Application of Deep Eutectic Solvents for the Extraction of Carnosic Acid and Carnosol from Sage (Salvia officinalis L.) with Response Surface Methodology Optimization
Abstract
1. Introduction
2. Results and Discussion
2.1. Influence of DESs on the Obtained Amount of Carnosic Acid and Carnosol in the Extracts
2.2. Comparison of the Used Extraction Methods
2.3. Influence of Various DES Extraction Parameters on the Content of Carnosol and Carnosic Acid
2.4. Comparison with Other Extraction Methods
3. Materials and Methods
3.1. Chemicals
3.2. Plant Material
3.3. Preparation of DES
3.4. Extraction of Carnosic Acid and Carnosol with DESs
3.5. Extraction of Carnosic Acid and Carnosol with Conventional Solvents
3.6. Chemical Characterization of the Extracts
3.7. Statistical Experimental Design
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Terms | Coefficients | Standard Error | F-Value | p-Value |
|---|---|---|---|---|
| Carnosic acid (stirring) | ||||
| Intercept | 4.93 | 0.42 | ||
| X1 | −3.97 | 0.33 | 145.19 | 0.0001 |
| X2 | 0.98 | 0.33 | 8.95 | 0.0202 |
| X3 | 0.40 | 0.33 | 1.49 | 0.2619 |
| X12 | 1.60 | 0.45 | 12.51 | 0.0095 |
| X22 | 0.71 | 0.45 | 2.46 | 0.1608 |
| X32 | 0.28 | 0.45 | 0.39 | 0.5530 |
| X1X2 | −1.31 | 0.47 | 7.94 | 0.0259 |
| X1X3 | 0.46 | 0.47 | 0.98 | 0.3562 |
| X2X3 | 0.47 | 0.47 | 1.00 | 0.3495 |
| R2= 0.9630 | ||||
| Carnosic acid (UAE) | ||||
| Intercept | 4.76 | 0.87 | ||
| X1 | −3.17 | 0.69 | 21.24 | 0.0025 |
| X2 | 1.08 | 0.69 | 2.45 | 0.1617 |
| X3 | 2.22 | 0.69 | 10.45 | 0.0144 |
| X12 | 1.39 | 0.95 | 2.14 | 0.1871 |
| X22 | −0.52 | 0.95 | 0.30 | 0.6019 |
| X32 | −0.57 | 0.95 | 0.37 | 0.5645 |
| X1X2 | −0.34 | 0.97 | 0.12 | 0.7349 |
| X1X3 | −2.39 | 0.97 | 6.06 | 0.0433 |
| X2X3 | 1.46 | 0.97 | 2.24 | 0.1780 |
| R2= 0.8660 | ||||
| Carnosic acid (MCE) | ||||
| Intercept | 3.44 | 0.28 | ||
| X1 | −1.34 | 0.22 | 35.66 | 0.006 |
| X2 | 0.63 | 0.22 | 7.82 | 0.0266 |
| X3 | 1.59 | 0.22 | 50.46 | 0.0002 |
| X12 | −0.38 | 0.31 | 1.50 | 0.2608 |
| X22 | 0.16 | 0.31 | 0.27 | 0.6209 |
| X32 | 1.05 | 0.31 | 11.49 | 0.0116 |
| X1X2 | 0.066 | 0.32 | 0.043 | 0.8409 |
| X1X3 | −0.93 | 0.32 | 8.56 | 0.0221 |
| X2X3 | 0.54 | 0.32 | 2.95 | 0.1294 |
| R2 = 0.9442 | ||||
| Terms | Coefficients | Standard Error | F-Value | p-Value |
|---|---|---|---|---|
| Carnosol (stirring) | ||||
| Intercept | 2.15 | 0.16 | ||
| X1 | −1.07 | 0.13 | 70.76 | 0.0001 |
| X2 | 0.72 | 0.13 | 31.74 | 0.0008 |
| X3 | 0.86 | 0.13 | 45.54 | 0.0003 |
| X12 | −0.093 | 0.18 | 0.28 | 0.6146 |
| X22 | 0.22 | 0.18 | 1.52 | 0.2580 |
| X32 | 0.26 | 0.18 | 2.26 | 0.1768 |
| X1X2 | −0.55 | 0.18 | 9.34 | 0.0184 |
| X1X3 | −0.22 | 0.18 | 1.48 | 0.2630 |
| X2X3 | 0.52 | 0.18 | 8.34 | 0.0234 |
| R2= 0.9607 | ||||
| Carnosol (MCE) | ||||
| Intercept | 1.09 | 0.077 | ||
| X1 | −0.24 | 0.061 | 15.65 | 0.0055 |
| X2 | 0.19 | 0.061 | 9.28 | 0.0187 |
| X3 | 0.32 | 0.061 | 27.85 | 0.0012 |
| X12 | −0.18 | 0.084 | 4.61 | 0.0690 |
| X22 | 0.029 | 0.084 | 0.12 | 0.7436 |
| X32 | 0.21 | 0.084 | 6.33 | 0.0401 |
| X1X2 | 6.381 × 10−3 | 0.086 | 5.511 × 10−3 | 0.9429 |
| X1X3 | −0.096 | 0.086 | 1.26 | 0.2988 |
| X2X3 | 0.074 | 0.086 | 0.74 | 0.4189 |
| R2= 0.9032 | ||||
| Source | Sum of Squares | Degree of Freedom | Mean Square | F-Value | p-Value * |
|---|---|---|---|---|---|
| Carnosic acid (mixing) | |||||
| The recovery | |||||
| Model | 157.64 | 9 | 17.52 | 20.22 | 0.0003 |
| Residual | 6.06 | 7 | 0.87 | ||
| Lack of fit | 3.92 | 3 | 1.31 | 2.44 | 0.2042 |
| Pure error | 2.14 | 4 | 0.54 | ||
| Total | 163.70 | 16 | |||
| Carnosic acid (UAE) | |||||
| The recovery | |||||
| Model | 171.12 | 9 | 19.01 | 5.03 | 0.0224 |
| Residual | 26.48 | 7 | 3.78 | ||
| Lack of fit | 13.99 | 3 | 4.66 | 1.49 | 0.3444 |
| Pure error | 12.49 | 4 | 3.12 | ||
| Total | 197.60 | 16 | |||
| Carnosic acid (MCE) | |||||
| The recovery | |||||
| Model | 47.49 | 9 | 5.28 | 13.17 | 0.0013 |
| Residual | 2.81 | 7 | 0.40 | ||
| Lack of fit | 2.75 | 3 | 0.92 | 64.44 | 0.008 |
| Pure error | 0.057 | 4 | 0.014 | ||
| Total | 50.30 | 16 |
| Source | Sum of Squares | Degree of Freedom | Mean Square | F-Value | p-Value * |
|---|---|---|---|---|---|
| Carnosol (mixing) | |||||
| The recovery | |||||
| Model | 22.28 | 9 | 2.48 | 19.04 | 0.0004 |
| Residual | 0.91 | 7 | 0.13 | ||
| Lack of fit | 0.41 | 3 | 0.14 | 1.09 | 0.4491 |
| Pure error | 0.50 | 4 | 0.13 | ||
| Total | 23.19 | 16 | |||
| Carnosol (UAE) | |||||
| The recovery | |||||
| Model | 18.29 | 9 | 2.03 | 3.18 | 0.0708 |
| Residual | 4.48 | 7 | 0.64 | ||
| Lack of fit | 2.81 | 3 | 0.94 | 2.24 | 0.2256 |
| Pure error | 1.67 | 4 | 0.42 | ||
| Total | 22.77 | 16 | |||
| Carnosol (MCE) | |||||
| The recovery | |||||
| Model | 1.93 | 9 | 0.21 | 7.26 | 0.0080 |
| Residual | 0.21 | 7 | 0.030 | ||
| Lack of fit | 0.17 | 3 | 0.055 | ||
| Pure error | 0.042 | 4 | 0.010 | ||
| Total | 2.14 | 16 |
| Carnosic Acid (µg mg−1) | Mean Value (µg mg−1) | SD | RSD (%) | Carnosol (µg mg−1) | Mean Value (µg mg−1) | SD | RSD (%) | ||
|---|---|---|---|---|---|---|---|---|---|
| Repeatability of measurements | 100.406 | 100.39 | 0.43 | 0.43% | 99.999 | ||||
| 100.042 | 100.004 | ||||||||
| 100.913 | 100.100 | ||||||||
| 100.842 | 99.947 | ||||||||
| 100.305 | 99.977 | ||||||||
| 99.831 | 99.987 | 100.00 | 0.05 | 0.05% | |||||
| Repeatability of solution preparation | 104.65 | 107.46 | 2.40 | 2.24% | 102.231 | ||||
| 108.45 | 101.996 | ||||||||
| 109.22 | 103.260 | ||||||||
| 109.09 | 102.161 | ||||||||
| 109.24 | 101.677 | ||||||||
| 104.13 | 101.996 | 102.22 | 0.54 | 0.53% | |||||
| Added amount of carnosic acid (µg mg−1) | Expected value of carnosic acid (µg mg−1) | Obtained value of carnosic acid (µg mg−1) | Recovery (%) | Added amount of carnosol (µg mg−1) | Expected value of carnosol (µg mg−1) | Obtained value of carnosol (µg mg−1) | Recovery (%) | ||
| Accuracy | Sample | 10.36 | 6.50 | ||||||
| Sample + std 1 (1:1) | 22.570 | 16.461 | 16.426 | 99% | 21.329 | 13.915 | 13.213 | 95% | |
| Sample + std 2 (1:1) | 100.406 | 55.385 | 55.797 | 99% | 99.947 | 53.223 | 52.999 | 99% | |
| Sample + std 3 (1:1) | 212.859 | 111.608 | 107.049 | 96% | 209.221 | 107.860 | 106.995 | 99% |

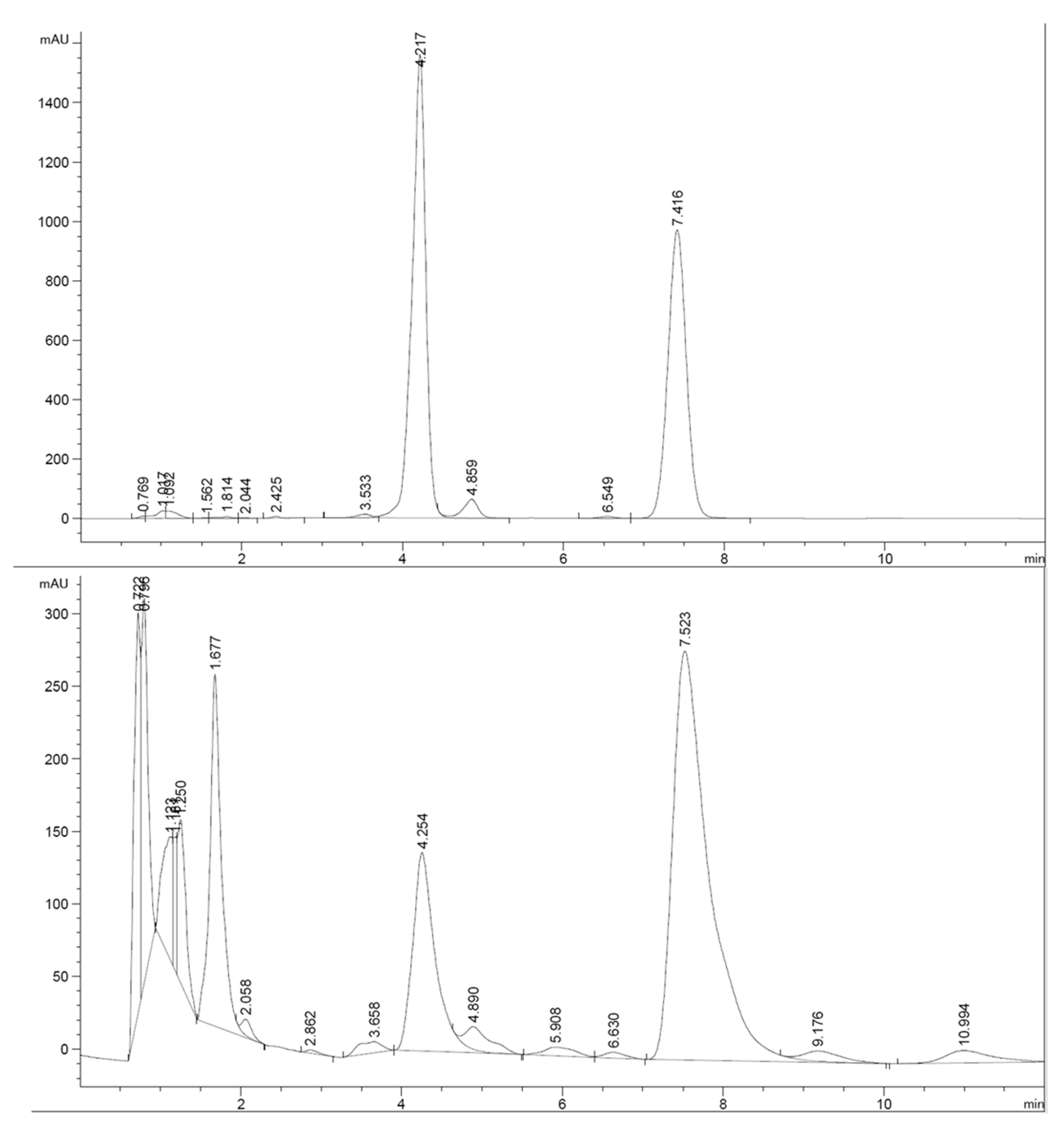
References
- Zainal-Abidin, M.H.; Hayyan, M.; Hayyan, A.; Jayakumar, N.S. New horizons in the extraction of bioactive compounds using deep eutectic solvents: A review. Anal. Chim. Acta 2017, 979, 1–23. [Google Scholar] [CrossRef]
- Bakirtzi, C.; Triantafyllidou, K.; Makris, D.P. Novel lactic acid-based natural deep eutectic solvents: Efficiency in the ultrasound-assisted extraction of antioxidant polyphenols from common native Greek medicinal plants. J. Appl. Res. Med. Aromat. Plants 2016, 3, 120–127. [Google Scholar] [CrossRef]
- Wang, L.; Weller, C.L. Recent advances in extraction of nutraceuticals from plants. Trends Food Sci. Technol. 2006, 17, 300–312. [Google Scholar] [CrossRef]
- Wang, M.; Wang, J.; Zhang, Y.; Xia, Q.; Bi, W.; Yang, X.; Chen, D.D.Y. Fast environment-friendly ball mill-assisted deep eutectic solvent-based extraction of natural products. J. Chromatogr. A 2016, 1443, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.L.; Rasheed, R.K. Deep eutectic solvents formed between choline chloride and carboxylic acids: Versatile alternatives to ionic liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 9, 70–71. [Google Scholar] [CrossRef]
- Radošević, K.; Ćurko, N.; Gaurina Srček, V.; Cvjetko Bubalo, M.; Tomašević, M.; Kovačević Ganić, K.; Radojčić Redovniković, I. Natural deep eutectic solvents as beneficial extractants for enhancement of plant extracts bioactivity. LWT Food Sci. Technol. 2016, 73, 45–51. [Google Scholar] [CrossRef]
- Zhang, C.W.; Xia, S.Q.; Ma, P.S. Facile pretreatment of lignocellulosic biomass using deep eutectic solvents. Bioresour. Technol. 2016, 219, 1–5. [Google Scholar] [CrossRef]
- Min, W.N.; Jing, Z.; Min, S.L.; Ji, H.J.; Lee, J. Enhanced extraction of bioactive natural products using tailor-made deep eutectic solvents: Application to flavonoid extraction from Flossophorae. Green Chem. 2015, 17, 1718–1727. [Google Scholar]
- Cvjetko Bubalo, M.; Vidović, S.; Radojčić Redovniković, I.; Jokić, S. New perspective in extraction of plant biologically active compounds by green solvents. Food Bioprod. Process. 2018, 109, 52–73. [Google Scholar] [CrossRef]
- Bi, W.; Tian, M.; Row, K.H. Evaluation of alcohol based deep eutectic solvent in extraction and determination of flavonoids with response surface methodology optimization. J. Chromatogr. A 2013, 1285, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Rozema, E.; Verpoorte, R.; Hae Choi, Y. Application of natural deep eutectic solvents to the extraction of anthocyanins from Catharanthus roseus with high extractability and stability replacing conventional organic solvents. J. Chromatogr. A 2016, 1434, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Jiao, J.; Gai, Q.Y.; Wang, P.; Guo, N.; Niu, L.L.; Fu, Y.J. Enhanced and green extraction polyphenols and furanocoumarins from Fig (Ficus carica L.) leaves using deep eutectic solvents. J. Pharm. Biomed. Anal. 2017, 145, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Bosiljkov, T.; Dujmić, F.; Cvjetko Bubalo, M.; Hribar, J.; Vidrih, R.; Brnčić, M.; Zlatić, E.; Radojčić Redovniković, I.; Jokić, S. Natural deep eutectic solvents and ultrasound-assisted extraction: Green approaches for extraction of wine lees anthocyanins. Food Bioprod. Process. 2017, 102, 195–203. [Google Scholar] [CrossRef]
- Wang, M.; Wang, J.; Zhou, Y.; Mingyue, Z.; Xia, Q.; Bi, W.; Chen, D.D.Y. Ecofriendly Mechanochemical Extraction of Bioactive Compounds from Plants with Deep Eutectic Solvents. ACS Sustain. Chem. Eng. 2017, 5, 6297–6303. [Google Scholar] [CrossRef]
- Aruoma, O.I.; Halliwell, B.; Aeschbach, R.; Loligers, J. Antioxidant and prooxidant properties of active rosemary constituents: Carnosol and carnosic acid. Xenobiotica 1992, 22, 257–268. [Google Scholar] [CrossRef]
- Tsai, C.W.; Lin, C.Y.; Wang, Y.J. Carnosic acid induces the NAD(P)H: Quinone oxidoreductase 1 expression in rat clone 9 cells through the p38/nuclear factor erythroid-2 related factor 2 pathway. J. Nutr. 2011, 141, 2119–2125. [Google Scholar] [CrossRef]
- Kontogianni, V.G.; Tomic, G.; Nikolic, I.; Nerantzaki, A.A.; Sayyad, N.; Stosic-Grujicic, S.; Stojanovic, I.; Gerothanassis, I.P.; Tzakos, A.G. Phytochemical profile of Rosmarinus officinalis and Salvia officinalis extracts and correlation to their antioxidant and anti-proliferative activity. Food Chem. 2013, 136, 120–129. [Google Scholar] [CrossRef]
- Lopez-Jimenez, A.; Garcia-Caballero, M.; Medina, M.A.; Quesada, A.R. Antiangiogenic properties of carnosol and carnosic acid, two major dietary compounds from rosemary. Eur. J. Nutr. 2013, 52, 85–95. [Google Scholar] [CrossRef]
- Georgantzi, C.; Lioliou, A.E.; Paterakis, N.; Makris Dimitris, P. Combination of Lactic Acid-Based Deep Eutectic Solvents (DES) with β-Cyclodextrin: Performance Screening Using Ultrasound-Assisted Extraction of Polyphenols from Selected Native Greek Medicinal Plants. Agronomy 2017, 7, 54. [Google Scholar] [CrossRef]
- Okamura, N.; Fujimoto, Y.; Kuwabara, S.; Yagi, A. High-performance liquid chromatographic determination of carnosic acid and carnosol in Rosmarinus oficinalis and Salvia oficinalis. J. Chromatogr. A 1994, 679, 381–386. [Google Scholar] [CrossRef]
- Vinatoru, M. An overview of the ultrasonically assisted extraction of bioactive principles from herbs. Ultrason. Sonochem. 2001, 8, 303–313. [Google Scholar] [CrossRef]
- Fatma Ebru, K.; Ayse, A.; Caglar, K. Extraction and HPLC Analysis of Sage (Salvia officinalis) Plant. Nat. Prod. Res. 2018, 5, 298. [Google Scholar]
- Durling, N.E.; Catchpole, O.J.; Grey, J.B.; Webby, R.F.; Mitchell, K.A.; Foo, L.Y.; Perry, N.B. Extraction of phenolics and essential oil from dried sage (Salvia officinalis) using ethanol–water mixtures. Food Chem. 2007, 101, 1417–1424. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green extraction of natural products: Concept and principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef] [PubMed]
- Castańeda-Ovando, A.; Pacheco-Hernández, M.; Páez-Hernández, M.E.; Rodríguez, J.A.; Galán-Vidal, C.A. Chemical studies of anthocyanins: A review. Food Chem. 2009, 113, 859–871. [Google Scholar] [CrossRef]
- Abreu, M.E.; Müller, M.; Alegre, L.; Munné-Bosch, S. Phenolic diterpene and α-tocopherol contents in leaf extracts of 60 Salvia species. J. Sci. Food Agric. 2008, 88, 2648–2653. [Google Scholar] [CrossRef]
- Shan, B.; Cai, Y.Z.; Sun, M.; Corke, H. Antioxidant Capacity of 26 Spice Extracts and Characterization of Their Phenolic Constituents. J. Sci. Food Agric. 2005, 53, 7749–7759. [Google Scholar] [CrossRef]
- Lamien-Meda, A.; Nell, M.; Lohwasser, U.; Börner, A.; Franz, C.; Novak, J. Investigation of Antioxidant and Rosmarinic Acid Variation in the Sage Collection of the Genebank in Gatersleben. J. Agric. Food Chem. 2010, 58, 3813–3819. [Google Scholar] [CrossRef]
- Juntachote, T.; Berghofer, E.; Bauer, F.; Siebenhand, S. The application of response surface methodology to the production of phenolic extracts of lemon grass, galangal, holy basil and rosemary. Int. J. Food Sci. Technol. 2006, 41, 121–133. [Google Scholar] [CrossRef]
- Tena, M.T.; Valcarcel, M.; Hidalgo, P.J.; Ubera, J.L. Supercritical Fluid Extraction of Natural Antioxidants from Rosemary: Comparison with Liquid Solvent Sonication. Anal. Chem. 1997, 69, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Cháfer, A.; Fornari, T.; Berna, A.; Ibañez, E.; Reglero, G. Solubility of solid carnosic acid in supercritical CO2 with ethanol as a co-solvent. J. Supercrit. Fluid 2005, 34, 323–329. [Google Scholar] [CrossRef]
- Pavić, V.; Jakovljević, M.; Molnar, M.; Jokić, S. Extraction of Carnosic Acid and Carnosol from Sage (Salvia officinalis L.) Leaves by Supercritical Fluid Extraction and Their Antioxidant and Antibacterial Activity. Plants 2019, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Babovic, N.; Djilas, S.; Jadranin, M.; Vajs, V.; Ivanovic, J.; Petrovic, S.; Zizovic, I. Supercritical carbon dioxide extraction of antioxidant fractions from selected Lamiaceae herbs and their antioxidant capacity. Innov. Food Sci. Emerg. Technol. 2010, 11, 98–107. [Google Scholar] [CrossRef]
- Jokić, S.; Molnar, M.; Jakovljević, M.; Aladić, K.; Jerković, I. Optimization of supercritical CO2 extraction of Salvia officinalis L. leaves targeted on Oxygenated monoterpenes, α-humulene, viridiflorol and manool. J. Supercrit. Fluid 2018, 133, 253–262. [Google Scholar] [CrossRef]
- Molnar, M.; Jakovljević, M.; Jokić, S. Optimization of the process conditions for the extraction of rutin from Ruta graveolens L. by choline chloride based deep eutectic solvents. Solvent Extr. Res. Dev. 2018, 25, 109–116. [Google Scholar] [CrossRef]
- Bas, D.; Boyaci, I.H. Modeling and optimization I: Usability of response surface methodology. J. Food Eng. 2007, 78, 836–845. [Google Scholar] [CrossRef]

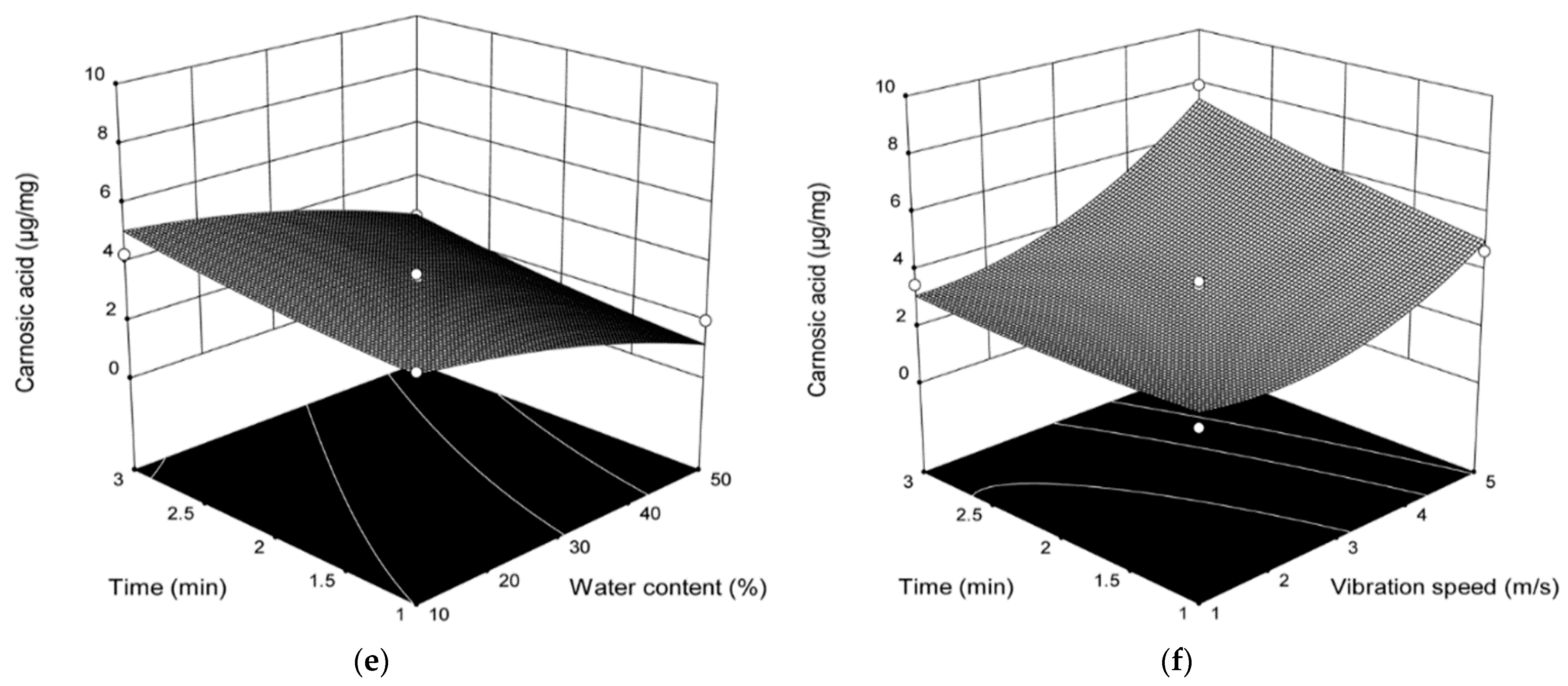

| Run | H2O (%) | Time (min) | Temperature (°C) | DES-MIXING | DES-UAE | ||
|---|---|---|---|---|---|---|---|
| Carnosic Acid (µg mg−1) | Carnosol (µg mg−1) | Carnosic Acid (µg mg−1) | Carnosol (µg mg−1) | ||||
| 1 | 30 | 60 | 50 | 3.86 ± 0.82 | 1.99 ± 0.05 | 7.37 ± 0.34 | 3.06 ± 0.21 |
| 2 | 10 | 90 | 50 | 14.43 ± 0.28 | 4.83 ± 0.09 | 9.94 ± 0.22 | 3.92 ± 0.09 |
| 3 | 10 | 60 | 70 | 10.16 ± 0.05 | 3.90 ± 0.37 | 14.00 ± 0.02 | 4.18 ± 0.05 |
| 4 | 30 | 60 | 50 | 4.53 ± 0.49 | 2.27 ± 0.09 | 4.18 ± 0.21 | 2.22 ± 0.14 |
| 5 | 30 | 60 | 50 | 5.16 ± 0.45 | 2.70 ± 0.17 | 5.04 ± 0.05 | 2.46 ± 0.29 |
| 6 | 10 | 30 | 50 | 8.71 ± 0.26 | 2.10 ± 0.06 | 8.48 ± 0.06 | 3.34 ± 0.10 |
| 7 | 30 | 30 | 70 | 5.64 ± 0.11 | 2.51 ± 0.22 | 1.62 ± 0.29 | 0.70 ± 0.02 |
| 8 | 50 | 90 | 50 | 3.16 ± 0.14 | 1.34 ± 0.21 | 2.09 ± 0.37 | 0.84 ± 0.29 |
| 9 | 30 | 90 | 70 | 7.41 ± 0.06 | 4.79 ± 0.38 | 8.06 ± 0.13 | 2.93 ± 0.02 |
| 10 | 10 | 60 | 30 | 10.68 ± 0.66 | 2.35 ± 0.03 | 2.66 ± 0.41 | 0.83 ± 0.38 |
| 11 | 50 | 60 | 70 | 3.87 ± 0.19 | 1.85 ± 0.15 | 3.69 ± 0.24 | 1.85 ± 0.11 |
| 12 | 50 | 30 | 50 | 2.68 ± 0.26 | 0.81 ± 0.01 | 2.00 ± 0.07 | 0.82 ± 0.13 |
| 13 | 30 | 60 | 50 | 5.65 ± 0.36 | 1.99 ± 0.09 | 4.37 ± 0.09 | 2.25 ± 0.17 |
| 14 | 50 | 60 | 30 | 2.55 ± 0.04 | 0.89 ± 0.03 | 1.94 ± 0.21 | 0.92 ± 0.09 |
| 15 | 30 | 60 | 50 | 5.44 ± 0.21 | 1.79 ± 0.11 | 2.46 ± 0.05 | 1.27 ± 0.16 |
| 16 | 30 | 90 | 30 | 5.27 ± 0.18 | 1.70 ± 0.08 | 2.80 ± 0.25 | 1.22 ± 0.12 |
| 17 | 30 | 30 | 30 | 5.37 ± 0.01 | 1.50 ± 0.19 | 2.18 ± 0.23 | 0.56 ± 0.02 |
| Run | H2O (%) | Time (min) | Vibration Speed (m s−1) | Carnosic Acid (µg mg−1) | Carnosol (µg mg−1) |
|---|---|---|---|---|---|
| 1 | 30 | 3 | 1 | 3.50 ± 0.35 | 1.25 ± 0.02 |
| 2 | 30 | 2 | 3 | 3.38 ± 0.20 | 1.23 ± 0.06 |
| 3 | 50 | 3 | 3 | 2.51 ± 0.34 | 0.86 ± 0.02 |
| 4 | 50 | 1 | 3 | 2.01 ± 0.16 | 0.71 ± 0.04 |
| 5 | 50 | 2 | 5 | 2.98 ± 0.02 | 1.03 ± 0.13 |
| 6 | 10 | 3 | 3 | 4.29 ± 0.25 | 1.16 ± 0.04 |
| 7 | 50 | 2 | 1 | 1.80 ± 0.02 | 0.57 ± 0.11 |
| 8 | 10 | 2 | 1 | 3.37 ± 0.17 | 1.02 ± 0.20 |
| 9 | 10 | 1 | 3 | 4.06 ± 0.26 | 1.04 ± 0.37 |
| 10 | 30 | 1 | 5 | 4.69 ± 0.61 | 1.27 ± 0.11 |
| 11 | 30 | 2 | 3 | 3.31 ± 0.42 | 1.01 ± 0.09 |
| 12 | 10 | 2 | 5 | 8.26 ± 0.45 | 1.87 ± 0.33 |
| 13 | 30 | 2 | 3 | 3.63 ± 0.18 | 1.14 ± 0.01 |
| 14 | 30 | 2 | 3 | 3.45 ± 0.28 | 1.09 ± 0.12 |
| 15 | 30 | 1 | 1 | 2.45 ± 0.07 | 0.79 ± 21 |
| 16 | 30 | 2 | 3 | 3.41 ± 0.24 | 0.98 ± 0.03 |
| 17 | 30 | 3 | 5 | 7.92 ± 0.29 | 2.02 ± 0.14 |
| Solvent | Time (min) | Temperature (°C) | Carnosic Acid (µg mg−1) | Carnosol (µg mg−1) |
|---|---|---|---|---|
| 30% ethanol (v/v) | 30 | 30 | 1.11 ± 0.03 | 2.63 ± 0.21 |
| 60 | 1.33 ± 0.55 | 2.26 ± 0.02 | ||
| 90 | 1.45 ± 0.53 | 2.18 ± 0.20 | ||
| 30 | 50 | 2.32 ± 0.07 | 2.04 ± 0.05 | |
| 60 | 2.64 ± 0.04 | 1.99 ± 0.04 | ||
| 90 | 2.26 ± 0.30 | 1.51 ± 0.00 | ||
| 30 | 70 | 2.82 ± 0.03 | 2.92 ± 0.16 | |
| 60 | 2.13 ± 0.14 | 2.92 ± 0.311 | ||
| 90 | 0.93 ± 0.17 | 2.27 ± 0.38 | ||
| 50% ethanol (v/v) | 30 | 30 | 5.91 ± 0.19 | 4.65 ± 0.22 |
| 60 | 3.07 ± 0.39 | 9.31 ± 0.29 | ||
| 90 | 3.02 ± 0.15 | 9.73 ± 0.86 | ||
| 30 | 50 | 7.17 ± 0.05 | 8.39 ± 0.47 | |
| 60 | 3.15 ± 0.02 | 9.06 ± 0.11 | ||
| 90 | 2.11 ± 0.15 | 11.25 ± 0.35 | ||
| 30 | 70 | 7.63 ± 0.44 | 6.73 ± 0.38 | |
| 60 | 4.43 ± 0.20 | 8.79 ± 0.79 | ||
| 90 | 1.91 ± 0.00 | 11.23 ± 0.13 | ||
| 70% ethanol (v/v) | 30 | 30 | 8.28 ± 0.53 | 3.04 ± 0.01 |
| 60 | 7.40 ± 0.05 | 4.82 ± 0.29 | ||
| 90 | 7.63 ± 0.0 | 5.93 ± 0.22 | ||
| 30 | 50 | 7.73 ± 0.22 | 6.17 ± 0.59 | |
| 60 | 8.54 ± 0.28 | 7.21 ± 0.44 | ||
| 90 | 8.71 ± 0.28 | 5.43 ± 0.51 | ||
| 30 | 70 | 8.73 ± 0.14 | 4.37 ± 0.07 | |
| 60 | 7.53 ± 0.06 | 6.55 ± 0.22 | ||
| 90 | 6.85 ± 0.32 | 6.89 ± 0.26 | ||
| ethanol | 30 | 30 | 11.21 ± 0.51 | 2.72 ± 0.27 |
| 60 | 11.13 ± 0.13 | 3.57 ± 0.25 | ||
| 90 | 12.77 ± 0.22 | 2.83 ± 0.06 | ||
| 30 | 50 | 11.74 ± 0.09 | 3.57 ± 0.47 | |
| 60 | 12.80 ± 0.19 | 3.14 ± 0.06 | ||
| 90 | 13.64 ± 0.10 | 3.47 ± 0.01 | ||
| 30 | 70 | 13.36 ± 0.37 | 4.46 ± 0.11 | |
| 60 | 12.27 ± 0.11 | 3.29 ± 0.21 | ||
| 90 | 11.27 ± 0.05 | 3.11 ± 0.09 | ||
| methanol | 30 | 30 | 8.71 ± 0.87 | 2.88 ± 0.48 |
| 60 | 9.26 ± 0.06 | 4.44 ± 0.19 | ||
| 90 | 10.50 ± 0.58 | 4.69 ± 0.58 | ||
| 30 | 50 | 9.68 ± 0.25 | 5.03 ± 0.08 | |
| 60 | 11.85 ± 0.05 | 5.24 ± 0.07 | ||
| 90 | 10.72 ± 0.30 | 4.85 ± 0.40 | ||
| 30 | 70 | 9.69 ± 0.58 | 3.67 ± 0.24 | |
| 60 | 10.11 ± 0.43 | 4.41 ± 0.10 | ||
| 90 | 10.41 ± 0.12 | 4.80 ± 0.03 | ||
| H2O | 30 | 30 | 0.00 | 0.00 |
| 60 | 0.00 | 0.24 ± 0.00 | ||
| 90 | 0.00 | 0.24 ± 0.00 | ||
| 30 | 0.00 | 0.29 ± 0.01 | ||
| 60 | 0.00 | 0.27 ± 0.00 | ||
| 90 | 0.75 ± 0.03 | 0.24 ± 0.00 | ||
| 30 | 0.00 | 0.26 ± 0.03 | ||
| 60 | 0.00 | 0.25 ± 0.01 | ||
| 90 | 0.74 ± 0.00 | 0.42 ± 0.02 |
| Name | Combination | Molar Ratio |
|---|---|---|
| ChClU | Choline chloride:urea | 1:2 |
| ChClmU | Choline chloride:N-methylurea | 1:3 |
| ChCltU | Choline chloride:thiourea | 1:2 |
| ChClG | Choline chloride:glucose | 1:1 |
| ChClF | Choline chloride:fructose | 1:1 |
| ChClX | Choline chloride:xylitol | 1:1 |
| ChClS | Choline chloride:sorbitol | 1:1 |
| ChClB | Choline chloride:butane-1,4-diol | 1:2 |
| ChClE | Choline chloride:ethane-1,2-diol | 1:2 |
| ChClGl | Choline chloride:glycerol | 1:2 |
| ChClA | Choline chloride:acetamide | 1:2 |
| ChClM | Choline chloride:malic acid | 1:1 |
| ChClC | Choline chloride:citric acid | 1:1 |
| ChClMa | Choline chloride:malonic acid | 1:1 |
| ChClO | Choline chloride:oxalic acid | 1:1 |
| ChClLa | Choline chloride:lactic acid | 1:2 |
| ChClL | Choline chloride:levulinic acid | 1:1 |
| Type of Extraction | Independent Variable | Symbol | Level | ||
|---|---|---|---|---|---|
| Low (−1) | Middle (0) | High (+1) | |||
| Stirring and heating | H2O (%) | X1 | 10 | 30 | 50 |
| Time (min) | X2 | 30 | 60 | 90 | |
| Temperature (°C) | X3 | 30 | 50 | 70 | |
| Ultrasound assisted extraction | H2O (%) | X1 | 10 | 30 | 50 |
| Time (min) | X2 | 30 | 60 | 90 | |
| Temperature (°C) | X3 | 30 | 50 | 70 | |
| Mechanochemical extraction | H2O (%) | X1 | 10 | 30 | 50 |
| Time (min) | X2 | 1 | 2 | 3 | |
| Vibration speed (ms−1) | X3 | 1 | 3 | 5 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakovljević, M.; Jokić, S.; Molnar, M.; Jerković, I. Application of Deep Eutectic Solvents for the Extraction of Carnosic Acid and Carnosol from Sage (Salvia officinalis L.) with Response Surface Methodology Optimization. Plants 2021, 10, 80. https://doi.org/10.3390/plants10010080
Jakovljević M, Jokić S, Molnar M, Jerković I. Application of Deep Eutectic Solvents for the Extraction of Carnosic Acid and Carnosol from Sage (Salvia officinalis L.) with Response Surface Methodology Optimization. Plants. 2021; 10(1):80. https://doi.org/10.3390/plants10010080
Chicago/Turabian StyleJakovljević, Martina, Stela Jokić, Maja Molnar, and Igor Jerković. 2021. "Application of Deep Eutectic Solvents for the Extraction of Carnosic Acid and Carnosol from Sage (Salvia officinalis L.) with Response Surface Methodology Optimization" Plants 10, no. 1: 80. https://doi.org/10.3390/plants10010080
APA StyleJakovljević, M., Jokić, S., Molnar, M., & Jerković, I. (2021). Application of Deep Eutectic Solvents for the Extraction of Carnosic Acid and Carnosol from Sage (Salvia officinalis L.) with Response Surface Methodology Optimization. Plants, 10(1), 80. https://doi.org/10.3390/plants10010080








