Cytotoxicity of Ficus Crocata Extract on Cervical Cancer Cells and Protective Effect against Hydrogen Peroxide-Induced Oxidative Stress in HaCaT Non-Tumor Cells
Abstract
:1. Introduction
2. Results
2.1. Ace-EFc Has Antiradical Activity Comparable or Superior to That of Ascorbic Acid
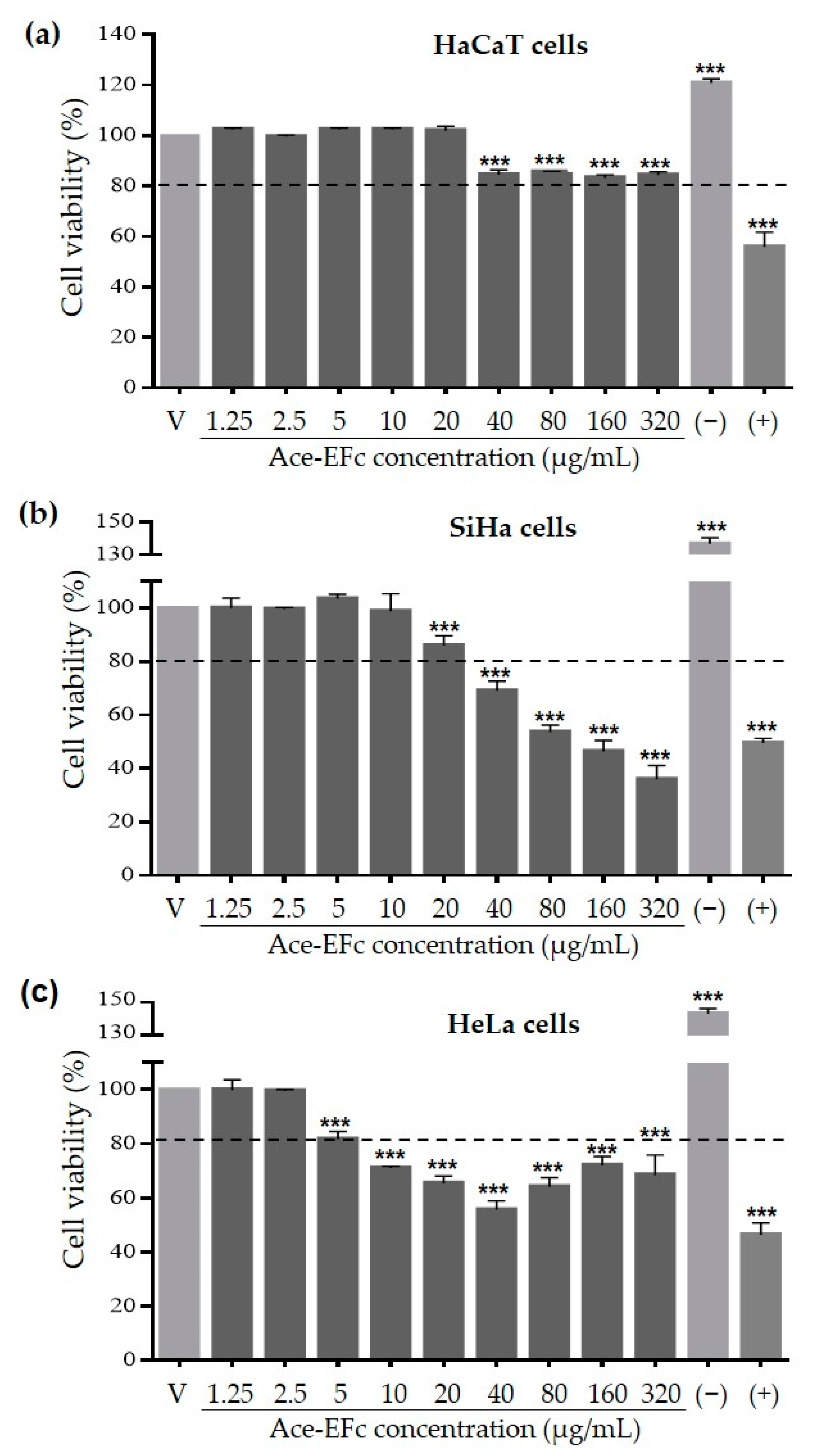
2.2. Ace-EFc Is Cytotoxic on SiHa and HeLa Cervical Cancer Cells but Not in HaCaT Non-Tumor Cells
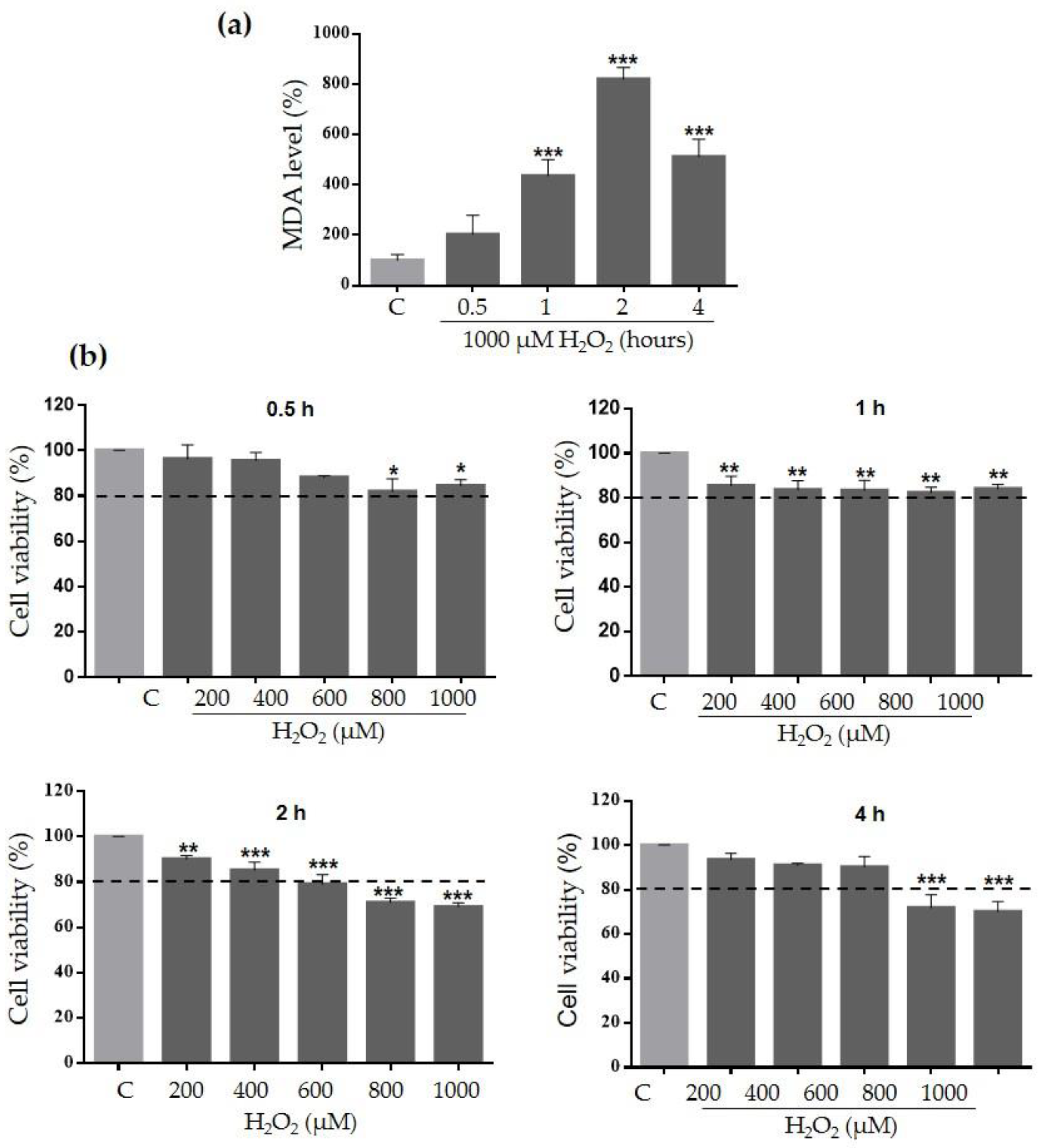
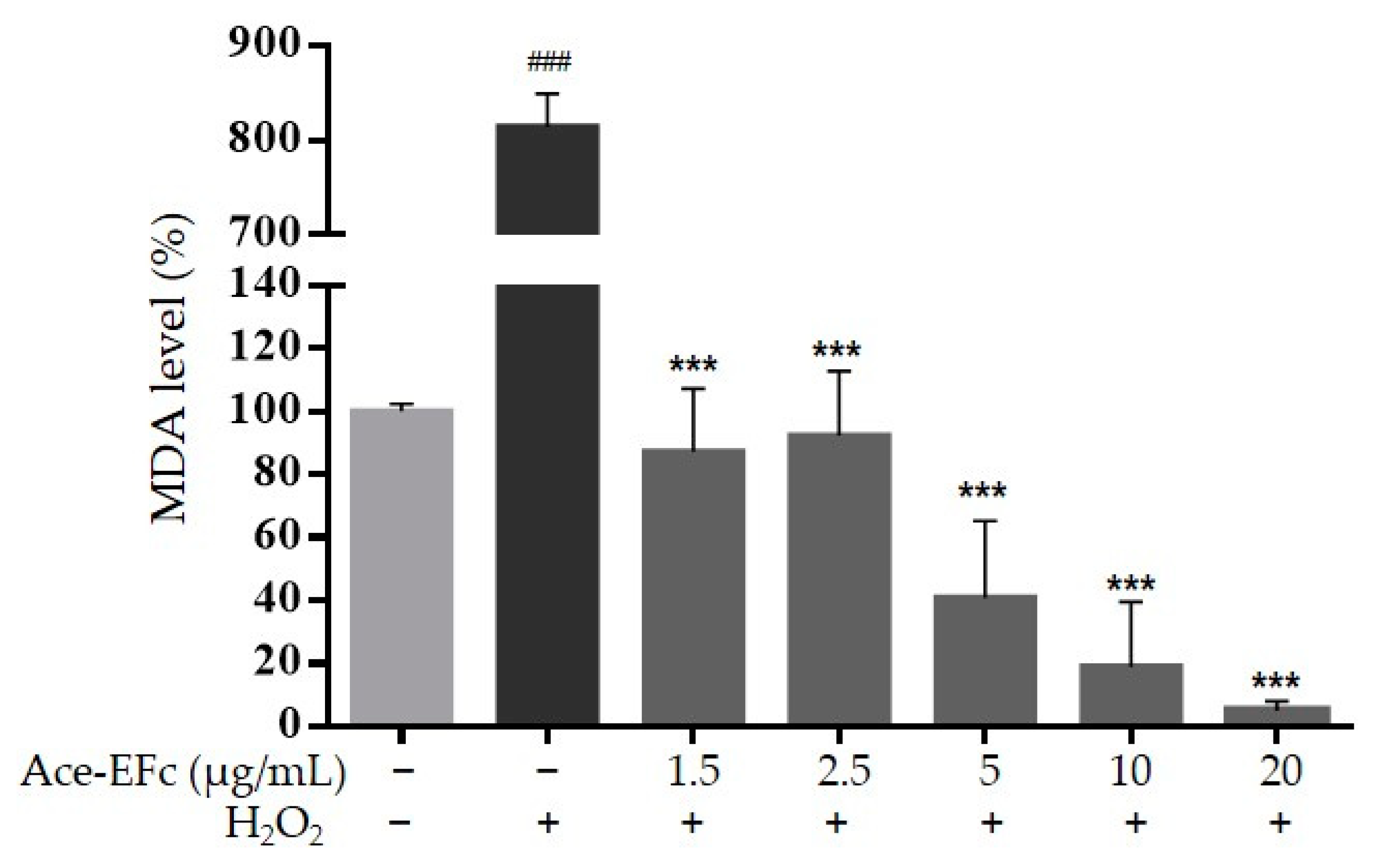
2.3. Ace-EFc Protects HaCaT Cells from H2O2-Induced Lipoperoxidation and Cytotoxicity
3. Discussion
4. Materials and Methods
4.1. Plant Material and Preparation of the Extract
4.2. Phytochemical Profile of Ace-EFc
4.3. DPPH• and ABTS•+ Radical Inhibition Assays
4.4. Cell Culture and H2O2-Induced Lipoperoxidation and Cytotoxicity Model
4.5. Cell Viability Assays
4.6. Oxidative Damage Determination (Lipoperoxidation)
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rahal, A.; Kumar, A.; Singh, V.; Yadav, B.; Tiwari, R.; Chakraborty, S.; Dhama, K. Oxidative stress, prooxidants, and antioxidants: The interplay. BioMed Res. Int. 2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Song, M.; Zhang, B.; Zhang, Y. Reactive oxygen species regulate T cell immune response in the tumor microenvironment. Oxidative Med. Cell. Longev. 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biswas, S.K. Does the interdependence between oxidative stress and inflammation explain the antioxidant paradox? Oxidative Med. Cell. Longev. 2016. [Google Scholar] [CrossRef] [Green Version]
- Zi, Y.; Zhang, B.; Jiang, B.; Yang, X.; Liang, Z.; Liu, W.; He, C.; Liu, L. Antioxidant action and protective and reparative effects of lentinan on oxidative damage in HaCaT cells. J. Cosmet. Dermatol. 2018, 17, 1108–1114. [Google Scholar] [CrossRef]
- Kumari, A.; Kakkar, P. Lupeol prevents Acetaminophen-induced in vivo hepatotoxicity by altering the Bax/Bcl-2 and oxidative stress-mediated mitochondrial signaling cascade. Life Sci. 2012, 90, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, E.; Tsuchiya, M.; Shimamoto, S.; Fujimoto, T.; Tokumitsu, H.; Tokuda, M. Oxidative stress impairs the stimulatory effect of S100 proteins on protein phosphatase 5 activity. Tohoku J. Exp. Med. 2016, 240, 67–78. [Google Scholar] [CrossRef] [Green Version]
- Hecht, F.; Pessoa, C.F.; Gentile, L.B.; Rosenthal, D.; Carvalho, D.P.; Fortunato, R.S. The role of oxidative stress on breast cancer development and therapy. Tumour Biol. 2016, 37, 4281–4291. [Google Scholar] [CrossRef]
- Gupta, A.K.; Rather, M.A.; Kumar Jha, A.; Shashank, A.; Singhal, S.; Sharma, M.; Pathak, U.; Sharma, D.; Mastinu, A. Artocarpus lakoocha Roxb. and Artocarpus heterophyllus Lam. Flowers: New Sources of Bioactive Compounds. Plants 2020, 9, 1329. [Google Scholar] [CrossRef]
- Mopuri, A.; Ganjayi, M.; Meriga, B.; Koorbanally, N.A.; Islam, M.S. The effects of Ficus carica on the activity of enzymes related to metabolic syndrome Ramgopal. J. Food Drug Anal. 2018, 26, 201–210. [Google Scholar] [CrossRef]
- Nurdiana, S.; Goh, Y.M.; Ahmad, H.; Dom, S.M.; Syimal’ain, N.; Noor Mohamad Zin, N.S.; Ebrahimi, M. Changes in pancreatic histology, insulin secretion and oxidative status in diabetic rats following treatment with Ficus deltoidea and vitexin. BMC Complement. Altern. Med. 2017, 17, 290. [Google Scholar] [CrossRef] [Green Version]
- Joshi, H.; Vaishnav, D.; Sanghvi, G.; Rabadia, S.; Airao, V.; Sharma, T. Ficus recemosa bark extract attenuates diabetic complications and oxidative stress in STZ-induced diabetic rats. Pharm. Biol. 2016, 54, 1586–1595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sumi, S.A.; Siraj, M.A.; Hossain, A.; Mia, M.S.; Afrin, S.; Rahman, M.M. Investigation of the key pharmacological activities of Ficus racemosa and analysis of its major bioactive polyphenols by HPLC-DAD. Evid. Based Complement. Altern. Med. 2016. [Google Scholar] [CrossRef] [PubMed]
- Ibarra-Manríquez, G.; Cornejo-Tenorio, G.; González-Castañeda, N.; Piedra-Malagón, E.M.; Luna, A. El género Ficus L. (Moraceae) en México. Bot. Sci. 2012, 90, 389–452. [Google Scholar] [CrossRef] [Green Version]
- Saba, N.F.; Mody, M.D.; Tan, E.S.; Gill, H.S.; Rinaldo, A.; Takes, R.P.; Strojan, P.; Hartl, D.M.; Vermorken, J.B.; Haigentz, M., Jr.; et al. Toxicities of systemic agents in squamous cell carcinoma of the head and neck (SCCHN); A new perspective in the era of immunotherapy. Crit. Rev. Oncol. Hematol. 2017, 115, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Valdeolivar, C.A.; Álvarez-Fitz, P.; Zacapala-Gómez, A.E.; Acevedo-Quiroz, M.; Cayetano-Salazár, L.; Olea-Flores, M.; Castillo-Reyes, J.U.; Navarro-Tito, N.; Ortuño-Pineda, C.; Leyva-Vázquez, M.A.; et al. Phytochemical profile and antiproliferative effect of Ficus crocata extracts on triple-negative breast cancer cells. BMC Complement. Med. Ther. 2020, 20, 1–15. [Google Scholar] [CrossRef]
- Gupta, N.; Verma, K.; Nalla, S.; Kulshreshtha, A.; Lall, R.; Prasad, S. Free Radicals as a Double-Edged Sword: The Cancer Preventive and Therapeutic Roles of Curcumin. Molecules 2020, 25, 5390. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, J.; Rangarajan, M.; Shao, Y.; LaVoie, E.J.; Huang, T.C. Antioxidative phenolic compounds from sage (Salvia officinalis). J. Agric. Food Chem. 1998, 46, 4869–4873. [Google Scholar] [CrossRef]
- Cerretani, L.; Bendini, A. Olives and Olive Oil in Health and Disease Prevention, 1st ed.; Academic Press: San Diego, CA, USA, 2010. [Google Scholar]
- Adebiyi, O.E.; Funsho, O.O.; Ning-Hua, T.; Guang-Zhi, Z. In vitro antioxidant activity, total phenolic and flavonoid contents of ethanol extract of stem and leaf of Grewia carpinifolia. Beni Suef Univ. J. Appl. Sci. 2017, 6, 10–14. [Google Scholar] [CrossRef]
- Zervos, I.A.; Nikolaidis, E.; Lavrentiadou, S.N.; Tsantarliotou, M.P.; Eleftheriadou, E.K.; Papapanagiotou, E.P.; Fletouris, D.J.; Georgiadis, M.; Taitzoglou, I.A. Endosulfan-induced lipid peroxidation in rat brain and its effect on t-PA and PAI-1: Ameliorating effect of vitamins C and E. J. Toxicol. Sci. 2011, 36, 423–433. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Zhou, C.; Huang, S.; Jiang, C. Selenium polysaccharide SPMP-2a from Pleurotus geesteranus alleviates H2O2-induced oxidative damage in HaCaT cells. BioMed Res. Int. 2017. [Google Scholar] [CrossRef] [Green Version]
- Xiao, X.; Liu, J.; Hu, J.; Zhu, X.; Yang, H.; Wang, C.; Zhang, Y. Protective effects of protopine on hydrogen peroxide-induced oxidative injury of PC12 cells via Ca(2+) antagonism and antioxidant mechanisms. Eur. J. Pharmacol. 2008, 591, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.J.; Jeong, J.W.; Choi, E.O.; Kim, M.J.; Hwang-Bo, H.; Kim, H.J.; Hong, S.H.; Park, C.; Lee, D.H.; Choi, Y.H. Protective effects of Scutellaria baicalensis Georgi against hydrogen peroxide-induced DNA damage and apoptosis in HaCaT human skin keratinocytes. EXCLI J. 2017, 16, 426–438. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, C.; Yu, N.; Si, L.; Zhu, L.; Zeng, A.; Liu, Z.; Wang, X. INF2 regulates oxidative stress-induced apoptosis in epidermal HaCaT cells by modulating the HIF1 signaling pathway. Biomed. Pharmacother. 2019, 111, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.; Lee, Y.M.; Song, S.; Lee, Y.Y.; Yeum, K.J. Black soybeans protect human keratinocytes from oxidative stress-induced cell death. Food Sci. Nutr. 2018, 6, 2423–2430. [Google Scholar] [CrossRef] [PubMed]
- Fuhrmann, D.C.; Brüne, B. Mitochondrial composition and function under the control of hypoxia. Redox Biol. 2017, 12, 208–215. [Google Scholar] [CrossRef]
- Zhou, H.; Shi, C.; Hu, S.; Zhu, H.; Ren, J.; Chen, Y. BI1 is associated with microvascular protection in cardiac ischemia reperfusion injury via repressing Syk-Nox2-Drp1-mitochondrial fission pathways. Angiogenesis 2018, 21, 599–615. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wang, J.; Zhu, P.; Zhu, H.; Toan, S.; Hu, S.; Ren, J.; Chen, Y. NR4A1 aggravates the cardiac microvascular ischemia reperfusion injury through suppressing FUNDC1-mediated mitophagy and promoting Mff-required mitochondrial fission by CK2alpha. Basic Res. Cardiol. 2018, 113, 23. [Google Scholar] [CrossRef]
- Zhu, H.; Jin, Q.; Li, Y.; Ma, Q.; Wang, J.; Li, D.; Zhou, H.; Chen, Y. Melatonin protected cardiac microvascular endothelial cells against oxidative stress injury via suppression of IP3R-[Ca2+]c/VDAC-[Ca2+]m axis by activation of MAPK/ERK signaling pathway. Cell Stress Chaperones 2018, 23, 101–113. [Google Scholar] [CrossRef]
- Perona, J.S.; Cabello-Moruno, R.; Ruiz-Gutierrez, V. The role of virgin olive oil components in the modulation of endothelial function. J. Nutr. Biochem. 2006, 17, 429–445. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.K.; Pan, Y.R.; Wang, H.F.; Wei, C.W.; Yu, Y.L. Vitamin E (α-tocopherol) ameliorates aristolochic acid-induced renal tubular epithelial cell death by attenuating oxidative stress and caspase3 activation. Mol. Med. Rep. 2018, 17, 31–36. [Google Scholar] [CrossRef]
- Adebiyi, O.E.; Olayemi, F.O.; Olopade, J.O.; Tan, N.H. Βeta-sitosterol enhances motor coordination, attenuates memory loss and demyelination in a vanadium-induced model of experimental neurotoxicity. Pathophysiology 2018, 26, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Suriyakalaa, U.; Ramachandran, R.; Usha, K.; Sankarganesh, D.; Praveenkumar, D.; Abinaya, S.; Tirupathi Pichiah, P.B.; Kamalakkannan, S.; Achiraman, S. Squalene is a potential endocrine modulator in rat: A proof-of-principle study with 3-methylcholanthrene-induced toxicity. Andrologia 2018, 50, e13117. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Kakkar, P. Lupeol protects against acetaminophen induced oxidative stress and cell death in rat primary hepatocytes. Food Chem. Toxicol. 2012, 50, 1781–1789. [Google Scholar] [CrossRef] [PubMed]
- Jamilian, M.; Hashemi Dizaji, S.; Bahmani, F.; Taghizadeh, M.; Memarzadeh, M.R.; Karamali, M.; Akbari, M.; Asemi, Z. A randomized controlled clinical trial investigating the effects of omega-3 fatty acids and vitamin E co-supplementation on biomarkers of oxidative stress, inflammation and pregnancy outcomes in gestational diabetes. Can. J. Diabetes 2017, 41, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C. Chemistry and biology of vitamin E. Mol. Nutr. Food Res. 2005, 49, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Milczarek, R.; Klimek, J.; Zelewski, L. The effects of ascorbate and alpha-tocopherol on the NADPH-dependent lipid peroxidation in human placental mitochondria. Mol. Cell. Biochem. 2000, 210, 65–73. [Google Scholar] [CrossRef]
- Brimson, J.M.; Brimson, S.J.; Brimson, C.A.; Rakkhitawatthana, V.; Tencomnao, T. Rhinacanthus nasutus extracts prevent glutamate and amyloid-β neurotoxicity in HT-22 mouse hippocampal cells: Possible active compounds include lupeol, stigmasterol and β-sitosterol. Int. J. Mol. Sci. 2012, 13, 5074–5097. [Google Scholar] [CrossRef] [Green Version]
- Papi Reddy, K.; Singh, A.B.; Puri, A.; Srivastava, A.K.; Narender, T. Synthesis of novel triterpenoid (lupeol) derivatives and their in vivo antihyperglycemic and antidyslipidemic activity. Bioorg. Med. Chem. Lett. 2009, 19, 4463–4466. [Google Scholar] [CrossRef]
- Takhshid, M.A.; Tavasuli, A.R.; Heidary, Y.; Keshavarz, M.; Kargar, H. Protective effect of vitamins E and C on endosulfan-induced reproductive toxicity in male rats. Iran. J. Med. Sci. 2012, 37, 173–180. [Google Scholar]
- Kim, W.S.; Kim, I.; Kim, W.K.; Choi, J.Y.; Kim, D.Y.; Moon, S.G. Mitochondria-targeted vitamin E protects skin from UVB-irradiation. Biomol. Ther. 2016, 24, 305–311. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.K.; Wei, C.W.; Pan, Y.R.; Cherng, S.H.; Chang, W.J.; Wang, H.F.; Yu, Y.L. Vitamin C attenuates the toxic effect of aristolochic acid on renal tubular cells via decreasing oxidative stress-mediated cell death pathways. Mol. Med. Rep. 2015, 12, 6086–6092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sudhahar, V.; Veena, C.K.; Varalakshmi, P. Antiurolithic effect of lupeol and lupeol linoleate in experimental hyperoxaluria. J. Nat. Prod. 2008, 71, 1509–1512. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, S.F.; Ooi, K.L. Polyphenolic and vitamin C contents and antioxidant activities of aqueous extracts from mature-green and ripe fruit fleshes of Mangifera sp. J. Agric. Food Chem. 2012, 60, 11832–11838. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Siddique, Y.H.; Ara, G.; Afzal, M. Estimation of lipid peroxidation induced by hydrogen peroxide in cultured human lymphocytes. Dose Response 2012, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]

| No. | Compound | PubChem CID | Family | Molecular Formula | Molecular Weight | Abundance (%) * |
|---|---|---|---|---|---|---|
| 1 | Phytol | 5366244 | Diterpene | C20H40O | 296.539 | 8.50 |
| 2 | Squalene | 638072 | Triterpene | C30H50 | 410.73 | 24.00 |
| 3 | Stigmastan-3,5-diene | 525918 | Sterol | C29H48 | 396.703 | 9.00 |
| 4 | Alpha-tocopherol | 14985 | Tocopherol | C29H50O2 | 430.717 | 42.40 |
| 5 | β-sitosterol | 222284 | Sterol | C29H50O | 414.718 | 7.50 |
| 6 | Lupeol | 259846 | Triterpene | C30H50O | 426.729 | 8.60 |
| Samples | IC50 (μg/mL) ± SD | |
|---|---|---|
| DPPH | ABTS | |
| Ace-EFc | 107.05 ± 2.61 * | 1.47 ± 1.21 * |
| Ascorbic Acid | 118.82 ± 2.48 | 2.22 ± 0.80 |
| Cell Line | IC50 (μg/mL) ± SD |
|---|---|
| HaCaT | 734.33 ± 2.20 |
| SiHa | 196.99 ± 2.70 *** |
| HeLa | 463.30 ± 3.23 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De la Cruz-Concepción, B.; Espinoza-Rojo, M.; Álvarez-Fitz, P.; Illades-Aguiar, B.; Acevedo-Quiroz, M.; Zacapala-Gómez, A.E.; Navarro-Tito, N.; Jiménez-Wences, H.; Torres-Rojas, F.I.; Mendoza-Catalán, M.A. Cytotoxicity of Ficus Crocata Extract on Cervical Cancer Cells and Protective Effect against Hydrogen Peroxide-Induced Oxidative Stress in HaCaT Non-Tumor Cells. Plants 2021, 10, 183. https://doi.org/10.3390/plants10010183
De la Cruz-Concepción B, Espinoza-Rojo M, Álvarez-Fitz P, Illades-Aguiar B, Acevedo-Quiroz M, Zacapala-Gómez AE, Navarro-Tito N, Jiménez-Wences H, Torres-Rojas FI, Mendoza-Catalán MA. Cytotoxicity of Ficus Crocata Extract on Cervical Cancer Cells and Protective Effect against Hydrogen Peroxide-Induced Oxidative Stress in HaCaT Non-Tumor Cells. Plants. 2021; 10(1):183. https://doi.org/10.3390/plants10010183
Chicago/Turabian StyleDe la Cruz-Concepción, Brenda, Mónica Espinoza-Rojo, Patricia Álvarez-Fitz, Berenice Illades-Aguiar, Macdiel Acevedo-Quiroz, Ana E. Zacapala-Gómez, Napoleón Navarro-Tito, Hilda Jiménez-Wences, Francisco I. Torres-Rojas, and Miguel A. Mendoza-Catalán. 2021. "Cytotoxicity of Ficus Crocata Extract on Cervical Cancer Cells and Protective Effect against Hydrogen Peroxide-Induced Oxidative Stress in HaCaT Non-Tumor Cells" Plants 10, no. 1: 183. https://doi.org/10.3390/plants10010183
APA StyleDe la Cruz-Concepción, B., Espinoza-Rojo, M., Álvarez-Fitz, P., Illades-Aguiar, B., Acevedo-Quiroz, M., Zacapala-Gómez, A. E., Navarro-Tito, N., Jiménez-Wences, H., Torres-Rojas, F. I., & Mendoza-Catalán, M. A. (2021). Cytotoxicity of Ficus Crocata Extract on Cervical Cancer Cells and Protective Effect against Hydrogen Peroxide-Induced Oxidative Stress in HaCaT Non-Tumor Cells. Plants, 10(1), 183. https://doi.org/10.3390/plants10010183








