Type IV Pili in Thermophilic Bacteria: Mechanisms and Ecological Implications
Abstract
1. Introduction
2. Pili for Bacterial Surface Motility
3. Function Diversity of T4P
4. T4P Function in T. thermophilus
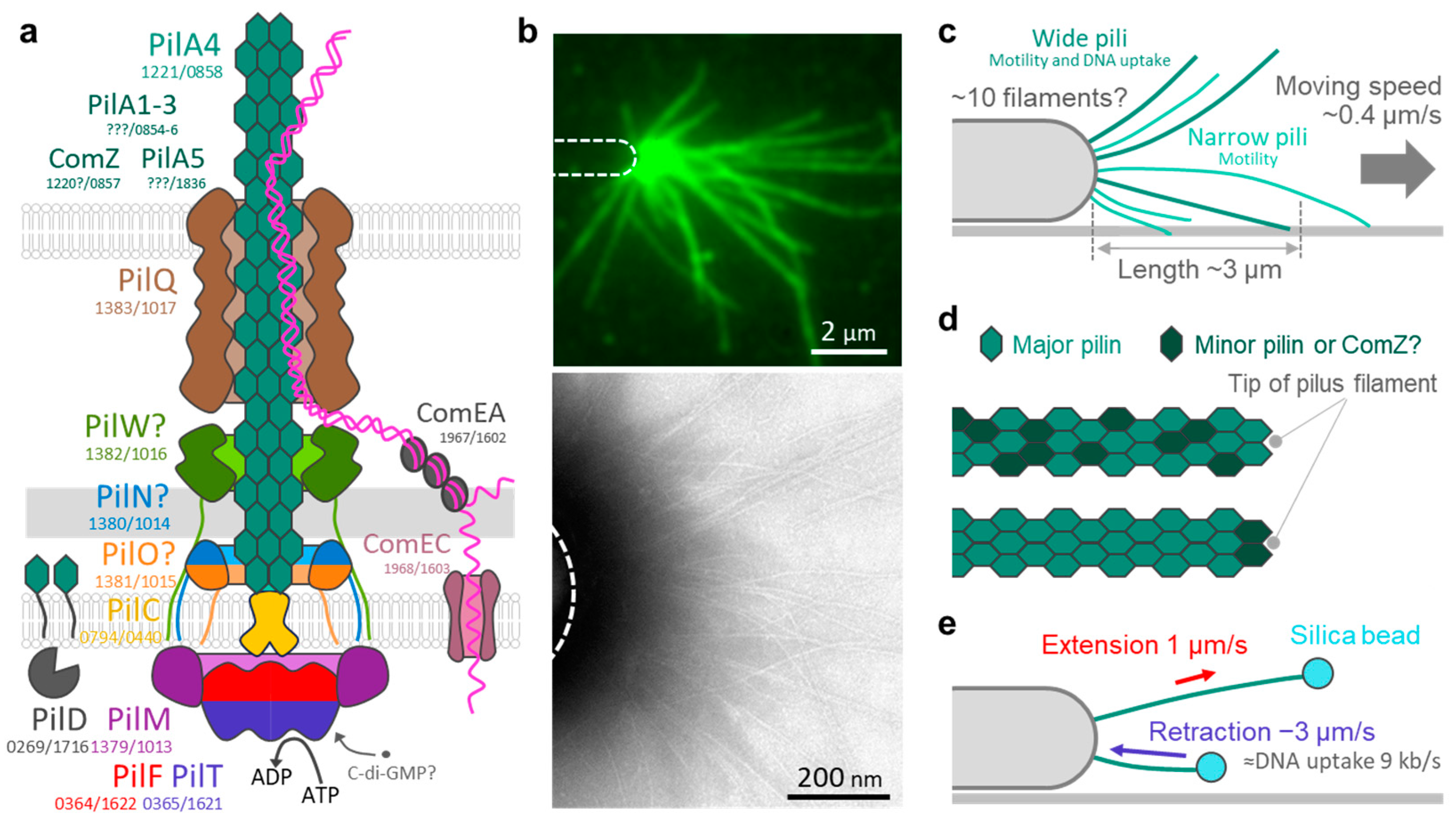
5. T4P Components in T. thermophilus
6. T4P Structure in T. thermophilus
7. T4P Dynamics in T. thermophilus
8. T4P Polarity and Signal Transduction in T. thermophilus
9. T4P and Surface Sensing in T. thermophilus
10. T4P Contributions to T. thermophilus Survival in Natural Habitats
11. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Macnab, R.M.; Koshland, D.E., Jr. The gradient-sensing mechanism in bacterial chemotaxis. Proc. Natl. Acad. Sci. USA 1972, 69, 2509–2512. [Google Scholar] [CrossRef] [PubMed]
- Beeby, M.; Ferreira, J.L.; Tripp, P.; Albers, S.V.; Mitchell, D.R. Propulsive nanomachines: The convergent evolution of archaella, flagella and cilia. FEMS Microbiol. Rev. 2020, 44, 253–304. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Minamino, T. Flagella-driven motility of bacteria. Biomolecules 2019, 9, 279. [Google Scholar] [CrossRef]
- Miyata, M.; Robinson, R.C.; Uyeda, T.Q.P.; Fukumori, Y.; Fukushima, S.I.; Haruta, S.; Homma, M.; Inaba, K.; Ito, M.; Kaito, C.; et al. Tree of motility—A proposed history of motility systems in the tree of life. Genes Cells 2020, 25, 6–21. [Google Scholar] [CrossRef] [PubMed]
- Craig, L.; Forest, K.T.; Maier, B. Type IV pili: Dynamics, biophysics and functional consequences. Nat. Rev. Microbiol. 2019, 17, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Faure, L.M.; Fiche, J.B.; Espinosa, L.; Ducret, A.; Anantharaman, V.; Luciano, J.; Lhospice, S.; Islam, S.T.; Tréguier, J.; Sotes, M.; et al. The mechanism of force transmission at bacterial focal adhesion complexes. Nature 2016, 539, 530–535. [Google Scholar] [CrossRef]
- Nan, B.; McBride, M.J.; Chen, J.; Zusman, D.R.; Oster, G. Bacteria that glide with helical tracks. Curr. Biol. 2014, 24, R169–R173. [Google Scholar] [CrossRef]
- Nakane, D.; Sato, K.; Wada, H.; Mcbride, M.J.; Nakayama, K. Helical flow of surface protein required for bacterial gliding motility. Proc. Natl. Acad. Sci. USA 2013, 110, 11145–11150. [Google Scholar] [CrossRef]
- Toyonaga, T.; Kato, T.; Kawamoto, A.; Miyata, T.; Kawakami, K.; Fujita, J.; Hamaguchi, T.; Namba, K.; Miyata, M. Dimeric assembly of F1-like ATPase for the gliding motility of Mycoplasma. Sci. Adv. 2025, 11, eadr9319. [Google Scholar] [CrossRef]
- Denise, R.; Abby, S.S.; Rocha, E.P.C. Diversification of the type IV filament superfamily into machines for adhesion, protein secretion, DNA uptake, and motility. PLoS Biol. 2019, 17, e3000390. [Google Scholar] [CrossRef]
- Oshima, T.; Imahori, K. Description of Thermus thermophilus (Yoshida and Oshima) comb. nov., a nonsporulating thermophilic bacterium from a Japanese thermal spa. Int. J. Syst. Evol. Microbiol. 1974, 24, 102–112. [Google Scholar] [CrossRef]
- Cava, F.; Hidalgo, A.; Berenguer, J. Thermus thermophilus as biological model. Extremophiles 2009, 13, 213–231. [Google Scholar] [CrossRef] [PubMed]
- Uemura, N.; Chiba, N.; Tamakoshi, M.; Nakane, D. Rapid water flow navigates long-distance migration of thermophilic bacteria. In Proceedings of the BLAST (Bacterial Locomotion and Signal Transduction) XVIII Meeting, Cancun, Mexico, 19–24 January 2025. [Google Scholar]
- Hospenthal, M.; Costa, T.; Waksman, G. A comprehensive guide to pilus biogenesis in Gram-negative bacteria. Nat. Rev. Microbiol. 2017, 15, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Busch, A.; Phan, G.; Waksman, G. Molecular mechanism of bacterial type 1 and P pili assembly. Philos. Trans. R. Soc. A 2015, 373, 20130153. [Google Scholar] [CrossRef] [PubMed]
- Ilangovan, A.; Connery, S.; Waksman, G. Structural biology of the Gram-negative bacterial conjugation systems. Trends Microbiol. 2015, 23, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Shoji, M.; Shibata, S.; Sueyoshi, T.; Naito, M.; Nakayama, K. Biogenesis of Type V pili. Microbiol. Immunol. 2020, 64, 643–656. [Google Scholar] [CrossRef]
- Korotkov, K.; Sandkvist, M.; Hol, W. The type II secretion system: Biogenesis, molecular architecture and mechanism. Nat. Rev. Microbiol. 2012, 10, 336–351. [Google Scholar] [CrossRef] [PubMed]
- Jarrell, K.F.; Albers, S.-V.; Machado, J.N.D.S. A comprehensive history of motility and Archaellation in Archaea. FEMS Microbes 2021, 2, xtab002. [Google Scholar] [CrossRef]
- Ellison, C.K.; Whitfield, G.B.; Brun, Y.V. Type IV Pili: Dynamic bacterial nanomachines. FEMS Microbiol. Rev. 2022, 46, fuab053. [Google Scholar] [CrossRef]
- Albers, S.; Jarrell, K. The Archaellum: An update on the unique archaeal motility structure. Trends Microbiol. 2018, 26, 351–362. [Google Scholar] [CrossRef]
- Korotkov, K.V.; Sandkvist, M. Architecture, function, and substrates of the type II secretion system. EcoSal Plus 2019, 8, 227–244. [Google Scholar] [CrossRef] [PubMed]
- Salzer, R.; Joos, F.; Averhoff, B. Type IV pilus biogenesis, twitching motility, and DNA uptake in Thermus thermophilus: Discrete roles of antagonistic ATPases PilF, PilT1, and PilT2. Appl. Environ. Microbiol. 2014, 80, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Tamakoshi, M.; Murakami, A.; Sugisawa, M.; Tsuneizumi, K.; Takeda, S.; Saheki, T.; Izumi, T.; Akiba, T.; Mitsuoka, K.; Toh, H.; et al. Genomic and proteomic characterization of the large Myoviridae bacteriophage ϕTMA of the extreme thermophile Thermus thermophilus. Bacteriophage 2011, 1, 152–164. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Liao, J.; Lin, G.; Lin, M.; Chang, Y.; Liang, S.; Yang, F.; Khoo, K.; Wu, S. Phosphoproteomic analysis reveals the effects of PilF phosphorylation on type IV pilus and biofilm formation in Thermus thermophilus HB27. Mol. Cell Proteom. 2013, 12, 2701–2713. [Google Scholar] [CrossRef]
- Hijikata, A.; Oshima, T.; Yura, K.; Bessho, Y. ThermusQ: Toward the cell simulation platform for Thermus thermophilus. J. Gen. Appl. Microbiol. 2023, 69, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Bessho, Y. The long journey of the Thermus thermophilus Whole-Cell project. J. Gen. Appl. Microbiol. 2023, 69, 57–58. [Google Scholar] [CrossRef]
- Averhoff, B.; Kirchner, L.; Pfefferle, K.; Yaman, D. Natural transformation in Gram-negative bacteria thriving in extreme environments: From genes and genomes to proteins, structures and regulation. Extremophiles 2021, 25, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Kirchner, L.; Averhoff, B. DNA binding by pilins and their interaction with the inner membrane platform of the DNA transporter in Thermus thermophilus. Biochim. Biophys. Acta Biomembr. 2022, 1864, 183818. [Google Scholar] [CrossRef]
- Averhoff, B. Shuffling genes around in hot environments: The unique DNA transporter of Thermus thermophilus. FEMS Microbiol. Rev. 2009, 33, 611–626. [Google Scholar] [CrossRef]
- Salzer, R.; Kern, T.; Joos, F.; Averhoff, B. The Thermus thermophilus comEA/comEC operon is associated with DNA binding and regulation of the DNA translocator and type IV pili. Environ. Microbiol. 2016, 18, 65–74. [Google Scholar] [CrossRef]
- Johnston, C.; Martin, B.; Fichant, G.; Polard, P.; Claverys, J. Bacterial transformation: Distribution, shared mechanisms and divergent control. Nat. Rev. Microbiol. 2014, 12, 181–196. [Google Scholar] [CrossRef] [PubMed]
- Dubnau, D.; Blokesch, M. Mechanisms of DNA uptake by naturally competent bacteria. Annu. Rev. Genet. 2019, 53, 217–237. [Google Scholar] [CrossRef] [PubMed]
- Taton, A.; Erikson, C.; Yang, Y.; Rubin, B.E.; Rifkin, S.A.; Golden, J.W.; Golden, S.S. The circadian clock and darkness control natural competence in cyanobacteria. Nat. Commun. 2020, 11, 1688. [Google Scholar] [CrossRef] [PubMed]
- Seitz, P.; Blokesch, M. DNA-uptake machinery of naturally competent Vibrio cholerae. Proc. Natl. Acad. Sci. USA 2013, 110, 17987–17992. [Google Scholar] [CrossRef] [PubMed]
- Krüger, N.J.; Stingl, K. Two steps away from novelty—Principles of bacterial DNA uptake. Mol. Microbiol. 2011, 80, 860–867. [Google Scholar] [CrossRef]
- Gold, V.A.M.; Salzer, R.; Averhoff, B.; Kühlbrandt, W. Structure of a type IV pilus machinery in the open and closed state. eLlife 2015, 4, e07380. [Google Scholar] [CrossRef] [PubMed]
- Yaman, D.; Averhoff, B. Functional dissection of structural regions of the Thermus thermophilus competence protein PilW: Implication in secretin complex stability, natural transformation and pilus functions. Biochim. Biophys. Acta Biomembr. 2021, 1863, 183666. [Google Scholar] [CrossRef]
- Karuppiah, V.; Collins, R.F.; Thistlethwaite, A.; Gao, Y.; Derrick, J.P. Structure and assembly of an inner membrane platform for initiation of type IV pilus biogenesis. Proc. Natl. Acad. Sci. USA 2013, 110, E4638–E4647. [Google Scholar] [CrossRef]
- Rumszauer, J.; Schwarzenlander, C.; Averhoff, B. Identification, subcellular localization and functional interactions of PilMNOWQ and PilA4 involved in transformation competency and pilus biogenesis in the thermophilic bacterium Thermus thermophilus HB27. FEBS J. 2006, 273, 3261–3272. [Google Scholar] [CrossRef]
- Neuhaus, A.; Selvaraj, M.; Salzer, R.; Langer, J.D.; Kruse, K.; Kirchner, L.; Sanders, K.; Daum, B.; Averhoff, B.; Gold, V.A.M. Cryo-electron microscopy reveals two distinct type IV pili assembled by the same bacterium. Nat. Commun. 2020, 11, 2231. [Google Scholar] [CrossRef]
- Craig, L.; Volkmann, N.; Arvai, A.S.; Pique, M.E.; Yeager, M.; Egelman, E.H.; Tainer, J.A. Type IV pilus structure by cryo-electron microscopy and crystallography: Implications for pilus assembly and functions. Mol. Cell 2006, 23, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Koch, M.D.; Black, M.E.; Han, E.; Shaevitz, J.W.; Gitai, Z. Pseudomonas aeruginosa distinguishes surfaces by stiffness using retraction of type IV pili. Proc. Natl. Acad. Sci. USA 2022, 119, e2119434119. [Google Scholar] [CrossRef] [PubMed]
- Koch, M.D.; Fei, C.Y.; Wingreen, N.S.; Shaevitz, J.W.; Gitai, Z. Competitive binding of independent extension and retraction motors explains the quantitative dynamics of type IV pili. Proc. Natl. Acad. Sci. USA 2021, 118, e2014926118. [Google Scholar] [CrossRef]
- Ellison, C.K.; Kan, J.; Dillard, R.S.; Kysela, D.T.; Ducret, A.; Berne, C.; Hampton, C.M.; Ke, Z.; Wright, E.R.; Biais, N.; et al. Obstruction of pilus retraction stimulates bacterial surface sensing. Science 2017, 358, 535–538. [Google Scholar] [CrossRef] [PubMed]
- Henne, A.; Brüggemann, H.; Raasch, C.; Wiezer, A.; Hartsch, T.; Liesegang, H.; Johann, A.; Lienard, T.; Gohl, O.; Martinez-Arias, R.; et al. The genome sequence of the extreme thermophile Thermus thermophilus. Nat. Biotechnol. 2004, 22, 547–553. [Google Scholar] [CrossRef]
- Karuppiah, V.; Thistlethwaite, A.; Derrick, J. Structures of type IV pilins from Thermus thermophilus demonstrate similarities with type II secretion system pseudopilins. J. Struct. Biol. 2016, 196, 375–384. [Google Scholar] [CrossRef]
- Friedrich, A.; Rumszauer, J.; Henne, A.; Averhoff, B. Pilin-like proteins in the extremely thermophilic bacterium Thermus thermophilus HB27: Implication in competence for natural transformation and links to type IV pilus biogenesis. Appl. Environ. Microbiol. 2003, 69, 3695–3700. [Google Scholar] [CrossRef] [PubMed]
- Salleh, M.Z.; Karuppiah, V.; Snee, M.; Thistlethwaite, A.; Levy, C.W.; Knight, D.; Derrick, J.P. Structure and properties of a natural competence-associated pilin suggest a unique pilus tip-associated DNA receptor. mBio 2019, 10, e00614–e00619. [Google Scholar] [CrossRef]
- Guo, S.; Chang, Y.; Brun, Y.V.; Howell, P.L.; Burrows, L.L.; Liu, J. PilY1 regulates the dynamic architecture of the type IV pilus machine in Pseudomonas aeruginosa. Nat. Commun. 2024, 15, 9382. [Google Scholar] [CrossRef]
- Chang, Y.W.; Rettberg, L.A.; Treuner-Lange, A.; Iwasa, J.; Sogaard-Andersen, L.; Jensen, G.J. Architecture of the type IVa pilus machine. Science 2016, 351, 6278. [Google Scholar] [CrossRef]
- D’Imprima, E.; Salzer, R.; Bhaskara, R.M.; Sánchez, R.; Rose, I.; Kirchner, L.; Hummer, G.; Kühlbrandt, W.; Vonck, J.; Averhoff, B. Cryo-EM structure of the bifunctional secretin complex of Thermus thermophilus. eLife 2017, 6, e30483. [Google Scholar] [CrossRef] [PubMed]
- Blesa, A.; Sánchez-Costa, M.; Berenguer, J. The PulE ATPase is required for twitching motility and DNA donation during Thermus thermophilus transjugation. Int. Microbiol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, T.; Bardiaux, B.; Francetic, O.; Izadi-Pruneyre, N.; Nilges, M. Structure and function of minor pilins of type IV pili. Med. Microbiol. Immunol. 2020, 209, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Giltner, C.L.; Nguyen, Y.; Burrows, L.L. Type IV pilin proteins: Versatile molecular modules. Microbiol. Mol. Biol. Rev. 2012, 76, 740–772. [Google Scholar] [CrossRef] [PubMed]
- Treuner-Lange, A.; Chang, Y.-W.; Glatter, T.; Herfurth, M.; Lindow, S.; Chreifi, G.; Jensen, G.J.; Søgaard-Andersen, L. PilY1 and minor pilins form a complex priming the type IVa pilus in Myxococcus xanthus. Nat. Commun. 2020, 11, 5054. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Y.; Sugiman-Marangos, S.; Harvey, H.; Bell, S.; Charlton, C.; Junop, M.; Burrows, L. Pseudomonas aeruginosa minor pilins prime type IVa pilus assembly and promote surface display of the PilY1 adhesin. J. Biol. Chem. 2015, 290, 601–611. [Google Scholar] [CrossRef]
- Piepenbrink, K.H. DNA uptake by type IV filaments. Front. Mol. Biosci. 2019, 6, 1. [Google Scholar] [CrossRef]
- Barnier, J.-P.; Meyer, J.; Kolappan, S.; Bouzinba-Ségard, H.; Gesbert, G.; Jamet, A.; Frapy, E.; Schönherr-Hellec, S.; Capel, E.; Virion, Z.; et al. The minor pilin PilV provides a conserved adhesion site throughout the antigenically variable meningococcal type IV pilus. Proc. Natl. Acad. Sci. USA 2021, 118, e2109364118. [Google Scholar] [CrossRef]
- Shen, Y.; Siryaporn, A.; Lecuyer, S.; Gitai, Z.; Stone, A.H. Flow directs surface-attached bacteria to twitch upstream. Biophys. J. 2012, 103, 146–151. [Google Scholar] [CrossRef]
- Nakane, D.; Enomoto, G.; Baehre, H.; Hirose, Y.; Wilde, A.; Nishizaka, T. Thermosynechococcus switches the direction of phototaxis by a c-di-GMP-dependent process with high spatial resolution. eLife 2022, 11, e73405. [Google Scholar] [CrossRef]
- Nakane, D.; Nishizaka, T. Asymmetric distribution of type IV pili triggered by directional light in unicellular cyanobacteria. Proc. Natl. Acad. Sci. USA 2017, 114, 6593–6598. [Google Scholar] [CrossRef] [PubMed]
- Marathe, R.; Meel, C.; Schmidt, N.C.; Dewenter, L.; Kurre, R.; Greune, L.; Schmidt, M.A.; Mueller, M.J.I.; Lipowsky, R.; Maier, B.; et al. Bacterial twitching motility is coordinated by a two-dimensional tug-of-war with directional memory. Nat. Commun. 2014, 5, 3759. [Google Scholar] [CrossRef]
- Schwarzenlander, C.; Averhoff, B. Characterization of DNA transport in the thermophilic bacterium Thermus thermophilus HB27. FEBS J. 2006, 273, 4210–4218. [Google Scholar] [CrossRef] [PubMed]
- Chlebek, J.L.; Denise, R.; Craig, L.; Dalia, A.B. Motor-independent retraction of type IV pili is governed by an inherent property of the pilus filament. Proc. Natl. Acad. Sci. USA 2021, 118, e2102780118. [Google Scholar] [CrossRef] [PubMed]
- Zöllner, R.; Cronenberg, T.; Maier, B. Motor properties of PilT-independent type 4 pilus retraction in Gonococci. J. Bacteriol. 2019, 201, e00778-18. [Google Scholar] [CrossRef]
- Clausen, M.; Jakovljevic, V.; Søgaard-Andersen, L.; Maier, B. High-force generation is a conserved property of type IV pilus systems. J. Bacteriol. 2009, 191, 4633–4638. [Google Scholar] [CrossRef] [PubMed]
- Colin, R.; Ni, B.; Laganenka, L.; Sourjik, V. Multiple functions of flagellar motility and chemotaxis in bacterial physiology. FEMS Microbiol. Rev. 2021, 45, fuab038. [Google Scholar] [CrossRef] [PubMed]
- Wuichet, K.; Zhulin, I.B. Origins and diversification of a complex signal transduction system in prokaryotes. Sci. Signal. 2010, 3, ra50. [Google Scholar] [CrossRef]
- Yarrington, K.D.; Shendruk, T.N.; Limoli, D.H. The type IV pilus chemoreceptor PilJ controls chemotaxis of one bacterial species towards another. PLoS Biol. 2024, 22, e3002488. [Google Scholar] [CrossRef]
- Oliveira, N.M.; Fostera, K.R.; Durham, W.M. Single-cell twitching chemotaxis in developing biofilms. Proc. Natl. Acad. Sci. USA 2016, 113, 6532–6537. [Google Scholar] [CrossRef]
- Han, Y.; Hammerl, J.; Flemming, F.E.; Schuergers, N.; Wilde, A. A cyanobacterial chemotaxis-like system controls phototactic orientation via phosphorylation of two antagonistic response regulators. microLife 2024, 5, uqae012. [Google Scholar] [CrossRef] [PubMed]
- Skerker, J.M.; Berg, H.C. Direct observation of extension and retraction of type IV pili. Proc. Natl. Acad. Sci. USA 2001, 98, 6901–6904. [Google Scholar] [CrossRef] [PubMed]
- Mercier, R.; Bautista, S.; Delannoy, M.; Gibert, M.; Guiseppi, A.; Herrou, J.; Mauriello, E.M.F.; Mignot, T. The polar Ras-like GTPase MglA activates type IV pilus via SgmX to enable twitching motility in Myxococcus xanthus. Proc. Natl. Acad. Sci. USA 2020, 117, 28366–28373. [Google Scholar] [CrossRef] [PubMed]
- Talà, L.; Fineberg, A.; Kukura, P.; Persat, A. Pseudomonas aeruginosa orchestrates twitching motility by sequential control of type IV pili movements. Nat. Microbiol. 2019, 4, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.Z.; Li, Y.X.; Galvani, C.D.; Hao, G.X.; Turner, J.N.; Burr, T.J.; Hoch, H.C. Upstream migration of Xylella fastidiosa via pilus-driven twitching motility. J. Bacteriol. 2005, 187, 5560–5567. [Google Scholar] [CrossRef]
- Gibiansky, M.L.; Conrad, J.C.; Jin, F.; Gordon, V.D.; Motto, D.A.; Mathewson, M.A.; Stopka, W.G.; Zelasko, D.C.; Shrout, J.D.; Wong, G.C. Bacteria use type IV pili to walk upright and detach from surfaces. Science 2010, 330, 197. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zusman, D.R.; Shi, W. Type IV pilus of Myxococcus xanthus is a motility apparatus controlled by the frz chemosensory system. Curr.Biol. 2000, 10, 1143–1146. [Google Scholar] [CrossRef] [PubMed]
- Dinet, C.; Mignot, T. Unorthodox regulation of the MglA Ras-like GTPase controlling polarity in Myxococcus xanthus. FEBS Lett. 2023, 597, 850–864. [Google Scholar] [CrossRef]
- Salzer, R.; Joos, F.; Averhoff, B. Different effects of MglA and MglB on pilus-mediated functions and natural competence in Thermus thermophilus. Extremophiles 2015, 19, 261–267. [Google Scholar] [CrossRef]
- Webster, S.S.; Lee, C.K.; Schmidt, W.C.; Wong, G.C.L.; O’Toole, G.A. Interaction between the type 4 pili machinery and a diguanylate cyclase fine-tune c-di-GMP levels during early biofilm formation. Proc. Natl. Acad. Sci. USA 2021, 118, e2105566118. [Google Scholar] [CrossRef]
- Skotnicka, D.; Petters, T.; Heering, J.; Hoppert, M.; Kaever, V.; Søgaard-Andersen, L. Cyclic di-GMP regulates type IV pilus-dependent motility in Myxococcus xanthus. J. Bacteriol. 2016, 198, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.C.; Chin, K.H.; Tu, Z.L.; He, J.; Jones, C.J.; Sanchez, D.Z.; Yildiz, F.H.; Galperin, M.Y.; Chou, S.H. Nucleotide binding by the widespread high-affinity cyclic di-GMP receptor MshEN domain. Nat. Commun. 2016, 7, 12481. [Google Scholar] [CrossRef] [PubMed]
- Neissner, K.; Keller, H.; Kirchner, L.; Dusterhus, S.; Duchardt-Ferner, E.; Averhoff, B.; Wohnert, J. The structural basis for high-affinity c-di-GMP binding to the GSPII-B domain of the traffic ATPase PilF from Thermus thermophilus. J. Biol. Chem. 2024, 301, 108041. [Google Scholar] [CrossRef]
- Kaczmarczyk, A.; van Vliet, S.; Jakob, R.P.; Teixeira, R.D.; Scheidat, I.; Reinders, A.; Klotz, A.; Maier, T.; Jenal, U. A genetically encoded biosensor to monitor dynamic changes of c-di-GMP with high temporal resolution. Nat. Commun. 2024, 15, 3920. [Google Scholar] [CrossRef] [PubMed]
- Cava, F.; De Pedro, M.A.; Blas-Galindo, E.; Waldo, G.S.; Westblade, L.F.; Berenguer, J. Expression and use of superfolder green fluorescent protein at high temperatures in vivo: A tool to study extreme thermophile biology. Environ. Microbiol. 2008, 10, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Persat, A.; Inclan, Y.F.; Engel, J.N.; Stone, H.A.; Gitai, Z. Type IV pili mechanochemically regulate virulence factors in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 2015, 112, 7563–7568. [Google Scholar] [CrossRef] [PubMed]
- Gerber, E.; Bernard, R.; Castang, S.; Chabot, N.; Coze, F.; Dreux-Zigha, A.; Hauser, E.; Hivin, P.; Joseph, P.; Lazarelli, C.; et al. Deinococcus as new chassis for industrial biotechnology: Biology, physiology and tools. J. Appl. Microbiol. 2015, 119, 1–10. [Google Scholar] [CrossRef]
- Miyazaki, K.; Tomariguchi, N.; Ueno, Y. Complete genome sequences of four halophilic Thermus thermophilus strains isolated from Arima Hot Spring in Japan. Microbiol. Resour. Announc. 2021, 10, e0087421. [Google Scholar] [CrossRef]
- Nelson, K.E.; Clayton, R.A.; Gill, S.R.; Gwinn, M.L.; Dodson, R.J.; Haft, D.H.; Hickey, E.K.; Peterson, J.D.; Nelson, W.C.; Ketchum, K.A.; et al. Evidence for lateral gene transfer between Archaea and Bacteria from genome sequence of Thermotoga maritima. Nature 1999, 399, 323–329. [Google Scholar] [CrossRef]
- Deckert, G.; Warren, P.V.; Gaasterland, T.; Young, W.G.; Lenox, A.L.; Graham, D.E.; Overbeek, R.; Snead, M.A.; Keller, M.; Aujay, M.; et al. The complete genome of the hyperthermophilic bacterium Aquifex aeolicus. Nature 1998, 392, 353–358. [Google Scholar] [CrossRef]
- Sexton, D.L.; Hashimi, A.; Beskrovnaya, P.; Sibanda, L.; Huan, T.; Tocheva, E.I. The cell envelope of Thermotogae suggests a mechanism for outer membrane biogenesis. Proc. Natl. Acad. Sci. USA 2023, 120, e2303275120. [Google Scholar] [CrossRef] [PubMed]
- Fujino, Y.; Kawatsu, R.; Inagaki, F.; Umeda, A.; Yokoyama, T.; Okaue, Y.; Iwai, S.; Ogata, S.; Ohshima, T.; Doi, K. Thermus thermophilus TMY isolated from silica scale taken from a geothermal power plant. J. Appl. Microbiol. 2008, 104, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Iwai, S.; Doi, K.; Fujino, Y.; Nakazono, T.; Fukuda, K.; Motomura, Y.; Ogata, S. Silica deposition and phenotypic changes to Thermus thermophilus cultivated in the presence of supersaturated silicia. ISME J. 2010, 4, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, F.; Yokoyama, T.; Doi, K.; Izawa, E.; Ogata, S. Bio-deposition of amorphous silica by an extremely thermophilic bacterium, Thermus spp. Biosci. Biotechnol. Biochem. 1998, 62, 1271–1272. [Google Scholar] [CrossRef] [PubMed]
- Chevallereau, A.; Pons, B.; van Houte, S.; Westra, E. Interactions between bacterial and phage communities in natural environments. Nat. Rev. Microbiol. 2022, 20, 49–62. [Google Scholar] [CrossRef]
- Mushegian, A.R. Are there 1031 virus particles on earth, or more, or fewer? J. Bacteriol. 2020, 202, 9. [Google Scholar] [CrossRef] [PubMed]
- Thongchol, J.; Yu, Z.; Harb, L.; Lin, Y.; Koch, M.; Theodore, M.; Narsaria, U.; Shaevitz, J.; Gitai, Z.; Wu, Y.; et al. Removal of Pseudomonas type IV pili by a small RNA virus. Science 2024, 384, eadl0635. [Google Scholar] [CrossRef]
- Bradley, D.E. Shortening of Pseudomonas aeruginosa pili after RNA-phage adsorption. Microbiology 1972, 72, 303–319. [Google Scholar] [CrossRef] [PubMed]
- Tamakoshi, M.; Hijikata, A.; Yura, K.; Oshima, K.; Toh, H.; Mitsuoka, K.; Oshima, T.; Bessho, Y. Isolation and genomic analysis of a type IV pili-independent Thermus thermophilus phage, φMN1 from a Japanese hot spring. J. Gen. Appl. Microbiol. 2023, 69, 117–124. [Google Scholar] [CrossRef]
- Naryshkina, T.; Liu, J.; Florens, L.; Swanson, S.K.; Pavlov, A.R.; Pavlova, N.V.; Inman, R.; Minakhin, L.; Kozyavkin, S.A.; Washburn, M.; et al. Thermus thermophilus bacteriophage phiYS40 genome and proteomic characterization of virions. J. Mol. Biol. 2006, 364, 667–677. [Google Scholar] [CrossRef]
- Rusconi, R.; Stocker, R. Microbes in flow. Curr. Opin. Microbiol. 2015, 25, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Schwendel, A.C.; Death, R.G.; Fuller, I.C. The assessment of shear stress and bed stability in stream ecology. Freshw. Biol. 2010, 55, 261–281. [Google Scholar] [CrossRef]
- Ohmura, T.; Nishigami, Y.; Taniguchi, A.; Nonaka, S.; Ishikawa, T.; Ichikawa, M. Near-wall rheotaxis of the ciliate Tetrahymena induced by the kinesthetic sensing of cilia. Sci. Adv. 2021, 7, eabi5878. [Google Scholar] [CrossRef] [PubMed]
- Miki, K.; Clapham, D.E. Rheotaxis guides mammalian sperm. Curr. Biol. 2013, 23, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Marcos; Fu, H.C.; Powers, T.R.; Stocker, R. Bacterial rheotaxis. Proc. Natl. Acad. Sci. USA 2012, 109, 4780–4785. [Google Scholar] [CrossRef]
- Kaya, T.; Koser, H. Direct upstream motility in Escherichia coli. Biophys. J. 2012, 102, 1514–1523. [Google Scholar] [CrossRef]
- Nakane, D. Rheotaxis in Mycoplasma gliding. Microbiol. Immunol. 2023, 67, 389–395. [Google Scholar] [CrossRef]
- Nakane, D.; Kabata, Y.; Nishizaka, T. Cell shape controls rheotaxis in small parasitic bacteria. PLoS Pathog. 2022, 18, e1010648. [Google Scholar] [CrossRef]
- Rosengarten, R.; Klein-Struckmeier, A.; Kirchhoff, H. Rheotactic behavior of a gliding mycoplasma. J. Bacteriol. 1988, 170, 989–990. [Google Scholar] [CrossRef]
- Yao, W.-W.; Chen, Y.; Zhong, Y.; Zhang, W.; Fan, H. Habitat models for assessing river ecosystems and their application to the development of river restoration strategies. J. Freshw. Ecol. 2017, 32, 601–617. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uemura, N.A.; Nakane, D. Type IV Pili in Thermophilic Bacteria: Mechanisms and Ecological Implications. Biomolecules 2025, 15, 459. https://doi.org/10.3390/biom15040459
Uemura NA, Nakane D. Type IV Pili in Thermophilic Bacteria: Mechanisms and Ecological Implications. Biomolecules. 2025; 15(4):459. https://doi.org/10.3390/biom15040459
Chicago/Turabian StyleUemura, Naoki A., and Daisuke Nakane. 2025. "Type IV Pili in Thermophilic Bacteria: Mechanisms and Ecological Implications" Biomolecules 15, no. 4: 459. https://doi.org/10.3390/biom15040459
APA StyleUemura, N. A., & Nakane, D. (2025). Type IV Pili in Thermophilic Bacteria: Mechanisms and Ecological Implications. Biomolecules, 15(4), 459. https://doi.org/10.3390/biom15040459






