Mechanistic Insight of Allantoin in Protecting Tomato Plants Against Ultraviolet C Stress
Abstract
1. Introduction
2. Results
2.1. Doses of UVC and Exogenous AT
2.2. AT Improved Growth, Water Relations, and Contents Photosynthetic Pigments
2.3. AT Differentially Maintained Water Content, Epicuticular Wax, Pro, FAA, Soluble Proteins, and Soluble Carbohydrates
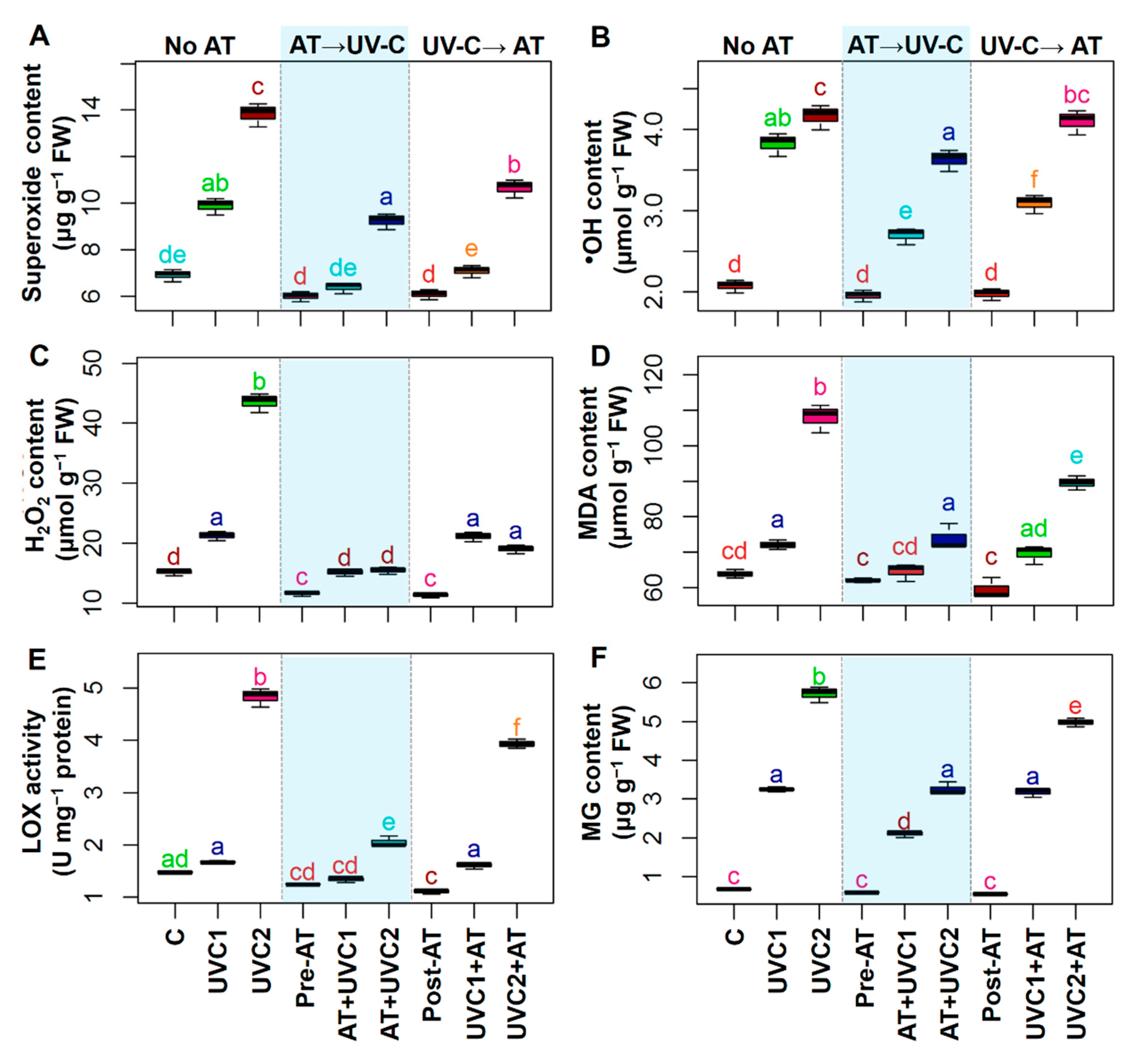
2.4. AT Attenuated Oxidative Damage
2.5. AT Improved Non-Enzymatic Antioxidant Status and Secondary Metabolites
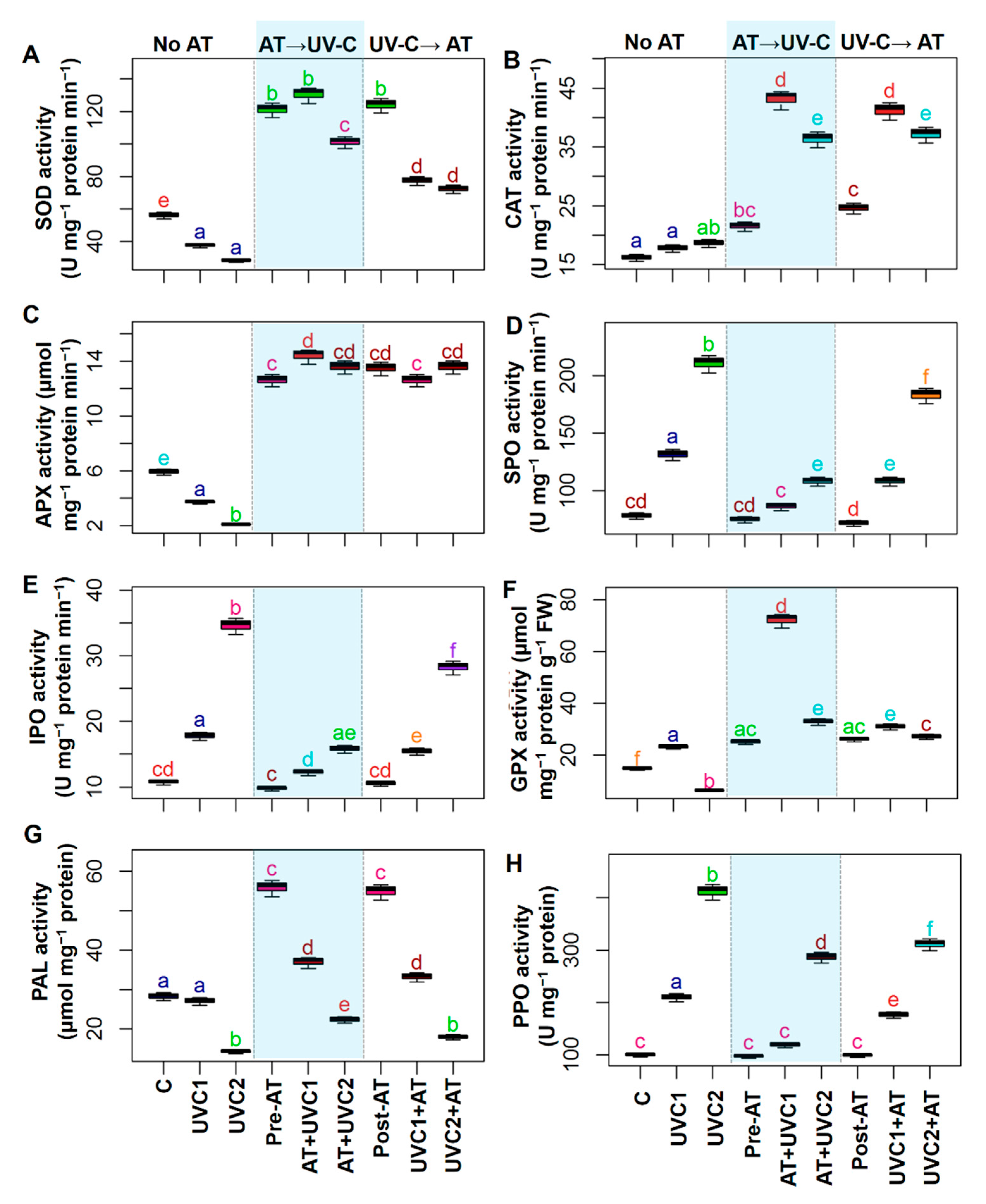
2.6. AT Improved Different Enzymatic Activities
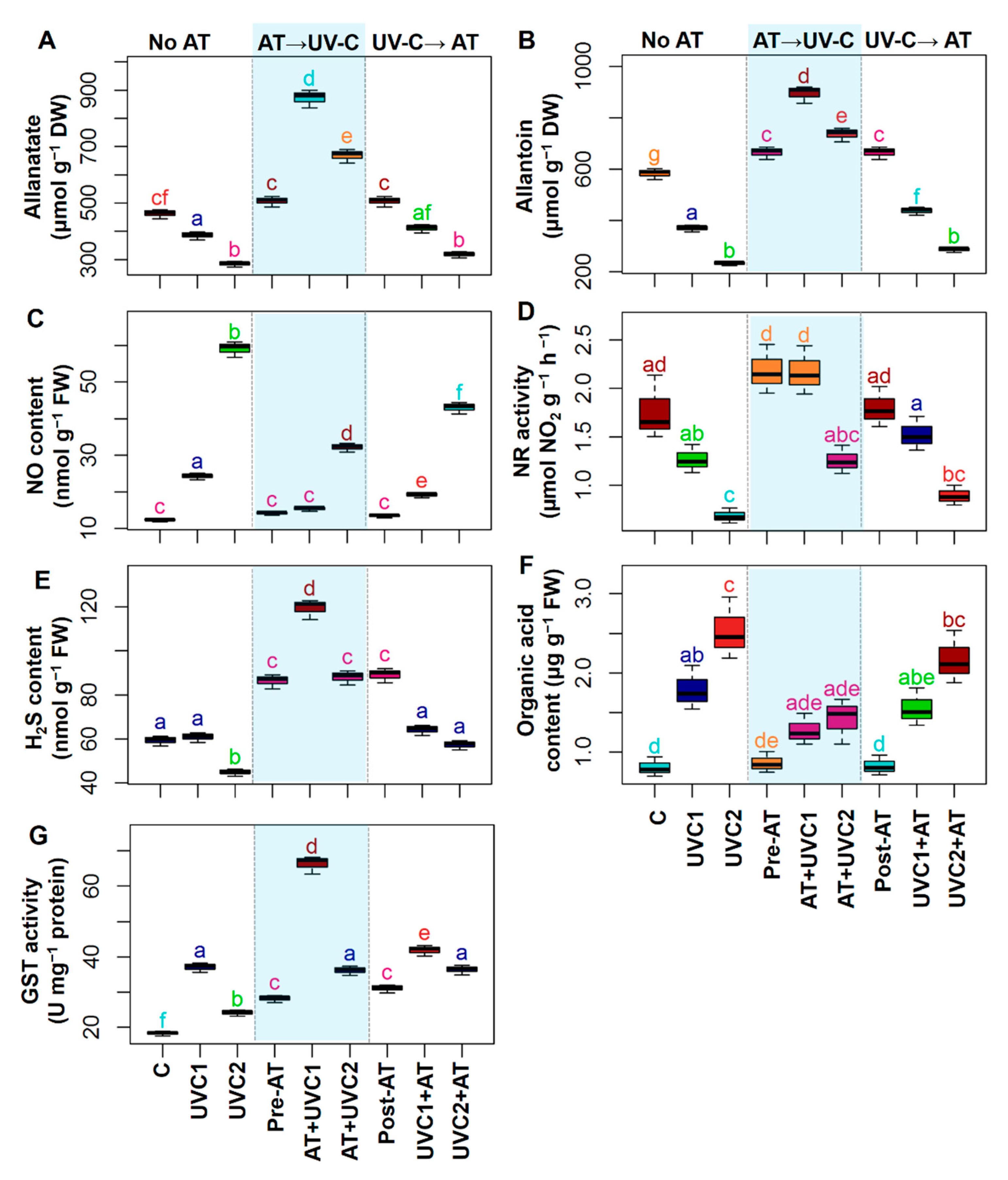
2.7. AT Regulates Endogenous Metabolites, Signaling Molecules, GST, and PPO Activity
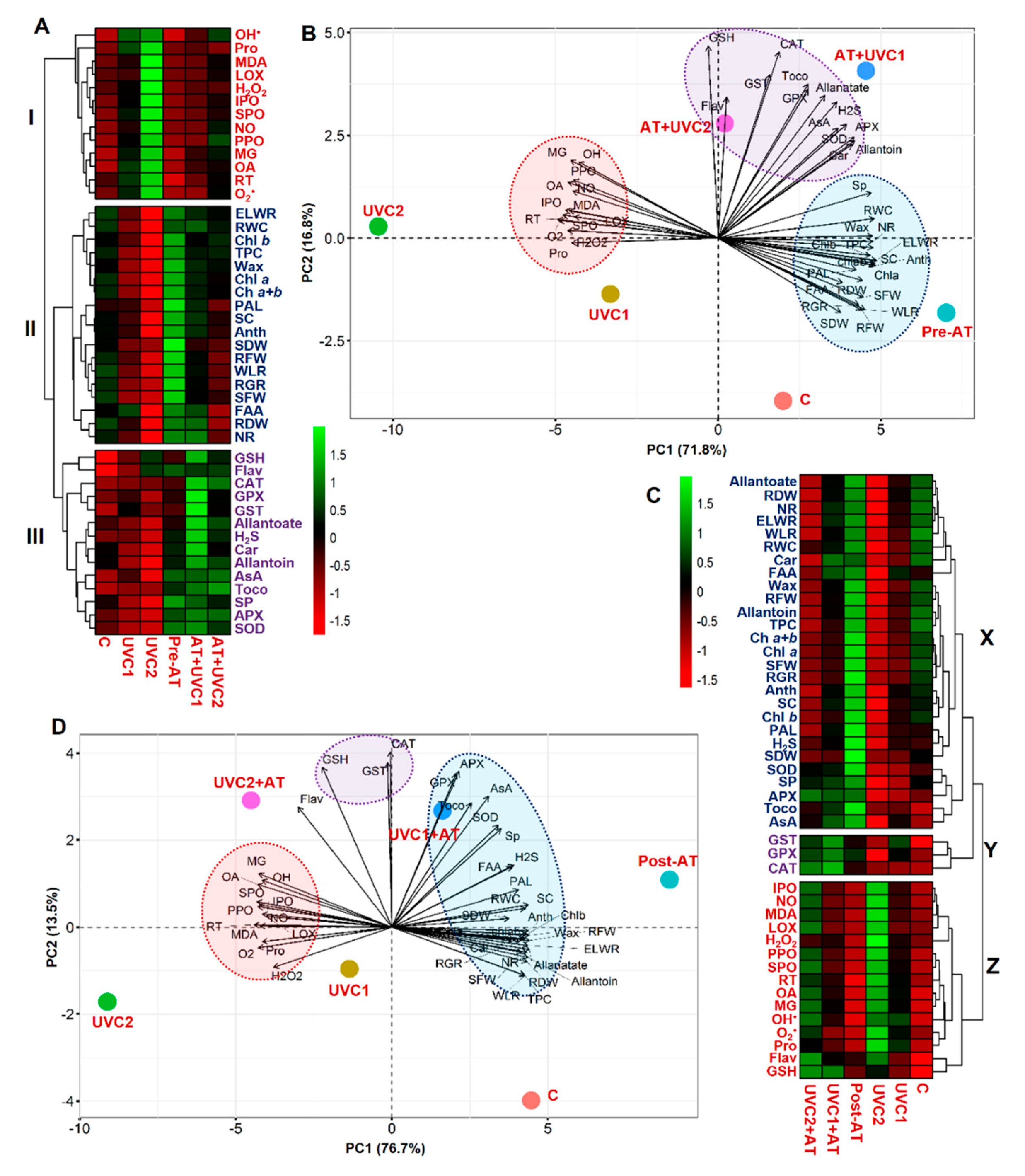
2.8. Treatment–Variable and Variable–Variable Interaction
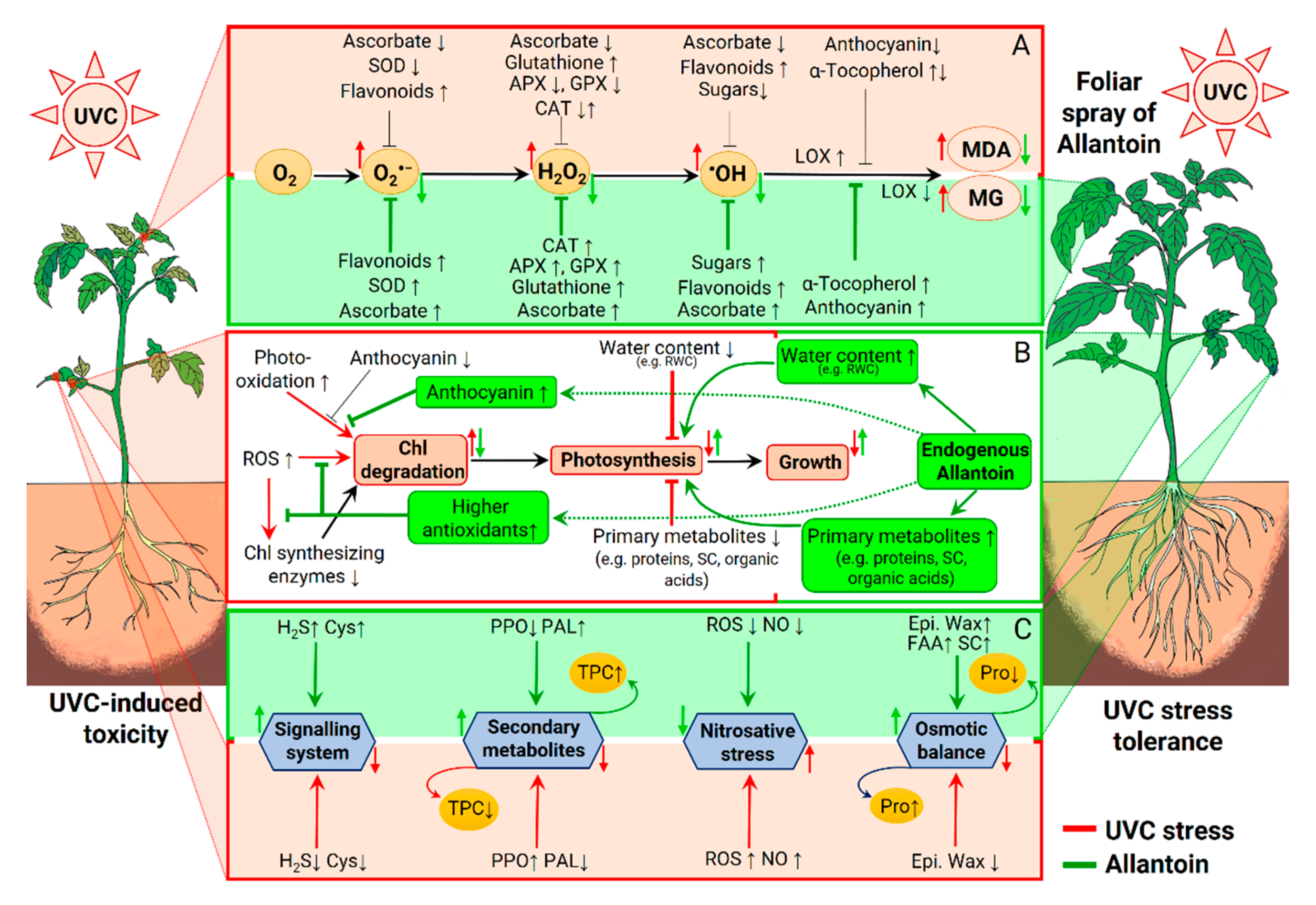
3. Discussion
4. Materials and Methods
4.1. Experimental Conditions, Crop, and UVC Stress
4.2. Evaluation of the Effect of the Optimal Dose of AT on UVC Stress
4.3. Growth Parameter Measurements
4.4. Photosynthetic Pigments Content Determination
4.5. SC, SP, FAA, and Pro Content Determination
4.6. Oxidative Stress Markers Determination
4.7. Non-Enzymatic Antioxidants and Secondary Metabolites Estimation
4.8. Enzymatic Antioxidants Extraction and Assay
4.9. H2S, Cysteine, Nitric Oxide Content, Nitrate Reductase Activity, AT, Allantoate, and Organic Acid Determination
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, S.; Chatterjee, S.; Kataria, S.; Joshi, J.; Datta, S.; Vairale, M.G.; Veer, V. A review on responses of plants to UV-B radiation related stress. In UV-B Radiation: From Environmental Stressor to Regulator of Plant Growth; John Wiley & Sons: West Sussex, UK, 2017; Volume 75. [Google Scholar]
- Pazuki, A.; Aflaki, F.; Pessarakli, M.; Gurel, E.; Gurel, S. Plant responses to extended photosynthetically active radiation (EPAR). Adv. Plants Agric. Res. 2017, 7, 313–318. [Google Scholar]
- Müller-Xing, R.; Xing, Q.; Goodrich, J. Footprints of the sun: Memory of UV and light stress in plants. Front. Plant Sci. 2014, 5, 474. [Google Scholar] [PubMed]
- Nawkar, G.M.; Maibam, P.; Park, J.H.; Sahi, V.P.; Lee, S.Y.; Kang, C.H. UV-induced cell death in plants. Int. J. Mol. Sci. 2013, 14, 1608–1628. [Google Scholar] [CrossRef]
- Čejka, Č.; Ardan, T.; Širc, J.; Michálek, J.; Beneš, J.; Brůnová, B.; Rosina, J. Hydration and transparency of the rabbit cornea irradiated with UVB-doses of 0.25 J/cm2 and 0.5 J/cm2 compared with equivalent UVB radiation exposure reaching the human cornea from sunlight. Curr. Eye Res. 2011, 36, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Tossi, V.E.; Regalado, J.J.; Iannicelli, J.; Laino, L.E.; Burrieza, H.P.; Escandón, A.S.; Pitta-Álvarez, S.I. Beyond Arabidopsis: Differential UV-B response mediated by UVR8 in diverse species. Front. Plant Sci. 2019, 10, 780. [Google Scholar] [CrossRef] [PubMed]
- Häder, D.P.; Kumar, H.D.; Smith, R.C.; Worrest, R.C. Effects of solar UV radiation on aquatic ecosystems and interactions with climate change. Photochem. Photobiol. Sci. 2007, 6, 267–285. [Google Scholar] [CrossRef]
- Sgroppo, S.C.; Sosa, C. Zapallo anco (Cucurbita moschata D.) Fresco cortado tratado con luz UV-C. Facena 2009, 25, 7–19. [Google Scholar]
- Vian, A.; Davies, E.; Gendraud, M.; Bonnet, P. Plant responses to high frequency electromagnetic fields. BioMed Res. Int. 2016, 2016, 1830262. [Google Scholar] [CrossRef]
- Urban, L.; Charles, F.; de Miranda, M.R.A.; Aarrouf, J. Understanding the physiological effects of UV-C light and exploiting its agronomic potential before and after harvest. Plant. Physiol. Biochem. 2016, 105, 1–11. [Google Scholar] [CrossRef]
- Karki, K.B.; Mishra, A.K.; Choi, S.-J.; Baek, K.-H. Effect of ultraviolet C irradiation on isoflavone concentrations in different cultivars of soybean (Glycine max). Plants 2020, 9, 1043. [Google Scholar] [CrossRef]
- Wargent, J.J.; Jordan, B.R. From ozone depletion to agriculture: Understanding the role of UV radiation in sustainable crop production. New Phytol. 2013, 197, 1058–1076. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Bravo, R.; Klinkhamer, P.G.L.; Leiss, K.A. Interactive effects of UV-B light with abiotic factors on plant growth and chemistry, and their consequences for defense against arthropod herbivores. Front. Plant Sci. 2017, 8, 278. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, R.P.; Kumar, A.; Tyagi, M.B.; Sinha, R.P. Molecular mechanisms of ultraviolet radiation-induced DNA damage and repair. J. Nucleic Acids 2010, 2010, 592980. [Google Scholar] [CrossRef] [PubMed]
- Yokawa, K.; Kagenishi, T.; Baluška, F. UV-B induced generation of reactive oxygen species promotes formation of BFA-induced compartments in cells of Arabidopsis root apices. Front. Plant Sci. 2016, 6, 1162. [Google Scholar] [CrossRef]
- Melis, A. Photosystem-II damage and repair cycle in chloroplasts: What modulates the rate of photodamage in vivo? Trends Plant Sci. 1999, 4, 130–135. [Google Scholar] [CrossRef]
- Castronuovo, D.; Sofo, A.; Lovelli, S.; Candido, V.; Scopa, A. Effects of UV-C radiation on common dandelion and purple coneflower: First results. Int. J. Plant Biol. 2017, 8. [Google Scholar] [CrossRef]
- Kataria, S.; Guruprasad, K.N. Solar UV-B and UV-A/B exclusion effects on intraspecific variations in crop growth and yield of wheat varieties. Field Crops Res. 2012, 125, 8–13. [Google Scholar] [CrossRef]
- Correia, C.M.; Areal, E.L.V.; Torres-Pereira, M.S.; Torres-Pereira, J.M.G. Intraspecific variation in sensitivity to ultraviolet-B radiation in maize grown under field conditions: II. Physiological and biochemical aspects. Field Crops Res. 1999, 62, 97–105. [Google Scholar] [CrossRef]
- Verdaguer, D.; Jansen, M.A.K.; Llorens, L.; Morales, L.O.; Neugart, S. UV-A radiation effects on higher plants: Exploring the known unknown. Plant Sci. 2017, 255, 72–81. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Akram, N.A.; Ashraf, M. Osmoprotection in plants under abiotic stresses: New insights into a classical phenomenon. Planta 2020, 251, 3. [Google Scholar] [CrossRef]
- You, J.; Chan, Z. ROS regulation during abiotic stress responses in crop plants. Front. Plant Sci. 2015, 6, 1092. [Google Scholar] [CrossRef] [PubMed]
- Hoque, T.S.; Hossain, M.A.; Mostofa, M.G.; Burritt, D.J.; Fujita, M.; Tran, L.-S.P. Methylglyoxal: An emerging signaling molecule in plant abiotic stress responses and tolerance. Front. Plant Sci. 2016, 7, 1341. [Google Scholar] [CrossRef] [PubMed]
- Isah, T. Stress and defense responses in plant secondary metabolites production. Biol. Res. 2019, 52, 39. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kumar, V.; Shahzad, B.; Ramakrishnan, M.; Sidhu, G.P.S.; Bali, A.S.; Handa, N.; Kapoor, D.; Yadav, P.; Khanna, K. Photosynthetic response of plants under different abiotic stresses: A review. J. Plant Growth Regul. 2020, 39, 509–531. [Google Scholar] [CrossRef]
- Khorobrykh, S.; Havurinne, V.; Mattila, H.; Tyystjärvi, E. Oxygen and ROS in photosynthesis. Plants 2020, 9, 91. [Google Scholar] [CrossRef]
- Singh, P.; Singh, I.; Shah, K. Reduced activity of nitrate reductase under heavy metal cadmium stress in rice: An in silico answer. Front. Plant Sci. 2019, 9, 1948. [Google Scholar] [CrossRef]
- Kumar, S.; Trivedi, P.K. Glutathione S-transferases: Role in combating abiotic stresses including arsenic detoxification in plants. Front. Plant Sci. 2018, 9, 751. [Google Scholar] [CrossRef]
- Boeckx, T.; Webster, R.; Winters, A.L.; Webb, K.J.; Gay, A.; Kingston-Smith, A.H. Polyphenol oxidase-mediated protection against oxidative stress is not associated with enhanced photosynthetic efficiency. Ann. Bot. 2015, 116, 529–540. [Google Scholar] [CrossRef]
- Werner, A.K.; Witte, C.-P. The biochemistry of nitrogen mobilization: Purine ring catabolism. Trends Plant Sci. 2011, 16, 381–387. [Google Scholar] [CrossRef]
- Nikiforova, V.J.; Kopka, J.; Tolstikov, V.; Fiehn, O.; Hopkins, L.; Hawkesford, M.J.; Hesse, H.; Hoefgen, R. Systems rebalancing of metabolism in response to sulfur deprivation, as revealed by metabolome analysis of Arabidopsis plants. Plant Physiol. 2005, 138, 304–318. [Google Scholar] [CrossRef]
- Rose, M.T.; Rose, T.J.; Pariasca-Tanaka, J.; Yoshihashi, T.; Neuweger, H.; Goesmann, A.; Frei, M.; Wissuwa, M. Root metabolic response of rice (Oryza sativa L.) genotypes with contrasting tolerance to zinc deficiency and bicarbonate excess. Planta 2012, 236, 959–973. [Google Scholar] [CrossRef] [PubMed]
- Alamillo, J.M.; DÍAz-Leal, J.L.; SÁNchez-Moran, M.V.; Pineda, M. Molecular analysis of ureide accumulation under drought stress in Phaseolus vulgaris L. Plant Cell Environ. 2010, 33, 1828–1837. [Google Scholar] [CrossRef] [PubMed]
- Yobi, A.; Wone, B.W.M.; Xu, W.; Alexander, D.C.; Guo, L.; Ryals, J.A.; Oliver, M.J.; Cushman, J.C. Metabolomic profiling in Selaginella lepidophylla at various hydration states provides new insights into the mechanistic basis of desiccation tolerance. Mol. Plant 2013, 6, 369–385. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Matsumoto, M.; Hakomori, Y.; Takagi, H.; Shimada, H.; Sakamoto, A. The purine metabolite allantoin enhances abiotic stress tolerance through synergistic activation of abscisic acid metabolism. Plant Cell Environ. 2014, 37, 1022–1036. [Google Scholar] [CrossRef]
- Kanani, H.; Dutta, B.; Klapa, M.I. Individual vs. combinatorial effect of elevated CO2 conditions and salinity stress on Arabidopsis thaliana liquid cultures: Comparing the early molecular response using time-series transcriptomic and metabolomic analyses. BMC Syst. Biol. 2010, 4, 177. [Google Scholar] [CrossRef]
- Brychkova, G.; Alikulov, Z.; Fluhr, R.; Sagi, M. A critical role for ureides in dark and senescence-induced purine remobilization is unmasked in the Atxdh1 Arabidopsis mutant. Plant. J. 2008, 54, 496–509. [Google Scholar] [CrossRef]
- Montalbini, P. Effect of rust infection on levels of uricase, allantoinase and ureides in susceptible and hypersensitive bean leaves. Physiol. Mol. Plant Pathol. 1991, 39, 173–188. [Google Scholar] [CrossRef]
- Smith, P.M.C.; Atkins, C.A. Purine biosynthesis. Big in cell division, even bigger in nitrogen assimilation. Plant Physiol. 2002, 128, 793–802. [Google Scholar] [CrossRef]
- Gus’ Kov, E.P.; Prokof’ev, V.N.; Kletskii, M.E.; Kornienko, I.V.; Gapurenko, O.A.; Olekhnovich, L.P.; Chistyakov, V.A.; Shestopalov, A.V.; Sazykina, M.A.; Markeev, A.V. Allantoin as a vitamin. Dokl. Biochem. Biophys. 2004, 398, 320–324. [Google Scholar] [CrossRef]
- Irani, S.; Todd, C.D. Exogenous allantoin increases Arabidopsis seedlings tolerance to NaCl stress and regulates expression of oxidative stress response genes. J. Plant Physiol. 2018, 221, 43–50. [Google Scholar] [CrossRef]
- Watanabe, S.; Nakagawa, A.; Izumi, S.; Shimada, H.; Sakamoto, A. RNA interference-mediated suppression of xanthine dehydrogenase reveals the role of purine metabolism in drought tolerance in Arabidopsis. FEBS Lett. 2010, 584, 1181–1186. [Google Scholar] [CrossRef]
- Wang, P.; Kong, C.-H.; Sun, B.; Xu, X.-H. Distribution and function of allantoin (5-ureidohydantoin) in rice grains. J. Agric. Food Chem. 2012, 60, 2793–2798. [Google Scholar] [CrossRef] [PubMed]
- Castronuovo, D.; Tataranni, G.; Lovelli, S.; Candido, V.; Sofo, A.; Scopa, A. UV-C irradiation effects on young tomato plants: Preliminary results. Pak. J. Bot. 2014, 46, 945–949. [Google Scholar]
- Sarghein, S.H.; Carapetian, J.; Khara, J. The effects of UV radiation on some structural and ultrastructural parameters in pepper (Capsicum longum A. DC.). Turk. J. Biol. 2011, 35, 69–77. [Google Scholar]
- Kakani, V.G.; Reddy, K.R.; Zhao, D.; Mohammed, A.R. Effects of ultraviolet-B radiation on cotton (Gossypium hirsutum L.) morphology and anatomy. Ann. Bot. 2003, 91, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Sohag, A.A.M.; Tahjib-Ul-Arif, M.; Afrin, S.; Khan, M.K.; Hannan, M.A.; Skalicky, M.; Mortuza, M.G.; Brestic, M.; Hossain, M.A.; Murata, Y. Insights into nitric oxide-mediated water balance, antioxidant defence and mineral homeostasis in rice (Oryza sativa L.) under chilling stress. Nitric Oxide 2020, 100–101, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, D.; Singh, S.; Kumar, V.; Romero, R.; Prasad, R.; Singh, J. Antioxidant enzymes regulation in plants in reference to reactive oxygen species (ROS) and reactive nitrogen species (RNS). Plant Gene 2019, 19, 100182. [Google Scholar] [CrossRef]
- Casartelli, A.; Melino, V.J.; Baumann, U.; Riboni, M.; Suchecki, R.; Jayasinghe, N.S.; Mendis, H.; Watanabe, M.; Erban, A.; Zuther, E. Opposite fates of the purine metabolite allantoin under water and nitrogen limitations in bread wheat. Plant Mol. Biol. 2019, 99, 477–497. [Google Scholar] [CrossRef]
- Guidi, L.; Lo Piccolo, E.; Landi, M. Chlorophyll fluorescence, photoinhibition and abiotic stress: Does it make any difference the fact to be a C3 or C4 species? Front. Plant Sci. 2019, 10, 174. [Google Scholar] [CrossRef]
- Tränkner, M.; Tavakol, E.; Jákli, B. Functioning of potassium and magnesium in photosynthesis, photosynthate translocation and photoprotection. Physiol. Plant. 2018, 163, 414–431. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.; Ahammed, G.J.; Li, Z.-X.; Wei, J.-P.; Shen, C.; Yan, P.; Zhang, L.-P.; Han, W.-Y. Stimulation in primary and secondary metabolism by elevated carbon dioxide alters green tea quality in Camellia sinensis L. Sci. Rep. 2017, 7, 7937. [Google Scholar] [CrossRef] [PubMed]
- Morales, F.; Ancín, M.; Fakhet, D.; González-Torralba, J.; Gámez, A.L.; Seminario, A.; Soba, D.; Ben Mariem, S.; Garriga, M.; Aranjuelo, I. Photosynthetic metabolism under stressful growth conditions as a bases for crop breeding and yield improvement. Plants 2020, 9, 88. [Google Scholar] [CrossRef] [PubMed]
- Tahjib-Ul-Arif, M.; Siddiqui, M.N.; Sohag, A.A.M.; Sakil, M.A.; Rahman, M.M.; Polash, M.A.S.; Mostofa, M.G.; Tran, L.-S.P. Salicylic acid-mediated enhancement of photosynthesis attributes and antioxidant capacity contributes to yield improvement of maize plants under salt stress. J. Plant Growth Regul. 2018, 37, 1318–1330. [Google Scholar] [CrossRef]
- Sohag, A.A.M.; Tahjib-Ul-Arif, M.; Polash, M.A.S.; Belal Chowdhury, M.; Afrin, S.; Burritt, D.J.; Murata, Y.; Hossain, M.A.; Afzal Hossain, M. Exogenous glutathione-mediated drought stress tolerance in rice (Oryza sativa L.) is associated with lower oxidative damage and favorable ionic homeostasis. Iran. J. Sci. Technol. Trans. A Sci. 2020, 44, 955–971. [Google Scholar] [CrossRef]
- Sohag, A.A.M.; Tahjib-Ul-Arif, M.; Brestic, M.; Afrin, S.; Sakil, M.A.; Hossain, M.T.; Hossain, M.A.; Hossain, M.A. Exogenous salicylic acid and hydrogen peroxide attenuate drought stress in rice. Plant Soil Environ. 2020, 66, 7–13. [Google Scholar] [CrossRef]
- Alhaithloul, H.A.S. Impact of combined heat and drought stress on the potential growth responses of the desert grass Artemisia sieberi alba: Relation to biochemical and molecular adaptation. Plants 2019, 8, 416. [Google Scholar] [CrossRef]
- Handayani, T.; Watanabe, K. The combination of drought and heat stress has a greater effect on potato plants than single stresses. Plant Soil Environ. 2020, 66, 175–182. [Google Scholar] [CrossRef]
- Slama, I.; Abdelly, C.; Bouchereau, A.; Flowers, T.; Savoure, A. Diversity, distribution and roles of osmoprotective compounds accumulated in halophytes under abiotic stress. Ann. Bot. 2015, 115, 433–447. [Google Scholar] [CrossRef]
- Czarnocka, W.; Karpiński, S. Friend or foe? Reactive oxygen species production, scavenging and signaling in plant response to environmental stresses. Free Radic. Biol. Med. 2018, 122, 4–20. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Anee, T.I.; Fujita, M. Glutathione in plants: Biosynthesis and physiological role in environmental stress tolerance. Physiol. Mol. Biol. Plants 2017, 23, 249–268. [Google Scholar] [CrossRef]
- Thoma, F.; Somborn-Schulz, A.; Schlehuber, D.; Keuter, V.; Deerberg, G. Effects of light on secondary metabolites in selected leafy greens: A review. Front. Plant Sci. 2020, 11, 497. [Google Scholar] [CrossRef] [PubMed]
- Naoumkina, M.A.; Zhao, Q.; Gallego-Giraldo, L.; Dai, X.; Zhao, P.X.; Dixon, R.A. Genome-wide analysis of phenylpropanoid defence pathways. Mol. Plant Pathol. 2010, 11, 829–846. [Google Scholar] [CrossRef] [PubMed]
- Mrázová, A.; Belay, S.A.; Eliášová, A.; Perez-Delgado, C.; Kaducová, M.; Betti, M.; Vega, J.M.; Paľove-Balang, P. Expression, activity of phenylalanine-ammonia-lyase and accumulation of phenolic compounds in Lotus japonicus under salt stress. Biologia 2017, 72, 36–42. [Google Scholar] [CrossRef]
- Mao, B.; Yin, H.; Wang, Y.; Zhao, T.-H.; Tian, R.-R.; Wang, W.; Ye, J.-S. Combined effects of O3 and UV radiation on secondary metabolites and endogenous hormones of soybean leaves. PLoS ONE 2017, 12, e0183147. [Google Scholar] [CrossRef]
- Coleto, I.; Pineda, M.; Rodiño, A.P.; De Ron, A.M.; Alamillo, J.M. Comparison of inhibition of N2 fixation and ureide accumulation under water deficit in four common bean genotypes of contrasting drought tolerance. Ann. Bot. 2014, 113, 1071–1082. [Google Scholar] [CrossRef]
- Malik, V.M.; Lobo, J.M.; Stewart, C.; Irani, S.; Todd, C.D.; Gray, G.R. Growth irradiance affects ureide accumulation and tolerance to photoinhibition in Eutrema salsugineum (Thellungiella salsuginea). Photosynthetica 2016, 54, 93–100. [Google Scholar] [CrossRef]
- Irani, S.; Lobo, J.M.; Gray, G.R.; Todd, C.D. Allantoin accumulation in response to increased growth irradiance in Arabidopsis thaliana. Biol. Plant. 2018, 62, 181–187. [Google Scholar] [CrossRef]
- Takagi, H.; Watanabe, S.; Tanaka, S.; Matsuura, T.; Mori, I.C.; Hirayama, T.; Shimada, H.; Sakamoto, A. Disruption of ureide degradation affects plant growth and development during and after transition from vegetative to reproductive stages. BMC Plant Biol. 2018, 18, 287. [Google Scholar] [CrossRef]
- Simontacchi, M.; Galatro, A.; Ramos-Artuso, F.; Santa-María, G.E. Plant survival in a changing environment: The role of nitric oxide in plant responses to abiotic stress. Front. Plant Sci. 2015, 6, 977. [Google Scholar] [CrossRef]
- Esringu, A.; Aksakal, O.; Tabay, D.; Kara, A.A. Effects of sodium nitroprusside (SNP) pretreatment on UV-B stress tolerance in lettuce (Lactuca sativa L.) seedlings. Environ. Sci. Pollut. Res. 2016, 23, 589–597. [Google Scholar] [CrossRef]
- Wang, Y.; Loake, G.J.; Chu, C. Cross-talk of nitric oxide and reactive oxygen species in plant programed cell death. Front. Plant Sci. 2013, 4, 314. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Gao, M.; Hu, J.; Zhang, X.; Wang, K.; Ashraf, M. Modulation role of abscisic acid (ABA) on growth, water relations and glycinebetaine metabolism in two maize (Zea mays L.) cultivars under drought stress. Int. J. Mol. Sci. 2012, 13, 3189–3202. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.-J.; Liu, J.-H. Nitric oxide is involved in dehydration/drought tolerance in Poncirus trifoliata seedlings through regulation of antioxidant systems and stomatal response. Plant Cell Rep. 2012, 31, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Astier, J.; Gross, I.; Durner, J. Nitric oxide production in plants: An update. J. Exp. Bot. 2018, 69, 3401–3411. [Google Scholar] [CrossRef] [PubMed]
- Lai, D.; Mao, Y.; Zhou, H.; Li, F.; Wu, M.; Zhang, J.; He, Z.; Cui, W.; Xie, Y. Endogenous hydrogen sulfide enhances salt tolerance by coupling the reestablishment of redox homeostasis and preventing salt-induced K+ loss in seedlings of Medicago sativa. Plant Sci. 2014, 225, 117–129. [Google Scholar] [CrossRef]
- Shi, H.; Ye, T.; Han, N.; Bian, H.; Liu, X.; Chan, Z. Hydrogen sulfide regulates abiotic stress tolerance and biotic stress resistance in Arabidopsis. J. Integr. Plant Biol. 2015, 57, 628–640. [Google Scholar] [CrossRef]
- Aroca, Á.; Serna, A.; Gotor, C.; Romero, L.C. S-sulfhydration: A cysteine posttranslational modification in plant systems. Plant Physiol. 2015, 168, 334–342. [Google Scholar] [CrossRef]
- Aroca, A.; Gotor, C.; Romero, L.C. Hydrogen sulfide signaling in plants: Emerging roles of protein persulfidation. Front. Plant Sci. 2018, 9, 1369. [Google Scholar] [CrossRef]
- Yavaş, İ.; Ünay, A.; Ali, S.; Abbas, Z. UV-B Radiations and Secondary Metabolites. Turk. J. Agric. Food Sci. Technol. 2020, 8, 147–157. [Google Scholar] [CrossRef]
- Morales, L.O.; Tegelberg, R.; Brosché, M.; Keinänen, M.; Lindfors, A.; Aphalo, P.J. Effects of solar UV-A and UV-B radiation on gene expression and phenolic accumulation in Betula pendula leaves. Tree Physiol. 2010, 30, 923–934. [Google Scholar] [CrossRef]
- Petersen, M.; Hans, J.; Matern, U. Biosynthesis of phenylpropanoids and related compounds. Ann. Plant Rev. 2018, 40, 182–257. [Google Scholar]
- Hoagland, D.; Arnon, D. The water-culture method for growing plants without soil. Circ. Calif. Agric. Exp. Stn. 1950, 347, 32. [Google Scholar]
- López Rubira, V.; Artés Hernández, F.d.A.; Artés Calero, F. Evaluación de la Calidad de Granadas Tratadas con UV-C y Almacenadas en Atmósfera Controlada; Universidad Politecnica de Cartagena: Cartagena, Spain, 2007. [Google Scholar]
- Hunt, R. Relative growth rates. In Basic Growth Analysis; Springer: Berlin, Germany, 1990; pp. 25–34. [Google Scholar]
- Clarke, J.M.; Richards, R.A.; Condon, A.G. Effect of drought stress on residual transpiration and its relationship with water use of wheat. Can. J. Plant Sci. 1991, 71, 695–702. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of Total Carotenoids and Chlorophylls A and B of Leaf Extracts in Different Solvents; Portland Press Ltd.: London, UK, 1983. [Google Scholar]
- Fales, F.W. The assimilation and degradation of carbohydrates by yeast cells. J. Biol. Chem. 1951, 193, 113–124. [Google Scholar]
- Schlegel, H.G. Die verwertung organischer säuren durch Chlorella im licht. Planta 1956, 47, 510–526. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar]
- Moore, S.; Stein, W.H. Photometric ninhydrin method for use in the ehromatography of amino acids. J. Biol. Chem. 1948, 176, 367–388. [Google Scholar]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Mukherjee, S.P.; Choudhuri, M.A. Implications of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol. Plant. 1983, 58, 166–170. [Google Scholar] [CrossRef]
- Elstner, E.F.; Heupel, A. Formation of hydrogen peroxide by isolated cell walls from horseradish (Armoracia lapathifolia Gilib.). Planta 1976, 130, 175–180. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C.; Aruoma, O.I. The deoxyribose method: A simple “test-tube” assay for determination of rate constants for reactions of hydroxyl radicals. Anal. Biochem. 1987, 165, 215–219. [Google Scholar] [CrossRef]
- Rao, K.V.M.; Sresty, T.V.S. Antioxidative parameters in the seedlings of pigeonpea (Cajanus cajan (L.) Millspaugh) in response to Zn and Ni stresses. Plant Sci. 2000, 157, 113–128. [Google Scholar]
- Minguez-Mosquera, M.I.; Jaren-Galan, M.; Garrido-Fernandez, J. Lipoxygenase activity during pepper ripening and processing of paprika. Phytochemistry 1993, 32, 1103–1108. [Google Scholar] [CrossRef]
- Gilbert, R.P.; Brandt, R.B. Spectrophotometric determination of methyl glyoxal with 2, 4-dinitrophenylhydrazine. Anal. Chem. 1975, 47, 2418–2422. [Google Scholar] [CrossRef] [PubMed]
- Jagota, S.K.; Dani, H.M. A new colorimetric technique for the estimation of vitamin C using Folin phenol reagent. Anal. Biochem. 1982, 127, 178–182. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Kivçak, B.; Mert, T. Quantitative determination of α-tocopherol in Arbutus unedo by TLC-densitometry and colorimetry. Fitoterapia 2001, 72, 656–661. [Google Scholar] [CrossRef]
- Kofalvi, S.A.; Nassuth, A. Influence of wheat streak mosaic virus infection on phenylpropanoid metabolism and the accumulation of phenolics and lignin in wheat. Physiol. Mol. Plant Pathol. 1995, 47, 365–377. [Google Scholar] [CrossRef]
- Zou, Y.; Lu, Y.; Wei, D. Antioxidant activity of a flavonoid-rich extract of Hypericum perforatum L. in vitro. J. Agric. Food Chem. 2004, 52, 5032–5039. [Google Scholar] [CrossRef]
- Krizek, D.T.; Kramer, G.F.; Upadhyaya, A.; Mirecki, R.M. UV-B response of cucumber seedlings grown under metal halide and high pressure sodium/deluxe lamps. Physiol. Plant. 1993, 88, 350–358. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1984; Volume 105, pp. 121–126. [Google Scholar]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Kumar, K.B.; Khan, P.A. Peroxidase and polyphenol oxidase in excised ragi (Eleusine corocana cv PR 202) leaves during senescence. Indian J. Exp. Biol. 1982, 20, 412–416. [Google Scholar]
- Havir, E.A.; Hanson, K.R. L-phenylalanine ammonia-lyase (maize and potato). Evidence that the enzyme is composed of four subunits. Biochemistry 1973, 12, 1583–1591. [Google Scholar] [CrossRef] [PubMed]
- Ghanati, F.; Morita, A.; Yokota, H. Induction of suberin and increase of lignin content by excess boron in tobacco cells. Soil Sci. Plant Nutr. 2002, 48, 357–364. [Google Scholar] [CrossRef]
- Nashef, A.S.; Osuga, D.T.; Feeney, R.E. Determination of hydrogen sulfide with 5, 5′-dithiobis-(2-nitrobenzoic acid), N-ethylmaleimide, and parachloromercuribenzoate. Anal. Biochem. 1977, 79, 394–405. [Google Scholar] [CrossRef]
- Gaitonde, M.K. A spectrophotometric method for the direct determination of cysteine in the presence of other naturally occurring amino acids. Biochem. J. 1967, 104, 627–633. [Google Scholar] [CrossRef]
- Ding, M.; Pierre, B.A.S.; Parkinson, J.F.; Medberry, P.; Wong, J.L.; Rogers, N.E.; Ignarro, L.J.; Merrill, J.E. Inducible nitric-oxide synthase and nitric oxide production in human fetal astrocytes and microglia A kinetic analysis. J. Biol. Chem. 1997, 272, 11327–11335. [Google Scholar] [CrossRef]
- Hu, X.; Neill, S.J.; Cai, W.; Tang, Z. NO-mediated hypersensitive responses of rice suspension cultures induced by incompatible elicitor. Chin. Sci. Bullet. 2003, 48, 358–363. [Google Scholar] [CrossRef]
- Downs, M.R.; Nadelhoffer, K.J.; Melillo, J.M.; Aber, J.D. Foliar and fine root nitrate reductase activity in seedlings of four forest tree species in relation to nitrogen availability. Trees 1993, 7, 233–236. [Google Scholar] [CrossRef]
- Vogels, G.D.; Van der Drift, C. Differential analyses of glyoxylate derivatives. Anal. Biochem. 1970, 33, 143–157. [Google Scholar] [CrossRef]
- Zhang, D.; Nie, S.; Xie, M.; Hu, J. Antioxidant and antibacterial capabilities of phenolic compounds and organic acids from Camellia oleifera cake. Food Sci. Biotechnol. 2019, 29, 17–25. [Google Scholar] [CrossRef] [PubMed]


| Treatments | RGR | SFW (g) | RFW (g) | SDW (g) | RDW (g) | Chl-a Content | Chl-b Content | Chl-a+b Content | Car Content |
|---|---|---|---|---|---|---|---|---|---|
| C | 0.050 ± 0.002 d | 8.64 ± 0.32 f | 3.08 ± 0.11 d | 0.933 ± 0.03 g | 0.206 ± 0.007 e | 2.43 ± 0.09 f | 0.670 ± 0.02 d | 3.11 ± 0.11 f | 1.30 ± 0.04 e |
| UVC1 | 0.025 ±0.003 a,b | 4.59 ± 0.17 a | 2.10 ± 0.07 a | 0.490 ± 0.01 a,b | 0.155 ± 0.005 a | 1.37 ± 0.05 a,b | 0.559 ± 0.02 a | 1.93 ± 0.07 a,b | 0.745 ± 0.02 a |
| UVC2 | 0.017 ± 0.000 a | 3.49 ± 0.12 b | 1.03 ± 0.03 b | 0.361 ± 0.01 c | 0.075 ± 0.002 b | 0.893 ± 0.03 c | 0.344 ± 0.01 b | 1.23 ± 0.04 c | 0.494 ± 0.01 b |
| Pre-AT | 0.076 ± 0.004 c | 11.77 ± 0.11 c | 4.56 ± 0.04 c | 1.927 ± 0.01 d | 0.230 ± 0.002 c | 3.18 ± 0.03 d | 0.874 ± 0.008 c | 4.06 ± 0.04 d | 1.55 ± 0.01 c |
| AT+ UVC1 | 0.044 ± 0.003 d | 7.40 ± 0.04 d | 2.83 ±0.01 d | 0.726 ± 0.00 e | 0.224 ± 0.001 c | 2.38 ± 0.01 e,f | 0.662 ± 0.004 d | 3.04 ± 0.01 e,f | 2.34 ± 0.01 d |
| AT+ UVC2 | 0.029 ± 0.001 b | 6.40 ± 0.33 e | 1.84 ± 0.09 a | 0.608 ± 0.03 a,e | 0.113 ± 0.005 d | 2.06 ± 0.10 e | 0.713 ± 0.03 d | 2.77 ± 0.14 e | 1.37 ± 0.07 e |
| Post-AT | 0.078 ± 0.003 c | 11.11 ± 0.57 c | 3.95 ± 0.20 e | 2.088 ± 0.10 f | 0.221 ± 0.011 c,e | 3.35 ± 0.17 d | 0.878 ± 0.04 c | 4.23 ± 0.21 d | 1.25 ± 0.06 e |
| UVC1+AT | 0.031 ± 0.002 b | 5.63 ± 0.21 e | 2.43 ± 0.09 f | 0.589 ± 0.02 a,b | 0.168 ± 0.006 a | 1.66 ± 0.06 a | 0.548 ± 0.003 a | 2.20 ± 0.06 a | 1.31 ± 0.05 e |
| UVC1+AT | 0.021 ± 0.002 a | 4.31 ± 0.15 a | 1.48 ± 0.05 g | 0.471 ± 0.01 b,c | 0.093 ± 0.003 f | 1.28 ± 0.04 b | 0.443 ± 0.004 e | 1.72 ± 0.04 b | 0.539 ± 0.01 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dawood, M.F.A.; Tahjib-Ul-Arif, M.; Sohag, A.A.M.; Abdel Latef, A.A.H.; Ragaey, M.M. Mechanistic Insight of Allantoin in Protecting Tomato Plants Against Ultraviolet C Stress. Plants 2021, 10, 11. https://doi.org/10.3390/plants10010011
Dawood MFA, Tahjib-Ul-Arif M, Sohag AAM, Abdel Latef AAH, Ragaey MM. Mechanistic Insight of Allantoin in Protecting Tomato Plants Against Ultraviolet C Stress. Plants. 2021; 10(1):11. https://doi.org/10.3390/plants10010011
Chicago/Turabian StyleDawood, Mona F. A., Md. Tahjib-Ul-Arif, Abdullah Al Mamun Sohag, Arafat Abdel Hamed Abdel Latef, and Marwa M. Ragaey. 2021. "Mechanistic Insight of Allantoin in Protecting Tomato Plants Against Ultraviolet C Stress" Plants 10, no. 1: 11. https://doi.org/10.3390/plants10010011
APA StyleDawood, M. F. A., Tahjib-Ul-Arif, M., Sohag, A. A. M., Abdel Latef, A. A. H., & Ragaey, M. M. (2021). Mechanistic Insight of Allantoin in Protecting Tomato Plants Against Ultraviolet C Stress. Plants, 10(1), 11. https://doi.org/10.3390/plants10010011








