Evaluation of Dittrichia viscosa (L.) Greuter Dried Biomass for Weed Management
Abstract
:1. Introduction
2. Results
2.1. Experiment 1: Effects of DB on Emergence of Cress and Wheat
2.2. Experiment 2: Effects on Emergence of the Natural Seed Bank in Greenhouse Conditions
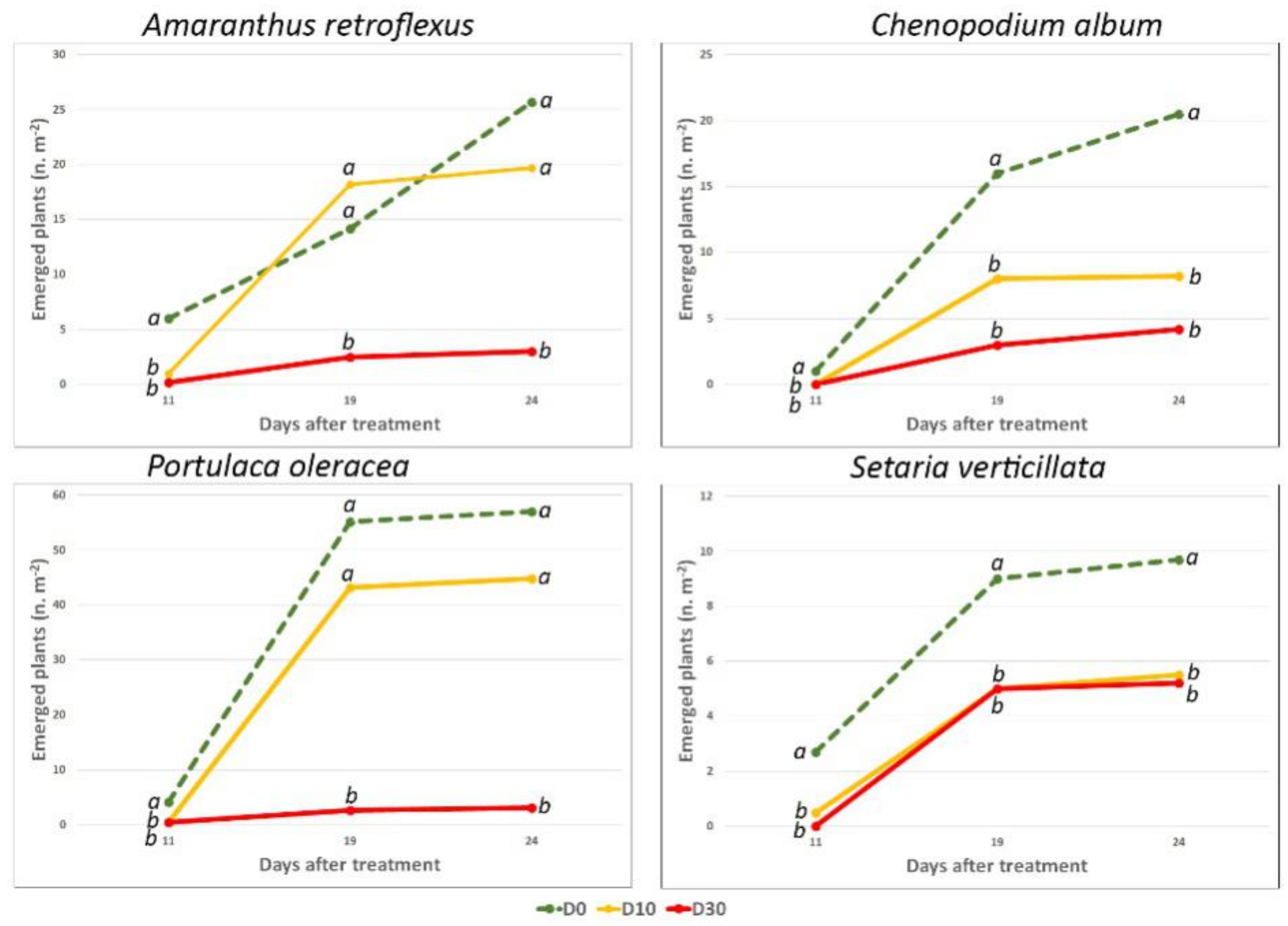
2.3. Experiment 3: Effects on Emergence of the Natural Seed Bank in Open Field Conditions
2.4. Experiment 4: Effects on Seedlings
2.5. Experiment 5: Reversibility of the Effects on Seed Germination
2.6. Experiment 6: DB Efficacy in Relation to Different Soil Texture
3. Discussion
4. Materials and Methods
4.1. Location and Site of the Experiments
4.2. Dried Biomass (DB)
4.3. Indicator Plant Species
4.4. Preparation of the Substrate Necessary for Seed Germination or Plant Growth
4.5. Experiments
4.5.1. Experiment 1: Effects of DB on Emergence of Indicator Plants
4.5.2. Experiment 2: Effects of DB on Emergence of the Natural Seed Bank in Greenhouse Conditions
4.5.3. Experiment 3: Effects on Emergence of the Natural Seed Bank in Open Field Conditions
4.5.4. Experiment 4: Effects on Seedlings
4.5.5. Experiment 5: Reversibility of the Effects on Seed Germination
- Treatment 1: D0 + D0 (i.e., control) = seeds buried in untreated soil (with no biomass) were then buried again in untreated soil.
- Treatment 2: D30 + D0 (i.e., inhibitory factor removed) = seeds previously buried in treated soil were buried in untreated soil to remove the inhibitory factor.
- Treatment 3: D30 + D30 (i.e., inhibitory factor not removed) = seeds buried in treated soil were buried again in treated soil.
4.5.6. Experiment 6: DB Efficacy in Relation to Different Soil Texture
4.6. Experimental Design and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Perotti, V.E.; Larran, A.S.; Palmieri, V.E.; Martinatto, A.K.; Permingeat, H.R. Herbicide resistant weeds: A call to integrate conventional agricultural practices, molecular biology knowledge and new technologies. Plant Sci. 2020, 290, 110255. [Google Scholar] [CrossRef]
- Duke, S. Proving Allelopathy in Crop–Weed Interactions. Weed Sci. 2015, 63, 121–132. [Google Scholar] [CrossRef] [Green Version]
- De Albuquerque, M.B.; Santos, R.C.; Lima, L.M.; Melo Filho, P.D.A.; Nogueira, R.J.M.C.; Câmara, C.A.G.; Ramos, A. Allelopathy, an alternative tool to improve cropping systems. A review. Agron. Sustain. Dev. 2011, 31, 379–395. [Google Scholar] [CrossRef] [Green Version]
- Chon, S.U.; Nelson, C.J. Allelopathy in Compositae plants. A review. Agron. Sustain. Dev. 2010, 30, 349–358. [Google Scholar] [CrossRef] [Green Version]
- Macıas, F.A.; Molinillo, J.M.G.; Varela, R.M.; Galindo, C.G. Allelopathy—A natural alternative for weed control. Pest Manag. Sci. 2007, 63, 327–348. [Google Scholar] [CrossRef]
- Belz, R.G. Allelopathy in crop/weed interactions—An update. Pest Manag. Sci. 2007, 63, 308–326. [Google Scholar] [CrossRef]
- Weston, L.A. Utilization of allelopathy for weed management in agroecosystems. Agron. J. 1996, 88, 860–866. [Google Scholar] [CrossRef]
- Weston, L.A.; Duke, S.O. Weed and crop allelopathy. Crit. Rev. Plant Sci. 2003, 22, 367–389. [Google Scholar] [CrossRef]
- Abbas, T.; Nadeem, M.A.; Tanveer, A.; Farooq, N.; Zohaib, A. Mulching with allelopathic crops to manage herbicide resistant littleseed canarygrass. Herbologia 2016, 16, 31–39. [Google Scholar] [CrossRef]
- Bilalis, D.; Sidiras, N.; Economou, G.; Vakali, C. Effect of different levels of wheat straw soil surface coverage on weed flora in Vicia faba crops. J. Agron. Crop Sci. 2003, 189, 233–241. [Google Scholar] [CrossRef]
- Vidotto, F.; Tesio, F.; Ferrero, A. Allelopathic effects of Helianthus tuberosus L. on germination and seedling growth of several crops and weeds. Biol. Agric. Hortic. 2008, 26, 55–68. [Google Scholar] [CrossRef]
- Lin, D.; Tsuzuki, E.; Dong, Y.; Terao, H.; Xuan, T.D. Potential biological control of weeds in rice fields by allelopathy of dwarf lilyturf plants. BioControl 2004, 49, 187–196. [Google Scholar] [CrossRef]
- Khanh, T.D.; Hong, N.H.; Xuan, T.D.; Chung, I.M. Paddy weed control by medicinal and leguminous plants from Southeast Asia. Crop Prot. 2005, 24, 421–431. [Google Scholar] [CrossRef]
- De Mastro, G.; Fracchiolla, M.; Verdini, L.; Montemurro, P. Oregano and its potential use as bioherbicide. Acta Hortic. 2006, 723, 335–346. [Google Scholar] [CrossRef]
- Om, H.; Dhiman, S.D.; Kumar, S.; Kumar, H. Allelopathic response of Phalaris minor to crop and weed plants in rice–wheat system. Crop Prot. 2002, 21, 699–705. [Google Scholar] [CrossRef]
- Stevens, K.L. Allelopathic polyacetylenes from Centaurea repens (Russian knapweed). J. Chem. Ecol. 1986, 12, 1205–1211. [Google Scholar] [CrossRef]
- Sisodia, S.; Siddiqui, M.B. Allelopathic effect by aqueous extracts of different parts of Croton bonplandianum Baill. on some crop and weed plants. J. Agric. Ext. Rural Dev. 2010, 2, 22–28. [Google Scholar] [CrossRef]
- Jabran, K.; Farooq, M.; Hussain, M.; Rehman, H.; Ali, M.A. Wild oat (Avena fatua L.) and canary grass (Phalaris minor Ritz.) management through allelopathy. J. Plant Prot. Res. 2010, 50, 41–44. [Google Scholar] [CrossRef]
- Andolfi, A.; Zermane, N.; Cimmino, A.; Avolio, F.; Boari, A.; Vurro, M.; Evidente, A. Inuloxins A–D, phytotoxic bi-and tri-cyclic sesquiterpene lactones produced by Inula viscosa: Potential for broomrapes and field dodder management. Phytochemistry 2013, 86, 112–120. [Google Scholar] [CrossRef]
- Masi, M.; Fernández-Aparicio, M.; Zatout, R.; Boari, A.; Cimmino, A.; Evidente, A. Inuloxin E, a new seco-eudesmanolideisolated from Dittrichia viscosa, stimulating Orobanche cumana seed germination. Molecules 2019, 24, 3479. [Google Scholar] [CrossRef] [Green Version]
- Dor, E.; Hershenhorn, J. Allelopathic effects of Inula viscosa leaf extracts on weeds. Allelopath. J. 2012, 30, 281–289. [Google Scholar]
- Grande, M.; Bellido, I.S.; Torres, P.; Piera, F. 9-Hydroxynerolidol esters and bicyclic sesquiterpenoids from Dittrichia viscosa. J. Nat. Prod. 1992, 55, 1074–1079. [Google Scholar] [CrossRef]
- Grande, M.; Piera, F.; Cuenca, A.; Torres, P.; Bellido, I. Flavonoids from Inula viscosa. Planta Med. 1985, 51, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Grande, M.; Torres, P.; Piera, F.; Bellido, I.S. Triterpenoids from Dittrichia viscos. Phytochemistry 1992, 31, 1826–1828. [Google Scholar] [CrossRef]
- Grauso, L.; Cesarano, G.; Zotti, M.; Ranesi, M.; Sun, W.; Bonanom, G.; Lanzotti, V. Exploring Dittrichia viscosa (L.) Greuter phytochemical diversity to explain its antimicrobial, nematicidal and insecticidal activity. Phytochem. Rev. 2019, 19, 659–689. [Google Scholar] [CrossRef]
- Levizou, E.; Karageorgou, P.; Psaras, G.K.; Manetas, Y. Inhibitory effects of water soluble leaf leachates from Dittrichia viscosa on lettuce root growth, statocyte development and graviperception. Flora 2002, 197, 152–157. [Google Scholar] [CrossRef] [Green Version]
- Omezzine, F.; Rinez, A.; Ladhari, A.; Farooq, M.; Haouala, R. Allelopathic potential of Inula viscosa against crops and weeds. Int. J. Agric. Biol. 2011, 13, 841–849. [Google Scholar]
- Kunz, C.; Sturm, D.J.; Varnholt, D.; Walker, F.; Gerhards, R. Allelopathic effects and weed suppressive ability of cover crops. Plant Soil Environ. 2016, 62, 60–66. [Google Scholar] [CrossRef] [Green Version]
- Sturm, D.J.; Peteinatos, G.; Gerhards, R. Contribution of allelopathic effects to the overall weed suppression by different cover crops. Weed Res. 2018, 58, 331–337. [Google Scholar] [CrossRef]
- Weaver, S.; Kropff, M.; Groeneveld, R. Use of Ecophysiological Models for Crop-Weed Interference: The Critical Period of Weed Interference. Weed Sci. 1992, 40, 302–307. [Google Scholar] [CrossRef] [Green Version]
- Pannacci, E.; Lattanzi, B.; Tei, F. Non-chemical weed management strategies in minor crops: A review. Crop Prot. 2017, 96, 44–58. [Google Scholar] [CrossRef]
- Parolin, P.; Ion Scotta, M.; Bresch, C. Biology of Dittrichia viscosa, a Mediterranean ruderal plant: A review. Phyton Int. J. Exp. Bot. 2014, 83, 251–262. [Google Scholar]


| Treatments ** | Emerged Plants * | |||||
|---|---|---|---|---|---|---|
| Lepidium sativum | Triticum durum | |||||
| DAS | D.W. | DAS | D.W. | |||
| 7 | 16 | 7 | 12 | |||
| D0 | 11.7 a | 15.5 a | 0.04 a | 14.5 a | 17.0 a | 0.31 a |
| D2 | 6.2 b | 16.2 a | 0.03 b | 8.7 b | 17.5 a | 0.25 b |
| D4 | 6.2 b | 16.5 a | 0.03 b | 2.7 c | 19.0 a | 0.23 b |
| D10 | 5.7 b | 17.5 a | 0.03 b | 4.7 bc | 18.0 a | 0.23 b |
| D20 | 0.0 c | 10.5 b | 0.02 c | 0.0 c | 19.0 a | 0.16 c |
| D40 | 0.0 c | 0.0 c | 0.0 d | 0.0 c | 6.2 b | 0.04 d |
| Scientific Name | Common Name | Emerged Plants (n.) * per Each Treatment ** | |||
|---|---|---|---|---|---|
| D0 | D10 | D20 | D30 | ||
| Amaranthus retroflexus L. a | redroot pigweed | 178.5 a | 128.2 b | 30.7 c | 9.2 c |
| Calendula arvensis (Vaill.) L. a | marigold | 3.7 a | 0.0 b | 0.0 b | 0.0 b |
| Chenopodium album L. a | common lambsquarters | 169.0 a | 52.5 b | 18.7 c | 7.5 c |
| Solanum nigrum L. a | black nightshade | 7.2 a | 4.0 b | 1.2 bc | 0.5 c |
| Lamium amplexicaule L. b | henbit | 75.7 a | 28.2 b | 13.5 c | 6.5 c |
| Veronica hederifolia L. b | veronica | 14.7 a | 3.7 b | 3.5 b | 1.0 b |
| Urtica dioica L. b | nettle | 59.5 a | 17.0 b | 6.5 c | 1.5 c |
| Treatments * | n. of Emerged Plants ** |
|---|---|
| D0 | 16.0 a |
| D10 | 14.0 a |
| D20 | 4.7 b |
| D30 | 4.0 b |
| Emerged Plants (n.) from Reburied Seeds * | ||||
|---|---|---|---|---|
| Treatments ** | Days After Reburial | |||
| 2 | 4 | 7 | 10 | |
| D0 + D0 | 9.0 a | 12.0 a | 13.7 a | 14.3 a |
| D30 + D0 | 4.0 b | 8.0 b | 9.3 b | 13.7 a |
| D30 + D30 | 0.0 c | 0.0 c | 0.0 c | 0.0 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boari, A.; Vurro, M.; Calabrese, G.J.; Mahmoud, M.N.Z.; Cazzato, E.; Fracchiolla, M. Evaluation of Dittrichia viscosa (L.) Greuter Dried Biomass for Weed Management. Plants 2021, 10, 147. https://doi.org/10.3390/plants10010147
Boari A, Vurro M, Calabrese GJ, Mahmoud MNZ, Cazzato E, Fracchiolla M. Evaluation of Dittrichia viscosa (L.) Greuter Dried Biomass for Weed Management. Plants. 2021; 10(1):147. https://doi.org/10.3390/plants10010147
Chicago/Turabian StyleBoari, Angela, Maurizio Vurro, Generosa Jenny Calabrese, Mohamed Nesma Zakaria Mahmoud, Eugenio Cazzato, and Mariano Fracchiolla. 2021. "Evaluation of Dittrichia viscosa (L.) Greuter Dried Biomass for Weed Management" Plants 10, no. 1: 147. https://doi.org/10.3390/plants10010147






