Plasma and Myocardial miRNomes Similarities and Differences during Cardiac Remodelling and Reverse Remodelling in a Murine Model of Heart Failure with Preserved Ejection Fraction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Design
2.3. Echocardiography
2.4. Exercise Capacity
2.5. RNA Isolation
2.6. RNA Preparation for Sequencing
2.7. Bulk microRNA-Sequencing
2.8. MicroRNA Analyses
2.9. Statistical Analysis
3. Results
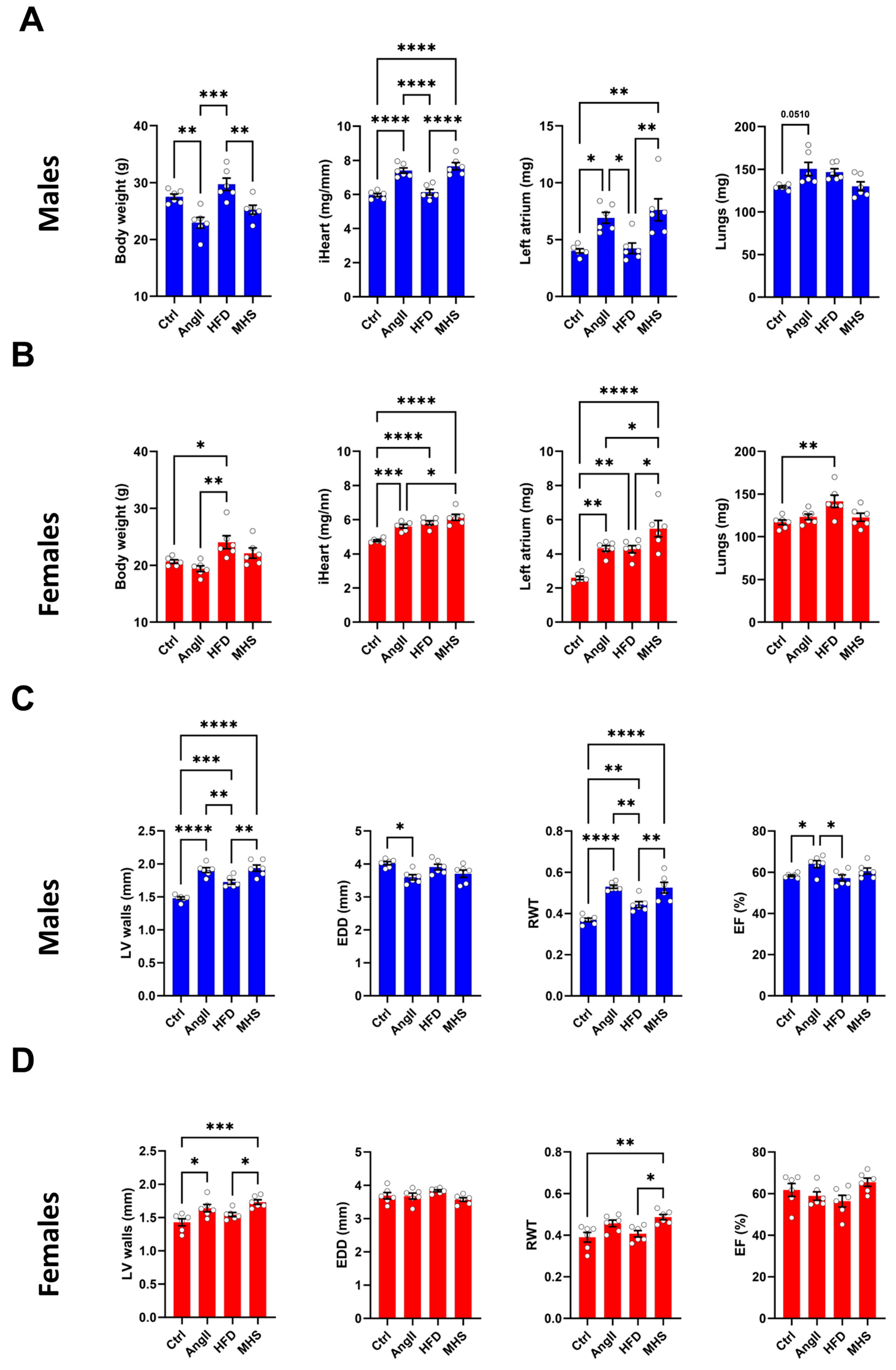
3.1. Angiotensin II Alone or Combined with a High-Fat Diet Induces Cardiac Hypertrophy in Male and Female Mice
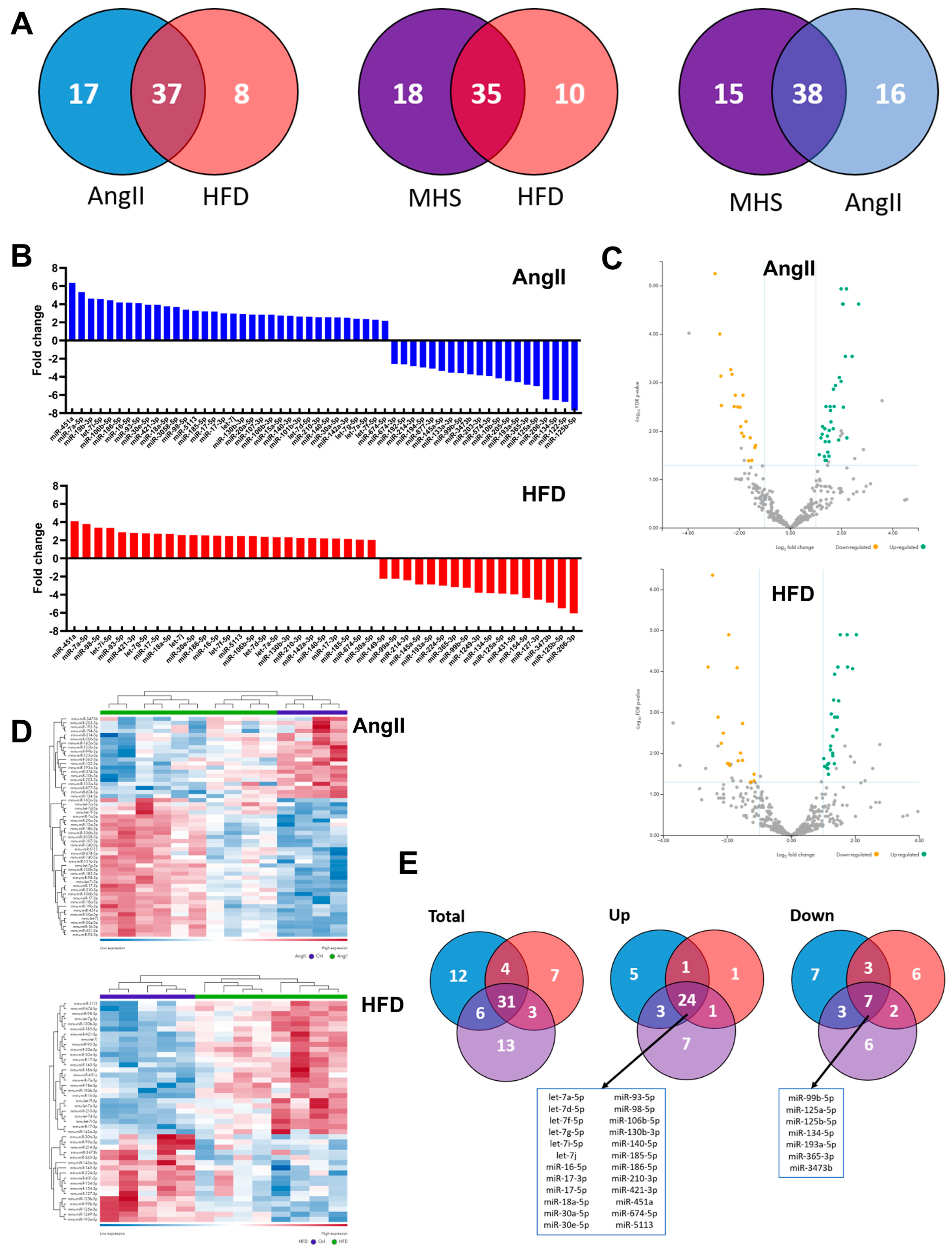
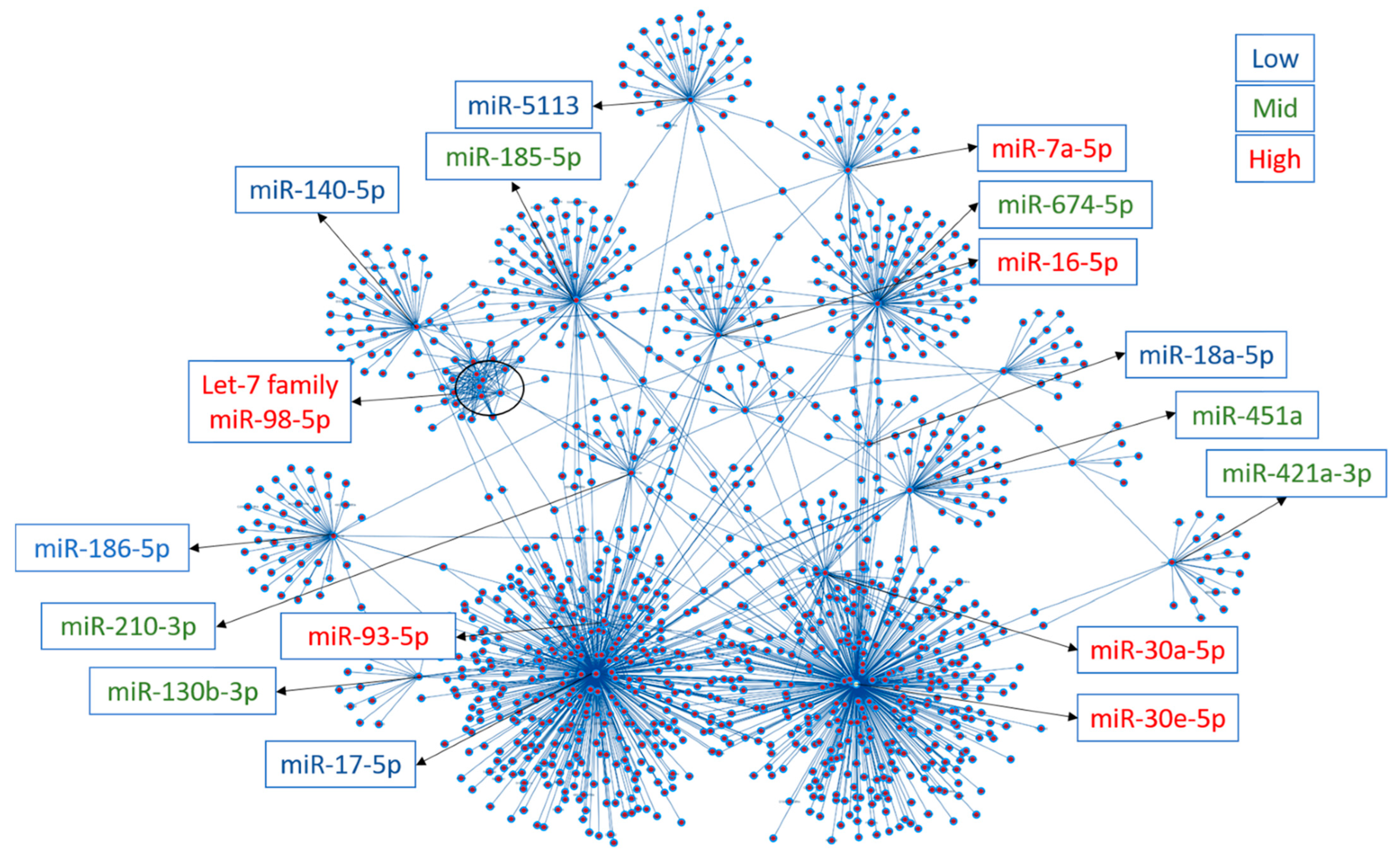
3.2. Plasma miRNomes of Mice Receiving Either AngII or HFD or a Combination of Both (MHS) Show Many Common Modulated microRNAs
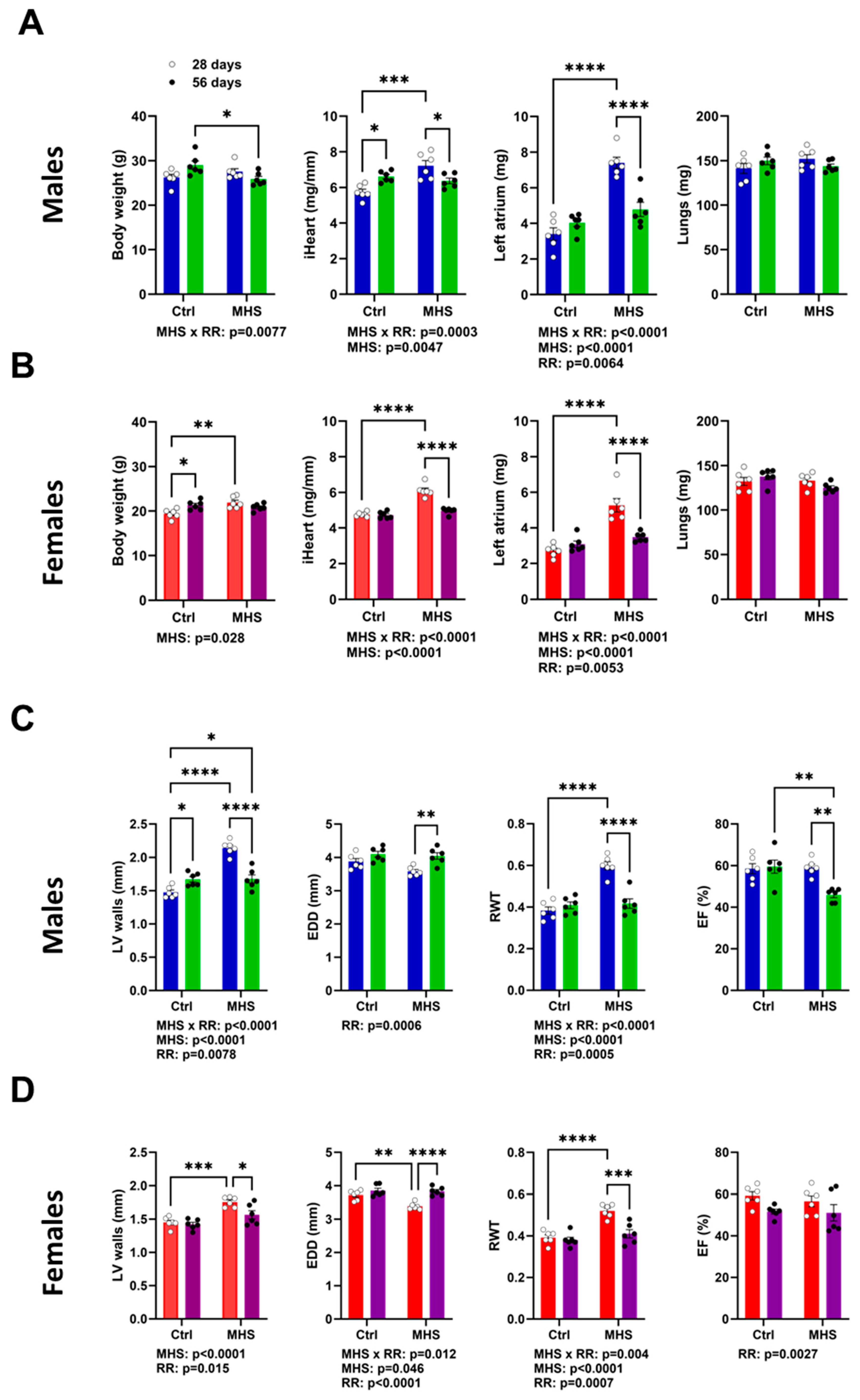
3.3. MHS Cardiac Hypertrophy is Reversed after RR
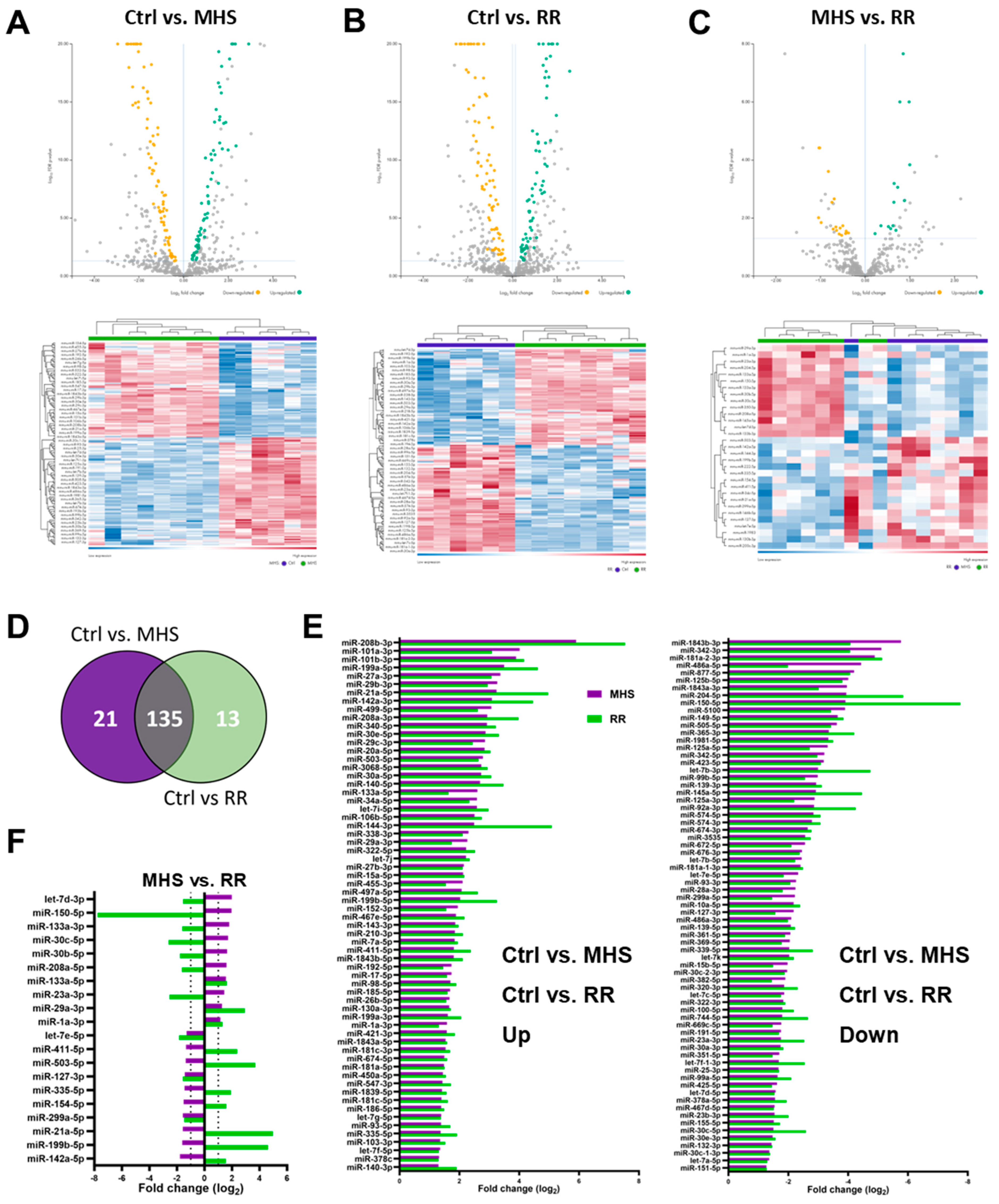
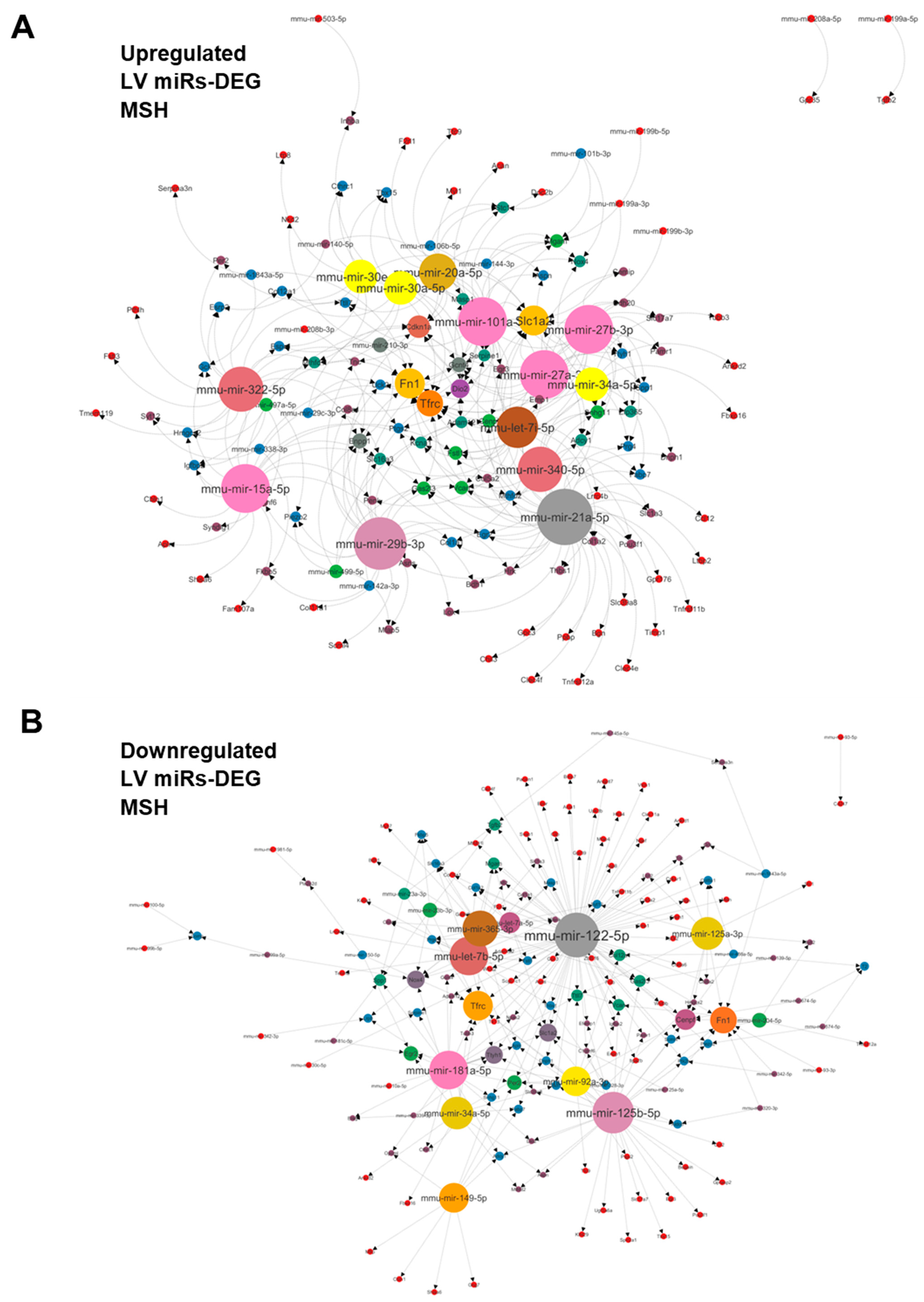
3.4. Plasma miRNome, But Not LV miRNome, Is Normalised after RR
4. Discussion
4.1. Future Perspectives
4.2. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [CrossRef] [PubMed]
- Kitzman, D.W.; Shah, S.J. The HFpEF Obesity Phenotype: The Elephant in the Room. J. Am. Coll. Cardiol. 2016, 68, 200–203. [Google Scholar] [CrossRef] [PubMed]
- Borlaug, B.A.; Sharma, K.; Shah, S.J.; Ho, J.E. Heart Failure With Preserved Ejection Fraction: JACC Scientific Statement. J. Am. Coll. Cardiol. 2023, 81, 1810–1834. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.E.; Enserro, D.; Brouwers, F.P.; Kizer, J.R.; Shah, S.J.; Psaty, B.M.; Bartz, T.M.; Santhanakrishnan, R.; Lee, D.S.; Chan, C.; et al. Predicting heart failure with preserved and reduced ejection fraction clinical perspective. Circ. Heart Fail. 2016, 9, e003116. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.J.; Kitzman, D.W.; Borlaug, B.A.; van Heerebeek, L.; Zile, M.R.; Kass, D.A.; Paulus, W.J. Phenotype-specific treatment of heart failure with preserved ejection fraction. Circulation 2016, 134, 73–90. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e876–e894. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Brunner-La Rocca, H.P.; Choi, D.J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef]
- Sachdev, V.; Sharma, K.; Keteyian, S.J.; Alcain, C.F.; Desvigne-Nickens, P.; Fleg, J.L.; Florea, V.G.; Franklin, B.A.; Guglin, M.; Halle, M.; et al. Supervised Exercise Training for Chronic Heart Failure with Preserved Ejection Fraction: A Scientific Statement from the American Heart Association and American College of Cardiology. Circulation 2023, 147, e699–e715. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.A.; Bhardwaj, A.; Munagala, M.; Abraham, S.; Adig, S.; Shen, A.; Hamad, E. Sex Differences in Circulating Biomarkers of Heart Failure. Curr. Heart Fail. Rep. 2024, 21, 11–21. [Google Scholar] [CrossRef]
- Jalink, E.A.; Schonk, A.W.; Boon, R.A.; Juni, R.P. Non-coding RNAs in the pathophysiology of heart failure with preserved ejection fraction. Front. Cardiovasc. Med. 2024, 10, 1300375. [Google Scholar] [CrossRef]
- Dzau, V.J.; Hodgkinson, C.P. RNA Therapeutics for the Cardiovascular System. Circulation 2024, 149, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Sayed, D.; Hong, C.; Chen, I.Y.; Lypowy, J.; Abdellatif, M. MicroRNAs play an essential role in the development of cardiac hypertrophy. Circ. Res. 2007, 100, 416–424. [Google Scholar] [CrossRef] [PubMed]
- van Rooij, E.; Sutherland, L.B.; Thatcher, J.E.; DiMaio, J.M.; Naseem, R.H.; Marshall, W.S.; Hill, J.A.; Olson, E.N. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc. Natl. Acad. Sci. USA 2008, 105, 13027–13032. [Google Scholar] [CrossRef] [PubMed]
- Bagnall, R.D.; Tsoutsman, T.; Shephard, R.E.; Ritchie, W.; Semsarian, C. Global microRNA profiling of the mouse ventricles during development of severe hypertrophic cardiomyopathy and heart failure. PLoS ONE 2012, 7, e44744. [Google Scholar] [CrossRef]
- Marfella, R.; Di Filippo, C.; Potenza, N.; Sardu, C.; Rizzo, M.R.; Siniscalchi, M.; Musacchio, E.; Barbieri, M.; Mauro, C.; Mosca, N.; et al. Circulating microRNA changes in heart failure patients treated with cardiac resynchronization therapy: Responders vs. non-responders. Eur. J. Heart Fail. 2013, 15, 1277–1288. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.Y.; Jiang, Y.N.; Guan, X.; Ren, F.F.; Wu, S.J.; Chu, M.P.; Wu, L.P.; Lai, T.F.; Li, L. Aerobic Exercise Attenuates Pressure Overload-Induced Myocardial Remodeling and Myocardial Inflammation via Upregulating miR-574-3p in Mice. Circ. Heart Fail. 2024, 17, e010569. [Google Scholar] [CrossRef]
- Rodrigues, P.G.; Miranda-Silva, D.; Li, X.; Sousa-Mendes, C.; Martins-Ferreira, R.; Elbeck, Z.; Leite-Moreira, A.F.; Knöll, R.; Falcão-Pires, I. The Degree of Cardiac Remodelling before Overload Relief Triggers Different Transcriptome and miRome Signatures during Reverse Remodelling (RR)-Molecular Signature Differ with the Extent of RR. Int. J. Mol. Sci. 2020, 21, 9687. [Google Scholar] [CrossRef]
- Iguchi, T.; Niino, N.; Tamai, S.; Sakurai, K.; Mori, K. Comprehensive Analysis of Circulating microRNA Specific to the Liver, Heart, and Skeletal Muscle of Cynomolgus Monkeys. Int. J. Toxicol. 2017, 36, 220–228. [Google Scholar] [CrossRef]
- Li, H.; Fan, J.; Yin, Z.; Wang, F.; Chen, C.; Wang, D.W. Identification of cardiac-related circulating microRNA profile in human chronic heart failure. Oncotarget 2016, 7, 33–45. [Google Scholar] [CrossRef]
- Morishima, M.; Ono, K. Serum microRNA-30d is a sensitive biomarker for angiotensin II-induced cardiovascular complications in rats. Heart Vessel. 2021, 36, 1597–1606. [Google Scholar] [CrossRef]
- Huang, D.; Chen, Z.; Wang, J.; Chen, Y.; Liu, D.; Lin, K. MicroRNA-221 is a potential biomarker of myocardial hypertrophy and fibrosis in hypertrophic obstructive cardiomyopathy. Biosci. Rep. 2020, 40, BSR20191234. [Google Scholar] [CrossRef] [PubMed]
- Withaar, C.; Lam, C.S.P.; Schiattarella, G.G.; de Boer, R.A.; Meems, L.M.G. Heart failure with preserved ejection fraction in humans and mice: Embracing clinical complexity in MOUSE models. Eur. Heart J. 2021, 42, 4420–4430. [Google Scholar] [CrossRef] [PubMed]
- Aidara, M.L.; Walsh-Wilkinson, É.; Thibodeau, S.È.; Labbé, E.A.; Morin-Grandmont, A.; Gagnon, G.; Boudreau, D.K.; Arsenault, M.; Bossé, Y.; Couet, J. Cardiac reverse remodeling in a mouse model with many phenotypical features of heart failure with preserved ejection fraction: Effects of modifying lifestyle. Am. J. Physiol. Heart Circ. Physiol. 2024, 326, H1017–H1036. [Google Scholar] [CrossRef] [PubMed]
- Walsh-Wilkinson, É.; Aidara, M.L.; Morin-Grandmont, A.; Thibodeau, S.È.; Gagnon, J.; Genest, M.; Arsenault, M.; Couet, J. Age and sex hormones modulate left ventricle regional response to angiotensin II in male and female mice. Am. J. Physiol. Heart Circ. Physiol. 2022, 323, H643–H658. [Google Scholar] [CrossRef] [PubMed]
- Walsh-Wilkinson, E.; Arsenault, M.; Couet, J. Segmental analysis by speckle-tracking echocardiography of the left ventricle response to isoproterenol in male and female mice. PeerJ 2021, 9, e11085. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Zhou, G.; Soufan, O.; Xia, J. miRNet 2.0—Network-based visual analytics for miRNA functional analysis and systems biology. Nucl. Acids Res. 2020, 48, W244–W251. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Siklenka, K.; Arora, S.K.; Ribeiro, P.; Kimmins, S.; Xia, J. miRNet—Dissecting miRNA-target interactions and functional associations through network-based visual analysis. Nucl. Acids Res. 2016, 44, W135–W141. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Xia, J. MicroRNA Regulatory Network Analysis Using miRNet 2.0 Transcription Factor Regulatory Networks, 185–204; Humana Press: New York, NY, USA, 2022. [Google Scholar]
- Fan, Y.; Xia, J. miRNet: Functional Analysis and Visual Exploration of miRNA-Target Interactions in a Network Context Computational Cell Biology; Humana Press: New York, NY, USA, 2018. [Google Scholar]
- Morin-Grandmont, A.; Walsh-Wilkinson, É.; Labbé, E.A.; Thibodeau, S.È.; Dupont, É.; Boudreau, D.K.; Arsenault, M.; Bossé, Y.; Couet, J. Biological sex, sex steroids and sex chromosomes contribute to mouse cardiac aging. Aging 2024, 16, 7553–7577. [Google Scholar] [CrossRef]
- Cai, Z.; Fang, L.; Jiang, Y.; Liang, M.; Wang, J.; Shen, Y.; Wang, Z.; Liang, F.; Huo, H.; Pan, C.; et al. Angiotensin II Promotes White Adipose Tissue Browning and Lipolysis in Mice. Oxid. Med. Cell Longev. 2022, 2022, 6022601. [Google Scholar] [CrossRef]
- Funcke, J.B.; Scherer, P.E. Beyond adiponectin and leptin: Adipose tissue-derived mediators of inter-organ communication. J. Lipid Res. 2019, 60, 1648–1684. [Google Scholar] [CrossRef] [PubMed]
- Crisci, G.; De Luca, M.; D’Assante, R.; Ranieri, B.; D’Agostino, A.; Valente, V.; Giardino, F.; Capone, V.; Chianese, S.; Rega, S.; et al. Effects of Exercise on Heart Failure with Preserved Ejection Fraction: An Updated Review of Literature. J. Cardiovasc. Dev. Dis. 2022, 9, 241. [Google Scholar] [CrossRef]
- Fukuta, H.; Goto, T.; Wakami, K.; Kamiya, T.; Ohte, N. Effects of exercise training on cardiac function, exercise capacity, and quality of life in heart failure with preserved ejection fraction: A meta-analysis of randomized controlled trials. Heart Fail. Rev. 2019, 24, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Kitzman, D.W.; Brubaker, P.; Morgan, T.; Haykowsky, M.; Hundley, G.; Kraus, W.E.; Eggebeen, J.; Nicklas, B.J. Effect of Caloric Restriction or Aerobic Exercise Training on Peak Oxygen Consumption and Quality of Life in Obese Older Patients with Heart Failure with Preserved Ejection Fraction: A Randomized Clinical Trial. JAMA 2016, 315, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Casanova-Vallve, N.; Duglan, D.; Vaughan, M.E.; Pariollaud, M.; Handzlik, M.K.; Fan, W.; Yu, R.T.; Liddle, C.; Downes, M.; Delezie, J.; et al. Daily running enhances molecular and physiological circadian rhythms in skeletal muscle. Mol. Metab. 2022, 61, 101504. [Google Scholar] [CrossRef] [PubMed]
- Maier, G.; Delezie, J.; Westermark, P.O.; Santos, G.; Ritz, D.; Handschin, C. Transcriptomic, proteomic and phosphoproteomic underpinnings of daily exercise performance and zeitgeber activity of training in mouse muscle. J. Physiol. 2022, 600, 769–796. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M.; Suzuki, S.; Moriya, A.; Nogami, K.; Uchida, R.; Saito, Y.; Saito, H. The Effects of Continuous and Withdrawal Voluntary Wheel Running Exercise on the Expression of Senescence-Related Genes in the Visceral Adipose Tissue of Young Mice. Int. J. Mol. Sci. 2020, 22, 264. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.G.; Juarros, M.A.; Leinwand, L.A. Regression of cardiac hypertrophy in health and disease: Mechanisms and therapeutic potential. Nat. Rev. Cardiol. 2023, 20, 347–363. [Google Scholar] [CrossRef] [PubMed]
- Généreux, P.; Pibarot, P.; Redfors, B.; Bax, J.J.; Zhao, Y.; Makkar, R.R.; Kapadia, S.; Thourani, V.H.; Mack, M.J.; Nazif, T.M.; et al. Evolution and Prognostic Impact of Cardiac Damage After Aortic Valve Replacement. J. Am. Coll. Cardiol. 2022, 80, 783–800. [Google Scholar] [CrossRef] [PubMed]
- Uchida, S.; Dimmeler, S. Long noncoding RNAs in cardiovascular diseases. Circ. Res. 2015, 116, 737–750. [Google Scholar] [CrossRef]
- Wang, X.; Wang, H.X.; Li, Y.L.; Zhang, C.C.; Zhou, C.Y.; Wang, L.; Xia, Y.L.; Du, J.; Li, H.H. MicroRNA Let-7i negatively regulates cardiac inflammation and fibrosis. Hypertension 2015, 66, 776–785. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Guo, H.Y.; Li, Y.Q.; Li, J.C.; Yang, X.H.; Liu, J.; Hu, Q.D.; Wang, H.L.; Wang, L. The Smad3-dependent microRNA let-7i-5p promoted renal fibrosis in mice with unilateral ureteral obstruction. Front. Physiol. 2022, 13, 937878. [Google Scholar] [CrossRef] [PubMed]
- Roush, S.; Slack, F.J. The let-7 family of microRNAs. Trends Cell Biol. 2008, 18, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Su, M.; Li, W.; Yuan, Y.; Liu, S.; Liang, C.; Liu, H.E.; Zhang, R.; Liu, Y.; Sun, L.I.; Wei, Y.; et al. Epididymal white adipose tissue promotes angiotensin II-induced cardiac fibrosis in an exosome-dependent manner. Transl. Res. 2022, 248, 51–67. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.R.; Ding, L.L.; Xu, L.; Huang, J.; Zhang, Z.B.; Chen, X.H.; Cheng, Y.W.; Ruan, C.C.; Gao, P.J. Brown Adipocyte ADRB3 Mediates Cardioprotection via Suppressing Exosomal iNOS. Circ. Res. 2022, 131, 133–147. [Google Scholar] [CrossRef]
- Novak, C.M.; Burghardt, P.R.; Levine, J.A. The use of a running wheel to measure activity in rodents: Relationship to energy balance, general activity, and reward. Neurosci. Biobehav. Rev. 2012, 36, 1001–1014. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thibodeau, S.-È.; Labbé, E.-A.; Walsh-Wilkinson, É.; Morin-Grandmont, A.; Arsenault, M.; Couet, J. Plasma and Myocardial miRNomes Similarities and Differences during Cardiac Remodelling and Reverse Remodelling in a Murine Model of Heart Failure with Preserved Ejection Fraction. Biomolecules 2024, 14, 892. https://doi.org/10.3390/biom14080892
Thibodeau S-È, Labbé E-A, Walsh-Wilkinson É, Morin-Grandmont A, Arsenault M, Couet J. Plasma and Myocardial miRNomes Similarities and Differences during Cardiac Remodelling and Reverse Remodelling in a Murine Model of Heart Failure with Preserved Ejection Fraction. Biomolecules. 2024; 14(8):892. https://doi.org/10.3390/biom14080892
Chicago/Turabian StyleThibodeau, Sara-Ève, Emylie-Ann Labbé, Élisabeth Walsh-Wilkinson, Audrey Morin-Grandmont, Marie Arsenault, and Jacques Couet. 2024. "Plasma and Myocardial miRNomes Similarities and Differences during Cardiac Remodelling and Reverse Remodelling in a Murine Model of Heart Failure with Preserved Ejection Fraction" Biomolecules 14, no. 8: 892. https://doi.org/10.3390/biom14080892
APA StyleThibodeau, S.-È., Labbé, E.-A., Walsh-Wilkinson, É., Morin-Grandmont, A., Arsenault, M., & Couet, J. (2024). Plasma and Myocardial miRNomes Similarities and Differences during Cardiac Remodelling and Reverse Remodelling in a Murine Model of Heart Failure with Preserved Ejection Fraction. Biomolecules, 14(8), 892. https://doi.org/10.3390/biom14080892






