How Transcription Factor Clusters Shape the Transcriptional Landscape
Abstract
:1. Introduction
2. Overview of Cluster Formation
2.1. Interaction between TF Molecules and TF Binding Sites in the DNA
2.2. Interaction of DNA Bound TF with Cofactor Molecules
2.3. Clusters Confer Information in Nuclear TF Concentration
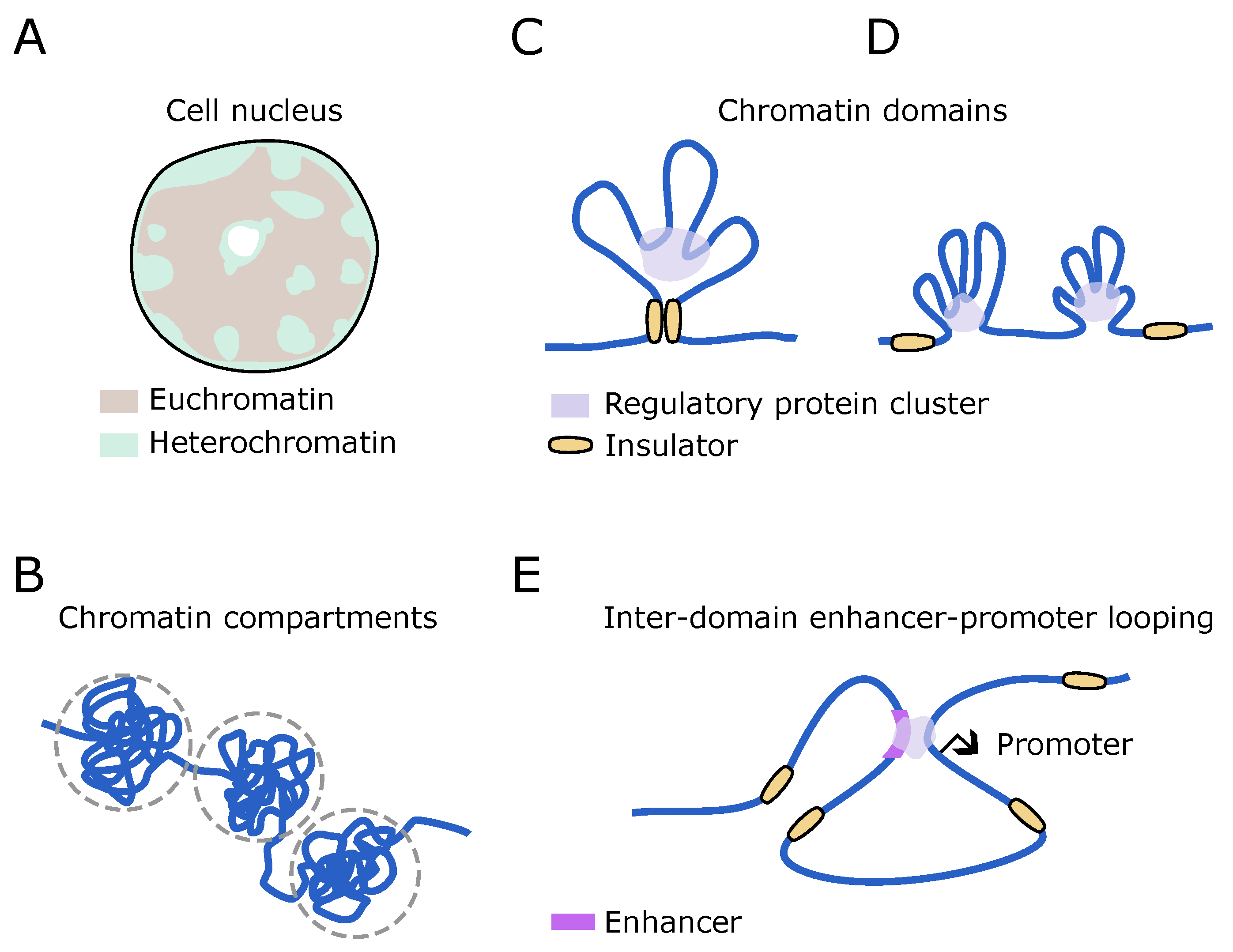
3. Interplay of 3D Chromatin Architecture and TF Clusters
3.1. Overview of High-Level 3D Chromatin Architecture
3.2. Organization of Chromatin Domains
3.3. Long-Range Enhancer–Promoter Association
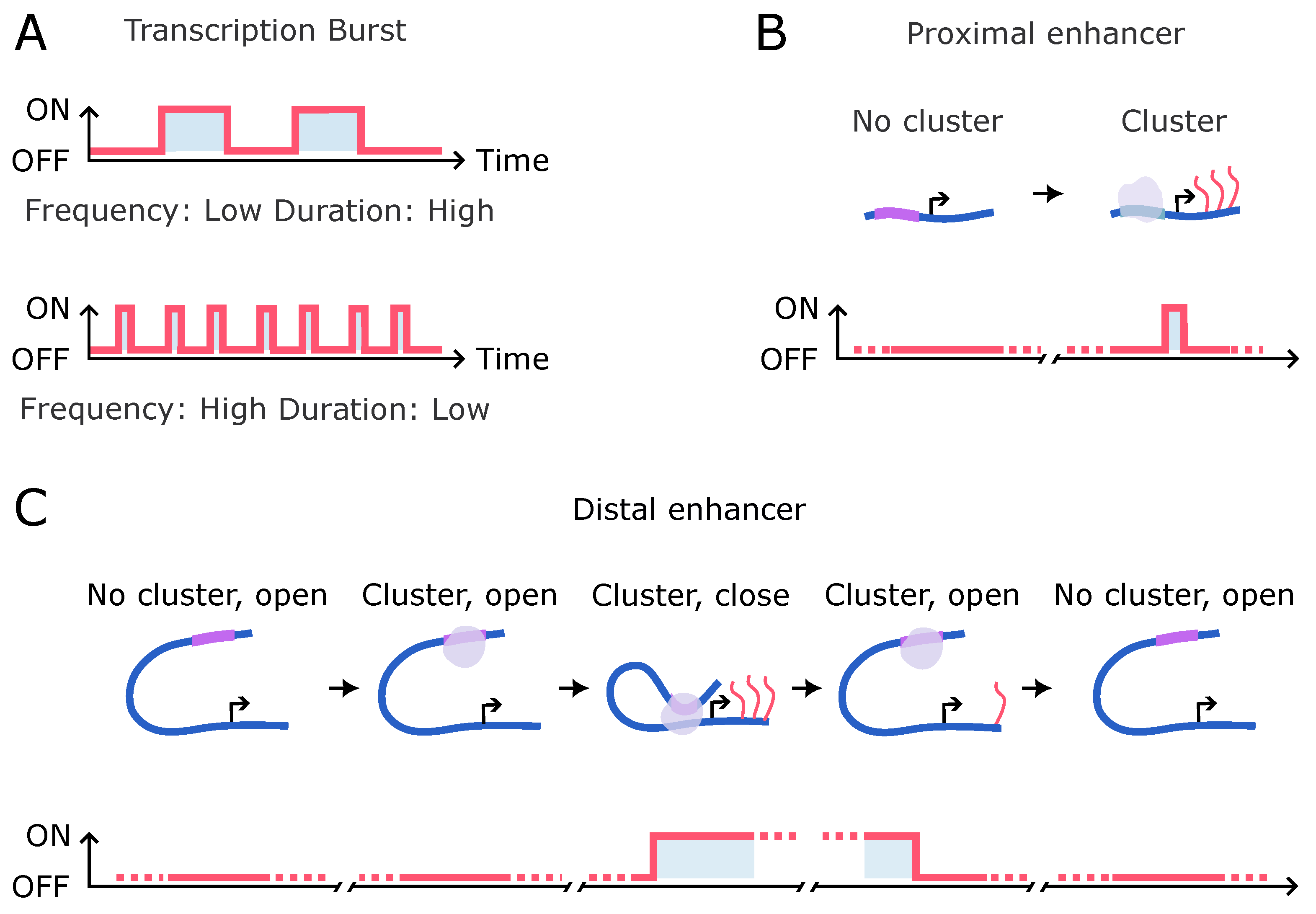
4. The Relationship between Clustering and Gene Transcription
5. Studying TF Cluster Dynamics and Transcriptional Bursts
5.1. Labeling Proteins and RNA
5.2. Imaging Clusters
5.3. Application Examples
6. Outlook
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| DNA | Deoxyribonucleic acid |
| RNA | Ribonucleic acid |
| TF | Transcription factor |
| DBD | DNA binding domain |
| AD | Activation domain |
| PIC | Pre-initiation complex |
| GTF | General transcription factor |
| IDR | Intrinsically disordered region |
| LLPS | Liquid–liquid phase separation |
| TAD | Topologically associated domain |
| SBS | Strings and binders switch |
| 2P | Two-photon |
| FCS | Fluorescence correlation spectroscopy |
| FRAP | Fluorescence Recovery After Photobleaching |
| GFP | Green fluorescent protein |
References
- Alberts, B.; Johnson, A.; Lewis, J.; Morgan, D.; Raff, M.; Roberts, K.; Walter, P. Molecular Biology of the Cell; W.W. Norton & Company: New York, NY, USA, 2014. [Google Scholar] [CrossRef]
- Haberle, V.; Stark, A. Eukaryotic core promoters and the functional basis of transcription initiation. Nat. Rev. Mol. Cell Biol. 2018, 19, 621–637. [Google Scholar] [CrossRef]
- Larson, D.R.; Zenklusen, D.; Wu, B.; Chao, J.A.; Singer, R.H. Real-Time Observation of Transcription Initiation and Elongation on an Endogenous Yeast Gene. Science 2011, 332, 475–478. [Google Scholar] [CrossRef] [PubMed]
- Jonkers, I.; Lis, J.T. Getting up to speed with transcription elongation by RNA polymerase II. Nat. Rev. Mol. Cell Biol. 2015, 16, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.X.; Smith, E.R.; Shilatifard, A. Born to run: Control of transcription elongation by RNA polymerase II. Nat. Rev. Mol. Cell Biol. 2018, 19, 464–478. [Google Scholar] [CrossRef] [PubMed]
- Fazal, F.M.; Meng, C.A.; Murakami, K.; Kornberg, R.D.; Block, S.M. Real-time observation of the initiation of RNA polymerase II transcription. Nature 2015, 525, 274–277. [Google Scholar] [CrossRef]
- Vos, S.M. Understanding transcription across scales: From base pairs to chromosomes. Mol. Cell 2021, 81, 1601–1616. [Google Scholar] [CrossRef] [PubMed]
- Raj, A.; Oudenaarden, A.V. Nature, Nurture, or Chance: Stochastic Gene Expression and Its Consequences. Cell 2008, 135, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Raj, A.; Peskin, C.S.; Tranchina, D.; Vargas, D.Y.; Tyagi, S. Stochastic mRNA Synthesis in Mammalian Cells. PLoS Biol. 2006, 4, e309. [Google Scholar] [CrossRef] [PubMed]
- Tunnacliffe, E.; Chubb, J.R. What Is a Transcriptional Burst? Trends Genet. 2020, 36, 288–297. [Google Scholar] [CrossRef]
- Meeussen, J.V.; Lenstra, T.L. Time will tell: Comparing timescales to gain insight into transcriptional bursting. Trends Genet. 2024, 40, 160–174. [Google Scholar] [CrossRef]
- Sainsbury, S.; Bernecky, C.; Cramer, P. Structural basis of transcription initiation by RNA polymerase II. Nat. Rev. Mol. Cell Biol. 2015, 16, 129–143. [Google Scholar] [CrossRef]
- Porello, E.A.L.; Trudeau, R.T.; Lim, B. Transcriptional bursting: Stochasticity in deterministic development. Development 2023, 150, dev201546. [Google Scholar] [CrossRef] [PubMed]
- Urban, E.A.; Johnston, R.J. Buffering and Amplifying Transcriptional Noise During Cell Fate Specification. Front. Genet. 2018, 9, 591. [Google Scholar] [CrossRef] [PubMed]
- Little, S.; Tikhonov, M.; Gregor, T. Precise Developmental Gene Expression Arises from Globally Stochastic Transcriptional Activity. Cell 2013, 154, 789–800. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Singh, A.; Kulkarni, R.V. Transcriptional Bursting in Gene Expression: Analytical Results for General Stochastic Models. PLoS Comput. Biol. 2015, 11, e1004292. [Google Scholar] [CrossRef] [PubMed]
- Dar, R.D.; Razooky, B.S.; Singh, A.; Trimeloni, T.V.; McCollum, J.M.; Cox, C.D.; Simpson, M.L.; Weinberger, L.S. Transcriptional burst frequency and burst size are equally modulated across the human genome. Proc. Natl. Acad. Sci. USA 2012, 109, 17454–17459. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.T.; Zoller, B.; Levo, M.; Gregor, T. Common bursting relationships underlie eukaryotic transcription dynamics. arXiv 2023, arXiv:2304.08770. [Google Scholar]
- Zoller, B.; Little, S.C.; Gregor, T. Diverse Spatial Expression Patterns Emerge from Unified Kinetics of Transcriptional Bursting. Cell 2018, 175, 835–847.e25. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Sepúlveda, L.A.; Figard, L.; Sokac, A.M.; Golding, I. Combining protein and mRNA quantification to decipher transcriptional regulation. Nat. Methods 2015, 12, 739–742. [Google Scholar] [CrossRef] [PubMed]
- Benabdallah, N.S.; Bickmore, W.A. Regulatory Domains and Their Mechanisms. Cold Spring Harb. Symp. Quant. Biol. 2015, 80, 45–51. [Google Scholar] [CrossRef]
- Giammartino, D.C.D.; Polyzos, A.; Apostolou, E. Transcription factors: Building hubs in the 3D space. Cell Cycle 2020, 19, 1–16. [Google Scholar] [CrossRef]
- Spitz, F.; Furlong, E.E.M. Transcription factors: From enhancer binding to developmental control. Nat. Rev. Genet. 2012, 13, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Rohs, R.; Jin, X.; West, S.M.; Joshi, R.; Honig, B.; Mann, R.S. Origins of Specificity in Protein-DNA Recognition. Annu. Rev. Biochem. 2010, 79, 233–269. [Google Scholar] [CrossRef]
- Gebhardt, J.C.M.; Suter, D.M.; Roy, R.; Zhao, Z.W.; Chapman, A.R.; Basu, S.; Maniatis, T.; Xie, X.S. Single-molecule imaging of transcription factor binding to DNA in live mammalian cells. Nat. Methods 2013, 10, 421–426. [Google Scholar] [CrossRef]
- Ptashne, M.; Gann, A. Transcriptional activation by recruitment. Nature 1997, 386, 569–577. [Google Scholar] [CrossRef]
- Bhat, P.; Honson, D.; Guttman, M. Nuclear compartmentalization as a mechanism of quantitative control of gene expression. Nat. Rev. Mol. Cell Biol. 2021, 22, 653–670. [Google Scholar] [CrossRef] [PubMed]
- Misteli, T. The Self-Organizing Genome: Principles of Genome Architecture and Function. Cell 2020, 183, 28–45. [Google Scholar] [CrossRef]
- Furlong, E.E.M.; Levine, M. Developmental enhancers and chromosome topology. Science 2018, 361, 1341–1345. [Google Scholar] [CrossRef]
- Laat, W.d.; Duboule, D. Topology of mammalian developmental enhancers and their regulatory landscapes. Nature 2013, 502, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Lee, J.; Wang, H.; Miller, J.; Reik, A.; Gregory, P.; Dean, A.; Blobel, G. Controlling Long-Range Genomic Interactions at a Native Locus by Targeted Tethering of a Looping Factor. Cell 2012, 149, 1233–1244. [Google Scholar] [CrossRef]
- Miele, A.; Dekker, J. Long-range chromosomal interactions and gene regulation. Mol. Biosyst. 2008, 4, 1046–1057. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Shendure, J. Mechanisms of Interplay between Transcription Factors and the 3D Genome. Mol. Cell 2019, 76, 306–319. [Google Scholar] [CrossRef]
- Stadhouders, R.; Filion, G.J.; Graf, T. Transcription factors and 3D genome conformation in cell-fate decisions. Nature 2019, 569, 345–354. [Google Scholar] [CrossRef]
- Popay, T.M.; Dixon, J.R. Coming full circle: On the origin and evolution of the looping model for enhancer—Promoter communication. J. Biol. Chem. 2022, 298, 102117. [Google Scholar] [CrossRef] [PubMed]
- Brückner, D.B.; Chen, H.; Barinov, L.; Zoller, B.; Gregor, T. Stochastic motion and transcriptional dynamics of pairs of distal DNA loci on a compacted chromosome. Science 2023, 380, 1357–1362. [Google Scholar] [CrossRef]
- Lampo, T.; Kennard, A.; Spakowitz, A. Physical Modeling of Dynamic Coupling between Chromosomal Loci. Biophys. J. 2016, 110, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Sakaue, T.; Schiessel, H. Slow chromatin dynamics enhances promoter accessibility to transcriptional condensates. Nucleic Acids Res. 2021, 49, 5017–5027. [Google Scholar] [CrossRef] [PubMed]
- Fukaya, T.; Lim, B.; Levine, M. Enhancer Control of Transcriptional Bursting. Cell 2016, 166, 358–368. [Google Scholar] [CrossRef]
- Schoenfelder, S.; Fraser, P. Long-range enhancer—Promoter contacts in gene expression control. Nat. Rev. Genet. 2019, 20, 437–455. [Google Scholar] [CrossRef]
- Vernimmen, D.; Gobbi, M.D.; Sloane-Stanley, J.A.; Wood, W.G.; Higgs, D.R. Long-range chromosomal interactions regulate the timing of the transition between poised and active gene expression. EMBO J. 2007, 26, 2041–2051. [Google Scholar] [CrossRef]
- Stadhouders, R.; Vidal, E.; Serra, F.; Stefano, B.D.; Dily, F.L.; Quilez, J.; Gomez, A.; Collombet, S.; Berenguer, C.; Cuartero, Y.; et al. Transcription factors orchestrate dynamic interplay between genome topology and gene regulation during cell reprogramming. Nat. Genet. 2018, 50, 238–249. [Google Scholar] [CrossRef] [PubMed]
- Orphanides, G.; Lagrange, T.; Reinberg, D. The general transcription factors of RNA polymerase II. Genes Dev. 1996, 10, 2657–2683. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.; Roeder, R.G. Regulation of the RNA polymerase II pre-initiation complex by its associated coactivators. Nat. Rev. Genet. 2023, 24, 767–782. [Google Scholar] [CrossRef]
- Boija, A.; Klein, I.A.; Sabari, B.R.; Dall’Agnese, A.; Coffey, E.L.; Zamudio, A.V.; Li, C.H.; Shrinivas, K.; Manteiga, J.C.; Hannett, N.M.; et al. Transcription Factors Activate Genes through the Phase-Separation Capacity of Their Activation Domains. Cell 2018, 175, 1842–1855.e16. [Google Scholar] [CrossRef] [PubMed]
- Tsai, A.; Muthusamy, A.K.; Alves, M.R.; Lavis, L.D.; Singer, R.H.; Stern, D.L.; Crocker, J. Nuclear microenvironments modulate transcription from low-affinity enhancers. eLife 2017, 6, e28975. [Google Scholar] [CrossRef] [PubMed]
- Hayward-Lara, G.; Fischer, M.D.; Mir, M. Dynamic microenvironments shape nuclear organization and gene expression. Curr. Opin. Genet. Dev. 2024, 86, 102177. [Google Scholar] [CrossRef] [PubMed]
- Zhu, I.; Song, W.; Ovcharenko, I.; Landsman, D. A model of active transcription hubs that unifies the roles of active promoters and enhancers. Nucleic Acids Res. 2021, 49, gkab235. [Google Scholar] [CrossRef]
- Allen, B.L.; Taatjes, D.J. The Mediator complex: A central integrator of transcription. Nat. Rev. Mol. Cell Biol. 2015, 16, 155–166. [Google Scholar] [CrossRef]
- Khattabi, L.E.; Zhao, H.; Kalchschmidt, J.; Young, N.; Jung, S.; Blerkom, P.V.; Kieffer-Kwon, P.; Kieffer-Kwon, K.R.; Park, S.; Wang, X.; et al. A Pliable Mediator Acts as a Functional Rather Than an Architectural Bridge between Promoters and Enhancers. Cell 2019, 178, 1145–1158.e20. [Google Scholar] [CrossRef]
- Palacio, M.; Taatjes, D.J. Merging Established Mechanisms with New Insights: Condensates, Hubs, and the Regulation of RNA Polymerase II Transcription. J. Mol. Biol. 2022, 434, 167216. [Google Scholar] [CrossRef]
- Banani, S.F.; Lee, H.O.; Hyman, A.A.; Rosen, M.K. Biomolecular condensates: Organizers of cellular biochemistry. Nat. Rev. Mol. Cell Biol. 2017, 18, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Gomes, E.; Shorter, J. The molecular language of membraneless organelles. J. Biol. Chem. 2019, 294, 7115–7127. [Google Scholar] [CrossRef]
- Cho, W.K.; Spille, J.H.; Hecht, M.; Lee, C.; Li, C.; Grube, V.; Cisse, I.I. Mediator and RNA polymerase II clusters associate in transcription-dependent condensates. Science 2018, 361, 412–415. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.K.; Jayanth, N.; English, B.P.; Inoue, T.; Andrews, J.O.; Conway, W.; Grimm, J.B.; Spille, J.H.; Lavis, L.D.; Lionnet, T.; et al. RNA Polymerase II cluster dynamics predict mRNA output in living cells. eLife 2016, 5, e13617. [Google Scholar] [CrossRef] [PubMed]
- Cisse, I.I.; Izeddin, I.; Causse, S.Z.; Boudarene, L.; Senecal, A.; Muresan, L.; Dugast-Darzacq, C.; Hajj, B.; Dahan, M.; Darzacq, X. Real-Time Dynamics of RNA Polymerase II Clustering in Live Human Cells. Science 2013, 341, 664–667. [Google Scholar] [CrossRef] [PubMed]
- McSwiggen, D.T.; Hansen, A.S.; Teves, S.S.; Marie-Nelly, H.; Hao, Y.; Heckert, A.B.; Umemoto, K.K.; Dugast-Darzacq, C.; Tjian, R.; Darzacq, X. Evidence for DNA-mediated nuclear compartmentalization distinct from phase separation. eLife 2019, 8, e47098. [Google Scholar] [CrossRef] [PubMed]
- Mittag, T.; Pappu, R.V. A conceptual framework for understanding phase separation and addressing open questions and challenges. Mol. Cell 2022, 82, 2201–2214. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Pugh, B.F. What do Transcription Factors Interact With? J. Mol. Biol. 2021, 433, 166883. [Google Scholar] [CrossRef] [PubMed]
- Suter, D.M. Transcription Factors and DNA Play Hide and Seek. Trends Cell Biol. 2020, 30, 491–500. [Google Scholar] [CrossRef]
- Ryu, K.; Park, G.; Cho, W.K. Emerging insights into transcriptional condensates. Exp. Mol. Med. 2024, 56, 820–826. [Google Scholar] [CrossRef]
- Park, P.J. ChIP—Seq: Advantages and challenges of a maturing technology. Nat. Rev. Genet. 2009, 10, 669–680. [Google Scholar] [CrossRef]
- Buenrostro, J.D.; Wu, B.; Chang, H.Y.; Greenleaf, W.J. ATAC-seq: A Method for Assaying Chromatin Accessibility Genome-Wide. Curr. Protoc. Mol. Biol. 2015, 109, 21.29.1–21.29.9. [Google Scholar] [CrossRef] [PubMed]
- Alberti, S.; Saha, S.; Woodruff, J.B.; Franzmann, T.M.; Wang, J.; Hyman, A.A. A User’s Guide for Phase Separation Assays with Purified Proteins. J. Mol. Biol. 2018, 430, 4806–4820. [Google Scholar] [CrossRef]
- Ren, B.; Robert, F.; Wyrick, J.J.; Aparicio, O.; Jennings, E.G.; Simon, I.; Zeitlinger, J.; Schreiber, J.; Hannett, N.; Kanin, E.; et al. Genome-Wide Location and Function of DNA Binding Proteins. Science 2000, 290, 2306–2309. [Google Scholar] [CrossRef] [PubMed]
- Irgen-Gioro, S.; Yoshida, S.; Walling, V.; Chong, S. Fixation can change the appearance of phase separation in living cells. eLife 2022, 11, e79903. [Google Scholar] [CrossRef]
- Mir, M.; Reimer, A.; Haines, J.E.; Li, X.Y.; Stadler, M.; Garcia, H.; Eisen, M.B.; Darzacq, X. Dense Bicoid hubs accentuate binding along the morphogen gradient. Genes Dev. 2017, 31, 1784–1794. [Google Scholar] [CrossRef]
- Izeddin, I.; Récamier, V.; Bosanac, L.; Cissé, I.I.; Boudarene, L.; Dugast-Darzacq, C.; Proux, F.; Bénichou, O.; Voituriez, R.; Bensaude, O.; et al. Single-molecule tracking in live cells reveals distinct target-search strategies of transcription factors in the nucleus. eLife 2014, 3, e02230. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Stitzinger, S.H.; Spille, J.H.; Cho, W.K.; Lee, C.; Hijaz, M.; Quintana, A.; Cissé, I.I. Direct observation of a condensate effect on super-enhancer controlled gene bursting. Cell 2024, 187, 331–344.e17. [Google Scholar] [CrossRef] [PubMed]
- Chong, S.; Dugast-Darzacq, C.; Liu, Z.; Dong, P.; Dailey, G.M.; Cattoglio, C.; Heckert, A.; Banala, S.; Lavis, L.; Darzacq, X.; et al. Imaging dynamic and selective low-complexity domain interactions that control gene transcription. Science 2018, 361, eaar2555. [Google Scholar] [CrossRef]
- Kim, Y.J.; Lee, M., Jr.; Lee, Y.T.; Jing, J.; Sanders, J.T.; Botten, G.A.; He, L.; Lyu, J.; Zhang, Y.; Mettlen, M.; et al. Light-activated macromolecular phase separation modulates transcription by reconfiguring chromatin interactions. Sci. Adv. 2023, 9, eadg1123. [Google Scholar] [CrossRef]
- Lee, M.; Moon, H.C.; Jeong, H.; Kim, D.W.; Park, H.Y.; Shin, Y. Optogenetic control of mRNA condensation reveals an intimate link between condensate material properties and functions. Nat. Commun. 2024, 15, 3216. [Google Scholar] [CrossRef] [PubMed]
- Taylor, N.O.; Wei, M.T.; Stone, H.A.; Brangwynne, C.P. Quantifying Dynamics in Phase-Separated Condensates Using Fluorescence Recovery after Photobleaching. Biophys. J. 2019, 117, 1285–1300. [Google Scholar] [CrossRef] [PubMed]
- Shimobayashi, S.F.; Ronceray, P.; Sanders, D.W.; Haataja, M.P.; Brangwynne, C.P. Nucleation landscape of biomolecular condensates. Nature 2021, 599, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.; Berry, J.; Pannucci, N.; Haataja, M.P.; Toettcher, J.E.; Brangwynne, C.P. Spatiotemporal Control of Intracellular Phase Transitions Using Light-Activated optoDroplets. Cell 2017, 168, 159–171.e14. [Google Scholar] [CrossRef] [PubMed]
- Donovan, B.T.; Huynh, A.; Ball, D.A.; Patel, H.P.; Poirier, M.G.; Larson, D.R.; Ferguson, M.L.; Lenstra, T.L. Live-cell imaging reveals the interplay between transcription factors, nucleosomes, and bursting. EMBO J. 2019, 38, e100809. [Google Scholar] [CrossRef] [PubMed]
- Garcia, H.; Tikhonov, M.; Lin, A.; Gregor, T. Quantitative Imaging of Transcription in Living Drosophila Embryos Links Polymerase Activity to Patterning. Curr. Biol. 2013, 23, 2140–2145. [Google Scholar] [CrossRef] [PubMed]
- Golding, I.; Paulsson, J.; Zawilski, S.M.; Cox, E.C. Real-Time Kinetics of Gene Activity in Individual Bacteria. Cell 2005, 123, 1025–1036. [Google Scholar] [CrossRef]
- Yunger, S.; Rosenfeld, L.; Garini, Y.; Shav-Tal, Y. Single-allele analysis of transcription kinetics in living mammalian cells. Nat. Methods 2010, 7, 631–633. [Google Scholar] [CrossRef]
- Kawasaki, K.; Fukaya, T. Functional coordination between transcription factor clustering and gene activity. Mol. Cell 2023, 83, 1605–1622.e9. [Google Scholar] [CrossRef] [PubMed]
- Bintu, B.; Mateo, L.J.; Su, J.H.; Sinnott-Armstrong, N.A.; Parker, M.; Kinrot, S.; Yamaya, K.; Boettiger, A.N.; Zhuang, X. Super-resolution chromatin tracing reveals domains and cooperative interactions in single cells. Science 2018, 362, eaau1783. [Google Scholar] [CrossRef]
- Gabriele, M.; Brandão, H.B.; Grosse-Holz, S.; Jha, A.; Dailey, G.M.; Cattoglio, C.; Hsieh, T.H.S.; Mirny, L.; Zechner, C.; Hansen, A.S. Dynamics of CTCF- and cohesin-mediated chromatin looping revealed by live-cell imaging. Science 2022, 376, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Levo, M.; Barinov, L.; Fujioka, M.; Jaynes, J.B.; Gregor, T. Dynamic interplay between enhancer—Promoter topology and gene activity. Nat. Genet. 2018, 50, 1296–1303. [Google Scholar] [CrossRef] [PubMed]
- Munshi, R.; Ling, J.; Ryabichko, S.; Wieschaus, E.F.; Gregor, T. Transcription factor clusters as information transfer agents. arXiv 2024, arXiv:2403.02943. [Google Scholar]
- Gregor, T.; Tank, D.W.; Wieschaus, E.F.; Bialek, W. Probing the Limits to Positional Information. Cell 2007, 130, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Petkova, M.D.; Tkačik, G.; Bialek, W.; Wieschaus, E.F.; Gregor, T. Optimal Decoding of Cellular Identities in a Genetic Network. Cell 2019, 176, 844–855.e15. [Google Scholar] [CrossRef] [PubMed]
- Tkačik, G.; Callan, C.G.; Bialek, W. Information flow and optimization in transcriptional regulation. Proc. Natl. Acad. Sci. USA 2008, 105, 12265–12270. [Google Scholar] [CrossRef] [PubMed]
- Bintu, L.; Buchler, N.E.; Garcia, H.G.; Gerland, U.; Hwa, T.; Kondev, J.; Phillips, R. Transcriptional regulation by the numbers: Models. Curr. Opin. Genet. Dev. 2005, 15, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Hippel, P.H.v.; Berg, O.G. Facilitated Target Location in Biological Systems. J. Biol. Chem. 1989, 264, 675–678. [Google Scholar] [CrossRef]
- Mirny, L.; Slutsky, M.; Wunderlich, Z.; Tafvizi, A.; Leith, J.; Kosmrlj, A. How a protein searches for its site on DNA: The mechanism of facilitated diffusion. J. Phys. A Math. Theor. 2009, 42, 434013. [Google Scholar] [CrossRef]
- Marklund, E.; Oosten, B.v.; Mao, G.; Amselem, E.; Kipper, K.; Sabantsev, A.; Emmerich, A.; Globisch, D.; Zheng, X.; Lehmann, L.C.; et al. DNA surface exploration and operator bypassing during target search. Nature 2020, 583, 858–861. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Z.; Li, L.; Chen, B.C.; Revyakin, A.; Hajj, B.; Legant, W.; Dahan, M.; Lionnet, T.; Betzig, E.; et al. Single-Molecule Dynamics of Enhanceosome Assembly in Embryonic Stem Cells. Cell 2014, 156, 1274–1285. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, C.; Rube, H.T.; Kribelbauer, J.F.; Crocker, J.; Loker, R.E.; Martini, G.D.; Laptenko, O.; Freed-Pastor, W.A.; Prives, C.; Stern, D.L.; et al. Accurate and sensitive quantification of protein-DNA binding affinity. Proc. Natl. Acad. Sci. USA 2018, 115, E3692–E3701. [Google Scholar] [CrossRef] [PubMed]
- Geertz, M.; Shore, D.; Maerkl, S.J. Massively parallel measurements of molecular interaction kinetics on a microfluidic platform. Proc. Natl. Acad. Sci. USA 2012, 109, 16540–16545. [Google Scholar] [CrossRef] [PubMed]
- Garcia, D.A.; Fettweis, G.; Presman, D.M.; Paakinaho, V.; Jarzynski, C.; Upadhyaya, A.; Hager, G. Power-law behavior of transcription factor dynamics at the single-molecule level implies a continuum affinity model. Nucleic Acids Res. 2021, 49, gkab072. [Google Scholar] [CrossRef] [PubMed]
- Berman, B.P.; Nibu, Y.; Pfeiffer, B.D.; Tomancak, P.; Celniker, S.E.; Levine, M.; Rubin, G.M.; Eisen, M.B. Exploiting transcription factor binding site clustering to identify cis-regulatory modules involved in pattern formation in the Drosophila genome. Proc. Natl. Acad. Sci. USA 2002, 99, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Wagh, K.; Stavreva, D.A.; Upadhyaya, A.; Hager, G.L. Transcription Factor Dynamics: One Molecule at a Time. Annu. Rev. Cell Dev. Biol. 2023, 39, 277–305. [Google Scholar] [CrossRef] [PubMed]
- Zaret, K.S. Pioneer Transcription Factors Initiating Gene Network Changes. Annu. Rev. Genet. 2020, 54, 367–385. [Google Scholar] [CrossRef] [PubMed]
- Zaret, K.S.; Carroll, J.S. Pioneer transcription factors: Establishing competence for gene expression. Genes Dev. 2011, 25, 2227–2241. [Google Scholar] [CrossRef]
- Zaret, K.S.; Mango, S.E. Pioneer transcription factors, chromatin dynamics, and cell fate control. Curr. Opin. Genet. Dev. 2016, 37, 76–81. [Google Scholar] [CrossRef]
- Felipe, C.; Shin, J.; Kolomeisky, A.B. How Pioneer Transcription Factors Search for Target Sites on Nucleosomal DNA. J. Phys. Chem. B 2022, 126, 4061–4068. [Google Scholar] [CrossRef]
- Donovan, B.T.; Chen, H.; Jipa, C.; Bai, L.; Poirier, M.G. Dissociation rate compensation mechanism for budding yeast pioneer transcription factors. eLife 2019, 8, e43008. [Google Scholar] [CrossRef] [PubMed]
- Udupa, A.; Kotha, S.R.; Staller, M.V. Commonly asked questions about transcriptional activation domains. Curr. Opin. Struct. Biol. 2024, 84, 102732. [Google Scholar] [CrossRef] [PubMed]
- Soto, L.F.; Li, Z.; Santoso, C.S.; Berenson, A.; Ho, I.; Shen, V.X.; Yuan, S.; Bass, J.I.F. Compendium of human transcription factor effector domains. Mol. Cell 2022, 82, 514–526. [Google Scholar] [CrossRef] [PubMed]
- Dyson, H.J.; Wright, P.E. Role of Intrinsic Protein Disorder in the Function and Interactions of the Transcriptional Coactivators CREB-binding Protein (CBP) and p300*. J. Biol. Chem. 2016, 291, 6714–6722. [Google Scholar] [CrossRef] [PubMed]
- Garcia, D.A.; Johnson, T.A.; Presman, D.M.; Fettweis, G.; Wagh, K.; Rinaldi, L.; Stavreva, D.A.; Paakinaho, V.; Jensen, R.A.; Mandrup, S.; et al. An intrinsically disordered region-mediated confinement state contributes to the dynamics and function of transcription factors. Mol. Cell 2021, 81, 1484–1498.e6. [Google Scholar] [CrossRef] [PubMed]
- Kent, S.; Brown, K.; Yang, C.h.; Alsaihati, N.; Tian, C.; Wang, H.; Ren, X. Phase-Separated Transcriptional Condensates Accelerate Target-Search Process Revealed by Live-Cell Single-Molecule Imaging. Cell Rep. 2020, 33, 108248. [Google Scholar] [CrossRef] [PubMed]
- Chappleboim, M.; Naveh-Tassa, S.; Carmi, M.; Levy, Y.; Barkai, N. Ordered and disordered regions of the Origin Recognition Complex direct differential in vivo binding at distinct motif sequences. Nucleic Acids Res. 2024, 52, 5720–5731. [Google Scholar] [CrossRef] [PubMed]
- Hurieva, B.; Kumar, D.K.; Morag, R.; Lupo, O.; Carmi, M.; Barkai, N.; Jonas, F. Disordered sequences of transcription factors regulate genomic binding by integrating diverse sequence grammars and interaction types. Nucleic Acids Res. 2024, gkae521. [Google Scholar] [CrossRef]
- Lu, F.; Lionnet, T. Transcription Factor Dynamics. Cold Spring Harb. Perspect. Biol. 2021, 13, a040949. [Google Scholar] [CrossRef]
- Meeussen, J.V.W.; Pomp, W.; Brouwer, I.; de Jonge, W.J.; Patel, H.P.; Lenstra, T.L. Transcription factor clusters enable target search but do not contribute to target gene activation. Nucleic Acids Res. 2023, 51, 5449–5468. [Google Scholar] [CrossRef]
- Shrinivas, K.; Sabari, B.R.; Coffey, E.L.; Klein, I.A.; Boija, A.; Zamudio, A.V.; Schuijers, J.; Hannett, N.M.; Sharp, P.A.; Young, R.A.; et al. Enhancer Features that Drive Formation of Transcriptional Condensates. Mol. Cell 2019, 75, 549–561.e7. [Google Scholar] [CrossRef] [PubMed]
- Hnisz, D.; Shrinivas, K.; Young, R.A.; Chakraborty, A.K.; Sharp, P.A. A Phase Separation Model for Transcriptional Control. Cell 2017, 169, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Henninger, J.E.; Oksuz, O.; Shrinivas, K.; Sagi, I.; LeRoy, G.; Zheng, M.M.; Andrews, J.O.; Zamudio, A.V.; Lazaris, C.; Hannett, N.M.; et al. RNA-Mediated Feedback Control of Transcriptional Condensates. Cell 2021, 184, 207–225.e24. [Google Scholar] [CrossRef] [PubMed]
- Mattick, J.S.; Amaral, P.P.; Carninci, P.; Carpenter, S.; Chang, H.Y.; Chen, L.L.; Chen, R.; Dean, C.; Dinger, M.E.; Fitzgerald, K.A.; et al. Long non-coding RNAs: Definitions, functions, challenges and recommendations. Nat. Rev. Mol. Cell Biol. 2023, 24, 430–447. [Google Scholar] [CrossRef] [PubMed]
- Pott, S.; Lieb, J.D. What are super-enhancers? Nat. Genet. 2015, 47, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Hnisz, D.; Abraham, B.; Lee, T.; Lau, A.; Saint-André, V.; Sigova, A.; Hoke, H.; Young, R. Super-Enhancers in the Control of Cell Identity and Disease. Cell 2013, 155, 934–947. [Google Scholar] [CrossRef] [PubMed]
- Sabari, B.R.; Dall’Agnese, A.; Boija, A.; Klein, I.A.; Coffey, E.L.; Shrinivas, K.; Abraham, B.J.; Hannett, N.M.; Zamudio, A.V.; Manteiga, J.C.; et al. Coactivator condensation at super-enhancers links phase separation and gene control. Science 2018, 361, eaar3958. [Google Scholar] [CrossRef]
- Trojanowski, J.; Frank, L.; Rademacher, A.; Mücke, N.; Grigaitis, P.; Rippe, K. Transcription activation is enhanced by multivalent interactions independent of phase separation. Mol. Cell 2022, 82, 1878–1893.e10. [Google Scholar] [CrossRef] [PubMed]
- Arnone, M.I.; Davidson, E.H. The hardwiring of development: Organization and function of genomic regulatory systems. Development 1997, 124, 1851–1864. [Google Scholar] [CrossRef]
- Calo, E.; Wysocka, J. Modification of Enhancer Chromatin: What, How, and Why? Mol. Cell 2013, 49, 825–837. [Google Scholar] [CrossRef]
- Rao, S.; Ahmad, K.; Ramachandran, S. Cooperative binding between distant transcription factors is a hallmark of active enhancers. Mol. Cell 2021, 81, 1651–1665.e4. [Google Scholar] [CrossRef] [PubMed]
- Mirny, L.A. Nucleosome-mediated cooperativity between transcription factors. Proc. Natl. Acad. Sci. USA 2010, 107, 22534–22539. [Google Scholar] [CrossRef] [PubMed]
- Morgunova, E.; Taipale, J. Structural perspective of cooperative transcription factor binding. Curr. Opin. Struct. Biol. 2017, 47, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Driever, W.; Nüsslein-Volhard, C. A gradient of bicoid protein in Drosophila embryos. Cell 1988, 54, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Driever, W.; Nüsslein-Volhard, C. The bicoid protein determines position in the Drosophila embryo in a concentration-dependent manner. Cell 1988, 54, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Blythe, S.A.; Wieschaus, E.F. Establishment and maintenance of heritable chromatin structure during early Drosophila embryogenesis. eLife 2016, 5, e20148. [Google Scholar] [CrossRef] [PubMed]
- Ling, J.; Umezawa, K.Y.; Scott, T.; Small, S. Bicoid-Dependent Activation of the Target Gene hunchback Requires a Two-Motif Sequence Code in a Specific Basal Promoter. Mol. Cell 2019, 75, 1178–1187.e4. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Chen, H.; Ling, J.; Yu, D.; Struffi, P.; Small, S. Impacts of the ubiquitous factor Zelda on Bicoid-dependent DNA binding and transcription in Drosophila. Genes Dev. 2014, 28, 608–621. [Google Scholar] [CrossRef] [PubMed]
- Burz, D.S.; Rivera-Pomar, R.; Jäckle, H.; Hanes, S.D. Cooperative DNA-binding by Bicoid provides a mechanism for threshold-dependent gene activation in the Drosophila embryo. EMBO J. 1998, 17, 5998–6009. [Google Scholar] [CrossRef]
- Lebrecht, D.; Foehr, M.; Smith, E.; Lopes, F.J.P.; Vanario-Alonso, C.E.; Reinitz, J.; Burz, D.S.; Hanes, S.D. Bicoid cooperative DNA binding is critical for embryonic patterning in Drosophila. Proc. Natl. Acad. Sci. USA 2005, 102, 13176–13181. [Google Scholar] [CrossRef]
- Yamada, S.; Whitney, P.H.; Huang, S.K.; Eck, E.C.; Garcia, H.G.; Rushlow, C.A. The Drosophila Pioneer Factor Zelda Modulates the Nuclear Microenvironment of a Dorsal Target Enhancer to Potentiate Transcriptional Output. Curr. Biol. 2019, 29, 1387–1393.e5. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hodgins, L.; Sakib, S.; Mahmood, A.; Perez-Romero, C.; Marmion, R.A.; Dostatni, N.; Fradin, C. Both the transcriptional activator, Bcd, and transcriptional repressor, Cic, form small mobile oligomeric clusters in early fly embryo nuclei. bioRxiv 2024. [Google Scholar] [CrossRef]
- Forés, M.; Ajuria, L.; Samper, N.; Astigarraga, S.; Nieva, C.; Grossman, R.; González-Crespo, S.; Paroush, Z.; Jiménez, G. Origins of Context-Dependent Gene Repression by Capicua. PLoS Genet. 2015, 11, e1004902. [Google Scholar] [CrossRef]
- Driever, W.; Nüsslein-Volhard, C. The bicoid protein is a positive regulator of hunchback transcription in the early Drosophila embryo. Nature 1989, 337, 138–143. [Google Scholar] [CrossRef]
- Singh, A.P.; Wu, P.; Ryabichko, S.; Raimundo, J.; Swan, M.; Wieschaus, E.; Gregor, T.; Toettcher, J.E. Optogenetic control of the Bicoid morphogen reveals fast and slow modes of gap gene regulation. Cell Rep. 2022, 38, 110543. [Google Scholar] [CrossRef]
- Weber, S.C.; Theriot, J.A.; Spakowitz, A.J. Subdiffusive motion of a polymer composed of subdiffusive monomers. Phys. Rev. E 2010, 82, 011913. [Google Scholar] [CrossRef]
- Câmara, A.S.; Mascher, M. Consistencies and contradictions in different polymer models of chromatin architecture. Comput. Struct. Biotechnol. J. 2023, 21, 1084–1091. [Google Scholar] [CrossRef]
- Grosberg, A.; Rabin, Y.; Havlin, S.; Neer, A. Crumpled Globule Model of the Three-Dimensional Structure of DNA. EPL (Europhys. Lett.) 1993, 23, 373–378. [Google Scholar] [CrossRef]
- Mirny, L.A. The fractal globule as a model of chromatin architecture in the cell. Chromosome Res. 2011, 19, 37–51. [Google Scholar] [CrossRef]
- Huisinga, K.L.; Brower-Toland, B.; Elgin, S.C.R. The contradictory definitions of heterochromatin: Transcription and silencing. Chromosoma 2006, 115, 110–122. [Google Scholar] [CrossRef]
- Bickmore, W.; van Steensel, B. Genome Architecture: Domain Organization of Interphase Chromosomes. Cell 2013, 152, 1270–1284. [Google Scholar] [CrossRef] [PubMed]
- Talbert, P.B.; Henikoff, S. Histone variants on the move: Substrates for chromatin dynamics. Nat. Rev. Mol. Cell Biol. 2017, 18, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Lieberman-Aiden, E.; Berkum, N.L.v.; Williams, L.; Imakaev, M.; Ragoczy, T.; Telling, A.; Amit, I.; Lajoie, B.R.; Sabo, P.J.; Dorschner, M.O.; et al. Comprehensive Mapping of Long-Range Interactions Reveals Folding Principles of the Human Genome. Science 2009, 326, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Rowley, M.J.; Nichols, M.H.; Lyu, X.; Ando-Kuri, M.; Rivera, I.S.M.; Hermetz, K.; Wang, P.; Ruan, Y.; Corces, V.G. Evolutionarily Conserved Principles Predict 3D Chromatin Organization. Mol. Cell 2017, 67, 837–852.e7. [Google Scholar] [CrossRef]
- Erdel, F.; Rippe, K. Formation of Chromatin Subcompartments by Phase Separation. Biophys. J. 2018, 114, 2262–2270. [Google Scholar] [CrossRef] [PubMed]
- McSwiggen, D.T.; Mir, M.; Darzacq, X.; Tjian, R. Evaluating phase separation in live cells: Diagnosis, caveats, and functional consequences. Genes Dev. 2019, 33, 1619–1634. [Google Scholar] [CrossRef] [PubMed]
- Hildebrand, E.M.; Dekker, J. Mechanisms and Functions of Chromosome Compartmentalization. Trends Biochem. Sci. 2020, 45, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Rowley, M.J.; Corces, V.G. Organizational principles of 3D genome architecture. Nat. Rev. Genet. 2018, 19, 789–800. [Google Scholar] [CrossRef] [PubMed]
- Szabo, Q.; Bantignies, F.; Cavalli, G. Principles of genome folding into topologically associating domains. Sci. Adv. 2019, 5, eaaw1668. [Google Scholar] [CrossRef]
- Dixon, J.R.; Selvaraj, S.; Yue, F.; Kim, A.; Li, Y.; Shen, Y.; Hu, M.; Liu, J.S.; Ren, B. Topological domains in mammalian genomes identified by analysis of chromatin interactions. Nature 2012, 485, 376–380. [Google Scholar] [CrossRef]
- Dixon, J.; Gorkin, D.; Ren, B. Chromatin Domains: The Unit of Chromosome Organization. Mol. Cell 2016, 62, 668–680. [Google Scholar] [CrossRef] [PubMed]
- Brasset, E.; Vaury, C. Insulators are fundamental components of the eukaryotic genomes. Heredity 2005, 94, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, L.; Kamakaka, R.T. Chromatin Insulators*. Genetics 2006, 40, 107–138. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, E.J.; Geyer, P.K. Genomic insulators: Connecting properties to mechanism. Curr. Opin. Cell Biol. 2003, 15, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Fujioka, M.; Mistry, H.; Schedl, P.; Jaynes, J.B. Determinants of Chromosome Architecture: Insulator Pairing in cis and in trans. PLoS Genet. 2016, 12, e1005889. [Google Scholar] [CrossRef] [PubMed]
- Eagen, K.P.; Aiden, E.L.; Kornberg, R.D. Polycomb-mediated chromatin loops revealed by a subkilobase-resolution chromatin interaction map. Proc. Natl. Acad. Sci. USA 2017, 114, 8764–8769. [Google Scholar] [CrossRef] [PubMed]
- Davidson, I.F.; Goetz, D.; Zaczek, M.P.; Molodtsov, M.I.; Veld, P.J.H.i.t.; Weissmann, F.; Litos, G.; Cisneros, D.A.; Ocampo-Hafalla, M.; Ladurner, R.; et al. Rapid movement and transcriptional re-localization of human cohesin on DNA. EMBO J. 2016, 35, 2671–2685. [Google Scholar] [CrossRef] [PubMed]
- Beagan, J.A.; Phillips-Cremins, J.E. On the existence and functionality of topologically associating domains. Nat. Genet. 2020, 52, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.E.; Corces, V.G. CTCF: Master Weaver of the Genome. Cell 2009, 137, 1194–1211. [Google Scholar] [CrossRef]
- Goloborodko, A.; Marko, J.; Mirny, L. Chromosome Compaction by Active Loop Extrusion. Biophys. J. 2016, 110, 2162–2168. [Google Scholar] [CrossRef]
- Chen, L.F.; Long, H.K.; Park, M.; Swigut, T.; Boettiger, A.N.; Wysocka, J. Structural elements promote architectural stripe formation and facilitate ultra-long-range gene regulation at a human disease locus. Mol. Cell 2023, 83, 1446–1461.e6. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.S.; Cattoglio, C.; Darzacq, X.; Tjian, R. Recent evidence that TADs and chromatin loops are dynamic structures. Nucleus 2018, 9, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Levo, M.; Raimundo, J.; Bing, X.Y.; Sisco, Z.; Batut, P.J.; Ryabichko, S.; Gregor, T.; Levine, M.S. Transcriptional coupling of distant regulatory genes in living embryos. Nature 2022, 605, 754–760. [Google Scholar] [CrossRef] [PubMed]
- Batut, P.J.; Bing, X.Y.; Sisco, Z.; Raimundo, J.; Levo, M.; Levine, M.S. Genome organization controls transcriptional dynamics during development. Science 2022, 375, 566–570. [Google Scholar] [CrossRef] [PubMed]
- Paliou, C.; Guckelberger, P.; Schöpflin, R.; Heinrich, V.; Esposito, A.; Chiariello, A.M.; Bianco, S.; Annunziatella, C.; Helmuth, J.; Haas, S.; et al. Preformed chromatin topology assists transcriptional robustness of Shh during limb development. Proc. Natl. Acad. Sci. USA 2019, 116, 12390–12399. [Google Scholar] [CrossRef] [PubMed]
- Bonev, B.; Cohen, N.M.; Szabo, Q.; Fritsch, L.; Papadopoulos, G.L.; Lubling, Y.; Xu, X.; Lv, X.; Hugnot, J.P.; Tanay, A.; et al. Multiscale 3D Genome Rewiring during Mouse Neural Development. Cell 2017, 171, 557–572.e24. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Levine, M. What are tethering elements? Curr. Opin. Genet. Dev. 2024, 84, 102151. [Google Scholar] [CrossRef] [PubMed]
- Nicodemi, M.; Prisco, A. Thermodynamic Pathways to Genome Spatial Organization in the Cell Nucleus. Biophys. J. 2009, 96, 2168–2177. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, M.; Chotalia, M.; Fraser, J.; Lavitas, L.M.; Dostie, J.; Pombo, A.; Nicodemi, M. Complexity of chromatin folding is captured by the strings and binders switch model. Proc. Natl. Acad. Sci. USA 2012, 109, 16173–16178. [Google Scholar] [CrossRef]
- Clark, D.J.; Kimura, T. Electrostatic mechanism of chromatin folding. J. Mol. Biol. 1990, 211, 883–896. [Google Scholar] [CrossRef]
- Lipfert, J.; Doniach, S.; Das, R.; Herschlag, D. Understanding Nucleic Acid—Ion Interactions. Annu. Rev. Biochem. 2014, 83, 813–841. [Google Scholar] [CrossRef]
- Simonson, T.; Brooks, C.L. Charge Screening and the Dielectric Constant of Proteins: Insights from Molecular Dynamics. J. Am. Chem. Soc. 1996, 118, 8452–8458. [Google Scholar] [CrossRef]
- Yokoshi, M.; Segawa, K.; Fukaya, T. Visualizing the Role of Boundary Elements in Enhancer-Promoter Communication. Mol. Cell 2020, 78, 224–235.e5. [Google Scholar] [CrossRef]
- Benabdallah, N.S.; Williamson, I.; Illingworth, R.S.; Kane, L.; Boyle, S.; Sengupta, D.; Grimes, G.R.; Therizols, P.; Bickmore, W.A. Decreased Enhancer-Promoter Proximity Accompanying Enhancer Activation. Mol. Cell 2019, 76, 473–484.e7. [Google Scholar] [CrossRef] [PubMed]
- Shaner, N.C.; Steinbach, P.A.; Tsien, R.Y. A guide to choosing fluorescent proteins. Nat. Methods 2005, 2, 905–909. [Google Scholar] [CrossRef]
- Chudakov, D.M.; Matz, M.V.; Lukyanov, S.; Lukyanov, K.A. Fluorescent Proteins and Their Applications in Imaging Living Cells and Tissues. Physiol. Rev. 2010, 90, 1103–1163. [Google Scholar] [CrossRef]
- Rodriguez, E.A.; Campbell, R.E.; Lin, J.Y.; Lin, M.Z.; Miyawaki, A.; Palmer, A.E.; Shu, X.; Zhang, J.; Tsien, R.Y. The Growing and Glowing Toolbox of Fluorescent and Photoactive Proteins. Trends Biochem. Sci. 2017, 42, 111–129. [Google Scholar] [CrossRef] [PubMed]
- Grimm, J.B.; Muthusamy, A.K.; Liang, Y.; Brown, T.A.; Lemon, W.C.; Patel, R.; Lu, R.; Macklin, J.J.; Keller, P.J.; Ji, N.; et al. A general method to fine-tune fluorophores for live-cell and in vivo imaging. Nat. Methods 2017, 14, 987–994. [Google Scholar] [CrossRef]
- Stagge, F.; Mitronova, G.Y.; Belov, V.N.; Wurm, C.A.; Jakobs, S. Snap-, CLIP- and Halo-Tag Labelling of Budding Yeast Cells. PLoS ONE 2013, 8, e78745. [Google Scholar] [CrossRef]
- Boersma, S.; Khuperkar, D.; Verhagen, B.M.; Sonneveld, S.; Grimm, J.B.; Lavis, L.D.; Tanenbaum, M.E. Multi-Color Single-Molecule Imaging Uncovers Extensive Heterogeneity in mRNA Decoding. Cell 2019, 178, 458–472.e19. [Google Scholar] [CrossRef]
- Wang, L.; Frei, M.S.; Salim, A.; Johnsson, K. Small-Molecule Fluorescent Probes for Live-Cell Super-Resolution Microscopy. J. Am. Chem. Soc. 2019, 141, 2770–2781. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, S.; Apaydin, S.; Bucevičius, J.; Gerasimaitė, R.; Kostiuk, G.; Lukinavičius, G. Sequence-specific DNA labelling for fluorescence microscopy. Biosens. Bioelectron. 2023, 230, 115256. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Hou, Y.; Zhang, X.E.; Gao, Y. Live cell imaging of DNA and RNA with fluorescent signal amplification and background reduction techniques. Front. Cell Dev. Biol. 2023, 11, 1216232. [Google Scholar] [CrossRef] [PubMed]
- Pichon, X.; Robert, M.C.; Bertrand, E.; Singer, R.H.; Tutucci, E. RNA Tagging, Methods and Protocols. Methods Mol. Biol. 2020, 2166, 121–144. [Google Scholar] [CrossRef] [PubMed]
- Durrieu, L.; Kirrmaier, D.; Schneidt, T.; Kats, I.; Raghavan, S.; Hufnagel, L.; Saunders, T.E.; Knop, M. Bicoid gradient formation mechanism and dynamics revealed by protein lifetime analysis. Mol. Syst. Biol. 2018, 14, e8355. [Google Scholar] [CrossRef] [PubMed]
- Los, G.V.; Encell, L.P.; McDougall, M.G.; Hartzell, D.D.; Karassina, N.; Zimprich, C.; Wood, M.G.; Learish, R.; Ohana, R.F.; Urh, M.; et al. HaloTag: A Novel Protein Labeling Technology for Cell Imaging and Protein Analysis. ACS Chem. Biol. 2008, 3, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Grimm, J.B.; English, B.P.; Chen, J.; Slaughter, J.P.; Zhang, Z.; Revyakin, A.; Patel, R.; Macklin, J.J.; Normanno, D.; Singer, R.H.; et al. A general method to improve fluorophores for live-cell and single-molecule microscopy. Nat. Methods 2015, 12, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.C.; Legant, W.R.; Wang, K.; Shao, L.; Milkie, D.E.; Davidson, M.W.; Janetopoulos, C.; Wu, X.S.; Hammer, J.A., III; Liu, Z.; et al. Lattice light-sheet microscopy: Imaging molecules to embryos at high spatiotemporal resolution. Science 2014, 346, 1257998. [Google Scholar] [CrossRef]
- Schermelleh, L.; Ferrand, A.; Huser, T.; Eggeling, C.; Sauer, M.; Biehlmaier, O.; Drummen, G.P.C. Super-resolution microscopy demystified. Nat. Cell Biol. 2019, 21, 72–84. [Google Scholar] [CrossRef]
- Mertz, J.; Kim, J. Scanning light-sheet microscopy in the whole mouse brain with HiLo background rejection. J. Biomed. Opt. 2010, 15, 016027. [Google Scholar] [CrossRef]
- Dunsby, C. Optically sectioned imaging by oblique plane microscopy. Opt. Express 2008, 16, 20306. [Google Scholar] [CrossRef] [PubMed]
- Stelzer, E.H.K.; Strobl, F.; Chang, B.J.; Preusser, F.; Preibisch, S.; McDole, K.; Fiolka, R. Light sheet fluorescence microscopy. Nat. Rev. Methods Prim. 2021, 1, 73. [Google Scholar] [CrossRef]
- Betzig, E.; Patterson, G.H.; Sougrat, R.; Lindwasser, O.W.; Olenych, S.; Bonifacino, J.S.; Davidson, M.W.; Lippincott-Schwartz, J.; Hess, H.F. Imaging Intracellular Fluorescent Proteins at Nanometer Resolution. Science 2006, 313, 1642–1645. [Google Scholar] [CrossRef] [PubMed]
- Huff, J. The Airyscan detector from ZEISS: Confocal imaging with improved signal-to-noise ratio and super-resolution. Nat. Methods 2015, 12, i–ii. [Google Scholar] [CrossRef]
- Benninger, R.K.; Piston, D.W. Two-Photon Excitation Microscopy for the Study of Living Cells and Tissues. Curr. Protoc. Cell Biol. 2013, 59, 4.11.1–4.11.24. [Google Scholar] [CrossRef] [PubMed]
- Becker, W.; Su, B.; Holub, O.; Weisshart, K. FLIM and FCS detection in laser-scanning microscopes: Increased efficiency by GaAsP hybrid detectors. Microsc. Res. Tech. 2011, 74, 804–811. [Google Scholar] [CrossRef]
- Luu, P.; Fraser, S.E.; Schneider, F. More than double the fun with two-photon excitation microscopy. Commun. Biol. 2024, 7, 364. [Google Scholar] [CrossRef]
- Engelbrecht, C.J.; Stelzer, E.H. Resolution enhancement in a light-sheet-based microscope (SPIM). Opt. Lett. 2006, 31, 1477. [Google Scholar] [CrossRef]
- Chen, B.; Chang, B.J.; Roudot, P.; Zhou, F.; Sapoznik, E.; Marlar-Pavey, M.; Hayes, J.B.; Brown, P.T.; Zeng, C.W.; Lambert, T.; et al. Resolution doubling in light-sheet microscopy via oblique plane structured illumination. Nat. Methods 2022, 19, 1419–1426. [Google Scholar] [CrossRef] [PubMed]
- Mir, M.; Stadler, M.R.; Ortiz, S.A.; Hannon, C.E.; Harrison, M.M.; Darzacq, X.; Eisen, M.B. Dynamic multifactor hubs interact transiently with sites of active transcription in Drosophila embryos. eLife 2018, 7, e40497. [Google Scholar] [CrossRef]
- Elson, E. Fluorescence Correlation Spectroscopy: Past, Present, Future. Biophys. J. 2011, 101, 2855–2870. [Google Scholar] [CrossRef] [PubMed]
- Athilingam, T.; Nelanuthala, A.V.S.; Breen, C.; Karedla, N.; Fritzsche, M.; Wohland, T.; Saunders, T.E. Long-range formation of the Bicoid gradient requires multiple dynamic modes that spatially vary across the embryo. Development 2024, 151, dev202128. [Google Scholar] [CrossRef] [PubMed]
- Axelrod, D.; Koppel, D.; Schlessinger, J.; Elson, E.; Webb, W. Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys. J. 1976, 16, 1055–1069. [Google Scholar] [CrossRef] [PubMed]
- Elf, J.; Li, G.W.; Xie, X.S. Probing Transcription Factor Dynamics at the Single-Molecule Level in a Living Cell. Science 2007, 316, 1191–1194. [Google Scholar] [CrossRef] [PubMed]
- Mazzocca, M.; Fillot, T.; Loffreda, A.; Gnani, D.; Mazza, D. The needle and the haystack: Single molecule tracking to probe the transcription factor search in eukaryotes. Biochem. Soc. Trans. 2021, 49, 1121–1132. [Google Scholar] [CrossRef] [PubMed]
- Lelek, M.; Gyparaki, M.T.; Beliu, G.; Schueder, F.; Griffié, J.; Manley, S.; Jungmann, R.; Sauer, M.; Lakadamyali, M.; Zimmer, C. Single-molecule localization microscopy. Nat. Rev. Methods Prim. 2021, 1, 39. [Google Scholar] [CrossRef] [PubMed]
- Dey, B.; Thukral, S.; Krishnan, S.; Chakrobarty, M.; Gupta, S.; Manghani, C.; Rani, V. DNA—Protein interactions: Methods for detection and analysis. Mol. Cell. Biochem. 2012, 365, 279–299. [Google Scholar] [CrossRef] [PubMed]
- Rengachari, S.; Schilbach, S.; Aibara, S.; Dienemann, C.; Cramer, P. Structure of the human Mediator—RNA polymerase II pre-initiation complex. Nature 2021, 594, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Grandi, F.C.; Modi, H.; Kampman, L.; Corces, M.R. Chromatin accessibility profiling by ATAC-seq. Nat. Protoc. 2022, 17, 1518–1552. [Google Scholar] [CrossRef] [PubMed]
- Sati, S.; Cavalli, G. Chromosome conformation capture technologies and their impact in understanding genome function. Chromosoma 2017, 126, 33–44. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munshi, R. How Transcription Factor Clusters Shape the Transcriptional Landscape. Biomolecules 2024, 14, 875. https://doi.org/10.3390/biom14070875
Munshi R. How Transcription Factor Clusters Shape the Transcriptional Landscape. Biomolecules. 2024; 14(7):875. https://doi.org/10.3390/biom14070875
Chicago/Turabian StyleMunshi, Rahul. 2024. "How Transcription Factor Clusters Shape the Transcriptional Landscape" Biomolecules 14, no. 7: 875. https://doi.org/10.3390/biom14070875
APA StyleMunshi, R. (2024). How Transcription Factor Clusters Shape the Transcriptional Landscape. Biomolecules, 14(7), 875. https://doi.org/10.3390/biom14070875





