Enkephalins and Pain Modulation: Mechanisms of Action and Therapeutic Perspectives
Abstract
:1. Introduction
2. Molecular Biology of the Enkephalins and Their Receptors
3. Distribution and Functions of Enkephalins
3.1. Central Nervous System (CNS)
- Pain modulation. One of the primary mechanisms by which ENKs modulate pain involves the inhibition of the transmission of nociceptive signals in the dorsal horn. ENKs, released by spinal interneurons, exert postsynaptic effects within the CNS (Figure 3), because they suppress the activity of ascending neurons (activated by excitatory neurotransmitters -such as glutamate, substance P, and calcitonin gene-related peptide or CGRP, secreted by primary afferent neurons) [84,85,86,87,88,89]. Furthermore, ENKs indirectly activate serotonergic neurons in the raphe nucleus and noradrenergic neurons in the locus coeruleus, thereby promoting the activation of the descending pathway, which in turn regulates pain intensity [85]. To summarize, ENKs can alter the function of GABAergic, noradrenergic, serotoninergic, and glutamatergic neurons, thereby affecting the equilibrium of excitatory and inhibitory neurotransmission within pain pathways [86].
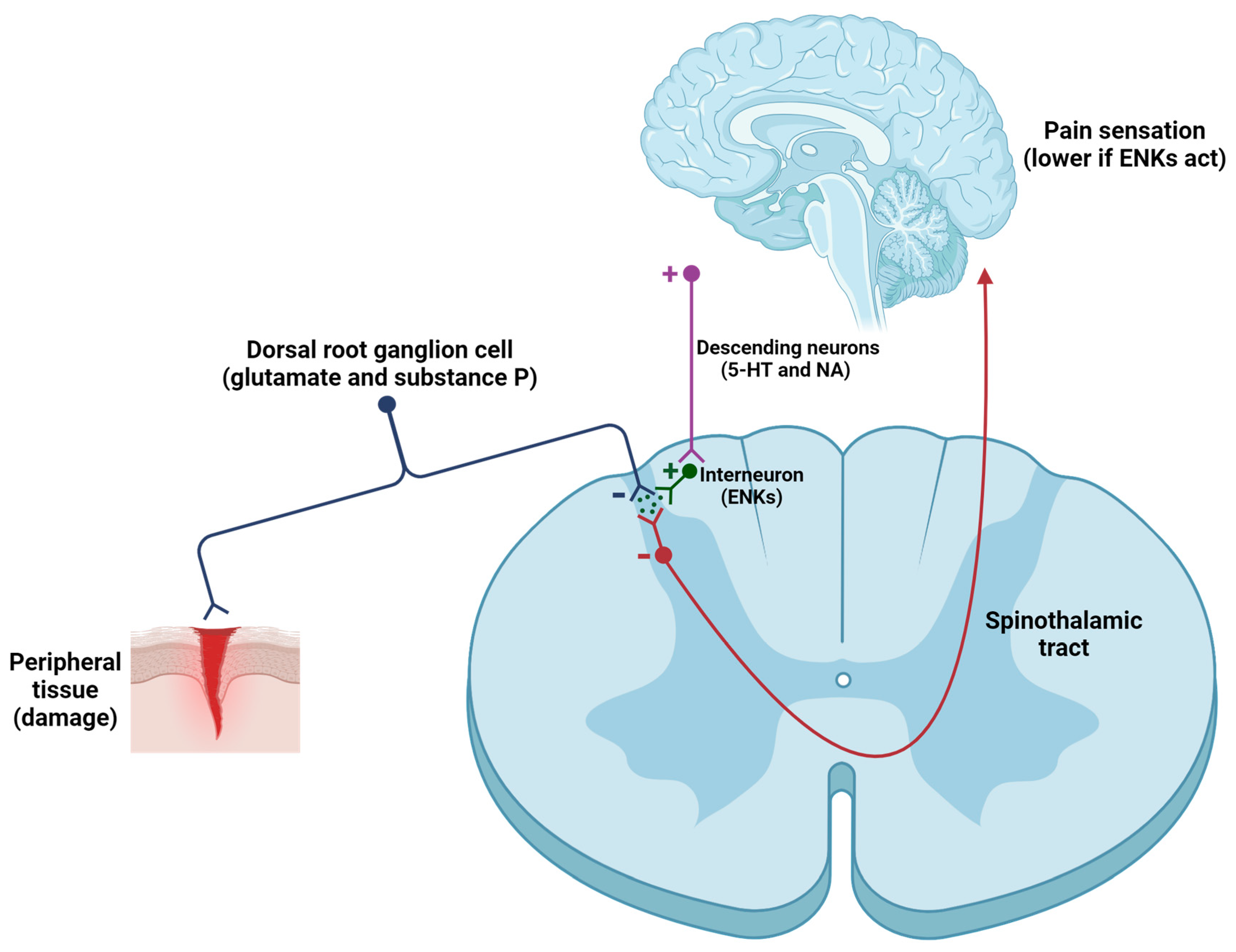
- 2.
- Emotional control. The mood-modulating effects evoked by ENKs are largely attributed to the alleviation of pain sensation. These effects are similar to those observed following the administration of exogenous opioids, like morphine or fentanyl [17]. Moreover, ENKs are involved in the stress response by modulating the hypothalamic-pituitary-adrenal (HPA) axis, thereby affecting cortisol secretion, the main stress hormone [90].
- 3.
- Neuroprotective effects. The role of ENKs in neuroprotection is an emerging field of interest. Several studies have demonstrated that ENKs can exert neuroprotective effects by reducing toxicity, particularly in those cases where excessive glutamate release leads to neuronal damage [98,99]. Another function induced by ENKs is the attenuation of oxidative stress in the CNS following pathological disorders, as seen in stroke [99], Alzheimer’s disease [100], or Parkinson’s disease [101,102].
3.2. Peripheral Nervous System (PNS)
- Control of inflammation and pain modulation. Inflammation is the physiological response to harmful stimuli such as pathogens, damaged cells, toxic compounds, or irradiation [112]. Inflammation is characterized by redness, heat, swelling, and pain [113]. Critical microcirculatory events in inflammation involve changes in vascular permeability, the release of pro-inflammatory mediators, and the recruitment of leukocytes [114]. During acute inflammatory responses, cellular and molecular events reduce the risk of injury and lead to the resolution of inflammation. Nevertheless, uncontrolled acute inflammation can progress to chronic inflammation, which contributes to the onset of multiple chronic conditions [115].
- 2.
- Regulation of gastrointestinal function. Endogenous opioid peptides (including ENKs) have been localized to both enteric neurons and mucosal endocrine cells within the gastrointestinal tract [124]. In the ENS, derivatives of pENK are predominantly localized within myenteric neurons projecting to the circular muscle and submucosal plexus [124]. Upon secretion, ENKs increase the gastrointestinal transit time by interacting with DOPr on the enteric circuitries that control motility and secretion [125].
4. Relationship of Enkephalins with Painful Disorders
- Fibromyalgia. Fibromyalgia is a chronic pain syndrome characterized by musculoskeletal pain accompanied by other non-specific symptoms [126]. Its prevalence in the United States is 6.4%, while in Europe and South America ranges from 2.4% to 3.3% [127,128,129]. Although the main cause of fibromyalgia remains unclear, it’s believed to involve a combination of many factors that involve genetics and/or environmental influences [130]. Symptoms of fibromyalgia differ among individuals but typically include pain (pain varies in intensity and location and is frequently accompanied by sensitivity in particular regions known as tender points, as defined by the American College of Rheumatology or ACR), fatigue (many people experience profound fatigue, even after sleeping for long periods), cognitive difficulties (issues with memory and concentration), frequent headaches, anxiety, depression, and sensitivity to noise or lights [130].
- 2.
- Migraine. Migraine is a condition identified by either occasional headaches accompanied by symptoms like sensitivity to light (photophobia), or sound (phonophobia) [139]. This circumstance, that impacts on 15% of the global population [140], can also be linked to different symptoms such as somnambulism, emesis, abdominal migraine, benign paroxysmal positional vertigo (BPPV), benign paroxysmal torticollis (BPT), and confusional migraine, each characterized by singular clinical presentations [141]. A migraine attack can be divided into four phases based on its temporal sequence and symptoms: (i) premonitory phase (involves symptoms like mood changes or food cravings); (ii) aura phase (not always present; includes sensory or visual disturbances); (iii) headache phase (strong head pain, generally unilateral and pulsating); (iv) postdrome phase (leave individuals exhausted or disoriented, lasting for hours to days after the pain relief) [141]. These symptoms are caused by the activation of the trigeminovascular system, which triggers the release of certain vasoactive peptides, such as substance P and/or CGRP. This secretion leads to neurogenic inflammation and vasodilation of cerebral blood vessels [142].
- 3.
- Complex Regional Pain Syndrome (CRPS). CRPS is a chronic pain characterized by allodynia and hyperalgesia, usually involving the limbs [152,153]. This term was proposed by the IASP in 1994, distinguishing it into type 1 (caused by injury) and type 2 (resulting from prior neurological damage) [152,153]. Signs and symptoms are disproportionate to the triggering event and include spontaneous or movement-induced pain, sensory changes (such as allodynia and hyperesthesia), autonomic dysfunctions (with many changes in biophysical properties of skin), and motor abnormalities (like tremors or dystonia) [152,153]. According to the European Medicine Agency (EMA) and the Food and Drug Administration (FDA), CRPS is designated as a rare disease, affecting fewer than 10,000 people in Europe or 200,000 individuals in the United States [154].
- 4.
- Rheumatoid arthritis. This disease, which affects 1% of the global population [163], is characterized by a chronic inflammatory process, which can lead to damage joints and extra-articular organs such as the heart, kidneys, lungs, gut, and brain [164]. Clinical symptoms of this pathology include morning stiffness, pain (shoulders, neck, and pelvic girdle), reduced mobility accompanied by fever, fatigue, weight loss, and the formation of rheumatoid nodules [164]. Rheumatoid arthritis is caused by crystal depositions (calcium pyrophosphate deposition disease or CPPD); by microbial agents (S. aureus, N. gonorrhoeae, several species of Borrelia -e.g., B. burgdorferi-, Parvovirus, and Enterovirus); or by autoimmune processes [164,165,166]. Risk factors for rheumatoid arthritis include age (older individuals show increased risk), sex (females exhibit higher incidence), smoke, obesity, and genetics [167].
- 5.
- Trigeminal neuralgia. According to the IASP, trigeminal neuralgia is a pathology identified by chronic facial pain [176,177,178]. Trigeminal neuralgia induces severe pain, defined by burning, sharp, and stabbing sensation [176,177,178]. Periods of pain are transient, with minor painful intervals between episodes [176,177,178].
- 6.
- Multiple sclerosis. Multiple sclerosis is an autoimmune disorder that affects the CNS, where demyelination and axonal transection occur [187,188]. This pathology is defined by a destroyed myelin sheath, at different levels, of myelinated neurons in discrete regions through the CNS [187,188]. The most characteristic lesions are focal areas in the white matter of CNS, identified with MRI [189]. The classical presentations of multiple sclerosis comprise depression; diplopia; sexual dysfunction; unilateral optic neuritis; myelitis (impaired sensation, weakness, and ataxia); focal sensory disturbance (limb paresthesias); brainstem syndromes (intranuclear ophthalmoplegia, vertigo, hearing loss, and facial sensory disturbance); and fatigue [187,188,190]. In most subjects, multiple sclerosis initially manifests as episodes of reversible neurological deficits, frequently followed by a progressive deterioration in neurological function [187,188,190].
- 7.
- Crohn’s disease. Crohn’s disease is an inflammatory bowel disease (IBD), typified by inflammation and ulceration of the digestive tract, being the most frequently affected part the distal ileum, but it can impact any portion of the intestine in a non-continuous manner [206,207]. The symptoms of this disease affect patients’ health and may comprise abdominal pain, diarrhea, weight loss, rectal bleeding, and fatigue [206,207]. In addition, Crohn’s disease can lead to severe complications such as intestinal obstruction, fistulas, and abscesses, which require hospitalization and surgery [206,207]. Crohn’s disease is more prevalent in the industrialized world, particularly in North America and Western Europe, although the incidence is rising in Asia and South America [208,209].
- 8.
- Cancer. Cancer develops as a result of multiple molecular events involving numerous interactions between genetics and environment [223]. This pathological process consists in a multistep phenomenon involving sequential mutations, resulting in uncontrolled cell growth [223]. These modifications alter the cellular metabolism, affect proliferation control, enable indefinite lifespan, alter intercellular communication, and provide the ability to avoid recognition by the immune cells [224]. Moreover, malignant cells become genetically damaged, but maintain their proliferative capacity [224].
5. Conclusions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Raja, S.N.; Carr, D.B.; Cohen, M.; Finnerup, N.B.; Flor, H.; Gibson, S.; Keefe, F.J.; Mogil, J.S.; Ringkamp, M.; Sluka, K.A.; et al. The revised International Association for the Study of Pain definition of pain: Concepts, challenges, and compromises. Pain 2020, 161, 1976–1982. [Google Scholar] [CrossRef]
- Vader, K.; Bostick, G.P.; Carlesso, L.C.; Hunter, J.; Mesaroli, G.; Perreault, K.; Tousignant-Laflamme, Y.; Tupper, S.; Walton, D.M.; Wideman, T.H.; et al. The Revised IASP Definition of Pain and Accompanying Notes: Considerations for the Physiotherapy Profession. Physiother. Can. 2021, 73, 103–106. [Google Scholar] [CrossRef]
- Lee, G.I.; Neumeister, M.W. Pain: Pathways and physiology. Clin. Plast. Surg. 2020, 47, 173–180. [Google Scholar] [CrossRef]
- Schumacher, M.A. Peripheral Neuroinflammation and Pain: How Acute Pain Becomes Chronic. Curr. Neuropharmacol. 2024, 22, 6–14. [Google Scholar] [CrossRef]
- Karcioglu, O.; Topacoglu, H.; Dikme, O.; Dikme, O. A systematic review of the pain scales in adults: Which to use? Am. J. Emerg. Med. 2018, 36, 707–714. [Google Scholar] [CrossRef]
- Zajacova, A.; Grol-Prokopczyk, H.; Zimmer, Z. Sociology of chronic pain. J. Health. Soc. Behav. 2021, 62, 302–317. [Google Scholar] [CrossRef]
- Zimmer, Z.; Fraser, K.; Grol-Prokopczyk, H.; Zajacova, A. A global study of pain prevalence across 52 countries: Examining the role of country-level contextual factors. Pain 2022, 163, 1740–1750. [Google Scholar] [CrossRef]
- Henschke, N.; Kamper, S.J.; Maher, C.G. The epidemiology and economic consequences of pain. Mayo Clin. Proc. 2015, 90, 139–147. [Google Scholar] [CrossRef]
- Macfarlane, G.J. The epidemiology of chronic pain. Pain 2016, 157, 2158–2159. [Google Scholar] [CrossRef]
- Todd, A.; McNamara, C.L.; Balaj, M.; Huijts, T.; Akhter, N.; Thomson, K.; Kasim, A.; Eikemo, T.A.; Bambra, C. The European epidemic: Pain prevalence and socioeconomic inequalities in pain across 19 European countries. Eur. J. Pain 2019, 23, 1425–1436. [Google Scholar] [CrossRef]
- Yong, R.J.; Mullins, P.M.; Bhattacharyya, N. Prevalence of chronic pain among adults in the United States. Pain 2022, 163, e328–e332. [Google Scholar] [CrossRef] [PubMed]
- Dagnino, A.P.; Campos, M.M. Chronic pain in the elderly: Mechanisms and perspectives. Front. Hum. Neurosci. 2022, 16, 736688. [Google Scholar] [CrossRef] [PubMed]
- Mills, S.E.E.; Nicolson, K.P.; Smith, B.H. Chronic pain: A review of its epidemiology and associated factors in population-based studies. Br. J. Anaesth. 2019, 123, e273–e283. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Dionne, R.A. Individualized pain medicine. Drug. Discov. Today Ther. Strateg. 2009, 6, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, J.T.; Seminowicz, D.A. Neuroimaging of pain in animal models: A review of recent literature. Pain Rep. 2019, 4, e732. [Google Scholar] [CrossRef] [PubMed]
- Howard, M.A.; Lawn, T.; Kowalczyk, O.S. Harnessing the power of endogenous pain control mechanisms for novel therapeutics: How might innovations in neuroimaging help? Curr. Opin. Support. Palliat. Care 2023, 17, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.K.; Smith, C.M.; Rahmatullah, M.; Nissapatorn, V.; Wilairatana, P.; Spetea, M.; Gueven, N.; Dietis, N. Opioid analgesia and opioid-induced adverse effects: A review. Pharmaceuticals 2021, 14, 1091. [Google Scholar] [CrossRef] [PubMed]
- Holden, J.E.; Jeong, Y.; Forrest, J.M. The endogenous opioid system and clinical pain management. AACN Clin. Issues 2005, 16, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Froehlich, J.C. Opioid peptides. Alcohol Health Res. World 1997, 21, 132–136. [Google Scholar]
- Nadeau, S.E.; Wu, J.K.; Lawhern, R.A. Opioids and Chronic Pain: An Analytic Review of the Clinical Evidence. Front. Pain Res. 2021, 2, 721357. [Google Scholar] [CrossRef]
- Hughes, J.; Smith, T.W.; Kosterlitz, H.W.; Fothergill, L.A.; Morgan, B.A.; Morris, H.R. Identification of two related pentapeptides from the brain with potent opiate agonist activity. Nature 1975, 258, 577–580. [Google Scholar] [CrossRef] [PubMed]
- Le Merrer, J.; Becker, J.A.; Befort, K.; Kieffer, B.L. Reward processing by the opioid system in the brain. Physiol. Rev. 2009, 89, 1379–1412. [Google Scholar] [CrossRef] [PubMed]
- Arvidsson, U.; Dado, R.J.; Riedl, M.; Lee, J.H.; Law, P.Y.; Loh, H.H.; Elde, R.; Wessendorf, M.W. delta-Opioid receptor immunoreactivity: Distribution in brainstem and spinal cord, and relationship to biogenic amines and enkephalin. J. Neurosci. 1995, 15, 1215–1235. [Google Scholar] [CrossRef] [PubMed]
- Bastiaensen, E.; De Potter, W. Enkephalin containing peptides in the peripheral sympathetic nervous system. Neurochem. Int. 1987, 11, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Soinila, S.; Mpitsos, G.J.; Soinila, J. Immunohistochemistry of enkephalins: Model studies on hapten-carrier conjugates and fixation methods. J. Histochem. Cytochem. 1992, 40, 231–239. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, P.J.; Zagon, I.S. Duration of opioid receptor blockade determines biotherapeutic response. Biochem. Pharmacol. 2015, 97, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Pathan, H.; Williams, J. Basic opioid pharmacology: An update. Br. J. Pain 2012, 6, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Remesic, M.; Ramos-Colon, C.; Wu, Z.; LaVigne, J.; Molnar, G.; Tymecka, D.; Misicka, A.; Streicher, J.M.; Hruby, V.J.; et al. Multifunctional Enkephalin Analogs with a New Biological Profile: MOR/DOR Agonism and KOR Antagonism. Biomedicines 2021, 9, 625. [Google Scholar] [CrossRef] [PubMed]
- Nieto, M.M.; Guen, S.L.; Kieffer, B.L.; Roques, B.P.; Noble, F. Physiological control of emotion-related behaviors by endogenous enkephalins involves essentially the delta opioid receptors. Neuroscience 2005, 135, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Berggren, A.; Rubenson, A.; Sillén, U. Involvement of opioid mechanisms in peripheral motor control of detrusor muscle. Pharmacol. Toxicol. 1992, 71 Pt 1, 179–184. [Google Scholar] [CrossRef]
- Nogueiras, R.; Romero-Picó, A.; Vazquez, M.J.; Novelle, M.G.; López, M.; Diéguez, C. The opioid system and food intake: Homeostatic and hedonic mechanisms. Obes. Facts. 2012, 5, 196–207. [Google Scholar] [CrossRef]
- Akil, H.; Watson, S.J.; Young, E.; Lewis, M.E.; Khachaturian, H.; Walker, J.M. Endogenous opioids: Biology and function. Annu. Rev. Neurosci. 1984, 7, 223–255. [Google Scholar] [CrossRef]
- Henry, M.S.; Gendron, L.; Tremblay, M.E.; Drolet, G. Enkephalins: Endogenous Analgesics with an Emerging Role in Stress Resilience. Neural Plast. 2017, 2017, 1546125. [Google Scholar] [CrossRef]
- Fricker, L.D.; Margolis, E.B.; Gomes, I.; Devi, L.A. Five Decades of Research on Opioid Peptides: Current Knowledge and Unanswered Questions. Mol. Pharmacol. 2020, 98, 96–108. [Google Scholar] [CrossRef]
- Bigliardi, P.L.; Dancik, Y.; Neumann, C.; Bigliardi-Qi, M. Opioids and skin homeostasis, regeneration and ageing—What’s the evidence? Exp. Dermatol. 2016, 25, 586–591. [Google Scholar] [CrossRef]
- Celik, M.Ö.; Labuz, D.; Henning, K.; Busch-Dienstfertig, M.; Gaveriaux-Ruff, C.; Kieffer, B.L.; Zimmer, A.; Machelska, H. Leukocyte opioid receptors mediate analgesia via Ca(2+)-regulated release of opioid peptides. Brain Behav. Immun. 2016, 57, 227–242. [Google Scholar] [CrossRef]
- Livett, B.G.; Dean, D.M.; Whelan, L.G.; Udenfriend, S.; Rossier, J. Co-release of enkephalin and catecholamines from cultured adrenal chromaffin cells. Nature 1981, 289, 317–319. [Google Scholar] [CrossRef]
- Corder, G.; Castro, D.C.; Bruchas, M.R.; Scherrer, G. Endogenous and exogenous opioids in pain. Annu. Rev. Neurosci. 2018, 41, 453–473. [Google Scholar] [CrossRef]
- Breslin, M.B.; Lindberg, I.; Benjannet, S.; Mathis, J.P.; Lazure, C.; Seidah, N.G. Differential processing of proenkephalin by prohormone convertases 1(3) and 2 and furin. J. Biol. Chem. 1993, 268, 27084–27093. [Google Scholar] [CrossRef]
- Fricker, L.D. Neuropeptide biosynthesis: Focus on the carboxypeptidase processing enzyme. Trends Neurosci. 1985, 8, 210. [Google Scholar] [CrossRef]
- Lu, W.D.; Funkelstein, L.; Toneff, T.; Reinheckel, T.; Peters, C.; Hook, V. Cathepsin H functions as an aminopeptidase in secretory vesicles for production of enkephalin and galanin peptide neurotransmitters. J. Neurochem. 2012, 122, 512–522. [Google Scholar] [CrossRef]
- Schwartz, J.C.; Malfroy, B.; De La Baume, S. Biological inactivation of enkephalins and the role of enkephalin-dipeptidyl-carboxypeptidase (“enkephalinase”) as neuropeptidase. Life Sci. 1981, 29, 1715–1740. [Google Scholar] [CrossRef]
- Grossman, A.; Clement-Jones, V. Opiate receptors: Enkephalins and endorphins. Clin. Endocrinol. Metab. 1983, 12, 31–56. [Google Scholar] [CrossRef]
- Peppin, J.F.; Raffa, R.B. Delta opioid agonists: A concise update on potential therapeutic applications. J. Clin. Pharm. Ther. 2015, 40, 155–166. [Google Scholar] [CrossRef]
- Claff, T.; Yu, J.; Blais, V.; Patel, N.; Martin, C.; Wu, L.; Han, G.W.; Holleran, B.J.; Van der Poorten, O.; White, K.L.; et al. Elucidating the active δ-opioid receptor crystal structure with peptide and small-molecule agonists. Sci. Adv. 2019, 5, eaax9115. [Google Scholar] [CrossRef]
- Wang, Y.; Zhuang, Y.; DiBerto, J.F.; Zhou, X.E.; Schmitz, G.P.; Yuan, Q.; Jain, M.K.; Liu, W.; Melcher, K.; Jiang, Y.; et al. Structures of the entire human opioid receptor family. Cell 2023, 186, 413–427. [Google Scholar] [CrossRef]
- Befort, K.; Mattéi, M.G.; Roeckel, N.; Kieffer, B. Chromosomal localization of the delta opioid receptor gene to human 1p34.3-p36.1 and mouse 4D bands by in situ hybridization. Genomics 1994, 20, 143–145. [Google Scholar] [CrossRef]
- Bzdega, T.; Chin, H.; Kim, H.; Jung, H.H.; Kozak, C.A.; Klee, W.A. Regional expression and chromosomal localization of the delta opiate receptor gene. Proc. Natl. Acad. Sci. USA 1993, 90, 9305–9309. [Google Scholar] [CrossRef]
- Kaufman, D.L.; Xia, Y.R.; Keith, D.E., Jr.; Newman, D.; Evans, C.J.; Lusis, A.J. Localization of the delta-opioid receptor gene to mouse chromosome 4 by linkage analysis. Genomics 1994, 19, 405–406. [Google Scholar] [CrossRef]
- Piltonen, M.; Krokhotin, A.; Parisien, M.; Bérubé, P.; Djambazian, H.; Sladek, R.; Dokholyan, N.V.; Shabalina, S.A.; Diatchenko, L. Alternative Splicing of Opioid Receptor Genes Shows a Conserved Pattern for 6TM Receptor Variants. Cell Mol. Neurobiol. 2021, 41, 1039–1055. [Google Scholar] [CrossRef]
- Piltonen, M.; Parisien, M.; Grégoire, S.; Chabot-Doré, A.J.; Jafarnejad, S.M.; Bérubé, P.; Djambazian, H.; Sladek, R.; Geneau, G.; Willett, P.; et al. Alternative Splicing of the Delta-Opioid Receptor Gene Suggests Existence of New Functional Isoforms. Mol. Neurobiol. 2019, 56, 2855–2869. [Google Scholar] [CrossRef]
- Quirion, B.; Bergeron, F.; Blais, V.; Gendron, L. The Delta-Opioid Receptor; a Target for the Treatment of Pain. Front. Mol. Neurosci. 2020, 13, 52. [Google Scholar] [CrossRef] [PubMed]
- Wittert, G.; Hope, P.; Pyle, D. Tissue distribution of opioid receptor gene expression in the rat. Biochem. Biophys. Res. Commun. 1996, 218, 877–881. [Google Scholar] [CrossRef] [PubMed]
- Galligan, J.J.; Akbarali, H.I. Molecular physiology of enteric opioid receptors. Am. J. Gastroenterol. Suppl. 2014, 10, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Gendron, L.; Mittal, N.; Beaudry, H.; Walwyn, W. Recent advances on the δ opioid receptor: From trafficking to function. Br. J. Pharmacol. 2015, 172, 403–419. [Google Scholar] [CrossRef] [PubMed]
- Al-Hasani, R.; Bruchas, M.R. Molecular mechanisms of opioid receptor-dependent signaling and behavior. Anesthesiology 2011, 115, 1363–1381. [Google Scholar] [CrossRef] [PubMed]
- Farsetti, A.; Illi, B.; Gaetano, C. How epigenetics impacts on human diseases. Eur. J. Intern. Med. 2023, 114, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Liu, T.; Wei, L.N.; Law, P.Y.; Loh, H.H. DNA methylation-related chromatin modification in the regulation of mouse delta-opioid receptor gene. Mol. Pharmacol. 2005, 67, 2032–2039. [Google Scholar] [CrossRef] [PubMed]
- Vucetic, Z.; Kimmel, J.; Totoki, K.; Hollenbeck, E.; Reyes, T.M. Maternal high-fat diet alters methylation and gene expression of dopamine and opioid-related genes. Endocrinology 2010, 151, 4756–4764. [Google Scholar] [CrossRef]
- Denning, G.M.; Ackermann, L.W.; Barna, T.J.; Armstrong, J.G.; Stoll, L.L.; Weintraub, N.L.; Dickson, E.W. Proenkephalin expression and enkephalin release are widely observed in non-neuronal tissues. Peptides 2008, 29, 83–92. [Google Scholar] [CrossRef]
- Jin, T.; Hao, J.; Fan, D. Nicotine induces aberrant hypermethylation of tumor suppressor genes in pancreatic epithelial ductal cells. Biochem. Biophys. Res. Commun. 2018, 499, 934–940. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Zmijewski, M.A.; Zbytek, B.; Brozyna, A.A.; Granese, J.; Pisarchik, A.; Szczesniewski, A.; Tobin, D.J. Regulated proenkephalin expression in human skin and cultured skin cells. J. Invest. Dermatol. 2011, 131, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Comb, M.; Goodman, H.M. CpG methylation inhibits proenkephalin gene expression and binding of the transcription factor AP-2. Nucleic Acids Res. 1990, 18, 3975–3982. [Google Scholar] [CrossRef] [PubMed]
- Nicoll, R.; Alger, B.; Jahr, C. Enkephalin blocks inhibitory pathways in the vertebrate CNS. Nature 1980, 287, 22–25. [Google Scholar] [CrossRef] [PubMed]
- McGinty, J.F.; van der Kooy, D.; Bloom, F.E. The distribution and morphology of opioid peptide immunoreactive neurons in the cerebral cortex of rats. J. Neurosci. 1984, 4, 1104–1117. [Google Scholar] [CrossRef]
- Duque-Díaz, E.; Alvarez-Ojeda, O.; Coveñas, R. Enkephalins and ACTH in the mammalian nervous system. Vitam. Horm. 2019, 111, 147–193. [Google Scholar] [CrossRef]
- Lucas, L.R.; Harlan, R.E. Cholinergic regulation of tachykinin- and enkephalin-gene expression in the rat striatum. Brain Res. Mol. Brain Res. 1995, 30, 181–195. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.X.; Li, W.; Zhao, X.; Yang, J.X.; Xu, H.Y.; Wang, Z.F.; Yue, G.X. Changes of mRNA expression of enkephalin and prodynorphin in hippocampus of rats with chronic immobilization stress. World J. Gastroenterol. 2004, 10, 2547–2549. [Google Scholar] [CrossRef]
- Hervert, E.A.; Birdsong, W. The opioid peptide met-enkephalin modulates thalamo-cortical excitation inhibition balance in a medial thalamus-anterior cingulate cortex circuit. Neuropharmacology 2024, 242, 109785. [Google Scholar] [CrossRef]
- Chang, G.Q.; Barson, J.R.; Karatayev, O.; Chang, S.Y.; Chen, Y.W.; Leibowitz, S.F. Effect of chronic ethanol on enkephalin in the hypothalamus and extra-hypothalamic areas. Alcohol Clin. Exp. Res. 2010, 34, 761–770. [Google Scholar] [CrossRef]
- Gioia, M.; Bianchi, R. The distribution of substance P and met-enkephalin in the periaqueductal gray matter of the rat. Basic Appl. Histochem. 1988, 32, 103–108. [Google Scholar]
- Senba, E.; Tohyama, M. Reticulo-facial enkephalinergic pathway in the rat: An experimental immunohistochemical study. Neuroscience 1983, 10, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Van Bockstaele, E.J.; Saunders, A.; Commons, K.G.; Liu, X.B.; Peoples, J. Evidence for coexistence of enkephalin and glutamate in axon terminals and cellular sites for functional interactions of their receptors in the rat locus coeruleus. J. Comp. Neurol. 2000, 31, 103–114. [Google Scholar] [CrossRef]
- Rutherfurd, S.D.; Gundlach, A.L. Opioid peptide gene expression in the nucleus tractus solitarius of rat brain and increases induced by unilateral cervical vagotomy: Implications for role of opioid neurons in respiratory control mechanisms. Neuroscience 1993, 57, 797–810. [Google Scholar] [CrossRef]
- Marvizón, J.C.; Chen, W.; Murphy, N. Enkephalins, dynorphins, and beta-endorphin in the rat dorsal horn: An immunofluorescence colocalization study. J. Comp. Neurol. 2009, 517, 51–68. [Google Scholar] [CrossRef]
- Fukushima, T.; Tsuda, M.; Kofuji, T.; Hori, Y. Physiological properties of enkephalin-containing neurons in the spinal dorsal horn visualized by expression of green fluorescent protein in BAC transgenic mice. BMC Neurosci. 2011, 12, 36. [Google Scholar] [CrossRef]
- Bouras, C.; Taban, C.H.; Constantinidis, J. Mapping of enkephalins in human brain. An immunohistofluorescence study on brains from patients with senile and presenile dementia. Neuroscience 1984, 12, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Haber, S.N.; Watson, S.J. The comparative distribution of enkephalin, dynorphin and substance P in the human globus pallidus and basal forebrain. Neuroscience 1985, 14, 1011–1024. [Google Scholar] [CrossRef] [PubMed]
- McCollum, L.A.; Roche, J.K.; Roberts, R.C. Immunohistochemical localization of enkephalin in the human striatum: A postmortem ultrastructural study. Synapse 2012, 66, 204–219. [Google Scholar] [CrossRef]
- Kubek, M.J.; Wilber, J.F. Regional distribution of leucine-enkephalin in hypothalamic and extrahypothalamic loci of the human nervous system. Neurosci. Lett. 1980, 18, 155–161. [Google Scholar] [CrossRef]
- Banghart, M.R.; Neufeld, S.Q.; Wong, N.C.; Sabatini, B.L. Enkephalin Disinhibits Mu Opioid Receptor-Rich Striatal Patches via Delta Opioid Receptors. Neuron 2015, 88, 1227–1239. [Google Scholar] [CrossRef] [PubMed]
- Tkaczynski, J.A.; Borodovitsyna, O.; Chandler, D.J. Delta Opioid Receptors and Enkephalinergic Signaling within Locus Coeruleus Promote Stress Resilience. Brain Sci. 2022, 12, 860. [Google Scholar] [CrossRef] [PubMed]
- de Lanerolle, N.C.; LaMotte, C.C. The human spinal cord: Substance P and methionine-enkephalin immunoreactivity. J. Neurosci. 1982, 2, 1369–1386. [Google Scholar] [CrossRef] [PubMed]
- Gomes, L.R.; Leão, P. Recent Approaches on Signal Transduction and Transmission in Acupuncture: A Biophysical Overview for Medical Sciences. J. Acupunct. Meridian. Stud. 2020, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Millan, M.J. Descending control of pain. Prog. Neurobiol. 2002, 66, 355–474. [Google Scholar] [CrossRef] [PubMed]
- Lubejko, S.T.; Graham, R.D.; Livrizzi, G.; Schaefer, R.; Banghart, M.R.; Creed, M.C. The role of endogenous opioid neuropeptides in neurostimulation-driven analgesia. Front. Syst. Neurosci. 2022, 16, 1044686. [Google Scholar] [CrossRef] [PubMed]
- Hori, Y.; Endo, K.; Takahashi, T. Presynaptic inhibitory action of enkephalin on excitatory transmission in superficial dorsal horn of rat spinal cord. J. Physiol. 1992, 450, 673–685. [Google Scholar] [CrossRef]
- Heinke, B.; Gingl, E.; Sandkühler, J. Multiple targets of μ-opioid receptor-mediated presynaptic inhibition at primary afferent Aδ- and C-fibers. J. Neurosci. 2011, 31, 1313–1322. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Li, M.Y.; Ma, J.B.; Li, J.N.; Teng, X.Y.; Chen, Y.B.; Yin, J.B.; Huang, J.; Chen, J.; Zhang, T.; et al. Enkephalinergic Circuit Involved in Nociceptive Modulation in the Spinal Dorsal Horn. Neuroscience 2020, 429, 78–91. [Google Scholar] [CrossRef]
- Redekopp, C.A.; Livesey, J.H.; Donald, R.A. Inhibition of spontaneous ACTH release and improved response to CRF in sheep premedicated with the enkephalin analogue DAMME. Horm. Metab. Res. 1985, 17, 646–649. [Google Scholar] [CrossRef]
- Nakamoto, K.; Tokuyama, S. Stress-Induced Changes in the Endogenous Opioid System Cause Dysfunction of Pain and Emotion Regulation. Int. J. Mol. Sci. 2023, 24, 11713. [Google Scholar] [CrossRef] [PubMed]
- Rysztak, L.G.; Jutkiewicz, E.M. The role of enkephalinergic systems in substance use disorders. Front. Syst. Neurosci. 2022, 16, 932546. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Manzo, G.; Asai, M.; Fernández-Guasti, A. Evidence for changes in brain enkephalin contents associated to male rat sexual activity. Behav. Brain. Res. 2002, 131, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Simmons, D.; Self, D.W. Role of mu- and delta-opioid receptors in the nucleus accumbens in cocaine-seeking behavior. Neuropsychopharmacology 2009, 34, 1946–1957. [Google Scholar] [CrossRef] [PubMed]
- Margolis, E.B.; Fields, H.L.; Hjelmstad, G.O.; Mitchell, J.M. Delta-opioid receptor expression in the ventral tegmental area protects against elevated alcohol consumption. J. Neurosci. 2008, 28, 12672–12681. [Google Scholar] [CrossRef]
- Mendez, I.A.; Ostlund, S.B.; Maidment, N.T.; Murphy, N.P. Involvement of Endogenous Enkephalins and β-Endorphin in Feeding and Diet-Induced Obesity. Neuropsychopharmacology 2015, 40, 2103–2112. [Google Scholar] [CrossRef] [PubMed]
- DiFeliceantonio, A.G.; Mabrouk, O.S.; Kennedy, R.T.; Berridge, K.C. Enkephalin surges in dorsal neostriatum as a signal to eat. Curr. Biol. 2012, 22, 1918–1924. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Haddad, G.G.; Xia, Y. delta-, but not mu- and kappa-, opioid receptor activation protects neocortical neurons from glutamate-induced excitotoxic injury. Brain. Res. 2000, 885, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fu, N.; Su, J.; Wang, X.; Li, X. Rapid Enkephalin Delivery Using Exosomes to Promote Neurons Recovery in Ischemic Stroke by Inhibiting Neuronal p53/Caspase-3. Biomed. Res. Int. 2019, 2019, 4273290. [Google Scholar] [CrossRef]
- Meilandt, W.J.; Yu, G.Q.; Chin, J.; Roberson, E.D.; Palop, J.J.; Wu, T.; Scearce-Levie, K.; Mucke, L. Enkephalin elevations contribute to neuronal and behavioral impairments in a transgenic mouse model of Alzheimer’s disease. J. Neurosci. 2008, 28, 5007–5017. [Google Scholar] [CrossRef]
- Coccia, R.; Foppoli, C.; Blarzino, C.; De Marco, C.; Rosei, M.A. Interaction of enkephalin derivatives with reactive oxygen species. Biochim. Biophys. Acta 2001, 1525, 43–49. [Google Scholar] [CrossRef]
- Buhidma, Y.; Hobbs, C.; Malcangio, M.; Duty, S. Periaqueductal grey and spinal cord pathology contribute to pain in Parkinson’s disease. NPJ Park. Dis. 2023, 9, 69. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.D.; Galligan, J.J. Function of opioids in the enteric nervous system. Neurogastroenterol. Motil. 2004, 16 (Suppl. 2), 17–28. [Google Scholar] [CrossRef]
- Sternini, C.; Patierno, S.; Selmer, I.S.; Kirchgessner, A. The opioid system in the gastrointestinal tract. Neurogastroenterol. Motil. 2004, 16 (Suppl. 2), 3–16. [Google Scholar] [CrossRef] [PubMed]
- Thompson, G.L.; Canals, M.; Poole, D.P. Biological redundancy of endogenous GPCR ligands in the gut and the potential for endogenous functional selectivity. Front. Pharmacol. 2014, 5, 262. [Google Scholar] [CrossRef]
- Wang, Y.N.; Wyatt, R.J. Comparative immunohistochemical demonstration of peptide F- and other enkephalin-containing neurons in the enteric nervous system of the rat. Synapse 1987, 1, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Gonkowski, S.; Makowska, K.; Calka, J. The influence of experimental inflammation and axotomy on leucine enkephalin (leuENK) distribution in intramural nervous structures of the porcine descending colon. BMC Vet. Res. 2018, 14, 169. [Google Scholar] [CrossRef]
- Drokhlyansky, E.; Smillie, C.S.; Van Wittenberghe, N.; Ericsson, M.; Griffin, G.K.; Eraslan, G.; Dionne, D.; Cuoco, M.S.; Goder-Reiser, M.N.; Sharova, T.; et al. The Human and Mouse Enteric Nervous System at Single-Cell Resolution. Cell 2020, 182, 1606–1622. [Google Scholar] [CrossRef]
- Schultzberg, M.; Hökfelt, T.; Terenius, L.; Elfvin, L.G.; Lundberg, J.M.; Brandt, J.; Elde, R.P.; Goldstein, M. Enkephalin immunoreactive nerve fibres and cell bodies in sympathetic ganglia of the guinea-pig and rat. Neuroscience 1979, 4, 249–270. [Google Scholar] [CrossRef]
- Benarroch, E.E. Neuropeptides in the sympathetic system: Presence, plasticity, modulation, and implications. Ann. Neurol. 1994, 36, 6–13. [Google Scholar] [CrossRef]
- Eiden, L.E. The enkephalin-containing cell: Strategies for polypeptide synthesis and secretion throughout the neuroendocrine system. Cell. Mol. Neurobiol. 1987, 7, 339–352. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, J.M.; Hökfelt, T.; Kewenter, J.; Pettersson, G.; Ahlman, H.; Edin, R.; Dahlström, A.; Nilsson, G.; Terenius, L.; Uvnäs-Wallensten, K.; et al. Substance P-, VIP-, and enkephalin-like immunoreactivity in the human vagus nerve. Gastroenterology 1979, 77, 468–471. [Google Scholar] [CrossRef] [PubMed]
- Soares, C.L.R.; Wilairatana, P.; Silva, L.R.; Moreira, P.S.; Vilar Barbosa, N.M.M.; da Silva, P.R.; Coutinho, H.D.M.; de Menezes, I.R.A.; Felipe, C.F.B. Biochemical aspects of the inflammatory process: A narrative review. Biomed. Pharmacother. 2023, 168, 115764. [Google Scholar] [CrossRef] [PubMed]
- Claesson-Welsh, L.; Dejana, E.; McDonald, D.M. Permeability of the Endothelial Barrier: Identifying and Reconciling Controversies. Trends Mol. Med. 2021, 27, 314–331. [Google Scholar] [CrossRef] [PubMed]
- Kiss, A.L. Inflammation in Focus: The Beginning and the End. Pathol. Oncol. Res. 2022, 27, 1610136. [Google Scholar] [CrossRef] [PubMed]
- Middleton, J.; Patterson, A.M.; Gardner, L.; Schmutz, C.; Ashton, B.A. Leukocyte extravasation: Chemokine transport and presentation by the endothelium. Blood 2002, 100, 3853–3860. [Google Scholar] [CrossRef] [PubMed]
- Speyer, C.L.; Ward, P.A. Role of endothelial chemokines and their receptors during inflammation. J. Investig. Surg. 2011, 24, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Stein, C.; Lang, L.J. Peripheral mechanisms of opioid analgesia. Curr. Opin. Pharmacol. 2009, 9, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Binder, W.; Mousa, S.A.; Sitte, N.; Kaiser, M.; Stein, C.; Schäfer, M. Sympathetic activation triggers endogenous opioid release and analgesia within peripheral inflamed tissue. Eur. J. Neurosci. 2004, 20, 92–100. [Google Scholar] [CrossRef]
- Schäfer, M.; Carter, L.; Stein, C. Interleukin 1 beta and corticotropin-releasing factor inhibit pain by releasing opioids from immune cells in inflamed tissue. Proc. Natl. Acad. Sci. USA 1994, 91, 4219–4223. [Google Scholar] [CrossRef]
- García-Domínguez, M.; Lastra, A.; Folgueras, A.R.; Cernuda-Cernuda, R.; Fernández-García, M.T.; Hidalgo, A.; Menéndez, L.; Baamonde, A. The Chemokine CCL4 (MIP-1β) Evokes Antinociceptive Effects in Mice: A Role for CD4+ Lymphocytes and Met-Enkephalin. Mol. Neurobiol. 2019, 56, 1578–1595. [Google Scholar] [CrossRef]
- Rittner, H.L.; Labuz, D.; Schaefer, M.; Mousa, S.A.; Schulz, S.; Schäfer, M.; Stein, C.; Brack, A. Pain control by CXCR2 ligands through Ca2+-regulated release of opioid peptides from polymorphonuclear cells. FASEB J. 2006, 20, 2627–2629. [Google Scholar] [CrossRef]
- Zoghbi, S.S.; Liow, J.S.; Yasuno, F.; Hong, J.; Tuan, E.; Lazarova, N.; Gladding, R.L.; Pike, V.W.; Innis, R.B. 11C-loperamide and its N-desmethyl radiometabolite are avid substrates for brain permeability-glycoprotein efflux. J. Nucl. Med. 2008, 49, 649–656. [Google Scholar] [CrossRef]
- Ferri, G.L.; Watkinson, A.; Dockray, G.J. Proenkephalin A-derived peptides in the human gut. Gastroenterology 1988, 95, 1011–1017. [Google Scholar] [CrossRef]
- Galligan, J.J.; Sternini, C. Insights into the Role of Opioid Receptors in the GI Tract: Experimental Evidence and Therapeutic Relevance. Handb. Exp. Pharmacol. 2017, 239, 363–378. [Google Scholar] [CrossRef]
- Cohen-Biton, L.; Buskila, D.; Nissanholtz-Gannot, R. Review of Fibromyalgia (FM) Syndrome Treatments. Int. J. Environ. Res. Public Health 2022, 19, 12016. [Google Scholar] [CrossRef]
- Vincent, A.; Lahr, B.D.; Wolfe, F.; Clauw, D.J.; Whipple, M.O.; Oh, T.H.; Barton, D.L.; St Sauver, J. Prevalence of fibromyalgia: A population-based study in Olmsted County, Minnesota, utilizing the Rochester Epidemiology Project. Arthritis Care Res. 2013, 65, 786–792. [Google Scholar] [CrossRef]
- Heidari, F.; Afshari, M.; Moosazadeh, M. Prevalence of fibromyalgia in general population and patients, a systematic review and meta-analysis. Rheumatol. Int. 2017, 37, 1527–1539. [Google Scholar] [CrossRef]
- Otón, T.; Messina, O.D.; Fernández Ávila, D.G.; Robles San Román, M.; Mata, D.; Arguissain, C.; Galindo Guzmán, J.M.; Pérez, M.; Carmona, L.; Grupo Fibrojourney Latam. The patient journey of fibromyalgia in Latin America. Reumatol. Clin. (Engl. Ed.) 2024, 20, 32–42. [Google Scholar] [CrossRef]
- Siracusa, R.; Paola, R.D.; Cuzzocrea, S.; Impellizzeri, D. Fibromyalgia: Pathogenesis, Mechanisms, Diagnosis and Treatment Options Update. Int. J. Mol. Sci. 2021, 22, 3891. [Google Scholar] [CrossRef]
- Figuerola, M.L.; Loe, W.; Sormani, M.; Barontini, M. Met-enkephalin increase in patients with fibromyalgia under local treatment. Funct. Neurol. 1998, 13, 291–295. [Google Scholar]
- Baraniuk, J.N.; Whalen, G.; Cunningham, J.; Clauw, D.J. Cerebrospinal fluid levels of opioid peptides in fibromyalgia and chronic low back pain. BMC Musculoskelet. Disord. 2004, 5, 48. [Google Scholar] [CrossRef]
- Martínez-Martos, J.M.; Correa-Rodríguez, M.; Rus, A.; Molina, F.; Ramírez-Expósito, M.J.; Aguilar-Ferrandiz, M.E. Altered Serum Oxytocinase and Enkephalin-Degrading Aminopeptidase Activities in Patients With Fibromyalgia. Biol. Res. Nurs. 2019, 21, 431–439. [Google Scholar] [CrossRef]
- Roy, D.; Ahad, H.A.; Chinthaginjala, H.; Kumar, G.A.; Reddy, G.G.; Teja, A.S.T. A possible alternative to Opiorphin and its stable analogues for treating fibromyalgia pain: A clinical hypothesis. North Clin. Istanb. 2023, 10, 122–126. [Google Scholar] [CrossRef]
- Wisner, A.; Dufour, E.; Messaoudi, M.; Nejdi, A.; Marcel, A.; Ungeheuer, M.N.; Rougeot, C. Human Opiorphin, a natural antinociceptive modulator of opioid-dependent pathways. Proc. Natl. Acad. Sci. USA 2006, 103, 17979–17984. [Google Scholar] [CrossRef]
- Toljan, K.; Vrooman, B. Low-Dose Naltrexone (LDN)-Review of Therapeutic Utilization. Med. Sci. 2018, 6, 82. [Google Scholar] [CrossRef]
- Metyas, S.; Chen, C.L.; Yeter, K.; Solyman, J.; Arkfeld, D.G. Low Dose Naltrexone in the Treatment of Fibromyalgia. Curr. Rheumatol. Rev. 2018, 14, 177–180. [Google Scholar] [CrossRef]
- Yang, J.; Shin, K.M.; Do, A.; Bierle, D.M.; Abu Dabrh, A.M.; Yin, Z.; Bauer, B.A.; Mohabbat, A.B. The Safety and Efficacy of Low-Dose Naltrexone in Patients with Fibromyalgia: A Systematic Review. J. Pain Res. 2023, 16, 1017–1023. [Google Scholar] [CrossRef]
- Amiri, P.; Kazeminasab, S.; Nejadghaderi, S.A.; Mohammadinasab, R.; Pourfathi, H.; Araj-Khodaei, M.; Sullman, M.J.M.; Kolahi, A.A.; Safiri, S. Migraine: A Review on Its History, Global Epidemiology, Risk Factors, and Comorbidities. Front. Neurol. 2022, 12, 800605. [Google Scholar] [CrossRef]
- Steiner, T.J.; Stovner, L.J. Global epidemiology of migraine and its implications for public health and health policy. Nat. Rev. Neurol. 2023, 19, 109–117. [Google Scholar] [CrossRef]
- Eigenbrodt, A.K.; Ashina, H.; Khan, S.; Diener, H.C.; Mitsikostas, D.D.; Sinclair, A.J.; Pozo-Rosich, P.; Martelletti, P.; Ducros, A.; Lantéri-Minet, M.; et al. Diagnosis and management of migraine in ten steps. Nat. Rev. Neurol. 2021, 17, 501–514. [Google Scholar] [CrossRef]
- Kamm, K. CGRP and Migraine: What Have We Learned From Measuring CGRP in Migraine Patients So Far? Front. Neurol. 2022, 13, 930383. [Google Scholar] [CrossRef]
- Mosnaim, A.D.; Wolf, M.E.; Chevesich, J.; Callaghan, O.H.; Diamond, S. Plasma methionine enkephalin levels. A biological marker for migraine? Headache 1985, 25, 259–261. [Google Scholar] [CrossRef]
- Mosnaim, A.D.; Chevesich, J.; Wolf, M.E.; Freitag, F.G.; Diamond, S. Plasma methionine enkephalin. Increased levels during a migraine episode. Headache 1986, 26, 278–281. [Google Scholar] [CrossRef]
- Sicuteri, F.; Renzi, D.; Geppetti, P. Substance P and enkephalins: A creditable tandem in the pathophysiology of cluster headache and migraine. Adv. Exp. Med. Biol. 1986, 198, 145–152. [Google Scholar] [CrossRef]
- De Marinis, M.; Feliciani, M.; Janiri, L.; Cerbo, R.; Agnoli, A. Increased reactivity to a met-enkephalin analogue in the control of autonomic responses in migraine patients. Clin. Neuropharmacol. 1990, 13, 507–521. [Google Scholar] [CrossRef]
- de Lourdes Figuerola, M.; Levin, G.; Leston, J.; Barontini, M. Plasma met-enkephalin and catecholamine changes during the menstrual cycle and pain episode in menstrual migraine. Funct. Neurol. 1997, 12, 69–75. [Google Scholar]
- Descheemaeker, A.; Poras, H.; Wurm, M.; Luccarini, P.; Ouimet, T.; Dallel, R. Dual enkephalinase inhibitor PL37 as a potential novel treatment of migraine: Evidence from a rat model. Brain 2022, 145, 2664–2670. [Google Scholar] [CrossRef]
- Mei, H.R.; Hu, Y.Y.; Kapadia, S.; Ouimet, T.; Poras, H.; Dussor, G. Efficacy of dual enkephalinase inhibition in a preclinical migraine model is mediated by activation of peripheral delta opioid receptors. Headache 2023, 63, 621–633. [Google Scholar] [CrossRef]
- Alvarez-Perez, B.; Poras, H.; Maldonado, R. The inhibition of enkephalin catabolism by dual enkephalinase inhibitor: A novel possible therapeutic approach for opioid use disorders. Br. J. Pharmacol. 2023, 180, 879–893. [Google Scholar] [CrossRef]
- Codino, H.; Hardin, A. Low Dose Naltrexone in Conjunction With the Wahls Protocol to Reduce the Frequency of Chronic Migraines in a Patient With Multiple Sclerosis: A Case Study. Integr. Med. 2021, 20, 30–34. [Google Scholar]
- Taylor, S.S.; Noor, N.; Urits, I.; Paladini, A.; Sadhu, M.S.; Gibb, C.; Carlson, T.; Myrcik, D.; Varrassi, G.; Viswanath, O. Complex Regional Pain Syndrome: A Comprehensive Review. Pain Ther. 2021, 10, 875–892. [Google Scholar] [CrossRef]
- Abd-Elsayed, A.; Stark, C.W.; Topoluk, N.; Isaamullah, M.; Uzodinma, P.; Viswanath, O.; Gyorfi, M.J.; Fattouh, O.; Schlidt, K.C.; Dyara, O. A brief review of complex regional pain syndrome and current management. Ann. Med. 2024, 56, 2334398. [Google Scholar] [CrossRef]
- Ferraro, M.C.; O’Connell, N.E.; Sommer, C.; Goebel, A.; Bultitude, J.H.; Cashin, A.G.; Moseley, G.L.; McAuley, J.H. Complex regional pain syndrome: Advances in epidemiology, pathophysiology, diagnosis, and treatment. Lancet Neurol. 2024, 23, 522–533. [Google Scholar] [CrossRef]
- Mangnus, T.J.P.; Dirckx, M.; Huygen, F.J.P.M. Different Types of Pain in Complex Regional Pain Syndrome Require a Personalized Treatment Strategy. J. Pain Res. 2023, 16, 4379–4391. [Google Scholar] [CrossRef]
- Wen, B.; Pan, Y.; Cheng, J.; Xu, L.; Xu, J. The Role of Neuroinflammation in Complex Regional Pain Syndrome: A Comprehensive Review. J. Pain Res. 2023, 16, 3061–3073. [Google Scholar] [CrossRef]
- Chu, J.; Bruyninckx, F.; Neuhauser, D.V. Autonomic components of Complex Regional Pain Syndrome (CRPS) are favourably affected by Electrical Twitch-Obtaining Intramuscular Stimulation (ETOIMS): Effects on blood pressure and heart rate. BMJ Innov. 2017, 3, 176–187. [Google Scholar] [CrossRef]
- Halicka, M.; Vittersø, A.D.; Proulx, M.J.; Bultitude, J.H. Neuropsychological Changes in Complex Regional Pain Syndrome (CRPS). Behav. Neurol. 2020, 2020, 4561831. [Google Scholar] [CrossRef]
- van den Brink, O.W.; Rowland, M.A.; Marasco, S.F.; Esmore, D.S.; Rosenfeldt, F.L.; Pepe, S. Complex regional pain syndrome and methionine-enkephalin. Clin. Chem. 2006, 52, 535. [Google Scholar] [CrossRef]
- Chopra, P.; Cooper, M.S. Treatment of Complex Regional Pain Syndrome (CRPS) using low dose naltrexone (LDN). J. Neuroimmune Pharmacol. 2013, 8, 470–476. [Google Scholar] [CrossRef]
- Soin, A.; Soin, Y.; Dann, T.; Buenaventura, R.; Ferguson, K.; Atluri, S.; Sachdeva, H.; Sudarshan, G.; Akbik, H.; Italiano, J. Low-Dose Naltrexone Use for Patients with Chronic Regional Pain Syndrome: A Systematic Literature Review. Pain Physician. 2021, 24, E393–E406. [Google Scholar]
- Cordero, M.D. The inflammasome in fibromyalgia and CRPS: A microglial hypothesis? Nat. Rev. Rheumatol. 2015, 11, 630. [Google Scholar] [CrossRef]
- Silva-Fernández, L.; Macía-Villa, C.; Seoane-Mato, D.; Cortés-Verdú, R.; Romero-Pérez, A.; Quevedo-Vila, V.; Fábregas-Canales, D.; Antón-Pagés, F.; Añez, G.; Brandy, A.; et al. The prevalence of rheumatoid arthritis in Spain. Sci. Rep. 2020, 10, 21551. [Google Scholar] [CrossRef]
- Jahid, M.; Khan, K.U.; Rehan-Ul-Haq; Ahmed, R.S. Overview of Rheumatoid Arthritis and Scientific Understanding of the Disease. Mediterr. J. Rheumatol. 2023, 34, 284–291. [Google Scholar] [CrossRef]
- Smolen, J.S.; Aletaha, D.; Barton, A.; Burmester, G.R.; Emery, P.; Firestein, G.S.; Kavanaugh, A.; Mclnnes, I.B.; Solomon, D.H.; Strand, V.; et al. Rheumatoid arthritis. Nat. Rev. Dis. Primers 2018, 4, 18001. [Google Scholar] [CrossRef]
- Kamel, S.R. Calcium pyrophosphate dihydrate and hydroxyapatite crystals in a patient with rheumatoid arthritis: A case report. Egypt Rheumatol. Rehabil. 2017, 44, 92–94. [Google Scholar] [CrossRef]
- Romão, V.C.; Fonseca, J.E. Etiology and Risk Factors for Rheumatoid Arthritis: A State-of-the-Art Review. Front. Med. 2021, 8, 689698. [Google Scholar] [CrossRef]
- Grönblad, M.; Konttinen, Y.T.; Korkala, O.; Liesi, P.; Hukkanen, M.; Polak, J.M. Neuropeptides in synovium of patients with rheumatoid arthritis and osteoarthritis. J. Rheumatol. 1988, 15, 1807–1810. [Google Scholar]
- Takeba, Y.; Suzuki, N.; Kaneko, A.; Asai, T.; Sakane, T. Endorphin and enkephalin ameliorate excessive synovial cell functions in patients with rheumatoid arthritis. J. Rheumatol. 2001, 28, 2176–2183. [Google Scholar]
- Mousa, S.A.; Straub, R.H.; Schäfer, M.; Stein, C. Beta-endorphin, Met-enkephalin and corresponding opioid receptors within synovium of patients with joint trauma, osteoarthritis and rheumatoid arthritis. Ann. Rheum. Dis. 2007, 66, 871–879. [Google Scholar] [CrossRef]
- Matucci-Cerinic, M.; Lombardi, A.; Leoncini, G.; Pignone, A.; Sacerdoti, L.; Spillantini, M.G.; Partsch, G. Neutral endopeptidase (3.4.24.11) in plasma and synovial fluid of patients with rheumatoid arthritis. A marker of disease activity or a regulator of pain and inflammation? Rheumatol. Int. 1993, 13, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Basso, L.; Garnier, L.; Bessac, A.; Boué, J.; Blanpied, C.; Cenac, N.; Laffont, S.; Dietrich, G. T-lymphocyte-derived enkephalins reduce Th1/Th17 colitis and associated pain in mice. J. Gastroenterol. 2018, 53, 215–226. [Google Scholar] [CrossRef]
- Braz, J.; Beaufour, C.; Coutaux, A.; Epstein, A.L.; Cesselin, F.; Hamon, M.; Pohl, M. Therapeutic efficacy in experimental polyarthritis of viral-driven enkephalin overproduction in sensory neurons. J. Neurosci. 2001, 21, 7881–7888. [Google Scholar] [CrossRef]
- Lu, Y.; McNearney, T.A.; Wilson, S.P.; Yeomans, D.C.; Westlund, K.N. Joint capsule treatment with enkephalin-encoding HSV-1 recombinant vector reduces inflammatory damage and behavioural sequelae in rat CFA monoarthritis. Eur. J. Neurosci. 2008, 27, 1153–1165. [Google Scholar] [CrossRef]
- Enkorten—A Potential Drug for the Treatment of Rheumatoid Arthritis. Available online: https://www.intechopen.com/chapters/26079 (accessed on 3 July 2024).
- Yadav, Y.R.; Nishtha, Y.; Sonjjay, P.; Vijay, P.; Shailendra, R.; Yatin, K. Trigeminal Neuralgia. Asian J. Neurosurg. 2017, 12, 585–597. [Google Scholar] [CrossRef]
- Khawaja, S.N.; Scrivani, S.J. Trigeminal Neuralgia. Dent. Clin. N. Am. 2023, 67, 99–115. [Google Scholar] [CrossRef]
- Ashina, S.; Robertson, C.E.; Srikiatkhachorn, A.; Di Stefano, G.; Donnet, A.; Hodaie, M.; Obermann, M.; Romero-Reyes, M.; Park, Y.S.; Cruccu, G.; et al. Trigeminal neuralgia. Nat. Rev. Dis. Primers 2024, 10, 39. [Google Scholar] [CrossRef]
- Lambru, G.; Zakrzewska, J.; Matharu, M. Trigeminal neuralgia: A practical guide. Pract. Neurol. 2021, 21, 392–402. [Google Scholar] [CrossRef]
- Mueller, D.; Obermann, M.; Yoon, M.S.; Poitz, F.; Hansen, N.; Slomke, M.A.; Dommes, P.; Gizewski, E.; Diener, H.C.; Katsarava, Z. Prevalence of trigeminal neuralgia and persistent idiopathic facial pain: A population-based study. Cephalalgia 2011, 31, 1542–1548. [Google Scholar] [CrossRef] [PubMed]
- De Stefano, G.; Litewczuk, D.; Mollica, C.; Di Pietro, G.; Galosi, E.; Leone, C.; Falco, P.; Tullo, M.G.; Caramia, F.; Truini, A.; et al. Sex differences in trigeminal neuralgia: A focus on radiological and clinical characteristics. Neurol. Sci. 2023, 44, 4465–4472. [Google Scholar] [CrossRef] [PubMed]
- Maarbjerg, S.; Di Stefano, G.; Bendtsen, L.; Cruccu, G. Trigeminal neuralgia—Diagnosis and treatment. Cephalalgia 2017, 37, 648–657. [Google Scholar] [CrossRef]
- Duransoy, Y.K.; Mete, M.; Akçay, E.; Selçuki, M. Differences in individual susceptibility affect the development of trigeminal neuralgia. Neural Regen. Res. 2013, 8, 1337–1342. [Google Scholar] [CrossRef]
- Meunier, A.; Latrémolière, A.; Mauborgne, A.; Bourgoin, S.; Kayser, V.; Cesselin, F.; Hamon, M.; Pohl, M. Attenuation of pain-related behavior in a rat model of trigeminal neuropathic pain by viral-driven enkephalin overproduction in trigeminal ganglion neurons. Mol. Ther. 2005, 11, 608–616. [Google Scholar] [CrossRef]
- Tzabazis, A.Z.; Klukinov, M.; Feliciano, D.P.; Wilson, S.P.; Yeomans, D.C. Gene therapy for trigeminal pain in mice. Gene. Ther. 2014, 21, 422–426. [Google Scholar] [CrossRef]
- de Oliveira, C.L.; Medeiros, L.F.; de Souza, V.S.; Lopes, B.C.; de Oliveira, F.F.; Marques, L.X.; da Silva Torres, I.L.; de Souza, A. Low-Dose Naltrexone Reverses Facial Mechanical Allodynia in a Rat Model of Trigeminal Neuralgia. Neurosci. Lett. 2020, 736, 135248. [Google Scholar] [CrossRef]
- Goldenberg, M.M. Multiple sclerosis review. P T. 2012, 37, 175–184. [Google Scholar]
- Dobson, R.; Giovannoni, G. Multiple sclerosis—A review. Eur. J. Neurol. 2019, 26, 27–40. [Google Scholar] [CrossRef]
- Hemond, C.C.; Bakshi, R. Magnetic Resonance Imaging in Multiple Sclerosis. Cold Spring. Harb. Perspect. Med. 2018, 8, a028969. [Google Scholar] [CrossRef]
- Haki, M.; Al-Biati, H.A.; Al-Tameemi, Z.S.; Ali, I.S.; Al-Hussaniy, H.A. Review of multiple sclerosis: Epidemiology, etiology, pathophysiology, and treatment. Medicine 2024, 103, e37297. [Google Scholar] [CrossRef]
- Leray, E.; Moreau, T.; Fromont, A.; Edan, G. Epidemiology of multiple sclerosis. Rev. Neurol. 2016, 172, 3–13. [Google Scholar] [CrossRef]
- Dighriri, I.M.; Aldalbahi, A.A.; Albeladi, F.; Tahiri, A.A.; Kinani, E.M.; Almohsen, R.A.; Alamoudi, N.H.; Alanazi, A.A.; Alkhamshi, S.J.; Althomali, N.A.; et al. An Overview of the History, Pathophysiology, and Pharmacological Interventions of Multiple Sclerosis. Cureus 2023, 15, e33242. [Google Scholar] [CrossRef] [PubMed]
- Titus, H.E.; Chen, Y.; Podojil, J.R.; Robinson, A.P.; Balabanov, R.; Popko, B.; Miller, S.D. Pre-clinical and Clinical Implications of “Inside-Out” vs. “Outside-In” Paradigms in Multiple Sclerosis Etiopathogenesis. Front. Cell Neurosci. 2020, 14, 599717. [Google Scholar] [CrossRef]
- Ludwig, M.D.; Zagon, I.S.; McLaughlin, P.J. Serum [Met5]-enkephalin levels are reduced in multiple sclerosis and restored by low-dose naltrexone. Exp. Biol. Med. 2017, 242, 1524–1533. [Google Scholar] [CrossRef] [PubMed]
- Patel, C.; Meadowcroft, M.D.; Zagon, I.S.; McLaughlin, P.J. [Met5]-enkephalin preserves diffusion metrics in EAE mice. Brain Res. Bull. 2020, 165, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Eisenstein, T.K. The Role of Opioid Receptors in Immune System Function. Front. Immunol. 2019, 10, 2904. [Google Scholar] [CrossRef] [PubMed]
- Rakanović-Todić, M.; Burnazović-Ristić, L.; Ibrulj, S.; Mulbegović, N. Effect of met-enkephalin on chromosomal aberrations in the lymphocytes of the peripheral blood of patients with multiple sclerosis. Bosn. J. Basic Med. Sci. 2014, 14, 75–80. [Google Scholar] [CrossRef]
- Zagon, I.S.; Rahn, K.A.; Turel, A.P.; McLaughlin, P.J. Endogenous opioids regulate expression of experimental autoimmune encephalomyelitis: A new paradigm for the treatment of multiple sclerosis. Exp. Biol. Med. 2009, 234, 1383–1392. [Google Scholar] [CrossRef] [PubMed]
- Zagon, I.S.; Rahn, K.A.; Bonneau, R.H.; Turel, A.P.; McLaughlin, P.J. Opioid growth factor suppresses expression of experimental autoimmune encephalomyelitis. Brain Res. 2010, 1310, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Rahn, K.A.; McLaughlin, P.J.; Zagon, I.S. Prevention and diminished expression of experimental autoimmune encephalomyelitis by low dose naltrexone (LDN) or opioid growth factor (OGF) for an extended period: Therapeutic implications for multiple sclerosis. Brain Res. 2011, 1381, 243–253. [Google Scholar] [CrossRef]
- Campbell, A.M.; Zagon, I.S.; McLaughlin, P.J. Opioid growth factor arrests the progression of clinical disease and spinal cord pathology in established experimental autoimmune encephalomyelitis. Brain Res. 2012, 1472, 138–148. [Google Scholar] [CrossRef]
- Campbell, A.M.; Zagon, I.S.; McLaughlin, P.J. Astrocyte proliferation is regulated by the OGF-OGFr axis in vitro and in experimental autoimmune encephalomyelitis. Brain Res. Bull. 2013, 90, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Hammer, L.A.; Zagon, I.S.; McLaughlin, P.J. Treatment of a relapse-remitting model of multiple sclerosis with opioid growth factor. Brain Res. Bull. 2013, 98, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, M.D.; Turel, A.P.; Zagon, I.S.; McLaughlin, P.J. Long-term treatment with low dose naltrexone maintains stable health in patients with multiple sclerosis. Mult. Scler. J. Exp. Transl. Clin. 2016, 2, 2055217316672242. [Google Scholar] [CrossRef] [PubMed]
- Enkephalin Therapy Improves Relapsing-Remitting Multiple Sclerosis. Available online: https://www.intechopen.com/chapters/70924 (accessed on 6 July 2024).
- Gajendran, M.; Loganathan, P.; Catinella, A.P.; Hashash, J.G. A comprehensive review and update on Crohn’s disease. Dis. Mon. 2018, 64, 20–57. [Google Scholar] [CrossRef]
- Roda, G.; Chien Ng, S.; Kotze, P.G.; Argollo, M.; Panaccione, R.; Spinelli, A.; Kaser, A.; Peyrin-Biroulet, L.; Danese, S. Crohn’s disease. Nat. Rev. Dis. Primers 2020, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Loftus, E.V., Jr. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology 2004, 126, 1504–1517. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C.; Bernstein, C.N.; Vatn, M.H.; Lakatos, P.L.; Loftus, E.V., Jr.; Tysk, C.; O’Morain, C.; Moum, B.; Colombel, J.F. Epidemiology and Natural History Task Force of the International Organization of Inflammatory Bowel Disease (IOIBD). Geographical variability and environmental risk factors in inflammatory bowel disease. Gut 2013, 62, 630–649. [Google Scholar] [CrossRef] [PubMed]
- Tsianos, E.V.; Katsanos, K.H.; Tsianos, V.E. Role of genetics in the diagnosis and prognosis of Crohn’s disease. World J. Gastroenterol. 2012, 18, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Dam, A.N.; Berg, A.M.; Farraye, F.A. Environmental influences on the onset and clinical course of Crohn’s disease-part 1: An overview of external risk factors. Gastroenterol. Hepatol. 2013, 9, 711–717. [Google Scholar]
- Li, N.; Shi, R.H. Updated review on immune factors in pathogenesis of Crohn’s disease. World J. Gastroenterol. 2018, 24, 15–22. [Google Scholar] [CrossRef]
- Sjölund, K.; Schaffalitzky, O.B.; Muckadell, D.E.; Fahrenkrug, J.; Håkanson, R.; Peterson, B.G.; Sundler, F. Peptide-containing nerve fibres in the gut wall in Crohn’s disease. Gut 1983, 24, 724–733. [Google Scholar] [CrossRef]
- Koch, T.R.; Carney, J.A.; Go, V.L. Distribution and quantitation of gut neuropeptides in normal intestine and inflammatory bowel diseases. Dig. Dis. Sci. 1987, 32, 369–376. [Google Scholar] [CrossRef]
- Fehér, E.; Kovács, A.; Gallatz, K.; Fehér, J. Direct morphological evidence of neuroimmunomodulation in colonic mucosa of patients with Crohn’s disease. Neuroimmunomodulation 1997, 4, 250–257. [Google Scholar] [CrossRef]
- Owczarek, D.; Cibor, D.; Mach, T.; Cieśla, A.; Pierzchała-Koziec, K.; Sałapa, K.; Kuśnierz-Cabała, B. Met-enkephalins in patients with inflammatory bowel diseases. Adv. Med. Sci. 2011, 6, 158–164. [Google Scholar] [CrossRef]
- Wilenska, B.; Tymecka, D.; Włodarczyk, M.; Sobolewska-Włodarczyk, A.; Wiśniewska-Jarosińska, M.; Dyniewicz, J.; Somogyi, Á.; Fichna, J.; Misicka, A. Enkephalin degradation in serum of patients with inflammatory bowel diseases. Pharmacol. Rep. 2019, 71, 42–47. [Google Scholar] [CrossRef]
- Zatorski, H.; Sałaga, M.; Fichna, J. Role of glucagon-like peptides in inflammatory bowel diseases-current knowledge and future perspectives. Naunyn Schmiedebergs Arch. Pharmacol. 2019, 392, 1321–1330. [Google Scholar] [CrossRef]
- Smith, J.P.; Stock, H.; Bingaman, S.; Mauger, D.; Rogosnitzky, M.; Zagon, I.S. Low-dose naltrexone therapy improves active Crohn’s disease. Am. J. Gastroenterol. 2007, 102, 820–828. [Google Scholar] [CrossRef]
- Parker, C.E.; Nguyen, T.M.; Segal, D.; MacDonald, J.K.; Chande, N. Low dose naltrexone for induction of remission in Crohn’s disease. Cochrane Database Syst. Rev. 2018, 4, CD010410. [Google Scholar] [CrossRef]
- Szymaszkiewicz, A.; Storr, M.; Fichna, J.; Zielinska, M. Enkephalinase inhibitors, potential therapeutics for the future treatment of diarrhea predominant functional gastrointestinal disorders. Neurogastroenterol. Motil. 2019, 31, e13526. [Google Scholar] [CrossRef]
- Eberlin, M.; Chen, M.; Mueck, T.; Däbritz, J. Racecadotril in the treatment of acute diarrhea in children: A systematic, comprehensive review and meta-analysis of randomized controlled trials. BMC Pediatr. 2018, 18, 124. [Google Scholar] [CrossRef]
- Upadhyay, A. Cancer: An unknown territory; rethinking before going ahead. Genes Dis. 2020, 8, 655–661. [Google Scholar] [CrossRef]
- Hiam-Galvez, K.J.; Allen, B.M.; Spitzer, M.H. Systemic immunity in cancer. Nat. Rev. Cancer 2021, 21, 345–359. [Google Scholar] [CrossRef] [PubMed]
- Diori Karidio, I.; Sanlier, S.H. Reviewing cancer’s biology: An eclectic approach. J. Egypt Natl. Canc. Inst. 2021, 33, 32. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Snijders, R.A.H.; Brom, L.; Theunissen, M.; van den Beuken-van Everdingen, M.H.J. Update on Prevalence of Pain in Patients with Cancer 2022: A Systematic Literature Review and Meta-Analysis. Cancers 2023, 15, 591. [Google Scholar] [CrossRef]
- Mattiuzzi, C.; Lippi, G. Current Cancer Epidemiology. J. Epidemiol. Glob. Health 2019, 9, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Haroun, R.; Wood, J.N.; Sikandar, S. Mechanisms of cancer pain. Front. Pain Res. 2023, 3, 1030899. [Google Scholar] [CrossRef]
- Mestdagh, F.; Steyaert, A.; Lavand’homme, P. Cancer Pain Management: A Narrative Review of Current Concepts, Strategies, and Techniques. Curr. Oncol. 2023, 30, 6838–6858. [Google Scholar] [CrossRef]
- Lin, J.; Hsieh, R.K.; Chen, J.S.; Lee, K.D.; Rau, K.M.; Shao, Y.Y.; Sung, Y.C.; Yeh, S.P.; Chang, C.S.; Liu, T.C.; et al. Satisfaction with pain management and impact of pain on quality of life in cancer patients. Asia Pac. J. Clin. Oncol. 2020, 16, e91–e98. [Google Scholar] [CrossRef]
- Schäfer, M.; Mousa, S.A. Opioid therapy and tumor progression. Adv. Pall. Med. 2009, 8, 53–56. [Google Scholar]
- Wang, X.; Li, S.; Yan, S.; Shan, Y.; Wang, X.; Jingbo, Z.; Wang, Y.; Shan, F.; Griffin, N.; Sun, X. Methionine enkephalin inhibits colorectal cancer by remodeling the immune status of the tumor microenvironment. Int. Immunopharmacol. 2022, 111, 109125. [Google Scholar] [CrossRef] [PubMed]
- Zagon, I.S.; Hytrek, S.D.; Lang, C.M.; Smith, J.P.; McGarrity, T.J.; Wu, Y.; McLaughlin, P.J. Opioid growth factor ([Met5]enkephalin) prevents the incidence and retards the growth of human colon cancer. Am. J. Physiol. 1996, 271 Pt 2, R780–R786. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, Y.; Shan, F. Interaction of opioid growth factor (OGF) and opioid antagonist and their significance in cancer therapy. Int. Immunopharmacol. 2019, 75, 105785. [Google Scholar] [CrossRef]
- Li, X.; Meng, Y.; Plotnikoff, N.P.; Youkilis, G.; Griffin, N.; Wang, E.; Lu, C.; Shan, F. Methionine enkephalin (MENK) inhibits tumor growth through regulating CD4+Foxp3+ regulatory T cells (Tregs) in mice. Cancer Biol. Ther. 2015, 16, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.M.; Jiao, X.; Plotnikoff, N.P.; Griffin, N.; Qi, R.Q.; Gao, X.H.; Shan, F.P. Killing effect of methionine enkephalin on melanoma in vivo and in vitro. Oncol. Rep. 2017, 38, 2132–2140. [Google Scholar] [CrossRef]
- Tuo, Y.; Tian, C.; Lu, L.; Xiang, M. The paradoxical role of methionine enkephalin in tumor responses. Eur. J. Pharmacol. 2020, 882, 173253. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Huang, H.; Handley, M.; Griffin, N.; Bai, X.; Shan, F. A novel mechanism of lung cancer inhibition by methionine enkephalin through remodeling the immune status of the tumor microenvironment. Int. Immunopharmacol. 2021, 99, 107999. [Google Scholar] [CrossRef]
- Blebea, J.; Mazo, J.E.; Kihara, T.K.; Vu, J.H.; McLaughlin, P.J.; Atnip, R.G.; Zagon, I.S. Opioid growth factor modulates angiogenesis. J. Vasc. Surg. 2000, 32, 364–373. [Google Scholar] [CrossRef]
- Scholar, E.M.; Violi, L.; Hexum, T.D. The antimetastatic activity of enkephalin-like peptides. Cancer Lett. 1987, 35, 133–138. [Google Scholar] [CrossRef]
- Zhang, S.; Geng, J.; Shan, F.; Shan, Y.; Griffin, N.; Wu, B.; Wang, X. Methionine enkephalin suppresses lung cancer metastasis by regulating the polarization of tumor-associated macrophages and the distribution of myeloid-derived suppressor cells in the tumor microenvironment and inhibiting epithelial-mesenchymal transition. Int. Immunopharmacol. 2023, 118, 110064. [Google Scholar] [CrossRef]
- Zhao, G.; Shi, Y.; Gong, C.; Liu, T.; Nan, W.; Ma, L.; Wu, Z.; Da, C.; Zhou, K.; Zhang, H. Curcumin Exerts Antinociceptive Effects in Cancer-Induced Bone Pain via an Endogenous Opioid Mechanism. Front. Neurosci. 2021, 15, 696861. [Google Scholar] [CrossRef]
- Sevcik, M.A.; Jonas, B.M.; Lindsay, T.H.; Halvorson, K.G.; Ghilardi, J.R.; Kuskowski, M.A.; Mukherjee, P.; Maggio, J.E.; Mantyh, P.W. Endogenous opioids inhibit early-stage pancreatic pain in a mouse model of pancreatic cancer. Gastroenterology 2006, 131, 900–910. [Google Scholar] [CrossRef]
- González-Rodríguez, S.; Poras, H.; Menéndez, L.; Lastra, A.; Ouimet, T.; Fournié-Zaluski, M.C.; Roques, B.P.; Baamonde, A. Synergistic combinations of the dual enkephalinase inhibitor PL265 given orally with various analgesic compounds acting on different targets, in a murine model of cancer-induced bone pain. Scand. J. Pain. 2017, 14, 25–38. [Google Scholar] [CrossRef]
- Wang, X.; Tian, J.; Jiao, X.; Geng, J.; Wang, R.; Liu, N.; Gao, X.; Griffin, N.; Gao, Y.; Shan, F. The novel mechanism of anticancer effect on gastric cancer through inducing G0/G1 cell cycle arrest and caspase-dependent apoptosis in vitro and in vivo by methionine enkephalin. Cancer Manag. Res. 2018, 10, 4773–4787. [Google Scholar] [CrossRef]
- Moulin, D.E.; Max, M.B.; Kaiko, R.F.; Inturrisi, C.E.; Maggard, J.; Yaksh, T.L.; Foley, K.M. The analgesic efficacy of intrathecal D-Ala2-D-Leu5-enkephalin in cancer patients with chronic pain. Pain 1985, 23, 213–221. [Google Scholar] [CrossRef]
- Meynadier, J.; Dalmas, S.; Lecomte, J.M.; Gros, C.I.; Schwartz, J.C. Potent analgesic effects of inhibitors of enkephalin metabolism administered intrathecally to cancer patients. Pain Clinic. 1988, 2, 201–205. [Google Scholar]
- Smith, J.P.; Bingaman, S.I.; Mauger, D.T.; Harvey, H.H.; Demers, L.M.; Zagon, I.S. Opioid growth factor improves clinical benefit and survival in patients with advanced pancreatic cancer. Open Access J. Clin. Trials 2010, 2010, 37–48. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Domínguez, M. Enkephalins and Pain Modulation: Mechanisms of Action and Therapeutic Perspectives. Biomolecules 2024, 14, 926. https://doi.org/10.3390/biom14080926
García-Domínguez M. Enkephalins and Pain Modulation: Mechanisms of Action and Therapeutic Perspectives. Biomolecules. 2024; 14(8):926. https://doi.org/10.3390/biom14080926
Chicago/Turabian StyleGarcía-Domínguez, Mario. 2024. "Enkephalins and Pain Modulation: Mechanisms of Action and Therapeutic Perspectives" Biomolecules 14, no. 8: 926. https://doi.org/10.3390/biom14080926




