The Hydrological-Hydrochemical Factors that Control the Invasion of the Black Locust (Robinia pseudoacacia L.) in Succession in Areas with Opencast Mines
Abstract
1. Introduction
2. Results
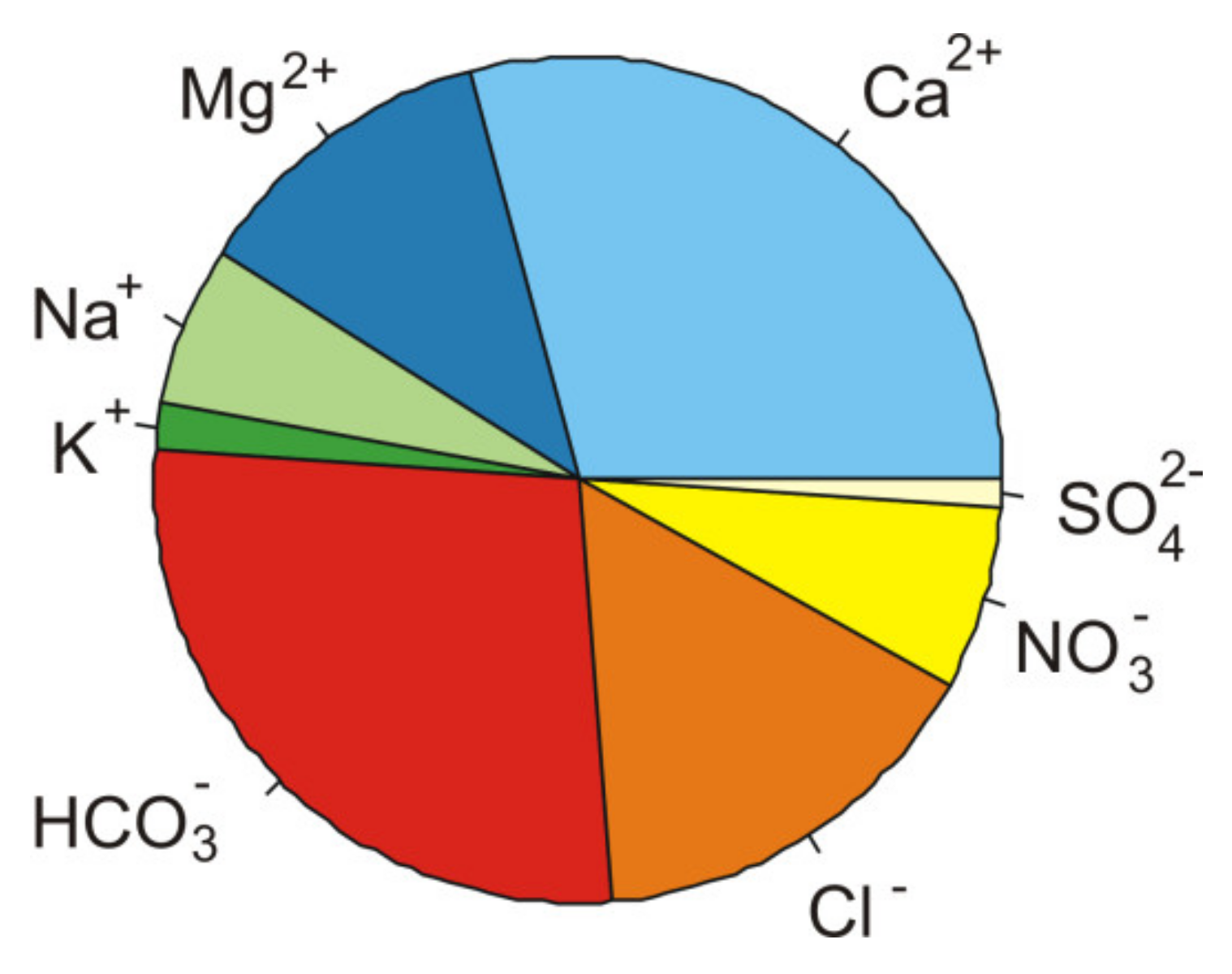
2.1. General Hydrochemical Characteristics of the Water
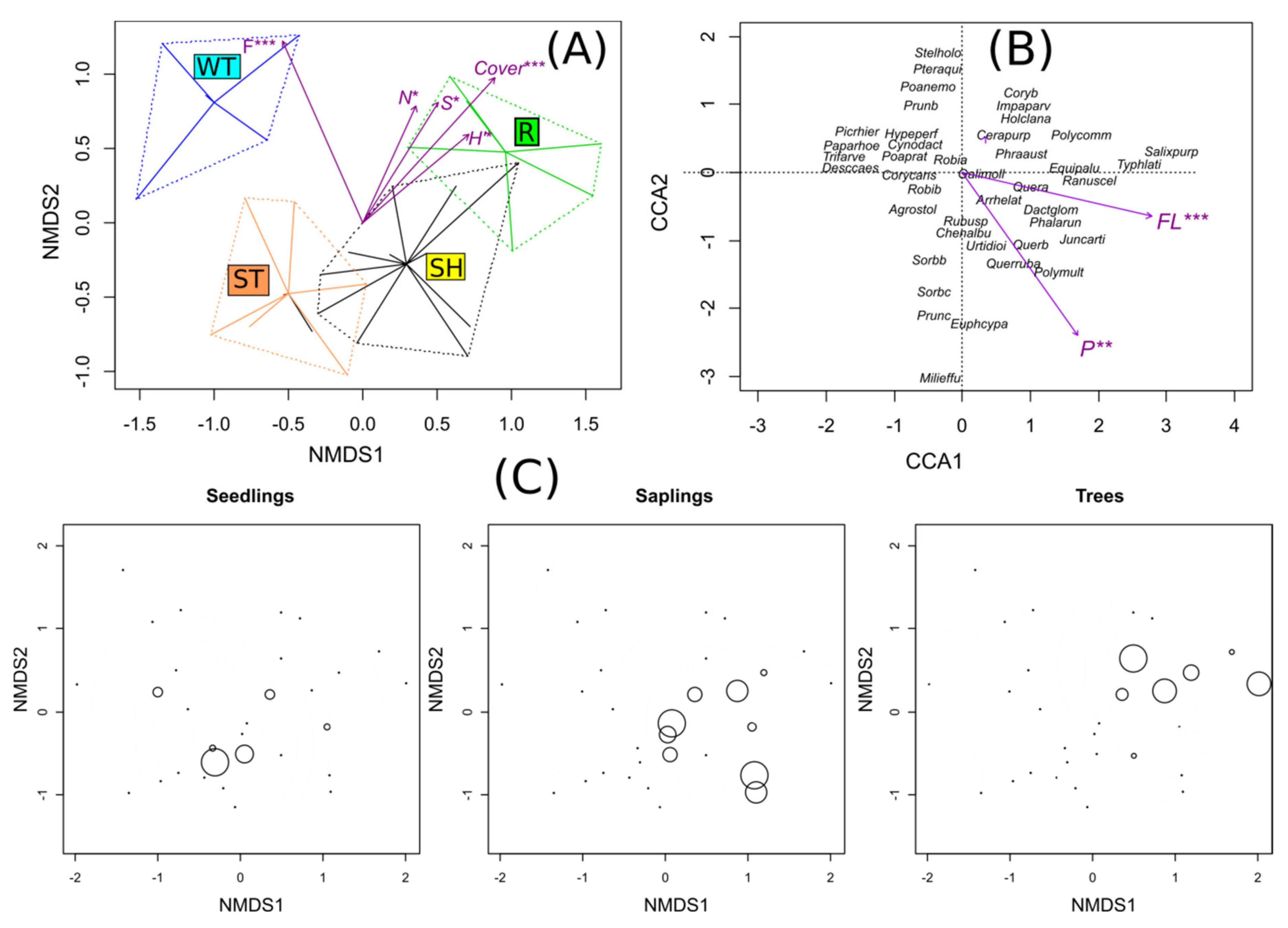
2.2. Characteristics of the Vegetation and Substratum on the Study Sites
3. Discussion
3.1. Hydrochemical Assesment of Water and Pulp
3.2. Type of Succession of the Vegetation in the Vicinity of the Open-cast Mine
3.3. Invasion of Robinia Pseudoacacia
4. Materials and Methods
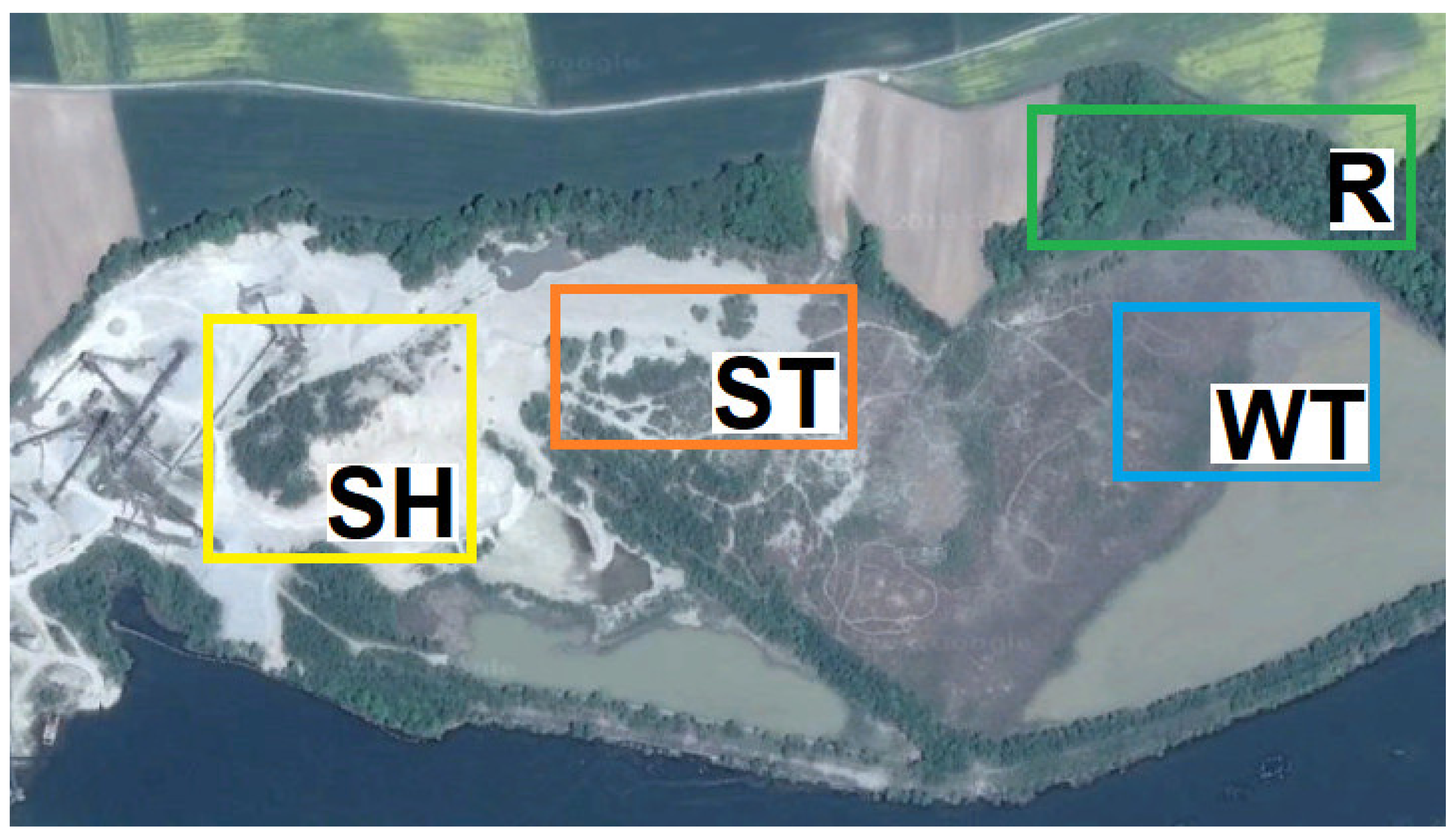
4.1. Description of the Study Area and Study Design
4.2. Methods of the Hydrological Studies
4.3. Methods of the Soil Analyses
4.4. Methods of the Botanical Studies
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A



References
- Šebelíková, L.; Řehounková, K.; Prach, K. Spontaneous revegetation vs. forestry reclamation in post-mining sand pits. Environ. Sci. Pollut. Res. 2016, 23, 13598–13605. [Google Scholar] [CrossRef] [PubMed]
- Kasprowska-Nowak, K.; Beczała, T. Przemiany krajobrazu w obszarze i otoczeniu piaskowni “Krasna-Bielowiec” (Pogórze Cieszyńskie). Prace Kom. Krajobraz. Kultur. 2014, 26, 139–154. [Google Scholar]
- Beczała, T.; Dorda, A. Tereny po eksploatacji surowców skalnych ostoją bioróżnorodności—na przykładzie wybranych wyrobisk na Pogórzu Cieszyńskim. Bezpieczeństwo Pracy i Ochron Środ w Górnictwie 2015, 9, 17–25. [Google Scholar]
- Molenda, T.; Błońska, A.; Chmura, D. Hydrochemical Diversity of Selected Anthropogenic Wetlands Developed in Disused Sandpits. In Proceedings of the 13th International Multidisciplinary Scientific, SGEM2013 Conference, Albena, Bulgaria, 16‒22 June 2013; Volume 1, pp. 547–554, ISBN 978-619-7105-04-9. [Google Scholar]
- Nowak, A. Anthropogenic habitats as sites of occurrence of endangered, rare and protected plants on the example of Opole Silesia, SW Poland. Thaiszia J. Bot. 2005, 15, 155–172. [Google Scholar]
- Nejfeld, P. Chronione i zagrożone gatunki roślin naczyniowych w nieczynnych kamieniołomach wapienia w Kotlinie Żywieckiej (zewnętrzne Karpaty Zachodnie, Południowa Polska). In Zapobieganie Zanieczyszczeniu, Przekształcaniu i Degradacji Środowiska; Kasza, H., Klama, H., Eds.; Wydawnictwo Akademii Techniczno-Humanistycznej: Bielsko-Biała, Poland, 2007; Volume 14, pp. 121–131. [Google Scholar]
- Nowak, A.; Hebda, G. The Biodiversity of Quarries and Pits; Opole Scientific: Opole, Poland, 2006. [Google Scholar]
- Woźniak, G.; Sierka, E. Importance of spontaneous succession in reclamation processes. Zeszyty Nauk. Inżynieria Środ. Uniwer. Zielonogórski 2004, 131(12), 391–398. [Google Scholar]
- Chmura, D.; Błońska, A.; Molenda, T. Hydrographic properties and vegetation differentiation in selected anthropogenic wetlands. In Proceedings of the 13th International Multidisciplinary Scientific, SGEM2013 Conference, Albena, Bulgaria, 16‒22 June 2013; Volume 1, pp. 555–562, ISBN 978-619-7105-04-9. [Google Scholar]
- Mota, J.F.; Sola, A.J.; Jiménez-Sánchez, M.L.; Pérez-García, F.J.; Merlo, M.E. Gypsicolous flora, conservation and restoration of quarries in the southeastern of the Iberian Peninsula. Biodivers. Conserv. 2004, 13, 1797–1808. [Google Scholar] [CrossRef]
- Pérez-García, F.J.; Salmerón-Sánchez, E.; Martínez-Hernández, F.; Mendoza-Fernandez, A.; Merlo, E.; Mota, J.F. Towards an Eco-Compatible Origin of Construction Materials. Case Study: Gypsum. In New Metropolitan Perspectives, Knowledge Dynamics and Innovation-Driven Polices towards Urban and Regional; SIST 178; Belilacqua, C., Calabrò, F., Della Spina, L., Eds.; Springer: Cham, Switzerland, 2021; Volume 2, pp. 1259–1267. [Google Scholar] [CrossRef]
- Trnkova, R.; Řehounková, K.; Prach, K. Spontaneous succession of vegetation on acidic bedrock in quarries in the Czech Republic. Preslia 2010, 82, 333–343. [Google Scholar]
- Czylok, A.; Szymczyk, A. Sand quarries as biotopes of rare and critically endangered plant species. In Rare, Relicts and Endangered Plants and Fungi in Poland; Mirek, Z., Nikiel, A., Eds.; Szafer Institute of Botany Polish Academy of Sciences: Kraków, Poland, 2009; pp. 187–192. [Google Scholar]
- Rahmonov, O.; Szymczyk, A. Relations between vegetation and soil and initial succession phases in post-sand excavations. Ekológia (Bratislava) 2010, 29, 412. [Google Scholar] [CrossRef]
- Madon, O.; Médail, F. The ecological significance of annuals on a Mediterranean grassland (Mt Ventoux, France). Plant Ecol. 1997, 129, 189–199. [Google Scholar] [CrossRef]
- Řehounková, K.; Řehounek, J. Sand pits and gravel-sand pits. In Near-Natural Restoration vs. Technical Reclamation of Mining Sites; Řehounková, K., Řehounek, J., Prach, K., Eds.; Faculty of Science, University of South Bohemia: České Budějovice, Czech Republic, 2011; pp. 51–68. [Google Scholar]
- Chmura, D.; Molenda, T. The anthropogenic mire communities of the Silesian Upland (S Poland): A case of selected exploitation hollows. Nat. Conserv. 2007, 64, 57–63. [Google Scholar]
- Řehounková, K.; Prach, K. Spontaneous vegetation succession in gravel–sand pits: A potential for restoration. Rest. Ecol. 2008, 16, 305–312. [Google Scholar] [CrossRef]
- Dulias, R. Landscape planning in areas of sand extraction in the Silesian Upland, Poland. Landsc. Urban Plan. 2010, 95, 91–104. [Google Scholar] [CrossRef]
- Vítková, M.; Müllerová, J.; Sádlo, J.; Pergl, J.; Pyšek, P. Black locust (Robinia pseudoacacia) beloved and despised: A story of an invasive tree in Central Europe. For. Ecol. Manag. 2017, 384, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Höfker, H. Jahresversammlung in Glogau, Sagan Und Muskau. Mitteilungen der Deutschen Dendrologischen Gesellschaft 1936, 48, 318–319. [Google Scholar]
- CABI. Invasive Species Compendium. 2019. Available online: http://www.cabi.org/isc/datasheet/47698 (accessed on 14 November 2020).
- Tokarska-Guzik, B.; Dajdok, Z.; Zając, M.; Zając, A.; Urbisz, A.; Danielewicz, W.; Hołdyński, C. Rośliny obcego pochodzenia w Polsce; Generalna Dyrekcja Ochrony Środowiska: Warsaw, Poland, 2012. [Google Scholar]
- Wojda, T.; Klisz, M.; Jastrzebowski, S.; Mionskowski, M.; Szyp-Borowska, I.; Szczygiel, K. The geographical distribution of the black locust (Robinia pseudoacacia L.) in Poland and its role on non-forest land. Pap. Glob. Chang. 2015, 22, 101–113. [Google Scholar] [CrossRef]
- Batzli, J.M.; Graves, W.R.; Van Berkum, P. Diversity among rhizobia effective with Robinia pseudoacacia L. Appl. Environ. Microbiol. 1992, 58, 2137–2143. [Google Scholar] [CrossRef]
- Rice, S.K.; Westerman, B.; Federici, R. Impacts of the exotic, nitrogen-fixing black locust (Robinia pseudoacacia) on nitrogen-cycling in a pine-oak ecosystem. Plant Ecol. 2004, 174, 97–107. [Google Scholar] [CrossRef]
- Benesperi, R.; Giuliani, C.; Zanetti, S.; Gennai, M.; Lippi, M.M.; Guidi, T.; Nascimbene, J.; Foggi, B. Forest plant diversity is threatened by Robinia pseudoacacia (black-locust) invasion. Biodiver. Conserv. 2012, 21, 3555–3568. [Google Scholar] [CrossRef]
- Von Holle, B.; Joseph, K.A.; Largay, E.F.; Lohnes, R.G. Facilitations between the introduced nitrogen-fixing tree, Robinia pseudoacacia, and nonnative plant species in the glacial outwash upland ecosystem of cape cod, MA. Biodiver. Conserv. 2006, 15, 2197–2215. [Google Scholar] [CrossRef]
- Hanzelka, J.; Reif, J. Responses to the black locust (Robinia pseudoacacia) invasion differ between habitat specialists and generalists in central European forest birds. J. Ornithol. 2015, 156, 1015–1024. [Google Scholar] [CrossRef]
- Wetzel, R.G. Limnology, 2nd ed.; Saunders College Publishing: Philadelphia, PA, USA, 2000. [Google Scholar]
- Molenda, T. Naturalne i Antropogeniczne Zmiany Właściwości Fizyczno-Chemicznych Wód w Pogórniczych Środowiskach Akwatycznych na Przykładzie Regionu Górnośląskiego i Obszarów ościennych; Wyd. UŚ: Katowice, Poland, 2011. [Google Scholar]
- Connell, J.H.; Slatyer, R.O. Mechanisms of succession in natural communities and their role in community stability and organization. Am. Nat. 1977, 111, 1119–1144. [Google Scholar] [CrossRef]
- Kujawa-Pawlaczyk, J. 6120 Ciepłolubne śródlądowe murawy napiaskowe. ed. Monitoring siedlisk przyrodniczych. Przewodnik metodyczny, cz. I.; Mróz, W., Ed.; Biblioteka Monitoringu Środowiska: Warsaw, Poland, 2010; pp. 106–118. Available online: http://siedliska.gios.gov.pl/images/pliki_pdf/publikacje/Monitoring-siedlisk-przyrodniczych.-Przewodnik-metodyczny.-Cz-I.pdf (accessed on 20 December 2020).
- Sienkiewicz-Paderewska, D. Zbiorowiska roślinne z klasy Koelerio glaucae-Corynephoretea canescentis Klika in Klika et Novak 1941 występujące na trwałych użytkach zielonych w Parku Krajobrazowym “Podlaski Przełom Bugu”. Łąkarstwo w Polsce 2010, 13, 137–155. [Google Scholar]
- Urbisz, A. Occurrence of Temporarily-Introduced Alien Plant Species (Ephemerophytes) in Poland-Scale and Assessment of the Phenomenon; Wyd. Uniwersytetu Śląskiego: Katowice, Poland, 2011. [Google Scholar]
- Fujita, M.; Koike, F. Landscape effects on ecosystems: Birds as active vectors of nutrient transport to fragmented urban forests versus forest-dominated landscapes. Ecosystems 2009, 12, 391–400. [Google Scholar] [CrossRef]
- Gwiazda, R.; Jarocha, K.; Szarek-Gwiazda, E. Impact of a small cormorant (Phalacrocorax carbo sinensis) roost on nutrients and phytoplankton assemblages in the littoral regions of a submontane reservoir. Biologia 2010, 65, 742–748. [Google Scholar] [CrossRef]
- Krywult, M.; Smykla, J.; Wincenciak, A. The presence of nitrates and the impact of ultraviolet radiation as factors that determine nitrate reductase activity and nitrogen concentrations in Deschampsia antarctica Desv. around penguin rookeries on King George Island, Maritime Antarctica. Water Air Soil Pollut. 2013, 224, 1563. [Google Scholar] [CrossRef]
- Grzelak, M.; Kryszak, A.; Spychalski, W. Charakterystyka geobotaniczna zbiorowisk szuwarowych związku Phragmition w wybranych dolinach rzecznych Wielkopolski. Rocz. Akad. Roln. w Poznaniu. Rolnictwo 2002, 62, 15–23. [Google Scholar]
- Aerts, R.; Chapin, F.S., III. The mineral nutrition of wild plants revisited: A re-evaluation of processes and patterns. Adv. Ecol. Res. 1999, 30, 1–67. [Google Scholar] [CrossRef]
- Řehounková, K.; Prach, K. Spontaneous vegetation succession in disused gravel-sand pits: Role of local site and landscape factors. J. Veg. Sci. 2006, 17, 583–590. [Google Scholar] [CrossRef]
- Dyderski, M.K.; Jagodziński, A.M. Impact of Invasive Tree Species on Natural Regeneration Species Composition, Diversity, and Density. Forests 2020, 11, 456. [Google Scholar] [CrossRef]
- Cierjacks, A.; Kowarik, I.; Joshi, J.; Hempel, S.; Ristow, M.; von der Lippe, M.; Weber, E. Biological flora of the British Isles: Robinia pseudoacacia. J. Ecol. 2013, 101, 1623–1640. [Google Scholar] [CrossRef]
- Dyderski, M.K.; Jagodziński, A.M. Context-dependence of urban forest vegetation invasion level and alien species’ ecological success. Forests 2019, 10, 26. [Google Scholar] [CrossRef]
- Rachmonov, O. Relacje Między Roślinnością i Glebą w Inicjalnej Fazie Sukcesji na Obszarach Piaszczystych; Wyd. Uniwersytetu Śląskiego: Katowice, Poland, 2007. [Google Scholar]
- Kompała-Bąba, A.; Bąba, W. The spontaneous succession in a sand-pit–the role of life history traits and species habitat preferences. Pol. J. Ecol. 2013, 61, 13–22. [Google Scholar]
- Gutry-Korycka, M.; Werner-Więckowska, H. (Eds.) Przewodnik do Hydrograficznych Badań Terenowych; PWN: Warsaw, Poland, 1996. [Google Scholar]
- Macioszczyk, A.; Dobrzyński, D. Hydrogeochemia Strefy Aktywnej Wymiany Wód Podziemnych; PWN: Warsaw, Poland, 2002. [Google Scholar]
- Drzymała, S.; Mocek, A. Uziarnienie roznych gleb Polski w swietle klasyfikacji PTG, PN-R-04033 i USDA. Roczniki Gleboznawcze 2004, 55, 107–115. [Google Scholar]
- Westhoff, V.; Van Der Maarel, E. The Braun-Blanquet approach. In Classification of Plant Communities; Springer: Dordrecht, The Netherlands, 1978; pp. 287–399. [Google Scholar] [CrossRef]
- Brown, N.; Jennings, S.; Wheeler, P.; Nabe-Nielsen, J. An improved method for the rapid assessment of forest understorey light environments. J. Appl. Ecol. 2000, 37, 1044–1053. [Google Scholar] [CrossRef]
- Chmura, D.; Salachna, A.; Sierka, E. Porównanie oceny zwarcia drzewostanu za pomocą metody wizualnej i zwarciomierza. Sylwan 2016, 160, 475–481. [Google Scholar] [CrossRef]
- Ellenberg, H.; Leuschner, C. Vegetation Mitteleuropas Mit den Alpen: In Ökologischer, Dynamischer Und Historischer Sicht; Utb: Stuttgart, Germany, 2020; Volume 8104. [Google Scholar]
- Euro+Med. Euro+MedPlantBase—The Information Resource for Euro-Mediterranean Plant Diversity. 2006. Available online: ww2.bgbm.org/EuroPlusMed/ (accessed on 20 December 2020).
- Matuszkiewicz, W. Guide for Identification of the Plant Communities of Poland; PWN: Warsaw, Poland, 2001. [Google Scholar]
- R Core Team. R: A language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 20 December 2020).
- Hothorn, T.; Hornik, K.; van de Wiel, M.A.; Zeileis, A. Implementing a class of permutation tests: The coin package. J. Stat. Softw. 2008, 28, 1–23. [Google Scholar] [CrossRef]


| Habitat | IndVal | Habitat | IndVal |
|---|---|---|---|
| Sand heaps (1) | Control (4) | ||
| Picris hieracioides L. | 0.603 * | Rubus sp | 0.683 * |
| Sandy bottom of the sedimentation tank (2) | Quercus robur a L. | 0.665 * | |
| Cynodon dactylon L. | 0.612 * | Arrhenatherum elatius (L.) P.Beauv. ex J.Presl and C.Presl | 0.578 * |
| Wetlands (3) | Convallaria majalis L. | 0.578 * | |
| Equisetum palustre L. | 0.894 *** | Corylus avellana b L. | 0.578 * |
| Epilobium cilatum Raf | 0.756 ** | Euphorbia cyparissias L. | 0.577 * |
| Phragmites australis (Cav.) Trin. ex Steud. | 0.731 * | Impatiens parviflora DC. | 0.577 * |
| Carex nigra (L.) Reichard | 0.632 * | Polygonatum multiflorum (L.) All. | 0.577 * |
| Lythrum salicaria L. | 0.632 * | Quercus rubra a L. | 0.577 * |
| 1 + 2 | 1 + 4 | ||
| Oenothera biennis L. | 0.761 * | Calamagrostis epigejos (L.) Roth | 0.686 * |
| Saponaria officinalis L. | 0.753 * | ||
| Habitat | Moisture | Grain Fraction | Species |
|---|---|---|---|
| Sand heaps (SH), n = 11 | low | coarse | Saponaria officinalis, Senecio viscosus, Corynephorus canescens, Oenothera sp., R. pseudoacacia |
| Sandy bottom of the sedimentation tank (ST), n = 8 | high | coarse to fine | Salix fragilis, S. purpurea, Corynephorus cansecens, Oenothera sp |
| Wetlands (WT), n = 5 | high | fine | Phragmites australis, Equisetum palustre, |
| Control (R), n = 6 | medium | medium | R. pseudoacacia, Quercus robur, Rubus sp. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kidawa, J.; Chmura, D.; Molenda, T. The Hydrological-Hydrochemical Factors that Control the Invasion of the Black Locust (Robinia pseudoacacia L.) in Succession in Areas with Opencast Mines. Plants 2021, 10, 40. https://doi.org/10.3390/plants10010040
Kidawa J, Chmura D, Molenda T. The Hydrological-Hydrochemical Factors that Control the Invasion of the Black Locust (Robinia pseudoacacia L.) in Succession in Areas with Opencast Mines. Plants. 2021; 10(1):40. https://doi.org/10.3390/plants10010040
Chicago/Turabian StyleKidawa, Joanna, Damian Chmura, and Tadeusz Molenda. 2021. "The Hydrological-Hydrochemical Factors that Control the Invasion of the Black Locust (Robinia pseudoacacia L.) in Succession in Areas with Opencast Mines" Plants 10, no. 1: 40. https://doi.org/10.3390/plants10010040
APA StyleKidawa, J., Chmura, D., & Molenda, T. (2021). The Hydrological-Hydrochemical Factors that Control the Invasion of the Black Locust (Robinia pseudoacacia L.) in Succession in Areas with Opencast Mines. Plants, 10(1), 40. https://doi.org/10.3390/plants10010040






