Effect of Aqueous Extracts from Solidago Canadensis L. Leaves on Germination and Early Growth Stages of Three Cultivars of Raphanus Sativus L. Var. Radicula Pers
Abstract
1. Introduction
2. Results
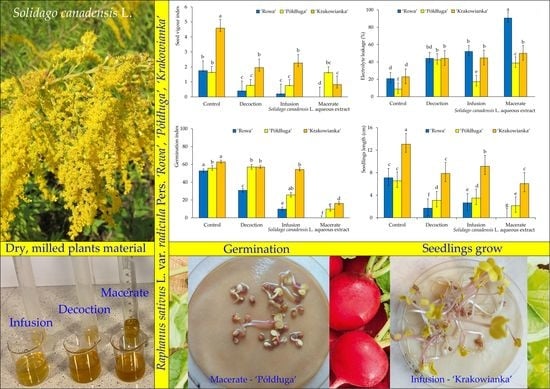
2.1. Germination Indexes
2.2. Length of Seedlings
2.3. Fresh, Dry Mass and Water Content
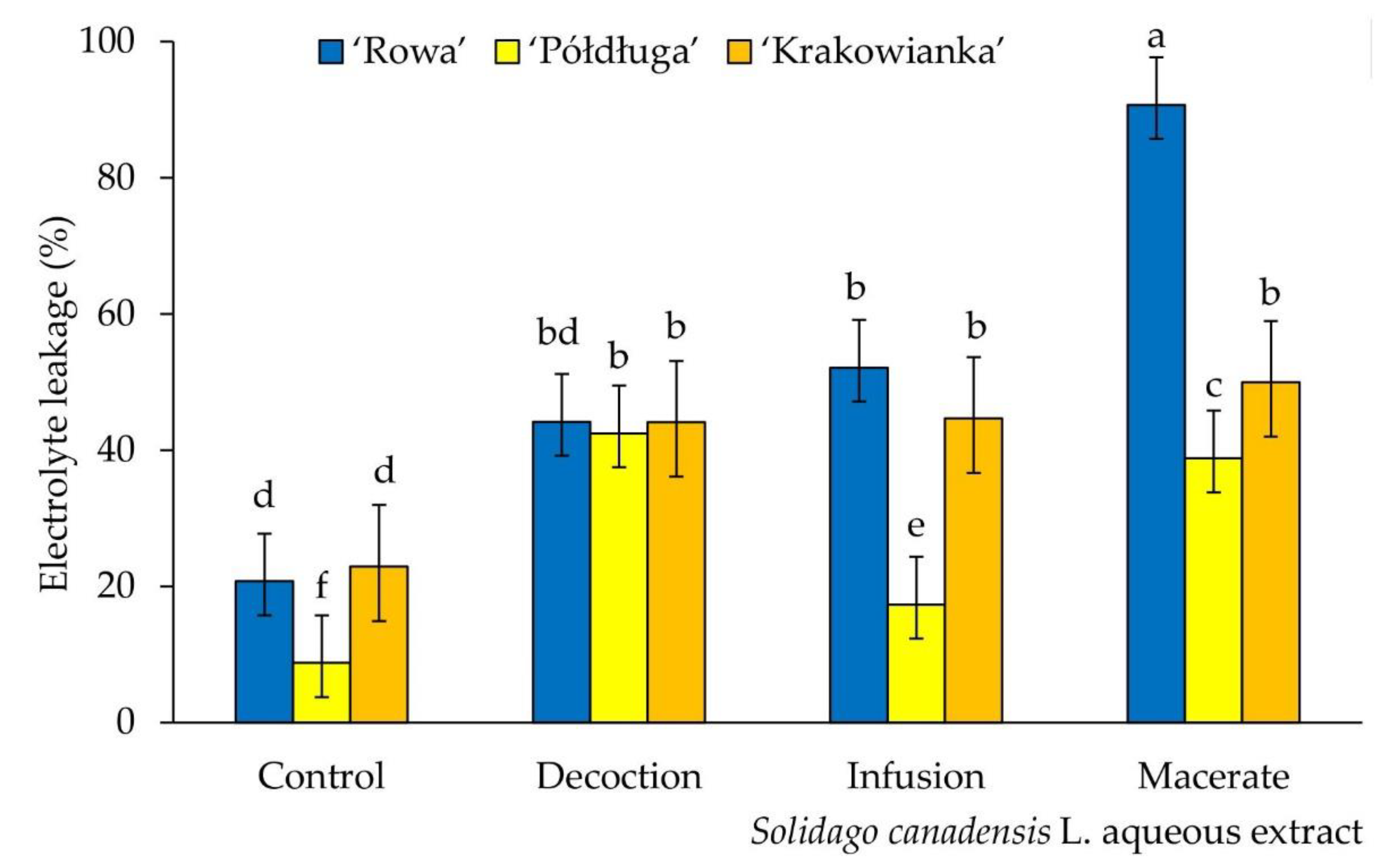
2.4. Chlorophyll Content and Electrolyte Leakage
3. Discussion
4. Materials and Methods
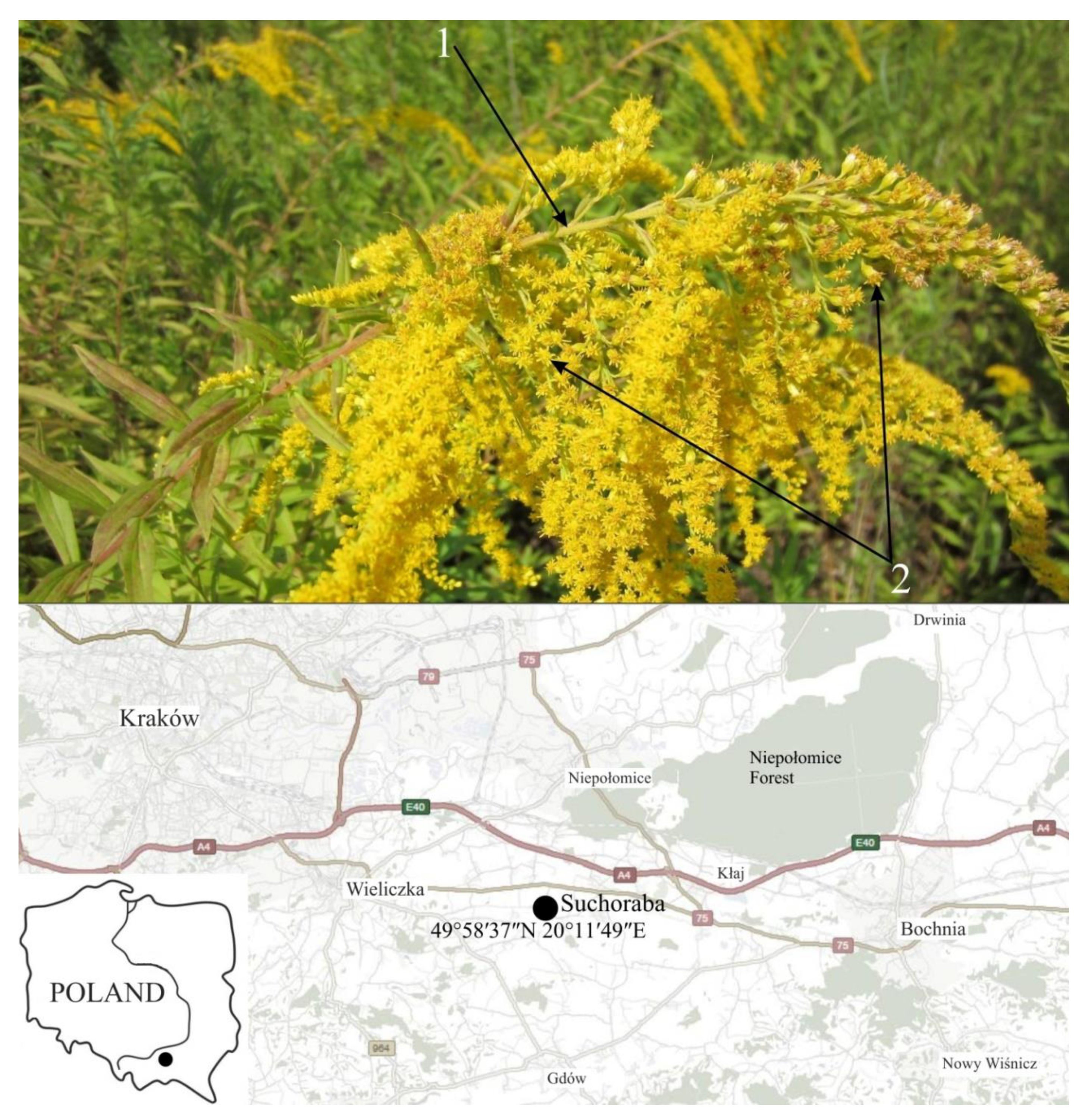
4.1. Plant Material
4.2. Extract Preparation
4.3. Germination Conditions
4.4. Germination Indexes
4.5. Seedlings Length
4.6. Fresh, Dry Mass and Water Content
4.7. Chlorophyll Content and Electrolyte Leakage
4.8. Statistic Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- McGeoch, M.A.; Butchart, S.H.M.; Spear, D.; Marais, E.; Kleynhans, E.J.; Symes, A.; Chanson, J.; Hoffmann, M. Global indicators of biological invasion: Species numbers, biodiversity impact and policy responses. Divers. Distrib. 2010, 16, 95–108. [Google Scholar] [CrossRef]
- Seebens, H.; Blackburn, T.M.; Dyer, E.E.; Genovesi, P.; Hulme, P.E.; Jeschke, J.M.; Essl, F. No saturation in the accumulation of alien species worldwide. Nat. Commun. 2017, 8, 1–9. [Google Scholar] [CrossRef]
- Crooks, J.A. Characterizing ecosystem-level consequences of biological invasions: The role of ecosystem engineers. Oikos 2002, 97, 153–166. [Google Scholar] [CrossRef]
- Jianzhong, L.; Wei, Q.; Jiakuan, C.; Bo, L. Impact of invasive species on soil properties: Canadian goldenrod (Solidago canadensis) as a case study. Chin. Biodivers. 2005, 13, 347–356. [Google Scholar] [CrossRef]
- Hulme, P.E.; Pyšek, P.; Nentwig, W.; Vilà, M. Will threat of biological invasions unite the European Union? Science 2009, 324, 40–41. [Google Scholar] [CrossRef]
- Tokarska-Guzik, B.; Dajdok, Z.; Urbisz, A.; Zając, M.; Danielewicz, W. Identification and categorisation of plants of foreign origin as the basis for practical activities. Acta Bot. Sil. 2011, 6, 23–53. [Google Scholar]
- Tang, L.; Gao, Y.; Wang, C.; Zhao, B.; Li, B. A plant invader declines through its modification to habitats: A case study of a 16-year chronosequence of Spartina alterniflora invasion in a salt marsh. Ecol. Eng. 2012, 49, 181–185. [Google Scholar] [CrossRef]
- Fenesi, A.; Vágási, C.I.; Beldean, M.; Földesi, R.; Kolcsár, L.P.; Shapiro, J.T.; Török, E.; Kovács-Hostyánszki, A. Solidago canadensis impacts on native plant and pollinator communities in different-aged old fields. Basic App. Ecol. 2015, 16, 335–346. [Google Scholar] [CrossRef]
- Tokarska-Guzik, B.E. The Establishment and Spread of Alien Plant Species (Kenophytes) in the Flora of Poland; Scientific Works no 2372; Silesian University Press: Katowice, Poland, 2005; Available online: http://www.sbc.org.pl/Content/39618/the_establishment_and_spread.pdf (accessed on 28 March 2020).
- Abhilasha, D.; Quintana, N.; Vivanco, J.; Joshi, J. Do allelopathic compounds in invasive Solidago canadensis restrain the native European flora. J. Ecol. 2008, 96, 993–1001. [Google Scholar] [CrossRef]
- Rola, J.; Rola, H. Solidago spp. as a bio-indicator of the occurrence of fallow lands on agricultural land. Frag. Agron. 2010, 27, 122–131. [Google Scholar]
- Aframowicz-Cieślak, R.; Bernacki, L.; Celka, Z.; Chmiel, J.; Cwener, A.; Dajdok, Z.; Danielewicz, W.; Kurnicki, B.; Łysko, A.; Marciniuk, J.; et al. Distribution atlas of vascular plants in Poland: Appendix. In Distribution Atlas of Vascular Plants in Poland; Zając, A., Zając, M., Eds.; Jagiellonian University Press: Warsaw, Poland, 2019; pp. 1–319. [Google Scholar]
- Pavek, P.L.S. Plant Guide for Canada Goldenrod (Solidago canadensis); USDA Natural Resources Conservation Service: Pullman, WA, USA, 2011.
- Chen, T.; Liu, W.L.; Zhang, C.B.; Wang, J. Effects of Solidago canadensis invasion on dynamics of native plant communities and their mechanisms. Chin. J. Plant Ecol. 2012, 36, 253–256. [Google Scholar] [CrossRef]
- Li, Y.Z.; Yin, X.; Wei, W.; Yang, E.Y.; Hang, Y.; Tian, Z.H.; Da, L.J. Inhibition of local plant Phragmites communis on the invasive plant Solidago canadensis. Acta Ecol. Sin. 2010, 30, 6881–6891. [Google Scholar]
- Huang, H.; Guo, S.L. Review on ecological studies on three invasive species of European genus Solidago. Guangxi Sci. 2004, 11, 69–74. [Google Scholar]
- Sun, B.Y.; Tan, J.Z.; Wan, Z.G.; Gu, F.G.; Zhu, M.D. Allelopathic effects of extracts from Solidago canadensis L. against seed germination and seedling growth of some plants. J. Environ. Sci. 2006, 18, 304–309. [Google Scholar]
- Yuan, Y.; Wang, B.; Zhang, S.; Tang, J.; Tu, C.; Hu, S.; Yong, J.W.H.; Xin, C. Enhanced allelopathy and competitive ability of invasive plant Solidago canadensis in its introduced range. Plant Ecol. 2013, 6, 253–263. [Google Scholar] [CrossRef]
- Ledger, K.J.; Pal, R.W.; Murphy, P.; Nagy, D.U.; Filep, R.; Callaway, R.M. Impact of an invader on species diversity is stronger in the non-native range than in the native range. Plant Ecol. 2015, 216, 1285–1295. [Google Scholar] [CrossRef]
- Powell, K.I.; Chase, J.M.; Knight, T.M. Invasive plants have scale-dependent effects on diversity by altering species-area relationships. Science 2013, 339, 316–318. [Google Scholar] [CrossRef]
- Zhang, F.; Wan, F. Canada goldenrod Solidago canadensis L. In Biological Invasions and Its Management in China; Wan, F., Jiang, M., Zhan, A., Eds.; Springer: Dordrecht, The Netherlands, 2017; Volume 13, pp. 143–151. [Google Scholar]
- Zhang, S.; Jin, Y.; Tang, J.; Chen, X. The invasive plant Solidago canadensis L. suppresses local soil pathogens through allelopathy. Appl. Soil Ecol. 2009, 41, 215–222. [Google Scholar] [CrossRef]
- Scharfy, D.; Güsewell, S.; Gessner, M.O.; Venterink, H.O. Invasion of Solidago gigantea in contrasting experimental plant communities: Effects on soil microbes, nutrients and plant–soil feedbacks. J. Ecol. 2010, 1379–1388. [Google Scholar] [CrossRef]
- Zhang, J.S.; Li, B.; Chen, J.K.; Zhou, T.S. Chemical constituents and antimicrobial activity of volatile oil from Solidago canadensis L. J. Found. Univ. Nat. Sci. 2006, 45, 412–415. [Google Scholar]
- Zhang, S.; Zhu, W.; Wang, B.; Tang, J.; Chen, X. Secondary metabolites from the invasive Solidago canadensis L. accumulation in soil and contribution to inhibition of soil pathogen Pythium ultimum. Appl. Soil Ecol. 2011, 48, 280–286. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, Y.; Padilla-Zakour, O.; Yang, G. Polyphenols, antioxidant and antimicrobial activities of leaf and bark extracts of Solidago canadensis L. Ind. Crop. Prod. 2015, 74, 803–809. [Google Scholar] [CrossRef]
- Fisher, R.F.; Woods, R.A.; Glavicic, M.R. Allelopathic effects of goldenrod and aster on young sugar maple. Can. J. For. Res. 1977, 8, 1–9. [Google Scholar] [CrossRef]
- Yang, R.Y.; Mei, L.X.; Tang, J.J.; Chen, X. Allelopathic effects of invasive Solidago canadensis L. on germination and growth of native Chinense plant species. Allelopath. J. 2007, 19, 241–248. [Google Scholar]
- Pisula, N.L.; Meiners, S.J. Allelopathic effects of goldenrod species on turnover in successional communities. Am. Midl. Nat. 2010, 163, 161–172. [Google Scholar] [CrossRef]
- Li, S.L.; Li, Z.H.; Wang, Y.F.; Ruan, X.; Pan, C.D.; Wang, Q. Preliminary study for the allelopathic effect of water extracts from Solidago canadensis leaves. Adv. Mat. Res. 2013, 699, 340–348. [Google Scholar] [CrossRef]
- Ye, X.Q.; Meng, J.L.; Wu, M. The effects of Solidago canadensis water extracts on maize seedling growth in association with the biomass allocation pattern. Peer J. 2019, 7, e6564. [Google Scholar] [CrossRef]
- Wang, C.; Jiang, K.; Wua, B.; Zhoua, J. The combined treatments of Canada goldenrod leaf extracts and cadmium pollution confer an inhibitory effect on seed germination and seedling development of lettuce. Aust. J. Bot. 2018, 66, 331–337. [Google Scholar] [CrossRef]
- Baličević, R.; Ravlić, M.; Živkovic, T. Allelopathic effect of invasive species giant goldenrod (Solidago gigantea Sit.) on craps and weeds. Herbologia 2015, 15, 19–29. [Google Scholar]
- Butcko, V.M.; Jensen, R.J. Evidence of tissue-specific allelopathic activity in Euthamia graminifolia and Solidago canadensis (Asteraceae). Am. Midl. Nat. 2002, 148, 253–262. [Google Scholar] [CrossRef]
- Petrášek, J.; Friml, J. Auxin transport routes in plant development. Development 2009, 136, 2675–2688. [Google Scholar] [CrossRef]
- Anžlovar, A.L.; Anžlovar, S. Allelopathic effect of aqueous extracts of Canadian goldenrod on germination and growth of radish. Acta Biol. Slov. 2019, 62, 27–34. [Google Scholar]
- Dżugan, M. Factors affecting the stability of green plant pigments. Soil Sci. Ann. 2006, 7, 27–33. [Google Scholar]
- Aarti, P.; Tanaka, R.; Tanaka, A. Effects of oxidative stress on chlorophyll biosynthesis in cucumber (Cucumis sativus) cotyledons. Physiol. Plant. 2006, 128, 186–197. [Google Scholar] [CrossRef]
- Rice, E.L. Allelopathy, 2nd ed.; Academic Press: Orlando, FL, USA, 1984; p. 422. [Google Scholar]
- Pandey, I.D.K. Inhibiton of najas (Najas graminea Del.) by parthenium (Parthenium hysterophorus L.). Allelopath. J. 1997, 4, 121–126. [Google Scholar]
- Baziramakenga, R.; Simard, R.R.; Leroux, G.D. Effects of benzoic and cinnamic acids on growth, mineral composition, and chlorophyll content of soybean. J. Chem. Ecol. 1994, 20, 2821–2833. [Google Scholar] [CrossRef] [PubMed]
- Yordanov, I.; Velikova, V.; Tsonev, T. Plant responses to drought, acclimation, and stress tolerance. Photosynthetica 2000, 38, 171–186. [Google Scholar] [CrossRef]
- Demidchik, V.; Straltsova, D.; Medvedev, S.S.; Pozhvanov, G.A.; Sokolik, A.; Yurin, V. Stress-induced electrolyte leakage: The role of K+-permeable channels and involvement in programmed cell death and metabolic adjustment. J. Exp. Bot. 2014, 65, 1259–1270. [Google Scholar] [CrossRef]
- Einhelling, F.A. Mechanism of action of allelochemicals in allelopathy. In Allelopathy, Organisms, Processes and Applications; Inderjit, K.M.M., Dakshini, F.A., Einhelling, Eds.; ASC Symp., Ser. 582; American Chemical Society: Washington, DC, USA, 1995; pp. 96–116. [Google Scholar]
- Balke, N.E. Effects of allelochemicals on mineral uptake and associated physiological processes. In The Chemistry of Allelopathy; Thompson, A.C., Ed.; American Chemical Society: Washington, DC, USA, 1985; pp. 161–178. [Google Scholar] [CrossRef]
- Vaughan, D.; Ord, B.G. Extraction of potential allelochemicals and their effects on root morphology and nutrient contents. In Plant Growth and Ecological Perspective; Atkinson, A., Ed.; Blackwell Scientific: Oxford, UK, 1991; pp. 399–421. [Google Scholar]
- Walter, M.H. Regulation of lignification in defense. In Plant Gene Res. Genes Involved in Plant Defense; Boller, T., Meins, F., Eds.; Springer-Verlag: Wien, Austria, 1992; pp. 27–352. [Google Scholar] [CrossRef]
- Wójcik-Wojtkowiak, D.; Politycka, B.; Weyman-Kaczmarkowa, W. Allelopathy; University of Life Sciences in Poznań Press: Poznań, Poland, 1998; pp. 1–92. [Google Scholar]
- Dong, L.J.; Yu, H.W.; He, W.M. What determines positive, neutral, and negative impacts of Solidago canadensis invasion on native plant species richness? Sci. Rep. 2015, 5, 16804. [Google Scholar] [CrossRef]
- Đikić, M. Allelopathic effect of aromatic and medicinal plants on the seed germination of Galinsoga parviflora. Echinochloa crusgalli and Galium molugo. Herbologia 2005, 6, 51–57. [Google Scholar]
- Baležentienė, L. Allelopathic activity of invasive species Solidago canadensis L. In Proceedings of the 7th international Scientific Conference Rural Development 2015: Towards the Transfer of Knowledge, Innovations and Social Progress, Aleksandras Stulginskis University, Akademija, Lithuania, 19–20 November 2015; Raupelienė, A., Ed.; Aleksandras Stulginskis University Press: Kaunas, Lithuania, 2015. [Google Scholar]
- Guo, S.L.; Fang, F. Physiological adaptation of the invasive plant Solidago canadensis to environments. Acta Phytoecol. Sin. 2003, 27, 47–52. [Google Scholar] [CrossRef]
- Tyszyńska-Kownacka, D.; Starek, T. Herbs in Polish House; Wydawnictwo Warta: Warszawa, Poland, 1989; p. 223. [Google Scholar]
- AOSA (Association of Official Seed Analysis). Seed vigour testing. In Handbook on Seed Testing; Anmol Publications Pvt. Ltd.: New Delhi, India, 1983. [Google Scholar]
- Islam, A.K.M.A.; Anuar, N.; Yaakob, Z. Effect of genotypes and pre-sowing treatments on seed germination behavior of Jatropha. Asian J. Plant Sci. 2009, 8, 433–439. [Google Scholar] [CrossRef]
- Chiapusio, G.; Sanchez, A.M.; Reigosa, M.J.; Gonzalez, L.; Pellissier, F. Do germination indices adequately reflect allelochemical effects on the germination process? J. Chem. Ecol. 1997, 23, 2445–2453. [Google Scholar] [CrossRef]
- Farooq, M.; Basra, S.M.A.; Afzal, I.; Khaliq, A. Optimization of hydropriming techniques for rice seed invigoration. Seed Sci. Technol. 2006, 34, 507–512. [Google Scholar] [CrossRef]
- Mominul Islam, A.K.M.; Kato-Noguchi, H. Allelopathic potentiality of medicinal plant Leucas aspera. Int. J. Agric. Sustain. 2012, 4, 1–7. [Google Scholar]
- Redmann, R.E.; Haraldson, J.; Gusta, L.V. Leakage of UV-absorbing substances as a measure of salt injury in leaf tissue of woody species. Acta Physiol. Plant. 1986, 67, 87–91. [Google Scholar] [CrossRef]

| Treatment | Days (24 h) | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| ‘Rowa’ | |||||||
| Control | 73.16 a ± 1.52 | 75.00 b ± 1.67 | 75.00 c ± 2.83 | 75.00 c ± 2.17 | 80.00 b ± 0.00 | 84.60 b ± 0.00 | 92.80 ab ± 0.00 |
| Decoction | 38.24 b ± 1.14 | 49.96 c ± 1.22 | 74.08 c ± 5.93 | 86.6 bc ± 1.58 | 92.68 ab ± 1.30 | 94.40 ab ± 1.34 | 92.68 ab ± 1.14 |
| Infusion | 13.92 d ± 0.55 | 20.32 d ± 1.22 | 23.52 d ± 0.84 | 28.32 e ± 0.71 | 33.12 e ± 0.84 | 35.52 e ± 0.84 | 37.84 d ± 0.89 |
| Macerate | 0.00 e ± 0.00 | 0.00 f ± 0.00 | 0.00 e ± 0.00 | 0.00 f ± 0.00 | 0.00 f ± 0.00 | 0.00 f ± 0.00 | 0.00 e ± 0.00 |
| ‘Półdługa’ | |||||||
| Control | 77.52 a ± 1.87 | 100.00 a ± 1.14 | 100.00 a ± 2.00 | 100.00 a ± 1.79 | 100.00 a ± 1.34 | 100.00 a ± 0.00 | 100.00 a ± 0.00 |
| Decoction | 38.28 b ± 0.71 | 46.28 c ± 0.71 | 83.88 bc ± 3.13 | 100.00 a ± 2.28 | 100.00 a ± 0.89 | 100.00 a ± 0.00 | 100.00 a ± 0.00 |
| Infusion | 32.76 b ± 1.14 | 40.76 c ± 0.89 | 73.56 c ± 2.30 | 86.36 bc ± 2.77 | 95.16 ab ± 2.55 | 100.00 a ± 2.30 | 100.00 a ± 2.07 |
| Macerate | 12.88 d ± 0.00 | 12.88 e ± 0.00 | 20.88 d ± 0.71 | 29.68 e ± 1.30 | 49.68 d ± 3.56 | 62.48 d ± 4.34 | 72.88 c ± 5.34 |
| ‘Krakowianka’ | |||||||
| Control | 74.80 a ± 0.89 | 86.00 b ± 1.14 | 92.40 b ± 0.00 | 92.40 b ± 0.00 | 92.40 ab ± 0.00 | 92.40 ab ± 0.00 | 92.40 ab ± 0.00 |
| Decoction | 77.12 a ± 0.71 | 81.12 b ± 1.14 | 81.12 bc ± 0.89 | 81.12 bc ± 1.22 | 81.12 b ± 0.55 | 81.12 b ± 0.45 | 81.12 b ± 0.45 |
| Infusion | 77.24 a ± 2.77 | 86.04 b ± 4.00 | 93.24 b ± 4.38 | 100.00 a ± 3.91 | 100.00 a ± 2.61 | 100.00 a ± 2.17 | 100.00 a ± 0.45 |
| Macerate | 22.12 c ± 0.84 | 30.92 cd ± 1.30 | 40.92 c ± 1.79 | 51.32 d ± 1.95 | 63.32 c ± 2.59 | 76.52 c ± 1.87 | 82.12 b ± 1.87 |
| Raphanus sativus Cultivars | Treatment | GI | CRG | T50 | SVI |
|---|---|---|---|---|---|
| ‘Rowa’ | Control | 52.70 b ± 2.17 | 20.71 b ± 0.25 | 0.47 a ± 0.001 | 1.75 b ± 0.66 |
| Decoction | 30.81 c ± 4.17 | 18.31 b ± 0.56 | 0.45 b ± 0.001 | 0.40 c ± 0.15 | |
| Infusion | 9.80 e ± 1.00 | 18.46 b ± 0.45 | 0.31 d ± 0.04 | 0.20 c ± 0.03 | |
| Macerate | 0.00 f ± 0.00 | 0.00 d ± 0.00 | 0.00 e ± 0.00 | 0.00 d ± 0.00 | |
| ‘Półdługa’ | Control | 55.76 b ± 2.88 | 21.05 ab ± 0.28 | 0.46 a ± 0.001 | 1.64 b ± 0.39 |
| Decoction | 57.03 b ± 2.29 | 18.17 c ± 0.33 | 0.46 a ± 0.001 | 0.77 c ± 0.12 | |
| Infusion | 25.70 ab ± 3.84 | 17.98 c ± 0.35 | 0.45 b ± 0.01 | 0.76 c ± 0.27 | |
| Macerate | 9.79 e ± 3.40 | 15.64 c ± 0.21 | 0.40 c ± 0.08 | 1.62 bc ± 0.01 | |
| ‘Krakowianka’ | Control | 62.75 a ± 1.44 | 21.74 b ± 0.15 | 0.46 a ± 0.001 | 4.59 a ± 1.57 |
| Decoction | 57.03 b ± 1.44 | 21.95 ab ± 0.15 | 0.46 a ± 0.001 | 1.95 b ± 0.50 | |
| Infusion | 54.21 b ± 7.71 | 20.92 ab ± 0.68 | 0.46 a ± 0.001 | 2.26 b ± 0.97 | |
| Macerate | 16.16 d ± 1.99 | 18.08 c ± 0.51 | 0.41 bc ± 0.02 | 0.82 c ± 0.13 |
| Raphanus sativus Cultivars | Control (cm) | Decoction | Infusion | Macerate | |||
|---|---|---|---|---|---|---|---|
| (cm) | IP (%) | (cm) | IP (%) | (cm) | IP (%) | ||
| ‘Rowa’ | 7.10 c ± 2.63 | 1.70 f ± 0.66 | 70.57 | 2.64 e ± 0.38 | 56.65 | 0.00 g ± 0.00 | 100.00 |
| ‘Półdługa’ | 6.56 c ± 1.58 | 3.08 d ± 0.48 | 50.55 | 3.50 d ± 1.17 | 44.48 | 2.14 e ± 1.25 | 69.50 |
| ‘Krakowianka’ | 13.06 a ± 2.16 | 7.86 c ± 1.94 | 39.02 | 9.14 b ± 3.99 | 27.54 | 6.06 c ± 1.44 | 51.83 |
| Raphanus sativus Cultivars | Control | Decoction | Infusion | Macerate |
|---|---|---|---|---|
| Fresh Mass (mg) | ||||
| ‘Rowa’ | 106.8 a ± 0.02 | 50.2 b ± 0.01 | 58.0 b ± 0.01 | 14.6 c ± 0.01 |
| ‘Półdługa’ | 116.2 a ± 0.06 | 48.4 b ± 0.01 | 67.8 ab ± 0.02 | 46.0 b ± 0.01 |
| ‘Krakowianka’ | 112.3 a ± 0.06 | 96.2 a ± 0.02 | 92.2 a ± 0.02 | 91.4 a ± 0.01 |
| Dry mass (mg) | ||||
| ‘Rowa’ | 5.0 b ± 0.002 | 4.8 bc ± 0.003 | 7.8 ab ± 0.003 | 7.2 ab ± 0.003 |
| ‘Półdługa’ | 5.6 ab ± 0.002 | 5.4 ab ± 0.002 | 7.0 ab ± 0.002 | 7.4 ab ± 0.001 |
| ‘Krakowianka’ | 8.4 a ± 0.003 | 7.0 ab ± 0.001 | 6.4 ab ± 0.002 | 8.6 a ± 0.002 |
| Water content (%) | ||||
| ‘Rowa’ | 95.30 a ± 2.35 | 90.91 a ± 4.55 | 86.28 a ± 5.78 | 50.85 c ± 11.08 |
| ‘Półdługa’ | 95.23 a ± 1.38 | 88.09 a ± 6.19 | 89.62 a ± 0.67 | 83.79 a ± 1.94 |
| ‘Krakowianka’ | 77.91 b ± 3.87 | 92.66 a ± 1.21 | 92.98 a ± 1.61 | 90.63 a ± 1.75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Możdżeń, K.; Barabasz-Krasny, B.; Zandi, P.; Kliszcz, A.; Puła, J. Effect of Aqueous Extracts from Solidago Canadensis L. Leaves on Germination and Early Growth Stages of Three Cultivars of Raphanus Sativus L. Var. Radicula Pers. Plants 2020, 9, 1549. https://doi.org/10.3390/plants9111549
Możdżeń K, Barabasz-Krasny B, Zandi P, Kliszcz A, Puła J. Effect of Aqueous Extracts from Solidago Canadensis L. Leaves on Germination and Early Growth Stages of Three Cultivars of Raphanus Sativus L. Var. Radicula Pers. Plants. 2020; 9(11):1549. https://doi.org/10.3390/plants9111549
Chicago/Turabian StyleMożdżeń, Katarzyna, Beata Barabasz-Krasny, Peiman Zandi, Angelika Kliszcz, and Joanna Puła. 2020. "Effect of Aqueous Extracts from Solidago Canadensis L. Leaves on Germination and Early Growth Stages of Three Cultivars of Raphanus Sativus L. Var. Radicula Pers" Plants 9, no. 11: 1549. https://doi.org/10.3390/plants9111549
APA StyleMożdżeń, K., Barabasz-Krasny, B., Zandi, P., Kliszcz, A., & Puła, J. (2020). Effect of Aqueous Extracts from Solidago Canadensis L. Leaves on Germination and Early Growth Stages of Three Cultivars of Raphanus Sativus L. Var. Radicula Pers. Plants, 9(11), 1549. https://doi.org/10.3390/plants9111549