The Inhibitory Effect of Peptide Hydrolysate of Type I Collagen Derived from Pig Skin on Melanogenesis in B16F10 Melanoma Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Extraction of Type I Collagen
2.2. Preparation of Collagen Peptide
2.3. Planar Bounded Point Set Model for Calculating the Length of Collagen Single Molecules
2.4. Characterization of Type I Collagen/Collagen Peptide
2.5. Cell Culture
2.6. Cell Viability Assay
2.7. Melanin Synthesis Assay
2.8. Inhibition of Tyrosinase Activation
2.9. B16F10 Fontana–Masson
2.10. Western Blot Analysis
2.11. Real-Time Quantitative-Polymerase Chain Reaction
2.12. Zebrafish Model
2.13. Identification of the CPH3 Peptide Sequence
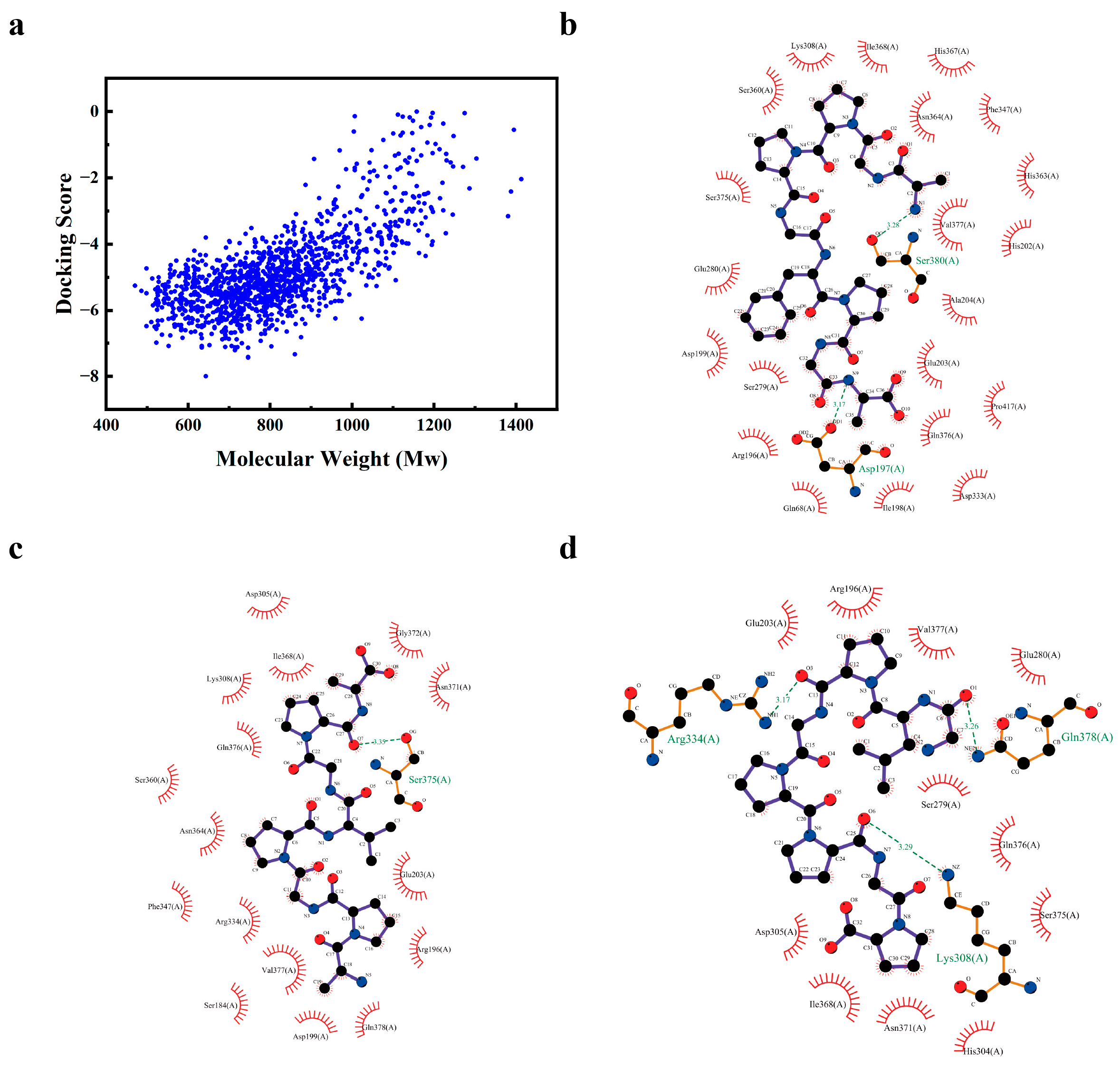
2.14. Molecular Docking
2.15. Statistical Analysis
3. Results
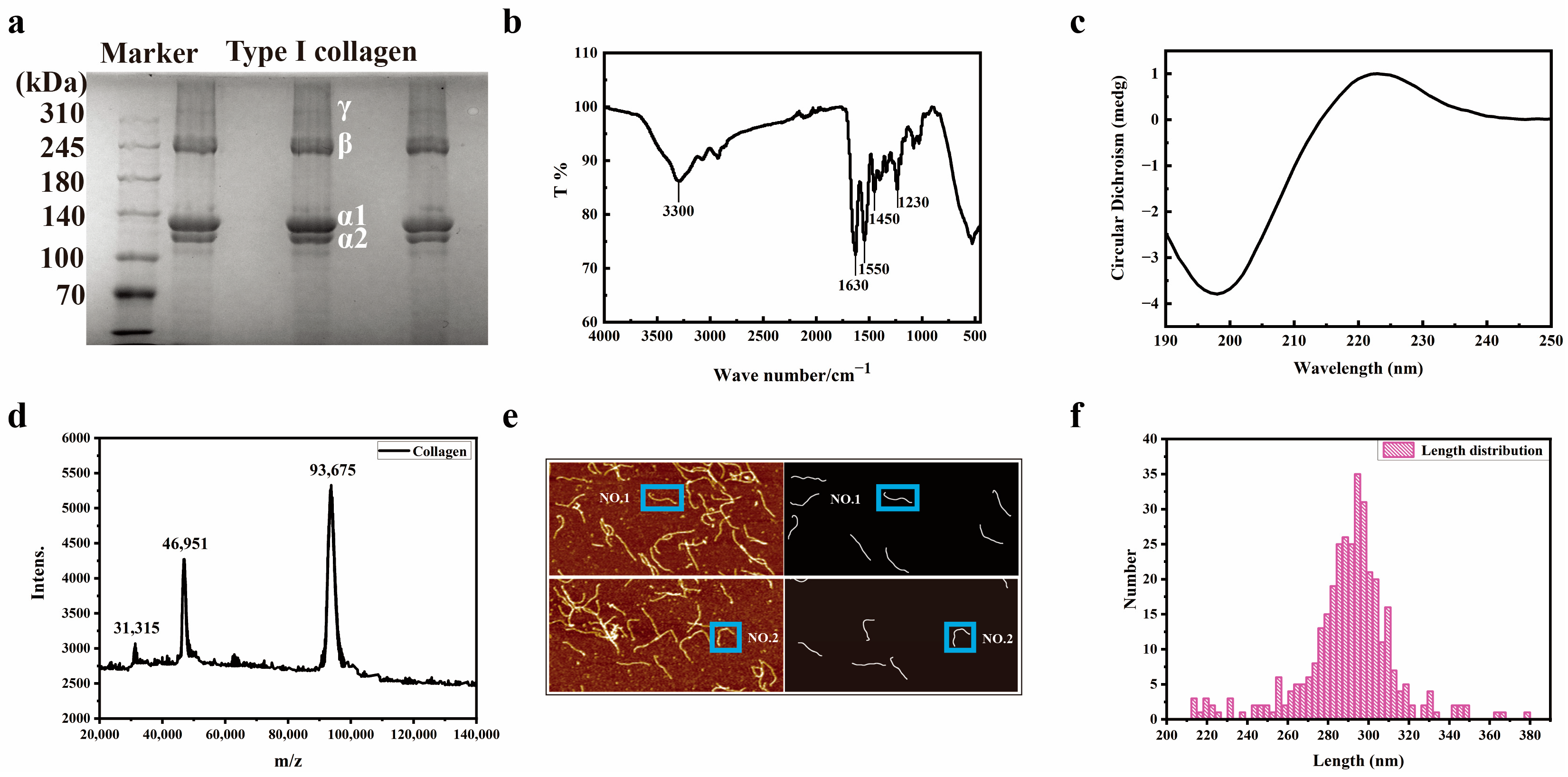
3.1. Structural Characterization of Collagen
3.2. Structural Characterization of Collagen Peptide
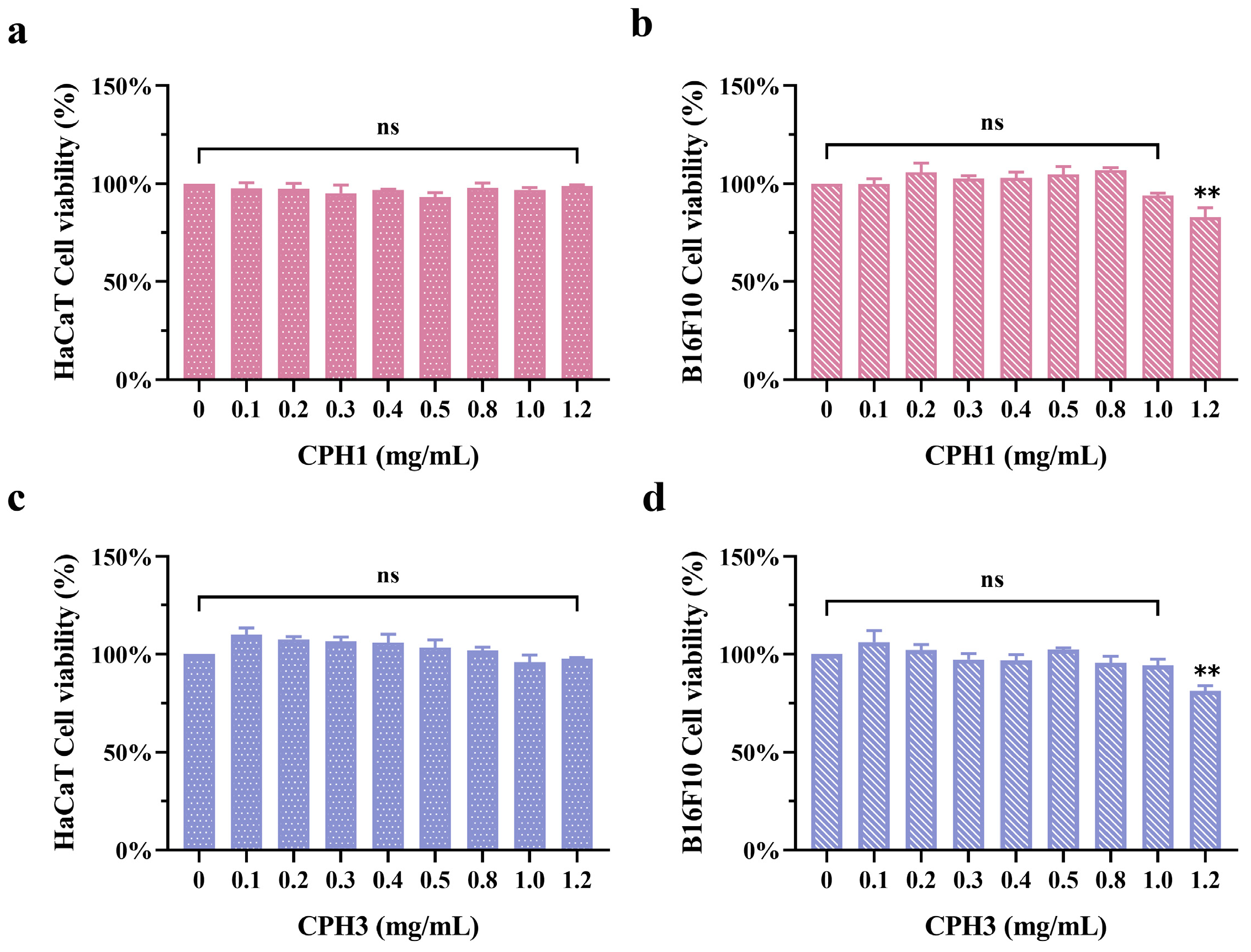
3.3. Effects of CPH1 and CPH3 on B16F10 and HaCaT Cells Viability
3.4. Effects of CPH1 and CPH3 on Cellular Melanogenesis
3.5. Effects of CPH3 on B16F10 Fontana–Masson Staining and Relative Protein Expression
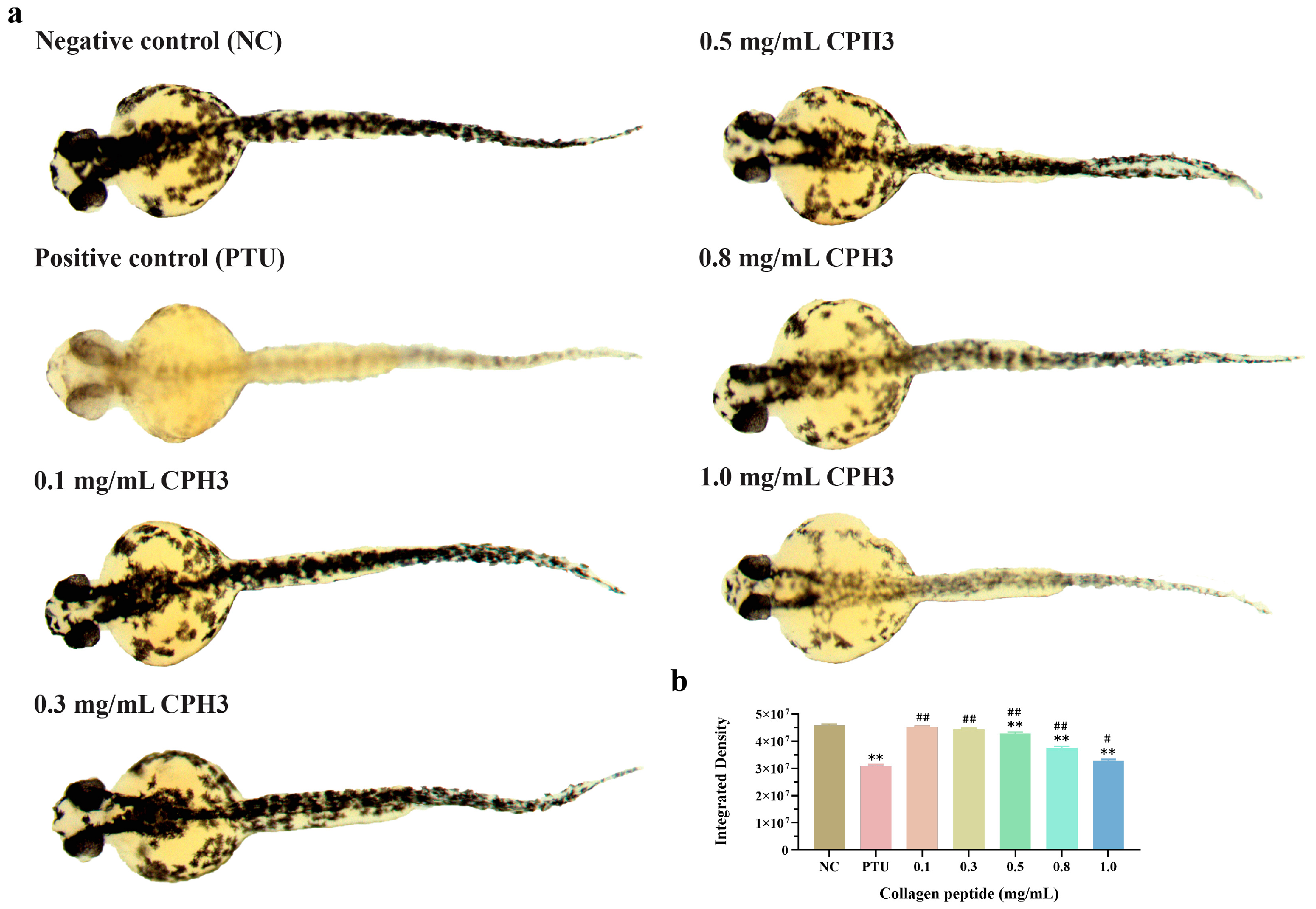
3.6. Effects of CPH3 on Pigmentation Model in Zebrafish
3.7. Screening of CPH3 Tyrosinase Inhibitory Peptides
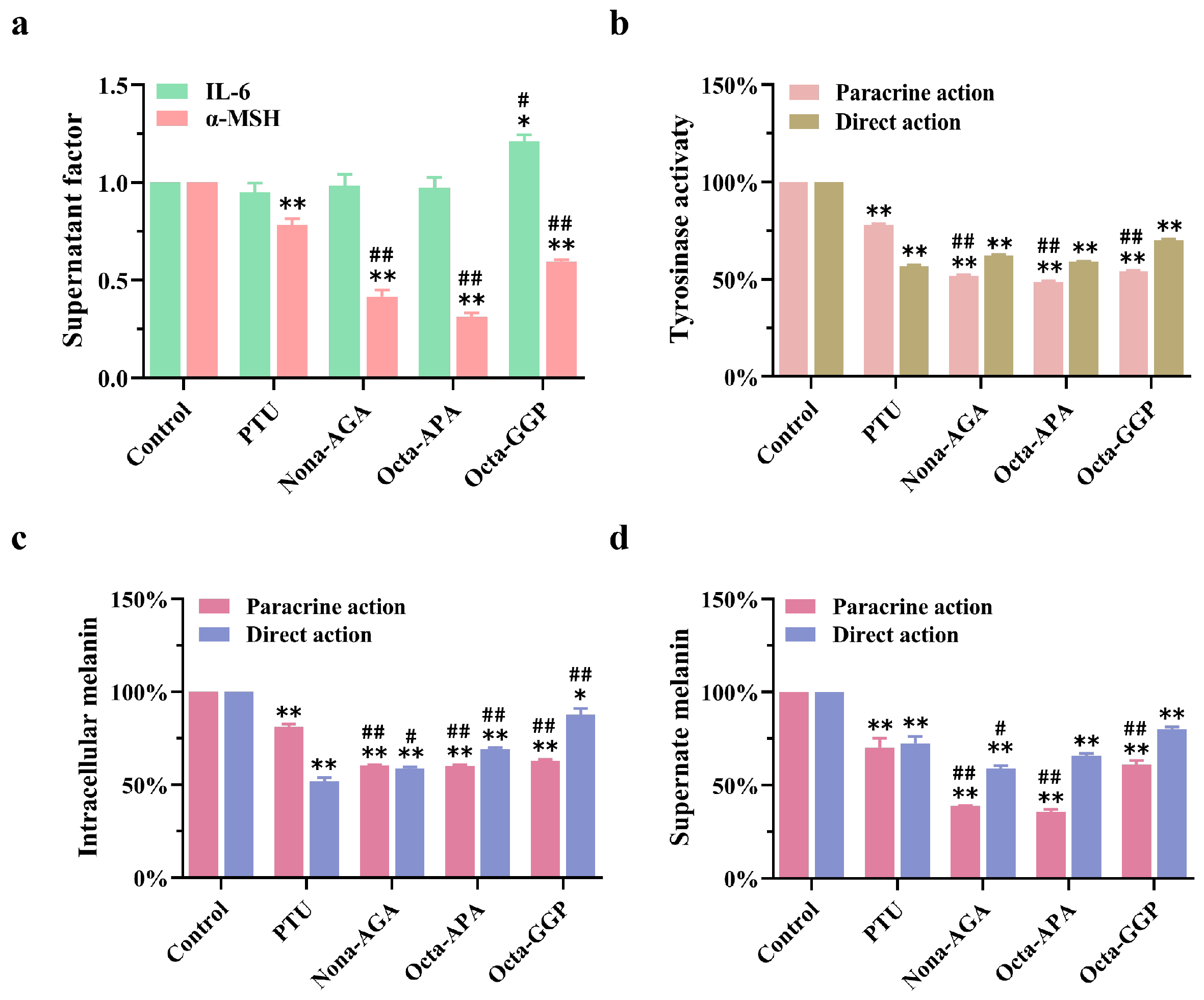
3.8. Inhibitory Effects of Nona-AGA, Octa-APA and Octa-GGP on Melanin
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shoulders, M.D.; Raines, R.T. Collagen structure and stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef]
- Tanrikulu, I.C.; Forticaux, A.; Jin, S.; Raines, R.T. Peptide tessellation yields micrometre-scale collagen triple helices. Nat. Chem. 2016, 8, 1008–1014. [Google Scholar] [CrossRef]
- Oblak, L.; van der Zaag, J.; Higgins-Chen, A.T.; Levine, M.E.; Boks, M.P. A systematic review of biological, social and environmental factors associated with epigenetic clock acceleration. Ageing Res. Rev. 2021, 69, 101348–101357. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, X.; Liu, D. Collagen peptides and the related synthetic peptides: A review on improving skin health. J. Funct. Foods 2021, 86, 104680–104688. [Google Scholar] [CrossRef]
- Hernández-Ruiz, K.L.; López-Cervantes, J.; Sánchez-Machado, D.I.; Campas-Baypoli, O.N.; Quintero-Guerrero, A.A.; de Lourdes Grijalva-Delgado, M.; Chávez-Almanza, A.F. Collagen peptide fractions from tilapia (Oreochromis aureus Steindachner, 1864) scales: Chemical characterization and biological activity. Food Biosci. 2023, 53, 102658–102668. [Google Scholar] [CrossRef]
- Woo, H.; Jeong, G.A.; Choi, H.; Lee, C.J. Characterization of low-molecular-weight collagen from Korean native chicken feet hydrolyzed using alcalase. J. Microbiol. Biotechnol. 2023, 33, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Delikanlı Kıyak, B.; İnan Çınkır, N.; Çelebi, Y.; Durgut Malçok, S.; Çalışkan Koç, G.; Adal, S.; Yüksel, A.N.; Süfer, Ö.; Özkan Karabacak, A.; Ramniwas, S.; et al. Advanced technologies for the collagen extraction from food waste—A review on recent progress. Microchem. J. 2024, 201, 110404–110418. [Google Scholar] [CrossRef]
- Li, J.; Zhai, Y.-N.; Xu, J.-P.; Zhu, X.-Y.; Yang, H.-R.; Che, H.-J.; Liu, C.-K.; Qu, J.-B. An injectable collagen peptide-based hydrogel with desirable antibacterial, self-healing and wound-healing properties based on multiple-dynamic crosslinking. Int. J. Biol. Macromol. 2024, 259, 129006–129018. [Google Scholar] [CrossRef]
- Liu, L.; Wen, H.; Rao, Z.; Zhu, C.; Liu, M.; Min, L.; Fan, L.; Tao, S. Preparation and characterization of chitosan-collagen peptide/oxidized konjac glucomannan hydrogel. Int. J. Biol. Macromol. 2018, 108, 376–382. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, X.; Hu, W.; Han, X.; Fan, L.; Tao, S. Preparation and characterization of carboxymethyl chitosan/collagen peptide/oxidized konjac composite hydrogel. Int. J. Biol. Macromol. 2020, 149, 31–40. [Google Scholar] [CrossRef]
- Echalier, C.; Jebors, S.; Laconde, G.; Brunel, L.; Verdié, P.; Causse, L.; Bethry, A.; Legrand, B.; Van Den Berghe, H.; Garric, X.; et al. So-gel synthesis of collagen-inspired peptide hydrogel. Mater. Today 2017, 20, 59–66. [Google Scholar] [CrossRef]
- Qian, Z.-J.; Jung, W.-K.; Kim, S.-K. Free radical scavenging activity of a novel antioxidative peptide purified from hydrolysate of bullfrog skin, rana catesbeiana shaw. Bioresour. Technol. 2008, 99, 1690–1698. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-J.; Kim, E.-K.; Hwang, J.-W.; Oh, H.-J.; Cheong, S.-H.; Moon, S.-H.; Jeon, B.-T.; Lee, S.M.; Park, P.-J. Purification and characterisation of an antioxidative peptide from enzymatic hydrolysates of duck processing by-products. Food Chem. 2010, 123, 216–220. [Google Scholar] [CrossRef]
- Lee, S.-J.; Kim, Y.-S.; Hwang, J.-W.; Kim, E.-K.; Moon, S.-H.; Jeon, B.-T.; Jeon, Y.-J.; Kim, J.M.; Park, P.-J. Purification and characterization of a novel antioxidative peptide from duck skin by-products that protects liver against oxidative damage. Food Res. Int. 2012, 49, 285–295. [Google Scholar] [CrossRef]
- Zhang, W.; Zheng, Z.; Wang, T.; Yang, X.; Zhao, J.; Zhong, Y.; Peng, X.; Zhou, Y. Succinylated type I collagen regulates ferroptosis to attenuate skin photoaging. ACS Appl. Mater. Interfaces 2024, 16, 56744–56761. [Google Scholar] [CrossRef] [PubMed]
- Zu, X.Y.; Li, M.J.; Xiong, G.Q.; Cai, J.; Liao, T.; Li, H.L. Silver Carp (Hypophthalmichthys molitrix) Scales Collagen Peptides (SCPs): Preparation, Whitening Activity Screening and Characterization. Foods 2023, 12, 1552. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Liu, X.; Zhao, W.; Yu, Z. Identification and molecular mechanism of novel tyrosinase inhibitory peptides from collagen. J. Food Sci. 2022, 87, 2744–2756. [Google Scholar] [CrossRef] [PubMed]
- Kongsompong, S.; E-kobon, T.; Chumnanpuen, P. K-Nearest Neighbor and Random Forest-Based Prediction of Putative Tyrosinase Inhibitory Peptides of Abalone Haliotis diversicolor. Molecules 2021, 26, 3671. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Tan, L.; Zhang, Q.; Chen, F.; Yu, Z. In silico identification and mechanistic evaluation of novel tyrosinase inhibitory peptides derived from coconut proteins. Food Biosci. 2024, 61, 104595–104602. [Google Scholar] [CrossRef]
- Zhang, L.; Zheng, Y.; Cheng, X.; Meng, M.; Luo, Y.; Li, B. The anti-photoaging effect of antioxidant collagen peptides from silver carp (Hypophthalmichthys molitrix) skin is preferable to tea polyphenols and casein peptides. Food Funct. 2017, 8, 1698–1707. [Google Scholar] [CrossRef]
- Iosageanu, A.; Ilie, D.; Craciunescu, O.; Seciu-Grama, A.M.; Oancea, A.; Zarnescu, O.; Moraru, I.; Oancea, F. Effect of Fish Bone Bioactive Peptides on Oxidative, Inflammatory and Pigmentation Processes Triggered by UVB Irradiation in Skin Cells. Molecules 2021, 26, 2691. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Oyama, T.; Yoshimori, A.; Ogawa, H.; Shirai, Y.; Abe, H.; Kamiya, T.; Tanuma, S.-i. The structural differences between mushroom and human tyrosinase cleared by investigating the inhibitory activities of stilbenes. J. Mol. Struct. 2023, 1272, 134180–134186. [Google Scholar] [CrossRef]
- Bonardi, A.; Gratteri, P. Computational studies of tyrosinase inhibitors. Enzymes 2024, 56, 191–229. [Google Scholar] [CrossRef]
- Nagai, T.; Araki, Y.; Suzuki, N. Collagen of the skin of ocellate puffer fish (Takifugu rubripes). Food Chem. 2002, 78, 173–177. [Google Scholar] [CrossRef]
- Unal, M.; Jung, H.; Akkus, O. Novel raman spectroscopic biomarkers indicate that postyield damage denatures bone’s collagen. J. Bone Miner. Res. 2016, 31, 1015–1025. [Google Scholar] [CrossRef] [PubMed]
- Li, L.C.; Yu, Y.; Wu, W.H.; Wang, P.P. Extraction, characterization and osteogenic activity of a type I collagen from starfish (Asterias amurensis). Mar. Drugs 2023, 21, 274. [Google Scholar] [CrossRef] [PubMed]
- Thingnes, J.; Lavelle, T.J.; Hovig, E.; Omholt, S.W. Understanding the melanocyte distribution in human epidermis: An agent-based computational model approach. PLoS ONE 2012, 7, e40377. [Google Scholar] [CrossRef]
- Swope, V.B.; Abdel-Malek, Z.; Kassem, L.M.; Nordlund, J.J. Interleukins 1 alpha and 6 and tumor necrosis factor-alpha are paracrine inhibitors of human melanocyte proliferation and melanogenesis. J. Investig. Dermatol. 1991, 96, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Kotobuki, Y.; Tanemura, A.; Yang, L.; Itoi, S.; Wataya-Kaneda, M.; Murota, H.; Fujimoto, M.; Serada, S.; Naka, T.; Katayama, I. Dysregulation of melanocyte function by Th17-related cytokines: Significance of Th17 cell infiltration in autoimmune vitiligo vulgaris. Pigment Cell Melanoma Res. 2012, 25, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Jeon, B.J.; Hwang, N.H.; Kim, M.S.; Park, S.H.; Dhong, E.S.; Yoon, E.S.; Lee, B.I. Adipose-derived stem cells inhibit epidermal melanocytes through an interleukin-6-mediated mechanism. Plast. Reconstr. Surg. 2014, 134, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Schaper, F.; Rose-John, S. Interleukin-6: Biology, signaling and strategies of blockade. Cytokine Growth Factor Rev. 2015, 26, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, K.; Kauser, S.; Pritchard, L.E.; Warhurst, A.; Oliver, R.L.; Slominski, A.; Wei, E.T.; Thody, A.J.; Tobin, D.J.; White, A. Proopiomelanocortin (POMC), the ACTH/melanocortin precursor, is secreted by human epidermal keratinocytes and melanocytes and stimulates melanogenesis. FASEB J. 2007, 21, 1844–1856. [Google Scholar] [CrossRef] [PubMed]
- Buscà, R.; Ballotti, R. Cyclic AMP a key messenger in the regulation of skin pigmentation. Pigment Cell Melanoma Res. 2000, 13, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Lee, J.; Jung, J.; Kim, G.H.; Yun, C.Y.; Jung, S.H.; Hwang, B.Y.; Hong, J.T.; Han, S.B.; Jung, J.K.; et al. Interruption of p38(MAPK)-MSK1-CREB-MITF-M pathway to prevent hyperpigmentation in the skin. Int. J. Biol. Sci. 2024, 20, 1688–1704. [Google Scholar] [CrossRef] [PubMed]
- León-López, A.; Fuentes-Jiménez, L.; Hernández-Fuentes, A.D.; Campos-Montiel, R.G.; Aguirre-Álvarez, G. Hydrolysed collagen from sheepskins as a source of functional peptides with antioxidant activity. Int. J. Mol. Sci. 2019, 20, 3931. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Jo, Y.-J. Static hydrothermal processing and fractionation for production of a collagen peptide with anti-oxidative and anti-aging properties. Process Biochem. 2019, 83, 176–182. [Google Scholar] [CrossRef]
- Rutschmann, C.; Baumann, S.; Cabalzar, J.; Luther, K.B.; Hennet, T. Recombinant expression of hydroxylated human collagen in Escherichia coli. Appl. Biochem. Microbiol. 2014, 98, 4445–4455. [Google Scholar] [CrossRef]
- Ramshaw, J.A.M.; Werkmeister, J.A.; Glattauer, V. Recent progress with recombinant collagens produced in Escherichia coli. Curr. Opin. Biomed. Eng. 2019, 10, 149–155. [Google Scholar] [CrossRef]
- Piórkowska, K.; Ropka-Molik, K. Pig genomics and genetics. Genes 2021, 12, 1692. [Google Scholar] [CrossRef] [PubMed]
- Lunney, J.K.; Van Goor, A.; Walker, K.E.; Hailstock, T.; Franklin, J.; Dai, C. Importance of the pig as a human biomedical model. Sci. Transl. Med. 2021, 13, eabd5758. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.; Min, S.; Jo, Y.-J. Functionality of porcine skin hydrolysates produced by hydrothermal processing for liposomal delivery system. J. Food Biochem. 2018, 42, e12464–e12474. [Google Scholar] [CrossRef]
- Hong, G.P.; Min, S.G.; Jo, Y.J. Anti-Oxidative and Anti-Aging Activities of Porcine By-Product Collagen Hydrolysates Produced by Commercial Proteases: Effect of Hydrolysis and Ultrafiltration. Molecules 2019, 24, 1104. [Google Scholar] [CrossRef]
- Je, J.-Y.; Qian, Z.-J.; Byun, H.-G.; Kim, S.-K. Purification and characterization of an antioxidant peptide obtained from tuna backbone protein by enzymatic hydrolysis. Process Biochem. 2007, 42, 840–846. [Google Scholar] [CrossRef]
- Chang, C.-Y.; Wu, K.-C.; Chiang, S.-H. Antioxidant properties and protein compositions of porcine haemoglobin hydrolysates. Food Chem. 2007, 100, 1537–1543. [Google Scholar] [CrossRef]
- Chen, H.; Yao, Y.; Xie, T.; Guo, H.; Chen, S.; Zhang, Y.; Hong, Z. Identification of Tyrosinase Inhibitory Peptides from Sea Cucumber (Apostichopus japonicus) Collagen by in silico Methods and Study of their Molecular Mechanism. Curr. Protein Pept. Sci. 2023, 24, 758–766. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Sha, X.; Zhang, L.; Huang, S.; Tu, Z. Effect of Grass Carp Scale Collagen Peptide FTGML on cAMP-PI3K/Akt and MAPK Signaling Pathways in B16F10 Melanoma Cells and Correlation between Anti-Melanin and Antioxidant Properties. Foods 2022, 11, 391. [Google Scholar] [CrossRef]
- Hou, H.; Zhao, X.; Li, B.; Zhang, Z.; Zhuang, Y. Inhibition of melanogenic activity by gelatin and polypeptides from pacific cod skin in b16 melanoma cells. J. Food Biochem. 2011, 35, 1099–1116. [Google Scholar] [CrossRef]
- Yuxiu, Z.; Haisheng, L.; Lei, D.; Jialong, G.; Wenhong, C.; Xiaoming, Q.; Zhongqin, C.; Huina, Z.; Saiyi, Z. A novel tyrosinase inhibitory peptide obtained from Sipunculus nudus gelatin hydrolysate: Preparation, identification, and action mechanism. LWT-Food Sci. Technol. 2024, 202, 116293–116302. [Google Scholar] [CrossRef]
- Hu, Z.Z.; Sha, X.M.; Zhang, L.; Zha, M.J.; Tu, Z.C. From fish scale gelatin to tyrosinase inhibitor: A novel peptides screening approach application. Front. Nutr. 2022, 9, 853442–853454. [Google Scholar] [CrossRef] [PubMed]



| 1 mg/mL | Supernatant Melanin | p Value | Intracellular Melanin | p Value | Tyrosinase Activity | p Value | |
|---|---|---|---|---|---|---|---|
| Paracrine action | CPH1 | 94.34 ± 0.54% | * | 81.84 ± 1.41% | *** | 85.53 ± 1.66 | *** |
| CPH3 | 82.96 ± 3.07% | 65.23 ± 1.30% | 61.50 ± 1.15 | ||||
| Direct action | CPH1 | 106.17 ± 3.56% | ns | 91.94 ± 1.81% | * | 108.60 ± 0.93 | *** |
| CPH3 | 103.65 ± 4.49% | 85.52 ± 0.83% | 92.09 ± 1.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, J.; Xu, D.; Li, T. The Inhibitory Effect of Peptide Hydrolysate of Type I Collagen Derived from Pig Skin on Melanogenesis in B16F10 Melanoma Cells. Biomolecules 2025, 15, 220. https://doi.org/10.3390/biom15020220
Zheng J, Xu D, Li T. The Inhibitory Effect of Peptide Hydrolysate of Type I Collagen Derived from Pig Skin on Melanogenesis in B16F10 Melanoma Cells. Biomolecules. 2025; 15(2):220. https://doi.org/10.3390/biom15020220
Chicago/Turabian StyleZheng, Jialin, Dandan Xu, and Tianduo Li. 2025. "The Inhibitory Effect of Peptide Hydrolysate of Type I Collagen Derived from Pig Skin on Melanogenesis in B16F10 Melanoma Cells" Biomolecules 15, no. 2: 220. https://doi.org/10.3390/biom15020220
APA StyleZheng, J., Xu, D., & Li, T. (2025). The Inhibitory Effect of Peptide Hydrolysate of Type I Collagen Derived from Pig Skin on Melanogenesis in B16F10 Melanoma Cells. Biomolecules, 15(2), 220. https://doi.org/10.3390/biom15020220





