Optimizing Immunotherapy: The Synergy of Immune Checkpoint Inhibitors with Artificial Intelligence in Melanoma Treatment
Abstract
1. Introduction
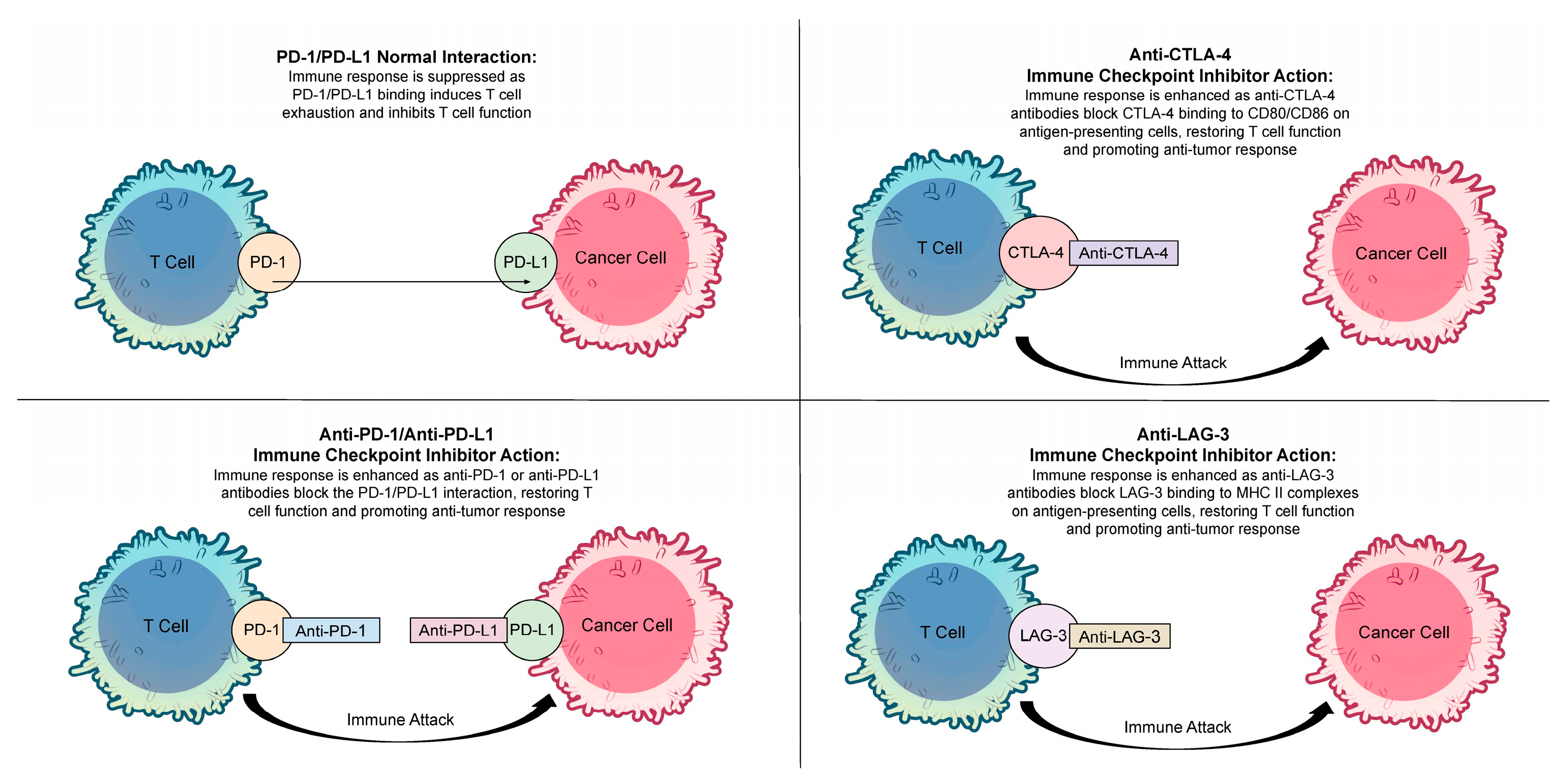
2. Immune Checkpoint Inhibitors
3. Artificial Intelligence and Prediction of Immune Responses with Immune Checkpoint Inhibitors
3.1. PD-L1 Expression Assessment
3.2. Assessment of Resistance to Immune Checkpoint Inhibitors
3.3. Clinical Management of Immune-Related Adverse Events
3.4. Deep Learning Applications in Treatment Response Prediction
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nierengarten, M.B. Cancer Statistics 2024: Deaths drop, incidences increase, prevention needed. Cancer 2024, 130, 1904. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef]
- Saginala, K.; Barsouk, A.; Aluru, J.S.; Rawla, P.; Barsouk, A. Epidemiology of Melanoma. Med. Sci. 2021, 20, 63. [Google Scholar] [CrossRef]
- Didier, A.J.; Nandwani, S.V.; Watkins, D.; Fahoury, A.M.; Campbell, A.; Craig, D.J.; Vijendra, D.; Parquet, N. Patterns and trends in melanoma mortality in the United States. BMC Cancer 2024, 27, 790. [Google Scholar] [CrossRef]
- Knight, A.; Karapetyan, L.; Kirkwood, J.M. Immunotherapy in Melanoma: Recent Advances and Future Directions. Cancers 2023, 15, 1106. [Google Scholar] [CrossRef]
- Alturki, N.A. Review of the Immune Checkpoint Inhibitors in the Context of Cancer Treatment. J. Clin. Med. 2023, 12, 4301. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi Rad, H.; Monkman, J.; Warkiani, M.E.; Ladwa, R.; O’Byrne, K.; Rezaei, N.; Kulasinghe, A. Understanding the tumor microenvironment for effective immunotherapy. Med. Res. Rev. 2021, 41, 1474–1498. [Google Scholar] [CrossRef] [PubMed]
- Keir, M.E.; Butte, M.J.; Freeman, G.J.; Sharpe, A.H. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 2008, 26, 677–704. [Google Scholar] [CrossRef]
- Barber, D.L.; Wherry, E.J.; Masopust, D.; Zhu, B.; Allison, J.P.; Sharpe, A.H.; Freeman, G.J.; Ahmed, R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 2006, 439, 682–687. [Google Scholar] [CrossRef]
- LaFleur, M.W.; Muroyama, Y.; Drake, C.G.; Sharpe, A.H. Inhibitors of the PD-1 Pathway in Tumor Therapy. J. Immunol. 2018, 200, 375–383. [Google Scholar] [CrossRef]
- Patel, S.P.; Kurzrock, R. PD-L1 Expression as a Predictive Biomarker in Cancer Immunotherapy. Mol. Cancer Ther. 2015, 14, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Mandai, M.; Hamanishi, J.; Abiko, K.; Matsumura, N.; Baba, T.; Konishi, I. Dual Faces of IFNgamma in Cancer Progression: A Role of PD-L1 Induction in the Determination of Pro- and Antitumor Immunity. Clin. Cancer Res. 2016, 22, 2329–2334. [Google Scholar] [CrossRef] [PubMed]
- Vaddepally, R.K.; Kharel, P.; Pandey, R.; Garje, R.; Chandra, A.B. Review of Indications of FDA-Approved Immune Checkpoint Inhibitors per NCCN Guidelines with the Level of Evidence. Cancers 2020, 12, 738. [Google Scholar] [CrossRef]
- Azarov, I.P.K.; Helmlinger, G.; Kosinsky, Y. Role of T cell-to-dendritic cell chemoattraction in T cell priming initiation in the lymph node: An agent-based modeling study. Front. Immunol. 2019, 10, 1289. [Google Scholar] [CrossRef]
- Buchbinder, E.I.; Desai, A. CTLA-4 and PD-1 Pathways: Similarities, Differences, and Implications of Their Inhibition. Am. J. Clin. Oncol. 2016, 39, 98–106. [Google Scholar] [CrossRef]
- Lin, X.; Kang, K.; Chen, P.; Zeng, Z.; Li, G.; Xiong, W.; Yi, M.; Xiang, B. Regulatory mechanisms of PD-1/PD-L1 in cancers. Mol. Cancer 2024, 23, 108. [Google Scholar] [CrossRef]
- Das, R.; Verma, R.; Sznol, M.; Boddupalli, C.S.; Gettinger, S.N.; Kluger, H.; Callahan, M.; Wolchok, J.D.; Halaban, R.; Dhodapkar, M.V.; et al. Combination therapy with anti-CTLA-4 and anti-PD-1 leads to distinct immunologic changes in vivo. J. Immunol. 2015, 194, 950–959. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Wu, L.; Han, L.; Zheng, X.; Tong, R.; Li, L.; Bai, L.; Bian, Y. Immune-related adverse events of immune checkpoint inhibitors: A review. Front. Immunol. 2023, 14, 1167975. [Google Scholar] [CrossRef]
- Wang, R.; Shao, X.; Zheng, J.; Saci, A.; Qian, X.; Pak, I.; Roy, A.; Bello, A.; Rizzo, J.I.; Hosein, F.; et al. A Machine-Learning Approach to Identify a Prognostic Cytokine Signature That Is Associated With Nivolumab Clearance in Patients with Advanced Melanoma. Clin. Pharmacol. Ther. 2020, 107, 978–987. [Google Scholar] [CrossRef]
- Johannet, P.; Coudray, N.; Donnelly, D.M.; Jour, G.; Illa-Bochaca, I.; Xia, Y.; Johnson, D.B.; Wheless, L.; Patrinely, J.R.; Nomikou, S.; et al. Using Machine Learning Algorithms to Predict Immunotherapy Response in Patients with Advanced Melanoma. Clin. Cancer Res. 2021, 27, 131–140. [Google Scholar] [CrossRef]
- Koelzer, V.H.; Gisler, A.; Hanhart, J.C.; Griss, J.; Wagner, S.N.; Willi, N.; Cathomas, G.; Sachs, M.; Kempf, W.; Thommen, D.S.; et al. Digital image analysis improves precision of PD-L1 scoring in cutaneous melanoma. Histopathology 2018, 73, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Huo, M.; Liu, X.; Li, S.C. Biomarkers and computational models for predicting efficacy to tumor ICI immunotherapy. Front. Immunol. 2024, 15, 1368749. [Google Scholar] [CrossRef] [PubMed]
- Zeng, T.; Zhang, J.Z.; Stromberg, A.; Chen, J.; Wang, C. Strategies for improving the performance of prediction models for response to immune checkpoint blockade therapy in cancer. BMC Res. Notes 2024, 17, 102. [Google Scholar] [CrossRef]
- Baxi, V.; Lee, G.; Duan, C.; Pandya, D.; Cohen, D.N.; Edwards, R.; Chang, H.; Li, J.; Elliott, H.; Pokkalla, H.; et al. Association of artificial intelligence-powered and manual quantification of programmed death-ligand 1 (PD-L1) expression with outcomes in patients treated with nivolumab ± ipilimumab. Mod. Pathol. 2022, 35, 1529–1539. [Google Scholar] [CrossRef]
- Sahni, S.; Wang, B.; Wu, D.; Dhruba, S.R.; Nagy, M.; Patkar, S.; Ferreira, I.; Day, C.P.; Wang, K.; Ruppin, E. A machine learning model reveals expansive downregulation of ligand-receptor interactions that enhance lymphocyte infiltration in melanoma with developed resistance to immune checkpoint blockade. Nat. Commun. 2024, 15, 8867. [Google Scholar] [CrossRef]
- Schonfeld, S.J.; Tucker, M.A.; Engels, E.A.; Dores, G.M.; Sampson, J.N.; Shiels, M.S.; Chanock, S.J.; Morton, L.M. Immune-Related Adverse Events After Immune Checkpoint Inhibitors for Melanoma Among Older Adults. JAMA Netw. Open 2022, 5, e223461. [Google Scholar] [CrossRef] [PubMed]
- Lippenszky, L.; Mittendorf, K.F.; Kiss, Z.; LeNoue-Newton, M.L.; Napan-Molina, P.; Rahman, P.; Ye, C.; Laczi, B.; Csernai, E.; Jain, N.M.; et al. Prediction of Effectiveness and Toxicities of Immune Checkpoint Inhibitors Using Real-World Patient Data. JCO Clin. Cancer Inform. 2024, 8, e2300207. [Google Scholar] [CrossRef]
- Burnette, H.; Pabani, A.; von Itzstein, M.S.; Switzer, B.; Fan, R.; Ye, F.; Puzanov, I.; Naidoo, J.; Ascierto, P.A.; Gerber, D.E.; et al. Use of artificial intelligence chatbots in clinical management of immune-related adverse events. J. Immunother. Cancer 2024, 12, e008599. [Google Scholar] [CrossRef]
- Hu, J.; Cui, C.; Yang, W.; Huang, L.; Yu, R.; Liu, S.; Kong, Y. Using deep learning to predict anti-PD-1 response in melanoma and lung cancer patients from histopathology images. Transl. Oncol. 2021, 14, 100921. [Google Scholar] [CrossRef]
- Higgins, H.; Nakhla, A.; Lotfalla, A.; Khalil, D.; Doshi, P.; Thakkar, V.; Shirini, D.; Bebawy, M.; Ammari, S.; Postow, M. Recent Advances in the Field of Artificial Intelligence for Precision Medicine in Patients with a Diagnosis of Metastatic Cutaneous Melanoma. Diagnostics 2023, 13, 3483. [Google Scholar] [CrossRef]
- Chatziioannou, E.; Roßner, J.; Aung, T.N.; Rimm, D.L.; Niessner, H.; Keim, U.; Serna-Higuita, L.M.; Bonzheim, I.; Cuellar, L.K.; Westphal, D.; et al. Deep learning-based scoring of tumour-infiltrating lymphocytes is prognostic in primary melanoma and predictive to PD-1 checkpoint inhibition in melanoma metastases. EBioMedicine 2023, 93, 104644. [Google Scholar] [CrossRef] [PubMed]
- Yeghaian, M.; Bodalal, Z.; Bucho, T.T.; Kurilova, I.; Blank, C.U.; Smit, E.F.; van der Heijden, M.S.; Nguyen-Kim, T.D.; van den Broek, D.; Beets-Tan, R.G.; et al. Integrated noninvasive diagnostics for prediction of survival in immunotherapy. Immunooncol. Technol. 2024, 24, 100723. [Google Scholar] [CrossRef] [PubMed]
- Luo, K.; Liu, S.; Shen, X.; Xu, J.; Shi, C.; Chao, Y.; Wen, Z.; Zhang, K.; Wang, R.; Liu, B.; et al. Integration of cancer stemness and neoantigen load to predict responsiveness to anti-PD1/PDL1 therapy. Front. Cell Dev. Biol. 2022, 10, 1003656. [Google Scholar] [CrossRef]
- Zhu, S.Y.; Yang, T.T.; Zhao, Y.Z.; Sun, Y.; Zheng, X.M.; Xu, H.B. Interpretable machine learning model predicting immune checkpoint inhibitor-induced hypothyroidism: A retrospective cohort study. Cancer Sci. 2024, 115, 3767–3775. [Google Scholar] [CrossRef]
- Chaddad, A.; Peng, J.; Xu, J.; Bouridane, A. Survey of Explainable AI Techniques in Healthcare. Sensors 2023, 23, 634. [Google Scholar] [CrossRef]
- Frenard, C.; Blanchet, K.; Lecerf, P.; Varey, E.; Khammari, A.; Dréno, B. Machine learning algorithm to predict response to immunotherapy in real-life settings for patients with advanced melanoma. Eur. J. Dermatol. 2023, 33, 75–80. [Google Scholar] [CrossRef]
- Faron, A.; Opheys, N.S.; Nowak, S.; Sprinkart, A.M.; Isaak, A.; Theis, M.; Mesropyan, N.; Endler, C.; Sirokay, J.; Pieper, C.C.; et al. Deep Learning-Based Body Composition Analysis Predicts Outcome in Melanoma Patients Treated with Immune Checkpoint Inhibitors. Diagnostics 2021, 11, 2314. [Google Scholar] [CrossRef]
- Tabari, A.; Cox, M.; D’Amore, B.; Mansur, A.; Dabbara, H.; Boland, G.; Gee, M.S.; Daye, D. Machine Learning Improves the Prediction of Responses to Immune Checkpoint Inhibitors in Metastatic Melanoma. Cancers 2023, 15, 2700. [Google Scholar] [CrossRef]
| Drug Name (Brand Name) | Target | Type | Initial FDA Approval | Key Indications for Melanoma | Common Side Effects |
|---|---|---|---|---|---|
| Pembrolizumab (Keytruda) | PD-1 | Monoclonal Antibody | 2014 | Unresectable or metastatic melanoma; adjuvant treatment of melanoma with involvement of lymph node(s) following complete resection | Fatigue, rash, diarrhea, pruritus, nausea, arthralgia, immune-mediated adverse events (e.g., colitis, pneumonitis) |
| Nivolumab (Opdivo) | PD-1 | Monoclonal Antibody | 2014 | Unresectable or metastatic melanoma; adjuvant treatment of melanoma with involvement of lymph node(s) following complete resection | Fatigue, rash, diarrhea, pruritus, nausea, arthralgia, immune-mediated adverse events (e.g., colitis, pneumonitis) |
| Atezolizumab (Tecentriq) | PD-L1 | Monoclonal Antibody | 2016 | Atezolizumab is not typically used as a single agent for melanoma. It is sometimes used in combination with other therapies in clinical trials. | Fatigue, nausea, decreased appetite, diarrhea, immune-mediated adverse events such as hepatitis, pneumonitis. |
| Avelumab (Bavencio) | PD-L1 | Monoclonal Antibody | 2017 | Avelumab is not typically used as a single agent for melanoma. It has been investigated in combination with other therapies in clinical trials. | Fatigue, infusion-related reactions, diarrhea, immune-mediated adverse events. |
| Durvalumab (Imfinzi) | PD-L1 | Monoclonal Antibody | 2017 | Durvalumab is not typically used as a single agent for melanoma. It has been investigated in combination with other therapies in clinical trials. | Fatigue, cough, nausea, immune-mediated adverse events. |
| Ipilimumab (Yervoy) | CTLA-4 | Monoclonal Antibody | 2011 | Unresectable or metastatic melanoma; adjuvant treatment of melanoma with involvement of lymph node(s) following complete resection | Fatigue, diarrhea, pruritus, rash, immune-mediated adverse events (e.g., colitis, hepatitis, endocrinopathies). |
| Tremelimumab (I judo) | CTLA-4 | Monoclonal Antibody | 2022 | In combination with durvalumab for unresectable hepatocellular carcinoma. It is not approved as a monotherapy for melanoma. | Fatigue, diarrhea, rash, decreased appetite, immune-mediated adverse events. |
| Relatlimab/Nivolumab (Opdualag) | LAG-3/PD-1 | Dual Monoclonal Antibody | 2022 | Unresectable or metastatic melanoma | Fatigue, musculoskeletal pain, rash, pruritus, diarrhea, nausea, decreased appetite, immune-mediated adverse events |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saleem, M.; Watson, A.E.; Anwaar, A.; Jasser, A.O.; Yusuf, N. Optimizing Immunotherapy: The Synergy of Immune Checkpoint Inhibitors with Artificial Intelligence in Melanoma Treatment. Biomolecules 2025, 15, 589. https://doi.org/10.3390/biom15040589
Saleem M, Watson AE, Anwaar A, Jasser AO, Yusuf N. Optimizing Immunotherapy: The Synergy of Immune Checkpoint Inhibitors with Artificial Intelligence in Melanoma Treatment. Biomolecules. 2025; 15(4):589. https://doi.org/10.3390/biom15040589
Chicago/Turabian StyleSaleem, Mohammad, Abigail E. Watson, Aisha Anwaar, Ahmad Omar Jasser, and Nabiha Yusuf. 2025. "Optimizing Immunotherapy: The Synergy of Immune Checkpoint Inhibitors with Artificial Intelligence in Melanoma Treatment" Biomolecules 15, no. 4: 589. https://doi.org/10.3390/biom15040589
APA StyleSaleem, M., Watson, A. E., Anwaar, A., Jasser, A. O., & Yusuf, N. (2025). Optimizing Immunotherapy: The Synergy of Immune Checkpoint Inhibitors with Artificial Intelligence in Melanoma Treatment. Biomolecules, 15(4), 589. https://doi.org/10.3390/biom15040589







