Melatonin MT1 Receptor Expression in Luminal Invasive Ductal Breast Carcinoma in Postmenopausal Women
Abstract
:1. Introduction
2. Materials and Methods
3. Results
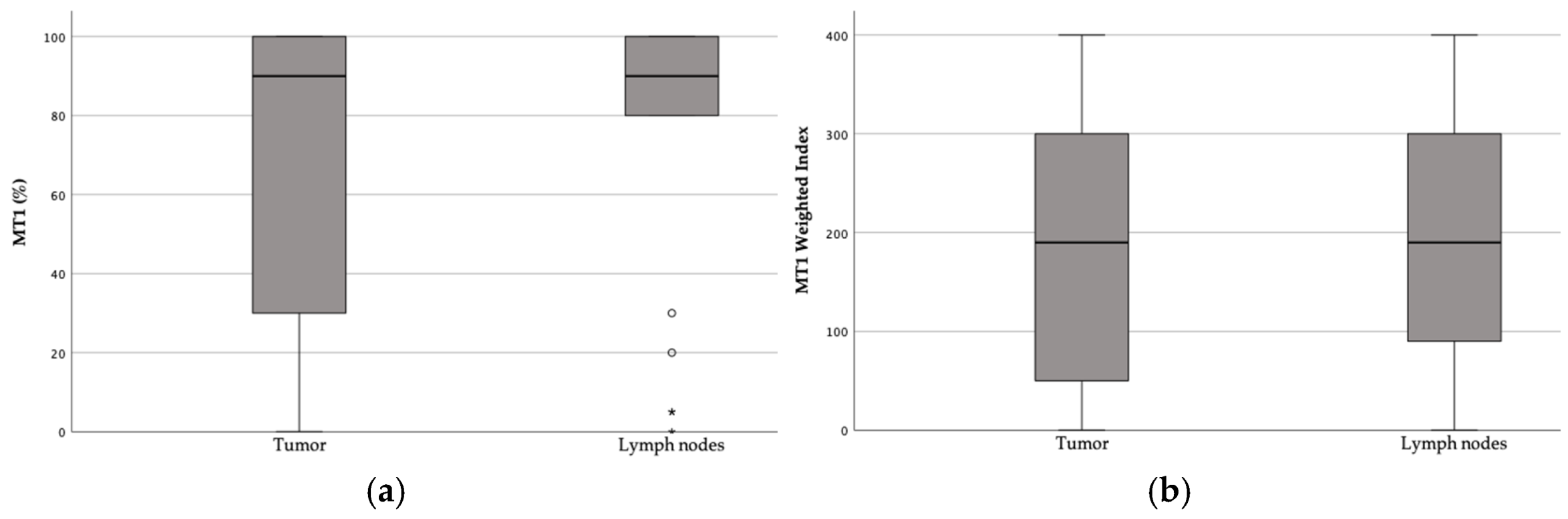
3.1. MT1 Receptor Expression
3.2. Correlation of MT1 Receptor Expression and WI with Patient and Tumor Characteristics
3.3. Survival Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tan, D.X.; Hardeland, R.; Manchester, L.C.; Paredes, S.D.; Korkmaz, A.; Sainz, R.M.; Mayo, J.C.; Fuentes-Broto, L.; Reiter, R.J. The changing biological roles of melatonin during evolution: From an antioxidant to signals of darkness, sexual selection and fitness. Biol. Rev. Camb. Philos. Soc. 2010, 85, 607–623. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Yu, Y.; Shen, Y.; Liu, Q.; Zhao, Z.; Sharma, R.; Reiter, R.J. Melatonin Synthesis and Function: Evolutionary History in Animals and Plants. Front. Endocrinol. 2019, 10, 249. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Galano, A. Melatonin: Exceeding expectations. Physiology 2014, 29, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Acuna-Castroviejo, D.; Escames, G.; Venegas, C.; Diaz-Casado, M.E.; Lima-Cabello, E.; Lopez, L.C.; Rosales-Corral, S.; Tan, D.-X.; Reiter, R.J. Extrapineal melatonin: Sources, regulation, and potential functions. Cell. Mol. Life Sci. 2014, 71, 2997–3025. [Google Scholar] [CrossRef]
- Samanta, S. Melatonin: An endogenous miraculous indolamine, fights against cancer progression. J. Cancer Res. Clin. Oncol. 2020, 146, 1893–1922. [Google Scholar] [CrossRef]
- Ma, H.; Kang, J.; Fan, W.; He, H.; Huang, F. ROR: Nuclear Receptor for Melatonin or Not? Molecules 2021, 26, 2693. [Google Scholar] [CrossRef]
- Dubocovich, M.L.; Delagrange, P.; Krause, D.N.; Sugden, D.; Cardinali, D.P.; Olcese, J. International Union of Basic and Clinical Pharmacology. LXXV. Nomenclature, classification, and pharmacology of G protein-coupled melatonin receptors. Pharmacol. Rev. 2010, 62, 343–380. [Google Scholar] [CrossRef]
- Jockers, R.; Delagrange, P.; Dubocovich, M.L.; Markus, R.P.; Renault, N.; Tosini, G.; Cecon, E.; Zlotos, D.P. Update on melatonin receptors: IUPHAR Review 20. Br. J. Pharmacol. 2016, 173, 2702–2725. [Google Scholar] [CrossRef]
- Stevens, R.G.; Davis, S. The melatonin hypothesis: Electric power and breast cancer. Environ. Health Perspect. 1996, 104 (Suppl. S1), 135–140. [Google Scholar]
- Schernhammer, E.S.; Laden, F.; Speizer, F.E.; Willett, W.C.; Hunter, D.J.; Kawachi, I.; Colditz, G.A. Rotating night shifts and risk of breast cancer in women participating in the nurses’ health study. J. Natl. Cancer Inst. 2001, 93, 1563–1568. [Google Scholar] [CrossRef]
- Manouchehri, E.; Taghipour, A.; Ghavami, V.; Ebadi, A.; Homaei, F.; Latifnejad Roudsari, R. Night-shift work duration and breast cancer risk: An updated systematic review and meta-analysis. BMC Women’s Health 2021, 21, 89. [Google Scholar] [CrossRef] [PubMed]
- Erren, T.C.; Reiter, R.J. Defining chronodisruption. J. Pineal Res. 2009, 46, 245–247. [Google Scholar] [CrossRef] [PubMed]
- Boutin, J.A.; Liberelle, M.; Yous, S.; Ferry, G.; Nepveu, F. Melatonin facts: Lack of evidence that melatonin is a radical scavenger in living systems. J. Pineal Res. 2024, 76, e12926. [Google Scholar] [CrossRef] [PubMed]
- Boutin, J.A.; Kennaway, D.J.; Jockers, R. Melatonin: Facts, Extrapolations and Clinical Trials. Biomolecules 2023, 13, 943. [Google Scholar] [CrossRef]
- Kennaway, D.J. Measuring melatonin by immunoassay. J. Pineal Res. 2020, 69, e12657. [Google Scholar] [CrossRef]
- Boutin, J.A.; de Almeida, V.H.; Coussay, N.; Legros, C.; Ferry, G.; Reybier, K. Melatonin facts: Melatonin lacks immuno-inflammation boosting capacities at the molecular and cellular levels. Biochimie 2024, 222, 195–202. [Google Scholar] [CrossRef]
- Kennaway, D.J. The mammalian gastro-intestinal tract is a NOT a major extra-pineal source of melatonin. J. Pineal Res. 2023, 75, e12906. [Google Scholar] [CrossRef]
- Bonnefond, A.; Froguel, P. Disentangling the Role of Melatonin and its Receptor MTNR1B in Type 2 Diabetes: Still a Long Way to Go? Curr. Diabetes Rep. 2017, 17, 122. [Google Scholar] [CrossRef]
- Nikolaev, G.; Robeva, R.; Konakchieva, R. Membrane Melatonin Receptors Activated Cell Signaling in Physiology and Disease. Int. J. Mol. Sci. 2021, 23, 471. [Google Scholar] [CrossRef]
- Bizzarri, M.; Proietti, S.; Cucina, A.; Reiter, R.J. Molecular mechanisms of the pro-apoptotic actions of melatonin in cancer: A review. Expert Opin. Ther. Targets 2013, 17, 1483–1496. [Google Scholar] [CrossRef]
- Chu, L.W.; John, E.M.; Yang, B.; Kurian, A.W.; Zia, Y.; Yu, K.; Ingles, S.A.; Stanczyk, F.Z.; Hsing, A.W. Measuring serum melatonin in postmenopausal women: Implications for epidemiologic studies and breast cancer studies. PLoS ONE 2018, 13, e0195666. [Google Scholar] [CrossRef] [PubMed]
- Boutin, J.A.; Jockers, R. Melatonin controversies, an update. J. Pineal Res. 2021, 70, e12702. [Google Scholar] [CrossRef] [PubMed]
- Harpsoe, N.G.; Andersen, L.P.; Gogenur, I.; Rosenberg, J. Clinical pharmacokinetics of melatonin: A systematic review. Eur. J. Clin. Pharmacol. 2015, 71, 901–909. [Google Scholar] [CrossRef]
- Andersen, L.P.; Gogenur, I.; Rosenberg, J.; Reiter, R.J. Pharmacokinetics of Melatonin: The Missing Link in Clinical Efficacy? Clin. Pharmacokinet. 2016, 55, 1027–1030. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Collins, A.R.; Dai, J.; Dubocovich, M.L.; Hill, S.M. MT(1) melatonin receptor overexpression enhances the growth suppressive effect of melatonin in human breast cancer cells. Mol. Cell. Endocrinol. 2002, 192, 147–156. [Google Scholar] [CrossRef]
- Jablonska, K.; Pula, B.; Zemla, A.; Owczarek, T.; Wojnar, A.; Rys, J.; Ambicka, A.; Podhorska-Okolow, M.; Ugorski, M.; Dziegiel, P. Expression of melatonin receptor MT1 in cells of human invasive ductal breast carcinoma. J. Pineal Res. 2013, 54, 334–345. [Google Scholar] [CrossRef]
- Goyal, R.; Gupta, T.; Bal, A.; Sahni, D.; Singh, G. Role of Melatonin in Breast Carcinoma: Correlation of Expression Patterns of Melatonin-1 Receptor With Estrogen, Progesterone, and HER2 Receptors. Appl. Immunohistochem. Mol. Morphol. 2019, 28, 518–523. [Google Scholar] [CrossRef]
- Dillon, D.C.; Easley, S.E.; Asch, B.B.; Cheney, R.T.; Brydon, L.; Jockers, R.; Winston, J.S.; Brooks, J.S.; Hurd, T.; Asch, H.L. Differential expression of high-affinity melatonin receptors (MT1) in normal and malignant human breast tissue. Am. J. Clin. Pathol. 2002, 118, 451–458. [Google Scholar] [CrossRef]
- Lai, L.; Yuan, L.; Cheng, Q.; Dong, C.; Mao, L.; Hill, S.M. Alteration of the MT1 melatonin receptor gene and its expression in primary human breast tumors and breast cancer cell lines. Breast Cancer Res. Treat. 2009, 118, 293–305. [Google Scholar] [CrossRef]
- Oprea-Ilies, G.; Haus, E.; Sackett-Lundeen, L.; Liu, Y.; McLendon, L.; Busch, R.; Adams, A.; Cohen, C. Expression of melatonin receptors in triple negative breast cancer (TNBC) in African American and Caucasian women: Relation to survival. Breast Cancer Res. Treat. 2013, 137, 677–687. [Google Scholar] [CrossRef]
- Cohen, M.; Lippman, M.; Chabner, B. Role of pineal gland in aetiology and treatment of breast cancer. Lancet 1978, 2, 814–846. [Google Scholar] [CrossRef] [PubMed]
- Tamarkin, L.; Danforth, D.; Lichter, A.; DeMoss, E.; Cohen, M.; Chabner, B.; Lippman, M. Decreased nocturnal plasma melatonin peak in patients with estrogen receptor positive breast cancer. Science 1982, 216, 1003–1005. [Google Scholar] [CrossRef] [PubMed]
- Wurtman, R.J. Age-related decreases in melatonin secretion--clinical consequences. J. Clin. Endocrinol. Metab. 2000, 85, 2135–2136. [Google Scholar]
- Lee, A.H.; Ellis, I.O. The Nottingham prognostic index for invasive carcinoma of the breast. Pathol. Oncol. Res. 2008, 14, 113–115. [Google Scholar] [CrossRef]
- de Castro, T.B.; Bordin-Junior, N.A.; de Almeida, E.A.; de Campos Zuccari, D.A.P. Evaluation of melatonin and AFMK levels in women with breast cancer. Endocrine 2018, 62, 242–249. [Google Scholar] [CrossRef]
- Danforth, D.N., Jr.; Tamarkin, L.; Mulvihill, J.J.; Bagley, C.S.; Lippman, M.E. Plasma melatonin and the hormone-dependency of human breast cancer. J. Clin. Oncol. 1985, 3, 941–948. [Google Scholar] [CrossRef]
- Sack, R.L.; Lewy, A.J.; Erb, D.L.; Vollmer, W.M.; Singer, C.M. Human melatonin production decreases with age. J. Pineal Res. 1986, 3, 379–388. [Google Scholar] [CrossRef]
- Tan, D.X.; Xu, B.; Zhou, X.; Reiter, R.J. Pineal Calcification, Melatonin Production, Aging, Associated Health Consequences and Rejuvenation of the Pineal Gland. Molecules 2018, 23, 301. [Google Scholar] [CrossRef]
- Reiter, R.J.; Sharma, R.; Ma, Q. Switching diseased cells from cytosolic aerobic glycolysis to mitochondrial oxidative phosphorylation: A metabolic rhythm regulated by melatonin? J. Pineal Res. 2021, 70, e12677. [Google Scholar] [CrossRef]
- Kontzoglou, K.; Palla, V.; Karaolanis, G.; Karaiskos, I.; Alexiou, I.; Pateras, I.; Konstantoudakis, K.; Stamatakos, M. Correlation between Ki67 and breast cancer prognosis. Oncology 2013, 84, 219–225. [Google Scholar] [CrossRef]
- Park, S.; Koo, J.S.; Kim, M.S.; Park, H.S.; Lee, J.S.; Lee, J.S.; Kim, S.I.; Park, B.-W. Characteristics and outcomes according to molecular subtypes of breast cancer as classified by a panel of four biomarkers using immunohistochemistry. Breast 2012, 21, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Colleoni, M.; Sun, Z.; Price, K.N.; Karlsson, P.; Forbes, J.F.; Thurlimann, B.; Gianni, L.; Castiglione, M.; Gelber, R.D.; Coates, A.S.; et al. Annual Hazard Rates of Recurrence for Breast Cancer During 24 Years of Follow-Up: Results From the International Breast Cancer Study Group Trials I to V. J. Clin. Oncol. 2016, 34, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Li, C.I.; Uribe, D.J.; Daling, J.R. Clinical characteristics of different histologic types of breast cancer. Br. J. Cancer 2005, 93, 1046–1052. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Sharma, R.; Tan, D.X.; Chuffa, L.G.A.; da Silva, D.G.H.; Slominski, A.T.; Steinbrink, K.; Kleszczynski, K. Dual sources of melatonin and evidence for different primary functions. Front. Endocrinol. 2024, 15, 1414463. [Google Scholar] [CrossRef]
- Bonmati-Carrion, M.A.; Tomas-Loba, A. Melatonin and Cancer: A Polyhedral Network Where the Source Matters. Antioxidants 2021, 10, 210. [Google Scholar] [CrossRef]
- Suofu, Y.; Li, W.; Jean-Alphonse, F.G.; Jia, J.; Khattar, N.K.; Li, J.; Baranov, S.V.; Leronni, D.; Mihalik, A.C.; He, Y.; et al. Dual role of mitochondria in producing melatonin and driving GPCR signaling to block cytochrome c release. Proc. Natl. Acad. Sci. USA 2017, 114, E7997–E8006. [Google Scholar] [CrossRef]
- Tan, D.X.; Manchester, L.C.; Esteban-Zubero, E.; Zhou, Z.; Reiter, R.J. Melatonin as a Potent and Inducible Endogenous Antioxidant: Synthesis and Metabolism. Molecules 2015, 20, 18886–18906. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef]
- Reiter, R.J.; Sharma, R.; Pires de Campos Zuccari, D.A.; de Almeida Chuffa, L.G.; Manucha, W.; Rodriguez, C. Melatonin synthesis in and uptake by mitochondria: Implications for diseased cells with dysfunctional mitochondria. Future Med. Chem. 2021, 13, 335–339. [Google Scholar] [CrossRef]
- Franco, P.I.R.; do Carmo Neto, J.R.; Milhomem, A.C.; Machado, J.R.; Miguel, M.P. Antitumor effect of melatonin on breast cancer in experimental models: A systematic review. Biochim. Biophys. Acta Rev. Cancer 2022, 1878, 188838. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Manchester, L.C.; Pilar Terron, M.; Flores, L.J.; Koppisepi, S. Medical implications of melatonin: Receptor-mediated and receptor-independent actions. Adv. Med. Sci. 2007, 52, 11–28. [Google Scholar] [PubMed]
- Dubocovich, M.L.; Rivera-Bermudez, M.A.; Gerdin, M.J.; Masana, M.I. Molecular pharmacology, regulation and function of mammalian melatonin receptors. Front. Biosci. 2003, 8, d1093–d1108. [Google Scholar] [CrossRef] [PubMed]
- Pariente, R.; Bejarano, I.; Espino, J.; Rodriguez, A.B.; Pariente, J.A. Participation of MT3 melatonin receptors in the synergistic effect of melatonin on cytotoxic and apoptotic actions evoked by chemotherapeutics. Cancer Chemother. Pharmacol. 2017, 80, 985–998. [Google Scholar] [CrossRef]
- Targhazeh, N.; Reiter, R.J.; Rahimi, M.; Qujeq, D.; Yousefi, T.; Shahavi, M.H.; Mir, S.M. Oncostatic activities of melatonin: Roles in cell cycle, apoptosis, and autophagy [Biochimie 200 (2022) 44–59]. Biochimie 2022, 200, 44–59. [Google Scholar] [CrossRef]
- Boutin, J.A. Quinone reductase 2 as a promising target of melatonin therapeutic actions. Expert Opin. Ther. Targets 2016, 20, 303–317. [Google Scholar] [CrossRef]
- Jin, Y.; Choi, Y.J.; Heo, K.; Park, S.J. Melatonin as an Oncostatic Molecule Based on Its Anti-Aromatase Role in Breast Cancer. Int. J. Mol. Sci. 2021, 22, 438. [Google Scholar] [CrossRef]
- Sanchez-Barcelo, E.J.; Cos, S.; Mediavilla, D.; Martinez-Campa, C.; Gonzalez, A.; Alonso-Gonzalez, C. Melatonin-estrogen interactions in breast cancer. J. Pineal Res. 2005, 38, 217–222. [Google Scholar] [CrossRef]
- Cos, S.; Gonzalez, A.; Martinez-Campa, C.; Mediavilla, M.D.; Alonso-Gonzalez, C.; Sanchez-Barcelo, E.J. Estrogen-signaling pathway: A link between breast cancer and melatonin oncostatic actions. Cancer Detect. Prev. 2006, 30, 118–128. [Google Scholar] [CrossRef]
- Mills, E.; Wu, P.; Seely, D.; Guyatt, G. Melatonin in the treatment of cancer: A systematic review of randomized controlled trials and meta-analysis. J. Pineal Res. 2005, 39, 360–366. [Google Scholar] [CrossRef]
- Wang, Y.M.; Jin, B.Z.; Ai, F.; Duan, C.H.; Lu, Y.Z.; Dong, T.F.; Fu, Q.-L. The efficacy and safety of melatonin in concurrent chemotherapy or radiotherapy for solid tumors: A meta-analysis of randomized controlled trials. Cancer Chemother. Pharmacol. 2012, 69, 1213–1220. [Google Scholar] [CrossRef]
- Lissoni, P.; Ardizzoia, A.; Barni, S.; Paolorossi, F.; Tancini, G.; Meregalli, S.; Esposti, D.; Zubelewicz, B.; Braczowski, R. A randomized study of tamoxifen alone versus tamoxifen plus melatonin in estrogen receptor-negative heavily pretreated metastatic breast-cancer patients. Oncol. Rep. 1995, 2, 871–873. [Google Scholar] [CrossRef] [PubMed]
- Lissoni, P.; Barni, S.; Meregalli, S.; Fossati, V.; Cazzaniga, M.; Esposti, D.; Tancini, G. Modulation of cancer endocrine therapy by melatonin: A phase II study of tamoxifen plus melatonin in metastatic breast cancer patients progressing under tamoxifen alone. Br. J. Cancer 1995, 71, 854–856. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Gonzalez, C.; Menendez-Menendez, J.; Gonzalez-Gonzalez, A.; Gonzalez, A.; Cos, S.; Martinez-Campa, C. Melatonin enhances the apoptotic effects and modulates the changes in gene expression induced by docetaxel in MCF7 human breast cancer cells. Int. J. Oncol. 2018, 52, 560–570. [Google Scholar] [PubMed]
- Najafi, M.; Salehi, E.; Farhood, B.; Nashtaei, M.S.; Hashemi Goradel, N.; Khanlarkhani, N.; Namjoo, Z.; Mortezaee, K. Adjuvant chemotherapy with melatonin for targeting human cancers: A review. J. Cell. Physiol. 2019, 234, 2356–2372. [Google Scholar] [CrossRef]
- Alonso-Gonzalez, C.; Gonzalez, A.; Martinez-Campa, C.; Gomez-Arozamena, J.; Cos, S. Melatonin sensitizes human breast cancer cells to ionizing radiation by downregulating proteins involved in double-strand DNA break repair. J. Pineal Res. 2015, 58, 189–197. [Google Scholar] [CrossRef]
- Alonso-Gonzalez, C.; Gonzalez, A.; Martinez-Campa, C.; Menendez-Menendez, J.; Gomez-Arozamena, J.; Garcia-Vidal, A.; Cos, S. Melatonin enhancement of the radiosensitivity of human breast cancer cells is associated with the modulation of proteins involved in estrogen biosynthesis. Cancer Lett. 2016, 370, 145–152. [Google Scholar] [CrossRef]
- Reiter, R.J.; Rosales-Corral, S.A.; Tan, D.X.; Acuna-Castroviejo, D.; Qin, L.; Yang, S.F.; Xu, K. Melatonin, a Full Service Anti-Cancer Agent: Inhibition of Initiation, Progression and Metastasis. Int. J. Mol. Sci. 2017, 18, 843. [Google Scholar] [CrossRef]
- Mao, L.; Cheng, Q.; Guardiola-Lemaitre, B.; Schuster-Klein, C.; Dong, C.; Lai, L.; Hill, S.M. In Vitro and In Vivo antitumor activity of melatonin receptor agonists. J. Pineal Res. 2010, 49, 210–221. [Google Scholar] [CrossRef]
- Hasan, M.; Marzouk, M.A.; Adhikari, S.; Wright, T.D.; Miller, B.P.; Matossian, M.D.; Elliott, S.; Wright, M.; Alzoubi, M.; Collins-Burow, B.M.; et al. Pharmacological, Mechanistic, and Pharmacokinetic Assessment of Novel Melatonin-Tamoxifen Drug Conjugates as Breast Cancer Drugs. Mol. Pharmacol. 2019, 96, 272–296. [Google Scholar] [CrossRef]
- Foley, H.M.; Steel, A.E. Adverse events associated with oral administration of melatonin: A critical systematic review of clinical evidence. Complement. Ther. Med. 2019, 42, 65–81. [Google Scholar] [CrossRef]
- Besag, F.M.C.; Vasey, M.J.; Lao, K.S.J.; Wong, I.C.K. Adverse Events Associated with Melatonin for the Treatment of Primary or Secondary Sleep Disorders: A Systematic Review. CNS Drugs 2019, 33, 1167–1186. [Google Scholar] [CrossRef] [PubMed]
- Kennaway, D.J. What do we really know about the safety and efficacy of melatonin for sleep disorders? Curr. Med. Res. Opin. 2022, 38, 211–227. [Google Scholar] [CrossRef] [PubMed]
- Andersen, L.P.; Gogenur, I.; Rosenberg, J.; Reiter, R.J. The Safety of Melatonin in Humans. Clin. Drug Investig. 2016, 36, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Sadoughi, F.; Dana, P.M.; Asemi, Z.; Shafabakhash, R.; Mohammadi, S.; Heidar, Z.; Mirzamoradi, M.; Targhazeh, N.; Mirzaei, H. Molecular and cellular mechanisms of melatonin in breast cancer. Biochimie 2022, 202, 26–33. [Google Scholar] [CrossRef]
- Giordano, F.; Paoli, A.; Forastiero, M.; Marsico, S.; De Amicis, F.; Marrelli, M.; Naimo, G.D.; Mauro, L.; Panno, M.L. Valproic acid inhibits cell growth in both MCF-7 and MDA-MB231 cells by triggering different responses in a cell type-specific manner. J. Transl. Med. 2023, 21, 165. [Google Scholar] [CrossRef]
- Jawed, S.; Kim, B.; Ottenhof, T.; Brown, G.M.; Werstiuk, E.S.; Niles, L.P. Human melatonin MT1 receptor induction by valproic acid and its effects in combination with melatonin on MCF-7 breast cancer cell proliferation. Eur. J. Pharmacol. 2007, 560, 17–22. [Google Scholar] [CrossRef]
- Kal Omar, R.; Hagstrom, A.; Stalhammar, G. Adjuvant melatonin for uveal melanoma (AMUM): Protocol for a randomized open-label phase III study. Trials 2023, 24, 230. [Google Scholar] [CrossRef]
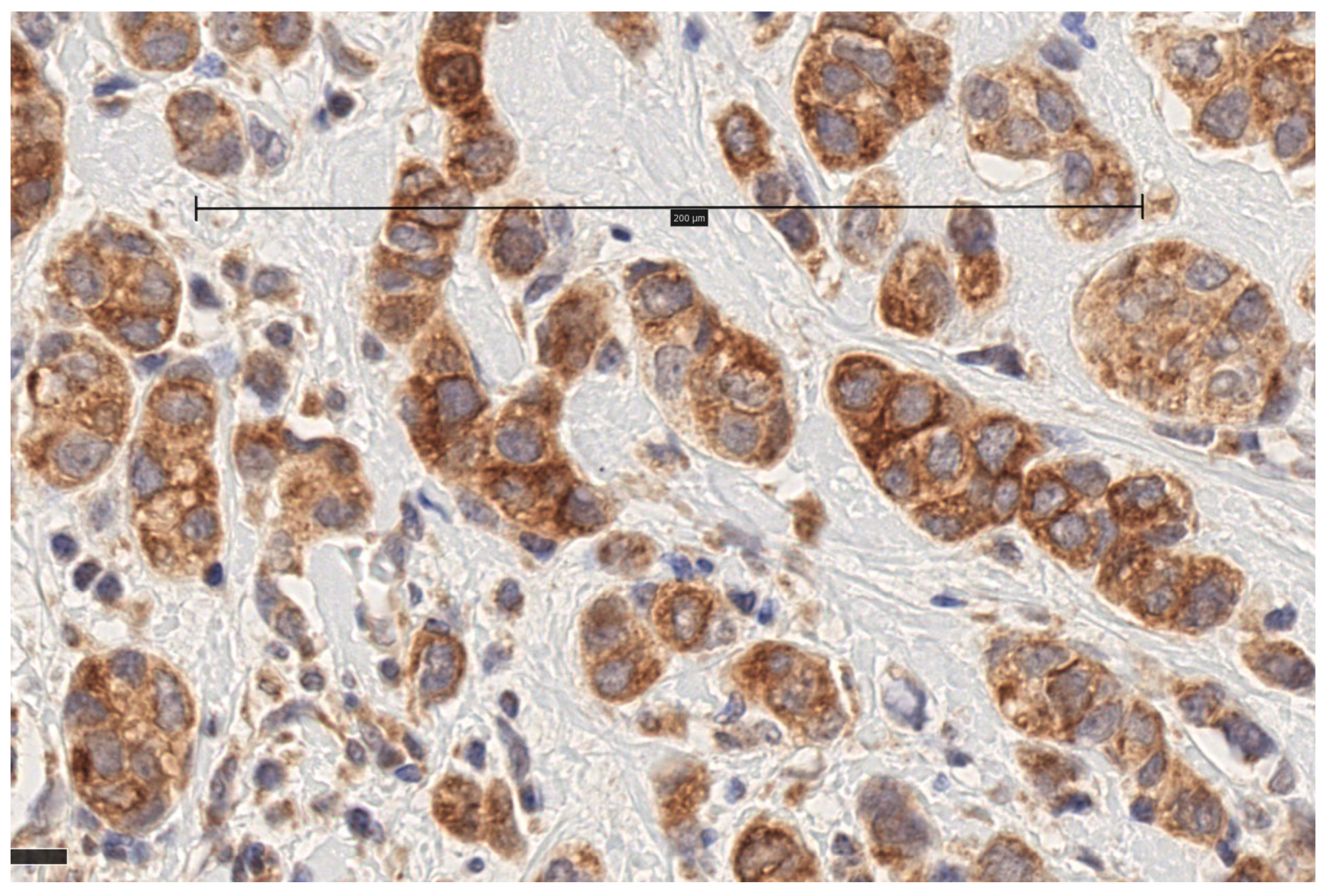



| Median (IQR) | Patients (%) | ||
|---|---|---|---|
| Age | 66 (61–79) | ||
| Breast surgery | Mastectomy | 45 (38.1%) | |
| Breast conservation | 73 (61.9%) | ||
| Axillary surgery | Not performed | 18 (15.3%) | |
| SLNB only 1 | 35 (29.7%) | ||
| ALND 2 | 65 (55.1%) | ||
| Tumor size (mm) | 17 (11–26) | ||
| NHG | Grade I | 40 (33.9%) | |
| Grade II | 55 (46.6%) | ||
| Grade III | 23 (19.5%) | ||
| ER (%) | 100 (100–100) | ||
| PR (%) | 70 (20–90) | ||
| Ki67 (%) | 10 (9–15) | ||
| TP53 (%) | 0 (0–10) | ||
| Axillary nodal status | Nx | 18 (15.3%) | |
| N0 | 67 (56.8%) | ||
| N1 | 21 (17.8%) | ||
| N2 | 5 (4.2%) | ||
| N3 | 7 (5.9%) | ||
| NPI | 3.39 (3.14–4.56) |
| Variable | Spearman’s r | p |
|---|---|---|
| Age | 0.073 | 0.433 |
| Tumor size | 0.416 | 0.416 |
| ER | −0.132 | 0.157 |
| PR | −0.037 | 0.693 |
| Ki67 | 0.101 | 0.278 |
| TP53 | 0.069 | 0.465 |
| NHG | 0.037 | 0.691 |
| NPI | −0.016 | 0.874 |
| MT1 nodes | −0.004 | 0.986 |
| Variable | Spearman’s r | p |
|---|---|---|
| Age | 0.084 | 0.368 |
| Tumor size | 0.137 | 0.138 |
| ER | −0.111 | 0.233 |
| PR | −0.054 | 0.563 |
| Ki67 | 0.154 | 0.096 |
| TP53 | 0.034 | 0.718 |
| NHG | 0.105 | 0.644 |
| NPI | 0.046 | 0.644 |
| WI nodes | −0.100 | 0.635 |
| OS | BCSS | RFI | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | |
| Patient age | 1.096 (1.067–1.126) | <0.001 | 1.052 (0.993–1.113) | 0.084 | 1.016 (0.971–1.063) | 0.495 |
| Tumor size | 1.015 (1.000–1.030) | 0.057 | 1.030 (1.004–1.056) | 0.022 | 1.032 (1.010–1.054) | 0.004 |
| NHG | ||||||
| Grade I | Ref | 0.068 | Ref | 0.175 | Ref | 0.126 |
| Grade II | 0.812 (0.490–1.344) | 0.417 | 6.929 (0.886–54.166) | 0.065 | 3.593 (1.032–12.509) | 0.044 |
| Grade III | 0.807 (0.410–1.588) | 0.534 | 6.851 (0.764–61.406) | 0.085 | 3.366 (0.803–14.099) | 0.097 |
| ER | 1.016 (0.995–1.036) | 0.138 | 1.019 (0.972–1.068) | 0.433 | 1.031 (0.978–1.087) | 0.255 |
| PR | 1.004 (0.998–1.011) | 0.194 | 0.999 (0.985–1.012) | 0.846 | 0.997 (0.986–1.008) | 0.593 |
| Ki67 | 0.998 (0.983–1.013) | 0.804 | 1.029 (1.008–1.050) | 0.006 | 1.020 (1.000–1.039) | 0.046 |
| TP53 | 0.987 (0.973–1.002) | 0.094 | 0.981 (0.941–1.022) | 0.362 | 0.969 (0.924–1.018) | 0.211 |
| N status | ||||||
| N0 | Ref | 0.402 | Ref | 0.584 | Ref | 0.029 |
| N1 | 1.065 (0.312–3.637) | 0.921 | 4.920 (0.307–78.812) | 0.260 | 29.767 (3.013–294.085) | 0.004 |
| N2 | 3.491 (0.770–15.831) | 0.105 | 0.000 (0.000–.) | 0.996 | 24.332 (1.412–419.289) | 0.028 |
| N3 | 0.755 (0.174–3.271) | 0.707 | 6.066 (0.379–97.190) | 0.203 | 7.595 (0.466–123.670) | 0.154 |
| NPI | 1.162 (0.934–1.445) | 0.179 | 2.803 (1.724–4.558) | <0.001 | 2.450 (1.667–3.602) | <0.001 |
| MT1 tumor | 1.001 (0.996–1.008) | 0.624 | 1.001 (0.988–1.014) | 0.877 | 1.002 (0.991–1.013) | 0.728 |
| WI tumor | 1.000 (0.998–1.002) | 0.851 | 1.000 (0.998–1.002) | 0.919 | 1.000 (0.997–1.003) | 0.903 |
| BCSS | RFI | |||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Patient age | 1.053 (0.990–1.120) | 0.099 | 1.036 (0.962–1.115) | 0.351 |
| NPI | 2.657 (1.659–4.258) | <0.001 | 1.572 (0.772–3.199) | 0.212 |
| MT1 tumor | 0.999 (0.984–1.013) | 0.844 | 1.001 (0.981–1.022) | 0.929 |
| BCSS | RFI | |||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Patient age | 1.055 (0.991–1.122) | 0.093 | 1.035 (0.965–1.110) | 0.330 |
| NPI | 2.688 (1.665–4.340) | <0.001 | 1.567 (0.772–3.182) | 0.214 |
| WI tumor | 0.999 (0.995–1.004) | 0.731 | 1.000 (0.996–1.005) | 0.830 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pistiolis, L.; Alawieh, S.; Halldorsdottir, T.; Kovács, A.; Olofsson Bagge, R. Melatonin MT1 Receptor Expression in Luminal Invasive Ductal Breast Carcinoma in Postmenopausal Women. Biomolecules 2025, 15, 581. https://doi.org/10.3390/biom15040581
Pistiolis L, Alawieh S, Halldorsdottir T, Kovács A, Olofsson Bagge R. Melatonin MT1 Receptor Expression in Luminal Invasive Ductal Breast Carcinoma in Postmenopausal Women. Biomolecules. 2025; 15(4):581. https://doi.org/10.3390/biom15040581
Chicago/Turabian StylePistiolis, Leda, Sahar Alawieh, Thorhildur Halldorsdottir, Anikó Kovács, and Roger Olofsson Bagge. 2025. "Melatonin MT1 Receptor Expression in Luminal Invasive Ductal Breast Carcinoma in Postmenopausal Women" Biomolecules 15, no. 4: 581. https://doi.org/10.3390/biom15040581
APA StylePistiolis, L., Alawieh, S., Halldorsdottir, T., Kovács, A., & Olofsson Bagge, R. (2025). Melatonin MT1 Receptor Expression in Luminal Invasive Ductal Breast Carcinoma in Postmenopausal Women. Biomolecules, 15(4), 581. https://doi.org/10.3390/biom15040581






