Cant1 Affects Cartilage Proteoglycan Properties: Aggrecan and Decorin Characterization in a Mouse Model of Desbuquois Dysplasia Type 1
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Model and Care
2.2. Chondrocyte Cultures
2.3. Protein Extraction from Chondrocytes
2.4. Proteoglycan Extraction from Cartilage Femoral Head and Skin
2.5. Purification of the Core Protein Standard from Cartilage Proteoglycans
2.6. Western Blot Analysis
2.7. Analysis of Collagen from Femoral Head and Skin
2.8. Immunohistochemical Analysis
3. Results
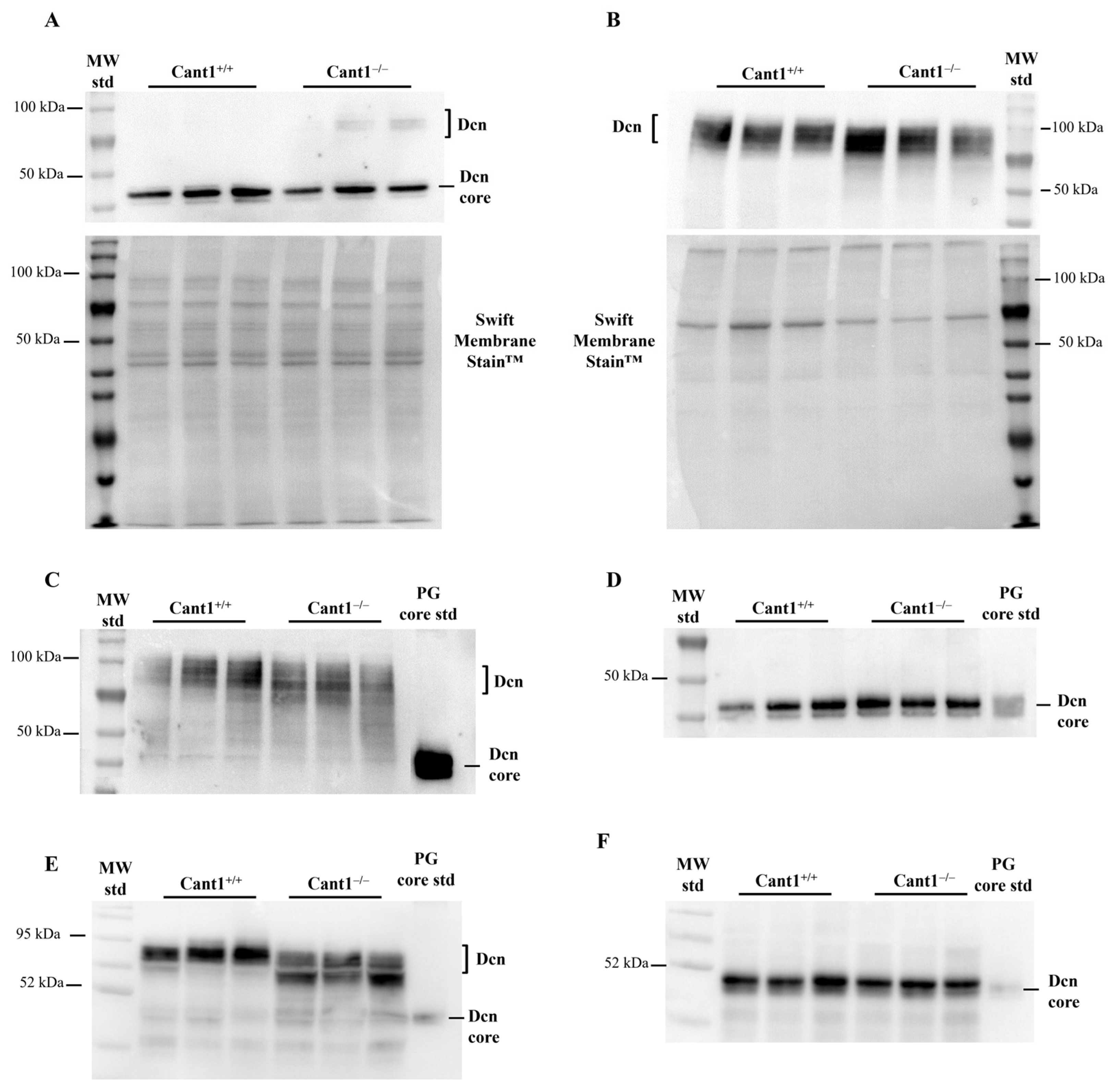
3.1. Characterization of Decorin in Cant1 Knock-Out Chondrocytes and Tissues
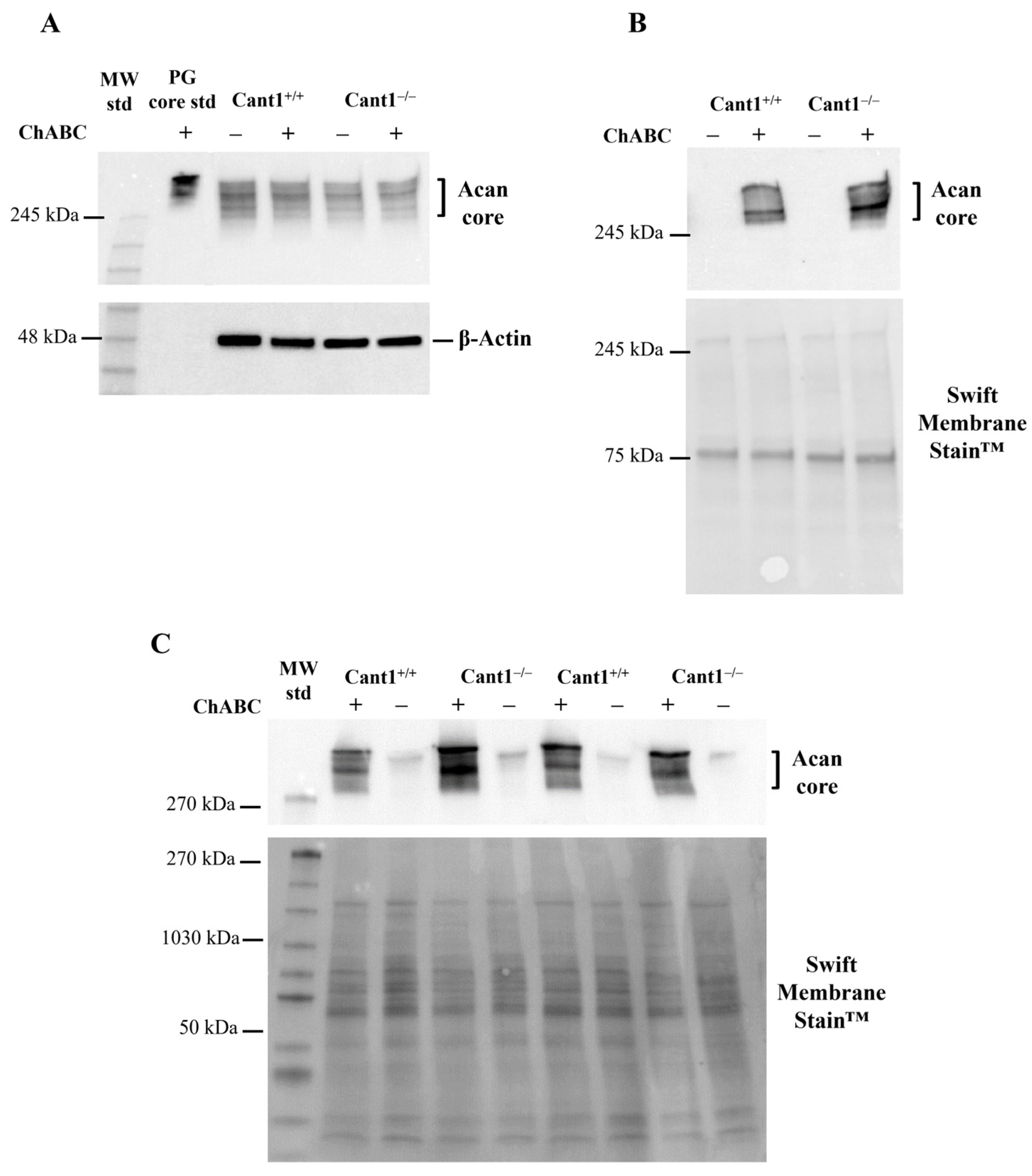
3.2. Characterization of Aggrecan in Cant1 Knock-Out Chondrocytes and Cartilage
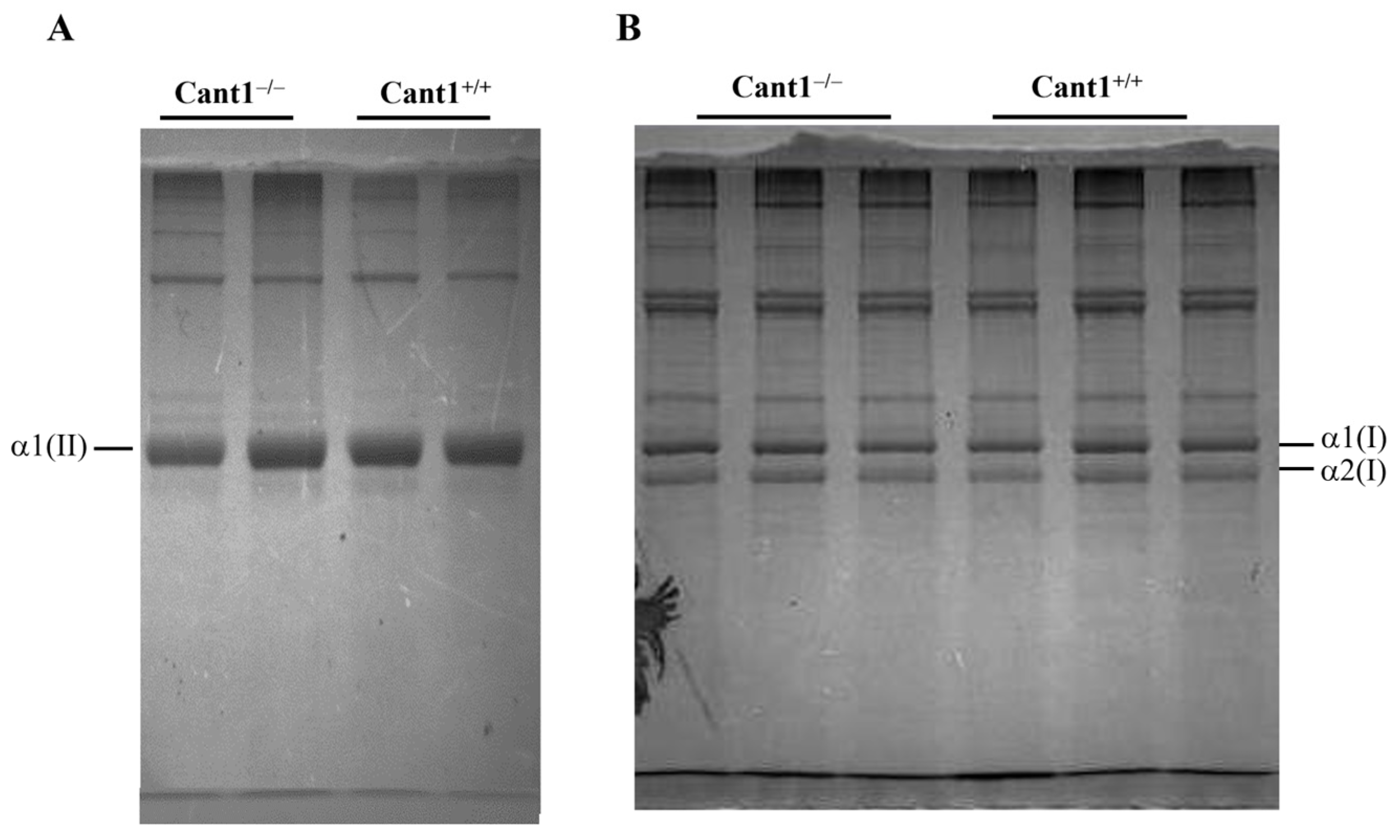
3.3. Collagen Analysis in Cartilage and Skin
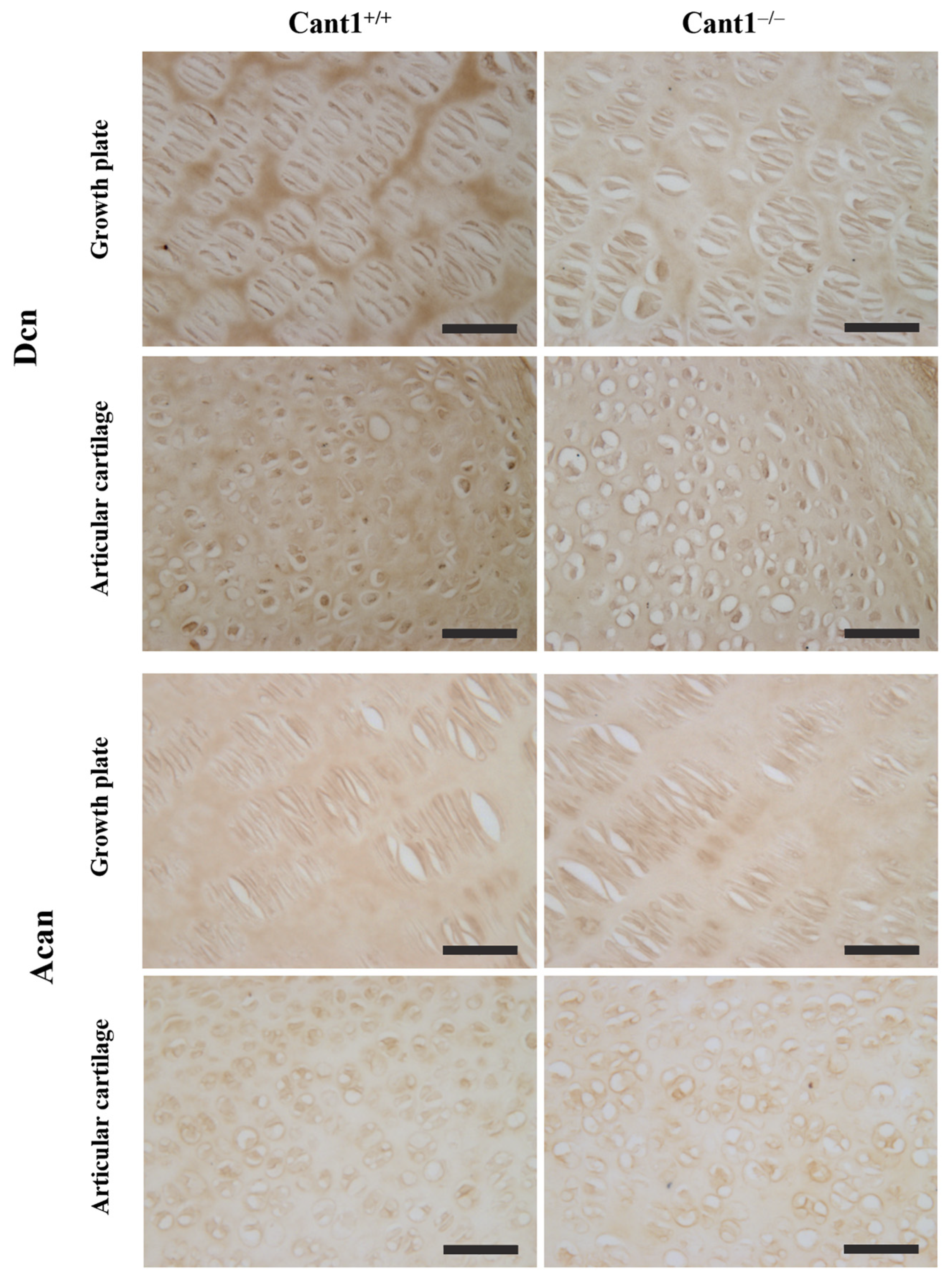
3.4. Immunohistochemical Localization of Decorin, Aggrecan, and Collagen Type II in the Tibia Epiphysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eyre, D.R.; Weis, M.A.; Wu, J.J. Articular cartilage collagen: An irreplaceable framework? Eur. Cells Mater. 2006, 12, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Roughley, P.J. The structure and function of cartilage proteoglycans. Eur. Cells Mater. 2006, 12, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Iozzo, R.V. Matrix proteoglycans: From molecular design to cellular function. Annu. Rev. Biochem. 1998, 67, 609–652. [Google Scholar] [CrossRef] [PubMed]
- Iozzo, R.V.; Schaefer, L. Proteoglycan form and function: A comprehensive nomenclature of proteoglycans. Matrix Biol. 2015, 42, 11–55. [Google Scholar] [CrossRef] [PubMed]
- Hellicar, J.; Stevenson, N.L.; Stephens, D.J.; Lowe, M. Supply chain logistics—The role of the Golgi complex in extracellular matrix production and maintenance. J. Cell Sci. 2022, 135, jcs258879. [Google Scholar] [CrossRef]
- Stevenson, N.L. The factory, the antenna and the scaffold: The three-way interplay between the Golgi, cilium and extracellular matrix underlying tissue function. Biol. Open 2023, 12, bio059719. [Google Scholar] [CrossRef]
- Paganini, C.; Costantini, R.; Superti-Furga, A.; Rossi, A. Bone and connective tissue disorders caused by defects in glycosaminoglycan biosynthesis: A panoramic view. FEBS J. 2019, 286, 3008–3032. [Google Scholar] [CrossRef]
- Faivre, L.; Le Merrer, M.; Zerres, K.; Ben Hariz, M.; Scheffer, D.; Young, I.D.; Maroteaux, P.; Munnich, A.; Cormier-Daire, V. Clinical and genetic heterogeneity in Desbuquois dysplasia. Am. J. Med. Genet. A 2004, 128A, 29–32. [Google Scholar] [CrossRef]
- Kim, O.H.; Nishimura, G.; Song, H.R.; Matsui, Y.; Sakazume, S.; Yamada, M.; Narumi, Y.; Alanay, Y.; Unger, S.; Cho, T.J.; et al. A variant of Desbuquois dysplasia characterized by advanced carpal bone age, short metacarpals, and elongated phalanges: Report of seven cases. Am. J. Med. Genet. A 2010, 152A, 875–885. [Google Scholar] [CrossRef]
- Failer, B.U.; Braun, N.; Zimmermann, H. Cloning, expression, and functional characterization of a Ca(2+)-dependent endoplasmic reticulum nucleoside diphosphatase. J. Biol. Chem. 2002, 277, 36978–36986. [Google Scholar] [CrossRef]
- Huber, C.; Oules, B.; Bertoli, M.; Chami, M.; Fradin, M.; Alanay, Y.; Al-Gazali, L.I.; Ausems, M.G.; Bitoun, P.; Cavalcanti, D.P.; et al. Identification of CANT1 mutations in Desbuquois dysplasia. Am. J. Hum. Genet. 2009, 85, 706–710. [Google Scholar] [CrossRef] [PubMed]
- Furuichi, T.; Dai, J.; Cho, T.J.; Sakazume, S.; Ikema, M.; Matsui, Y.; Baynam, G.; Nagai, T.; Miyake, N.; Matsumoto, N.; et al. CANT1 mutation is also responsible for Desbuquois dysplasia, type 2 and Kim variant. J. Med. Genet. 2011, 48, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Nizon, M.; Huber, C.; De Leonardis, F.; Merrina, R.; Forlino, A.; Fradin, M.; Tuysuz, B.; Abu-Libdeh, B.Y.; Alanay, Y.; Albrecht, B.; et al. Further delineation of CANT1 phenotypic spectrum and demonstration of its role in proteoglycan synthesis. Hum. Mutat. 2012, 33, 1261–1266. [Google Scholar] [CrossRef]
- Paganini, C.; Monti, L.; Costantini, R.; Besio, R.; Lecci, S.; Biggiogera, M.; Tian, K.; Schwartz, J.M.; Huber, C.; Cormier-Daire, V.; et al. Calcium activated nucleotidase 1 (CANT1) is critical for glycosaminoglycan biosynthesis in cartilage and endochondral ossification. Matrix Biol. 2019, 81, 70–90. [Google Scholar] [CrossRef] [PubMed]
- Kodama, K.; Takahashi, H.; Oiji, N.; Nakano, K.; Okamura, T.; Niimi, K.; Takahashi, E.; Guo, L.; Ikegawa, S.; Furuichi, T. CANT1 deficiency in a mouse model of Desbuquois dysplasia impairs glycosaminoglycan synthesis and chondrocyte differentiation in growth plate cartilage. FEBS Open Bio 2020, 10, 1096–1103. [Google Scholar] [CrossRef]
- Gramegna Tota, C.; Valenti, B.; Forlino, A.; Rossi, A.; Paganini, C. Phenotypic Characterization of Immortalized Chondrocytes from a Desbuquois Dysplasia Type 1 Mouse Model: A Tool for Studying Defects in Glycosaminoglycan Biosynthesis. Int. J. Mol. Sci. 2021, 22, 9304. [Google Scholar] [CrossRef]
- Gramegna Tota, C.; Leone, A.; Rossi, A.; Paganini, C. Analysis of Aggrecan Glycanation by Western Blot in Cell Culture. Methods Mol. Biol. 2023, 2619, 141–151. [Google Scholar] [CrossRef]
- Unger, S.; Ferreira, C.R.; Mortier, G.R.; Ali, H.; Bertola, D.R.; Calder, A.; Cohn, D.H.; Cormier-Daire, V.; Girisha, K.M.; Hall, C.; et al. Nosology of genetic skeletal disorders: 2023 revision. Am. J. Med. Genet. Part A 2023, 191, 1164–1209. [Google Scholar] [CrossRef]
- Trombetta, E.S.; Helenius, A. Glycoprotein reglucosylation and nucleotide sugar utilization in the secretory pathway: Identification of a nucleoside diphosphatase in the endoplasmic reticulum. EMBO J. 1999, 18, 3282–3292. [Google Scholar] [CrossRef]
- Read, R.; Hansen, G.; Kramer, J.; Finch, R.; Li, L.; Vogel, P. Ectonucleoside triphosphate diphosphohydrolase type 5 (Entpd5)-deficient mice develop progressive hepatopathy, hepatocellular tumors, and spermatogenic arrest. Vet. Pathol. 2009, 46, 491–504. [Google Scholar] [CrossRef]
- Gerhardt, J.; Steinbrech, C.; Büchi, O.; Behnke, S.; Bohnert, A.; Fritzsche, F.; Liewen, H.; Stenner, F.; Wild, P.; Hermanns, T.; et al. The androgen-regulated Calcium-Activated Nucleotidase 1 (CANT1) is commonly overexpressed in prostate cancer and is tumor-biologically relevant in vitro. Am. J. Pathol. 2011, 178, 1847–1860. [Google Scholar] [CrossRef]
- Liu, T.; Li, Z.Z.; Sun, L.; Yang, K.; Chen, J.M.; Han, X.Y.; Qi, L.M.; Zhou, X.G.; Wang, P. Upregulated CANT1 is correlated with poor prognosis in hepatocellular carcinoma. BMC Cancer 2023, 23, 1007. [Google Scholar] [CrossRef]
- Qiao, G.; Wang, H.B.; Duan, X.N.; Yan, X.F. The effect and mechanism of miR-607/CANT1 axis in lung squamous carcinoma. Anti-Cancer Drugs 2021, 32, 693–702. [Google Scholar] [CrossRef]
- Cali, T.; Fedrizzi, L.; Ottolini, D.; Gomez-Villafuertes, R.; Mellstrom, B.; Naranjo, J.R.; Carafoli, E.; Brini, M. Ca2+-activated nucleotidase 1, a novel target gene for the transcriptional repressor DREAM (downstream regulatory element antagonist modulator), is involved in protein folding and degradation. J. Biol. Chem. 2012, 287, 18478–18491. [Google Scholar] [CrossRef]
- Yang, M.; Horii, K.; Herr, A.B.; Kirley, T.L. Calcium-dependent dimerization of human soluble calcium activated nucleotidase: Characterization of the dimer interface. J. Biol. Chem. 2006, 281, 28307–28317. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.D.; Guo, L.J.; Feng, Z.Q.; Zhang, D.W.; Zhang, M.T.; Gao, Y.; Chen, C.L.; Zhu, B.F. Cloning, expression and enzyme activity delineation of two novel CANT1 mutations: The disappearance of dimerization may indicate the change of protein conformation and even function. Orphanet J. Rare Dis. 2020, 15, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Machamer, C.E. The Golgi complex in stress and death. Front. Neurosci. 2015, 9, 421. [Google Scholar] [CrossRef]
- Marzin, P.; Cormier-Daire, V. New perspectives on the treatment of skeletal dysplasia. Ther. Adv. Endocrinol. Metab. 2020, 11, 2042018820904016. [Google Scholar] [CrossRef] [PubMed]
- Mullan, L.A.; Mularczyk, E.J.; Kung, L.H.; Forouhan, M.; Wragg, J.M.; Goodacre, R.; Bateman, J.F.; Swanton, E.; Briggs, M.D.; Boot-Handford, R.P. Increased intracellular proteolysis reduces disease severity in an ER stress-associated dwarfism. J. Clin. Investig. 2017, 127, 3861–3865. [Google Scholar] [CrossRef] [PubMed]
- Ornitz, D.M.; Legeai-Mallet, L. Achondroplasia: Development, pathogenesis, and therapy. Dev. Dyn. 2017, 246, 291–309. [Google Scholar] [CrossRef]
- Paganini, C.; Gramegna Tota, C.; Monti, L.; Monti, I.; Maurizi, A.; Capulli, M.; Bourmaud, M.; Teti, A.; Cohen-Solal, M.; Villani, S.; et al. Improvement of the skeletal phenotype in a mouse model of diastrophic dysplasia after postnatal treatment with N-acetylcysteine. Biochem. Pharmacol. 2021, 185, 114452. [Google Scholar] [CrossRef]
- Sabir, A.; Irving, M. Clinical trials in skeletal dysplasia: A paradigm for treating rare diseases. Br. Med. Bull. 2021, 139, 16–35. [Google Scholar] [CrossRef] [PubMed]
- Hecht, J.T.; Veerisetty, A.C.; Patra, D.; Hossain, M.G.; Chiu, F.; Mobed, C.; Gannon, F.H.; Posey, K.L. Early Resveratrol Treatment Mitigates Joint Degeneration and Dampens Pain in a Mouse Model of Pseudoachondroplasia (PSACH). Biomolecules 2023, 13, 1553. [Google Scholar] [CrossRef] [PubMed]
- Heinegård, D. Fell-Muir Lecture: Proteoglycans and more-from molecules to biology. Int. J. Exp. Pathol. 2009, 90, 575–586. [Google Scholar] [CrossRef]
- Poole, A.R.; Rosenberg, L.C.; Reiner, A.; Ionescu, M.; Bogoch, E.; Roughley, P.J. Contents and distributions of the proteoglycans decorin and biglycan in normal and osteoarthritic human articular cartilage. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 1996, 14, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Quentin, E.; Gladen, A.; Rodén, L.; Kresse, H. A genetic defect in the biosynthesis of dermatan sulfate proteoglycan: Galactosyltransferase I deficiency in fibroblasts from a patient with a progeroid syndrome. Proc. Natl. Acad. Sci. USA 1990, 87, 1342–1346. [Google Scholar] [CrossRef]
- Seidler, D.G.; Faiyaz-Ul-Haque, M.; Hansen, U.; Yip, G.W.; Zaidi, S.H.; Teebi, A.S.; Kiesel, L.; Götte, M. Defective glycosylation of decorin and biglycan, altered collagen structure, and abnormal phenotype of the skin fibroblasts of an Ehlers-Danlos syndrome patient carrying the novel Arg270Cys substitution in galactosyltransferase I (beta4GalT-7). J. Mol. Med. 2006, 84, 583–594. [Google Scholar] [CrossRef]
- Chan, W.L.; Steiner, M.; Witkos, T.; Egerer, J.; Busse, B.; Mizumoto, S.; Pestka, J.M.; Zhang, H.; Hausser, I.; Khayal, L.A.; et al. Impaired proteoglycan glycosylation, elevated TGF-beta signaling, and abnormal osteoblast differentiation as the basis for bone fragility in a mouse model for gerodermia osteodysplastica. PLoS Genet. 2018, 14, e1007242. [Google Scholar] [CrossRef]
- Witkos, T.M.; Chan, W.L.; Joensuu, M.; Rhiel, M.; Pallister, E.; Thomas-Oates, J.; Mould, A.P.; Mironov, A.A.; Biot, C.; Guerardel, Y.; et al. GORAB scaffolds COPI at the trans-Golgi for efficient enzyme recycling and correct protein glycosylation. Nat. Commun. 2019, 10, 127. [Google Scholar] [CrossRef]
- Ferreira, C.R.; Xia, Z.J.; Clément, A.; Parry, D.A.; Davids, M.; Taylan, F.; Sharma, P.; Turgeon, C.T.; Blanco-Sánchez, B.; Ng, B.G.; et al. A Recurrent De Novo Heterozygous COG4 Substitution Leads to Saul-Wilson Syndrome, Disrupted Vesicular Trafficking, and Altered Proteoglycan Glycosylation. Am. J. Hum. Genet. 2018, 103, 553–567. [Google Scholar] [CrossRef]
- Forlino, A.; Marini, J.C. Osteogenesis imperfecta. Lancet 2016, 387, 1657–1671. [Google Scholar] [CrossRef] [PubMed]
- Miosge, N.; Flachsbart, K.; Goetz, W.; Schultz, W.; Kresse, H.; Herken, R. Light and electron microscopical immunohistochemical localization of the small proteoglycan core proteins decorin and biglycan in human knee joint cartilage. Histochem. J. 1994, 26, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Kavanagh, E.; Ashhurst, D.E. Development and aging of the articular cartilage of the rabbit knee joint: Distribution of biglycan, decorin, and matrilin-1. J. Histochem. Cytochem. Off. J. Histochem. Soc. 1999, 47, 1603–1615. [Google Scholar] [CrossRef] [PubMed]
- Douglas, T.; Heinemann, S.; Bierbaum, S.; Scharnweber, D.; Worch, H. Fibrillogenesis of collagen types I, II, and III with small leucine-rich proteoglycans decorin and biglycan. Biomacromolecules 2006, 7, 2388–2393. [Google Scholar] [CrossRef]
- Hagg, R.; Bruckner, P.; Hedbom, E. Cartilage fibrils of mammals are biochemically heterogeneous: Differential distribution of decorin and collagen IX. J. Cell Biol. 1998, 142, 285–294. [Google Scholar] [CrossRef]
- Han, B.; Li, Q.; Wang, C.; Patel, P.; Adams, S.M.; Doyran, B.; Nia, H.T.; Oftadeh, R.; Zhou, S.; Li, C.Y.; et al. Decorin Regulates the Aggrecan Network Integrity and Biomechanical Functions of Cartilage Extracellular Matrix. ACS Nano 2019, 13, 11320–11333. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gramegna Tota, C.; Leone, A.; Khan, A.; Forlino, A.; Rossi, A.; Paganini, C. Cant1 Affects Cartilage Proteoglycan Properties: Aggrecan and Decorin Characterization in a Mouse Model of Desbuquois Dysplasia Type 1. Biomolecules 2024, 14, 1064. https://doi.org/10.3390/biom14091064
Gramegna Tota C, Leone A, Khan A, Forlino A, Rossi A, Paganini C. Cant1 Affects Cartilage Proteoglycan Properties: Aggrecan and Decorin Characterization in a Mouse Model of Desbuquois Dysplasia Type 1. Biomolecules. 2024; 14(9):1064. https://doi.org/10.3390/biom14091064
Chicago/Turabian StyleGramegna Tota, Chiara, Alessandra Leone, Asifa Khan, Antonella Forlino, Antonio Rossi, and Chiara Paganini. 2024. "Cant1 Affects Cartilage Proteoglycan Properties: Aggrecan and Decorin Characterization in a Mouse Model of Desbuquois Dysplasia Type 1" Biomolecules 14, no. 9: 1064. https://doi.org/10.3390/biom14091064




