Importance of Circadian Rhythms in the Ocular Surface
Abstract
:1. Introduction
2. The Molecular Clock and Clock-Driven Gene Expression in the Mammalian Ocular Surface
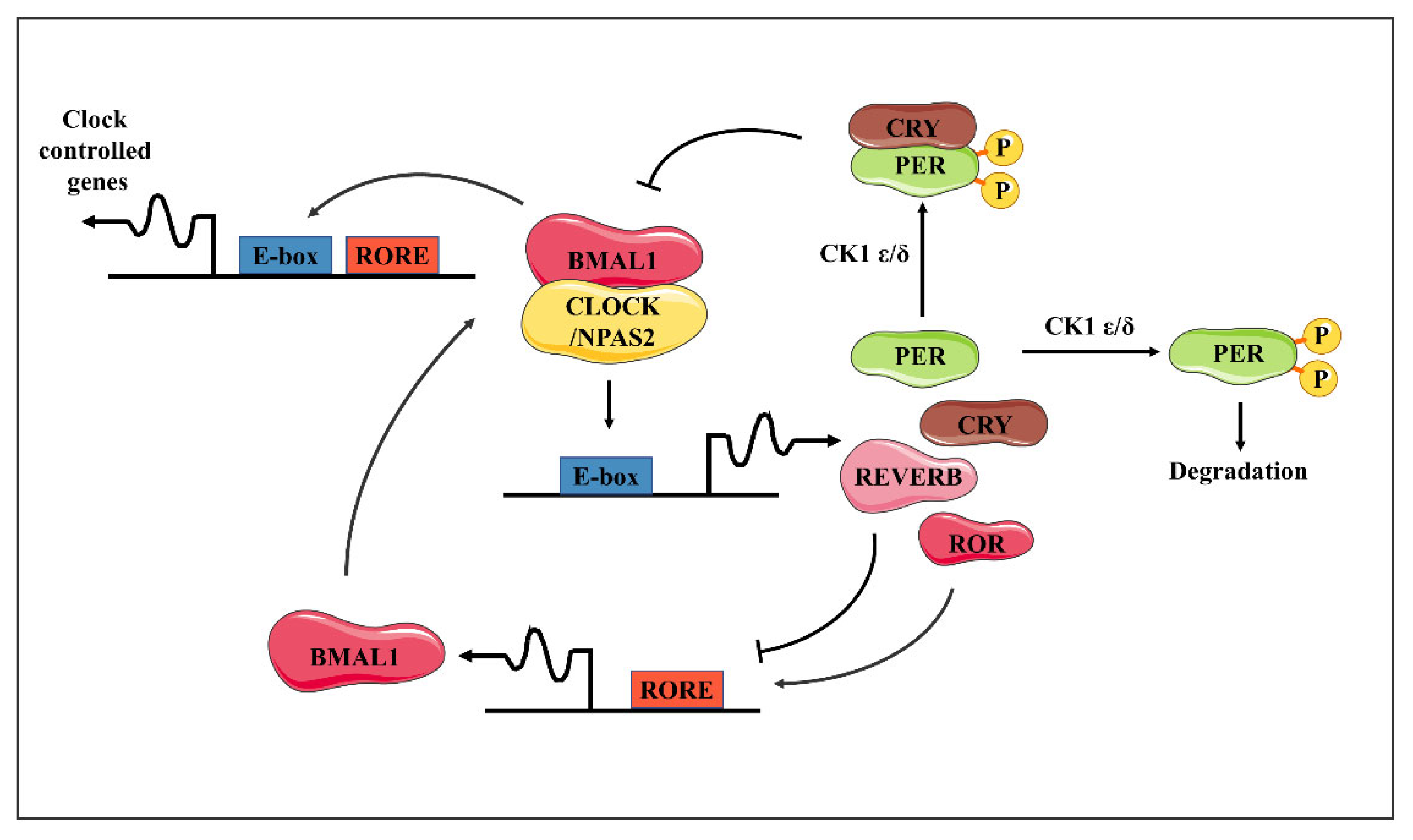
2.1. The Molecular Clock
2.2. Clock-Driven Gene Expression in the Mammalian Ocular Surface
3. The Diurnal Oscillations in the Mammalian Ocular Surface
4. The Factors of Entraining Circadian Oscillators in the Ocular Surface the Circadian Rhythm of the Ocular Surface Is Influenced by Diabetes
5. Normal Circadian Rhythms Are Important for Ocular Physiology and Health
6. Conclusions and Future Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rijo-Ferreira, F.; Takahashi, J.S.; Figueiredo, L.M. Circadian rhythms in parasites. PLoS Pathog. 2017, 13, e1006590. [Google Scholar] [CrossRef] [PubMed]
- Koronowski, K.B.; Sassone-Corsi, P. Communicating clocks shape circadian homeostasis. Science 2021, 371. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.Y.; Eichler, V.B. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. 1972, 42, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Rodríguez, V.A.; Rijo-Ferreira, F.; Green, C.B.; Takahashi, J.S. Importance of circadian timing for aging and longevity. Nat. Commun. 2021, 12, 2862. [Google Scholar] [CrossRef]
- Baba, K.; Tosini, G. Aging Alters Circadian Rhythms in the Mouse Eye. J. Biol. Rhythm. 2018, 33, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Wiechmann, A.F.; Summers, J.A. Circadian rhythms in the eye: The physiological significance of melatonin receptors in ocular tissues. Prog. Retin. Eye Res. 2008, 27, 137–160. [Google Scholar] [CrossRef] [PubMed]
- Besharse, J.C.; McMahon, D.G. The Retina and Other Light-sensitive Ocular Clocks. J. Biol. Rhythm. 2016, 31, 223–243. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, R.; Ostrin, L.A.; Nickla, D.L.; Iuvone, P.M.; Pardue, M.T.; Stone, R.A. Circadian rhythms, refractive development, and myopia. Ophthalmic Physiol. Opt. 2018, 38, 217–245. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.H.; Yamazaki, S.; Lowrey, P.L.; Shimomura, K.; Ko, C.H.; Buhr, E.D.; Siepka, S.M.; Hong, H.K.; Oh, W.J.; Yoo, O.J.; et al. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc. Natl. Acad. Sci. USA 2004, 101, 5339–5346. [Google Scholar] [CrossRef] [PubMed]
- Buhr, E.D.; Yue, W.W.; Ren, X.; Jiang, Z.; Liao, H.W.; Mei, X.; Vemaraju, S.; Nguyen, M.T.; Reed, R.R.; Lang, R.A.; et al. Neuropsin (OPN5)-mediated photoentrainment of local circadian oscillators in mammalian retina and cornea. Proc. Natl. Acad. Sci. USA 2015, 112, 13093–13098. [Google Scholar] [CrossRef] [PubMed]
- Baba, K.; Davidson, A.J.; Tosini, G. Melatonin Entrains PER2::LUC Bioluminescence Circadian Rhythm in the Mouse Cornea. Investig. Ophthalmol. Vis. Sci. 2015, 56, 4753–4758. [Google Scholar] [CrossRef] [PubMed]
- Read, S.A.; Collins, M.J. Diurnal variation of corneal shape and thickness. Optom. Vis. Sci. 2009, 86, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Howland, H.C.; Troilo, D. Diurnal illumination patterns affect the development of the chick eye. Vis. Res. 2000, 40, 2387–2393. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Howland, H.C. The effects of constant and diurnal illumination of the pineal gland and the eyes on ocular growth in chicks. Investig. Ophthalmol. Vis. Sci. 2003, 44, 3692–3697. [Google Scholar] [CrossRef] [PubMed]
- Wahl, C.; Li, T.; Choden, T.; Howland, H. Morphometrics of corneal growth in chicks raised in constant light. J. Anat. 2009, 214, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Doughty, M.J. Morphometric analysis of the surface cells of rabbit corneal epithelium by scanning electron microscopy. Am. J. Anat. 1990, 189, 316–328. [Google Scholar] [CrossRef]
- Buffa, A.; Rizzi, E.; Falconi, M.; Matteucci, A.; Baratta, B.; Fantazzini, A.; Lattanzi, G.; Rizzoli, R. Bromodeoxyuridine incorporation in corneal epithelium: An immunocytochemical study in rats. Boll. Soc. Ital. Biol. Sper. 1993, 69, 767–773. [Google Scholar] [PubMed]
- Xue, Y.; Liu, P.; Wang, H.; Xiao, C.; Lin, C.; Liu, J.; Dong, D.; Fu, T.; Yang, Y.; Wang, Z.; et al. Modulation of Circadian Rhythms Affects Corneal Epithelium Renewal and Repair in Mice. Investig. Ophthalmol. Vis. Sci. 2017, 58, 1865–1874. [Google Scholar] [CrossRef] [PubMed]
- Sandvig, K.U.; Haaskjold, E.; Refsum, S.B. Time dependency in the regenerative response to injury of the rat corneal epithelium. Chronobiol. Int. 1994, 11, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Felder-Schmittbuhl, M.P.; Buhr, E.D.; Dkhissi-Benyahya, O.; Hicks, D.; Peirson, S.N.; Ribelayga, C.P.; Sandu, C.; Spessert, R.; Tosini, G. Ocular Clocks: Adapting Mechanisms for Eye Functions and Health. Investig. Ophthalmol. Vis. Sci. 2018, 59, 4856–4870. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, J.S. Transcriptional architecture of the mammalian circadian clock. Nat. Rev. Genet. 2017, 18, 164–179. [Google Scholar] [CrossRef] [PubMed]
- Koike, N.; Yoo, S.H.; Huang, H.C.; Kumar, V.; Lee, C.; Kim, T.K.; Takahashi, J.S. Transcriptional architecture and chromatin landscape of the core circadian clock in mammals. Science 2012, 338, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Aryal, R.P.; Kwak, P.B.; Tamayo, A.G.; Gebert, M.; Chiu, P.L.; Walz, T.; Weitz, C.J. Macromolecular Assemblies of the Mammalian Circadian Clock. Mol. Cell. 2017, 67, 770–782.e776. [Google Scholar] [CrossRef] [PubMed]
- Partch, C.L.; Green, C.B.; Takahashi, J.S. Molecular architecture of the mammalian circadian clock. Trends Cell Biol. 2014, 24, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Papazyan, R.; Zhang, Y.; Lazar, M.A. Genetic and epigenomic mechanisms of mammalian circadian transcription. Nat. Struct. Mol. Biol. 2016, 23, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Jiao, X.; Wu, M.; Lu, D.; Gu, J.; Li, Z. Transcriptional Profiling of Daily Patterns of mRNA Expression in the C57BL/6J Mouse Cornea. Curr. Eye Res. 2019, 44, 1054–1066. [Google Scholar] [CrossRef] [PubMed]
- Yamajuku, D.; Shibata, Y.; Kitazawa, M.; Katakura, T.; Urata, H.; Kojima, T.; Takayasu, S.; Nakata, O.; Hashimoto, S. Cellular DBP and E4BP4 proteins are critical for determining the period length of the circadian oscillator. FEBS Lett. 2011, 585, 2217–2222. [Google Scholar] [CrossRef]
- Mure, L.S.; Le, H.D.; Benegiamo, G.; Chang, M.W.; Rios, L.; Jillani, N.; Ngotho, M.; Kariuki, T.; Dkhissi-Benyahya, O.; Cooper, H.M.; et al. Diurnal transcriptome atlas of a primate across major neural and peripheral tissues. Science 2018, 359, eaao0318. [Google Scholar] [CrossRef] [PubMed]
- Alenezi, H.; Ozkan, J.; Willcox, M.; Parnell, G.; Carnt, N. Differential gene expression of the healthy conjunctiva during the day. Contact Lens Anterior Eye 2022, 45, 101494. [Google Scholar] [CrossRef] [PubMed]
- Hughes, M.E.; DiTacchio, L.; Hayes, K.R.; Vollmers, C.; Pulivarthy, S.; Baggs, J.E.; Panda, S.; Hogenesch, J.B. Harmonics of circadian gene transcription in mammals. PLoS Genet. 2009, 5, e1000442. [Google Scholar] [CrossRef]
- Hoogerwerf, W.A.; Sinha, M.; Conesa, A.; Luxon, B.A.; Shahinian, V.B.; Cornélissen, G.; Halberg, F.; Bostwick, J.; Timm, J.; Cassone, V.M. Transcriptional profiling of mRNA expression in the mouse distal colon. Gastroenterology 2008, 135, 2019–2029. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Lahens, N.F.; Ballance, H.I.; Hughes, M.E.; Hogenesch, J.B. A circadian gene expression atlas in mammals: Implications for biology and medicine. Proc. Natl. Acad. Sci. USA 2014, 111, 16219–16224. [Google Scholar] [CrossRef] [PubMed]
- Westermark, P.O.; Herzel, H. Mechanism for 12 hr rhythm generation by the circadian clock. Cell Rep. 2013, 3, 1228–1238. [Google Scholar] [CrossRef]
- Zhu, B.; Zhang, Q.; Pan, Y.; Mace, E.M.; York, B.; Antoulas, A.C.; Dacso, C.C.; O’Malley, B.W. A Cell-Autonomous Mammalian 12 hr Clock Coordinates Metabolic and Stress Rhythms. Cell Metab. 2017, 25, 1305–1319.e9. [Google Scholar] [CrossRef]
- Eckel-Mahan, K.; Sassone-Corsi, P. Phenotyping Circadian Rhythms in Mice. Curr. Protoc. Mouse Biol. 2015, 5, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Ripperger, J.A.; Jud, C.; Albrecht, U. The daily rhythm of mice. FEBS Lett. 2011, 585, 1384–1392. [Google Scholar] [CrossRef] [PubMed]
- Ebihara, S.; Marks, T.; Hudson, D.J.; Menaker, M. Genetic control of melatonin synthesis in the pineal gland of the mouse. Science 1986, 231, 491–493. [Google Scholar] [CrossRef] [PubMed]
- Roseboom, P.H.; Namboodiri, M.A.; Zimonjic, D.B.; Popescu, N.C.; Rodriguez, I.R.; Gastel, J.A.; Klein, D.C. Natural melatonin ‘knockdown’ in C57BL/6J mice: Rare mechanism truncates serotonin N-acetyltransferase. Mol. Brain Res. 1998, 63, 189–197. [Google Scholar] [CrossRef]
- Hablitz, L.M.; Molzof, H.E.; Abrahamsson, K.E.; Cooper, J.M.; Prosser, R.A.; Gamble, K.L. GIRK Channels Mediate the Nonphotic Effects of Exogenous Melatonin. J. Neurosci. 2015, 35, 14957–14965. [Google Scholar] [CrossRef] [PubMed]
- Benito, M.J.; González-García, M.J.; Tesón, M.; García, N.; Fernández, I.; Calonge, M.; Enríquez-de-Salamanca, A. Intra- and inter-day variation of cytokines and chemokines in tears of healthy subjects. Exp. Eye Res. 2014, 120, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Uchino, E.; Sonoda, S.; Kinukawa, N.; Sakamoto, T. Alteration pattern of tear cytokines during the course of a day: Diurnal rhythm analyzed by multicytokine assay. Cytokine 2006, 33, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Kaji, Y. Prevention of diabetic keratopathy. Br. J. Ophthalmol. 2005, 89, 254–255. [Google Scholar] [CrossRef] [PubMed]
- Quadrado, M.J.; Popper, M.; Morgado, A.M.; Murta, J.N.; Van Best, J.A. Diabetes and corneal cell densities in humans by in vivo confocal microscopy. Cornea 2006, 25, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Peschke, E.; Wolgast, S.; Bazwinsky, I.; Pönicke, K.; Muhlbauer, E. Increased melatonin synthesis in pineal glands of rats in streptozotocin induced type 1 diabetes. J. Pineal Res. 2008, 45, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Thoft, R.A.; Friend, J. The X, Y, Z hypothesis of corneal epithelial maintenance. Investig. Ophthalmol. Vis. Sci. 1983, 24, 1442–1443. [Google Scholar]
- Lavker, R.M.; Dong, G.; Cheng, S.Z.; Kudoh, K.; Cotsarelis, G.; Sun, T.T. Relative proliferative rates of limbal and corneal epithelia. Implications of corneal epithelial migration, circadian rhythm, and suprabasally located DNA-synthesizing keratinocytes. Investig. Ophthalmol. Vis. Sci. 1991, 32, 1864–1875. [Google Scholar]
- Zagon, I.S.; Sassani, J.W.; McLaughlin, P.J. Insulin treatment ameliorates impaired corneal reepithelialization in diabetic rats. Diabetes 2006, 55, 1141–1147. [Google Scholar] [CrossRef] [PubMed]
- Arjona, A.; Silver, A.C.; Walker, W.E.; Fikrig, E. Immunity’s fourth dimension: Approaching the circadian-immune connection. Trends Immunol. 2012, 33, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Zagon, I.S.; Klocek, M.S.; Sassani, J.W.; McLaughlin, P.J. Use of topical insulin to normalize corneal epithelial healing in diabetes mellitus. Arch. Ophthalmol. 2007, 125, 1082–1088. [Google Scholar] [CrossRef] [PubMed]
- Alotaibi, S.; Ozkan, J.; Papas, E.; Markoulli, M. Diurnal Variation of Corneal Dendritic Cell Density. Curr. Eye Res. 2022, 47, 1239–1245. [Google Scholar] [CrossRef]
- Scheiermann, C.; Kunisaki, Y.; Frenette, P.S. Circadian control of the immune system. Nat. Rev. Immunol. 2013, 13, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Chakravarthy, U.; Hayes, R.G.; Stitt, A.W.; Douglas, A. Endothelin expression in ocular tissues of diabetic and insulin-treated rats. Investig. Ophthalmol. Vis. Sci. 1997, 38, 2144–2151. [Google Scholar]
- Karima, M.; Kantarci, A.; Ohira, T.; Hasturk, H.; Jones, V.L.; Nam, B.H.; Malabanan, A.; Trackman, P.C.; Badwey, J.A.; Van Dyke, T.E. Enhanced superoxide release and elevated protein kinase C activity in neutrophils from diabetic patients: Association with periodontitis. J. Leukoc. Biol. 2005, 78, 862–870. [Google Scholar] [CrossRef] [PubMed]
- Omori, K.; Ohira, T.; Uchida, Y.; Ayilavarapu, S.; Batista, E.L., Jr.; Yagi, M.; Iwata, T.; Liu, H.; Hasturk, H.; Kantarci, A.; et al. Priming of neutrophil oxidative burst in diabetes requires preassembly of the NADPH oxidase. J. Leukoc. Biol. 2008, 84, 292–301. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Jiao, X.; Sun, X.; Huang, Y.; Xu, P.; Xue, Y.; Fu, T.; Liu, J.; Li, Z. Short-Term High Fructose Intake Impairs Diurnal Oscillations in the Murine Cornea. Investig. Ophthalmol. Vis. Sci. 2021, 62, 22. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Lin, C.; Jiao, X.; Song, Z.; Wang, L.; Gu, J.; Li, Z. Short-term High Fructose Intake Reprograms the Transcriptional Clock Rhythm of the Murine Extraorbital Lacrimal Gland. Investig. Ophthalmol. Vis. Sci. 2019, 60, 2038–2048. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Hanzawa, F.; Umeki, M.; Matsuyama, Y.; Nishimura, N.; Ikeda, S.; Mochizuki, S.; Oda, H. Impacts of high-sucrose diet on circadian rhythms in the small intestine of rats. Chronobiol. Int. 2019, 36, 826–837. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Jie, Y. Importance of Circadian Rhythms in the Ocular Surface. Biomolecules 2024, 14, 796. https://doi.org/10.3390/biom14070796
Zhang X, Jie Y. Importance of Circadian Rhythms in the Ocular Surface. Biomolecules. 2024; 14(7):796. https://doi.org/10.3390/biom14070796
Chicago/Turabian StyleZhang, Xiaozhao, and Ying Jie. 2024. "Importance of Circadian Rhythms in the Ocular Surface" Biomolecules 14, no. 7: 796. https://doi.org/10.3390/biom14070796



