Mechanisms of Hormonal, Genetic, and Temperature Regulation of Germ Cell Proliferation, Differentiation, and Death During Spermatogenesis
Abstract
1. Introduction
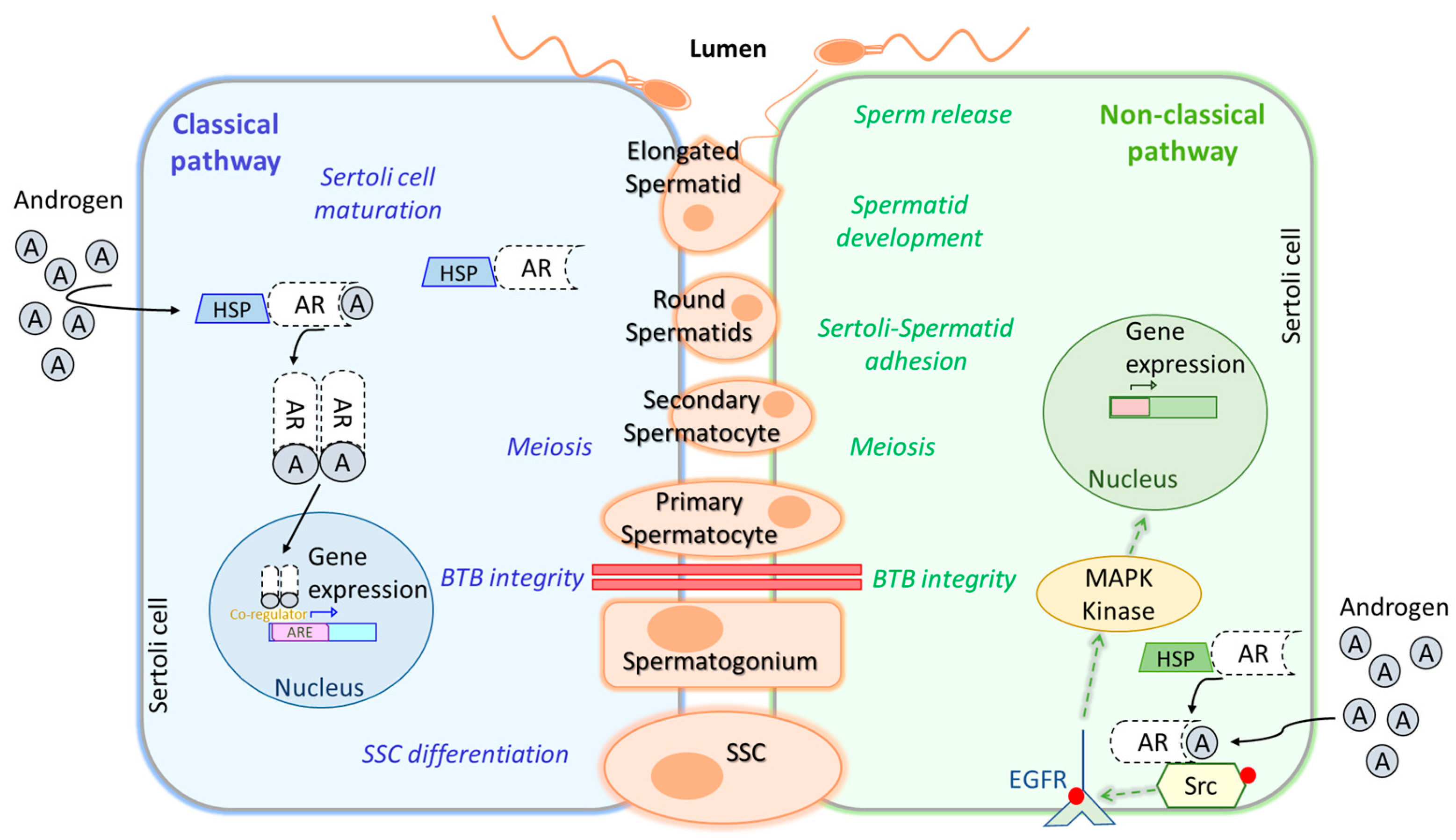
2. Hormonal Regulation in Spermatogenesis
3. Genetic Regulation in Spermatogenesis
4. Regulation by Temperature
5. Cellular Mechanisms of Proliferation and Differentiation of Germ Cells
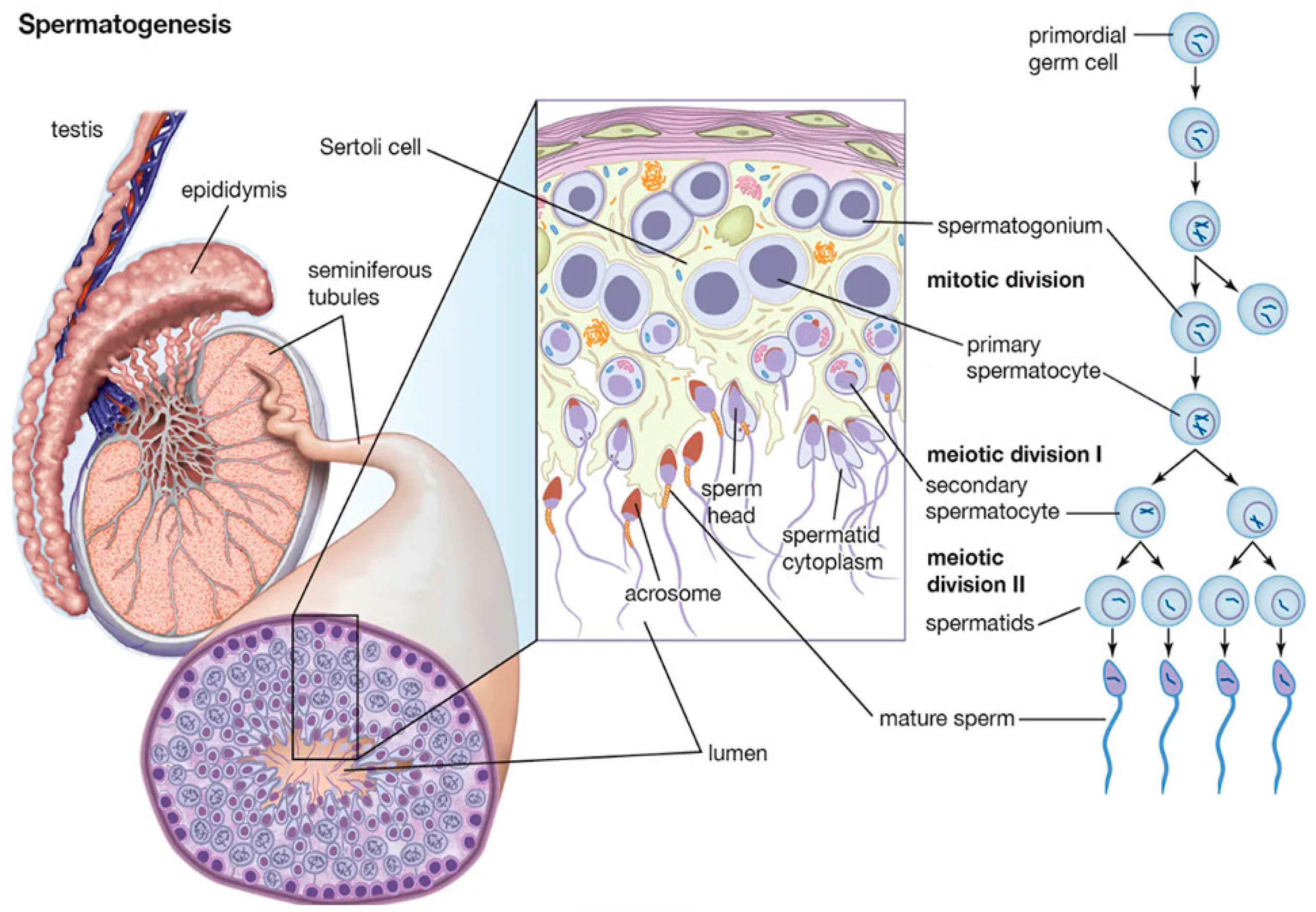
5.1. Phases of Spermatogenesis
5.1.1. Mitotic Phase
5.1.2. Meiosis
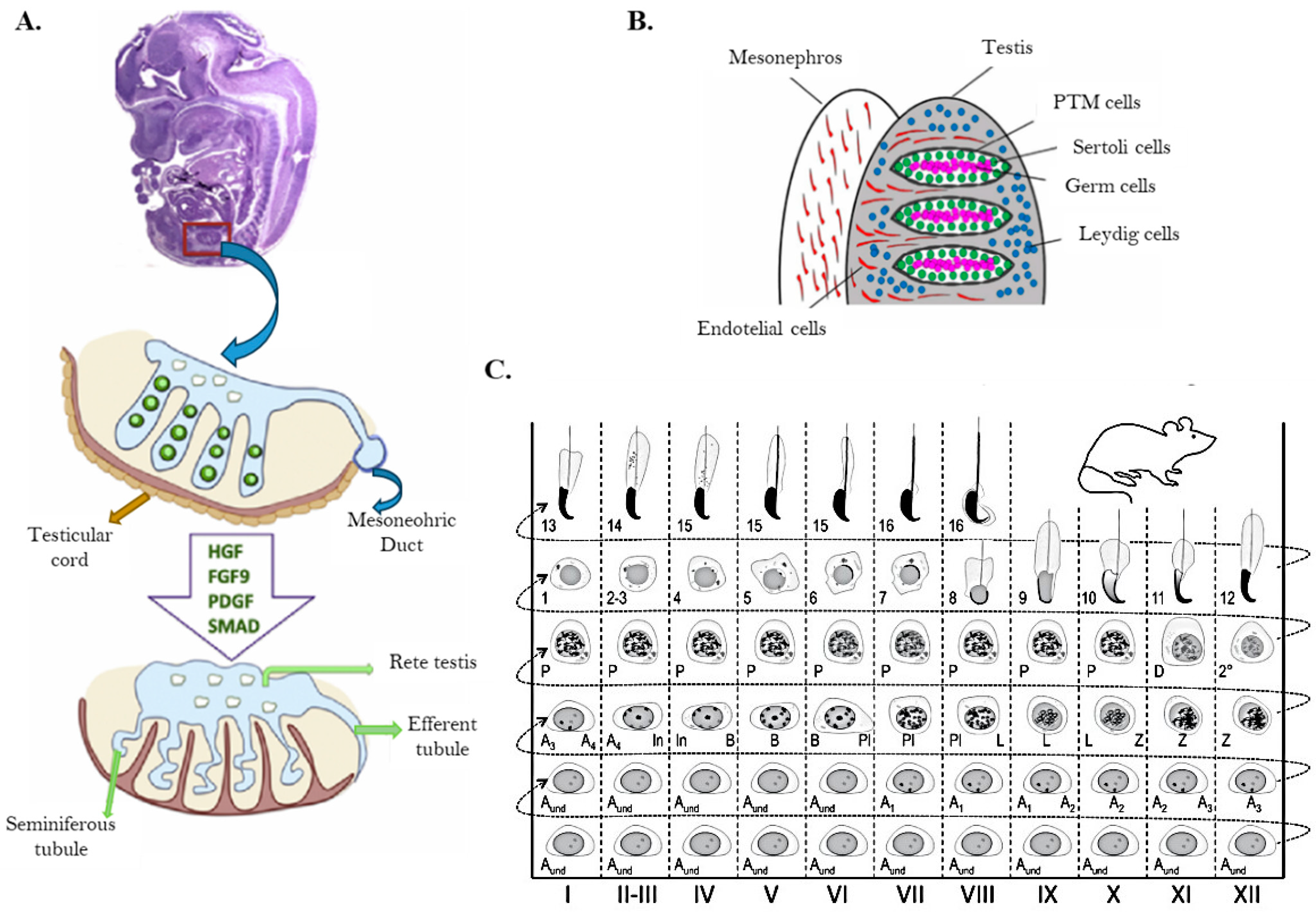
5.1.3. Testis Cells and Seminiferous Tubule Structure
5.1.4. Spermiogenesis
5.1.5. Mechanisms for Maintaining DNA Integrity in Spermatozoa
5.1.6. Importance of Apoptosis in Spermatogenesis
6. New Discoveries and Future Perspectives
6.1. Advancements in the Study of Male Hormonal Reproductive Disorders
6.2. The Testicular Renin–Angiotensin System (RAS)
6.3. Insights in Male Contraception
6.4. Advancements in the Gene Regulation of Spermatogenesis
6.5. Advancements in In Vitro Spermatogenesis
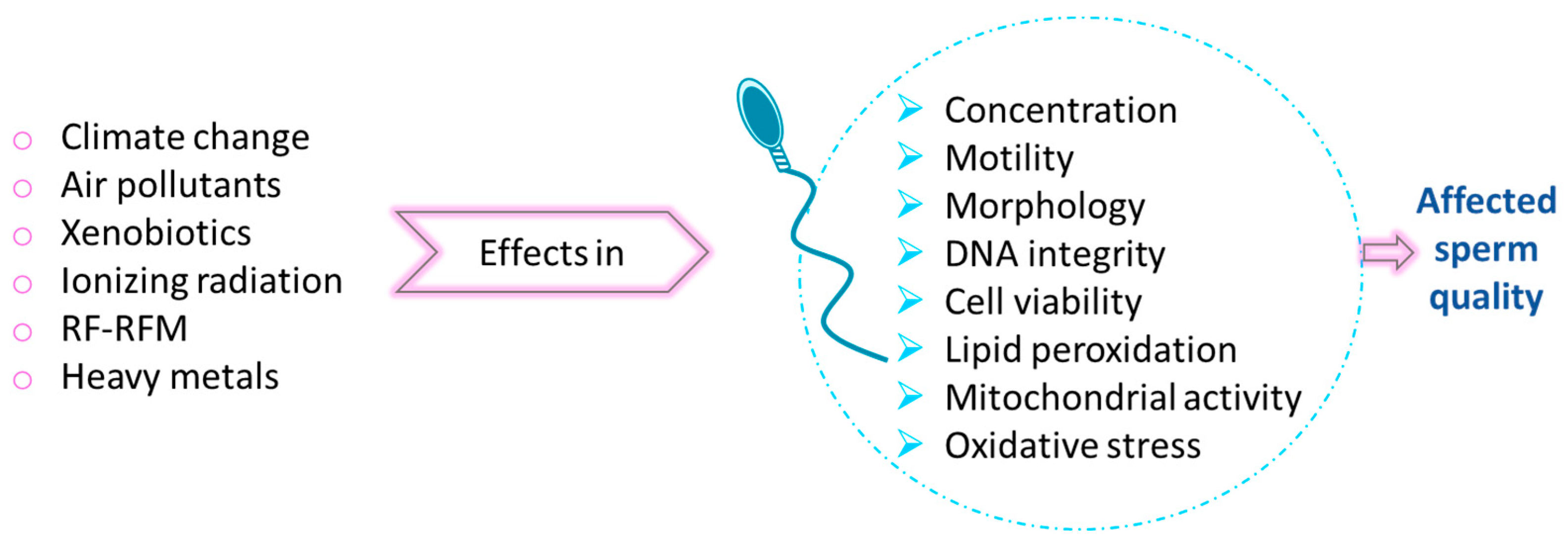
6.6. Environmental Threats for Male Fertility
6.7. The Gut Microbiota–Testis Axis
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Suede, S.H.; Malik, A.; Sapra, A. Histology, Spermatogenesis. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Oatley, J.M.; Brinster, R.L. The germline stem cell niche unit in mammalian testes. Physiol. Rev. 2012, 92, 577–595. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, H.; L’hernault, S.W. Spermatogenesis. Curr. Biol. 2017, 27, R988–R994. [Google Scholar] [CrossRef]
- Zakariah, M.; Molele, R.; Mahdy, M.A.; Ibrahim, M.I.; McGaw, L. Regulation of spermatogenic cell apoptosis by the pro-apoptotic proteins in the testicular tissues of mammalian and avian species. Anim. Reprod. Sci. 2022, 247, 107158. [Google Scholar] [CrossRef] [PubMed]
- Encyclopaedia Britannica. Spermatogenesis. Encyclopaedia Britannica Inc. 2024. Available online: www.britannica.com (accessed on 3 December 2024).
- Oduwole, O.O.; Huhtaniemi, I.T.; Misrahi, M. The roles of luteinizing hormone, follicle-stimulating hormone and testosterone in spermatogenesis and folliculogenesis revisited. Int. J. Mol. Sci. 2021, 22, 12735. [Google Scholar] [CrossRef]
- Sower, S.A. Landmark discoveries in elucidating the origins of the hypothalamic-pituitary system from the perspective of a basal vertebrate, sea lamprey. Gen. Comp. Endocrinol. 2018, 264, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Sower, S.A.; Freamat, M.; Kavanaugh, S.I. The origins of the vertebrate hypothalamic–pituitary–gonadal (HPG) and hypothalamic–pituitary–thyroid (HPT) endocrine systems: New insights from lampreys. Gen. Comp. Endocrinol. 2009, 161, 20–29. [Google Scholar] [CrossRef]
- Servier Medical Art. Graphical Material. Licensed Under CC BY 4.0. Available online: https://smart.servier.com/ (accessed on 5 February 2025).
- Ulloa-Aguirre, A.; Reiter, E.; Crepieux, P. FSH receptor signaling: Complexity of interactions and signal diversity. Endocrinology 2018, 159, 3020–3035. [Google Scholar] [CrossRef]
- Oduwole, O.O.; Peltoketo, H.; Huhtaniemi, I.T. Role of follicle-stimulating hormone in spermatogenesis. Front. Endocrinol. 2018, 9, 763. [Google Scholar] [CrossRef]
- Ramaswamy, S.; Weinbauer, G.F. Endocrine control of spermatogenesis: Role of FSH and LH/testosterone. Spermatogenesis 2014, 4, e996025. [Google Scholar] [CrossRef]
- Nieschlag, E.; Simoni, M.; Gromoll, J.; Weinbauer, G.F. Role of FSH in the regulation of spermatogenesis: Clinical aspects. Clin. Endocrinol. 1999, 51, 139–146. [Google Scholar] [CrossRef]
- Abel, M.H.; Wootton, A.N.; Wilkins, V.; Huhtaniemi, I.; Knight, P.G.; Charlton, H.M. The Effect of a Null Mutation in the Follicle-Stimulating Hormone Receptor Gene on Mouse Reproduction. Endocrinology 2000, 141, 1795–1803. [Google Scholar] [CrossRef] [PubMed]
- Dierich, A.; Ram Sairam, M.; Monaco, L.; Fimia, G.M.; Gansmuller, A.; Lemeur, M.; Sassone-Corsi, P. Impairing Follicle-Stimulating Hormone (FSH) Signaling In Vivo: Targeted Disruption of the FSH Receptor Leads to Aberrant Gametogenesis and Hormonal Imbalance. Proc. Natl. Acad. Sci. USA 1998, 95, 13612–13617. [Google Scholar]
- Schaison, G.; Young, J.; Pholsena, M.; Nahoul, K.; Couzinet, B. Failure of combined follicle-stimulating hormone-testosterone administration to initiate and/or maintain spermatogenesis in men with hypogonadotropic hypogonadism. J. Clin. Endocrinol. Metab. 1993, 77, 1545–1549. [Google Scholar]
- Li, L.; Lin, W.; Wang, Z.; Huang, R.; Xia, H.; Li, Z.; Deng, J.; Ye, T.; Huang, Y.; Yang, Y. Hormone Regulation in Testicular Development and Function. Int. J. Mol. Sci. 2024, 25, 5805. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-M.; Li, Z.-F.; Yang, W.-X. What Does Androgen Receptor Signaling Pathway in Sertoli Cells During Normal Spermatogenesis Tell Us? Front. Endocrinol. 2022, 13, 838858. [Google Scholar] [CrossRef]
- Zhou, R.; Wu, J.; Liu, B.; Jiang, Y.; Chen, W.; Li, J.; He, Q.; He, Z. The roles and mechanisms of Leydig cells and myoid cells in regulating spermatogenesis. Cell. Mol. Life Sci. 2019, 76, 2681–2695. [Google Scholar] [CrossRef]
- Wang, J.-M.; Li, Z.-F.; Yang, W.-X.; Tan, F.-Q. Follicle-stimulating hormone signaling in Sertoli cells: A licence to the early stages of spermatogenesis. Reprod. Biol. Endocrinol. 2022, 20, 97. [Google Scholar] [CrossRef]
- Lin, P.H.; Kuo, T.H.; Chen, C.C.; Jian, C.Y.; Chen, C.W.; Wang, K.L.; Kuo, Y.C.; Shen, H.Y.; Hsia, S.M.; Wang, P.S.; et al. Downregulation of testosterone production through luteinizing hormone receptor regulation in male rats exposed to 17α-ethynylestradiol. Sci. Rep. 2020, 10, 1576. [Google Scholar] [CrossRef]
- Kumar, A.; Raut, S.; Balasinor, N.H. Endocrine regulation of sperm release. Reprod. Fertil. Dev. 2018, 30, 1595–1603. [Google Scholar] [CrossRef]
- O’Donnell, L.; Pratis, K.; Wagenfeld, A.; Gottwald, U.; Müller, J.; Leder, G.; McLachlan, R.I.; Stanton, P.G. Transcriptional profiling of the hormone-responsive stages of spermatogenesis reveals cell-, stage-, and hormone-specific events. Endocrinology 2009, 150, 5074–5084. [Google Scholar] [CrossRef]
- Bole-Feysot, C.; Goffin, V.; Edery, M.; Binart, N.; Kelly, P.A. Prolactin (PRL) and Its Receptor: Actions, Signal Transduction Pathways and Phenotypes Observed in PRL Receptor Knockout Mice. Endocr. Rev. 1998, 19, 225–268. [Google Scholar] [PubMed]
- Raut, S.; Deshpande, S.; Balasinor, N.H. Unveiling the Role of Prolactin and its Receptor in Male Reproduction. Horm. Metab. Res. 2019, 51, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Binart, N.; Melaine, N.; Pineau, C.; Kercret, H.; Touzalin, A.M.; Imbert-Bolloré, P.; Kelly, P.A.; Jégou, B. Male reproductive function is not affected in prolactin receptor-deficient mice. Endocrinology 2003, 144, 3779–3782. [Google Scholar] [CrossRef]
- Steger, R.W.; Chandrashekar, V.; Zhao, W.; Bartke, A.; Horseman, N.D. Neuroendocrine and Reproductive Functions in Male Mice with Targeted Disruption of the Prolactin Gene. Endocrinology 1998, 139, 3691–3695. [Google Scholar] [PubMed]
- Krege, J.H.; Hodgin, J.B.; Couse, J.F.; Enmark, E.; Warner, M.; Mahler, J.F.; Sar, M.; Korach, K.S.; Gustafsson, J.-Å.; Smithies, O. Generation and reproductive phenotypes of mice lacking estrogen receptor β. Proc. Natl. Acad. Sci. USA 1998, 95, 15677–15682. [Google Scholar]
- Fisher, C.R.; Graves, K.H.; Parlow, A.F.; Simpson, E.R. Characterization of mice deficient in aromatase (ArKO) because of targeted disruption of the cyp19 gene. Proc. Natl. Acad. Sci. USA 1998, 95, 6965–6970. [Google Scholar] [CrossRef]
- Lui, W.-Y.; Cheng, C.Y. Transcription Regulation in Spermatogenesis. Adv. Exp. Med. Biol. 2009, 636, 115–136. [Google Scholar] [CrossRef]
- Neto, F.T.L.; Bach, P.V.; Najari, B.B.; Li, P.S.; Goldstein, M. Spermatogenesis in humans and its affecting factors. Semin. Cell Dev. Biol. 2016, 59, 10–26. [Google Scholar] [CrossRef]
- MacLean, J.A.; Wilkinson, M.F. Gene Regulation in Spermatogenesis. Curr. Top. Dev. Biol. 2005, 71, 131–197. [Google Scholar] [CrossRef]
- Sharma, M.; Srivastava, A.; Fairfield, H.E.; Bergstrom, D.; Flynn, W.F.; Braun, R.E. Identification of slow-cycling germline stem cells and their regulation by PLZF. bioRxiv 2018. [Google Scholar] [CrossRef]
- Song, W.; Shi, X.; Xia, Q.; Yuan, M.; Liu, J.; Hao, K.; Qian, Y.; Zhao, X.; Zou, K. PLZF suppresses differentiation of mouse spermatogonial progenitor cells via binding of differentiation associated genes. J. Cell. Physiol. 2020, 235, 3033–3042. [Google Scholar] [CrossRef] [PubMed]
- Costoya, J.A.; Hobbs, R.M.; Barna, M.; Cattoretti, G.; Manova, K.; Sukhwani, M.; Orwig, K.E.; Wolgemuth, D.J.; Pandolfi, P.P. Essential role of Plzf in maintenance of spermatogonial stem cells. Nat. Genet. 2004, 36, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Ahn, H.W.; Chu, T.; Bowden, W.; Gassei, K.; Orwig, K.; Rajkovic, A. SOHLH1 and SOHLH2 coordinate spermatogonial differentiation. Dev. Biol. 2012, 361, 301–312. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Zhang, X.; Sun, J.; Hao, J. BMP4/Smad signaling pathway induces the differentiation of mouse spermatogonial stem cells via upregulation of Sohlh2. Anat. Rec. 2014, 297, 749–757. [Google Scholar] [CrossRef]
- Endo, T.; Romer, K.A.; Anderson, E.L.; Baltus, A.E.; de Rooij, D.G.; Page, D.C. Periodic retinoic acid-STRA8 signaling intersects with periodic germ-cell competencies to regulate spermatogenesis. Proc. Natl. Acad. Sci. USA 2015, 112, E2347–E2356. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Grow, E.J.; Mlcochova, H.; Maher, G.J.; Lindskog, C.; Nie, X.; Guo, Y.; Takei, Y.; Yun, J.; Cai, L.; et al. The adult human testis transcriptional cell atlas. Cell Res. 2018, 28, 1141–1157. [Google Scholar] [CrossRef]
- Du, L.; Chen, W.; Cheng, Z.; Wu, S.; He, J.; Han, L.; He, Z.; Qin, W. Novel gene regulation in normal and abnormal spermatogenesis. Cells 2021, 10, 666. [Google Scholar] [CrossRef]
- Gill, M.E.; Hu, Y.C.; Lin, Y.; Page, D.C. Licensing of gametogenesis, dependent on RNA binding protein DAZL, as a gateway to sexual differentiation of fetal germ cells. Proc. Natl. Acad. Sci. USA 2011, 108, 7443–7448. [Google Scholar] [CrossRef]
- Seligman, J.; Page, D.C. TheDazhGene Is Expressed in Male and Female Embryonic Gonads before Germ Cell Sex Differentiation. Biochem. Biophys. Res. Commun. 1998, 245, 878–882. [Google Scholar] [CrossRef]
- Ruggiu, M.; Speed, R.; Taggart, M.; McKay, S.J.; Kilanowski, F.; Saunders, P.; Dorin, J.; Cooke, H.J. The mouse Dazla gene encodes a cytoplasmic protein essential for gametogenesis. Lett. Nat. 1997, 389, 73–77. [Google Scholar] [CrossRef]
- Mikedis, M.M.; Fan, Y.; Nicholls, P.K.; Endo, T.; Jackson, E.K.; Cobb, S.A.; de Rooij, D.G.; Page, D.C. Dazl mediates a broad translational program regulating expansion and differentiation of spermatogonial progenitors. eLife 2020, 9, e56523. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, H.; Xin, D.; Cheng, H.; Zhou, R. A novel ncRNA gene from mouse chromosome 5 trans-splices with Dmrt1 on chromosome 19. Biochem. Biophys. Res. Commun. 2010, 400, 696–700. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Yuan, P.; Yang, H.; Zhang, J.; Soh, B.S.; Li, P.; Lim, S.L.; Cao, S.; Tay, J.; Orlov, Y.L.; et al. Tbx3 improves the germ-line competency of induced pluripotent stem cells. Nature 2010, 463, 1096–1100. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.P.; Kotaja, N. Small RNAs in spermatogenesis. Mol. Cell. Endocrinol. 2014, 382, 498–508. [Google Scholar] [CrossRef]
- Saga, Y. Function of Nanos2 in the male germ cell lineage in mice. Cell. Mol. Life Sci. 2010, 67, 3815–3822. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Igarashi, K.; Aisaki, K.-I.; Kanno, J.; Saga, Y. NANOS2 interacts with the CCR4-NOT deadenylation complex and leads to suppression of specific RNAs. Proc. Natl. Acad. Sci. USA 2010, 107, 3594–3599. [Google Scholar] [CrossRef]
- Tsuda, M.; Sasaoka, Y.; Kiso, M.; Abe, K.; Haraguchi, S.; Kobayashi, S.; Saga, Y. Conserved Role of Nanos Proteins in Germ Cell Development. Science 2003, 301, 1239–1241. [Google Scholar] [CrossRef]
- Beck, A.R.P.; Miller, I.J.; Anderson, P.; Streuli, M. RNA-binding protein TIAR is essential for primordial germ cell development. Proc. Natl. Acad. Sci. USA 1998, 95, 2331–2336. [Google Scholar] [CrossRef]
- Youngren, K.K.; Coveney, D.; Peng, X.; Bhattacharya, C.; Schmidt, L.S.; Nickerson, M.L.; Lamb, B.T.; Deng, J.M.; Behringer, R.R.; Capel, B.; et al. The Ter mutation in the dead end gene causes germ cell loss and testicular germ cell tumours. Nature 2005, 435, 360–364. [Google Scholar] [CrossRef]
- Lv, Y.; Lu, G.; Cai, Y.; Su, R.; Liang, L.; Wang, X.; Mu, W.; He, X.; Huang, T.; Ma, J.; et al. RBM46 is essential for gametogenesis and functions in post-transcriptional roles affecting meiotic cohesin subunits. Protein Cell 2023, 14, 51–63. [Google Scholar] [CrossRef]
- Qian, B.; Li, Y.; Yan, R.; Han, S.; Bu, Z.; Gong, J.; Zheng, B.; Yuan, Z.; Ren, S.; He, Q.; et al. RNA binding protein RBM46 regulates mitotic-to-meiotic transition in spermatogenesis. Sci. Adv. 2022, 8, 2945. [Google Scholar] [CrossRef] [PubMed]
- Walker, W.H. Regulation of mammalian spermatogenesis by miRNAs. Semin. Cell Dev. Biol. 2021, 121, 24–31. [Google Scholar] [CrossRef]
- Kim, G.J.; Georg, I.; Scherthan, H.; Merkenschlager, M.; Guillou, F.; Scherer, G.; Barrionuevo, F. Dicer is required for Sertoli cell function and survival. Int. J. Dev. Biol. 2010, 54, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Maatouk, D.M.; Loveland, K.L.; McManus, M.T.; Moore, K.; Harfe, B.D. Dicer1 is required for differentiation of the mouse male germline. Biol. Reprod. 2008, 79, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Papaioannou, M.D.; Pitetti, J.L.; Ro, S.; Park, C.; Aubry, F.; Schaad, O.; Vejnar, C.E.; Kühne, F.; Descombes, P.; Zdobnov, E.M.; et al. Sertoli cell Dicer is essential for spermatogenesis in mice. Dev. Biol. 2009, 326, 250–259. [Google Scholar] [CrossRef]
- Wu, Q.; Song, R.; Ortogero, N.; Zheng, H.; Evanoff, R.; Small, C.L.; Griswold, M.D.; Namekawa, S.H.; Royo, H.; Turner, J.M.; et al. The RNase III enzyme DROSHA is essential for MicroRNA production and spermatogenesis. J. Biol. Chem. 2012, 287, 25173–25190. [Google Scholar] [CrossRef]
- Klees, C.; Alexandri, C.; Demeestere, I.; Lybaert, P. The Role of microRNA in Spermatogenesis: Is There a Place for Fertility Preservation Innovation? Int. J. Mol. Sci. 2024, 25, 460. [Google Scholar] [CrossRef]
- Huszar, J.M.; Payne, C.J. MicroRNA 146 (Mir146) modulates spermatogonial differentiation by retinoic acid in mice. Biol. Reprod. 2013, 88, 15. [Google Scholar] [CrossRef]
- Yang, Q.-E.; Racicot, K.E.; Kaucher, A.V.; Oatley, M.J.; Oatley, J.M. MicroRNAs 221 and 222 regulate the undifferentiated state in mammalian male germ cells. Development 2013, 140, 280–290. [Google Scholar] [CrossRef]
- Chen, J.; Gao, C.; Lin, X.; Ning, Y.; He, W.; Zheng, C.; Zhang, D.; Yan, L.; Jiang, B.; Zhao, Y.; et al. The microRNA miR-202 prevents precocious spermatogonial differentiation and meiotic initiation during mouse spermatogenesis. Development 2021, 148, dev199799. [Google Scholar] [CrossRef]
- Soumillon, M.; Necsulea, A.; Weier, M.; Brawand, D.; Zhang, X.; Gu, H.; Barthès, P.; Kokkinaki, M.; Nef, S.; Gnirke, A.; et al. Cellular Source and Mechanisms of High Transcriptome Complexity in the Mammalian Testis. Cell Rep. 2013, 3, 2179–2190. [Google Scholar] [CrossRef] [PubMed]
- Kayyar, B.; Kataruka, S.; Akhade, V.S.; Rao, M.R.S. REPRODUCTION REVIEW Molecular functions of Mrhl lncRNA in mouse spermatogenesis. Reproduction 2023, 166, R39–R50. [Google Scholar] [CrossRef]
- Anguera, M.C.; Ma, W.; Clift, D.; Namekawa, S.; Kelleher, R.J.; Lee, J.T. Tsx produces a long noncoding RNA and has general functions in the germline, stem cells, and brain. PLoS Genet. 2011, 7, e1002248. [Google Scholar] [CrossRef]
- Liang, M.; Li, W.; Tian, H.; Hu, T.; Wang, L.; Lin, Y.; Li, Y.; Huang, H.; Sun, F. Sequential expression of long noncoding RNA as mRNA gene expression in specific stages of mouse spermatogenesis. Sci. Rep. 2014, 4, srep05966. [Google Scholar] [CrossRef]
- Weng, B.; Ran, M.; Chen, B.; He, C.; Dong, L.; Peng, F. Genome-wide analysis of long non-coding RNAs and their role in postnatal porcine testis development. Genomics 2017, 109, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Hu, K.; He, C.; Zhou, J.; Liao, Y. Upregulated lncRNA Gm2044 inhibits male germ cell development by acting as miR-202 host gene. Anim. Cells Syst. 2019, 23, 128–134. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Wang, K.; Gao, Y.; Wang, C.; Li, L.; Liao, Y.; Hu, K.; Liang, M. Roles of Noncoding RNA in Reproduction. Front. Genet. 2021, 12, 777510. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.L. Linking Long Noncoding RNA Localization and Function. Trends Biochem. Sci. 2016, 41, 761–772. [Google Scholar] [CrossRef]
- Mukherjee, A.; Koli, S.; Reddy, K.V.R. Regulatory non-coding transcripts in spermatogenesis: Shedding light on ‘dark matter’. Andrology 2014, 2, 360–369. [Google Scholar] [CrossRef]
- Zhou, G.; Zhang, M.; Zhang, J.; Feng, Y.; Xie, Z.; Liu, S.; Zhu, D.; Luo, Y. The gene regulatory role of non-coding RNAs in non-obstructive azoospermia. Front. Endocrinol. 2022, 13, 959487. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, H.; Zheng, L.; Zhang, H.T.; Yang, Y.Z.; Mao, J.M.; Liu, D.F.; Zhao, L.M.; Liang, H.; Jiang, H. Identification and characterization of circular RNAs in the testicular tissue of patients with non-obstructive azoospermia. Asian J. Androl. 2022, 24, 660–665. [Google Scholar] [CrossRef]
- Saitou, M.; Hayashi, K. Mammalian in vitro gametogenesis. Science 2021, 374, 47. [Google Scholar]
- Fang, K.; Li, Q.; Wei, Y.; Zhou, C.; Guo, W.; Shen, J.; Wu, R.; Ying, W.; Yu, L.; Zi, J.; et al. Prediction and validation of mouse meiosis-essential genes based on spermatogenesis proteome dynamics. Mol. Cell. Proteom. 2021, 20, 100014. [Google Scholar] [CrossRef]
- Trasler, J.M. Epigenetics in spermatogenesis. Mol. Cell. Endocrinol. 2009, 306, 33–36. [Google Scholar] [CrossRef]
- Kaneda, M.; Okano, M.; Hata, K.; Sado, T.; Tsujimoto, N.; Li, E.; Sasaki, H. Essential role for de novo DNA methyltransferase Dnmt3a in paternal and maternal imprinting. Nature 2004, 429, 900–903. [Google Scholar] [PubMed]
- La Salle, S.; Mertineit, C.; Taketo, T.; Moens, P.B.; Bestor, T.H.; Trasler, J.M. Windows for sex-specific methylation marked by DNA methyltransferase expression profiles in mouse germ cells. Dev. Biol. 2004, 268, 403–415. [Google Scholar] [CrossRef]
- Salle, S.L.; Trasler, J.M. Dynamic expression of DNMT3a and DNMT3b isoforms during male germ cell development in the mouse. Dev. Biol. 2006, 296, 71–82. [Google Scholar] [CrossRef]
- Blanco, M.; Cocquet, J. Genetic factors affecting sperm chromatin structure. In Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2019; Volume 1166, pp. 1–28. [Google Scholar] [CrossRef]
- Cho, C.; Jung-Ha, H.; Willis, W.D.; Goulding, E.H.; Stein, P.; Xu, Z.; Schultz, R.M.; Hecht, N.B.; Eddy, E.M. Protamine 2 deficiency leads to sperm DNA damage and embryo death in mice. Biol. Reprod. 2003, 69, 211–217. [Google Scholar] [CrossRef]
- Cho, C.; Willis, W.D.; Goulding, E.H.; Jung-Ha, H.; Choi, Y.-C.; Hecht, N.B.; Eddy, E.M. Haploinsufficiency of Protamine-1 or -2 Causes Infertility in Mice. Nat. Genet. 2001, 28, 82–86. [Google Scholar] [CrossRef]
- Carrell, D.T.; Emery, B.R.; Hammoud, S. Altered protamine expression and diminished spermatogenesis: What is the link? Hum. Reprod. Update 2007, 13, 313–327. [Google Scholar] [CrossRef]
- Zhou, H.; Grubisic, I.; Zheng, K.; He, Y.; Wang, P.J.; Kaplan, T.; Tjian, R. Taf7l cooperates with Trf2 to regulate spermiogenesis. Proc. Natl. Acad. Sci. USA 2013, 110, 16886–16891. [Google Scholar] [CrossRef]
- Syrjänen, J.L.; Pellegrini, L.; Davies, O.R. A molecular model for the role of SYCP3 in meiotic chromosome organisation. eLife 2014, 3, e02963. [Google Scholar] [CrossRef]
- Yuan, L.; Liu, J.-G.; Zhao, J.; Brundell, E.; Daneholt, B.; Höög, C. The Murine SCP3 Gene Is Required for Synaptonemal Complex Assembly, Chromosome Synapsis, and Male Fertility. Mol. Cell 2000, 5, 73–83. [Google Scholar] [PubMed]
- Mieusset, R.; Fouda, P.J.; Vaysse, P.; Guitard, J.; Moscovici, J.; Juskiewenski, S. Increase in testicular temperature in case of cryptorchidism in boys. Fertil. Steril. 1993, 59, 1319–1321. [Google Scholar] [CrossRef] [PubMed]
- Ivell, R. Lifestyle impact and the biology of the human scrotum. Reprod. Biol. Endocrinol. RBE 2007, 5, 15. [Google Scholar] [CrossRef]
- Dierauf, L.; Gulland, F.M.D. CRC Handbook of Marine Mammal Medicine: Health, Disease, and Rehabilitation (Second); CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Miller, D.L. (Ed.) Reproductive Biology and Phylogeny of Cetacea: Whales, Porpoises and Dolphins (First); CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Rommel, S.A.; Pabst, D.A.; McLellan, W.A.; Mead, J.G.; Potter, C.W. Anatomical evidence for a countercurrent heat exchanger associated with dolphin testes. Anat. Rec. 1992, 232, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Scholander, P.F.; Schevill, W.E. Counter-current vascular heat exchange in the fins of whales. J. Appl. Physiol. 1955, 8, 279–282. [Google Scholar] [CrossRef]
- Pérez-Crespo, M.; Pintado, B.; Gutiérrez-Adán, A. Scrotal heat stress effects on sperm viability, sperm DNA integrity, and the offspring sex ratio in mice. Mol. Reprod. Dev. 2008, 75, 40–47. [Google Scholar] [CrossRef]
- Ziaeipour, S.; Piryaei, A.; Aliaghaei, A.; Nazarian, H.; Naserzadeh, P.; Ebrahimi, V.; Abdi, S.; Shahi, F.; Ahmadi, H.; Fathabadi, F.F.; et al. Chronic scrotal hyperthermia induces azoospermia and severe damage to testicular tissue in mice. Acta Histochem. 2021, 123, 151712. [Google Scholar] [CrossRef]
- Rockett, J.C.; Mapp, F.L.; Garges, J.B.; Luft, J.C.; Mori, C.; Dix, D.J. Effects of hyperthermia on spermatogenesis, apoptosis, gene expression, and fertility in adult male mice. Biol. Reprod. 2001, 65, 229–239. [Google Scholar] [CrossRef]
- Yin, J.; Ni, B.; Tian, Z.; Yang, F.; Liao, W.; Gao, Y. Regulatory effects of autophagy on spermatogenesis. Biol. Reprod. 2017, 96, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Kesari, K.K.; Agarwal, A.; Henkel, R. Radiations and male fertility. Reprod. Biol. Endocrinol. 2018, 16, 118. [Google Scholar] [CrossRef] [PubMed]
- Morrell, J.M. Heat stress and bull fertility. Theriogenology 2020, 153, 62–67. [Google Scholar] [CrossRef]
- Gao, P.; Gao, J.; Dou, X.; Peng, D.; Zhang, Y.; Li, H.; Zhu, T.; Jiang, H.; Zhang, X. The relationship between vascular endothelial growth factor and spermatogenesis disturbance in an experimentally-induced unilateral cryptorchidism murine model. Mol. Biol. Rep. 2020, 47, 3605–3613. [Google Scholar] [CrossRef] [PubMed]
- Ge, W.L.; Chen, J.N.; Ji, L.H.; Zhao, J.; Xian, H.; Xu, Y.Z. NEK2 gene expression in mouse cryptorchidism model and its mechanism involved in apoptosis. Zhonghua Yi Xue Za Zhi 2020, 100, 3534–3538. [Google Scholar]
- Lue, Y.; Hikim, A.P.S.; Wang, C.; Im, M.; Leung, A.; Swerdloff, R.S. Testicular Heat Exposure Enhances the Suppression of Spermatogenesis by Testosterone in Rats: The “Two-Hit” Approach to Male Contraceptive Development. Endocrinology 2000, 141, 1414–1424. [Google Scholar] [CrossRef]
- Qin, D.; Tang, Y.; Wang, X.; Mao, Y.; Feng, Z. Antagonistic Effect of Cuscuta chinensis on a Rat Model with Unilateral Cryptorchidism. Med. Sci. Monit. 2019, 25, 6727–6735. [Google Scholar] [CrossRef]
- Banks, S.; King, S.A.; Irvine, D.S.; Saunders, P.T.K. Impact of a mild scrotal heat stress on DNA integrity in murine spermatozoa. Reproduction 2005, 129, 505–514. [Google Scholar] [CrossRef]
- Mahdivand, N.; Shalizar-Jalali, A.; Nejati, V.; Najafi, G.; Rahmani, F. Adaptogenic potential of royal jelly in reproductive system of heat stress-exposed male rats. J. Therm. Biol. 2021, 96, 102827. [Google Scholar] [CrossRef]
- Yin, Y.; Hawkins, K.L.; DeWolf, W.C.; Morgentaler, A. Heat stress causes testicular germ cell apoptosis in adult mice. J. Androl. 1997, 18, 159–165. [Google Scholar] [CrossRef]
- de Dios Hourcade, J.; Pérez-Crespo, M.; Pintado, B.; Gutierrez-Adan, A. Screening Ability of Female Reproductive Tract for Fragmented DNA Spermatozoa Depends on the Source of Damage. Biol. Reprod. 2008, 78 (Suppl. 1), 181–182. [Google Scholar] [CrossRef]
- Gong, Y.; Guo, H.; Zhang, Z.; Zhou, H.; Zhao, R.; He, B. Heat Stress Reduces Sperm Motility via Activation of Glycogen Synthase Kinase-3α and Inhibition of Mitochondrial Protein Import. Front. Physiol. 2017, 8, 718. [Google Scholar] [CrossRef]
- Thundathil, J.; Rajamanickam, G.; Kastelic, J.; Newton, L. The Effects of Increased Testicular Temperature on Testis-Specific Isoform of Na+/K+-ATPase in Sperm and its Role in Spermatogenesis and Sperm Function. Reprod. Domest. Anim. 2012, 47, 170–177. [Google Scholar] [CrossRef]
- Somanath, P.R.; Jack, S.L.; Vijayaraghavan, S. Changes in Sperm Glycogen Synthase Kinase-3 Serine Phosphorylation and Activity Accompany Motility Initiation and Stimulation. J. Androl. 2004, 25, 605–617. [Google Scholar] [CrossRef]
- Vijayaraghavan, S.; Mohan, J.; Gray, H.; Khatra, B.; Carr, D.W. A Role for Phosphorylation of Glycogen Synthase Kinase-3α in Bovine Sperm Motility Regulation1. Biol. Reprod. 2000, 62, 1647–1654. [Google Scholar] [CrossRef] [PubMed]
- Houston, B.J.; Nixon, B.; Martin, J.H.; De Iuliis, G.N.; Trigg, N.A.; Bromfield, E.G.; McEwan, K.E.; Aitken, R.J. Heat exposure induces oxidative stress and DNA damage in the male germ line. Biol. Reprod. 2018, 98, 593–606. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Park, K.; Rhee, K. Heat stress response of male germ cells. Cell. Mol. Life Sci. 2013, 70, 2623–2636. [Google Scholar] [CrossRef]
- Allen, R.L.; O’Brien, D.A.; Eddy, E.M. A Novel hsp70-Like Protein (P70) is Present in Mouse Spermatogenic Cells. Mol. Cell. Biol. 1988, 8, 828–832. [Google Scholar] [CrossRef]
- Zakeri, Z.F.; Wolgemuth, D.J. Developmental-Stage-Specific Expression of the hsp70 Gene Family During Differentiation of the Mammalian Male Germ Line. Mol. Cell. Biol. 1987, 7, 1791–1796. [Google Scholar] [CrossRef]
- Zhu, B.; Setchell, B.P. Effects of paternal heat stress on the in vivo development of preimplantation embryos in the mouse. Reprod. Nutr. Dev. 2004, 44, 617–629. [Google Scholar] [CrossRef][Green Version]
- Jannes, P.; Spiessens, C.; Van der Auwera, I.; D’Hooghe, T.; Verhoeven, G.; Vanderschueren, D. Male subfertility induced by acute scrotal heating affects embryo quality in normal female mice. Hum. Reprod. 1998, 13, 372–375. [Google Scholar] [CrossRef] [PubMed]
- Setchell, B.P.; Ekpe, G.; Zupp, J.L.; Surani, M.A.H. Transient retardation in embryo growth in normal female mice made pregnant by males whose testes had been heated. Hum. Reprod. 1998, 13, 342–347. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yaeram, J.; Setchell, B.P.; Maddocks, S. Effect of heat stress on the fertility of male mice in vivo and in vitro. Reprod. Fertil. Dev. 2006, 18, 647–653. [Google Scholar] [CrossRef]
- Mieusset, R.; Quintana Casares, P.I.; Sánchez-Partida, L.G.; Sowerbutts, S.F.; Zupp, J.L.; Setchell, B.P. The Effects of Moderate Heating of the Testes and Epididymides of Rams by Scrotal Insulation on Body Temperature, Respiratory Rate, Spermatozoa Output and Motility, and on Fertility and Embryonic Survival in Ewes Inseminated with Frozen Semen. Ann. N. Y. Acad. Sci. 1991, 637, 445–458. [Google Scholar] [CrossRef]
- Zhu, B.; Maddocks, S. The effect of paternal heat stress on protein profiles of pre-implantation embryos in the mouse. Int. J. Androl. 2005, 28, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T.K.; Giwercman, A.; Carlsen, E.; Scheike, T.; Skakkebaek, N.E. Semen quality among members of organic food associations in Zealand, Denmark. Lancet 1996, 347, 1844. [Google Scholar] [CrossRef]
- Laven’, J.S.E.; Haverkorn, M.J.; Bots, R.S.G.M. Influence of occupation and living habits on semen quality in men (scrotal insulation and semen quality). Eur. J. Obstet. Gynecol. Reprod. Biol. 1988, 29, 137–141. [Google Scholar] [CrossRef]
- Lynch, R.; Iwan Lewis-Jones, D.; Machin, D.G.; Desmond, A.D. Improved seminal characteristics in infertile men after a conservative treatment regimen based on the avoidance of testicular hyperthermia. Fertil. Steril. 1986, 46, 476–479. [Google Scholar] [CrossRef]
- Bujan, L.; Daudin, M.; Charlet, J.-P.; Thonneau, P.; Mieusset, R. Increase in scrotal temperature in car drivers. Hum. Reprod. 2000, 15, 1355–1357. [Google Scholar] [CrossRef]
- Henrik, N.; Hjollund, I.; Storgaard, L.; Ernst, E.; Bonde, J.P.; Olsen, J. The relation between daily activities and scrotal temperature. Reprod. Toxicol. 2002, 16, 209–214. [Google Scholar]
- Kort, H.I.; Massey, J.B.; Elsner, C.W.; Mitchell-Leef, D.; Shapiro, D.B.; Witt, M.A.; Roudebush, W.E. Impact of body mass index values on sperm quantity and quality. J. Androl. 2006, 27, 450–452. [Google Scholar] [CrossRef]
- Garolla, A.; Torino, M.; Sartini, B.; Cosci, I.; Patassini, C.; Carraro, U.; Foresta, C. Seminal and molecular evidence that sauna exposure affects human spermatogenesis. Hum. Reprod. 2013, 28, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Shefi, S.; Tarapore, P.E.; Walsh, T.J.; Croughan, M.; Turek, P.J. Wet heat exposure: A potentially reversible cause of low semen quality in infertile men. Urol. Int. Braz. J. Urol. 2007, 33, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Mieusset, R.; Bujan, L.; Mondinat, C.; Mansat, A.; Pontonnier, F.; Grandjean, H. Association of scrotal hyperthermia with impaired spermatogenesis in infertile men. Fertil. Steril. 1987, 48, 1006–1011. [Google Scholar] [PubMed]
- Naughton, C.K.; Nangia, A.K.; Agarwal, A. Varicocele and male infertility: Part II: Pathophysiology of varicoceles in male infertility. Hum. Reprod. Updat. 2001, 7, 473–481. [Google Scholar]
- Sergerie, M.; Mieusset, R.; Croute, F.; Daudin, M.; Bujan, L. High risk of temporary alteration of semen parameters after recent acute febrile illness. Fertil. Steril. 2007, 88, 970.e1–970.e7. [Google Scholar] [CrossRef]
- Kabukçu, C.; Çil, N.; Turan, T.; Özlülerden, Y.; Çabuş, Ü.; Abban Mete, G. Do seasonal variations in ambient temperature, humidity and daylight duration affect semen parameters? A retrospective analysis over eight years. Andrologia 2020, 52, e13777. [Google Scholar] [CrossRef]
- Pakmanesh, H.; Nazarirobati, N.; Dabiri, S.; Mirshekari, T.R.; Eslami, N.; Torabinavid, P.; Rouientan, H.; Narouie, B. Impact of Season Variation on Semen Quality: A Comprehensive Retrospective Analysis of Data From Patients at an Eastern Iranian Tertiary Care Fertility Center Over a Decade. Am. J. Men’s Health 2024, 18, 15579883241237505. [Google Scholar] [CrossRef]
- Yogev, L.; Paz, G.; Kleiman, S.E.; Shabtai, E.; Gamzu, R.; Botchan, A.; Lehavi, O.; Yavetz, H.; Hauser, R. Freezability and semen parameters in candidates of sperm bank donors: 1992-2010. J. Androl. 2012, 33, 999–1006. [Google Scholar] [CrossRef]
- Zhou, Y.; Meng, T.; Wu, L.; Duan, Y.; Li, G.; Shi, C.; Zhang, H.; Peng, Z.; Fan, C.; Ma, J.; et al. Association between ambient temperature and semen quality: A longitudinal study of 10 802 men in China. Environ. Int. 2020, 135, 105364. [Google Scholar] [CrossRef]
- White-Cooper, H.; Bausek, N. Evolution and spermatogenesis. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 1465–1480. [Google Scholar] [CrossRef]
- Yildirim, E.; Aksoy, S.; Onel, T.; Yaba, A. Gonadal development and sex determination in mouse. Reprod. Biol. 2020, 20, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Pelosi, E.; Koopman, P. Development of the Testis. In Reference Module in Biomedical Sciences; Elsevier: Amsterdam, Netherlands, 2017. [Google Scholar] [CrossRef]
- Mäkelä, J.-A.; Cisneros-Montalvo, S.; Lehtiniemi, T.; Olotu, O.; La, H.M.; Toppari, J.; Hobbs, R.M.; Parvinen, M.; Kotaja, N. Transillumination-Assisted Dissection of Specific Stages of the Mouse Seminiferous Epithelial Cycle for Downstream Immunostaining Analyses. J. Vis. Exp. JoVE 2020, 164, e61800. [Google Scholar] [CrossRef]
- Hess, R.A.; De França, L.R. Spermatogenesis and cycle of the seminiferous epithelium. In Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2008; Volume 636, pp. 1–15. [Google Scholar] [CrossRef]
- Leblond, C.P.; Clermont, Y. De-finition of the stages of the cycle of the seminiferous epithelium in the rat. Ann. N. Y. Acad. Sci. 1952, 55, 548–573. [Google Scholar]
- Clouthier, D.E.; Avarbock, M.R.; Maika, S.D.; Hammer, R.E.; Brinster, R.L. Rat spermatogenesis in mouse testis. Nature 1996, 381, 418–421. [Google Scholar] [CrossRef] [PubMed]
- Oakberg, E.F. Duration of Spermatogenesis in the Mouse. Nature 1957, 180, 1137–1138. [Google Scholar] [CrossRef]
- Clermont, Y.; Trott, M. Duration of the Cycle of the Seminiferous Epithelium in the Mouse and Hamster Determined by Means of 8H-Thymidine and Radioautography. Fertil. Steril. 1969, 20, 805–817. [Google Scholar] [PubMed]
- Soares, J.M.; Avelar, G.F.; França, L.R. The seminiferous epithelium cycle and its duration in different breeds of dog (Canis familiaris). J. Anat. 2009, 215, 462–471. [Google Scholar] [CrossRef]
- Staub, C.; Johnson, L. Review: Spermatogenesis in the bull. Animal 2018, 12, s27–s35. [Google Scholar] [CrossRef]
- Amann, R.P. A Critical Review of Methods for Evaluation of Spermatogenesis from Seminal Characteristics. J. Androl. 1981, 2, 37–58. [Google Scholar] [CrossRef]
- França, L.R.; Cardoso, F.M. Duration of spermatogenesis and sperm transit time through the epididymis in the Piau boar. Tissue Cell 1998, 30, 573–582. [Google Scholar] [CrossRef]
- Blesbois, E. Biological features of the avian male gamete and their application to biotechnology of conservation. J. Poult. Sci. 2012, 49, 141–149. [Google Scholar] [CrossRef]
- Sousa, A.L.; Campos-Junior, P.H.A.; Costa, G.M.J.; de França, L.R. Spermatogenic cycle length and sperm production in the freshwater turtle Kinosternon scorpioides. Biol. Reprod. 2014, 90, 35. [Google Scholar] [CrossRef]
- Schulz, R.W.; de França, L.R.; Lareyre, J.J.; LeGac, F.; Chiarini-Garcia, H.; Nobrega, R.H.; Miura, T. Spermatogenesis in fish. Gen. Comp. Endocrinol. 2010, 165, 390–411. [Google Scholar] [CrossRef]
- Billard, R. Spermatogenesis and spermatology of some teleost fish species. Reprod. Nutr. Dev. 1986, 26, 877–920. [Google Scholar] [CrossRef]
- Mruk, D.D.; Cheng, C.Y. The Mammalian Blood-Testis Barrier: Its Biology and Regulation. Endocr. Rev. 2015, 36, 564–591. [Google Scholar] [CrossRef] [PubMed]
- Holstein, A.-F.; Schulze, W.; Davidoff, M. Understanding Spermatogenesis Is a Prerequisite for Treatment. Reprod. Biol. Endocrinol. 2003, 1, 107. [Google Scholar] [CrossRef]
- Chocu, S.; Calvel, P.; Rolland, A.D.; Pineau, C. Spermatogenesis in mammals: Proteomic insights. Syst. Biol. Reprod. Med. 2012, 58, 179–190. [Google Scholar] [CrossRef] [PubMed]
- De Kretser, D.M.; Loveland, K.L.; Meinhardt, A.; Simorangkir, D.; Wreford, N. Spermatogenesis. Hum Reprod. 1998, 13 (Suppl. 1), 1–8. [Google Scholar] [CrossRef]
- Griswold, M.D. Spermatogenesis: The commitment to Meiosis. Physiol. Rev. 2016, 96, 1–17. [Google Scholar] [CrossRef]
- Wang, J.-H.; Li, Y.; Deng, S.-L.; Liu, Y.-X.; Lian, Z.-X.; Yu, K. Recent research advances in mitosis during mammalian gametogenesis. Cells 2019, 8, 567. [Google Scholar] [CrossRef]
- Alavattam, K.G.; Maezawa, S.; Andreassen, P.R.; Namekawa, S.H. Meiotic sex chromosome inactivation and the XY body: A phase separation hypothesis. Cell. Mol. Life Sci. 2022, 79, 18. [Google Scholar] [CrossRef]
- Han, C. Gene expression programs in mammalian spermatogenesis. Development 2024, 151, dev202033. [Google Scholar] [CrossRef] [PubMed]
- Ishiguro, K.I. Mechanisms of meiosis initiation and meiotic prophase progression during spermatogenesis. Mol. Asp. Med. 2024, 97, 101282. [Google Scholar] [CrossRef]
- Lim, S.; Kaldis, P. Cdks, cyclins and CKIs: Roles beyond cell cycle regulation. Development 2013, 140, 3079–3093. [Google Scholar] [CrossRef]
- Turner, J.M.A. Meiotic Silencing in Mammals. Annu. Rev. Genet. 2015, 49, 395–412. [Google Scholar] [CrossRef]
- Ogawa, T.; Ohmura, M.; Ohbo, K. The niche for spermatogonial stem cells in the mammalian testis. Int. J. Hematol. 2005, 82, 381–388. [Google Scholar] [CrossRef]
- Rajender, S.; Rahul, P.; Mahdi, A.A. Mitochondria, spermatogenesis and male infertility. Mitochondrion 2010, 10, 419–428. [Google Scholar] [CrossRef]
- Wen, Q.; Tang, E.I.; Xiao, X.; Gao, Y.; Chu, D.S.; Mruk, D.D.; Silvestrini, B.; Cheng, C.Y. Transport of germ cells across the seminiferous epithelium during spermatogenesis—The involvement of both actin- and microtubule-based cytoskeletons. Tissue Barriers 2016, 4, e1265042. [Google Scholar] [CrossRef]
- Freitas, M.J.; Vijayaraghavan, S.; Fardilha, M. Signaling mechanisms in mammalian sperm motility. Biol. Reprod. 2017, 96, 2–12. [Google Scholar] [CrossRef]
- O’Donnell, L.; O’Bryan, M.K. Microtubules and spermatogenesis. Semin. Cell Dev. Biol. 2014, 30, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Teves, M.E.; Roldan, E.R.S.; Krapf, D.; Iii, J.F.S.; Bhagat, V.; Sapao, P. Sperm differentiation: The role of trafficking of proteins. Int. J. Mol. Sci. 2020, 21, 3702. [Google Scholar] [CrossRef]
- Anderson, M.J.; Dixon, A.F. Motility and the midpiece in primates. Nature 2002, 416, 496. [Google Scholar] [CrossRef] [PubMed]
- Gunes, S.; Al-Sadaan, M.; Agarwal, A. Spermatogenesis, DNA damage and DNA repair mechanisms in male infertility. Reprod. BioMed. Online 2015, 31, 309–319. [Google Scholar] [CrossRef]
- Suresh, B.; Lee, J.; Hong, S.-H.; Kim, K.-S.; Ramakrishna, S. The role of deubiquitinating enzymes in spermatogenesis. Cell. Mol. Life Sci. 2015, 72, 4711–4720. [Google Scholar] [CrossRef] [PubMed]
- Bejarano, I.; Rodríguez, A.B.; Pariente, J.A. Apoptosis is a demanding selective tool during the development of fetal male germ cells. Front. Cell Dev. Biol. 2018, 6, 65. [Google Scholar] [CrossRef]
- Nef, S.; Parada, L.F. Cryptorchidism in mice mutant for Insl3. Nat. Genet. 1999, 22, 295–299. [Google Scholar] [CrossRef]
- Acién, P.; Sánchez Del Campo, F.; Mayol, M.J.; Acién, M. The female gubernaculum: Role in the embryology and development of the genital tract and in the possible genesis of malformations. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011, 159, 426–432. [Google Scholar] [CrossRef]
- Xie, Q.; Kang, Y.; Zhang, C.; Xie, Y.; Wang, C.; Liu, J.; Yu, C.; Zhao, H.; Huang, D. The Role of Kisspeptin in the Control of the Hypothalamic-Pituitary-Gonadal Axis and Reproduction. Front. Endocrinol. 2022, 13, 925206. [Google Scholar] [CrossRef]
- Ishikawa, K.; Tanaka, A.; Kogame, A.; Watanabe, T.; Tagawa, Y.; Matsui, H. Usefulness of pharmacokinetic/efficacy analysis of an investigational kisspeptin analog, TAK-448, in quantitatively evaluating anti-tumor growth effect in the rat VCaP androgen-sensitive prostate cancer model. Eur. J. Pharmacol. 2018, 828, 126–134. [Google Scholar] [CrossRef]
- Matsui, H.; Tanaka, A.; Yokoyama, K.; Takatsu, Y.; Ishikawa, K.; Asami, T.; Nishizawa, N.; Suzuki, A.; Kumano, S.; Terada, M.; et al. Chronic administration of the metastin/kisspeptin analog KISS1-305 or the investigational agent TAK-448 suppresses hypothalamic pituitary gonadal function and depletes plasma testosterone in adult male rats. Endocrinology 2012, 153, 5297–5308. [Google Scholar] [CrossRef]
- Gianzo, M.; Subirán, N. Regulation of male fertility by the renin-angiotensin system. Int. J. Mol. Sci. 2020, 21, 7943. [Google Scholar] [CrossRef]
- Shibata, T.; Bhat, S.A.; Cao, D.; Saito, S.; Bernstein, E.A.; Nishi, E.; Medenilla, J.D.; Wang, E.T.; Chan, J.L.; Pisarska, M.D.; et al. Testicular ACE regulates sperm metabolism and fertilization through the transcription factor PPARγ. J. Biol. Chem. 2024, 300, 105486. [Google Scholar] [CrossRef]
- Edenfield, R.C.; Easley, C.A. Implications of testicular ACE2 and the renin–angiotensin system for SARS-CoV-2 on testis function. Nat. Rev. Urol. 2022, 19, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Zou, Y.; Li, H.; Zhang, Y.; Liu, X.; Zhao, X.; Wu, X.; Fei, W.; Xu, Z.; Yang, X. Decreased angiotensin receptor 1 expression in ± AT1 Knockout mice testis results in male infertility and GnRH reduction. Reprod. Biol. Endocrinol. 2021, 19, 120. [Google Scholar] [CrossRef]
- Nieschlag, E. Clinical trials in male hormonal contraception. Contraception 2010, 82, 457–470. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, C.; Meriggiola, M.C.; Behre, H.M.; Page, S.T. Hormonal male contraception. Andrology 2024, 12, 1551–1557. [Google Scholar] [CrossRef]
- Thirumalai, A.; Page, S.T. Androgens in male contraception. Best Pract. Res. Clin. Endocrinol. Metab. 2022, 36, 101627. [Google Scholar] [CrossRef]
- Wang, C.; Swerdloff, R.S. Hormonal approaches to male contraception. Curr. Opin. Urol. 2010, 20, 520–524. [Google Scholar] [CrossRef]
- Kandeel, F.R.; Swerdloff, R.S. Role of temperature in regulation of spermatogenesis and the use of heating as a method for contraception. Fertil. Steril. 1988, 49, 1–23. [Google Scholar]
- Liu, Y.-X. Temperature control of spermatogenesis and prospect of male contraception. Front. Biosci. 2010, S2, 730–755. [Google Scholar]
- Wang, R.H.; Wang, H.H.; Cheng, C.P.; Hsu, H.Y.; Lin, S.Y. Testing a model of contraception use behavior among sexually active female adolescents in Taiwan. Res. Nurs. Health 2007, 30, 628–640. [Google Scholar] [CrossRef] [PubMed]
- Mieusset, R.; Grandjean, H.; Mansat, A.; Pontonnier, F. Inhibiting effect of artificial cryptorchidism on spermatogenesis. Fertil. Steril. 1985, 43, 589–594. [Google Scholar] [CrossRef]
- Fahim, M.S.; Fahim, Z.; Harman, J.; Thomson, I.; Montie, J.; Hall, D.G. Ultrasound as a new method of male contraception. Fertil. Steril. 1977, 28, 823–831. [Google Scholar]
- Green, C.D.; Ma, Q.; Manske, G.L.; Shami, A.N.; Zheng, X.; Marini, S.; Moritz, L.; Sultan, C.; Gurczynski, S.J.; Moore, B.B.; et al. A Comprehensive Roadmap of Murine Spermatogenesis Defined by Single-Cell RNA-Seq. Dev. Cell 2018, 46, 651–667.e10. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Liu, X.; Chang, G.; Chen, Y.; An, G.; Yan, L.; Gao, S.; Xu, Y.; Cui, Y.; Dong, J.; et al. Single-Cell RNA Sequencing Analysis Reveals Sequential Cell Fate Transition during Human Spermatogenesis. Cell Stem Cell 2018, 23, 599–614.e4. [Google Scholar] [CrossRef]
- Guo, J.; Nie, X.; Giebler, M.; Mlcochova, H.; Wang, Y.; Grow, E.J.; DonorConnect; Kim, R.; Tharmalingam, M.; Matilionyte, G.; et al. The Dynamic Transcriptional Cell Atlas of Testis Development during Human Puberty. Cell Stem Cell 2020, 26, 262–276.e4. [Google Scholar] [CrossRef]
- Zhang, X.; Cao, Q.; Rajachandran, S.; Grow, E.J.; Evans, M.; Chen, H. Dissecting mammalian reproduction with spatial transcriptomics. Hum. Reprod. Updat. 2023, 29, 794–810. [Google Scholar] [CrossRef]
- Cao, C.; Ma, Q.; Mo, S.; Shu, G.; Liu, Q.; Ye, J.; Gui, Y. Single-Cell RNA Sequencing Defines the Regulation of Spermatogenesis by Sertoli-Cell Androgen Signaling. Front. Cell Dev. Biol. 2021, 9, 763267. [Google Scholar] [CrossRef]
- Guo, J.; Grow, E.J.; Yi, C.; Mlcochova, H.; Maher, G.J.; Lindskog, C.; Murphy, P.J.; Wike, C.L.; Carrell, D.T.; Goriely, A.; et al. Chromatin and Single-Cell RNA-Seq Profiling Reveal Dynamic Signaling and Metabolic Transitions during Human Spermatogonial Stem Cell Development. Cell Stem Cell 2017, 21, 533–546.e6. [Google Scholar] [CrossRef]
- Guo, J.; Sosa, E.; Chitiashvili, T.; Nie, X.; Rojas, E.J.; Oliver, E.; DonorConnect; Plath, K.; Hotaling, J.M.; Stukenborg, J.B.; et al. Single-cell analysis of the developing human testis reveals somatic niche cell specification and fetal germline stem cell establishment. Cell Stem Cell 2021, 28, 764–778.e4. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.B.; Zhang, M.F.; Yang, F.; Hua, J.L. Applications of single-cell RNA sequencing in spermatogenesis and molecular evolution. Zool. Res. 2024, 45, 575–585. [Google Scholar] [CrossRef]
- Wang, H.-Q.; Wu, X.-L.; Zhang, J.; Wang, S.-T.; Sang, Y.-J.; Li, K.; Yang, C.-F.; Sun, F.; Li, C.-J. Meiotic transcriptional reprogramming mediated by cell-cell communications in humans and mice revealed by scATAC-seq and scRNA-seq. Zool. Res. 2024, 45, 601–616. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Cheng, X.; Huang, J.; Gao, Y.; Wang, D.; Feng, Z.; Zhai, G.; Lou, Q.; He, J.; Wang, Z.; et al. Rbm46, a novel germ cell-specific factor, modulates meiotic progression and spermatogenesis. Biol. Reprod. 2021, 104, 1139–1153. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-Q.; Wang, T.; Gao, F.; Ren, W.-Z. Application of CRISPR/Cas Technology in Spermatogenesis Research and Male Infertility Treatment. Genes 2022, 13, 1000. [Google Scholar] [CrossRef]
- Robles, V.; Valcarce, D.G.; Riesco, M.F. Non-coding RNA regulation in reproduction: Their potential use as biomarkers. Non-Coding RNA Res. 2019, 4, 54–62. [Google Scholar] [CrossRef]
- Li, X.; Sun, T.; Wang, X.; Tang, J.; Liu, Y. Restore natural fertility of Kitw/Kitwv mouse with nonobstructive azoospermia through gene editing on SSCs mediated by CRISPR-Cas9. Stem Cell Res. Ther. 2019, 10, 271. [Google Scholar] [CrossRef]
- Wang, Y.H.; Yan, M.; Zhang, X.; Liu, X.Y.; Ding, Y.F.; Lai, C.P.; Tong, M.H.; Li, J.S. Rescue of male infertility through correcting a genetic mutation causing meiotic arrest in spermatogonial stem cells. Asian J. Androl. 2021, 23, 590–599. [Google Scholar] [CrossRef]
- Chen, J.; Cai, T.; Zheng, C.; Lin, X.; Wang, G.; Liao, S.; Wang, X.; Gan, H.; Zhang, D.; Hu, X.; et al. MicroRNA-202 maintains spermatogonial stem cells by inhibiting cell cycle regulators and RNA binding proteins. Nucleic Acids Res. 2017, 45, 4142–4157. [Google Scholar] [CrossRef]
- Kulibin, A.Y.u.; Malolina, E.A. In vitro spermatogenesis: In search of fully defined conditions. Front. Cell Dev. Biol. 2023, 11, 1106111. [Google Scholar] [CrossRef]
- Sato, T.; Katagiri, K.; Gohbara, A.; Inoue, K.; Ogonuki, N.; Ogura, A.; Kubota, Y.; Ogawa, T. In vitro production of functional sperm in cultured neonatal mouse testes. Nature 2011, 471, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Stopel, A.; Lev, C.; Dahari, S.; Adibi, O.; Armon, L.; Gonen, N. Towards a “Testis in a Dish”: Generation of Mouse Testicular Organoids that Recapitulate Testis Structure and Expression Profiles. Int. J. Biol. Sci. 2024, 20, 1024–1041. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, M.; Yuan, Y.; Wang, X.; Fu, R.; Wan, H.; Xie, M.; Liu, M.; Guo, X.; Zheng, Y.; et al. Complete Meiosis from Embryonic Stem Cell-Derived Germ Cells In Vitro. Cell Stem Cell 2016, 18, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Singh, A.K. Impact of environmental factors on human semen quality and male fertility: A narrative review. Environ. Sci. Eur. 2022, 34, 6. [Google Scholar] [CrossRef]
- Wdowiak, N.; Wójtowicz, K.; Wdowiak-Filip, A.; Pucek, W.; Wróbel, A.; Wróbel, J.; Wdowiak, A. Environmental Factors as the Main Hormonal Disruptors of Male Fertility. J. Clin. Med. 2024, 13, 1986. [Google Scholar] [CrossRef]
- Gallo, A.; Boni, R.; Tosti, E. Gamete quality in a multistressor environment. Environ. Int. 2020, 138, 105627. [Google Scholar] [CrossRef]
- Szabó, A.; Váncsa, S.; Hegyi, P.; Váradi, A.; Forintos, A.; Filipov, T.; Ács, J.; Ács, N.; Szarvas, T.; Nyirády, P.; et al. Lifestyle-, environmental-, and additional health factors associated with an increased sperm DNA fragmentation: A systematic review and meta-analysis. Reprod. Biol. Endocrinol. 2023, 21, 5. [Google Scholar] [CrossRef]
- Nassan, F.L.; Chavarro, J.E.; Tanrikut, C. Diet and men’s fertility: Does diet affect sperm quality? Fertil. Steril. 2018, 110, 570–577. [Google Scholar] [CrossRef]
- Salas-Huetos, A.; James, E.R.; Aston, K.I.; Jenkins, T.G.; Carrell, D.T. Diet and sperm quality: Nutrients, foods and dietary patterns. Reprod. Biol. 2019, 19, 219–224. [Google Scholar] [CrossRef]
- Agarwal, A.; Singh, A.; Hamada, A.; Kesari, K. Cell Phones and Male Infertility: A Review of Recent Innovations in Technology and Consequences. Int. Braz. J. Urol. 2011, 37, 432–454. [Google Scholar] [CrossRef]
- Pall, M.L. Wi-Fi is an important threat to human health. Environ. Res. 2018, 164, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Sciorio, R.; Tramontano, L.; Esteves, S.C. Effects of mobile phone radiofrequency radiation on sperm quality. Zygote 2022, 30, 159–168. [Google Scholar] [CrossRef]
- Campbell, A.L.; Levitan, D.R.; Hosken, D.J.; Lewis, C. Ocean acidification changes the male fitness landscape. Sci. Rep. 2016, 6, 31250. [Google Scholar] [CrossRef]
- Hao, Y.; Feng, Y.; Yan, X.; Chen, L.; Ma, X.; Tang, X.; Zhong, R.; Sun, Z.; Agarwal, M.; Zhang, H.; et al. Gut Microbiota-Testis Axis: FMT Mitigates High-Fat Diet-Diminished Male Fertility via Improving Systemic and Testicular Metabolome. Microbiol. Spectr. 2022, 10, e0002822. [Google Scholar] [CrossRef]
- Hao, Y.; Feng, Y.; Yan, X.; Chen, L.; Zhong, R.; Tang, X.; Shen, W.; Sun, Q.; Sun, Z.; Ren, Y.; et al. Gut microbiota-testis axis: FMT improves systemic and testicular micro-environment to increase semen quality in type 1 diabetes. Mol. Med. 2022, 28, 45. [Google Scholar] [CrossRef]
- Lv, S.; Huang, J.; Luo, Y.; Wen, Y.; Chen, B.; Qiu, H.; Chen, H.; Yue, T.; He, L.; Feng, B.; et al. Gut microbiota is involved in male reproductive function: A review. Front. Microbiol. 2024, 15, 1371667. [Google Scholar] [CrossRef]
- Chen, W.; Zou, H.; Xu, H.; Cao, R.; Zhang, H.; Zhang, Y.; Zhao, J. The potential influence and intervention measures of gut microbiota on sperm: It is time to focus on testis-gut microbiota axis. Front. Microbiol. 2024, 15, 1478082. [Google Scholar] [CrossRef]
- Cai, H.; Cao, X.; Qin, D.; Liu, Y.; Liu, Y.; Hua, J.; Peng, S. Gut microbiota supports male reproduction via nutrition, immunity, and signaling. Front. Microbiol. 2022, 13, 977574. [Google Scholar] [CrossRef]
- Ishikura, Y.; Ohta, H.; Sato, T.; Murase, Y.; Yabuta, Y.; Kojima, Y.; Yamashiro, C.; Nakamura, T.; Yamamoto, T.; Ogawa, T.; et al. In vitro reconstitution of the whole male germ-cell development from mouse pluripotent stem cells. Cell Stem Cell 2021, 28, 2167–2179.e9. [Google Scholar] [CrossRef]
- Bourdon, G.; Cadoret, V.; Charpigny, G.; Couturier-Tarrade, A.; Dalbies-Tran, R.; Flores, M.-J.; Froment, P.; Raliou, M.; Reynaud, K.; Saint-Dizier, M.; et al. Progress and challenges in developing organoids in farm animal species for the study of reproduction and their applications to reproductive biotechnologies. Vet. Res. 2021, 52, 42. [Google Scholar] [CrossRef]
- Cham, T.-C.; Chen, X.; Honaramooz, A. Current progress, challenges, and future prospects of testis organoids. Biol. Reprod. 2021, 104, 942–961. [Google Scholar] [CrossRef]
- Sakib, S.; Yu, Y.; Voigt, A.; Ungrin, M.; Dobrinski, I. Generation of Porcine Testicular Organoids with Testis Specific Architecture using Microwell Culture. J. Vis. Exp. JoVE 2019, 152, e60387. [Google Scholar] [CrossRef]
- Cham, T.-C.; Ibtisham, F.; Al-Dissi, A.; Honaramooz, A. An in vitro testicular organoid model for the study of testis morphogenesis, somatic cell maturation, endocrine function, and toxicological assessment of endocrine disruptors. Reprod. Toxicol. 2024, 128, 108645. [Google Scholar] [CrossRef] [PubMed]
- Cho, I.K.; Easley, C.A. Recent Developments in In Vitro Spermatogenesis and Future Directions. Reprod. Med. 2023, 4, 215–232. [Google Scholar] [CrossRef]
- Fang, Y.; Akhtar, H.; Wang, J. The application of organoids in toxicity test of environmental pollutants. Cell Organoid. 2024. [Google Scholar] [CrossRef]
- Garcia AL, C.; Arlt, V.M.; Phillips, D.H. Organoids for toxicology and genetic toxicology: Applications with drugs and prospects for environmental carcinogenesis. Mutagenesis 2022, 37, 143–154. [Google Scholar] [CrossRef]
- Hu, C.; Yang, S.; Zhang, T.; Ge, Y.; Chen, Z.; Zhang, J.; Pu, Y.; Liang, G. Organoids and organoids-on-a-chip as the new testing strategies for environmental toxicology-applications & advantages. Environ. Int. 2024, 184, 108415. [Google Scholar] [CrossRef]
- Ibtisham, F.; Wu, J.; Xiao, M.; An, L.; Banker, Z.; Nawab, A.; Zhao, Y.; Li, G. Progress and future prospect of in vitro spermatogenesis. Oncotarget 2017, 8, 66709–66727. [Google Scholar] [CrossRef]


| Type | Elements | Description |
|---|---|---|
| Genes and transcription factors | Sry | Master regulator initiating testicular differentiation; activates SOX9 |
| Sox9 | Promotes seminiferous tubule formation and maintenance | |
| Plzf | Critical for spermatogonial stem cell maintenance; binds and represses differentiation-related genes | |
| Sohlh1, Sohlh2 | Spermatogonia regulation by suppresing or inducing related genes | |
| Gfra1, Ngn3, and Sox3 | Directly involved in spermatogenesis regulation | |
| Kit | Regulates spermatogonial differentiation | |
| Stra8 | Retinoid acid target; regulates spermatogonial differentiation | |
| Dmrt1 | Differentiating marker; prevents meiosis and promotes spermatogonia development | |
| Rbmy1, Pry, Daz, Dby | Directly involved in essential spermatogenesis processes | |
| Dazl | Promotes germ cell development, determination, and meiotic progression | |
| Tbx3, Utf1 | Regulate spermatogonial stem cell differentiation | |
| CREMt | Controls post-meiotic germ cell differentiation | |
| Post-Transcriptional Regulation | Genes | |
| Nanos2, Nanos3 | Encode RNA-binding protein (RBP) to regulate primordial germ cells and germ cell development via mRNA downregulation | |
| Tial1, Dnd1 | Encode RBP to regulate primordial germ cells | |
| Rbm46 | Encode RBP to regulate mRNA translation and stability; essential for meiosis and spermatogenesis | |
| Gdnf, Fgf, Bclb6, Etv5 | Promote SSC self-renewal and maintain the niche | |
| miRNA | ||
| miR-146 | Inhibits RA-mediated differentiation | |
| miR-221/222 | Maintain undifferentiated state | |
| miR-202-3p | Maintain undifferentiated state | |
| miR-202 | Related to lncRNA Gm2044 and spermatogonial arrest; regulates Rbfox2 | |
| lncRNA | ||
| Mrhl | Regulates Wnt and spermatogonia development | |
| Tsx | Regulates pachytene spermatocyte development | |
| Dmr | Negative regulator of Dmrt1 in male development | |
| Rbfox2 | Regulates cell proliferation and differentiation in spermatogenesis | |
| LNC_003902 | Regulation of key genes essential for spermatogenesis and testis development | |
| LNC_007258 | Regulation of key genes essential for spermatogenesis and testis development | |
| lncRNA Gm2044 | Related to miR-202 and spermatogonial arrest; regulates Rbfox2 | |
| PiRNAs | Silence transposable elements (TEs) during meiosis | |
| circRNAs | MiRNA sponges; inhibit miRNA functions and regulate gene expression network | |
| Epigenetic Regulation | DNA methylation | |
| N6-methyladenosine | Plays a crucial role in gametogenesis | |
| Dnmt1 | Essential for imprinting and germline-specific methylation | |
| Dnmt3a, Dnmt3l | Novo methylation during prenatal development | |
| Specialized chromatin structures | ||
| Protamines | ||
| Prm1/Prm2 | Required for meiosis and spermiogenesis to ensure proper DNA condensation and stability | |
| Taf7l, Tarbp2 | Protamine regulators | |
| Sycp3 | Meiotic chromosome compaction |
| Group | Specie | Spermatogenesis Length |
|---|---|---|
| Mammals | Human | 74 days [30] |
| Rat | 52 days [143] | |
| Mouse | ~35 days [144] | |
| Sirian hamster | ~35 days [145] | |
| Dog | ~61 days [146] | |
| Bovine cattle | 61 days [147] | |
| Horse | ~55–57 days [148] | |
| Pig | 40 days [149] | |
| Birds | Chicken | ~14 days [150] |
| Reptiles | Ex. Scorpion | 53 days [151] |
| Fish | Guppy | 36 days [152] |
| Trout (seasonal) | Weeks to months [153] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maroto, M.; Torvisco, S.N.; García-Merino, C.; Fernández-González, R.; Pericuesta, E. Mechanisms of Hormonal, Genetic, and Temperature Regulation of Germ Cell Proliferation, Differentiation, and Death During Spermatogenesis. Biomolecules 2025, 15, 500. https://doi.org/10.3390/biom15040500
Maroto M, Torvisco SN, García-Merino C, Fernández-González R, Pericuesta E. Mechanisms of Hormonal, Genetic, and Temperature Regulation of Germ Cell Proliferation, Differentiation, and Death During Spermatogenesis. Biomolecules. 2025; 15(4):500. https://doi.org/10.3390/biom15040500
Chicago/Turabian StyleMaroto, María, Sara N. Torvisco, Cristina García-Merino, Raúl Fernández-González, and Eva Pericuesta. 2025. "Mechanisms of Hormonal, Genetic, and Temperature Regulation of Germ Cell Proliferation, Differentiation, and Death During Spermatogenesis" Biomolecules 15, no. 4: 500. https://doi.org/10.3390/biom15040500
APA StyleMaroto, M., Torvisco, S. N., García-Merino, C., Fernández-González, R., & Pericuesta, E. (2025). Mechanisms of Hormonal, Genetic, and Temperature Regulation of Germ Cell Proliferation, Differentiation, and Death During Spermatogenesis. Biomolecules, 15(4), 500. https://doi.org/10.3390/biom15040500






