Toxicity Mechanism of Low Doses of NaGdF4:Yb3+,Er3+ Upconverting Nanoparticles in Activated Macrophage Cell Lines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Lanthanide Nanocrystal Synthesis and Characterization
2.2. Cell Culture and Materials
2.3. Viability Assay
2.4. Apoptosis Assays
2.5. Mitochondrial Content Assay
2.6. Cell Acidification
2.7. Superoxide Production
2.8. Western Blotting
2.9. Microscopic Images
3. Results
3.1. Physical Properties and Characterization of Luminescent Yb3+,Er3+ Co-Doped NaGdF4 Nanoparticles
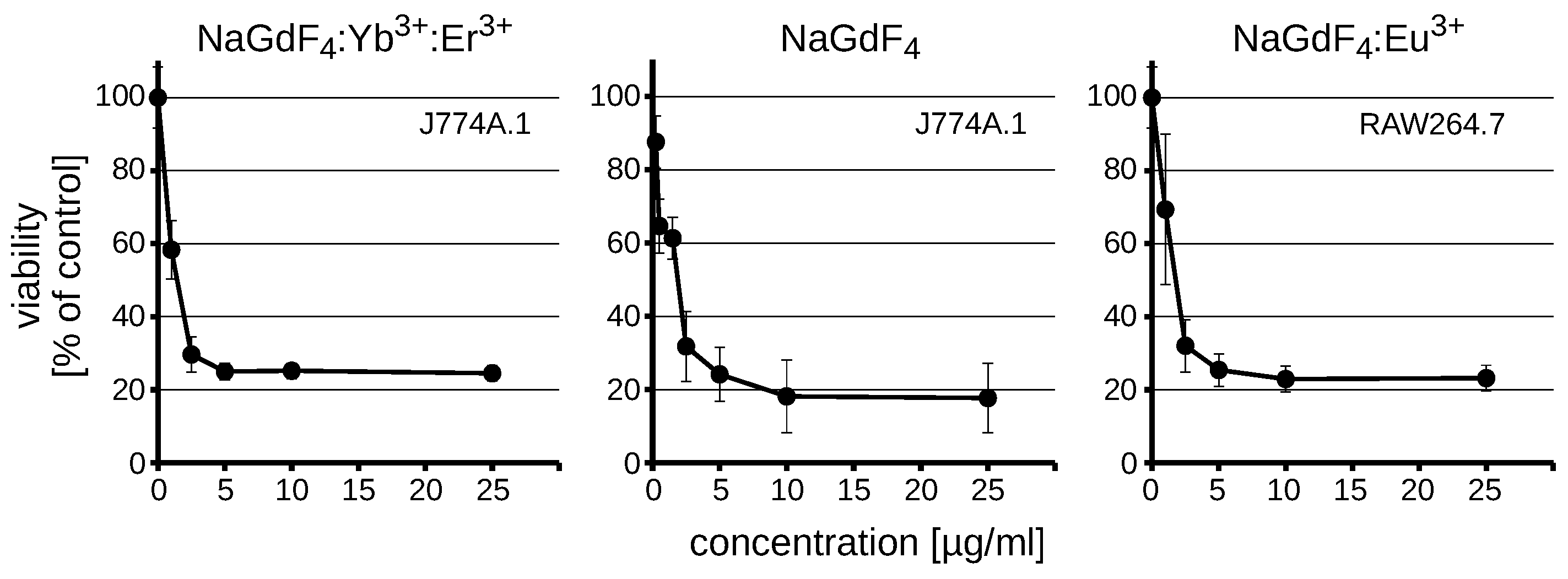
3.2. NaGdF4 Nanoparticles are Toxic Regardless of the Dopant
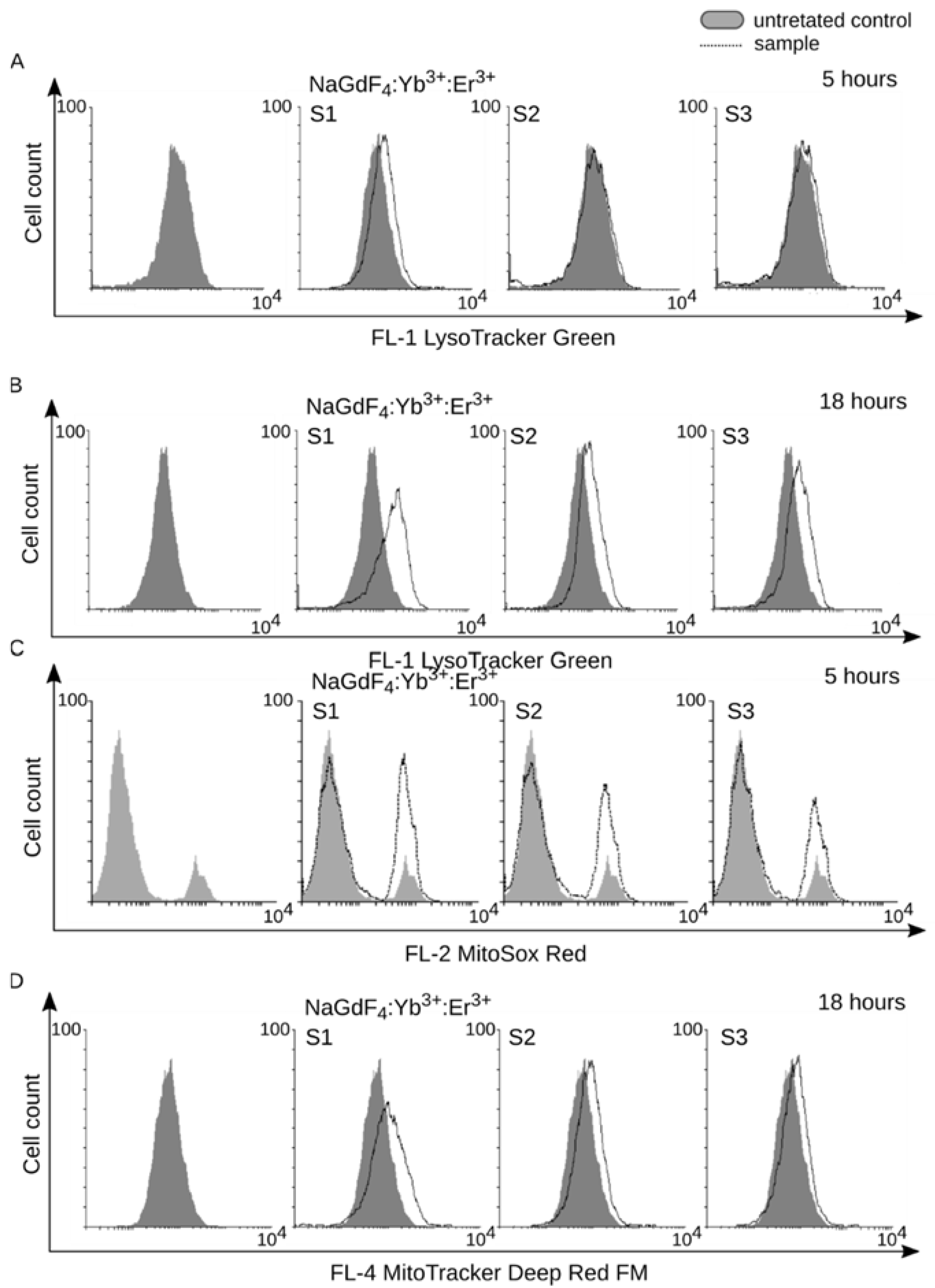
3.3. Internalization of NaGdF4 Nanoparticles is Accompanied by Enlargenemt of Lysosomal Compartment and Mitochondrial Homeostasis Disruption
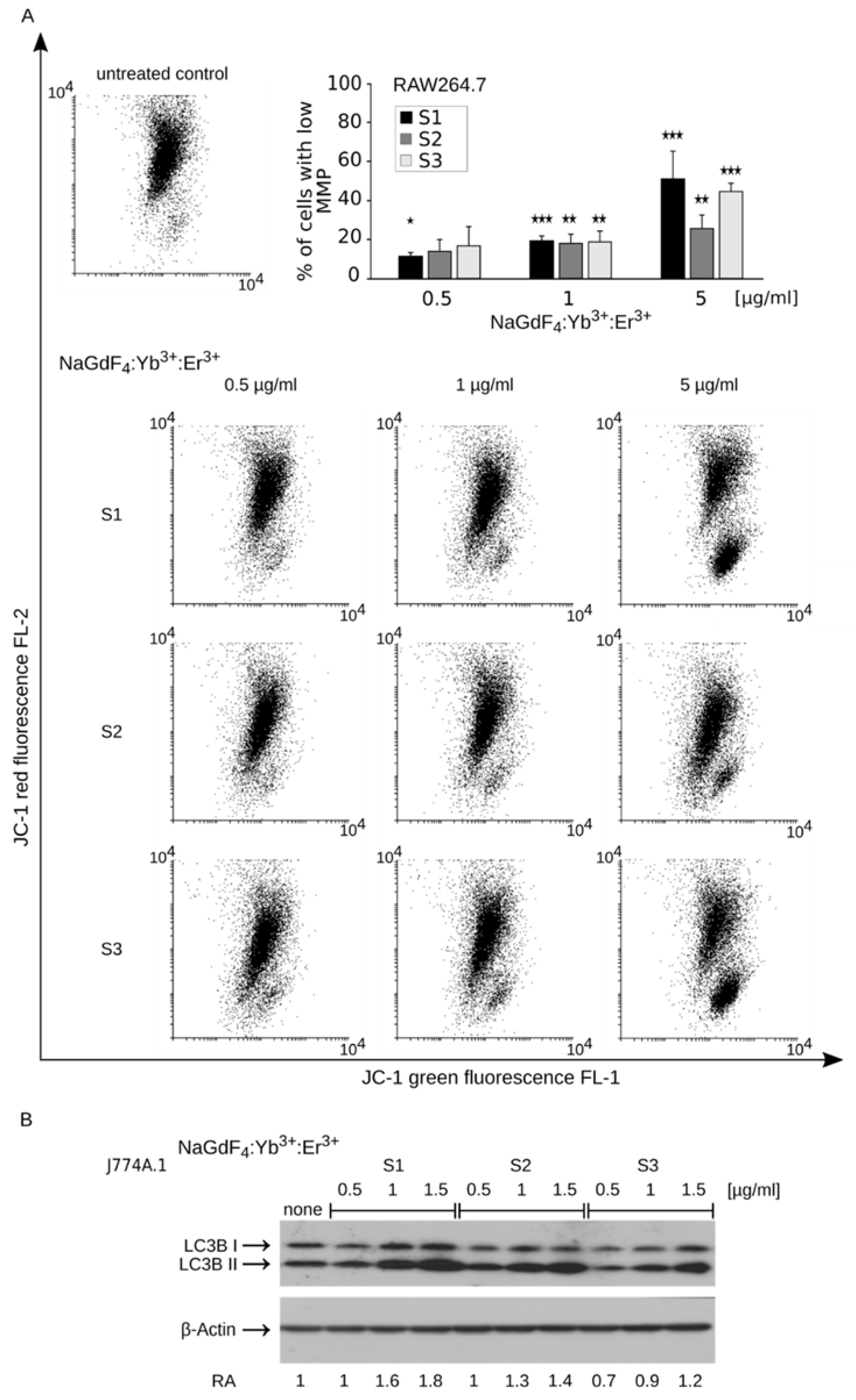
3.4. Disruption of Mitochondrial Homeostasis Induces Apoptotic Cell Death
3.5. Exogenous Soluble Gadolinium or Fluoride Did Not Decrease the Viability of RAW264.7 Macrophages
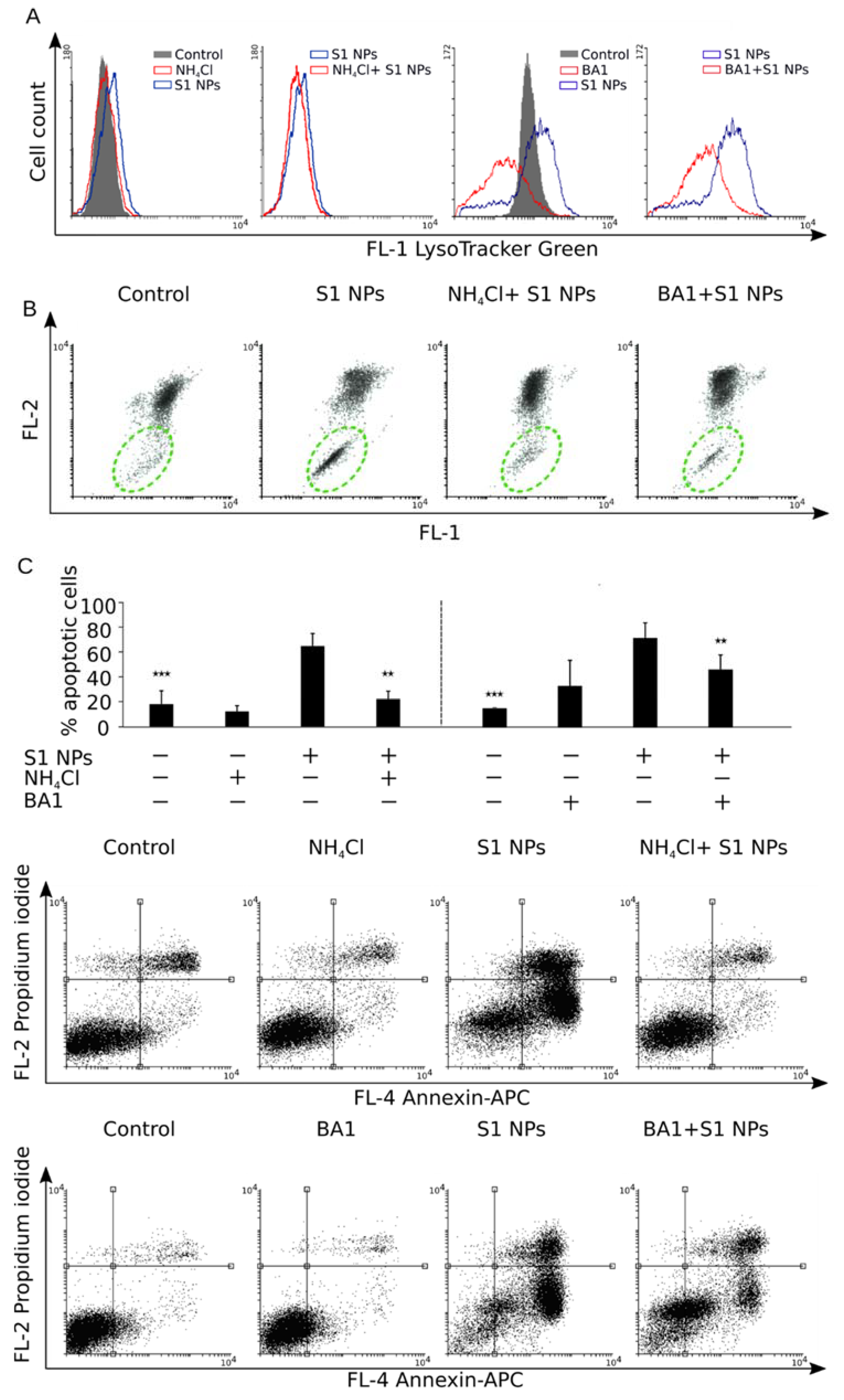
3.6. Inhibition of Cell Acidification Prevents Cytotoxicity of NaGdF4 Nanoparticles
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hao, D.; Ai, T.; Goerner, F.; Hu, X.; Runge, V.M.; Tweedle, M. MRI contrast agents: Basic chemistry and safety. J. Magn. Reson. Imaging JMRI 2012, 36, 1060–1071. [Google Scholar] [CrossRef] [PubMed]
- Nagy, G.; Baksa, V.; Kiss, A.; Turani, M.; Banfalvi, G. Gadolinium induced effects on mammalian cell motility, adherence and chromatin structure. Apoptosis 2017, 22, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Spencer, A.; Wilson, S.; Harpur, E. Gadolinium chloride toxicity in the mouse. Hum. Exp. Toxicol. 1998, 17, 633–637. [Google Scholar] [CrossRef] [PubMed]
- Trifuoggi, M.; Pagano, G.; Guida, M.; Palumbo, A.; Siciliano, A.; Gravina, M.; Lyons, D.M.; Burić, P.; Levak, M.; Thomas, P.J.; et al. Comparative toxicity of seven rare earth elements in sea urchin early life stages. Environ. Sci. Poll. Res. 2017, 24, 20803–20810. [Google Scholar] [CrossRef] [PubMed]
- Ramalho, J.; Ramalho, M.; Jay, M.; Burke, L.M.; Semelka, R.C. Gadolinium toxicity and treatment. Magn. Reson. Imaging 2016, 34, 1394–1398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogosnitzky, M.; Branch, S. Gadolinium-based contrast agent toxicity: A review of known and proposed mechanisms. BioMetals 2016, 29, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, S. Gadolinium toxicity: Iron and ferroportin as central targets. Magn. Reson. Imaging 2016, 34, 1373–1376. [Google Scholar] [CrossRef]
- Abdukayum, A.; Yang, C.-X.; Zhao, Q.; Chen, J.-T.; Dong, L.-X.; Yan, X.-P. Gadolinium Complexes Functionalized Persistent Luminescent Nanoparticles as a Multimodal Probe for Near-Infrared Luminescence and Magnetic Resonance Imaging in Vivo. Anal. Chem. 2014, 86, 4096–4101. [Google Scholar] [CrossRef]
- Santelli, J.; Lechevallier, S.; Baaziz, H.; Vincent, M.; Martinez, C.; Mauricot, R.; Parini, A.; Verelst, M.; Cussac, D. Multimodal gadolinium oxysulfide nanoparticles: A versatile contrast agent for mesenchymal stem cell labeling. Nanoscale 2018, 10, 16775–16786. [Google Scholar] [CrossRef]
- Yang, C.; Liu, Q.; He, D.; Na, N.; Zhao, Y.; Ouyang, J. Dual-modal imaging and photodynamic therapy using upconversion nanoparticles for tumor cells. Analyst 2014, 139, 6414–6420. [Google Scholar] [CrossRef]
- Dellinger, A.; Olson, J.; Link, K.; Vance, S.; Sandros, M.G.; Yang, J.; Zhou, Z.; Kepley, C.L. Functionalization of gadolinium metallofullerenes for detecting atherosclerotic plaque lesions by cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2013, 15, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, L.; Zheng, X.; Gu, Z.; Yin, W.; Zhang, X.; Ruan, L.; Yang, Y.; Hu, Z.; Zhao, Y. Mesoporous NaYbF4@NaGdF4 core-shell up-conversion nanoparticles for targeted drug delivery and multimodal imaging. Biomaterials 2014, 35, 7666–7678. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, T.S.; Ryu, J.; Hong, S.; Kang, M.; Im, K.; Kang, J.H.; Lim, S.M.; Park, S.; Song, R. RGD Peptide-Conjugated Multimodal NaGdF4:Yb3+/Er3+ Nanophosphors for Upconversion Luminescence, MR, and PET Imaging of Tumor Angiogenesis. J. Nucl. Med. 2013, 54, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Xia, F.; Alfranca, G.; Yan, H.; Zhi, X.; Liu, Y.; Peng, C.; Zhang, C.; de la Fuente, J.M.; Cui, D. Nanoparticles for multi-modality cancer diagnosis: Simple protocol for self-assembly of gold nanoclusters mediated by gadolinium ions. Biomaterials 2017, 120, 103–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, Y.; Zhang, N.; Li, C.; Pu, K.; Ding, C.; Zhu, Y. Nanosystem composed with MSNs, gadolinium, liposome and cytotoxic peptides for tumor theranostics. Colloids Surf. B Biointerfaces 2017, 151, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Ghalandarlaki, N.; Latifi, N.; Mirzaei, M.; Joghatei, M.T.; Safari, S.; Ashtari, K.; Mehravi, B. In Vitro Evaluation of Gadolinium-Silica Mesoporous Nanoparticles-Monoclonal Antibody: Potential Nanoprobe for Prostate Cancer Cell Imaging. Available online: http://www.eurekaselect.com/135498/article (accessed on 14 December 2018).
- Hajdu, I.; Trencsényi, G.; Bodnár, M.; Emri, M.; Bánfalvi, G.; Sikula, J.; Márián, T.; Kollár, J.; Vámosi, G.; Borbély, J. Tumor-specific localization of self-assembled nanoparticle PET/MR modalities. Anticancer Res. 2014, 34, 49–59. [Google Scholar] [PubMed]
- Gnach, A.; Lipinski, T.; Bednarkiewicz, A.; Rybka, J.; Capobianco, J.A. Upconverting nanoparticles: Assessing the toxicity. Chem. Soc. Rev. 2015, 44, 1561–1584. [Google Scholar] [CrossRef]
- Sun, Y.; Feng, W.; Yang, P.; Huang, C.; Li, F. The biosafety of lanthanide upconversion nanomaterials. Chem. Soc. Rev. 2015, 44, 1509–1525. [Google Scholar] [CrossRef]
- Liu, H.; Yuan, L.; Yang, X.; Wang, K. La3+, Gd3+ and Yb3+ induced changes in mitochondrial structure, membrane permeability, cytochrome c release and intracellular ROS level. Chem. Biol. Interact. 2003, 146, 27–37. [Google Scholar] [CrossRef]
- Sabella, S.; Carney, R.P.; Brunetti, V.; Malvindi, M.A.; Al-Juffali, N.; Vecchio, G.; Janes, S.M.; Bakr, O.M.; Cingolani, R.; Stellacci, F.; et al. A general mechanism for intracellular toxicity of metal-containing nanoparticles. Nanoscale 2014, 6, 7052. [Google Scholar] [CrossRef] [Green Version]
- Cho, W.-S.; Duffin, R.; Howie, S.E.; Scotton, C.J.; Wallace, W.A.; MacNee, W.; Bradley, M.; Megson, I.L.; Donaldson, K. Progressive severe lung injury by zinc oxide nanoparticles; the role of Zn2+ dissolution inside lysosomes. Part. Fibre Toxicol. 2011, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G.; Schaible, U.E. Macrophage defense mechanisms against intracellular bacteria. Immunol. Rev. 2015, 264, 182–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morrison, D.E.; Aitken, J.B.; de Jonge, M.D.; Ioppolo, J.A.; Harris, H.H.; Rendina, L.M. High mitochondrial accumulation of new gadolinium(III) agents within tumour cells. Chem. Commun. 2014, 50, 2252–2254. [Google Scholar] [CrossRef] [PubMed]
- Sohaebuddin, S.K.; Thevenot, P.T.; Baker, D.; Eaton, J.W.; Tang, L. Nanomaterial cytotoxicity is composition, size, and cell type dependent. Part. Fibre Toxicol. 2010, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Mohammad, A.; Patil, G.; Naqvi, S.A.H.; Chauhan, L.K.S.; Ahmad, I. Induction of ROS, mitochondrial damage and autophagy in lung epithelial cancer cells by iron oxide nanoparticles. Biomaterials 2012, 33, 1477–1488. [Google Scholar] [CrossRef] [PubMed]
- McDonald, R.J.; McDonald, J.S.; Kallmes, D.F.; Jentoft, M.E.; Murray, D.L.; Thielen, K.R.; Williamson, E.E.; Eckel, L.J. Intracranial Gadolinium Deposition after Contrast-enhanced MR Imaging. Radiology 2015, 275, 772–782. [Google Scholar] [CrossRef]
- Kanda, T.; Fukusato, T.; Matsuda, M.; Toyoda, K.; Oba, H.; Kotoku, J.; Haruyama, T.; Kitajima, K.; Furui, S. Gadolinium-based Contrast Agent Accumulates in the Brain Even in Subjects without Severe Renal Dysfunction: Evaluation of Autopsy Brain Specimens with Inductively Coupled Plasma Mass Spectroscopy. Radiology 2015, 276, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Wysokińska, E.; Cichos, J.; Zioło, E.; Bednarkiewicz, A.; Strządała, L.; Karbowiak, M.; Hreniak, D.; Kałas, W. Cytotoxic interactions of bare and coated NaGdF4:Yb(3+):Er(3+) nanoparticles with macrophage and fibroblast cells. Toxicol. Vitro Int. J. Publ. Assoc. BIBRA 2016, 32, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Yu, L.; Monopoli, M.P.; Sandin, P.; Mahon, E.; Salvati, A.; Dawson, K.A. The biomolecular corona is retained during nanoparticle uptake and protects the cells from the damage induced by cationic nanoparticles until degraded in the lysosomes. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 1159–1168. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Deng, R.; Liu, X. Preparation of core-shell NaGdF4 nanoparticles doped with luminescent lanthanide ions to be used as upconversion-based probes. Nat. Protoc. 2014, 9, 1634–1644. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Collins, J.E.; Kang, Y.; Chen, J.; Chen, D.T.N.; Yodh, A.G.; Murray, C.B. Morphologically controlled synthesis of colloidal upconversion nanophosphors and their shape-directed self-assembly. Proc. Natl. Acad. Sci. USA 2010, 107, 22430–22435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mech, A.; Karbowiak, M.; Kpiński, L.; Bednarkiewicz, A.; Strk, W. Structural and luminescent properties of nano-sized NaGdF4:Eu3+ synthesised by wet-chemistry route. J. Alloys Compd. 2004, 380, 315–320. [Google Scholar] [CrossRef]
- Cichos, J.; Karbowiak, M. Spectroscopic characterization of ligands on the surface of water dispersible NaGdF4:Ln3+ nanocrystals. Appl. Surf. Sci. 2012, 258, 5610–5618. [Google Scholar] [CrossRef]
- Cichos, J.; Marciniak, L.; Hreniak, D.; Strek, W.; Karbowiak, M. The effect of surface ligand, solvent and Yb3+ co-doping on the luminescence properties of Er3+ in colloidal NaGdF4 nanocrystals. J. Mater. Chem. C 2014, 2, 8244–8251. [Google Scholar] [CrossRef]
- Zhao, J.; Zhou, Z.-Q.; Jin, J.-C.; Yuan, L.; He, H.; Jiang, F.-L.; Yang, X.-G.; Dai, J.; Liu, Y. Mitochondrial dysfunction induced by different concentrations of gadolinium ion. Chemosphere 2014, 100, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.; Deng, X.; Zhou, W.; Tan, E.-K. Flow Cytometry-Based Assessment of Mitophagy Using MitoTracker. Front. Cell. Neurosci. 2016, 10. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-C.; Wei, Y.-H. Mitochondrial biogenesis and mitochondrial DNA maintenance of mammalian cells under oxidative stress. Int. J. Biochem. Cell Biol. 2005, 37, 822–834. [Google Scholar] [CrossRef]
- Tsai, Y.-F.; Huang, C.-W.; Chiang, J.-H.; Tsai, F.-J.; Hsu, Y.-M.; Lu, C.-C.; Hsiao, C.-Y.; Yang, J.-S. Gadolinium chloride elicits apoptosis in human osteosarcoma U-2 OS cells through extrinsic signaling, intrinsic pathway and endoplasmic reticulum stress. Oncol. Rep. 2016, 36, 3421–3426. [Google Scholar] [CrossRef]
- Ye, L.; Shi, Z.; Liu, H.; Yang, X.; Wang, K. Gadolinium induced apoptosis of human embryo liver L02 cell line by ROS-mediated AIF pathway. J. Rare Earths 2011, 29, 178–184. [Google Scholar] [CrossRef]
- Alarifi, S.; Ali, H.; Saad Alkahtani, M.S.A. Regulation of apoptosis through bcl-2/bax proteins expression and DNA damage by nano-sized gadolinium oxide. Int. J. Nanomed. 2017, 12, 4541–4551. [Google Scholar] [CrossRef]
- Zhang, Y. Nano rare-earth oxides induced size-dependent vacuolization: An independent pathway from autophagy. Int. J. Nanomed. 2010, 601. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, R.; Zhang, F.; Zhou, L.; Shen, D.; Yao, C.; Zhao, D. Nd3+ Sensitized Up/Down Converting Dual-Mode Nanomaterials for Efficient In-vitro and In-vivo Bioimaging Excited at 800 nm. Sci. Rep. 2013, 3. [Google Scholar] [CrossRef]
- Liu, C.; Gao, Z.; Zeng, J.; Hou, Y.; Fang, F.; Li, Y.; Qiao, R.; Shen, L.; Lei, H.; Yang, W.; et al. Magnetic/Upconversion Fluorescent NaGdF4:Yb,Er Nanoparticle-Based Dual-Modal Molecular Probes for Imaging Tiny Tumors in Vivo. ACS Nano 2013, 7, 7227–7240. [Google Scholar] [CrossRef] [PubMed]
- Akinc, A.; Battaglia, G. Exploiting endocytosis for nanomedicines. Cold Spring Harb. Perspect. Biol. 2013, 5, a016980. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, D.A.; Vanhecke, D.; Michen, B.; Blank, F.; Gehr, P.; Petri-Fink, A.; Rothen-Rutishauser, B. Different endocytotic uptake mechanisms for nanoparticles in epithelial cells and macrophages. Beilstein J. Nanotechnol. 2014, 5, 1625–1636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tedeschi, E.; Caranci, F.; Giordano, F.; Angelini, V.; Cocozza, S.; Brunetti, A. Gadolinium retention in the body: What we know and what we can do. Radiol. Med. (Torino) 2017, 122, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Stern, S.T.; Adiseshaiah, P.P.; Crist, R.M. Autophagy and lysosomal dysfunction as emerging mechanisms of nanomaterial toxicity. Part. Fibre Toxicol. 2012, 9, 20. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Yu, Y.; Lu, K.; Yang, M.; Li, Y.; Zhou, X.; Sun, Z. Silica nanoparticles induce autophagy dysfunction via lysosomal impairment and inhibition of autophagosome degradation in hepatocytes. Int. J. Nanomed. 2017, 12, 809–825. [Google Scholar] [CrossRef]
- Herd, H.L.; Malugin, A.; Ghandehari, H. Silica nanoconstruct cellular toleration threshold in vitro. J. Control. Release 2011, 153, 40–48. [Google Scholar] [CrossRef] [Green Version]
- Seleverstov, O.; Zabirnyk, O.; Zscharnack, M.; Bulavina, L.; Nowicki, M.; Heinrich, J.-M.; Yezhelyev, M.; Emmrich, F.; O’Regan, R.; Bader, A. Quantum Dots for Human Mesenchymal Stem Cells Labeling. A Size-Dependent Autophagy Activation. Nano Lett. 2006, 6, 2826–2832. [Google Scholar] [CrossRef]
- Adams, J.M.; Cory, S. Life-or-death decisions by the Bcl-2 protein family. Trends Biochem. Sci. 2001, 26, 61–66. [Google Scholar] [CrossRef]





| 20 nm | 20 nm | S1 | S2 | S3 | |
|---|---|---|---|---|---|
| NaGdF4 | NaGdF4:Eu | NaGdF4:Yb;Er | NaGdF4:Yb;Er | NaGdF4:Yb;Er | |
| RAW264.7 | n.a. | 1.87 ± 0.07 | 0.81 ± 0.06 | 1.33 ± 0.07 | 1.58 ± 0.05 |
| J774A.1 | 1.13 ± 0.09 | n.a. | 0.52 ± 0.04 | 1.81 ± 0.05 | 4.04 ± 0.20 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wysokińska, E.; Cichos, J.; Kowalczyk, A.; Karbowiak, M.; Strządała, L.; Bednarkiewicz, A.; Kałas, W. Toxicity Mechanism of Low Doses of NaGdF4:Yb3+,Er3+ Upconverting Nanoparticles in Activated Macrophage Cell Lines. Biomolecules 2019, 9, 14. https://doi.org/10.3390/biom9010014
Wysokińska E, Cichos J, Kowalczyk A, Karbowiak M, Strządała L, Bednarkiewicz A, Kałas W. Toxicity Mechanism of Low Doses of NaGdF4:Yb3+,Er3+ Upconverting Nanoparticles in Activated Macrophage Cell Lines. Biomolecules. 2019; 9(1):14. https://doi.org/10.3390/biom9010014
Chicago/Turabian StyleWysokińska, Edyta, Jakub Cichos, Agnieszka Kowalczyk, Mirosław Karbowiak, Leon Strządała, Artur Bednarkiewicz, and Wojciech Kałas. 2019. "Toxicity Mechanism of Low Doses of NaGdF4:Yb3+,Er3+ Upconverting Nanoparticles in Activated Macrophage Cell Lines" Biomolecules 9, no. 1: 14. https://doi.org/10.3390/biom9010014
APA StyleWysokińska, E., Cichos, J., Kowalczyk, A., Karbowiak, M., Strządała, L., Bednarkiewicz, A., & Kałas, W. (2019). Toxicity Mechanism of Low Doses of NaGdF4:Yb3+,Er3+ Upconverting Nanoparticles in Activated Macrophage Cell Lines. Biomolecules, 9(1), 14. https://doi.org/10.3390/biom9010014





