Shallot Species and Subtypes Discrimination Based on Morphology Descriptors
Abstract
:1. Introduction
2. Results
2.1. Comprehensive Morphological Data of the Ex Situ Collection of Croatian Shallot Landraces
2.1.1. Flower and Inflorescence Morphology
2.1.2. Vegetative and Bulb Morphological Traits
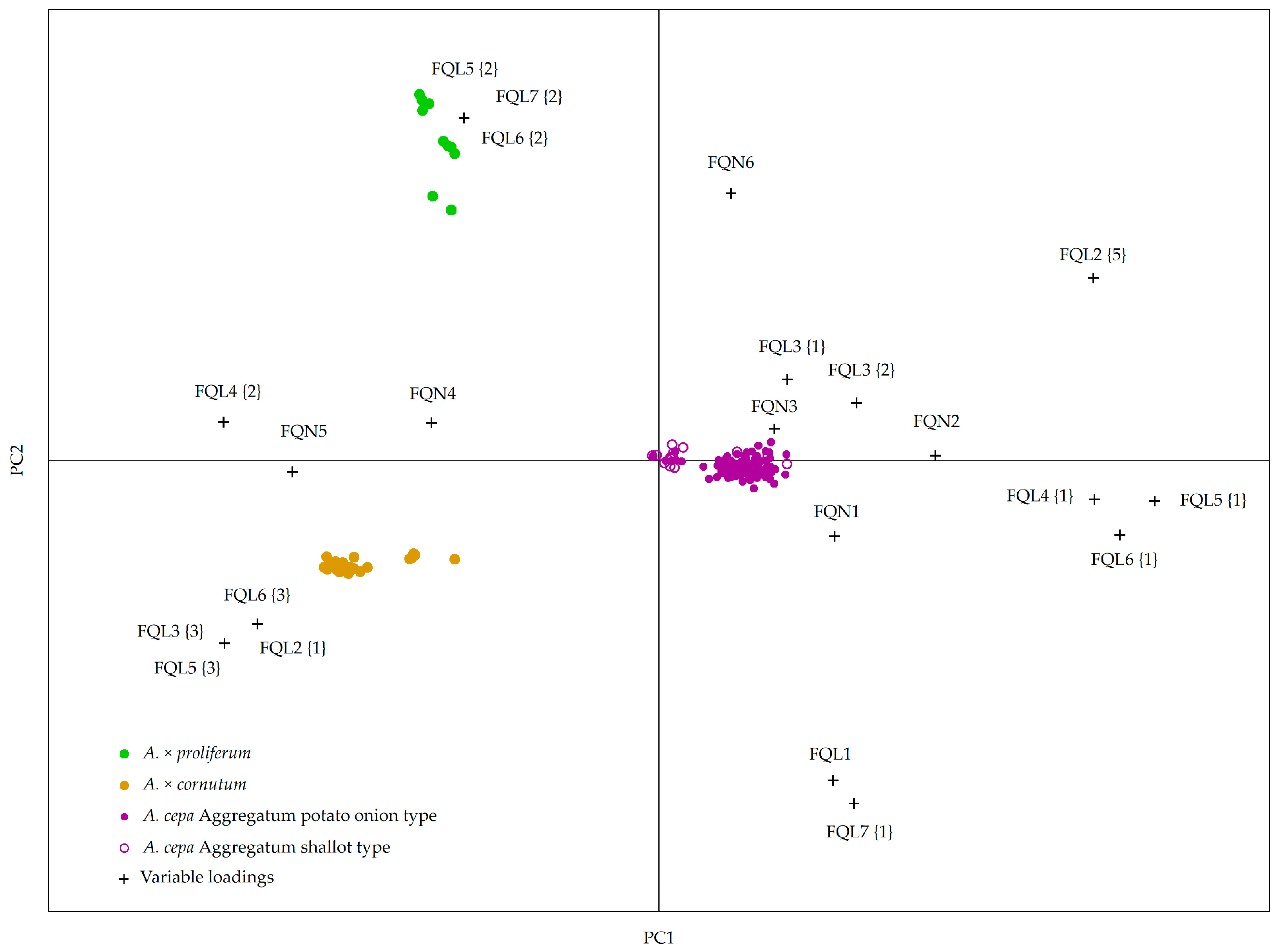
2.2. Creating a Model for Accession Grouping Based on Bulb and Vegetative Organ Morphological Descriptors
3. Discussion
4. Materials and Methods
Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- FAOSTAT—Food and Agriculture Organization Corporate Statistical Database. Available online: http://www.fao.org/faostat/en/#data/QC/visualize (accessed on 7 September 2017).
- Fritsch, R.M.; Friesen, N. Evolution, domestication and taxonomy. In Allium Crop Science: Recent Advances; Rabinowitch, H.D., Currah, L., Eds.; CABI: Wallingford, UK, 2002; pp. 5–30. [Google Scholar]
- Hanelt, P. Taxonomy, Evolution, and History of Alliums. In Onions and Allied Crops; Rabinowitch, H.D., Brewster, J.L., Eds.; CRC Press: Boca Raton, FL, USA, 1990; pp. 1–26. [Google Scholar]
- Lešić, R.; Borošić, J.; Buturac, I.; Ćustić, M.H.; Poljak, M.; Romić, D. Povrćarstvo [Vegetable Crops], 3rd ed.; Zrinski d.d.: Čakovec, Croatia, 2016. [Google Scholar]
- Puizina, J. Shallots in Croatia—Genetics, morphology and nomenclature. Acta Bot. Croat. 2013, 72, 387–398. [Google Scholar] [CrossRef] [Green Version]
- Rabinowitch, H.D.; Kamenetsky, R. Shallot (Allium cepa, Aggregatum group). In Allium Crop Science: Recent Advances; CABI: Wallingford, UK, 2002; pp. 409–430. [Google Scholar]
- Cohat, J.; Chauvin, J.E.; Le Nard, M. Shallot (Allium cepa var. aggregatum) production and breeding in France. In Acta Horticulturae, Proceedings of the II International Symposium on Edible Alliaceae, Adelaide, Australia, 10 November 1997; Armstrong, J., Ed.; International Society for Horticultural Science: Leuven, Belgium, 2001; Volume DLV, pp. 221–225. [Google Scholar]
- Esnault, F. Allium genetic resources at INRA-Ploudaniel, France. In European Collections of Vegetatively Propagated Allium: Report of a Workshop, Gatersleben, Germany, 21–22 May 2001; Maggioni, L., Keller, J., Astley, D., Eds.; IPGRI: Rome, Italy, 2001; pp. 28–29. [Google Scholar]
- Cottignies, A.; Cohat, J.; Le Floch, G.; Delpierre, N.; Le Nard, M. L’unité biologique du bulbe d’Échalote au cours du temps. Acta Bot. Gall. 1999, 146, 169–178. [Google Scholar] [CrossRef]
- Cottignies, A.; Cohat, J.; Le Nard, M.; Hourmant, A. Cycle cultural et floraison de l’échalote, Allium cepa L. var. aggregatum (cvs Mikor et Jermor). Acta Bot. Gall. 1997, 144, 209–216. [Google Scholar] [CrossRef] [Green Version]
- Khosa, J.; Dhatt, A.; Negi, K. Morphological Characterization of Allium spp. Using Multivariate Analysis. Indian J. Plant Genet. Resour. 2014, 27, 24–27. [Google Scholar]
- Petropoulos, S.A.; Fernandes, Â.; Barros, L.; Ferreira, I.C.F.R.; Ntatsi, G. Morphological, nutritional and chemical description of “Vatikiotiko”, an onion local landrace from Greece. Food Chem. 2015, 182, 156–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maggioni, L.; Keller, J.; Astley, D. European Collections of Vegetatively Propagated Allium: Report of a Workshop, Gatersleben, Germany, 21–22 May 2001; IPGRI: Rome, Italy, 2002; ISBN 9789290435280. [Google Scholar]
- Leino, M.W.; Solberg, S.; Tunset, H.M.; Fogelholm, J.; Strese, E.M.K.; Hagenblad, J. Patterns of Exchange of Multiplying Onion (Allium cepa L. Aggregatum-Group) in Fennoscandian Home Gardens. Econ. Bot. 2018, 72, 346–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruņǵis, D.; Leino, M.W.; Lepse, L.; Ban, S.G.; de Vahl, E.; Annamaa, K.; Põldma, P.; Suojala-Ahlfors, T.; Juškevičienė, D.; Kik, C.; et al. Genetic characterization of European potato onion (Allium cepa var. Aggregatum G. Don) collections. Genet. Resour. Crop Evol. 2020, 1–9. [Google Scholar] [CrossRef]
- Jones, H.A.; Mann, L.K. Onion and Their Allies; Leonard Hill, Ltd.: London, UK, 1963. [Google Scholar]
- Brewster, J.L. Onions and Other Vegetable Alliums, 2nd ed.; CABI: Wallingford, UK, 2008; Volume 2, ISBN 9781845933999. [Google Scholar]
- Aldén, B.; Ryman, S.; Hjertson, M. Våra Kulturväxters Namn: Ursprung och Användning; Formas: Stockholm, Sweden, 2009; ISBN 9789154060269. [Google Scholar]
- IPGRI; ECP/GR; AVRDC. Descriptors for Allium (Allium spp.); Asian Vegetable Research and Development Center: Tainan, Taiwan, 2001. [Google Scholar]
- Fredotović, Ž.; Šamanić, I.; Weiss-Schneeweiss, H.; Kamenjarin, J.; Jang, T.-S.; Puizina, J. Triparental origin of triploid onion, Allium × cornutum (Clementi ex Visiani, 1842), as evidenced by molecular, phylogenetic and cytogenetic analyses. BMC Plant Biol. 2014, 14, 24. [Google Scholar] [CrossRef] [Green Version]
- Major, N.; Ban, S.G.; Urlić, B.; Ban, D.; Dumičić, G.; Perković, J. Morphological and Biochemical Diversity of Shallot Landraces Preserved Along the Croatian Coast. Front. Plant Sci. 2018, 9, 1749. [Google Scholar] [CrossRef]
- Krontal, Y.; Kamenetsky, R.; Rabinowitch, H.D. Lateral development and florogenesis of a tropical shallot: A comparison with bulb onion. Int. J. Plant Sci. 1998, 159, 57–64. [Google Scholar] [CrossRef]
- Bogdanović, S.; Brullo, S.; Del Galdo, G.G.; Salmeri, C. A new autumn-flowering species of Allium (Alliaceae) from Croatia. Folia Geobot. 2009, 44, 83–93. [Google Scholar] [CrossRef]
- Rashid, M.; Islam, A.; Mian, M.; Hossain, T.; Kabir, M. Multivariate analysis in onion (Allium cepa L.). Bangladesh J. Agric. Res. 2013, 37, 573–582. [Google Scholar] [CrossRef] [Green Version]
- Yousaf, Z.; Shinwari, Z.K.; Qureshi, R.; SA, G. Can Complexity of Genus Allium L. Be Resolved Through Some Numerical Techniques? Pak. J. Bot. 2004, 36, 487–501. [Google Scholar]
- da Silva, A.R.; Cecon, P.R.; Dias, C.T.; Puiatti, M.; Finger, F.L.; Carneiro, A.P.S. Morphological phenotypic dispersion of garlic cultivars by cluster analysis and multidimensional scaling. Sci. Agric. 2014, 71, 38–43. [Google Scholar] [CrossRef] [Green Version]
- Aryakia, E.; Karimi, H.R.; Naghavi, M.R.; Fazeli, S.A.S. Morphological characterization of intra-and interspecific diversity in some Iranian wild Allium species. Euphytica 2016, 211, 185–200. [Google Scholar] [CrossRef]
- Fitriana, N.; Susandarini, R. Short Communication: Morphology and taxonomic relationships of shallot (Allium cepa L. group aggregatum) cultivars from Indonesia. Biodiversitas J. Biol. Divers. 2019, 20, 2809–2814. [Google Scholar] [CrossRef] [Green Version]
- Garthwaite, P.H. An interpretation of partial least squares. J. Am. Stat. Assoc. 1994, 89, 122–127. [Google Scholar] [CrossRef]
- Helland, I. Partial Least Squares Regression. In Wiley StatsRef: Statistics Reference Online; Wiley: Chichester, UK, 2014. [Google Scholar]
- Krontal, Y.; Kamenetsky, R.; Rabinowitch, H.D. Flowering physiology and some vegetative traits of short-day shallot: A comparison with bulb onion. J. Hortic. Sci. Biotechnol. 2000, 75, 35–41. [Google Scholar] [CrossRef]
- Lakshmi, G.V.; Raja, M.M.; Naik, M.L.; Kumar, S.P.; Khan, P.S.S.V. Florogenesis and Female Gametophyte Development in Allium cepa L. cv. Krishnapuram. Am. J. Plant Sci. 2017, 8, 2268–2281. [Google Scholar] [CrossRef] [Green Version]
- Marlin, M.; Maharijaya, A.; Purwito, A.; Sobir, R. Molecular Diversity of the Flowering Related Gene (LEAFY) on Shallot (Allium cepa var. aggregatum) and Alium Relatives. SABRAO J. Breed. Genet. 2018, 50, 313–328. [Google Scholar]
- Bernier, G.; Havelange, A.; Houssa, C.; Petitjean, A.; Lejeune, P. Physiological signals that induce flowering. Plant Cell 1993, 5, 1147–1155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halevy, A.H. Recent Advances in Control of Flowering and Growth Habit of Geophytes. Acta Hortic. 1990, 266, 35–42. [Google Scholar] [CrossRef]
- Ahmed, N.; Khan, S.H.; Afroza, B.; Hussain, K.; Qadri, S.; Nazir, G. Morphological characterization in onion (Allium cepa L.) for preparation and implementation of plant variety protection (PVP) legislation and distinctness, uniformity and stability (DUS) testing under temperate conditions of Kashmir. Afr. J. Agric. Res. 2013, 8, 1270–1276. [Google Scholar] [CrossRef] [Green Version]
- Laila, A.; Endang, S.; Wibowo, A. Morphogenetic Variation of Shallot (Allium cepa L. Aggregatum Group). Ilmu Pertanian. 2013, 16, 1–11. [Google Scholar] [CrossRef]
- Puizina, J.; Papeš, D. Cytogenetical evidences for hybrid structure and origin of diploid and triploid shallots (Allium cepa var.viviparum, Liliaceae) from Dalmatia (Croatia). Plant Syst. Evol. 1996, 199, 203–215. [Google Scholar] [CrossRef]
- Schwinn, K.E.; Ngo, H.; Kenel, F.; Brummell, D.A.; Albert, N.W.; McCallum, J.A.; Pither-Joyce, M.; Crowhurst, R.N.; Eady, C.; Davies, K.M. The onion (Allium cepa L.) R2R3-MYB gene MYB1 regulates anthocyanin biosynthesis. Front. Plant Sci. 2016, 7, 1865. [Google Scholar] [CrossRef] [Green Version]
- Khandagale, K.; Gawande, S. Genetics of bulb colour variation and flavonoids in onion. J. Hortic. Sci. Biotechnol. 2019, 94, 522–532. [Google Scholar] [CrossRef]
- De Vahl, E. Potato Onion, Johannes Onion or Nordic Shallot?—Historical Growing Systems, Denomination and Introduction for Allium cepa Aggregatum Group; Swedish University of Agricultural Sciences: Uppsala, Sweden, 2020. [Google Scholar]
- Dahlen, M. A Cook’s Guide to Chinese Vegetables; Wokman Press: Boston, MA, USA, 2001; 138p, ISBN 10 9627502545. [Google Scholar]
- Arifin, N.S.; Miyajima, I.; Okubo, H. Variation of pigments in the bulbs of shallot (Allium cepa var. ascalonicum) and Allium × wakegi. J. Fac. Agric. 1999, 43, 303–308. [Google Scholar]
- Sulistyaningsih, E.; Yamashita, K.I.; Tashiro, Y. Genetic characteristics of the Indonesian white shallot. J. Jpn. Soc. Hortic. Sci. 2002, 71, 504–508. [Google Scholar] [CrossRef] [Green Version]
- Wold, H. Estimation of principal components and related models by iterative least squares. In Multivariate Analysis; Krishnaiah, P.R., Ed.; Academic Press: New York, NY, USA, 1966; pp. 391–420. [Google Scholar]
- Wold, H. Path models with latent variables: The NIPALS approach. In Quantitative Sociology: International Perspectives on Mathematical and Statistical Model Building; Blalock, H.M., Ed.; Academic Press: New York, NY, USA, 1975; pp. 307–357. [Google Scholar]
- Potdar, K.; Pardawala, T.S.; Pao, C. A Comparative Study of Categorical Variable Encoding Techniques for Neural Network Classifiers. Int. J. Comput. Appl. 2017, 175, 7–9. [Google Scholar] [CrossRef]
- Jury, E. Theory and Application of the Z-Transform Method; Wiley: New York, NY, USA, 1964. [Google Scholar]

| Flowering Time in 2018 (WAP) 1 | Accessions 2 | |
|---|---|---|
| 31 WAP | IPT176, from IPT226 to IPT229, from IPT237 to IPT245 | |
| 32 WAP | A. × proliferum accessions, IPT208, IPT216, IPT217, IPT218, IPT225, from IPT230 to IPT236 | |
| 33 WAP | A. × cornutum accessions | |
| Ability to flower | Percentage of flowering in 2018 3 | Accessions |
| No or very rare flowering | 10% or less | IPT215, IPT022, IPT216, IPT239, IPT240 |
| Rare flowering | 15–30% | IPT021, IPT212, IPT231, IPT237 |
| Most accessions flower | 40–60% | IPT211, IPT213, IPT217, IPT218, IPT225, IPT228, IPT229, IPT235, IPT238, IPT241 |
| Obligatory flowering | 70–100% | A. × proliferum accessions, IPT214, IPT176, IPT208, IPT226, IPT227, IPT230, from IPT232 to IPT234, IPT236, from IPT242 to IPT245 |
| Accession | Species | 1 Flower Number in Inflorescence (FQL 1) 3 | Anther Color (FQL 2) | Stamen Type 4 (FQL 3) | Pistil Type 5 (FQL 4) | Perianth Type 6 (FQL 5) | Inflorescence Type 7 (FQL 6) | Scape Type 8 (FQL 7) |
|---|---|---|---|---|---|---|---|---|
| IPT023 | A. × proliferum | Few < 30 {1} | Green {5} 2 | {1} | {2} | {2} | {2} | {2} |
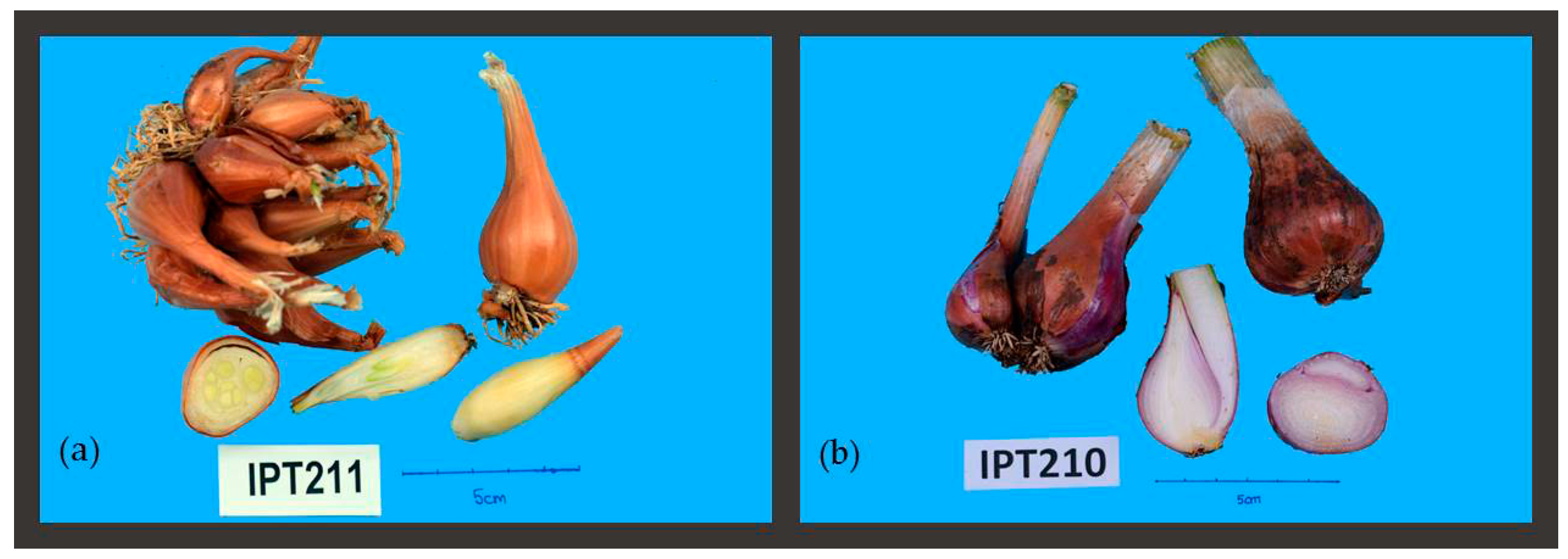
| IPT210 | Few < 30 {1} | Green {5} | {1} | {2} | {2} | {2} | {2} | |
| IPT021 | A. × cornutum | Many > 30 {2} | Yellow {1} | {3} | {2} | {3} | {1} | {1} |
| IPT211 | Many > 30 {2} | Yellow {1} | {3} | {2} | {3} | {3} | {1} | |
| IPT212 | Many > 30 {2} | Yellow {1} | {3} | {2} | {3} | {3} | {1} | |
| IPT213 | Many > 30 {2} | Yellow {1} | {3} | {2} | {3} | {3} | {1} | |
| IPT214 | Many > 30 {2} | Yellow {1} | {3} | {2} | {3} | {3} | {1} | |
| IPT176 | A. cepa Aggregatum group | Many > 30 {2} | Green {5} | {2} | {1} | {1} | {1} | {1} |
| IPT208 | Many > 30 {2} | Green {5} | {1} | {1} | {1} | {1} | {1} | |
| IPT217 | Many > 30 {2} | Green {5} | {1} | {1} | {1} | {1} | {1} | |
| IPT218 | Many > 30 {2} | Green {5} | {1} | {1} | {1} | {1} | {1} | |
| IPT225 | Many > 30 {2} | Green {5} | {2} | {1} | {1} | {1} | {1} | |
| IPT226 | Many > 30 {2} | Green {5} | {2} | {1} | {1} | {1} | {1} | |
| IPT227 | Many > 30 {2} | Green {5} | {2} | {1} | {1} | {1} | {1} | |
| IPT228 | Many > 30 {2} | Green {5} | {2} | {1} | {1} | {1} | {1} | |
| IPT229 | Many > 30{2} | Green {5} | {2} | {1} | {1} | {1} | {1} | |
| IPT230 | A. cepa Aggregatum group | Many > 30 {2} | Green {5} 2 | {1} | {1} | {1} | {1} | {1} |
| IPT231 | Many > 30 {2} | Green {5} | {2} | {1} | {1} | {1} | {1} | |
| IPT232 | Many > 30 {2} | Green {5} | {2} | {1} | {1} | {1} | {1} | |
| IPT233 | Many > 30 {2} | Green {5} | {2} | {1} | {1} | {1} | {1} | |
| IPT234 | Many > 30 {2} | Green {5} | {2} | {1} | {1} | {1} | {1} | |
| IPT235 | Many > 30 {2} | Green {5} | {1} | {1} | {1} | {1} | {1} | |
| IPT236 | Many > 30 {2} | Green {5} | {2} | {1} | {1} | {1} | {1} | |
| IPT237 | Many > 30 {2} | Green {5} | {2} | {1} | {1} | {1} | {1} | |
| IPT238 | Many > 30 {2} | Green {5} | {2} | {1} | {1} | {1} | {1} | |
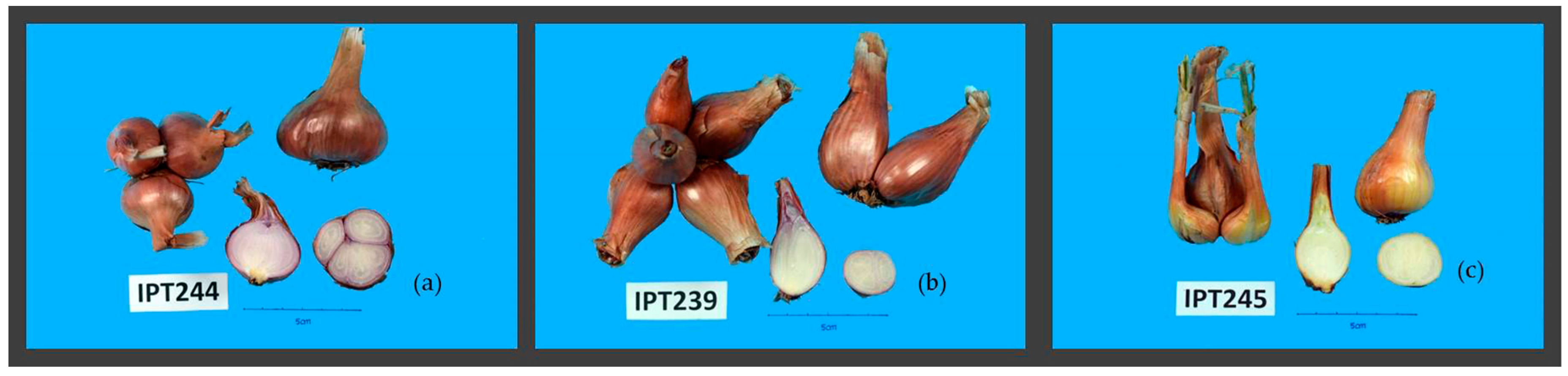
| IPT239 | Many > 30 {2} | Green {5} | {2} | {1} | {1} | {1} | {1} | |
| IPT240 | Many > 30 {2} | Green {5} | {2} | {2} | {1} | {1} | {1} | |
| IPT241 | Many > 30 {2} | Green {5} | {2} | {2} | {1} | {1} | {1} | |
| IPT242 | Many > 30 {2} | Green {5} | {2} | {2} | {1} | {1} | {1} | |
| IPT243 | Many > 30 {2} | Green {5} | {1} | {1} | {1} | {1} | {1} | |
| IPT244 | Many > 30 {2} | Green {5} | {1} | {1} | {1} | {1} | {1} | |
| IPT245 | Many > 30 {2} | Green {5} | {1} | {1} | {1} | {1} | {1} |
| Species | Inflorescence Diameter (mm) (FQN1) | Flower Pedicle Length (mm) (FQN 2) | Stamen Length (mm) (FQN 3) | Petal Length (mm) (FQN 4) | Petal Diameter (mm) (FQN 5) | Scape Diameter (mm) (FQN6) |
|---|---|---|---|---|---|---|
| A. × proliferum | 30.69 1 ± 3.39 b 2 | 11.62 ± 1.37 ab | 5.80 ± 0.24 | 4.21 ± 0.15 a | 2.78 ± 0.11 a | 24.87 ± 1.21 a |
| A. cepa Aggregatum group | 42.83 ± 0.69 a | 15.46 ± 0.39 a | 5.93 ± 0.07 | 3.58 ± 0.04 b | 2.12 ± 0.03 b | 14.87 ± 0.3 b |
| A. × cornutum | 38.64 ± 2.15 ab | 10.15 ± 0.87 b | 5.46 ± 0.15 | 4.10 ± 0.10 a | 3.04 ± 0.07 a | 9.69 ± 0.76 c |
| p-value 3 | 0.001 | <0.001 | ns | <0.001 | <0.01 | <0.01 |
| Accession | Species | 1 Bulb Shape (BQL 1) | Bulblets (BQL 3) | Bulbs Per Cluster (BQL 2) | Bulb Skin Color (BQL 4) | Bulb Skin Thickness (BQL 5) | Bulb Flesh Color (BQL 6) | Bulbs in Cluster (BQN 1) | Cluster Weight (g) (BQN 2) | Bulb Diameter (mm) (BQN 3) |
|---|---|---|---|---|---|---|---|---|---|---|
| IPT023 | A. × proliferum | Ovate {7} 2 | Present {1} | Scarce {1} | Brown {5} | Medium | Violet/white {4} | 2.00 ± 0.67 | 150.69 ± 32.78 | 42.10 ± 3.12 |
| IPT210 | Broad oval {4} | Absent {0} | Scarce {1} | Brown {5} | Medium | Violet/white {4} | 2.20 ± 0.84 | 82.70 ± 26.69 | 55.55 ± 8.36 | |
| IPT021 | A. × cornutum | Ovate {7} | Absent {0} | Medium {5} | Light brown {4} | Thick | Green/white {3} | 14.89 ± 10.46 | 284.92 ± 95.53 | 44.19 ± 4.97 |
| IPT022 | Ovate {7} | Absent {0} | Few {3} | Light brown {4} | Thick | Green/white {3} | 5.90 ± 2.51 | 149.50 ± 37.26 | 43.03 ± 4.89 | |
| IPT211 | Ovate {7} | Absent {0} | Medium {5} | Light brown {4} | Thick | Green/white {3} | 11.40 ± 6.17 | 218.30 ± 36.96 | 42.37 ± 5.88 | |
| IPT212 | Ovate {7} | Absent {0} | Few {3} | Light brown {4} | Thick | Green/white {3} | 7.70 ± 3.59 | 185.40 ± 52.83 | 41.93 ± 5.17 | |
| IPT213 | Ovate {7} | Absent {0} | Few {3} | Light brown {4} | Thick | Green/white {3} | 5.20 ± 1.14 | 196.65 ± 15.92 | 47.83 ± 4.35 | |
| IPT214 | Ovate {7} | Absent {0} | Few {3} | Light brown {4} | Thick | Green/white {3} | 9.40 ± 4.97 | 208.70 ± 37.27 | 43.77 ± 4.01 | |
| IPT215 | Ovate {7} | Absent {0} | Few {3} | Light brown {4} | Thick | Green/white {3} | 7.20 ± 3.71 | 163.85 ± 31.50 | 42.4 ± 4.11 | |
| IPT176 | A. cepa Aggregatum group | Broad oval {4} | Present {1} | Medium {5} | Light brown {4} | Thin | Violet/white {4} | 8.70 ± 2.87 | 293.20 ± 66.66 | 57.55 ± 8.01 |
| IPT208 | Rhomboid {3} | Absent {0} | Medium {5} | Light brown {4} | Thin | Violet/white {4} | 14.70 ± 5.96 | 277.80 ± 94.32 | 40.23 ± 7.13 | |
| IPT216 | Broad oval {4} | Present {1} | Medium {5} | Light brown {4} | Thin | Violet/white {4} | 15.00 ± 4.32 | 242.00 ± 56.85 | 44.41 ± 4.83 | |
| IPT217 | Broad oval {4} | Present {1} | Medium {5} | Light violet {8} | Thin | Violet/white {4} | 14.50 ± 8.09 | 352.55 ± 91.85 | 52.92 ± 9.69 | |
| IPT218 | Broad oval {4} | Present {1} | Medium {5} | Light violet {8} | Thin | Violet/white {4} | 11.90 ± 3.21 | 274.70 ± 53.94 | 53.29 ± 7.04 | |
| IPT225 | Broad oval {4} | Present {1} | Medium {5} | Light violet {8} | Thin | Violet/white {4} | 13.80 ± 5.90 | 327.06 ± 163.17 | 54.75 ± 11.43 | |
| IPT226 | Broad oval {4} | Present {1} | Medium {5} | Light violet {8} | Thin | Violet/white {4} | 15.50 ± 4.45 | 254.45 ± 75.00 | 42.41 ± 5.50 | |
| IPT227 | Ovate {7} | Present {1} | Scarce {1} | Yellow {2} | Thin | White {1} | 5.10 ± 2.38 | 128.95 ± 34.12 | 42.57 ± 9.26 | |
| IPT228 | Broad oval {4} | Present {1} | Medium {5} | Light violet {8} | Thin | Violet/white {4} | 11.60 ± 3.10 | 267.50 ± 67.80 | 47.90 ± 5.52 | |
| IPT229 | Broad oval {4} | Present {1} | Medium {5} | Light violet {8} | Thin | Violet/white {4} | 8.78 ± 2.30 | 353.89 ± 94.77 | 55.23 ± 10.02 |
| Species | Leaf Length (cm) (VQN 1) | Leaf Diameter (mm) (VQN 2) | Bulbs in Cluster (BQN 1) 1 | Cluster Weight (g) (BQN 2) | Bulb Diameter (mm) (BQN 3) |
|---|---|---|---|---|---|
| A. × proliferum | 38.99 1 ± 1.39 a 2 | 18.71 ± 0.64 a | 2.01 ± 1.26 c | 128.03 ± 22.22 c | 51.06 ± 2.16 a |
| A. cepa Aggregatum PO 3 | 36.29 ± 0.37 ab | 10.66 ± 0.17 b | 12.10 ± 0.34 a | 273.12 ± 5.94 a | 48.63 ± 0.58 a |
| A. cepa Aggregatum SH | 34.60 ± 0.76 b | 9.24 ± 0.35 c | 7.67 ± 0.69 b | 171.62 ± 12.17 bc | 40.19 ± 1.18 b |
| A. × cornutum | 35.53 ± 0.64 ab | 8.63 ± 0.30 c | 8.81 ± 0.52 b | 201.05 ± 10.28 b | 43.65 ± 1.00 b |
| p-value 4 | 0.029 | <0.01 | <0.01 | <0.01 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perković, J.; Major, N.; Ban, D.; Cvitan, D.; Ban, S.G. Shallot Species and Subtypes Discrimination Based on Morphology Descriptors. Plants 2021, 10, 60. https://doi.org/10.3390/plants10010060
Perković J, Major N, Ban D, Cvitan D, Ban SG. Shallot Species and Subtypes Discrimination Based on Morphology Descriptors. Plants. 2021; 10(1):60. https://doi.org/10.3390/plants10010060
Chicago/Turabian StylePerković, Josipa, Nikola Major, Dean Ban, Danko Cvitan, and Smiljana Goreta Ban. 2021. "Shallot Species and Subtypes Discrimination Based on Morphology Descriptors" Plants 10, no. 1: 60. https://doi.org/10.3390/plants10010060
APA StylePerković, J., Major, N., Ban, D., Cvitan, D., & Ban, S. G. (2021). Shallot Species and Subtypes Discrimination Based on Morphology Descriptors. Plants, 10(1), 60. https://doi.org/10.3390/plants10010060






