Molecules in or on Human Skin. Mass. Spectrometry 2017, 6, S0071. [Google Scholar] [CrossRef]
- Morato, N.M.; Cooks, R.G. Desorption Electrospray Ionization Mass Spectrometry: 20 Years. Acc. Chem. Res. 2023, 56, 2526–2536. [Google Scholar] [CrossRef]
- Cotte-Rodríguez, I.; Mulligan, C.C.; Cooks, R.G. Non-Proximate Detection of Small and Large Molecules by Desorption Electrospray Ionization and Desorption Atmospheric Pressure Chemical Ionization Mass Spectrometry: Instrumentation and Applications in Forensics, Chemistry, and Biology. Anal. Chem. 2007, 79, 7069–7077. [Google Scholar] [CrossRef]
- Abbassi-Ghadi, N.; Jones, E.A.; Gomez-Romero, M.; Golf, O.; Kumar, S.; Huang, J.; Kudo, H.; Goldin, R.D.; Hanna, G.B.; Takats, Z. A Comparison of DESI-MS and LC-MS for the Lipidomic Profiling of Human Cancer Tissue. J. Am. Soc. Mass. Spectrom. 2016, 27, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Cooks, R.G.; Ouyang, Z.; Takats, Z.; Wiseman, J.M. Ambient Mass Spectrometry. Science 2006, 311, 1566–1570. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Siems, W.F.; Hill, H.H. Secondary Electrospray Ionization Ion Mobility Spectrometry/Mass Spectrometry of Illicit Drugs. Anal. Chem. 2000, 72, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Hill, H.H.; Wittmer, D.P. Analytical Merit of Electrospray Ion Mobility Spectrometry as a Chromatographic Detector. J. Microcolumn Sep. 1994, 6, 515–524. [Google Scholar] [CrossRef]
- Kaeslin, J.; Wüthrich, C.; Giannoukos, S.; Zenobi, R. How Soft Is Secondary Electrospray Ionization? J. Am. Soc. Mass. Spectrom. 2022, 33, 1967–1974. [Google Scholar] [CrossRef]
- Gould, O.; Drabińska, N.; Ratcliffe, N.; de Lacy Costello, B. Hyphenated Mass Spectrometry versus Real-time Mass Spectrometry Techniques for the Detection of Volatile Compounds from the Human Body. Molecules 2021, 26, 7185. [Google Scholar] [CrossRef]
- Wang, H.; Liu, J.; Graham Cooks, R.; Ouyang, Z. Paper Spray for Direct Analysis of Complex Mixtures Using Mass Spectrometry. Angew. Chem.—Int. Ed. 2010, 49, 877–880. [Google Scholar] [CrossRef]
- Wleklinski, M.; Li, Y.; Bag, S.; Sarkar, D.; Narayanan, R.; Pradeep, T.; Cooks, R.G. Zero Volt Paper Spray Ionization and Its Mechanism. Anal. Chem. 2015, 87, 6786–6793. [Google Scholar] [CrossRef]
- Riboni, N.; Quaranta, A.; Motwani, H.V.; Österlund, N.; Gräslund, A.; Bianchi, F.; Ilag, L.L. Solvent-Assisted Paper Spray Ionization Mass Spectrometry (SAPSI-MS) for the Analysis of Biomolecules and Biofluids. Sci. Rep. 2019, 9, 10296. [Google Scholar] [CrossRef]
- Smith, C.A.; O’maille, G.; Want, E.J.; Qin, C.; Trauger, S.A.; Brandon, T.R.; Custodio, D.E.; Abagyan, R.; Siuzdak, G. METLIN: A Metabolite Mass Spectral Database. Ther. Drug Monit. 2005, 27, 747–751. [Google Scholar] [CrossRef]
- Horai, H.; Arita, M.; Kanaya, S.; Nihei, Y.; Ikeda, T.; Suwa, K.; Ojima, Y.; Tanaka, K.; Tanaka, S.; Aoshima, K.; et al. MassBank: A Public Repository for Sharing Mass Spectral Data for Life Sciences. J. Mass. Spectrom. 2010, 45, 703–714. [Google Scholar] [CrossRef]
- Wishart, D.S.; Tzur, D.; Knox, C.; Eisner, R.; Guo, A.C.; Young, N.; Cheng, D.; Jewell, K.; Arndt, D.; Sawhney, S.; et al. HMDB: The Human Metabolome Database. Nucleic Acids Res. 2007, 35 (Suppl. S1), D521–D526. [Google Scholar] [CrossRef]
- Ni, Z.; Angelidou, G.; Lange, M.; Hoffmann, R.; Fedorova, M. LipidHunter Identifies Phospholipids by High-Throughput Processing of LC-MS and Shotgun Lipidomics Datasets. Anal. Chem. 2017, 89, 8800–8807. [Google Scholar] [CrossRef]
- Herzog, R.; Schwudke, D.; Schuhmann, K.; Sampaio, J.L.; Bornstein, S.R.; Schroeder, M.; Shevchenko, A. A Novel Informatics Concept for High-Throughput Shotgun Lipidomics Based on the Molecular Fragmentation Query Language. Genome Biol. 2011, 12, R8. [Google Scholar] [CrossRef]
- Conroy, M.J.; Andrews, R.M.; Andrews, S.; Cockayne, L.; Dennis, E.A.; Fahy, E.; Gaud, C.; Griffiths, W.J.; Jukes, G.; Kolchin, M.; et al. LIPID MAPS: Update to Databases and Tools for the Lipidomics Community. Nucleic Acids Res. 2024, 52, D1677–D1682. [Google Scholar] [CrossRef]
- Välikangas, T.; Suomi, T.; Elo, L.L. A Systematic Evaluation of Normalization Methods in Quantitative Label-Free Proteomics. Brief. Bioinform. 2018, 19, 1–11. [Google Scholar] [CrossRef]
- Chua, A.E.; Pfeifer, L.D.; Sekera, E.R.; Hummon, A.B.; Desaire, H. Workflow for Evaluating Normalization Tools for Omics Data Using Supervised and Unsupervised Machine Learning. J. Am. Soc. Mass. Spectrom. 2023, 34, 2775–2784. [Google Scholar] [CrossRef]
- Zhang, Z.; Castelló, A. Principal Components Analysis in Clinical Studies. Ann. Transl. Med. 2017, 5, 351. [Google Scholar] [CrossRef]
- Liebal, U.W.; Phan, A.N.T.; Sudhakar, M.; Raman, K.; Blank, L.M. Machine Learning Applications for Mass Spectrometry-Based Metabolomics. Metabolites 2020, 10, 243. [Google Scholar] [CrossRef]
- Song, Y.Y.; Lu, Y. Decision Tree Methods: Applications for Classification and Prediction. Shanghai Arch. Psychiatry 2015, 27, 130–135. [Google Scholar] [CrossRef]
- Hua, D.; Desaire, H. Improved Discrimination of Disease States Using Proteomics Data with the Updated Aristotle Classifier. J. Proteome Res. 2021, 20, 2823–2829. [Google Scholar] [CrossRef]
- Pfeifer, L.D.; Patabandige, M.W.; Desaire, H. Leveraging R (LevR) for Fast Processing of Mass Spectrometry Data and Machine Learning: Applications Analyzing Fingerprints and Glycopeptides. Front. Anal. Sci. 2022, 2, 961592. [Google Scholar] [CrossRef]
- Hajian-Tilaki, K. Receiver Operating Characteristic (ROC) Curve Analysis for Medical Diagnostic Test Evaluation. Casp. J. Intern. Med. 2013, 4, 627–635. [Google Scholar]
- Desaire, H. How (Not) to Generate a Highly Predictive Biomarker Panel Using Machine Learning. J. Proteome Res. 2022, 21, 2071–2074. [Google Scholar] [CrossRef]
- Hart, A. Mann-Whitney test is not just a test of medians: Differences in spread can be important. BMJ 2001, 323, 391–393. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, S.; Lee, D.K. What Is the Proper Way to Apply the Multiple Comparison Test? Korean J. Anesthesiol. 2018, 71, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Liebisch, G.; Vizcaíno, J.A.; Köfeler, H.; Trötzmüller, M.; Griffiths, W.J.; Schmitz, G.; Spener, F.; Wakelam, M.J.O. Shorthand Notation for Lipid Structures Derived from Mass Spectrometry. J. Lipid Res. 2013, 54, 1523–1530. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.T.; Su, H.; Wu, C.Y.; Jeng, J.; Lee, C.W.; Wu, D.C.; Huang, T.L.; Shiea, J. The Study of Distribution of Ingested Terbinafine on Skin with Ambient Ionization Tandem Mass Spectrometry. J. Food Drug Anal. 2022, 30, 303–315. [Google Scholar] [CrossRef]

| Collection Substrate | Anatomical Location | Solvents | Separations and MS Analysis | Primary ESI-MS Lipid Analytes | Lipidomics Statistical Comparisons/Machine Learning | Purpose of Study | Reference |
|---|---|---|---|---|---|---|---|
| Glass slides | Fingerprints, forehead | N/A | DESI-LTQ Orbitrap (−) | FAs, TGs, DGs | GDBT, feature importance, cross-validation | Forensic applications | [3] |
| D-Squame | Forearm, forehead, cheek, chest abdomen, groin, thigh, heel, calf, shoulder, buttock, palm, hand, top of foot | MeOH, TCM, IPA | TriVersa NanoMate ion source, Q-Exactive (+/−) | CEs, DGs, TGs, CERs, Chol | Linear regression via least squares method, two-tailed Pearson and Spearman correlation, RF, cross-validation, PCA | Clinical applications | [4] |
| Sebutape | Cheeks | IPA, MeOH, water, toluene | Filtration, RP-LC-ESI-Qtrap (+/−) | CERs, FAs, oxylipins, nitrolipids, | Cauchy distribution, probabilistic PCA, full factorial MANOVA, Ward’s method for hierarchical clustering, Benjamini–Hochberg, Student’s t-test, Spearman’s rank correlation coefficient, Feltz and Miller test | Atopic dermatitis | [5] |
| Cotton swabs and phone surfaces (glass and plastic) | Hands | EtOH, water | RP-UPLC-ESI-QTOF (+) | FAs | Bray–Curtis dissimilarity analysis, RF, cross-validation, MSCluster, cosine scores, frequency histogram, heat mapping, 2D spatial molecular mapping | Forensic applications | [7] |
| Scintillation vials | Groomed fingerprints (forehead) | TCM | ESI-QTOF (−/+) | FAs | N/A | Forensic applications | [8] |
| Aluminum foil | Groomed fingerprints (forehead, nose, chin, and scalp) | TCM, water, IPA | RP-LC-ESI-QTOF (+) | TGs | In-house MIRION software package | Forensic applications | [9] |
| Cotton gauze | Back | MeOH, EtOH | Filtration, RP-LC- Synapt G2-Si QTOF (+) | CERs, TGs, DGs, MGs, SPs, FAs, STs | PLS-DA, AUC, ROC, bootstrapping, Pearson’s correlation matrix | Parkinson’s disease | [10] |
| Q-tip swabs | Back | N/A | IM-PSI (Synapt G2 Si) (+) | DGs, TGs, FAs, PLs | One-way ANOVA, probabilistic (Bayesian) method, RF, PCA, SVM | Parkinson’s disease | [11] |
| Sebutape | Forehead | TCM, MeOH, ACE, IPA | RP-UPLC-ESI-QTOF (+) | CERs, FAs, PLs, TGs, PRs | PLS-DA, PCA, VIP scores, heatmap analysis, Mann–Whitney U-test, ROC, AUC, topical overlap measurement | Type 2 diabetes | [12] |
| Silica plates | Back | MeOH, water | ESI-Q-Exactive Orbitrap (+) | FA amides, DGs, TGs, GPs, STs | Chi-squared independent test, Fisher’s exact test, Shapiro–Wilk test, Mann–Whitney hypothesis test, PLS-DA, VIP scores, permutation tests, t-test, volcano plots, linear SVM, ROC | COVID-19 | [13] |
| Gauze swabs | Back | MeOH, EtOH | Filtration, RP-LC-ESI Orbitrap Q-Exactive Plus (+) | CERs, TGs, DGs | PLS-DA, LOOCV, VIP scores, two-tailed Mann–Whitney U-test, PCA, ROC, AUC, volcano plots | COVID-19 | [14] |
| Gauze swabs | Back | MeOH, EtOH | RP-LC-ESI-Orbitrap Q-Exactive Plus (+) | TGs, DGs, CERs | PLS-DA, LOOCV, SVM, RF, K-nearest neighbors, Pearson correlation coefficient, VIP scores, PCA, logistic regression, recursive feature elimination with cross-validation, heatmapping | COVID-19 | [15] |
| Silica plates | Back | MeOH, water | ESI-LTQ Oribtrap (−) | CERs, DGs, PLs | OPLS-DA, VIP scores | Cystic fibrosis | [16] |
| Glass slides | Forehead | N/A | DESI-LTQ Orbitrap (−) | FAs, DGs | GBDT, 6-fold rotational cross-validation, bootstrapping, ROC, AUC | Cystic fibrosis | [17] |
| Tape strips | Forearm | MeOH, TCM, n-hexane, IPA | SPE, NP-HPLC-ESI-Q | CERs | Bonferroni’s post hoc multiple comparison test | Atopic dermatitis | [18] |
| Sebutape | Back | MeOH, water, TCM, IPA | RP-LC-ESI-Q-Exactive (+/−) | TGs, MGs, DGs, PLs, CERs, FAs | PCA, OPLS-DA, R, Spearman’s correlation coefficient, t-test | Atopic dermatitis | [19] |
| D-Squame | Forearm | MeOH, water, TCM | RP-LC-ESI-Qtrap (+) | CERs, SMs | Student’s t-test, one-way ANOVA, Benjamini–Hochberg | Atopic dermatitis | [20] |
| D-Squame | Forearm | Water, MeOH, TCM | RP-LC-ESI-QTRAP (+) | CERs | Logistic regression analysis, chi-square test, Fisher’s exact test, Shapiro–Wilk test, Mann–Whitney U-test, ROC, Youden index method, Firth’s penalized method | Atopic dermatitis | [21] |
| Silica plates | Back | MeOH | ESI-LTQ Orbitrap (+) | PLs, CERs, FAs | PCA | Leprosy | [22] |
| Aluminum foil | Groomed fingerprints (cheek, neck, forehead) | DCM, MeOH, water | ESI-Orbitrap (+) | TGs, WEs | ROC, AUC, XGBoost, PCA, in-house R script for matrix building | Biomarker exploration | [24] |
| D-Squame | Forearm, chest, forehead | EtOH, MeOH, ACE, IPA | RR-RP-HPLC-ESI-QTOF/QqQ (−/+) | FAs, cholesterol sulfate, CERs | One-way ANOVA, Tukey’s multiple comparisons test, PCA, C-C plots, volcano plots, hierarchical clustering | Clinical applications | [34] |
| Sampling probes | Entire body | N/A | TD-ESI-LTQ (+/−) | SQ | Temperature color gradient | Clinical applications | [38] |
| Cyanoacrylate resin | Cheek, forearm, thigh, leg, back, palm | Hexane, EtOH, MeOH | Filtration, RP-HPLC-ESI-MS (+) | CERs | Student’s t-test, Mann–Whitney U-test | Skin sensitivity | [39] |
| Tape strips | Scalp, forehead, cheek, lip, arm, hand, palm, finger, buttock, leg | MeOH, TCM, n-hexane, IPA | SPE, NP-LC-ESI-Q (+) | CERs | Tukey’s test, Pearson’s correlation coefficient | Clinical applications | [40] |
| Tape strips | Forearm, cheek | MeOH, TCM, n-hexane, IPA | SPE, NP-HPLC-ESI-Q (+) | CERs | Student’s t-test, correlation coefficient | Clinical applications | [41] |
| Glass, paper, plastic, metal; followed by tape lifting | Groomed fingerprints (forehead) | N/A | DESI-LTQ Linear IonTrap (−) | FAs | N/A | Forensic applications | [42] |
| D-Squame | Cheek | MeOH | RP/HILIC-UPLC-ESI- Orbitrap Q-Exactive (+/−) | FAs, DGs, MGs, Chol, CERs, STs | Wilcoxon test, PCA, heat mapping, Benjamini–Hochberg, boxplots, volcano plots, random forest, VIMP scores, sparse Canonical Correlation Analysis, Pearson correlation coefficient, PLS regression, multiblock analysis, v-test | Environmental pollutant exposure | [45] |
| Tape strips | Forearm | MeOH, TCM, hexane, IPA | SPE, NP-LC-ESI-QqQtrap (+/−) | CERs | N/A | Clinical applications | [70] |
| N/A | Hand | N/A | SESI-API-QTOF (−) | FAs | N/A | Mosquito attractants | [56] |
| PDMS sampling patches | Axilla | N/A | TD-SESI-QTOF (−) | FAs | PCA | Clinical applications | [57] |
| Tape strips | Inner thighs and diaper-covered buttocks | MeOH, TCM, hexane, IPA | NP-LC-ESI-Q | CERs | N/A | Infantile skin | [59] |
| Tape strips | Cheek, buttock | MeOH, hexane, IPA, water, TCM | SPE, RP-UPLC-ESI-QqQ (+) | CERs, SMs | Mann –Whitney hypothesis test, one-way ANOVA, Tukey’s test, Spearman Rank, Pearson’s R correlations | Menopause | [60] |
| Sebutape | Cheek | TCM, MeOH, ACE, IPA | RP-UPLC-ESI-QTOF (+) | CERs, FAs, GLs, GPs, SPs, STs, PRs, SLs, PKs, SQ-related compounds, Chol, WEs | PCA, score plots, OPLS-DA, VIPs, Student’s t-test | Acne | [61] |
| Sebutape | Cheek | TCM, MEOH, ACE, IPA | RR-UPLC-ESI-QTOF (+) | FAs, GLs, GPs, SPs, STs, PRs, SLs, PKs | VIP, t-test, PCA score plots, OPLS-DA score plots | Blue light therapy acne treatment | [62] |
| Glass slides | Forehead | TCM, MeOH, IPA, Methyl-tert-butyl ether | RP-LC-Nano-ESI- Q-Exactive (+/−) | TGs, CEs, CERs DGs, Chol, PLs, FAs | Heat mapping, Venn diagrams | Inflammation | [63] |
| Sebutape | Cheek | TCM, MeOH, ACE, IPA | RP-UPLC-ESI-QTOF (+) | CERs, FAs, GPs, TGs, DGs, | PLS-DA score plots, S-plots, VIP scores | Skin sensitivity | [64] |
| Tape strips | Forearm | MeOH, TCM | RP-UPLC-ESI-QqQ (+/−) | CERs | Student’s t-test | Clinical applications | [65] |
| Sebutape | Forehead | EtOH, butylated hydroxytoluene, ethyl acetate, MeOH, Me2O, IPA | RR-RP-HPLC-ESI-TOF (+/−) | TGs, DGs, MGs, FAs, WEs, PRs, triterpenes, SLs, STs | Mass Profiler Professional volcano plots, in-house statistical method, Bonferroni’s correction, PLS, PLS weights plot, Student’s t-test, ANOVA, post hoc Tukey’s honestly significant difference test | Acne | [66] |
| Sebutape | Forehead | EtOH, ethyl acetate, ACE, MeOH, IPA | RR-RP-HPLC-ESI-TOF/QqQ (+/−) | FAs, TGs, DGs, CEs, WEs, SQ | N/A | Understanding the sebum lipidome | [67] |
| D-Squame | Forearm | Cyclohexane, EtOH, TCM, MeOH | Silica acid column chromatography-nanoESI-LTQ (−) | CERs | N/A | Clinical applications | [68] |
| Tape strips | Forearm | MeOH, IPA, TCM, hexane | SPE, RP-LC-ESI-QTOF (+/−) | CERs | N/A | Clinical applications | [69] |
| D-Squame | Forearm | Water, MeOH, TCM, ACE, ACN | RP-LC-ESI-QqQ (+) | Derivatized FAs | Shapiro–Wilk normality test, two-tailed Mann–Whitney test | Atopic dermatitis | [71] |
| Cyanoacrylate resin and Teflon extraction funnel | Forearm | ACE, EtOH, TCM, MeOH, hexane, EtOH | RP-LC-ESI-Ion Trap (−) | CERs | N/A | Clinical applications | [72] |
| Cyanoacrylate resin | Forearm | Hexane, EtOH, diethylether, MeOH | RP-LC-ESI-Ion Trap (−) | CERs | N/A | Clinical applications | [73] |
| D-Squame | Forearm | Water, MeOH, TCM | RP-LC-ESI-Qtrap (+) | CERs | Benjamini–Hochberg, Tukey’s type boxplots, random forest, Gini index | Dupilumab therapy for atopic dermatitis | [74] |
| Leukoflex tape strips | Cheek | Ethyl acetate, IPA, water, MeOH | Filtration, RP-UPLC-ESI-QqQ (+) | CERs | Correlation coefficients, Cook’s score, unpaired t-tests corrected for multiple comparisons | Acne | [75] |
| N/A | Hand | N/A | SESI-Q-Exactive Orbitrap (+) | SQ | Clustergram hierarchical cluster analysis, OPLS-DA, VIP scores, S-plot | Air quality | [76] |
| Scraping method | Foot sole | TCM, MeOH | RP-HPLC-ESI-ion trap (−) | CERs | N/A | Clinical applications | [77] |
| N/A | Finger | EtOH, water | DESI-LTQ Orbitrap/LCQ Duo Quadrupole Ion Trap (+/−) | FAs | N/A | Direct skin analysis | [78] |
| Filter slip | cheek | MeOH | zvPSI-Orbitrap (+/−) | FAs, TGs, DGs, MGs, WEs, CEs, CERs, SMs, GPs | N/A | Voltage-free analysis of diverse samples | [79] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Isom, M.; Desaire, H. Skin Surface Sebum Analysis by ESI-MS. Biomolecules 2024, 14, 790. https://doi.org/10.3390/biom14070790
Isom M, Desaire H. Skin Surface Sebum Analysis by ESI-MS. Biomolecules. 2024; 14(7):790. https://doi.org/10.3390/biom14070790
Chicago/Turabian StyleIsom, Madeline, and Heather Desaire. 2024. "Skin Surface Sebum Analysis by ESI-MS" Biomolecules 14, no. 7: 790. https://doi.org/10.3390/biom14070790







