Detection and Quantification of Rhizoctonia solani and Rhizoctonia solani AG1-IB Causing the Bottom Rot of Lettuce in Tissues and Soils by Multiplex qPCR
Abstract
:1. Introduction
2. Results
2.1. Characterization of Rhizoctonia solani Isolates
2.2. Development of the Rhizoctonia solani- and Rhizoctonia solani AG1-IB-Specific qPCR Assays
2.2.1. Primer and Probe Design
2.2.2. Primer and Probe Specificity
2.2.3. Primer and Probe Efficiency and Sensitivity
2.2.4. Validation in Artificially Inoculated Soil with R. solani AG 1-IB and R. solani Sclerotia
2.3. Investigation of the Relationship between R. solani AG 1-IB Soil Inoculum and Disease Development
2.4. Validation Using Naturally Infested Plant and Soil Samples
2.4.1. Rhizoctonia solani and Rhizoctonia solani AG1-IB Detection in Plant Tissue
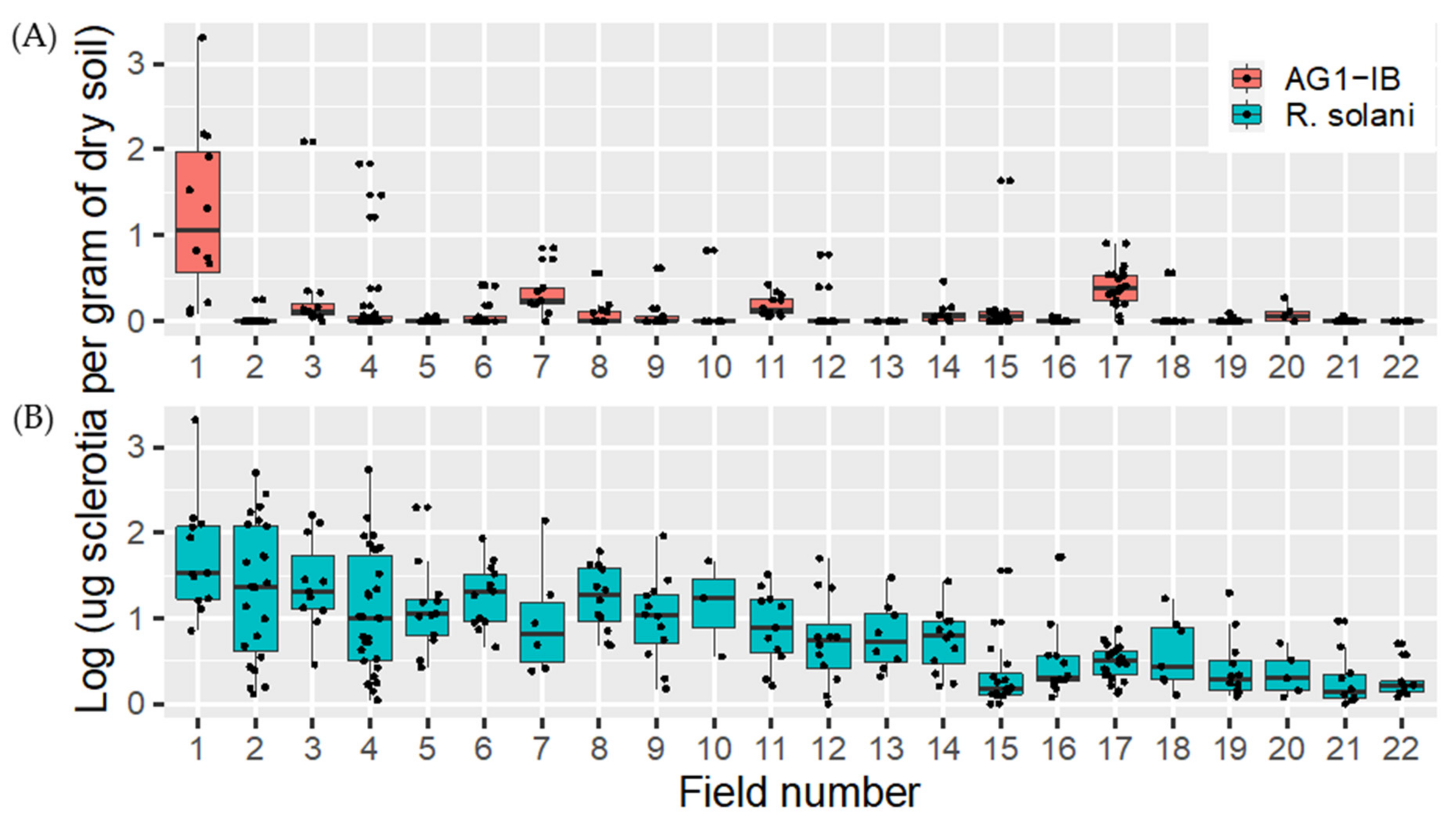
2.4.2. Rhizoctonia solani and Rhizoctonia solani AG1-IB Soil Distribution
3. Discussion
4. Materials and Methods
4.1. Field Sampling
4.2. Rhizoctonia Solani Isolation and Culture Method
4.3. Fungal, Plant, and Soil DNA Extraction
4.4. Rhizoctonia Solani Isolate Characterization
4.5. Design of Rhizoctonia solani and Rhizoctonia solani AG1-IB qPCR Assays on ITS
4.6. qPCR Conditions of Rhizoctonia solani and Rhizoctonia solani AG-1-IB Assays
4.7. Specificity and Sensitivity of the Rhizoctonia solani and Rhizoctonia solani AG1-IB qPCR Assays
4.8. Validation with Artificially Inoculated Soil
4.9. Growth Chamber Assays
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mailvaganam, S. Seasonal Fruit and Vegetable Annual Summary Reports; Fruit and Vegetable Survey, Statistics Canada: Ottawa, ON, Canada, 2017.
- Van der Heyden, H.; Wallon, T.; Lévesque, C.A.; Carisse, O. Detection and quantification of Pythium tracheiphilum in soil by multiplex real-Time qPCR. Plant Dis. 2019, 103, 475–483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sneh, B.; Burpee, L.; Ogoshi, A. Identification of Rhizoctonia Species; APS Press: St-Paul, MN, USA, 1998. [Google Scholar]
- Anderson, N.A. The genetics and pathology of Rhizoctonia solani. Annu. Rev. Phytopathol. 1982, 20, 329–347. [Google Scholar] [CrossRef]
- Herr, L.J. Characteristics of Rhizoctonia isolates associated with botton rot of lettuce in organic soils in Ohio. Phytopathology 1992, 82, 1046–1050. [Google Scholar] [CrossRef]
- Davis, M.; Subbarao, K.; Raid, R.; Kurtz, E. Compendium of Lettuce Diseases; Davis, M., Ed.; APS Press: St Paul, MN, USA, 1997. [Google Scholar]
- Grosch, R.; Schneider, J.H.M.; Kofoet, A. Characterisation of Rhizoctonia solani anastomosis groups causing bottom rot in field-Grown lettuce in Germany. Eur. J. Plant Pathol. 2004, 110, 53–62. [Google Scholar] [CrossRef]
- Kuramae, E.E.; Buzeto, A.L.; Ciampi, M.B.; Souza, N.L. Identification of Rhizoctonia solani AG 1-IB in Lettuce, AG 4 HG-I in tomato and melon, and AG 4 HG-III in broccoli and spinach, in Brazil. Eur. J. Plant Pathol. 2003, 109, 391–395. [Google Scholar] [CrossRef]
- Muzhinji, N.; Truter, M.; Woodhall, J.W.; van der Waals, J.E. Anastomosis groups and pathogenicity of Rhizoctonia solani and binucleate Rhizoctonia from potato in South Africa. Plant Dis. 2015, 99, 1790–1802. [Google Scholar] [CrossRef] [Green Version]
- Woodhall, J.; Webb, K.; Harper, G.; Peters, J.; Rodriguez-Carres, M.; Cubeta, M. First report of a new binucleate Rhizoctonia on potato tubers in the UK. New Dis. Rep. 2011, 23. [Google Scholar] [CrossRef] [Green Version]
- Jabaji-Hare, S.; Neate, S.M. Nonpathogenic binucleate Rhizoctonia spp. and benzothiadiazole protect cotton seedlings against Rhizoctonia damping-off and Alternaria leaf spot in cotton. Phytopathology 2005, 95, 1030–1036. [Google Scholar] [CrossRef] [Green Version]
- Carling, D.E.; Kuninaga, S.; Brainard, K.A. Hyphal anastomosis reactions, rDNA-internal transcribed spacer sequences, and virulence levels among subsets of Rhizoctonia solani anastomosis group-2 (AG-2) and AG-BI. Phytopathology 2002, 92, 43–50. [Google Scholar] [CrossRef] [Green Version]
- Toda, T.; Mghalu, J.; Priyatmojo, A.; Hyakumachi, M. Comparison of sequences for the internal transcribed spacer region in Rhizoctonia solani AG 1ID and other subgroups of AG 1. J. Gen. Plant Pathol. 2004, 70, 270–272. [Google Scholar] [CrossRef]
- Sharon, M.; Kuninaga, S.; Hyakumachi, M.; Naito, S.; Sneh, B. Classification of Rhizoctonia spp. using rDNA-ITS sequence analysis supports the genetic basis of the classical anastomosis grouping. Mycoscience 2008, 49, 93–114. [Google Scholar] [CrossRef]
- Broders, K.D.; Parker, M.L.; Melzer, M.S.; Boland, G.J. Phylogenetic diversity of Rhizoctonia solani associated with canola and wheat in Alberta, Manitoba, and Saskatchewan. Plant Dis. 2014, 98, 1695–1701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Claerbout, J.; Decombel, A.; Volckaert, A.; Venneman, S.; Vandevelde, I.; Bleyaert, P.; Neukermans, J.; Viaene, N.; Höfte, M. Glasshouse-specific occurrence of basal rot pathogens and the seasonal shift of Rhizoctonia solani anastomosis groups in lettuce. Eur. J. Plant Pathol. 2019, 155, 841–858. [Google Scholar] [CrossRef]
- Beneden, S.; Pannecoucque, J.; Debode, J.; Backer, G.; Höfte, M. Characterisation of fungal pathogens causing basal rot of lettuce in Belgian greenhouses. Eur. J. Plant Pathol. 2008, 124, 9–19. [Google Scholar] [CrossRef]
- Wareing, P.W.; Wang, Z.N.; Coley-Smith, J.R.; Jeves, T.M. Fungal pathogens in rotted basal leaves of lettuce in Humberside and Lancashire with particular reference to Rhizoctonia solani. Plant Pathol. 1986, 35, 390–395. [Google Scholar] [CrossRef]
- Grosch, R.; Kofoet, A. Influence of temperature, pH and inoculum density on bottom rot on lettuce caused by Rhizoctonia solani. Z. Pflanzenkrankh. Pflanzenschutz 2003, 110, 366–378. [Google Scholar]
- Ajayi-Oyetunde, O.O.; Bradley, C.A. Identification and characterization of Rhizoctonia species associated with soybean seedling disease. Plant Dis. 2016, 101, 520–533. [Google Scholar] [CrossRef] [Green Version]
- Boller, E.F.; Avilla, J.; Joerg, E.; Malavolta, C.; Wijnands, F.G.; Esbjerg, P.B. Integrated production: Principles and technical guidelines. Bull. OILB/SROP 2004, 27, 1–12. [Google Scholar]
- Panth, M.; Hassler, S.; Gurel, F.B. Methods for Management of Soilborne Diseases in Crop Production. Agriculture 2020, 10, 16. [Google Scholar] [CrossRef] [Green Version]
- Chellemi, D.O.; Gamliel, A.; Katan, J.; Subbarao, K.V. Development and deployment of systems-based approaches for the management of soilborne plant pathogens. Phytopathology 2015, 106, 216–225. [Google Scholar] [CrossRef] [Green Version]
- Bilodeau, G.J. Quantitative polymerase chain reaction for the detection of organisms in soil. CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2011, 6, 1–14. [Google Scholar] [CrossRef]
- Bilodeau, G.J.; Koike, S.T.; Uribe, P.; Martin, F.N. Development of an assay for rapid detection and quantification of Verticillium dahliae in soil. Phytopathology 2012, 102, 331–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sayler, R.; Yang, Y. Detection and quantification of Rhizoctonia solani AG1 IA, the rice sheath blight pathogen, in rice using real-time PCR. Plant Dis. 2007, 91, 1663–1668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boine, B.; Renner, A.-C.; Zellner, M.; Nechwatal, J. Quantitative methods for assessment of the impact of different crops on the inoculum density of Rhizoctonia solani AG2-2IIIB in soil. Eur. J. Plant Pathol. 2014, 140, 745–756. [Google Scholar] [CrossRef]
- Bartholomäus, A.; Mittler, S.; Märländer, B.; Varrelmann, M. Control of Rhizoctonia solani in sugar beet and effect of fungicide application and plant cultivar on inoculum potential in the soil. Plant Dis. 2017, 101, 941–947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Cointe, R.; Simon, T.E.; Delarue, P.; Hervé, M.; Leclerc, M.; Poggi, S. Reducing the use of pesticides with site-specific application: The chemical control of Rhizoctonia solani as a case of study for the management of soil-borne diseases. PLoS ONE 2016, 11, e0163221. [Google Scholar] [CrossRef]
- Brierley, J.L.; Hilton, A.J.; Wale, S.J.; Woodhall, J.W.; Lees, A.K. The relative importance of seed- and soil-borne Inoculum of Rhizoctonia solani AG-3 in causing black scurf on potato. Potato Res. 2016, 59, 181–193. [Google Scholar] [CrossRef]
- Grosch, R.; Schneider, J.H.M.; Kofoet, A.; Feller, C. Impact of continuous cropping of lettuce on the disease dynamics of bottom rot and genotypic diversity of Rhizoctonia solani AG 1-IB. J. Phytopathol. 2010, 159, 35–44. [Google Scholar] [CrossRef]
- Lees, A.K.; Cullen, D.W.; Sullivan, L.; Nicolson, M.J. Development of conventional and quantitative real-time PCR assays for the detection and identification of Rhizoctonia solani AG-3 in potato and soil. Plant Pathol. 2002, 51, 293–302. [Google Scholar] [CrossRef]
- Budge, G.E.; Shaw, M.W.; Colyer, A.; Pietravalle, S.; Boonham, N. Molecular tools to investigate Rhizoctonia solani distribution in soil. Plant Pathol. 2009, 58, 1071–1080. [Google Scholar] [CrossRef]
- Okubara, P.A.; Schroeder, K.L.; Paulitz, T.C. Identification and quantification of Rhizoctonia solani and R. oryzae using real-time polymerase chain reaction. Phytopathology 2008, 98, 837–847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sauvageau, A.; Gravel, V.; Van der Heyden, H. Soilborne inoculum density and environmental parameters influence the development of Pythium stunt caused by Pythium tracheiphilum in head lettuce crops. Plant Dis. 2019, 103, 1685–1692. [Google Scholar] [CrossRef] [PubMed]
- Johanson, A.; Turner, H.C.; McKay, G.J.; Brown, A.E. A PCR-based method to distinguish fungi of the rice sheath-blight complex, Rhizoctonia solani, R. oryzae and R. oryzae-sativae. FEMS Microbiol. Lett. 1998, 162, 289–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hyakumachi, M.; Sumino, A. New morphological type (IC) in Rhizoctonia solani AG-1 isolated from the sugarbeet-manufactory-waste-soils and some of its characteristics (in Japanese with English summary). Jpn. J. Phytopathol. 1984, 50, 507–514. [Google Scholar] [CrossRef]
- Grosch, R.; Schneider, J.H.M.; Peth, A.; Waschke, A.; Franken, P.; Kofoet, A.; Jabaji-Hare, S.H. Development of a specific PCR assay for the detection of Rhizoctonia solani AG 1-IB using SCAR primers. J. Appl. Microbiol. 2007, 102, 806–819. [Google Scholar] [CrossRef]
- Copley, T.R.; Aliferis, K.A.; Jabaji, S. Maple bark biochar affects Rhizoctonia solani metabolism and increases damping-off severity. Phytopathology 2015, 105, 1334–1346. [Google Scholar] [CrossRef]
- Dubey, S.; Tripathi, A.; Upadhyay, B.; Atul, K. Development of conventional and real time PCR assay for detection and quantification of Rhizoctonia solani infecting pulse crops. Biologia 2016, 71, 133–138. [Google Scholar] [CrossRef]
- Paulitz, T.C. Spatial distribution of Rhizoctonia solani and Rhizoctonia oryzae at three different scales in direct-seeded wheat. Can. J. Plant Pathol. 2004, 26, 419. [Google Scholar]
- Ophel-Keller, K.; McKay, A.; Hartley, D.; Curran, J. Development of a routine DNA-based testing service for soilborne diseases in Australia. Australas. Plant Pathol. 2008, 37, 243–253. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: New York, NY, USA, 1990. [Google Scholar]
- Hua, G.K.H.; Bertier, L.; Soltaninejad, S.; Höfte, M. Cropping systems and cultural practices determine the Rhizoctonia anastomosis groups associated with Brassica spp. in Vietnam. PLoS ONE 2014, 9, e111750. [Google Scholar] [CrossRef] [Green Version]
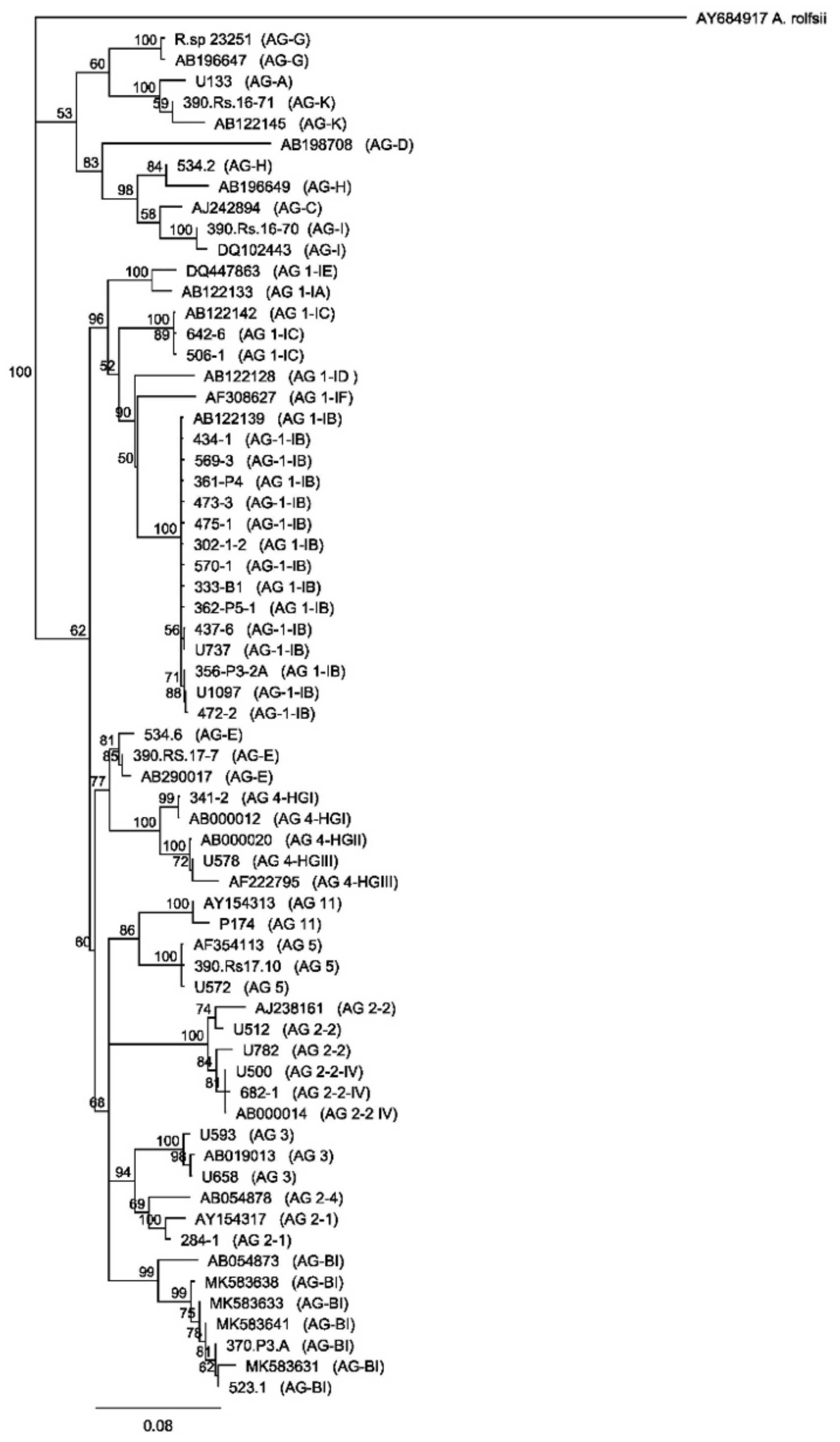

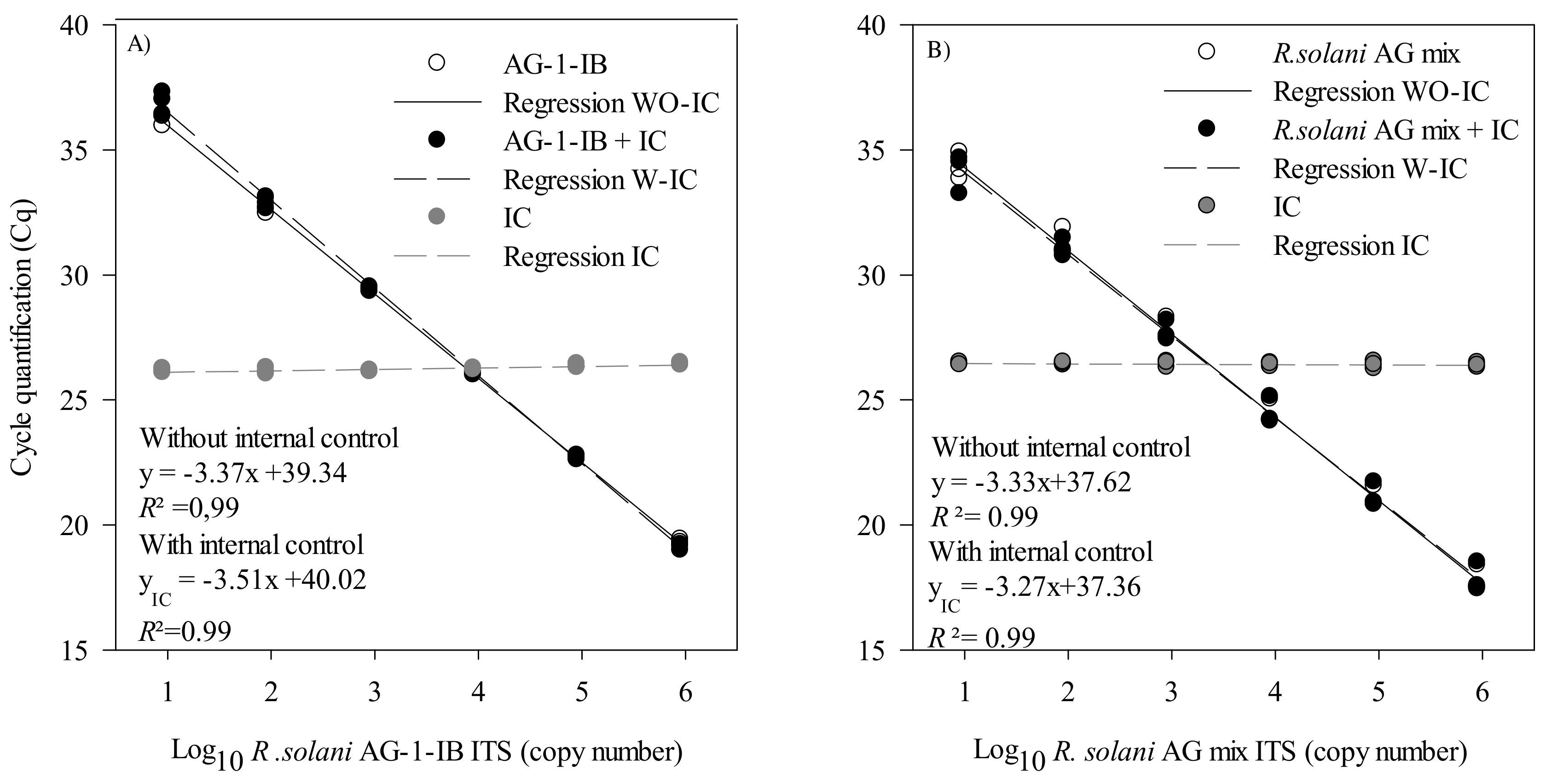
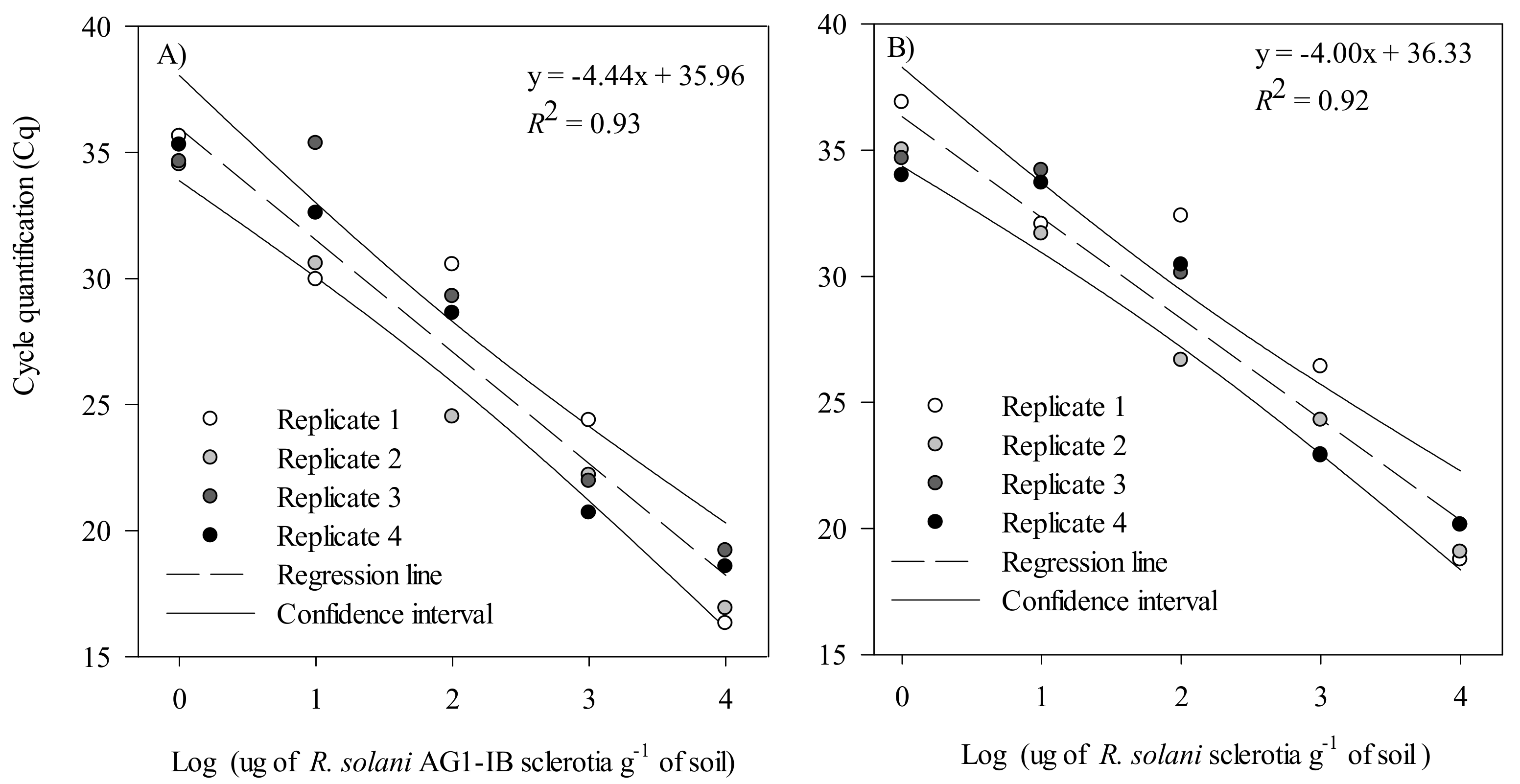
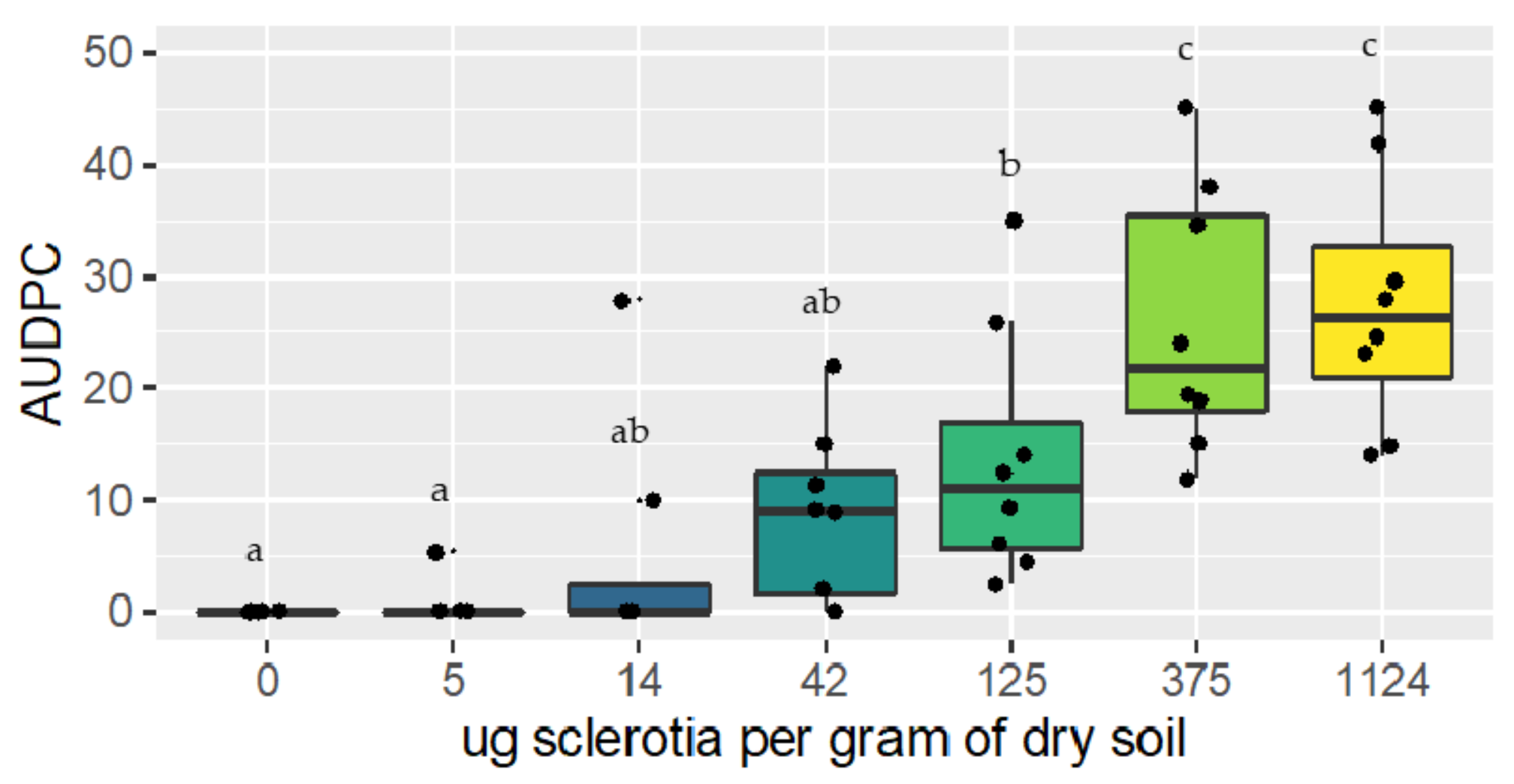
| Isolate | Host | Species a | AG | Genbank Accession | qPCR Assays e | |
|---|---|---|---|---|---|---|
| R. solani | R. solani AG 1-IB | |||||
| U737 b | Lactuca sativa | Rhizoctonia solani | 1-IB | MT177242 | 22.70 | 22.42 |
| U1097 b | Lactuca sativa | R. solani | 1-IB | MT177243 | 23.86 | 23.79 |
| 302-1-2 | Lactuca sativa | R. solani | 1-IB | MT177230 | 23.73 | 23.97 |
| 333-B-1 b | Lactuca sativa | R. solani | 1-IB | MT177231 | 25.12 | 25.20 |
| 356-P3-2A b | Lactuca sativa | R. solani | 1-IB | MT177232 | 23.00 | 23.38 |
| 361-P4 b | Lactuca sativa | R. solani | 1-IB | MT177233 | 23.62 | 23.70 |
| 362-P5-1 b | Lactuca sativa | R. solani | 1-IB | MT177234 | 23.14 | 23.67 |
| 434-1 b | Lactuca sativa | R. solani | 1-IB | MT177235 | 23.56 | 23.71 |
| 437-6 b | Lactuca sativa | R. solani | 1-IB | MT177236 | 22.30 | 22.92 |
| 472-2 b | Lactuca sativa | R. solani | 1-IB | MT177237 | 21.99 | 21.64 |
| 473-3 b | Lactuca sativa | R. solani | 1-IB | MT177238 | 21.58 | 21.59 |
| 475-1 b | Lactuca sativa | R. solani | 1-IB | MT177239 | 21.79 | 21.72 |
| 569-3 b | Lactuca sativa | R. solani | 1-IB | MT177240 | 23.56 | 23.38 |
| 570-1 b | Lactuca sativa | R. solani | 1-IB | MT177241 | 22.35 | 22.48 |
| 506-1 b | Lactuca sativa | R. solani | 1-IC | MT177244 | 23.47 | – |
| 642-6 b | Spinacia oleracea | R. solani | 1-IC | MT177245 | 26.77 | – |
| 370-P3-A b | Lactuca sativa | R. solani | BI | MT177259 | 24.92 | – |
| 523-1 b | Lactuca sativa | R. solani | BI | MT177260 | 24.53 | – |
| 284-1 b | Raphanus sativus | R. solani | 2-1 | MT177246 | 25.62 | – |
| U782 b | Spinacia oleracea | R. solani | 2-2 | MT177248 | 23.93 | – |
| U512 b | Daucus carota | R. solani | 2-2 | MT177247 | 24.22 | – |
| U500 b | Daucus carota | R. solani | 2-2-IV | MT177250 | 23.02 | – |
| 682-1 b | Daucus carota | R. solani | 2-2-IV | MT177249 | 28.91 | – |
| U593 b | Solanum tuberosum | R. solani | 3 | MT177251 | 24.90 | – |
| U658 b | Solanum tuberosum | R. solani | 3 | MT177252 | 24.53 | – |
| 341-2 b | Raphanus sativus | R. solani | 4-HGI | MT177253 | 22.64 | – |
| U578 b | Daucus carota | R. solani | 4-HGII | MT177254 | 30.45 | – |
| U572 b | Daucus carota | R. solani | 5 | MT177256 | 25.76 | – |
| 390.Rs17-10 d | Glycine max | R. solani | 5 | MT177255 | 29.81 | – |
| P174 b | Pisum sativum L. | R. solani | 11 | MT177257 | 25.09 | – |
| U133 b | Lactuca sativa | Binucleate Rhizoctonia | A | MT177258 | – | – |
| 390.Rs17-7 d | Glycine max | Binucleate Rhizoctonia | E | MT177261 | 25.69 | – |
| 534-6 d | Triticum L. | Binucleate Rhizoctonia | E | MT177262 | 27.24 | – |
| Rs.23251 c | Fragaria × ananassa | Binucleate Rhizoctonia | G | MT177263 | – | – |
| 534-2 d | Triticum L. | Binucleate Rhizoctonia | H | MT177264 | – | – |
| 390.Rs16-70 d | Glycine max | Binucleate Rhizoctonia | I | MT177265 | – | – |
| 390.Rs16-71 d | Glycine max | Binucleate Rhizoctonia | K | MT177266 | – | – |
| 343-5A-12 b | Apium graveolens L. | Alternaria sp. | MT177217 | – | – | |
| 381-B2 b | Daucus carota | Botrytis cinerea | MT177218 | – | – | |
| LDCC01 c | ND | Colletotrichum coccodes | MT177219 | – | – | |
| U301 b | Lactuca sativa | Fusarium equiseti | MT177220 | – | – | |
| U1416 b | Daucus carota | F. graminearum | MT177221 | – | – | |
| U197 b | Lactuca sativa | F. oxysporum | MT177222 | – | – | |
| 357-P12-4B b | Lactuca sativa | F. solani | MT177223 | – | – | |
| U709 b | Lactuca sativa | Mortierella sp. | MT177224 | – | – | |
| 280-3 b | Lactuca sativa | Pythium sylvaticum | MT177227 | – | – | |
| U199 b | Lactuca sativa | P. irregulare | MT177225 | – | – | |
| U191 b | Spinacia oleracea | P. oopapillum | MT177226 | – | – | |
| U415 b | Daucus carota | P. sulcatum | MH023358 | – | – | |
| 647-3 b | Lactuca sativa | P. tracheiphilum | MT177228 | – | – | |
| 278-4 b | Spinacia oleracea | P. ultimum | MT177229 | – | – | |
| 381-C1 b | Daucus carota | Sclerotinia sclerotiorum | MT177267 | – | – | |
| U795 b | Glycine max | Trichoderma sp. | MT177268 | – | – | |
| Vda-18481 c | ND | Verticillium dahliae | MT177269 | – | – | |
| Target | Primers and Probes | 5′-3′ Nucleotide Sequence | Reference |
|---|---|---|---|
| ITS | ITS1 | TCC GTA GGT GAA CCT GCG G | [30] |
| ITS4 | TCC TCC GCT TAT TGA TAT GC | ||
| EF | EF1 | ATG GGT AAG GAR GAC AAG AC | [31] |
| EF2 | GGA RGT ACC AGT SAT CAT GTT | ||
| GMRS3-R | AGT GTT ATG CTT GGT TCC ACT | ||
| R. solani | GRSM4M | CGG TTC RTC TGC ATT TAC CTT | Modified from [32] |
| GRMP | FAM-CRG CGT GAT AAR TTA TCT ATC GC | This study | |
| AG 1-IB-F3 | TGG CCT TTT AAC ATT GGC ATG T | This study | |
| R. solani AG 1-IB | AG 1-IB-R | CCA ACC CCA AAG GAC CTT GA | |
| AG 1-IB-P | VIC-CAC ACA CCC CTG TGC ACT TGT GAG AC | ||
| EIPC1mt100F | AGG CTA GCT AGG ACC GAT CAA TAGG | [33] | |
| IC-EIPC100 | EIPC1mt100R | AGT GCT TCG TTA CGA AAG TGA CCT TA | |
| EIPC1mt100P | ABY-CCT ATG CGT TCC GAG GTG ACG ACC TTG CC |
| Grower | Field | Sample | AG a Isolated | Lettuce Type b | qPCR Assays c | |
|---|---|---|---|---|---|---|
| R. solani | R. solani AG 1-IB | |||||
| 1 | A | 437 | 1-IB | R | + | + |
| 439 | 1-IB | R | + | + | ||
| 472 | 1-IB | R | + | + | ||
| 2 | B | 438 | 1-IB | R | + | + |
| 469 | 1-IB | R | + | + | ||
| C | 434 | 1-IB | I | + | + | |
| D | 564 | 1-IB | I | + | + | |
| 566 | 1-IB | I | + | + | ||
| 567 | 1-IB | I | + | + | ||
| 568 | 1-IB | I | + | + | ||
| 569 | 1-IB | I | + | + | ||
| 570 | 1-IB | I | + | + | ||
| 571 | 1-IB | I | + | + | ||
| 370-P4 | 1-IB | R | + | + | ||
| 370-P3 | BI | R | + | − | ||
| E | 523 | BI | R | + | − | |
| F | 369-P2 | 1-IB | R | + | + | |
| 369-P7 | 1-IB | R | + | + | ||
| 3 | G | 362-P5-1 | 1-IB | I | + | + |
| H | 337-P2 | 1-IB | I | + | + | |
| I | 506 | 1-IC | I | + | − | |
| J | 551 | 1-IB | I | + | + | |
| 552 | 1-IB | I | + | + | ||
| 4 | K | 333-B | 1-IB | ND | + | + |
| 333-E | 1-IB | ND | + | + | ||
| 333-J | 1-IB | ND | + | + | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wallon, T.; Sauvageau, A.; Van der Heyden, H. Detection and Quantification of Rhizoctonia solani and Rhizoctonia solani AG1-IB Causing the Bottom Rot of Lettuce in Tissues and Soils by Multiplex qPCR. Plants 2021, 10, 57. https://doi.org/10.3390/plants10010057
Wallon T, Sauvageau A, Van der Heyden H. Detection and Quantification of Rhizoctonia solani and Rhizoctonia solani AG1-IB Causing the Bottom Rot of Lettuce in Tissues and Soils by Multiplex qPCR. Plants. 2021; 10(1):57. https://doi.org/10.3390/plants10010057
Chicago/Turabian StyleWallon, Thérèse, Andréanne Sauvageau, and Hervé Van der Heyden. 2021. "Detection and Quantification of Rhizoctonia solani and Rhizoctonia solani AG1-IB Causing the Bottom Rot of Lettuce in Tissues and Soils by Multiplex qPCR" Plants 10, no. 1: 57. https://doi.org/10.3390/plants10010057
APA StyleWallon, T., Sauvageau, A., & Van der Heyden, H. (2021). Detection and Quantification of Rhizoctonia solani and Rhizoctonia solani AG1-IB Causing the Bottom Rot of Lettuce in Tissues and Soils by Multiplex qPCR. Plants, 10(1), 57. https://doi.org/10.3390/plants10010057





